Abstract
Gut dysbacteriosis is closely related to various intestinal and extraintestinal diseases. Fecal microbiota transplantation (FMT) is a biological therapy that entails transferring the gut microbiota from healthy individuals to patients in order to reconstruct the intestinal microflora in the latter. It has been proved to be an effective treatment for recurrent Clostridium difficile infection. Studies show that the gut microbiota plays an important role in the pathophysiology of neurological and psychiatric disorders through the microbiota-gut-brain axis. Therefore, reconstruction of the healthy gut microbiota is a promising new strategy for treating cerebral diseases. We have reviewed the latest research on the role of gut microbiota in different nervous system diseases as well as FMT in the context of its application in neurological, psychiatric, and other nervous system-related diseases (Parkinson's disease, Alzheimer's disease, multiple sclerosis, epilepsy, autism spectrum disorder, bipolar disorder, hepatic encephalopathy, neuropathic pain, etc.).
1. Introduction
The gut microbiota is often considered an “invisible organ” that significantly affects human health and disease. More than 100 trillion microorganisms have been found in the human gastrointestinal (GI) tract, which encodes close to 3,000,0000 genes compared to the 23,000 genes within the human genome [1, 2], and are crucial for maintaining the balance between different physiological activities. The cross-talk between the GI tract and the central nervous system, commonly known as the gut-brain axis, plays an important role in the pathophysiology of neurological diseases. Studies increasingly show that dysbacteriosis can lead to or exacerbate various neurological and psychiatric disorders, such as Parkinson's disease [3, 4], Alzheimer's disease [5–7], autism spectrum disorder [8], multiple sclerosis [9], and epilepsy [10]. In addition, patients with neurological dysfunction often present GI symptoms [11], which underscores the causative role of the gut in neuropathological progression and provides a solid rationale for therapeutically targeting the gut microbiota in these diseases (Table 1). The current therapies targeting the intestinal microbiota include the use of antibiotics, probiotics, prebiotics, synbiotics, and fecal microbiota transplantation (FMT) that entails transplanting functional microbiota from healthy individuals into the GI tracts of patients. FMT can reconstruct the healthy gut microecology and improve clinical symptoms. Apart from its direct therapeutic effect in GI diseases, FMT has also been shown to improve neurological and psychological symptoms by modulating the gut-brain axis (Figure 1) [12]. In this review, we have summarized the gut microbiota in different nervous system diseases as well as the current applications of FMT in various neurological and psychiatric diseases and discussed the potential mechanisms and future directions.
Table 1.
Characteristics, consequences, and application level of FMT in neuropsychological diseases.
| Disease types | Alterations of gut microbiota | Altered substances caused by microbial dysbiosis | Application level of FMT | References |
|---|---|---|---|---|
| Neurological diseases | ||||
| Parkinson's disease | Increase in Verrucomicrobiaceae, Ruminococcaceae, Proteobacteria, Clostridiaceae, Enterobacteriaceae, Bifidobacteriaceae, Lactobacillaceae, Pasteurellaceae, Christensenellaceae, Lactobacilli, Akkermansia, Ralstonia Decrease in Firmicutes, Prevotellaceae, Coprococcus, Bacteroides fragilis, Blauti, Roseburia, Faecalibacterium |
α-Synuclein, LPS, SCFAs, hydrogen production | Patient & animal | [4, 13, 14] |
| Alzheimer's disease | Increase in Escherichia, Shigella, Chlamydia pneumoniae, Borrelia burgdorferi, Treponema pallidum, Burkholderiaceae, Staphylococcaceae, Porphyromonas gingivalis, Propionibacterium acnes Decrease in Eubacterium rectale, Bacteroides fragilis |
Inflammatory cytokines (IL-6, CXCL2, NLRP3, IL-1β, IL-10), Aβ, GABA, BDNF, DHA | Patient & animal | [5, 6, 13, 15] |
| Multiple sclerosis | Increase in Firmicutes, Clostridium, Escherichia Shigella Decrease in Bacteroides, Faecalibacterium, Eubacterium rectale, Corynebacterium, Fusobacteria |
Proinflammatory cytokines, butyrate, lipid 654 | Patient & animal | [13] |
| Epilepsy | Increase in Firmicutes, Proteobacteria, Clostridium, Cronobacter, Akkermansia, Ruminococcus, Coprobacillus, Clostridium XVIII, Atopobium, Holdemania, Dorea, Saccharibacteria, Delftia, Paraprevotella, Gemmiger, Neisseria, Coprococcus, Fusobacterium, Methanobrevibacter, Phascolarctobacterium, Roseburia Decrease in Bacteroidetes, Actinobacteria, Prevotella, Bifidobacterium |
Proinflammatory cytokines (TNFα, IL-6, IL-1β), dopamine receptors D1 and D2 | Patient & animal | [16, 17] |
| Tourette Syndrome | Increase in Bacteroidetes; in particular, Bacteroides, Odoribacter, and Oscillospira were identified as potential microbial biomarkers | SCFAs, D-alanine, tyrosine, dopamine | Patient & animal | [18] |
| Myalgic encephalomyelitis/chronic fatigue syndrome | Increase in Roseburia, Holdemania, Enterococcus, Streptococcus spp. Decrease in most Bacteroidetes genera. |
Lactic acid, LPS, LPS-binding protein, soluble CD14, oxidative stress | Patient & animal | [19–21] |
| Guillain-Barré Syndrome | Campylobacter jejuni infection is associated with GBS while Enterococcus faecalis as a potential protective role | LPS, peripheral nerve gangliosides | Animal | [22, 23] |
| Stroke | Decreased neuronal injury and improved cognitive performance were observed in diabetic mice with bilateral common carotid arteries occlusion after receiving Clostridium butyricum | Trimethylamine N-oxide | Animal | [24, 25] |
| Amyotrophic lateral sclerosis | Increase in Dorea Decrease in Butyrivibrio fibrisolvens, Firmicutes, Peptostreptococcus, Escherichia coli, Oscillibacter, Anaerostipes, Lachnospira |
Butyrate | Animal | [26–28] |
| Huntington's disease | Increase in Bacteroidetes Decrease in Firmicutes, Lachnospiraceae, Akkermansiaceae |
Methionine, glycine | Animal | [29, 30] |
| Psychiatric diseases | ||||
| Autism spectrum disorder | Increase in Bacteroides, Barnesiella, Clostridium, Roseburia Decrease in Bifidobacterium, Coprococcus, Dialister, Faecalibacterium, Prevotella, Streptococcus |
Butyrate, lactate | Patient & animal | [31–33] |
| Bipolar disorder | Increase in Bacteroidetes, Actinobacteria, Coriobacteria, Lachnospira, Enterobacteriaceae, Flavonifractor Decrease in Firmicutes, Ruminococcaceae, Roseburia, Faecalibacterium, Coprococcus |
Butyrate | Patient & animal | [34–37] |
| Depression | Increase in Enterobacteriaceae, Prevotella, Klebsiella, Alistipes Decrease in Lachnospiraceae, Faecalibacterium, Coprococcus, Dialister, Ruminococcus, Lactobacillus, Bifidobacterium |
Butyrate, inflammatory cytokines | Patient & animal | [36, 38, 39] |
| Anxiety | Increase in Fusobacterium, Ruminococcus, Escherichia Shigella Decrease in Faecalibacterium, Eubacterium, Sutterella |
Animal | [40, 41] | |
| Other system-related neurological diseases | ||||
| Hepatic encephalopathy | Increases in Enterobacteriaceae, Streptococcaceae, Porphyromonadaceae, Staphylococcaceae, Enterococcaceae Decrease in Lachnospiraceae, Ruminococcaceae, Rikenellaceae, Clostridium XIV, Phascolarctobacterium |
Ammonia, urease, SCFAs, aromatic amino acids | Patient & Animal | [42, 43] |
| Neuropathic pain | Associated: Lactobacillus fermentum KBL374 & KBL375, Bacteroides fragilis, Escherichia coli, Lactobacillus, Streptococcus spp., Enterococcus spp., Corynebacterium glutamicum, Peptostreptococcus, Clostridium sporogenes | LPS, bacterial flagellin, indole, SCFAs, PUFAs, BAs | Patient & animal | [44] |
| Sepsis-associated encephalopathy | Associated: absence of anaerobes, including Staphylococcus species and Escherichia coli, with CDI, high relative abundance of pathogenic gram negatives, and Enterococci | LPS, SCFAs, BAs | Patient & animal | [45] |
LPS: lipopolysaccharide; SCFAs: short-chain fatty acids; IL-6: interleukin-6; CXCL2: C-X-C motif chemokine ligand 2; NLRP3: recombinant NLR family, pyrin domain containing protein 3; IL-1β: interleukin-1β; IL-10: interleukin-10; Aβ: amyloid β-protein; GABA: γ-aminobutyric acid; BDNF: brain-derived neurotrophic factor; DHA: docosahexaenoic acid; TNFα: tumor necrosis factor-α; PUFAs: polyunsaturated fatty acid; Bas: bile acids.
Figure 1.
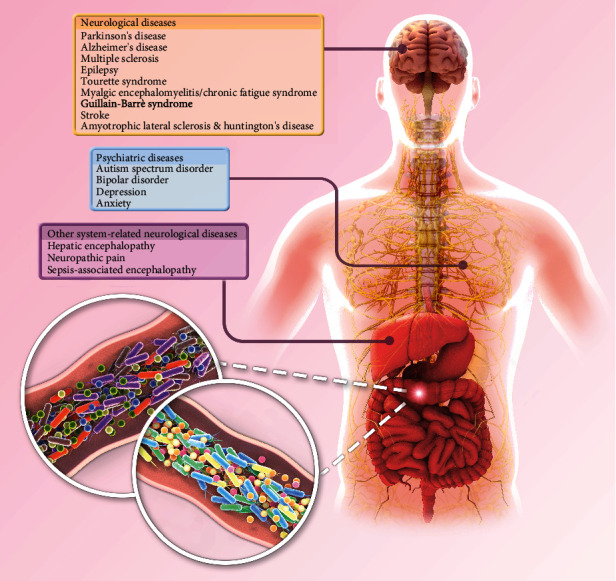
Current applications of FMT in various neurological and psychiatric diseases. Normal gut microbiota plays an important role in maintaining the functional stability of the gut-brain axis. Excessive reproduction of pathogenic bacteria or reduction of probiotics can lead to gut microbiota disorder and mediate a variety of neurological and psychological diseases. As an important therapeutic method to reconstruct gut microbiota, FMT has been tried to be applied to a variety of diseases related to gut-brain axis.
2. Neurological Diseases
Studies [46–48] show that the GI tract and resident microbiota are susceptible to the neurological dysfunction associated with Parkinson's disease, Alzheimer's disease, multiple sclerosis, epilepsy, and stroke. The gut-brain axis is adversely affected by the destruction of intestinal epithelial barrier, loss of intestinal neurons, and overproduction of proinflammatory cytokines. In addition, gut microbial abundance and diversity undergo significant changes during neurological disorders, especially that of bacteria producing anti-inflammatory factors. FMT can significantly adjust the richness of intestinal species and restore the proportion of anti-inflammatory bacteria and is therefore increasingly being considered for treating diseases of the nervous system (Table 2).
Table 2.
Clinical application for FMT based on the gut-brain axis.
| Disease type | Studies | Study type | N | Location | Age | Sex | Complication | Administration route | FMT frequency | Donor | Clinical outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurological diseases | |||||||||||
| Parkinson's disease | Huang et al. [49] | Case report | 1 | China | 71 | M | PD, constipation | TET tube (colon) | 3 times | Healthy volunteer | Constipation cured, PD symptoms relieved for 2 months |
| Alzheimer's disease | Hazan [50] | Case report | 1 | USA | 82 | M | AD, rCDI | Colonoscopy | Once | His wife | MMSE score increased from 20 to 29 |
| Multiple sclerosis | Borody et al. [51] | Case series | 3 | Australia | 30/29/80 | M/M/F | MS, constipation, vertigo, impaired concentration/MS, constipation/MS, constipation, proctalgia fugax, difficulty in walking | NA | 5 FMTs/10 FMTs/5 FMTs | NA | Constipation resoluted, MS improved, 15 years post-FMT without relapse/constipation resolved, neurological symptoms improved, 3 years maintained normal motor/bowel symptoms resoluted, neurological improved |
| Makkawi et al. [52] | Case report | 1 | Canada | 61 | F | MS, rCDI | Enema | Once | Her partner | rCDI resolved, prevented MS progression for over 10 years | |
| Epilepsy | He et al. [53] | Case report | 1 | China | 22 | F | Epilepsy, CD | TET tube (colon) | 3 times | Healthy volunteer | Seizure-free without antiepileptic drugs, decreasing CDAI to 104 points after 12 months and maintained until the end of 20-month follow-up |
| Tourette Syndrome | Zhao et al. [54] | Case report | 1 | China | 9 | M | TS | Gastroscope & colonoscopy | Once | Healthy volunteer | YGTSS-total tic score decreased from 31 to 5, motor severity score fell from 16 to 5, vocal severity score fell from 15 to 0, shifting from severe to mild |
| Ding et al. [55] | Open-label clinical trial | 11 | China | 19.2 ± 7.4 | M | TS | TET tube (nasojejunal) | 3 times | Healthy volunteer | 45.5% (5/11), 45.5% (5/11) and 36.4% (4/11) of patients achieved improvement (≥30% reduction in YGTSS-total tic score) at week 1, week 4, and week 8 post-FMT, respectively. GTS-QoL score decreased at week 8 post-FMT | |
| Myalgic encephalomyelitis/chronic fatigue syndrome | Borody et al. [56] | Larger cohort study | 60 | Australia | 55.0 ± 11.5 | 36 F, 24 M | CFS (52 with IBS, 4 with constipation) | Single TC infusion (n = 5), two-day infusion (TC and enema, n = 52), three-day infusion (TC, 2-day enema, n = 3) | Once/twice/3 times | 13 nonpathogenic enteric bacteria from healthy individual | 35/60 patients responded after single FMT while7 patients responded after secondary FMT, giving a total of 42/60 improved patients |
| Psychiatric diseases | |||||||||||
| Autism spectrum disorder | Ward et al. [57] | Case series | 9 | Canada | 7.7 ± 5.4 | NA | ASD | Capsules & enema | Twice | Healthy volunteer | ASD symptoms were not changed in the 21-year-old subject, while markedly improving in 1 of two 8-year-old subjects |
| Zhao et al. [58] | Open-label, randomized waitlist-controlled trial | 48 | China | NA | NA | ASD | Gastroscope & colonoscopy | Twice | Healthy volunteer | CARS score in the FMT group showed a statistically 10.8% decrease compared to a 0.8% decrease in the waitlist group after the first FMT and remained marginally reduced after the second FMT | |
| Kang et al. [32] | Open-label clinical trial | 18 | USA | 7 to 16 years | NA | ASD | Oral vs. enema | For 7–8 weeks | Healthy volunteer | 80% reduction of GI symptoms post-FMT, ASD symptoms improved significantly and remained improved 8 weeks post-FMT | |
| Kang et al. [32] | Follow-up of a clinical trial | 18 | USA | 7 to 16 years | NA | ASD | Oral vs. enema | For 7–8 weeks | Healthy volunteer | Two years post-FMT, most GI symptom improvements continued, and autism-related symptoms improved even more | |
| Bipolar disorder | Hinton [59] | Case report | 1 | Australia | 33 | F | BD | NA | 9 FMTs over a period of 2 months | Her husband | Symptom-free from depression |
| Depression | Cai et al. [60] | Case report | 1 | China | 79 | F | MDD | Gastroscope | Once | Her grandson | PHQ-9 scores improved |
| Other system-related neurological diseases | |||||||||||
| Hepatic encephalopathy | Kao et al. [61] | Case report | 1 | Canada | 57 | M | Liver cirrhosis, HE | Colonoscopy & enema | 5 FMTs | Healthy volunteer | Stoop test, serum ammonia, and quality of life all significantly improved; appetite, alertness and overall well-being improved |
| Bajaj et al. [62] | Open-label, randomized clinical trial | 20 | USA | 64.5 ± 5.1 (FMT) vs. 62.9 ± 9.8 (SOC) | M | Liver cirrhosis, HE | Enema | Once | Healthy volunteer | Significantly improved in PHES total score and EncephalApp Stroop in the FMT group | |
| Bajaj et al. [63] | A phase 1, randomized, placebo-controlled trial | 20 | USA | 63.3 ± 4.2 (FMT) vs. 64.2 ± 6.2 (SOC) | 16 M, 4 F | Liver cirrhosis, HE | Capsules | 15 capsules of FMT/placebo | Healthy volunteer | EncephalApp improved | |
| Neuropathic pain | Cai et al. [64] | Case report | 1 | China | 46 | F | Diabetic neuropathy | Colonoscopy | Twice | Healthy volunteer | The glycemic control improved, with a remarkable relief of the symptoms of painful DN |
| Sepsis | Li et al. [65] | Case report | 1 | China | 29 | F | Bacteremia, shock | Nasoduodenal tube | Once | Healthy volunteer | Fever went down, and the stool output had a marked reduction |
| Li et al. [66] | Case report | 1 | China | 44 | F | Shock, respiratory failure, AKI | Nasoduodenal tube | Once | Healthy volunteer | Patient's septic symptoms and severe diarrhea were successfully controlled | |
| Wei et al. [67] | Case report | 2 | China | 65/84 | M | Shock, respiratory failure, bacteremia, AKI | Nasoduodenal tube | Once | Healthy volunteer | MODS and severe diarrhea were alleviated in both patients | |
| Gopalsamy et al. [68] | Case report | 1 | Georgia | 57 | M | MDRO infection, respiratory failure | PEG tube | Once | NA | Death (not due to FMT) | |
FMT: fecal microbiota transplantation; M: male; F: female; PD: Parkinson's disease; TET: transendoscopic enteral tubing; MMSE score: minimental state examination score; AD: Alzheimer's disease; rCDI: recurrent clostridium difficile infection; CDAI: Crohn's disease activity index; MS: multiple sclerosis; CD: Crohn's disease; YGTSS-total tic score: Yale Global Tic Severity Scale-total tic score; TS: Tourette Syndrome; GTS-QoL score: Gilles de la Tourette Syndrome-quality of life score; CFS: chronic fatigue syndrome; IBS: Irritable bowel syndrome; TC: transcolonoscopic; ASD: autism spectrum disorder; CARS score: childhood autism rating scale score; BD: bipolar disorder; MDD: major depressive disorder; PHQ-9 scores: Patient Health Questionnaire-9 scores; HE: hepatic encephalopathy; PHES total score: psychometric hepatic encephalopathy score total score; DN: diabetic neuropathy; AKI: acute kidney injury; MODS: multiple organ dysfunction syndrome; MDRO infection: multidrug-resistant organism infection; PEG tube: polyethylene glycol tube.
2.1. Parkinson's Disease
Parkinson's disease (PD) is a progressive neurodegenerative disease characterized by accumulation of Lewy bodies [69]. PD patients often present with GI symptoms such as constipation [70]. According to the theory of intestinal origin of PD, a prion-like neurotrophic protein is misfolded into α-synuclein (α-syn) and transported from the GI tract to the central nervous system (CNS) [71]. Studies on the mouse model of PD have confirmed that α-syn can indeed be transferred from the gut to the brain by crossing the blood-brain barrier [72]. Consistent with this, several studies [3, 4, 73, 74] have reported considerable differences between the gut microbial composition and metabolites of healthy individuals and PD patients. Scheperjans et al. [73] compared the fecal microbiome of PD patients with that of 72 healthy controls and detected 77.6% lower prevalence of Prevotellaceae in the former. In addition, PD patients' postural instability and gait difficulty were positively associated with the higher abundance of Enterobacteriaceae, suggesting a causative association between the microbiota-gut-brain axis and progression of disease. Furthermore, Keshavarzian et al. [4] observed a greater proportion of LPS-producing proinflammatory bacteria (e.g., Ralstonia) and fewer bacteria producing the anti-inflammatory short-chain fatty acids (SCFAs) (e.g., Blautia, Coprococcus, Roseburia, and Faecalibacterium) in the gut of PD patients. Studies [75, 76] have shown that L-dopa can be metabolized into dopamine by gut microbial tyrosine decarboxylase, which is not easily affected by aromatic amino acid decarboxylase inhibitors such as carbidopa. In addition, the germ-free α-syn overexpressing (ASO) mice exhibited less severe motor and digestive symptoms (constipation), as well as lower microglia activation compared to their SPF counterparts [77], indicating that the gut microbiota is directly involved in PD's development.
Consistent with the above, Sun et al. [78] showed that FMT from healthy mice significantly improved the motor function in PD mice by mitigating intestinal inflammation and neuroinflammation and increasing the levels of dopamine and 5-hydroxytryptamine. The anti-inflammatory effects were mediated via TLR4/bk1/NF-κB/TNF-α pathway blockade, reduced activity of microglia and astrocytes, and increased producing SCFAs. In contrast, FMT from PD mice had a pathological effect on healthy recipients. Huang et al. [49] recently reported that three rounds of FMT over a period one week improved constipation and motor symptoms such as leg tremors in a PD patient. However, the tremors recurred 2 months after FMT, whereas constipation was relieved even after 3 months.
2.2. Alzheimer's Disease
Alzheimer's disease (AD) is a neurodegenerative disease characterized by cognitive decline due to the loss of neurons and synapses following deposition of neurofibrillary tangles (NFT) and misfolded amyloid β (Aβ) protein plaques [79]. Several studies [5, 6] have shown that the gut microbiota composition in AD patients differs considerably from that of healthy elderly individuals. For instance, the AD patients have a higher relative abundance of LPS-producing bacteria such as Burkholderiaceae, Staphylococcaceae, Porphyromonas gingivalis, and Propionibacterium acnes, as well as fungi in their intestine compared to healthy controls. In addition, patients with cerebral amyloidosis (Amy+) and cognitive impairment have more proinflammatory bacteria in their feces and higher levels of circulating inflammatory cytokines (IL-6, IL-1β, etc.) compared to healthy individuals and Amy- patients [80–84]. Likewise, Cattaneo et al. [85] also detected higher circulating levels of IL-6, IL-1β, and other inflammation-related factors like CXCL2 and NLRP3, along with reduced levels of the anti-inflammatory IL-10 in Amy+ patients relative to that in controls and Amy- patients. Furthermore, the Amy+ patients showed lower abundance of Eubacterium rectale and a higher abundance of Escherichia/Shigella compared to both healthy controls and Amy- patients. A significantly positive correlation was observed between the levels of proinflammatory factors and the abundance of Escherichia/Shigella. Several bacterial species are known to secrete neurotransmitters and alter the expression of synaptic plasticity, which may play a role in the pathogenesis of AD [86]. In addition to these direct effects, some changes in gut microbiota may indirectly promote AD by triggering neuroinflammation [87]. Consistent with this hypothesis, there are reports that probiotics can improve cognitive function in not only animal models but also AD patients or adults with cognitive impairment [88–90]. Furthermore, the age-related decline in cognitive ability may also be related to the concomitant decrease in the number of anti-inflammatory bacteria in the human gut [91, 92].
Recent studies [93, 94] have shown that antibiotic-mediated depletion of the gut microbiota alleviated Aβ-pathology and neuroinflammation in a mouse model of AD, and the therapeutic effect of antibiotics was partially reversed following FMT from AD mice. In addition, germ-free mice receiving feces from healthy old mice had worse cognitive function compared to the recipients of feces from younger mice due to lower fecal levels of nervous system-related metabolites (such as GABA) in the former [95]. Kim et al. [96] transplanted the fecal microbiota from health control mice into the recently developed AD-like pathology with amyloid and neurofibrillary tangle (ADLPAPT) transgenic mouse model and observed a significant reduction in cerebral amyloid plaques, NFTs and reactive gliosis, which correlated to improve cognitive and memory function. Hazan [50] reported the case of an 82-year-old AD patient who showed remission of Clostridium difficile infection (CDI) symptoms after receiving a single FMT from his 85-year-old wife and a negative stool test 2 months later. Interestingly, the minimental state examination (MMSE) score of the patient increased from 20 (mild cognitive impairment) to 26 (normal cognitive function) 2 months after FMT, and he reported memory retention and significant improvement in mood (MMSE score 29) after 4 and 6 months, respectively.
2.3. Multiple Sclerosis
Multiple sclerosis (MS) is a demyelinating disease of the CNS with uncertain etiology, although genetics, infection, and environmental factors have been implicated as key pathological factors [97]. The gut microbiota regulates the production of myelin sheath in the prefrontal cortex of mice [98, 99] and maintains the integrity of the blood-brain barrier [100] by producing SCFAs [101]. This is suggestive of a dysregulated gut microbiome in MS since the loss of blood-brain barrier integrity is also a cardinal sign of this disorder. In addition to the direct role in demyelination and blood-brain barrier disruption, the gut microbiota and its metabolites also regulate neuroinflammation [102–104], although the exact relationship between gut microorganisms and MS-related neuroinflammation needs a further study. The intestinal microbiota of MS patients have a lower relative abundance of Treg cell-inducing bacteria [9, 105], which may increase the proportion of peripheral Th1 and Th17 cells [98]. In addition, the risk of relapse in MS patients is associated with the depletion of Fusobacteria, expansion of the phylum Firmicutes, and presence of Archaea (Euryarchaeota) [106]. Oral gavage with Prevotella histicola not only reduced the severity of symptoms in a mouse model of MS but also decreased the number of Th1 and Th17 cells, while increasing that of Treg cells [107]. A randomized control trial (RCT) on 40 MS patients showed that probiotic (Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum) supplementation for 12 weeks significantly increased the circulating levels of IL-8 and TNF-α and improved the expanded disability status scale (EDSS) scores [108].
The clinical and pathophysiological characteristics of MS are best simulated in the experimental autoimmune encephalomyelitis (EAE) mouse model [109]. Oral gavage of the fecal microbiota from MS patients exacerbated the symptoms in EAE mice and decreased the levels of the anti-inflammatory cytokine IL-10 [98, 110]. Li et al. similarly showed that FMT from healthy mice alleviated the symptoms in EAE mice by reducing activity of microglia and astrocytes and restoring the blood-brain barrier integrity and axonal myelination [111]. The therapeutic effects of FMT in MS have been reported in only two studies so far [51, 52]. In one patient with secondary progressive MS complicated with recurrent CDI, FMT mitigated the recurrent infection and prevented disease progression of MS. However, the EDSS score of the patient stabilized without any improvements in the symptoms. Therefore, although FMT has limited therapeutic effect; it has the potential to provide long-term benefits for MS patients [52]. Furthermore, 3 MS patients with severe constipation were able to defecate normally after FMT, and their exercising ability was also improved significantly [51].
2.4. Epilepsy
Epilepsy is a chronic disease characterized by the sudden abnormal discharge from cerebral neurons, which leads to transient brain dysfunction. The individual susceptibility to epilepsy is associated with genetic and environmental factors, although the exact etiology of most cases remains unclear [16]. Nevertheless, the composition and distribution of gut microbes in patients with intractable epilepsy are distinct from that in healthy controls [17, 112, 113]. Peng et al. [17] found that compared to drug-sensitive patients, the intestinal Firmicutes/Bacteroides ratio and α-diversity were significantly higher in the drug-resistant patients. Interestingly, the α-diversity of the latter was similar to that of healthy controls, most likely due to an aberrant increase in the number of rare bacterial genera such as Clostridium XVIII, Atopobium, Holdemania, Dorea, Saccharibacteria, Delftia, Coprobacillus, Paraprevotella, Ruminococcus, Gemmiger, Akkermansia, Neisseria, Coprococcus, Fusobacterium, Methanobrevibacter, Phascolarctobacterium, and Roseburia. In addition, the increased abundance of Bifidobacterium and Lactobacillus was associated with fewer seizures per year, and a ketogenic diet reduced the frequency of seizures by modulating the gut microbiota [114]. Sewal et al. [115] further observed that intraperitoneal injection of LPS increased the frequency of epileptic symptoms, which was accompanied by an increase in the blood-brain barrier permeability and in the cerebral levels of proinflammatory cytokines. Antibiotics can protect against epileptic seizures by altering the bacterial population, although there is evidence that they may even induce epilepsy [16]. In addition, probiotic strains such as Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei, Lactobacillus helveticus, Lactobacillus brevis, Bifidobacterium lactis, Streptococcus salivarius subsp., and Thermophilus have also shown a positive effect in epilepsy patients [116, 117]. Olson et al. [118] observed that transplantation of ketogenic microbiota decreased the number of seizures in mice at a higher threshold. He et al. [53] reported a case of epilepsy complicated with Crohn's disease in a 17-year-old patient who showed improvements in neurological and intestinal symptoms following three rounds of FMT. Antiepileptic therapy with sodium valproate was discontinued after 20 months, and no epileptic seizures were observed.
2.5. Tourette Syndrome
Tourette Syndrome (TS) is a neurodevelopmental disorder characterized by motor and speech tics in childhood [119]. Liao et al. [120] found that probiotic supplementation improved tic-like behavior in mice, which coincided with an increased level of dopamine and norepinephrine. A study on 30 pediatric acute-onset neuropsychiatric syndrome and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections syndrome patients revealed a significantly different gut microbial composition compared to that of healthy controls [18]. Another study found that [121] antibiotics that effectively reduce streptococcal infections can also mitigate the associated tic disorders. Zhao et al. [54] reported that FMT eliminated involuntary articulation, reduced involuntary shrugging, and increased attention span in a pediatric case of TS over a period of 8 weeks. In an open label clinical trial [55], 11 TS patients experienced a transient decrease in seizure severity following three rounds of FMT.
2.6. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is characterized by unexplained persistent fatigue, disturbed sleep, cognitive impairment, fever, postural intolerance, lymphadenopathy, and irritable bowel syndrome. The gut microbiota is significantly altered in patients with ME/CFS [19], and the extent of microbial dysbiosis affects disease severity [122]. Sheedy et al. [123] observed increased relative abundance of gram-positive lactic acid-producing bacteria in the gut of ME/CFS patients, which may lower the mucosal pH and increase permeability. Moreover, the transfer of lactic acid from intestine to the blood may be one of the reasons for the increase of lactate level in cerebrospinal fluid of ME/CFS patients [124–126]. Selective transplantation of 13 nonpathogenic enteric bacteria through colonoscopy [56] significantly improved intestinal and other symptoms in 42/60 ME/CFS patients. In addition, 7/12 patients who were followed up for 15 to 20 years showed complete remission, indicating FMT is a promising treatment for ME/CFS.
2.7. Guillain-Barré Syndrome
Guillain-Barré Syndrome (GBS) is a paralytic autoimmune neuropathy caused by infection, especially Campylobacter jejuni infection in the GI tract, or other immune stimulation [127]. The innate immune response to campylobacteriosis is characterized by the accumulation of neutrophils and macrophages, inflammatory damage to the mucosa, gut barrier defects, and malabsorption, which eventually lead to bloody diarrhea [23]. Mice inoculated with Campylobacter jejuni from GBS patients showed increased levels of autoantibodies and peripheral nerve injury [128, 129], indicating a close association between gut dysbiosis and GBS pathogenesis. In fact, the cross-reaction between LPS produced by Campylobacter jejuni, and the peripheral gangliosides is one of the causative factors of GBS [130]. The combination of antibiotics and FMT significantly expedited Campylobacter jejuni clearance from the infected mice [131]. In addition, Brooks et al. [132] observed that human FMT increased the Th2 and autoimmune response in mice infected with Campylobacter jejuni. Finally, the outer core LPS of Campylobacter jejuni can directly initiate the peripheral neuropathy of GBS by inducing production of neurotoxic antiganglioside autoantibodies [133].
2.8. Stroke
Stroke is an acute cerebrovascular accident characterized by muscular and sensory weakness. Studies show that the composition of gut microbiota of stroke patients differs considerably from that in healthy controls [134, 135], although there are some reports indicating transient or no change [136]. Furthermore, the possible role of gut dysbiosis in stroke is ambiguous [137]. One study showed that a stroke episode decreased intestinal motility and α-diversity and led to bacterial overgrowth, intestinal barrier damage, and increased infiltration of inflammatory immune cells in the gut-associated lymphoid tissue and brain, eventually increasing the infarct volume [138]. In addition, the translocation of gut microbiota and their metabolites may also be involved in the pathogenesis of stroke [139]. For instance, trimethylamine-N-oxide produced by gut microbiota may be associated with a higher risk of atherosclerosis-mediated cardiovascular events, including stroke [140, 141]. Prebiotic treatment exacerbated the functional damage and inflammation in a mouse model of stroke, which increased the infarct volume [138, 142]. However, transplantation of healthy microbiota reduced infarct volume [138], indicating that FMT can be considered for treating stroke patients.
2.9. Amyotrophic Lateral Sclerosis and Huntington's Disease
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND), is a neurodegenerative disorder characterized by progressive atrophy of the limb, trunk, chest, and abdomen muscles following upper and lower motor neuron injury [143]. The mouse model of ALS shows an altered gut microbiota structure compared to healthy mice, such as a lower relative abundance of butyrate-producing bacteria [144]. Although a definitive pathological role of the gut microbiota in ALS has not been reported in humans [26], the clinical potential of FMT is still being explored [145]. Huntington's disease is caused by an autosomal dominant mutation in the huntingtin gene and is inherited in most cases. Nevertheless, several studies have implicated nongenetic factors in the development of Huntington's disease, such as the gut microbiota. Metabonomics analysis of the sera of preonset and early onset Huntington's disease patients and healthy controls showed significant differences in the gut microbiota metabolites across all groups [146], indicating that changes in the microflora determine disease course. The role of gut dysbiosis in the pathogenesis of Huntington's disease has also been confirmed in a murine transgenic model [29]. However, further studies are needed to fully understand the causative role of the gut microbiota and its metabolites in the genesis, progression, and severity of Huntington's disease.
3. Psychiatric Diseases
There is growing evidence that gut dysbiosis also contributes to mental health and psychiatric disorders, such as autism spectrum disorder, bipolar disorder, depression, anxiety, obsessive-compulsive disorder, posttraumatic stress disorder, schizophrenia, and dementia through the gut-brain axis [147, 148]. FMT has gained attention as a viable therapeutic option for these conditions (Table 2).
3.1. Autism Spectrum Disorder
Autism spectrum disorder (ASD) is a group of neurodevelopmental disorders characterized by changes in social interaction and repetitive, stereotypical behavior [149]. Recent studies show that gut microbial community and metabolites of ASD patients are distinct from that of healthy individuals [8, 150]. Although a putative relationship between gut dysbiosis and ASD behavior has been established in rodent models and human subjects, studies have not been sufficient to confirm the causal relationship between gut microbiota and ASD symptoms. The predominant phyla of the healthy adult human gut are Bacteroidetes (e.g., Bacteroides and Prevotella), Firmicutes (e.g., Clostridium, Lactobacillus, and Ruminococcus), Proteobacteria (e.g., Enterobacter), and Actinobacteria (e.g., Bifidobacterium) and constitute more than 90% of the gut microbiota [151, 152]. Since germ-free mice are socially dysfunctional compared to wild-type mice, the gut microbiota likely play an important role in normal behavior [153]. Regular administration of probiotics including Lactobacillus reuteri, Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus rhamnosus, and Bifidobacterium longum over a period of 3 weeks to 6 months improved autistic symptoms significantly in ASD children [154–156]. Lactobacillus rhamnosus and a placebo were, respectively, administered to 40 and 35 infants for the first 6 months of life in an RCT, and all subjects were followed over 13 years. Six infants of the placebo group were diagnosed with Asperger's syndrome or attention deficit hyperactivity disorder during the follow-up period whereas none in the probiotic group exhibited any signs of autism, indicating that early administration of probiotics can potentially reduce the risk of developing ASD [155]. A recent study has shown that there is a causal relationship between maternal diet, changes in gut microbiota, and social behavior. Among female neonatal rats fed with a high-fat diet, female rats born on a high-fat diet and for more than 4 weeks Lactobacillus reuteri restored gut microbial diversity and significantly improved their social behavior [157].
Sharon et al. [158] found that germ-free mice transplanted with the feces from children with ASD exhibited similar symptoms. In addition, the offspring of these FMT recipients also experienced these symptoms and showed alternative splicing of ASD-related genes in the brain. Likewise, FMT from healthy hamsters alleviated the ASD-like symptoms in the autism hamster model [159] by alleviating the brain oxidative stress response. In an open clinical trial on 18 children with ASD, FMT for 7 to 8 weeks could significantly improve digestive symptoms (abdominal pain, constipation, diarrhea, and indigestion) and the behavioral symptoms [32]. Furthermore, FMT also improved the bacterial diversity by significantly increasing the abundance of Bifidobacterium, Desulfovibrio, and Prevotella. The therapeutic effects persisted for 8 weeks after ceasing treatment. In another study, the ASD symptoms improved in 8/9 recipients of FMT and antibiotic treatment [57]. Zhao et al. [58] conducted an open label RCT on 24 autistic and 24 normal children that were treated with FMT for 2 months. Although FMT improved the behavioral and GI symptoms, the effects were transient.
3.2. Bipolar Disorder
Bipolar disorder (BD) is a type of mood disorder that clinical manifests as distinct episodes of depression, manic seizures, and their combination. Both the diversity and taxonomic composition of the gut microbiota in BD patients are significantly different from that of healthy individuals [36]. Painold et al. [160] further showed that the phylum Actinobacteria and class Coriobacteria were significantly more abundant in the gut of BD patients, whereas Ruminococcaceae and Faecalibacterium were more abundant in the healthy controls as per 16S rRNA gene sequencing and LEfSE analysis. They also observed a negative correlation between microbial α-diversity and duration of BD and identified bacterial clades associated with inflammatory status, serum lipids, depressive symptoms, oxidative stress, anthropometrics, and metabolic syndrome in the BD patients. Hu et al. [35] analyzed the gut microflora of 52 BD patients and 45 controls and found that the α-diversity of untreated BD patients was lower than that of the control group, and the predominant phyla were Bacteroidetes and Firmicutes, respectively. In addition, butyrate-producing bacteria were less abundant in the untreated patients, which was restored following quetiapine treatment. Furthermore, probiotics supplementation for a period of 3 months improved the cognitive and executive functions of 20 BD patients [161].
Hinton [59] reported a case of a 29-year-old female patient diagnosed with type I DSM-IV BD who had been treated with various drugs, including lithium, lamotrigine, valproate, quetiapine, olanzapine, and various benzodiazepines, that led to significant weight gain and poor quality of life. Nine rounds of FMT in 11 months not only alleviated depression and mania but also helped her lose the excess weight and remain asymptomatic without using other drugs.
3.3. Depression
Depression is a common mental disease typically characterized by persistent feelings of sadness and loss of interest in daily activities. It results from a combination of both genetic and environmental factors, and a major cause is stress [162]. Studies increasingly show that the gut microbiota can shape cognition through the microbiota gut-brain axis, and mice with altered microbiota usually exhibit depression-related behaviors [163]. Kelly and Borre [164] analyzed the intestinal flora of 34 patients with depression and 33 matched healthy subjects and found that the microbial abundance and biodiversity were decreased in the patient group. FMT from these patients into germ-free rats induced depression-like behavior such as lack of pleasure and anxiety in the latter, along with increased levels of tryptophan. A meta-analysis of 71 studies published between 2003 and 2019 [165] further revealed that probiotics and prebiotics can significantly improve symptoms of anxiety and depression compared to untreated or placebo-treated controls and provide additional benefits to patients with other diseases such as irritable bowel syndrome.
Zhang et al. [163] found that FMT from depressed patients into germ-free mice led to depressive behavior in the latter. Similar results were observed after antibiotic treatment as well. Furthermore, FMT from the NLRP3-knockout mice significantly improved the behavioral symptoms in a mouse model of depression. Likewise, Xie [166] also found that the fecal microbiota of healthy mice alleviated depressive symptoms. In a recent case report [60] of an older woman diagnosed with depression, a single FMT improved sleep cycle, appetite, and general mood within 4 days of treatment. The patient was able to live independently after 2 weeks and showed an increase in weight. Six months later, her weight had returned to normal, constipation symptoms had improved, and the Patient Health Questionnaire-9 score decreased from 21 to 4.
3.4. Anxiety
Anxiety is one of the most common types of neurosis and is characterized by feelings of tension/worry without a clear objective, restlessness, and autonomic nerve dysfunction. Clinically, it is classified into chronic/generalized anxiety and acute anxiety or panic attack [167]. A large case-control study [168] showed that the use of antibiotics increased the risk of anxiety and depression, and the risk increased with the frequency of usage, suggesting a causative or ancillary role of the gut microbiota. Furthermore, there is evidence that depression can lead to secondary changes in the composition of the gut microbiota, resulting in a regulatory feedback loop between depression and dysbacteriosis [169]. Compared to the SPF mice, sterile mice showed significantly higher anxiety in the elevated maze test, and oral administration of the JB-1 probiotic strain effectively reduced the anxious behavior and improved performance. Furthermore, a systematic review of 21 studies including 1503 subjects with anxiety disorders concluded that microbiota-targeted therapies [41], including probiotics supplements, single probiotics, double probiotics, multiple probiotics, dietary fiber supplement, and low FODMAP diet, can alleviate symptoms of anxiety by regulating the gut microbiota.
De Palma et al. [170] transplanted fecal microbiota from healthy control and diarrhea-predominant irritable bowel syndrome (IBS) patients with (IBS-A) or without anxiety into germ-free mice and analyzed the changes in intestinal function and behavior. The gut microbiota of mice transplanted with the feces of IBS patients showed unique clustering characteristics compared to that of control fecal recipients. Anxiety-like behavior was determined with the light/dark preference test and platform jumping test, which showed that the IBS-A recipient mice had the least preference for light and showed the delay in jumping off a high platform, both of which are indicative of a higher degree of anxiety. These studies clearly indicate the involvement of gut dysbiosis in the severity of anxiety symptoms.
4. Other System-Related Neurological Diseases
Several neurological and psychological diseases are frequently complicated with digestive system symptoms. Likewise, some diseases predominantly affecting the nonnervous systems may also have a neurological component and are commonly manifested as encephalopathies. For instance, decompensated hepatic encephalopathy and peripheral neuropathy are severe complications of cirrhosis and diabetes, respectively, and sepsis patients often present delirium, coma, and other neurological symptoms. The role of the gut microbiota in these encephalopathies is increasingly being recognized, thereby indicating the therapeutic potential of FMT for these diseases (Table 2).
4.1. Hepatic Encephalopathy
Hepatic encephalopathy (HE) is a serious complication of cirrhosis and is caused by brain dysfunction. The increased content of hepatic ammonia in cirrhosis patients with mild HE indicates the pathological involvement of intestinal dysbiosis. For instance, the intestinal tract of cirrhotic patients with/without mild HE frequently harbors urease-positive Streptococcus salivarius, which is absent in healthy individuals [171]. Thus, S. salivarius is a promising therapeutic target in liver cirrhosis patients with mild HE. Sung et al. [43] confirmed that fecal microbiota can predict the clinical prognosis of patients with liver cirrhosis and HE, such as Lactobacillus, Bacteroides, Clostridium_incertae_sedisof, and Clostridium XI, which were associated with patients' mortality. Furthermore, Kawaguchi et al. [172] showed that rifaximin improved both liver and neuropsychological function in liver cirrhosis patients with HE by adjusting the gut microbial structure.
A promising case study of a 57-year-old patient with HE due to alcoholic and hepatitis C cirrhosis [61] showed that FMT in addition to lactulose objectively improved reaction time, serum ammonia, and quality of life scores. However, these improvements were transient and subsided to the baseline levels within 7 weeks of FMT cessation. Furthermore, Bajaj et al. [62] conducted an RCT on male cirrhotic patients diagnosed with recurrent HE and found that FMT reduced hospitalization rate and improved cognitive ability in these patients during the 5-month follow-up. In another clinical trial conducted by Bajaj et al. [63], administration of FMT capsules to HE patients restored the gut microflora by significantly increasing the abundance of Bifidobacterium and Ruminococcaceae and decreasing that of pathogenic genera like Streptococcus and Veillonella. The FMT-induced changes in the gut microbiota led to an increase in duodenal E-cadherin and defensin-α5 expression and reduced serum levels of IL-6 and LBP.
4.2. Neuropathic Pain
Neuropathic pain is caused by peripheral or CNS injury (such as nerve injury or chemotherapy injury) or diabetes and is characterized by abnormal sensations or pain even after normal stimulations [173]. The composition and function of the gut microbiota in diabetic patients differ significantly from that of healthy controls [174]. FMT from conventionally reared mice increased the insulin resistance in germ-free mice [175], whereas subjects with metabolic syndrome showed increased insulin sensitivity following FMT [176]. Gut microbiota can also directly regulate the excitability of spinal dorsal root neurons or indirectly regulate inflammation in the peripheral and central nervous system [177]. Oxaliplatin can cause peripheral neuropathy and pain, but this phenomenon is not obvious in mice with antibiotic cleaning or in mice with complete loss of gut microbiota. Furthermore, if FMT was performed on the appellate mice, the pain would be restored, indicating that the gut microbiota has an effect on neuropathic pain [178]. Another study found that probiotics alleviated the characteristics of paclitaxel-induced neuropathic pain in vitro [179], although their efficacy is dependent on the type of neuropathic pain. For instance, Lactobacillus Reuteri or Bifidobacterium were not effective against the neuropathic pain induced by chronic compression injury in rats [180].
A case study [64] of a woman with type 2 diabetes mellitus and diabetic neuropathy showed that two rounds of FMT improved limb pain and paresthesia, which was manifested as decreased visual analogue pain score (VAS) and increased tibial nerve motor conduction velocity, without any significant improvement in EMG sensory dysfunction. In addition, the fasting blood glucose level also decreased and stabilized, and glycosylated hemoglobin content decreased post-FMT.
4.3. Sepsis-Associated Encephalopathy
Sepsis is an acute systemic infection caused by various pathogenic bacteria that invade the bloodstream and rapidly proliferate and produce life-threatening toxins. Sepsis-associated encephalopathy is a key neurological manifestation of sepsis, with symptoms ranging from delirium to coma. It occurs in almost 70% of the ICU patients and is associated with higher ICU and hospital mortality, as well as poor long-term outcomes (including cognitive and functional outcomes) [181, 182]. The toxins and other harmful antigens secreted by pathogenic bacteria or viruses can be neutralized by the antibodies produced by antigen-primed B cells. Intestinal microorganisms have been shown to induce the clonal expansion of specific B cell populations and increase production of antibodies to prevent the spread of infection [183]. Li et al. found that FMT effectively improved the spatial memory and EEG abnormalities in an LPS-induced rat model of sepsis combined with cervical vagotomy, and the therapeutic effect of FMT was likely mediated through the vagus nerve [184]. In addition, several case reports indicate that non-CDI sepsis patients with prolonged ICU stay and complications including bacteremia, MDR bacterial infection, respiratory failure, and organ dysfunction significantly benefitted from FMT. A total of 5 patients received FMT, of which 4 showed clinical improvement and 1 died from non-FMT-related causes [65–68].
5. Discussion
Nervous system diseases are highly complex and show cognitive, motor, and even systemic manifestations. Given that gut dysbiosis is a potential causative factor of neurological dysfunction, FMT-mediated restoration of the gut microbiota can stall the symptoms or progression of nervous system diseases through immune, endocrine, metabolic, and/or neural pathways. The metabolites and cytokines produced by gut bacteria determine intestinal and systemic inflammation and, therefore, the intestinal barrier function. However, there are several limitations of using FMT in treating neurological, mental, and psychological diseases: (1) for many diseases, the therapeutic effects of FMT are limited to animal models and isolated cases. Although transplantation of human feces to animal models has shown encouraging results, the GI and physiological differences between humans and animals preclude the extrapolation of the results to sick or healthy humans. (2) The fecal feeding behavior often observed in mice [185] may also affect the microbiota analysis and the efficacy of FMT. In addition, animals housed in the same cage may have a closer gut microbial structure, which can also affect the results. (3) The efficacy of FMT depends on the types of antibiotics, microbial composition, intervention procedure, and donors. The exact influence of these factors and the potential adverse effects of FMT are currently unknown due to lack of long-term follow-up and appropriate controls. Therefore, it is crucial to establish scientific standards in order to gauge the therapeutic efficacy of FMT [186]. (4) The role of the gut microbiota in the early development of nervous system also needs to be elucidated. For instance, a study on 39 infants showed that the α-diversity of gut microbiota was also associated with functional connectivity between the auxiliary motor area and the inferior parietal lobule, and this functional connectivity affects the cognitive level at 2 years of age [187]. (5) Many successful cases of FMT in the treatment of neurological diseases/psychiatric diseases often have obvious GI symptoms, and the improvement of neurological symptoms/mental symptoms is also related to the GI symptoms. For patients with neurological diseases/psychiatric diseases but without obvious GI symptoms, whether the curative effect of FMT will be reduced or unchanged is also worth our concern.
Despite the promising results, the rationale for the clinical application of FMT is currently based on animal models and a few case reports and clinical studies. Large-scale randomized double-blind controlled trials are still needed to clarify the role of FMT in neurological diseases. At present, 33 clinical trials are ongoing on the potential therapeutic effects of FMT on mental and nervous system diseases (Table 3). Furthermore, the modes of delivering fecal microbiota also need to be improved. While a capsular form is more comfortable for the patients, fecal bacterial liquid in the form of washed/selective microbiota transplantation [188, 189] may be more effective in reducing the potential side effects.
Table 3.
Clinical trials of FMT involving in nervous and mental disease.
| NCT number | Conditions | FMT route | Phases | Status | Locations |
|---|---|---|---|---|---|
| NCT02255617 | Hepatic encephalopathy | Colonoscopy & enema | Phase 1, phase 2 | Completed | Canada |
| NCT02636647 | Hepatic encephalopathy | Enema | Phase 1 | Completed | United States |
| NCT03420482 | Hepatic encephalopathy | Capsules | Phase 2 | Recruiting | United States |
| NCT03152188 | Hepatic encephalopathy | Capsules | Phase 1 | Completed | United States |
| NCT03439982 | Hepatic encephalopathy | Colonoscopy & enema | Phase 1, phase 2 | Recruiting | Canada |
| NCT03796598 | Hepatic encephalopathy | Capsules & enema | Phase 1, phase 2 | Recruiting | United States |
| NCT03408886 | Autism spectrum disorder | Pill (no detail) | Phase 2 | Recruiting | United States |
| NCT03426826 | Autism spectrum disorder | Gastroscope | Phase 1 | Recruiting | United States |
| NCT03829878 | Autism spectrum disorder | Capsules | Phase 2 | Not yet recruiting | United States |
| NCT04182633 | Autism spectrum disorder | Oral administration of FM (no detail) | Phase 2 | Recruiting | United States |
| NCT04246398 | Children with autism | Capsules | Not applicable | Not yet recruiting | Israel |
| NCT03026231 | Parkinson's disease | Capsules | Phase 1, phase 2 | Withdrawn | United States |
| NCT03671785 | Parkinson disease | Capsules | Phase 1 | Recruiting | United States |
| NCT03808389 | Parkinson disease | Nasojejunal | Not applicable | Recruiting | Belgium |
| NCT03876327 | Parkinson disease | Not applicable | Phase 2, phase 3 | Completed | Israel |
| NCT03183869 | Multiple sclerosis | Enema | Phase 2 | Terminated | Canada |
| NCT03594487 | Multiple sclerosis | Colonoscopy | Phase 1 | Recruiting | United States |
| NCT03975413 | Multiple sclerosis | Not applicable | Not applicable | Active, not recruiting | United States |
| NCT04203017 | Multiple sclerosis | Capsules | Phase 1 | Recruiting | Russian Federation |
| NCT03691987 | Chronic fatigue syndrome/myalgic encephalomyelitis | Enema | Phase 2 | Recruiting | Norway |
| NCT04158427 | Chronic fatigue syndrome/myalgic encephalomyelitis | Colonoscopy | Not applicable | Enrolling by invitation | Finland |
| NCT03233100 | Depressive symptoms, anxiety symptoms, gut-brain disorders | Not applicable | Not applicable | Unknown status∗ | China |
| NCT03281044 | Major depressive disorder | Capsules | Phase 2 | Terminated | Switzerland |
| NCT04001439 | Depression in schizophrenia | Capsules | Not applicable | Not yet recruiting | France |
| NCT03998423 | Alzheimer disease | Capsules | Phase 1 | Terminated | United States |
| NCT02889627 | Epilepsy | Microbiota suspension infused into midgut or lower gut (no detail) | Phase 2, phase 3 | Recruiting | China |
| NCT03279224 | Bipolar depression | Colonoscopy | Phase 2, phase 3 | Recruiting | Canada |
| NCT03766321 | Amyotrophic lateral sclerosis | Nasojejunal | Not applicable | Recruiting | Italy |
| NCT04132427 | Pitt-Hopkins syndrome | Oral (no detail) | Phase 2 | Recruiting | United States |
| NCT03416751 | Alcohol abuse | Enema | Phase 1 | Completed | United States |
| NCT03928808 | Anorexia nervosa | Nasogastric tube | Early phase 1 | Suspended | United States |
| NCT02336789 | Disorientation as to people, time and place | Colonoscopy | Not applicable | Unknown status∗ | Israel |
| NCT04014413 | Hepatic encephalopathy, multiple sclerosis, autism, alcohol dependence | Not applicable | Not applicable | Recruiting | China |
∗Study has passed its completion date, and status has not been verified in more than two years. Date from https://clinicaltrials.gov/.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (81700487, 81871905), Guangdong Medical Science and Technology Research Fund (A2019243), Guangzhou Planned Project of Science and Technology (202002020012, 202002030288, and 202002030293), and Innovative Clinical Technique of Guangzhou (2019GX05).
Contributor Information
Yong-Jian Zhou, Email: eyzhouyongjian@scut.edu.cn.
Yu-Qiang Nie, Email: eynieyuqiang@scut.edu.cn.
Data Availability
Data from the review are available upon request from the corresponding authors (Y.Q.N. and Y.J.Z.).
Conflicts of Interest
The authors declare no competing financial interests.
Authors' Contributions
H.M.X., H.L.H., and Y.L.Z. are involved in the design of the study and drafting of the article. H.L.Z. and J.X. are involved in the statistical analysis and interpretation of the data. D.W.S. and Y.D.L. are involved in the design of the figure and tables. Y.J.Z. and Y.Q.N. planned and directed the project and interpreted the results. All authors read and approved the final manuscript. H.M.X., H.L.H., and Y.L.Z. contributed equally to this article.
References
- 1.Bull M. J., Plummer N. T. Part 1: the human gut microbiome in health and disease. Integrative Medicine: A Clinician's Journal. 2014;13(6):17–22. [PMC free article] [PubMed] [Google Scholar]
- 2.Rath C. M., Dorrestein P. C. The bacterial chemical repertoire mediates metabolic exchange within gut microbiomes. Current Opinion in Microbiology. 2012;15(2):147–154. doi: 10.1016/j.mib.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasegawa S., Goto S., Tsuji H., et al. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson's disease. PLoS One. 2015;10(11, article e0142164) doi: 10.1371/journal.pone.0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keshavarzian A., Green S. J., Engen P. A., et al. Colonic bacterial composition in Parkinson's disease. Movement Disorders. 2015;30(10):1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 5.Liu P., Wu L., Peng G., et al. Altered microbiomes distinguish Alzheimer's disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain, Behavior, and Immunity. 2019;80:633–643. doi: 10.1016/j.bbi.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Li B., He Y., Ma J., et al. Mild cognitive impairment has similar alterations as Alzheimer's disease in gut microbiota. Alzheimer's & Dementia. 2019;15(10):1357–1366. doi: 10.1016/j.jalz.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Haran J. P., Bhattarai S. K., Foley S. E., et al. Alzheimer's disease microbiome is associated with dysregulation of the anti-inflammatory P-glycoprotein pathway. mBio. 2019;10(3) doi: 10.1128/mBio.00632-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma B., Liang J., Dai M., et al. Altered gut microbiota in Chinese children with autism spectrum disorders. Frontiers in Cellular and Infection Microbiology. 2019;9:p. 40. doi: 10.3389/fcimb.2019.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosorich I., Dalla-Costa G., Sorini C., et al. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Science Advances. 2017;3(7, article e1700492) doi: 10.1126/sciadv.1700492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan Y., Wang H., Liu X., Zhang J., Liu G. Crosstalk between the ketogenic diet and epilepsy: from the perspective of gut microbiota. Mediators of Inflammation. 2019;2019:9. doi: 10.1155/2019/8373060.8373060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stasi C., Caserta A., Nisita C., et al. The complex interplay between gastrointestinal and psychiatric symptoms in irritable bowel syndrome: a longitudinal assessment. Journal of Gastroenterology and Hepatology. 2019;34(4):713–719. doi: 10.1111/jgh.14375. [DOI] [PubMed] [Google Scholar]
- 12.Wang J. W., Kuo C. H., Kuo F. C., et al. Fecal microbiota transplantation: review and update. Journal of the Formosan Medical Association. 2019;118(Suppl 1):S23–S31. doi: 10.1016/j.jfma.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Grochowska M., Laskus T., Radkowski M. Gut microbiota in neurological disorders. Archivum Immunologiae et Therapiae Experimentalis (Warsz) 2019;67(6):375–383. doi: 10.1007/s00005-019-00561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adler C. H., Beach T. G. Neuropathological basis of nonmotor manifestations of Parkinson's disease. Movement Disorders. 2016;31(8):1114–1119. doi: 10.1002/mds.26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angelucci F., Cechova K., Amlerova J., Hort J. Antibiotics, gut microbiota, and Alzheimer's disease. Journal of Neuroinflammation. 2019;16(1):p. 108. doi: 10.1186/s12974-019-1494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lum G. R., Olson C. A., Hsiao E. Y. Emerging roles for the intestinal microbiome in epilepsy. Neurobiology of Disease. 2020;135:p. 104576. doi: 10.1016/j.nbd.2019.104576. [DOI] [PubMed] [Google Scholar]
- 17.Peng A., Qiu X., Lai W., et al. Altered composition of the gut microbiome in patients with drug-resistant epilepsy. Epilepsy Research. 2018;147:102–107. doi: 10.1016/j.eplepsyres.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Quagliariello A., Del Chierico F., Russo A., et al. Gut microbiota profiling and gut-brain crosstalk in children affected by pediatric acute-onset neuropsychiatric syndrome and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Frontiers in Microbiology. 2018;9:p. 675. doi: 10.3389/fmicb.2018.00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fremont M., Coomans D., Massart S., De Meirleir K. High-throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients. Anaerobe. 2013;22:50–56. doi: 10.1016/j.anaerobe.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Giloteaux L., Goodrich J. K., Walters W. A., Levine S. M., Ley R. E., Hanson M. R. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2016;4(1):p. 30. doi: 10.1186/s40168-016-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Missailidis D., Annesley S. J., Fisher P. R. Pathological mechanisms underlying myalgic encephalomyelitis/chronic fatigue syndrome. Diagnostics (Basel) 2019;9(3) doi: 10.3390/diagnostics9030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks P. T., Mansfield L. S. Effects of antibiotic resistance (AR) and microbiota shifts on Campylobacter jejuni-mediated diseases. Animal Health Research Reviews. 2017;18(2):99–111. doi: 10.1017/S1466252318000014. [DOI] [PubMed] [Google Scholar]
- 23.Mousavi S., Bereswill S., Heimesaat M. M. Novel clinical Campylobacter jejuni infection models based on sensitization of mice to lipooligosaccharide, a major bacterial factor triggering innate immune responses in human campylobacteriosis. Microorganisms. 2020;8(4) doi: 10.3390/microorganisms8040482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun J., Wang F., Ling Z., et al. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Research. 2016;1642:180–188. doi: 10.1016/j.brainres.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 25.Tang W. W., Wang Z., Levison B. S., et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. The New England Journal of Medicine. 2013;368(17):1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenner D., Hiergeist A., Adis C., et al. The fecal microbiome of ALS patients. Neurobiology of Aging. 2018;61:132–137. doi: 10.1016/j.neurobiolaging.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Fang X., Wang X., Yang S., et al. Evaluation of the microbial diversity in amyotrophic lateral sclerosis using high-throughput sequencing. Frontiers in Microbiology. 2016;7:p. 1479. doi: 10.3389/fmicb.2016.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright M. L., Fournier C., Houser M. C., Tansey M., Glass J., Hertzberg V. S. Potential role of the gut microbiome in ALS: a systematic review. Biological Research for Nursing. 2018;20(5):513–521. doi: 10.1177/1099800418784202. [DOI] [PubMed] [Google Scholar]
- 29.Kong G., Lê Cao K. A., Judd L. M., Li S., Renoir T., Hannan A. J. Microbiome profiling reveals gut dysbiosis in a transgenic mouse model of Huntington's disease. Neurobiology of Disease. 2020;135:p. 104268. doi: 10.1016/j.nbd.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Wasser C. I., Mercieca E. C., Kong G., et al. Gut dysbiosis in Huntington's disease: associations among gut microbiota, cognitive performance and clinical outcomes. Brain communications. 2020;2(2, article fcaa110) doi: 10.1093/braincomms/fcaa110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bundgaard-Nielsen C., Knudsen J., Leutscher P. D., et al. Gut microbiota profiles of autism spectrum disorder and attention deficit/hyperactivity disorder: a systematic literature review. Gut Microbes. 2020;11(5):1172–1187. doi: 10.1080/19490976.2020.1748258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang D. W., Adams J. B., Coleman D. M., et al. Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Scientific Reports. 2019;9(1):p. 5821. doi: 10.1038/s41598-019-42183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L., Christophersen C. T., Sorich M. J., Gerber J. P., Angley M. T., Conlon M. A. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Digestive Diseases and Sciences. 2012;57(8):2096–2102. doi: 10.1007/s10620-012-2167-7. [DOI] [PubMed] [Google Scholar]
- 34.Flowers S. A., Ward K. M., Clark C. T. The gut microbiome in bipolar disorder and pharmacotherapy management. Neuropsychobiology. 2020;79(1):43–49. doi: 10.1159/000504496. [DOI] [PubMed] [Google Scholar]
- 35.Hu S., Li A., Huang T., et al. Gut microbiota changes in patients with bipolar depression. Advancement of Science. 2019;6(14, article 1900752) doi: 10.1002/advs.201900752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang T. T., Lai J. B., Du YL X. Y., Ruan L. M., Hu S. H. Current understanding of gut microbiota in mood disorders: an update of human studies. Frontiers in Genetics. 2019;10:p. 98. doi: 10.3389/fgene.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rucklidge J. J., Harrison R. Successful treatment of bipolar disorder II and ADHD with a micronutrient formula: a case study. CNS Spectrums. 2010;15(5):289–295. doi: 10.1017/s1092852900027516. [DOI] [PubMed] [Google Scholar]
- 38.Fond G. B., Lagier J. C., Honore S., et al. Microbiota-orientated treatments for major depression and schizophrenia. Nutrients. 2020;12(4) doi: 10.3390/nu12041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valles-Colomer M., Falony G., Darzi Y., et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nature Microbiology. 2019;4(4):623–632. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- 40.Jiang H. Y., Zhang X., Yu Z. H., et al. Altered gut microbiota profile in patients with generalized anxiety disorder. Journal of Psychiatric Research. 2018;104:130–136. doi: 10.1016/j.jpsychires.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Yang B., Wei J., Ju P., Chen J. Effects of regulating intestinal microbiota on anxiety symptoms: a systematic review. General psychiatry. 2019;32(2, article e100056) doi: 10.1136/gpsych-2019-100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bajaj J. S., Khoruts A. Microbiota changes and intestinal microbiota transplantation in liver diseases and cirrhosis. Journal of Hepatology. 2020;72(5):1003–1027. doi: 10.1016/j.jhep.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 43.Sung C. M., Lin Y. F., Chen K. F., et al. Predicting clinical outcomes of cirrhosis patients with hepatic encephalopathy from the fecal microbiome. Cellular and Molecular Gastroenterology and Hepatology. 2019;8(2):301–318. doi: 10.1016/j.jcmgh.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin B., Wang Y., Zhang P., Yuan Y., Zhang Y., Chen G. Gut microbiota regulates neuropathic pain: potential mechanisms and therapeutic strategy. The Journal of Headache and Pain. 2020;21(1):p. 103. doi: 10.1186/s10194-020-01170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adelman M. W., Woodworth M. H., Langelier C., et al. The gut microbiome's role in the development, maintenance, and outcomes of sepsis. Critical Care. 2020;24(1):p. 278. doi: 10.1186/s13054-020-02989-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cerdo T., Dieguez E., Campoy C. Impact of gut microbiota on neurogenesis and neurological diseases during infancy. Current Opinion in Pharmacology. 2020;50:33–37. doi: 10.1016/j.coph.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Gubert C., Kong G., Renoir T., Hannan A. J. Exercise, diet and stress as modulators of gut microbiota: implications for neurodegenerative diseases. Neurobiology of Disease. 2020;134:p. 104621. doi: 10.1016/j.nbd.2019.104621. [DOI] [PubMed] [Google Scholar]
- 48.Pusceddu M. M., Del Bas J. M. The role of the gut microbiota in the pathophysiology of mental and neurological disorders. Psychiatric Genetics. 2020;30(4):87–100. doi: 10.1097/YPG.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 49.Huang H., Xu H., Luo Q., et al. Fecal microbiota transplantation to treat Parkinson's disease with constipation: a case report. Medicine. 2019;98(26, article e16163) doi: 10.1097/MD.0000000000016163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hazan S. Rapid improvement in Alzheimer's disease symptoms following fecal microbiota transplantation: a case report. The Journal of International Medical Research. 2020;48(6, article 300060520925930) doi: 10.1177/0300060520925930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borody T., Leis S., Campbell J., Torres M., Nowak A. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS): 942. Official journal of the American College of Gastroenterology. 2011;106 [Google Scholar]
- 52.Makkawi S., Camara-Lemarroy C., Metz L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurology-Neuroimmunology Neuroinflammation. 2018;5(4, article e459) doi: 10.1212/NXI.0000000000000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He Z., Cui B. T., Zhang T., et al. Fecal microbiota transplantation cured epilepsy in a case with Crohn's disease: the first report. World Journal of Gastroenterology. 2017;23(19):3565–3568. doi: 10.3748/wjg.v23.i19.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao H., Shi Y., Luo X., Peng L., Yang Y., Zou L. The effect of fecal microbiota transplantation on a child with Tourette syndrome. Case Reports in Medicine. 2017;2017:3. doi: 10.1155/2017/6165239.6165239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding X., Zhang F., Li Q., Ting Z., Cui B., Li P. Sa1926 – selective microbiota transplantation is effective for controlling Tourette’s syndrome. Gastroenterology. 2019;156, article S-456 [Google Scholar]
- 56.Borody T., Nowak A., Finlayson S. The GI microbiome and its role in chronic fatigue syndrome: a summary of bacteriotherapy. Journal of the Australasian College of Nutritional and Environmental Medicine. 2012;31:3–8. [Google Scholar]
- 57.Ward L., O'Grady H., Wu K., Cannon K., Workentine M., Louie T. Combined oral fecal capsules plus fecal enema as treatment of late-onset autism spectrum disorder in children: report of a small case series. Open Forum Infectious Diseases. 2016;3 [Google Scholar]
- 58.Zhao H., Gao X., Xi L., et al. Mo1667 fecal microbiota transplantation for children with autism spectrum disorder. Gastrointestinal Endoscopy. 2019;89:AB512–AB513. [Google Scholar]
- 59.Hinton R. A case report looking at the effects of faecal microbiota transplantation in a patient with bipolar disorder. The Australian and New Zealand Journal of Psychiatry. 2020;54(6):649–650. doi: 10.1177/0004867420912834. [DOI] [PubMed] [Google Scholar]
- 60.Cai T., Shi X., Yuan L. Z., Tang D., Wang F. Fecal microbiota transplantation in an elderly patient with mental depression. International Psychogeriatrics. 2019;31(10):1525–1526. doi: 10.1017/S1041610219000115. [DOI] [PubMed] [Google Scholar]
- 61.Kao D., Roach B., Park H., et al. Fecal microbiota transplantation in the management of hepatic encephalopathy. Hepatology. 2016;63(1):339–340. doi: 10.1002/hep.28121. [DOI] [PubMed] [Google Scholar]
- 62.Bajaj J. S., Kassam Z., Fagan A., et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology. 2017;66(6):1727–1738. doi: 10.1002/hep.29306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bajaj J. S., Salzman N. H., Acharya C., et al. Fecal microbial transplant capsules are safe in hepatic encephalopathy: a phase 1, randomized, placebo-controlled trial. Hepatology. 2019;70(5):1690–1703. doi: 10.1002/hep.30690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai T. T., Ye X. L., Yong H. J., et al. Fecal microbiota transplantation relieve painful diabetic neuropathy: a case report. Medicine. 2018;97(50, article e13543) doi: 10.1097/MD.0000000000013543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Q., Wang C., Tang C., et al. Therapeutic modulation and reestablishment of the intestinal microbiota with fecal microbiota transplantation resolves sepsis and diarrhea in a patient. The American Journal of Gastroenterology. 2014;109(11):1832–1834. doi: 10.1038/ajg.2014.299. [DOI] [PubMed] [Google Scholar]
- 66.Li Q., Wang C., Tang C., et al. Successful treatment of severe sepsis and diarrhea after vagotomy utilizing fecal microbiota transplantation: a case report. Critical care. 2015;19 doi: 10.1186/s13054-015-0738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei Y., Yang J., Wang J., et al. Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Critical care. 2016;20 doi: 10.1186/s13054-016-1491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gopalsamy S. N., Sherman A., Woodworth M. H., Lutgring J. D., Kraft C. S. Fecal microbiota transplant for multidrug-resistant organism decolonization administered during septic shock. Infection Control and Hospital Epidemiology. 2018;39(4):490–492. doi: 10.1017/ice.2017.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pakkenberg B., Moller A., Gundersen H. J., Mouritzen Dam A., Pakkenberg H. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson's disease estimated with an unbiased stereological method. Journal of Neurology, Neurosurgery, and Psychiatry. 1991;54(1):30–33. doi: 10.1136/jnnp.54.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Picillo M., Palladino R., Erro R., et al. The PRIAMO study: age- and sex-related relationship between prodromal constipation and disease phenotype in early Parkinson's disease. Journal of Neurology. 2020 doi: 10.1007/s00415-020-10156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 72.Kim S., Kwon S. H., Kam T. I., et al. Transneuronal propagation of pathologic alpha-synuclein from the gut to the brain models Parkinson's disease. Neuron. 2019;103(4):627–641. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scheperjans F., Aho V., Pereira P. A., et al. Gut microbiota are related to Parkinson's disease and clinical phenotype. Movement Disorders. 2015;30(3):350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 74.Unger M. M., Spiegel J., Dillmann K. U., et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism & Related Disorders. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 75.Maini Rekdal V., Bess E. N., Bisanz J. E., Turnbaugh P. J., Balskus E. P. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 2019;364(6445) doi: 10.1126/science.aau6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Kessel S. P., Frye A. K., El-Gendy A. O., et al. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson's disease. Nature Communications. 2019;10(1):p. 310. doi: 10.1038/s41467-019-08294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sampson T. R., Debelius J. W., Thron T., et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. 2016;167(6):1469–1480. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun M. F., Zhu Y. L., Zhou Z. L., et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson's disease mice: gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain, Behavior, and Immunity. 2018;70:48–60. doi: 10.1016/j.bbi.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 79.Andrews S. J., Fulton-Howard B., Goate A. Interpretation of risk loci from genome-wide association studies of Alzheimer's disease. Lancet Neurology. 2020;19(4):326–335. doi: 10.1016/S1474-4422(19)30435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dominy S. S., Lynch C., Ermini F., et al. Porphyromonas gingivalis in Alzheimer's disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Science Advances. 2019;5(1, article eaau3333) doi: 10.1126/sciadv.aau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pisa D., Alonso R., Fernandez-Fernandez A. M., Rabano A., Carrasco L. Polymicrobial infections in brain tissue from Alzheimer's disease patients. Scientific Reports. 2017;7(1, article 5559) doi: 10.1038/s41598-017-05903-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao Y., Jaber V., Lukiw W. J. Secretory products of the human GI tract microbiome and their potential impact on Alzheimer's disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus. Frontiers in Cellular and Infection Microbiology. 2017;7:p. 318. doi: 10.3389/fcimb.2017.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alonso R., Pisa D., Fernandez-Fernandez A. M., Carrasco L. Infection of fungi and bacteria in brain tissue from elderly persons and patients with Alzheimer's disease. Frontiers in Aging Neuroscience. 2018;10:p. 159. doi: 10.3389/fnagi.2018.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Emery D. C., Shoemark D. K., Batstone T. E., et al. 16S rRNA next generation sequencing analysis shows bacteria in Alzheimer's post-mortem brain. Frontiers in Aging Neuroscience. 2017;9:p. 195. doi: 10.3389/fnagi.2017.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cattaneo A., Cattane N., Galluzzi S., et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiology of Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 86.Maqsood R., Stone T. W. The gut-brain axis, BDNF, NMDA and CNS disorders. Neurochemical Research. 2016;41(11):2819–2835. doi: 10.1007/s11064-016-2039-1. [DOI] [PubMed] [Google Scholar]
- 87.Marques C., Meireles M., Faria A., Calhau C. High-fat diet-induced dysbiosis as a cause of neuroinflammation. Biological Psychiatry. 2016;80(1):e3–e4. doi: 10.1016/j.biopsych.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 88.Kobayashi Y., Kinoshita T., Matsumoto A., Yoshino K., Saito I., Xiao J. Z. Bifidobacterium breve A1 supplementation improved cognitive decline in older adults with mild cognitive impairment: an open-label, single-arm study. The Journal of Prevention of Alzheimer's Disease. 2019;6(1):70–75. doi: 10.14283/jpad.2018.32. [DOI] [PubMed] [Google Scholar]
- 89.Rezaei Asl Z., Sepehri G., Salami M. Probiotic treatment improves the impaired spatial cognitive performance and restores synaptic plasticity in an animal model of Alzheimer's disease. Behavioural Brain Research. 2019;376:p. 112183. doi: 10.1016/j.bbr.2019.112183. [DOI] [PubMed] [Google Scholar]
- 90.Tamtaji O. R., Heidari-Soureshjani R., Mirhosseini N., et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer's disease: a randomized, double-blind, controlled trial. Clinical Nutrition. 2019;38(6):2569–2575. doi: 10.1016/j.clnu.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 91.Biagi E., Nylund L., Candela M., et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5(5, article e10667) doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Franceschi C., Bonafè M., Valensin S., et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 93.Harach T., Marungruang N., Duthilleul N., et al. Erratum: reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Scientific Reports. 2017;7:p. 46856. doi: 10.1038/srep46856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dodiya H. B., Kuntz T., Shaik S. M., et al. Sex-specific effects of microbiome perturbations on cerebral Abeta amyloidosis and microglia phenotypes. The Journal of Experimental Medicine. 2019;216(7):1542–1560. doi: 10.1084/jem.20182386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fujii Y., Nguyen T. T., Fujimura Y., et al. Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with Alzheimer's disease. Bioscience, Biotechnology, and Biochemistry. 2019;83(11):2144–2152. doi: 10.1080/09168451.2019.1644149. [DOI] [PubMed] [Google Scholar]
- 96.Kim M. S., Kim Y., Choi H., et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer's disease animal model. Gut. 2020;69(2):283–294. doi: 10.1136/gutjnl-2018-317431. [DOI] [PubMed] [Google Scholar]
- 97.Chan V. S. Epigenetics in multiple sclerosis. Advances in Experimental Medicine and Biology. 2020;1253:309–374. doi: 10.1007/978-981-15-3449-2_12. [DOI] [PubMed] [Google Scholar]
- 98.Cekanaviciute E., Yoo B. B., Runia T. F., et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(40):10713–10718. doi: 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoban A. E., Stilling R. M., Ryan F. J., et al. Regulation of prefrontal cortex myelination by the microbiota. Translational Psychiatry. 2016;6, article e774 doi: 10.1038/tp.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Braniste V., Al-Asmakh M., Kowal C., et al. The gut microbiota influences blood-brain barrier permeability in mice. Science Translational Medicine. 2014;6(263, article 263ra158) doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Libbey J. E., Sanchez J. M., Doty D. J., et al. Variations in diet cause alterations in microbiota and metabolites that follow changes in disease severity in a multiple sclerosis model. Beneficial microbes. 2018;9(3):495–513. doi: 10.3920/BM2017.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fransen F., van Beek A. A., Borghuis T., et al. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Frontiers in Immunology. 2017;8:p. 1385. doi: 10.3389/fimmu.2017.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rea K., Dinan T. G., Cryan J. F. The microbiome: a key regulator of stress and neuroinflammation. Neurobiol Stress. 2016;4:23–33. doi: 10.1016/j.ynstr.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tankou S. K., Regev K., Healy B. C., et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Annals of Neurology. 2018;83(6):1147–1161. doi: 10.1002/ana.25244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen J., Chia N., Kalari K. R., et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Scientific Reports. 2016;6:p. 28484. doi: 10.1038/srep28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tremlett H., Fadrosh D. W., Faruqi A. A., et al. Gut microbiota composition and relapse risk in pediatric MS: a pilot study. Journal of the Neurological Sciences. 2016;363:153–157. doi: 10.1016/j.jns.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mangalam A., Shahi S. K., Luckey D., et al. Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Reports. 2017;20(6):1269–1277. doi: 10.1016/j.celrep.2017.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tamtaji O. R., Kouchaki E., Salami M., et al. The effects of probiotic supplementation on gene expression related to inflammation, insulin, and lipids in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Journal of the American College of Nutrition. 2017;36(8):660–665. doi: 10.1080/07315724.2017.1347074. [DOI] [PubMed] [Google Scholar]
- 109.Goverman J., Woods A., Larson L., Weiner L. P., Hood L., Zaller D. M. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72(4):551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 110.Berer K., Gerdes L. A., Cekanaviciute E., et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(40):10719–10724. doi: 10.1073/pnas.1711233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li K., Wei S., Hu L., et al. Protection of fecal microbiota transplantation in a mouse model of multiple sclerosis. Mediators of Inflammation. 2020;2020:13. doi: 10.1155/2020/2058272.2058272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lindefeldt M., Eng A., Darban H., et al. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. npj Biofilms and Microbiomes. 2019;5:p. 5. doi: 10.1038/s41522-018-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xie G., Zhou Q., Qiu C. Z., et al. Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World Journal of Gastroenterology. 2017;23(33):6164–6171. doi: 10.3748/wjg.v23.i33.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dahlin M., Prast-Nielsen S. The gut microbiome and epilepsy. eBioMedicine. 2019;44:741–746. doi: 10.1016/j.ebiom.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sewal R. K., Modi M., Saikia U. N., Chakrabarti A., Medhi B. Increase in seizure susceptibility in sepsis like condition explained by spiking cytokines and altered adhesion molecules level with impaired blood brain barrier integrity in experimental model of rats treated with lipopolysaccharides. Epilepsy Research. 2017;135:176–186. doi: 10.1016/j.eplepsyres.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 116.Gomez-Eguilaz M., Ramon-Trapero J. L., Perez-Martinez L., Blanco J. R. The beneficial effect of probiotics as a supplementary treatment in drug-resistant epilepsy: a pilot study. Beneficial Microbes. 2018;9(6):875–881. doi: 10.3920/BM2018.0018. [DOI] [PubMed] [Google Scholar]
- 117.Yeom J. S., Park J. S., Kim Y. S., et al. Neonatal seizures and white matter injury: role of rotavirus infection and probiotics. Brain and Development. 2019;41(1):19–28. doi: 10.1016/j.braindev.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 118.Olson C. A., Vuong H. E., Yano J. M., Liang Q. Y., Nusbaum D. J., Hsiao E. Y. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173(7):1728–1741. doi: 10.1016/j.cell.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seideman M. F., Seideman T. A. A review of the current treatment of Tourette syndrome. Journal of Pediatric Pharmacology and Therapeutics. 2020;25(5):401–412. doi: 10.5863/1551-6776-25.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liao J. F., Cheng Y. F., Li S. W., et al. Lactobacillus plantarum PS128 ameliorates 2,5-dimethoxy-4-iodoamphetamine-induced tic-like behaviors via its influences on the microbiota-gut-brain-axis. Brain Research Bulletin. 2019;153:59–73. doi: 10.1016/j.brainresbull.2019.07.027. [DOI] [PubMed] [Google Scholar]
- 121.Snider L. A., Lougee L., Slattery M., Grant P., Swedo S. E. Antibiotic prophylaxis with azithromycin or penicillin for childhood-onset neuropsychiatric disorders. Biological Psychiatry. 2005;57(7):788–792. doi: 10.1016/j.biopsych.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 122.Nagy-Szakal D., Williams B. L., Mishra N., et al. Fecal metagenomic profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2017;5(1):p. 44. doi: 10.1186/s40168-017-0261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sheedy J. R., Wettenhall R. E., Scanlon D., et al. Increased d-lactic acid intestinal bacteria in patients with chronic fatigue syndrome. In Vivo. 2009;23(4):621–628. [PubMed] [Google Scholar]
- 124.Mathew S. J., Mao X., Keegan K. A., et al. Ventricular cerebrospinal fluid lactate is increased in chronic fatigue syndrome compared with generalized anxiety disorder: an in vivo 3.0 T (1)H MRS imaging study. NMR in Biomedicine. 2009;22(3):251–258. doi: 10.1002/nbm.1315. [DOI] [PubMed] [Google Scholar]
- 125.Murrough J. W., Mao X., Collins K. A., et al. Increased ventricular lactate in chronic fatigue syndrome measured by 1H MRS imaging at 3.0 T. II: comparison with major depressive disorder. NMR in Biomedicine. 2010;23(6):643–650. doi: 10.1002/nbm.1512. [DOI] [PubMed] [Google Scholar]
- 126.Shungu D. C., Weiduschat N., Murrough J. W., et al. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR in Biomedicine. 2012;25(9):1073–1087. doi: 10.1002/nbm.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Storti B., Vedovello M., Riva R., et al. Posterior reversible encephalopathy and Guillain-Barré syndrome: which came first, the chicken or the egg? A review of literature. Neurological Sciences. 2020;41(12):3663–3666. doi: 10.1007/s10072-020-04496-1. [DOI] [PubMed] [Google Scholar]
- 128.Malik A., Sharma D., Charles J. S., Dybas L. A., Mansfield L. S. Contrasting immune responses mediate Campylobacter jejuni-induced colitis and autoimmunity. Mucosal Immunology. 2014;7(4):802–817. doi: 10.1038/mi.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.St Charles J. L., Bell J. A., Gadsden B. J., et al. Guillain Barre syndrome is induced in non-obese diabetic (NOD) mice following Campylobacter jejuni infection and is exacerbated by antibiotics. Journal of Autoimmunity. 2017;77:11–38. doi: 10.1016/j.jaut.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 130.Ang C. W., Laman J. D., Willison H. J., et al. Structure of Campylobacter jejuni lipopolysaccharides determines antiganglioside specificity and clinical features of Guillain-Barre and Miller Fisher patients. Infection and Immunity. 2002;70(3):1202–1208. doi: 10.1128/IAI.70.3.1202-1208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bereswill S., Fischer A., Plickert R., et al. Novel murine infection models provide deep insights into the "menage a trois" of Campylobacter jejuni, microbiota and host innate immunity. PLoS One. 2011;6(6, article e20953) doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brooks P. T., Brakel K. A., Bell J. A., et al. Transplanted human fecal microbiota enhanced Guillain Barre syndrome autoantibody responses after Campylobacter jejuni infection in C57BL/6 mice. Microbiome. 2017;5(1):p. 92. doi: 10.1186/s40168-017-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brooks P. T., Bell J. A., Bejcek C. E., Malik A., Mansfield L. S. An antibiotic depleted microbiome drives severe Campylobacter jejuni-mediated type 1/17 colitis, type 2 autoimmunity and neurologic sequelae in a mouse model. Journal of Neuroimmunology. 2019;337:p. 577048. doi: 10.1016/j.jneuroim.2019.577048. [DOI] [PubMed] [Google Scholar]
- 134.Karlsson F. H., Fåk F., Nookaew I., et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nature Communications. 2012;3:p. 1245. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yin J., Liao S. X., He Y., et al. Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. Journal of the American Heart Association. 2015;4(11) doi: 10.1161/JAHA.115.002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koren O., Spor A., Felin J., et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li N., Wang X., Sun C., et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiology. 2019;19(1, article 191) doi: 10.1186/s12866-019-1552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Singh V., Roth S., Llovera G., et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. The Journal of Neuroscience. 2016;36(28):7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen R., Wu P., Cai Z., et al. Puerariae Lobatae Radix with chuanxiong Rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain-gut barriers. The Journal of Nutritional Biochemistry. 2019;65:101–114. doi: 10.1016/j.jnutbio.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 140.Nam H. S., Ha J., Ji D., et al. Elevation of the gut microbiota metabolite trimethylamine N-oxide predicts stroke outcome. Journal of stroke. 2019;21(3):350–352. doi: 10.5853/jos.2019.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang S., Li X., Yang F., et al. Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Frontiers in Pharmacology. 2019;10:p. 1360. doi: 10.3389/fphar.2019.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xia G. H., You C., Gao X. X., et al. Stroke dysbiosis index (SDI) in gut microbiome are associated with brain injury and prognosis of stroke. Frontiers in Neurology. 2019;10:p. 397. doi: 10.3389/fneur.2019.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hergesheimer R., Lanznaster D., Vourc’h P., et al. Advances in disease-modifying pharmacotherapies for the treatment of amyotrophic lateral sclerosis. Expert Opinion on Pharmacotherapy. 2020;21(9):1103–1110. doi: 10.1080/14656566.2020.1746270. [DOI] [PubMed] [Google Scholar]
- 144.Minter M. R., Hinterleitner R., Meisel M., et al. Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APPSWE/PS1DeltaE9 murine model of Alzheimer's disease. Scientific Reports. 2017;7(1, article 10411) doi: 10.1038/s41598-017-11047-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mandrioli J., Amedei A., Cammarota G., et al. FETR-ALS study protocol: a randomized clinical trial of fecal microbiota transplantation in amyotrophic lateral sclerosis. Frontiers in Neurology. 2019;10:p. 1021. doi: 10.3389/fneur.2019.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rosas H. D., Doros G., Bhasin S., et al. A systems-level "misunderstanding": the plasma metabolome in Huntington's disease. Annals of Clinical Translational Neurology. 2015;2(7):756–768. doi: 10.1002/acn3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cheng S., Han B., Ding M., et al. Identifying psychiatric disorder-associated gut microbiota using microbiota-related gene set enrichment analysis. Briefings in Bioinformatics. 2020;21(3):1016–1022. doi: 10.1093/bib/bbz034. [DOI] [PubMed] [Google Scholar]
- 148.Yang Z., Li J., Gui X., et al. Updated review of research on the gut microbiota and their relation to depression in animals and human beings. Molecular Psychiatry. 2020;25(11):2759–2772. doi: 10.1038/s41380-020-0729-1. [DOI] [PubMed] [Google Scholar]
- 149.Bhandari R., Paliwal J. K., Kuhad A. Neuropsychopathology of autism spectrum disorder: complex interplay of genetic, epigenetic, and environmental factors. Advances in Neurobiology. 2020;24:97–141. doi: 10.1007/978-3-030-30402-7_4. [DOI] [PubMed] [Google Scholar]
- 150.De Angelis M., Francavilla R., Piccolo M., De Giacomo A., Gobbetti M. Autism spectrum disorders and intestinal microbiota. Gut Microbes. 2015;6(3):207–213. doi: 10.1080/19490976.2015.1035855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Principi N., Esposito S. Gut microbiota and central nervous system development. The Journal of Infection. 2016;73(6):536–546. doi: 10.1016/j.jinf.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 152.Qin J., Li R., Raes J., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Desbonnet L., Clarke G., Shanahan F., Dinan T. G., Cryan J. F. Microbiota is essential for social development in the mouse. Molecular Psychiatry. 2014;19(2):146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Golnik A. E., Ireland M. Complementary alternative medicine for children with autism: a physician survey. Journal of Autism and Developmental Disorders. 2009;39(7):996–1005. doi: 10.1007/s10803-009-0714-7. [DOI] [PubMed] [Google Scholar]
- 155.Partty A., Kalliomaki M., Wacklin P., Salminen S., Isolauri E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatric Research. 2015;77(6):823–828. doi: 10.1038/pr.2015.51. [DOI] [PubMed] [Google Scholar]
- 156.Urbanska M., Gieruszczak-Bialek D., Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Alimentary Pharmacology & Therapeutics. 2016;43(10):1025–1034. doi: 10.1111/apt.13590. [DOI] [PubMed] [Google Scholar]
- 157.Buffington S. A., Di Prisco G. V., Auchtung T. A., Ajami N. J., Petrosino J. F., Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. 2016;165(7):1762–1775. doi: 10.1016/j.cell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sharon G., Cruz N. J., Kang D. W., et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177(6):1600–1618. doi: 10.1016/j.cell.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Aabed K., Bhat R. S., Moubayed N., et al. Ameliorative effect of probiotics (Lactobacillus paracaseii and Protexin(R)) and prebiotics (propolis and bee pollen) on clindamycin and propionic acid-induced oxidative stress and altered gut microbiota in a rodent model of autism. Cellular and Molecular Biology (Noisy-le-Grand, France) 2019;65(1):1–7. [PubMed] [Google Scholar]
- 160.Painold A., Mörkl S., Kashofer K., et al. A step ahead: exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disorders. 2019;21(1):40–49. doi: 10.1111/bdi.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Reininghaus E. Z., Wetzlmair L. C., Fellendorf F. T., et al. The impact of probiotic supplements on cognitive parameters in euthymic individuals with bipolar disorder: a pilot study. Neuropsychobiology. 2018;79:63–70. doi: 10.1159/000492537. [DOI] [PubMed] [Google Scholar]
- 162.Simpson C. A., Schwartz O. S., Simmons J. G. The human gut microbiota and depression: widely reviewed, yet poorly understood. Journal of Affective Disorders. 2020;274:73–75. doi: 10.1016/j.jad.2020.05.115. [DOI] [PubMed] [Google Scholar]
- 163.Zhang Y., Huang R., Cheng M., et al. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome. 2019;7(1):p. 116. doi: 10.1186/s40168-019-0733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kelly J. R., Borre Y., O'Brien C., et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. Journal of Psychiatric Research. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 165.Noonan S., Zaveri M., Macaninch E., Martyn K. Food & mood: a review of supplementary prebiotic and probiotic interventions in the treatment of anxiety and depression in adults. BMJ Nutrition, Prevention & Health. 2020. [DOI] [PMC free article] [PubMed]
- 166.Xie P. Alterating the gut microbiome by microbiota transplantation from depressed patients into germ-free mice results in depressive-like behaviors through a pathway mediated by the host’s metabolism. European Neuropsychopharmacology. 2017;27:S478–S479. [Google Scholar]
- 167.Frankiensztajn L. M., Elliott E., Koren O. The microbiota and the hypothalamus-pituitary-adrenocortical (HPA) axis, implications for anxiety and stress disorders. Current Opinion in Neurobiology. 2020;62:76–82. doi: 10.1016/j.conb.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 168.Lurie I., Yang Y. X., Haynes K., Mamtani R., Boursi B. Antibiotic exposure and the risk for depression, anxiety, or psychosis: a nested case-control study. The Journal of Clinical Psychiatry. 2015;76(11):1522–1528. doi: 10.4088/JCP.15m09961. [DOI] [PubMed] [Google Scholar]
- 169.Bravo J. A., Forsythe P., Chew M. V., et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.De Palma G., Lynch M. D., Lu J., et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Science Translational Medicine. 2017;9(379) doi: 10.1126/scitranslmed.aaf6397. [DOI] [PubMed] [Google Scholar]
- 171.Zhang Z., Zhai H., Geng J., et al. Large-scale survey of gut microbiota associated with MHE via 16S rRNA-based pyrosequencing. The American Journal of Gastroenterology. 2013;108(10):1601–1611. doi: 10.1038/ajg.2013.221. [DOI] [PubMed] [Google Scholar]
- 172.Kawaguchi T., Suzuki F., Imamura M., et al. Rifaximin-altered gut microbiota components associated with liver/neuropsychological functions in patients with hepatic encephalopathy: an exploratory data analysis of phase II/III clinical trials. Hepatology Research. 2019;49(4):404–418. doi: 10.1111/hepr.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rosenberger D. C., Blechschmidt V., Timmerman H., Wolff A., Treede R. D. Challenges of neuropathic pain: focus on diabetic neuropathy. Journal of Neural Transmission (Vienna) 2020;127(4):589–624. doi: 10.1007/s00702-020-02145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jamshidi P., Hasanzadeh S., Tahvildari A., et al. Is there any association between gut microbiota and type 1 diabetes? A systematic review. Gut pathogens. 2019;11:p. 49. doi: 10.1186/s13099-019-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bäckhed F., Ding H., Wang T., et al. The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vrieze A., Van Nood E., Holleman F., et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–916. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 177.Guo R., Chen L. H., Xing C., Liu T. Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. British Journal of Anaesthesia. 2019;123(5):637–654. doi: 10.1016/j.bja.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 178.Shen S., Lim G., You Z., et al. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nature Neuroscience. 2017;20(9):1213–1216. doi: 10.1038/nn.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Castelli V., Palumbo P., d'Angelo M., et al. Probiotic DSF counteracts chemotherapy induced neuropathic pain. Oncotarget. 2018;9(46):27998–28008. doi: 10.18632/oncotarget.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang J., Zhang C., Wang J., Guo Q., Zou W. Oral Lactobacillus reuteri LR06 or Bifidobacterium BL5b supplement do not produce analgesic effects on neuropathic and inflammatory pain in rats. Brain and Behavior: A Cognitive Neuroscience Perspective. 2019;9(4, article e01260) doi: 10.1002/brb3.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mazeraud A., Righy C., Bouchereau E., Benghanem S., Bozza F. A., Sharshar T. Septic-associated encephalopathy: a comprehensive review. Neurotherapeutics. 2020;17(2):392–403. doi: 10.1007/s13311-020-00862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Czempik P. F., Pluta M. P., Krzych L. J. Sepsis-associated brain dysfunction: a review of current literature. International Journal of Environmental Research and Public Health. 2020;17(16) doi: 10.3390/ijerph17165852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li H., Limenitakis J. P., Greiff V., et al. Mucosal or systemic microbiota exposures shape the B cell repertoire. Nature. 2020;584(7820):274–278. doi: 10.1038/s41586-020-2564-6. [DOI] [PubMed] [Google Scholar]
- 184.Li S., Lv J., Li J., et al. Intestinal microbiota impact sepsis associated encephalopathy via the vagus nerve. Neuroscience Letters. 2018;662:98–104. doi: 10.1016/j.neulet.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 185.Ebino K. Y., Amao H., Suwa T., Kuwabara Y., Saito T. R., Takahashi K. W. Coprophagy in the germfree mouse. Jikken Dobutsu. 1987;36(1):33–37. doi: 10.1538/expanim1978.36.1_33. [DOI] [PubMed] [Google Scholar]
- 186.Chen H. T., Huang H. L., Xu H. M., et al. Fecal microbiota transplantation ameliorates active ulcerative colitis. Experimental and Therapeutic Medicine. 2020;19(4):2650–2660. doi: 10.3892/etm.2020.8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gao W., Salzwedel A. P., Carlson A. L., et al. Gut microbiome and brain functional connectivity in infants-a preliminary study focusing on the amygdala. Psychopharmacology. 2019;236(5):1641–1651. doi: 10.1007/s00213-018-5161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang F. M., Liu Y. F. Evidence and decision of the choice of delivery way in washed microbiota transplantation. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23(Z1):45–47. doi: 10.3760/cma.j.cn.441530-20200507-00259. [DOI] [PubMed] [Google Scholar]
- 189.Zhang T., Lu G., Zhao Z., et al. Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein & Cell. 2020;11(4):251–266. doi: 10.1007/s13238-019-00684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from the review are available upon request from the corresponding authors (Y.Q.N. and Y.J.Z.).


