Abstract
Alzheimer’s disease (AD) is one of the major neurodegenerative diseases whose underlying risk factors are yet to be fully understood. However, reduced cellular level of cholinesterase, as well as formation and deposition of amyloid plaques (Aβ) are thought to play critical roles in the pathogenesis of AD. Therefore, increases in cholinergic transmitter levels via cholinesterase (ChE) inhibitors as well as inhibition of amyloid plaques formation and aggregation via beta secretase-1 (BACE1) inhibitors have been proposed as treatment for this disease. This study was aimed at investigating the BACE1 and ChE inhibitory properties of compounds from Cajanus cajan and Citrus reticulata based on their traditional connection with the management of neurodegenerative diseases, coupled with their protective effects on chemical-induced cognitive impairment. Using in silico methods, one hundred and nineteen compounds from C. cajan and C. reticulata were docked with acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and BACE1 using Vina. Molecular interactions of the top-ranked compounds for the 3 protein targets were viewed with Discovery Studio, followed by characterization of their ADME properties using the Swiss online ADME web tool. Among the one hundred and ninety nine compounds screened, 3 compounds, genistin (76), naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester (94) and vitexin (119) have remarkable binding affinity for the three protein targets and passed the oral drugability test, while only naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester (94) exhibited BBB permeation property. Genistin and vitexin from C. cajan and naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester from C. reticulata possibly contributed, at least in part, to the neurotherapeutic potentials of these plants.
Keywords: In silico, Binding affinity, ADME, Alzheimer’s disease, Cajanus cajan, Citrus reticulata, Cholinesterase, Beta secretase-1
Introduction
Alzheimer’s disease (AD) is one of the major neurodegenerative diseases whose underlying risk factors are yet to be fully understood and for which no effective treatment is available. It is characterized by memory loss, behavioral imbalance and impaired cognitive function. It is estimated that about 74.7 million will be affected by this disease in 2030 and over 131.5 million by 2050 (Ijaopo 2017). Alzheimer’s disease is associated with the presence of senile plaques in the brain hippocampal area, leading to reduced cellular level of the neurotransmitter acetylcholine, deposition of amyloid plaques (Aβ) and formation of neurofibrillary tangles (NFT) (Murphy and LeVine 2010; Borah et al. 2019). Symptoms modifying therapeutics available for the treatment of AD involve the use of cholinesterase inhibitors like donepezil, rivastigmine, memantine and galantamine (Molinuevo et al. 2013; Borah et al. 2019). There are two major hypotheses regarding AD, namely the amyloid cascade and cholinergic hypotheses, that are widely accepted and the focus of many scientific investigations (Jung et al. 2015). The amyloid hypothesis suggests that the accumulation and oligomerization of Aβ peptide in the brain plays a critical role in AD pathogenesis while the cholinergic hypothesis proposes a massive loss of cholinergic neurons as a downstream phenotypic consequence in the pathogenesis of AD (Henry et al. 2010). Therefore, the upturn of cholinergic transmitter levels via acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitors (Rao et al. 2007), as well as inhibition of Aβ formation and aggregation via beta secretase-1 (BACE1) inhibitors (Yan et al. 1999; Vassar 2002), have been proposed as treatment options for AD.
Over the centuries, the importance of plant-based traditional medicine has been established, and in recent years there has been an increasing interest in plant products as important sources of new drugs for the treatment of neurodegenerative diseases such as AD. In line with this, computational studies, such as molecular docking approach, are now being used for screening plant-derived compounds to identify potential drug/targets (Walters et al. 1998; McConkey et al. 2002; Meng et al. 2011). Therefore, this molecular docking study was aimed at investigating the therapeutic potentials of some plant-derived compounds as drug agents for the treatment of AD. Potential lead compounds were sought from 2 medicinal plants; Cajanus cajan and Citrus reticulata, because of their traditional connection with the management of neurodegenerative diseases and their protective effects on chemical-induced cognitive impairment (Ruan et al. 2009; Seki et al. 2013; Ademosun and Oboh 2014; Ahmad et al. 2019; Wang et al. 2019). Cajanus cajan has been reported to have various medicinal properties, including antioxidative, anti-inflammatory, hypoglycemic, hypocholesterolemic, antibacterial, and antiproliferative properties (Gro et al. 2002; Luo et al. 2010; Schuster et al. 2016). Similarly, Citrus reticulata peels are used as a dietary source for the management of AD in some countries, including China, Korea, and Japan (Qin et al. 2013; Seki et al. 2013). Studies have shown that flavonoids from C. reticulata have neuroprotective effects via antioxidative, anticholinesterase, and anti-inflammatory mechanisms (Gopinath and Sudhandiran 2012; Ademosun and Oboh 2014). From extensive literature searches, we identified a list of C. cajan and C. reticulata-derived compounds and evaluated their interaction and binding affinity for AChE, BChE and BACE1, three popular AD target proteins.
Materials and methods
Pose validation
Crystal structure of AChE (6O4W) with its co-crystallized ligand (donepezil) was visualized using the structure editor in Discovery Studio Visualizer 2020. Thereafter, donepezil was docked to AChE (1QTI) and donepezil-1QTI complex was aligned to 6O4W. Subsequently the root mean square deviation (RMSD) was calculated between native pose and re-docked pose of ligand. RMSD values up to 2 Å are quite acceptable (Ganai et al. 2015) and the closer the value is to 0 the better the algorithm in reproducing native pose.
Protein preparation
The crystal structures of AChE, BChE, BACE1 with PDB IDs of 1QTI (Bartolucci et al. 2001), 1P0P (Nicolet et al. 2003) and, 6EJ3 (Johansson et al. 2018), respectively were retrieved from the protein databank (http://www.rcsb.org). All the crystal structures were prepared individually by removing existing ligands and water molecules while missing hydrogen atoms were added using Autodock v4.2 program, Scripps Research Institute. Thereafter, non-polar hydrogens were merged while polar hydrogen where added to each enzyme. The process was repeated for each protein and subsequently saved into dockable Protein Data Bank, Partial Charge, and Atom Type (PDBQT) format in preparation for molecular docking.
Ligand preparation
The structure data file (SDF) format of the standard inhibitors donepezil, elenbecestat and 119 compounds derived from C. cajan and C. reticulata were retrieved from the PubChem database (http://www.pubchem.ncbi.nlm.nih.gov). The compounds were converted to Protein Data Bank (PDB) format using Discovery Studio Visualizer, BIOVIA, 2016. Polar hydrogen charges of the Gasteiger-type were assigned and the nonpolar hydrogens were merged with the carbons and the internal degrees of freedom and torsions were set. The protein and ligand molecules were further converted to the dockable PDBQT format using Autodock tools.
Molecular docking
Docking of the ligands to various protein targets and determination of binding affinities was carried out using Vina (Trott and Olson 2010). PDBQT form of individual enzyme and ligands were dragged into their respective columns and the software was run. A cluster analysis based on root mean square deviation (RMSD) values, with reference to the starting geometry was subsequently performed and the lowest energy conformation of the more populated cluster was considered the most trustable solution. The binding affinities of compounds for the three targets were recorded. The compounds were then ranked by their affinity scores. Molecular interactions between compounds with multiple remarkable binding affinities for the three targets were viewed with Discovery Studio Visualizer, BIOVIA, 2020. The binding profiles were determined using receptor cavities identified using Discovery Studio Visualizer. The average RMSD for the complexes were determined using Docking Pose Distance Calculation (DockRMSD) program (Bell and Zhang 2019).
Druglikeness and prediction of absorption distribution metabolism excretion
In silico methods for the determination of Absorption–Distribution–Metabolism–Excretion (ADME) parameters rely on theoretically derived statistical models, which have been generated by relating the structural characteristics of compounds that have been measured in a given assay to their biological responses, and is now widely used due to its low resource requirement (Gleeson et al. 2011). Consequently, the top 5 compounds that showed reasonable binding modes with AChE, BChE and BACE1 (based on higher negative binding energy than respective standard inhibitors) were selected for druglikeness study. ADME studies were carried out using the Swiss online ADME web tool (Daina et al. 2014, 2017; Daina and Zoete 2016) to evaluate the drug-likeness of the phytochemicals that were selective for the three protein drug targets. A graph of WLOGP against TPSA was plotted using GraphPad Prism 6 software (GraphPad Software, California, USA) to determine the blood-brain barrier (BBB) properties of the compounds as described by (Ishola and Adewole 2019).
Results
Molecular docking
Prior to molecular docking of test ligands, docked-donepezil—1QTI complex was aligned with 6O4W PDB. The root mean square deviation was calculated between native (ash) and re-docked (yellow) pose of ligand atoms (Fig. 1). The root mean square deviation value was found to be 0.37 Å which was very low and acceptable. The binding affinities of the one hundred and nineteen compounds to AChE, BChE, and BACE1 are shown in Table 1. Compounds having higher binding affinity than the corresponding standard drugs are indicated in boldface. From the results, 7 out of the 119 compounds have remarkable (more) negative binding affinity for AChE compared to donepezil, the standard cholinesterase inhibitor, with naringin (95) being the most prominent AChE inhibitor (binding affinity of − 12.8 Kcal/mol) compared to donepezil (− 12.2 Kcal/mol) (Table 1). Limonexic acid (89) exhibited the most negative binding affinity (− 13.0 Kcal/mol) for BChE compared to donepezil (− 10.5 Kcal/mol). Naringin (95) was also the best ligand for BACE1 with a binding affinity of (− 12.6 Kcal/mol) compared to − 9.7 Kcal/mol for elenbecestat, the standard BACE1 inhibitor (Table 1). Of all the one hundred and ninety-nine compounds only genistin (76), naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester (94), naringin (95), orientin (99) and vitexin (119) have multiple specificities for the three protein targets, considering their remarkable binding affinity.
Fig. 1.

Crystal structure of AChE (6O4W) and docked donepezil-1QTI complex. The root mean square deviation was calculated between native (ash) and re-docked (yellow) pose of ligand
Table 1.
Binding affinity of compounds identified in Cajanus cajan and Citrus reticulata for AChE, BChE and BACE1
| Binding affinity (Kcal/mol) | |||||
|---|---|---|---|---|---|
| S/N | Compounds | Plants | AChE | BChE | BACE1 |
| S1 | Donepezil | Citrus reticulata | − 12.2 | − 10.5 | – |
| S2 | Elenbecestat | Citrus reticulata | – | – | − 9.7 |
| 1 | 1-(2-Acetoxyethyl)-3,6-diazahomoadamantan-9-one oxime | Citrus reticulata | − 9.0 | − 8.4 | − 7.4 |
| 2 | 1,5,5-Trimethyl-6-methylene-cyclohexene | Citrus reticulata | − 7.1 | − 6.0 | − 6.0 |
| 3 | 1-Heptatriacotanol | Citrus reticulata | − 6.9 | − 7.0 | − 7.5 |
| 4 | 2-(2-Azepan-1-yl-2-oxoethyl)-1-hydroxy-1-phenyl-octahydro-pyrido[1,2-a]azepin-4-one | Citrus reticulata | − 12.0 | − 11.2 | − 10.1 |
| 5 | 2,2,4,4-Tetramethyl-6-(1-oxo-3-phenylprop-2-enyl)-cyclohexane-1,3,5-trione | Citrus reticulata | − 12.4 | − 9.4 | − 9.0 |
| 6 | 2,4-diisopropenyl-1-methyl-1-vinyl | Citrus reticulata | − 9.2 | − 7.9 | − 7.4 |
| 7 | 2,5-Octadecadienoic acid, methyl ester | Citrus reticulata | − 9.4 | − 7.3 | − 7.5 |
| 8 | 2-Hydroxygenistein | Cajanus cajan | − 10.9 | − 9.1 | − 9.4 |
| 9 | 3-(5-Benzyloxy-3-methylpent-3-enyl)-2,2-dimethyloxirane | Citrus reticulata | − 9.4 | − 7.9 | − 7.2 |
| 10 | 2-Methoxy-4-vinylphenol | Citrus reticulata | − 7.0 | − 6.8 | − 6.0 |
| 11 | 2H-Indeno[1,2-b]furan-2-one, 3,3a,4,5,6,7, 8,8b-octahydro-8,8-dimethyl | Citrus reticulata | − 8.7 | − 7.7 | − 7.3 |
| 12 | 3’ Hydroxy-5,6,7,8,4’-pentamethoxyflavone | Citrus reticulata | − 11.1 | − 10.1 | − 10.3 |
| 13 | 3-Hydroxyquinalbarbitone | Citrus reticulata | − 8.9 | − 8.5 | − 7.1 |
| 14 | 4,4-Dimethyl-3-(3-methylbut-3-enylidene)-2-methylenebicyclo[4.1.0]heptane | Citrus reticulata | − 8.8 | − 8.0 | − 7.3 |
| 15 | 4,5-Di-epi-aristolochene | Citrus reticulata | − 9.3 | − 8.3 | − 7.4 |
| 16 | 4,7,10-Hexadecatrienoic acid, methyl ester | Citrus reticulata | − 8.8 | − 7.1 | − 7.3 |
| 17 | 5,6,7,3’,4’-pentamethoxyflavanone | Citrus reticulata | − 11.2 | − 9.7 | − 9.9 |
| 18 | 5,8,11-Eicosatrienoic acid, methyl ester | Citrus reticulata | − 9.5 | − 7.5 | − 8.1 |
| 19 | 5-Demethylcitromitine | Citrus reticulata | − 10.6 | − 10.1 | − 9.6 |
| 20 | 5-Demethylnobiletin | Citrus reticulata | − 11.0 | − 9.9 | − 9.8 |
| 21 | 6,9,12-Octadecatrienoic acid, methyl ester | Citrus reticulata | − 8.9 | − 7.3 | − 7.4 |
| 22 | 7-Epi-cis-sesquisabinene hydrate | Citrus reticulata | − 8.7 | − 7.8 | − 7.7 |
| 23 | 7-Hydroxy-6,9a-dimethyl-3-methylene-decahydro-azuleno[4,5-b]furan-2,9-dione | Citrus reticulata | − 10.0 | − 9.2 | − 8.2 |
| 24 | 9-Hexadecenoic acid | Citrus reticulata | − 9.2 | − 7.5 | − 7.4 |
| 25 | 10,13-Octadecadienoic acid, methyl ester | Citrus reticulata | − 9.1 | − 7.5 | − 7.5 |
| 26 | 10-Methyl-E-11-tridecen-1-ol propionate | Citrus reticulata | − 9.4 | − 7.2 | − 7.6 |
| 27 | 12,14,14-Trimethyl-3,6,9-trioxapentadecan-1-ol | Citrus reticulata | − 8.5 | − 7.5 | − 7.6 |
| 28 | 12,15-Octadecadienoic acid, methyl ester | Citrus reticulata | − 9.3 | − 7.2 | − 8.0 |
| 29 | Acetic acid, octyl ester | Citrus reticulata | − 7.1 | − 5.8 | − 6.3 |
| 30 | α-Copaene | Citrus reticulata | − 8.9 | − 7.8 | − 6.7 |
| 31 | α-Cadinol | Citrus reticulata | − 9.1 | − 8.1 | − 7.3 |
| 32 | α-Ionol | Citrus reticulata | − 8.0 | − 7.3 | − 7.0 |
| 33 | α-Phellandrene | Citrus reticulata | − 7.3 | − 6.0 | − 5.8 |
| 34 | α-Pinene | Citrus reticulata | − 6.8 | − 5.8 | − 5.5 |
| 35 | α-Sinensal | Citrus reticulata | − 8.5 | − 7.5 | − 7.2 |
| 36 | α-Terpinene | Citrus reticulata | − 7.6 | − 6.2 | − 5.9 |
| 37 | α-Terpineol | Citrus reticulata | − 7.4 | − 6.6 | − 6.6 |
| 38 | Aromadendrene oxide-(2) | Citrus reticulata | − 9.3 | − 7.9 | − 8.2 |
| 39 | β-Myrcene | Citrus reticulata | − 7.6 | − 6.4 | − 6.0 |
| 40 | β-Caryophyllene | Citrus reticulata | − 8.9 | − 7.8 | − 7.6 |
| 41 | β-Copaene | Citrus reticulata | − 8.9 | − 7.9 | − 6.8 |
| 42 | β-Elemene | Citrus reticulata | − 9.0 | − 8.0 | − 7.6 |
| 43 | β-Guaiene | Citrus reticulata | − 9.6 | − 7.8 | − 7.7 |
| 44 | β-Ocimene | Citrus reticulata | − 7.1 | − 6.2 | − 5.9 |
| 45 | β-Spathulenol | Citrus reticulata | − 9.4 | − 8.1 | − 7.1 |
| 46 | β-Vatirenene | Citrus reticulata | − 9.3 | − 8.2 | − 7.3 |
| 47 | Betulinic acid | Cajanus cajan | − 10.4 | − 12.0 | − 11.3 |
| 48 | Biochanin A | Cajanus cajan | − 10.6 | − 9.2 | − 9.4 |
| 49 | Cajanol | Cajanus cajan | − 10.3 | − 9.5 | − 9.8 |
| 50 | Cajanolactone A | Cajanus cajan | -11.0 | − 11.0 | − 10.0 |
| 51 | Camphene | Citrus reticulata | − 6.7 | − 5.7 | − 5.4 |
| 52 | Caryophyllene oxide | Citrus reticulata | − 10.0 | − 8.0 | − 7.5 |
| 53 | Cis Limonene oxide | Citrus reticulata | − 6.8 | − 6.0 | − 5.9 |
| 54 | Cis-p-Mentha-1(7),8-dien-2-ol | Citrus reticulata | − 7.2 | − 6.5 | − 6.1 |
| 55 | Citronellal | Citrus reticulata | − 7.0 | − 6.2 | − 6.2 |
| 56 | Citronellol | Citrus reticulata | − 6.9 | − 6.0 | − 6.1 |
| 57 | Cubenol | Citrus reticulata | − 9.4 | − 7.9 | − 8.0 |
| 58 | Cyclohexane | Citrus reticulata | − 4.7 | − 4.1 | − 3.8 |
| 59 | Cyclopentaneundecanoic acid | Citrus reticulata | − 9.2 | − 7.2 | − 7.5 |
| 60 | D-limonene | Citrus reticulata | − 7.4 | − 6.1 | − 5.8 |
| 61 | D-Verbenone | Citrus reticulata | − 7.0 | − 6.1 | − 5.6 |
| 62 | Deacetylnomilin | Citrus reticulata | − 10.5 | − 12.1 | − 11.3 |
| 63 | Decanal | Citrus reticulata | − 6.7 | − 5.5 | − 5.9 |
| 64 | δ-Guaiene | Citrus reticulata | − 9.6 | − 8.0 | − 7.3 |
| 65 | Desulphosinigrin | Citrus reticulata | − 9.2 | − 8.6 | − 8.1 |
| 66 | Dimethyl anthranilate | Citrus reticulata | − 7.2 | − 6.5 | − 6.1 |
| 67 | Doconexent | Citrus reticulata | -12.7 | − 11.2 | − 9.4 |
| 68 | Dodecanal | Citrus reticulata | − 7.6 | − 6.1 | − 6.0 |
| 69 | Ethylbenzene | Citrus reticulata | − 6.4 | − 5.4 | − 5.2 |
| 70 | γ-Elemene | Citrus reticulata | − 8.9 | − 7.9 | − 7.5 |
| 71 | γ-Pyronene | Citrus reticulata | − 7.1 | − 6.1 | − 6.0 |
| 72 | γ-Terpinene | Citrus reticulata | − 7.7 | − 6.3 | − 6.0 |
| 73 | Gardenin B | Citrus reticulata | − 10.8 | − 9.7 | − 9.6 |
| 74 | Gemacrene A | Citrus reticulata | − 9.0 | − 7.8 | − 7.5 |
| 75 | Genistein | Cajanus cajan | − 10.6 | − 9.4 | − 9.2 |
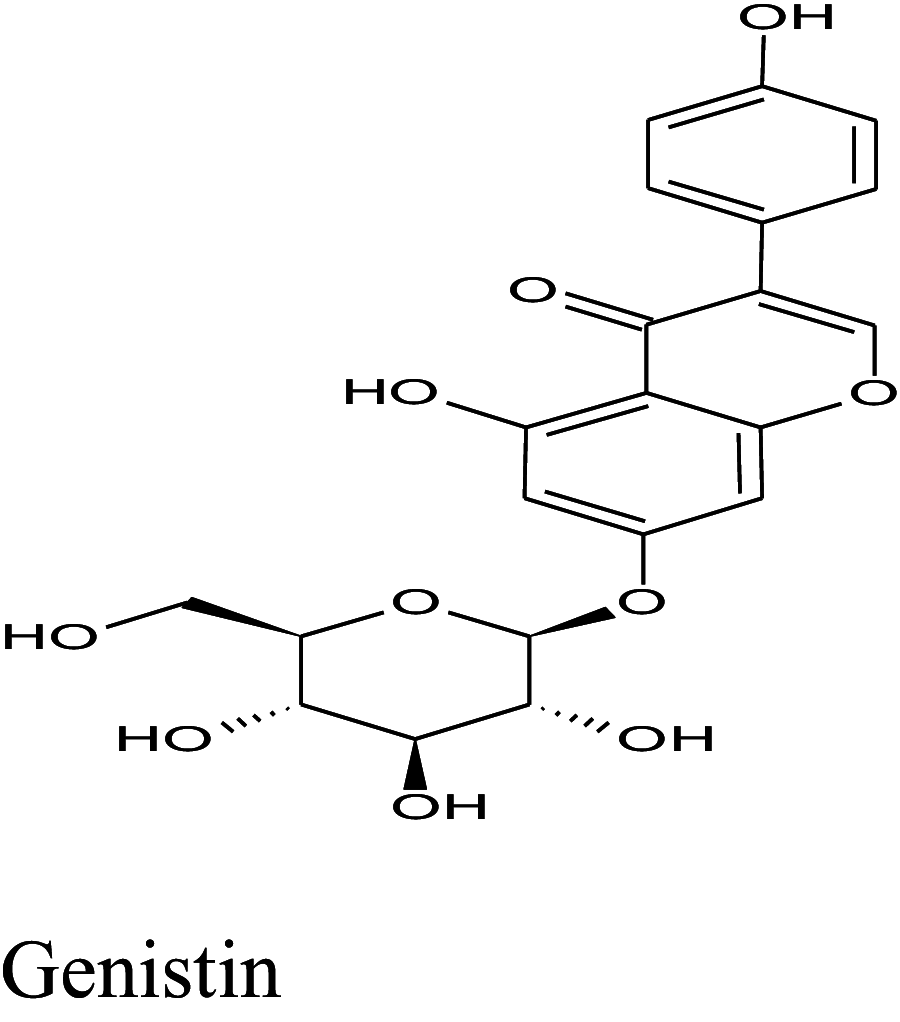
| 76 | Genistin | Cajanus cajan | − 12.5 | − 12.3 | − 11.4 |
| 77 | Gentamicin | Citrus reticulata | − 10.5 | − 10.4 | − 10.3 |
| 78 | Geranyl acetate | Citrus reticulata | − 8.1 | − 6.3 | − 7.2 |
| 79 | Geranyl isovalerate | Citrus reticulata | − 8.9 | − 7.5 | − 7.3 |
| 80 | Geranyl vinyl ether | Citrus reticulata | − 7.8 | − 6.4 | − 6.8 |
| 81 | Globulol | Citrus reticulata | − 8.9 | − 8.0 | − 7.2 |
| 82 | Heptamethoxyflavone | Citrus reticulata | − 10.3 | − 9.2 | − 10.2 |
| 83 | Humulene | Citrus reticulata | − 9.2 | − 7.9 | − 7.5 |
| 84 | Isocalamendiol | Citrus reticulata | − 9.3 | − 8.4 | − 7.6 |
| 85 | Isoledene | Citrus reticulata | − 9.2 | − 8.1 | − 7.4 |
| 86 | Isoterpinene | Citrus reticulata | − 7.6 | − 6.2 | − 5.8 |
| 87 | Kihadanin A | Citrus reticulata | − 11.4 | − 12.6 | − 11.8 |
| 88 | Limonen-6-ol, pivalate | Citrus reticulata | − 9.4 | − 8.9 | − 7.9 |
| 89 | Limonexic acid | Citrus reticulata | − 11.3 | − 13.0 | − 11.8 |
| 90 | Limonin | Citrus reticulata | − 10.2 | − 11.9 | − 11.2 |
| 91 | Linalool | Citrus reticulata | − 7.0 | − 6.3 | − 5.9 |
| 92 | Longistylin A | Cajanus cajan | -11.5 | − 9.7 | − 9.0 |
| 93 | Longistylin C | Cajanus cajan | − 11.3 | − 9.8 | − 9.6 |
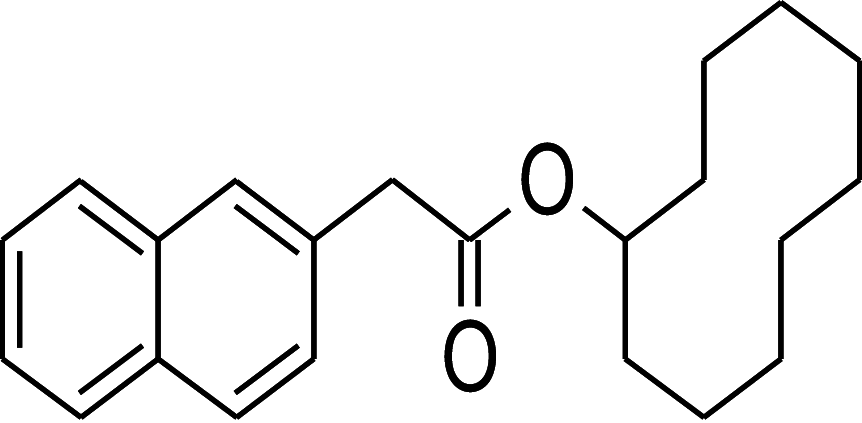
| 94 | Naphthalen-2-yl-acetic acid, 6-hydroxy-6- methyl-cyclodecyl ester | Citrus reticulata | − 12.7 | − 10.9 | − 10.0 |
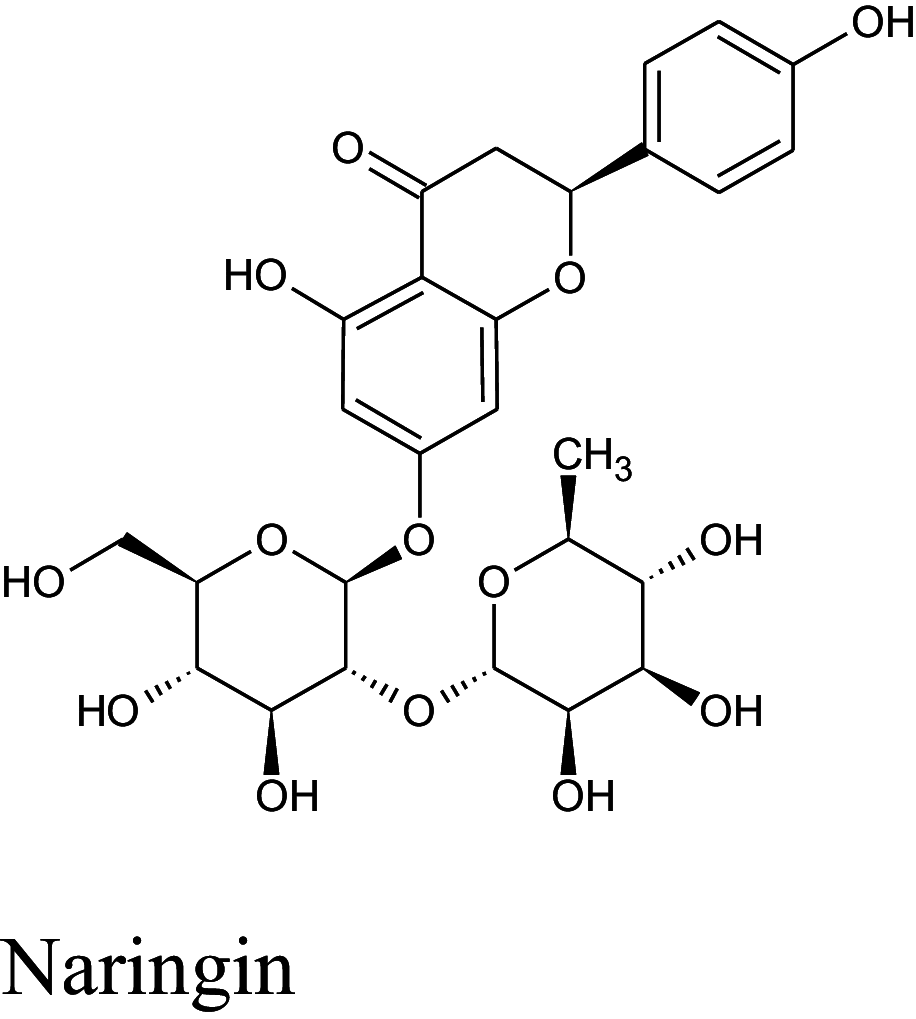
| 95 | Naringin | Citrus reticulata | − 12.8 | − 11.6 | − 12.6 |
| 96 | Neryl acetate | Citrus reticulata | − 8.1 | − 7.4 | − 7.0 |
| 97 | Nobiletin | Citrus reticulata | − 10.9 | − 9.9 | − 10.4 |
| 98 | O-Cymene | Citrus reticulata | − 7.3 | − 6.3 | − 6.2 |
| 99 | Orientin | Cajanus cajan | − 12.6 | − 10.8 | − 12.3 |
| 100 | Palmitic acid | Citrus reticulata | − 9.2 | − 7.5 | − 7.4 |
| 101 | Perillaldehyde | Citrus reticulata | − 7.3 | − 6.5 | − 5.8 |
| 102 | Peyonine | Citrus reticulata | − 9.3 | − 8.8 | − 8.9 |
| 103 | Pinostrobin | Cajanus cajan | − 10.3 | − 9.3 | − 8.6 |
| 104 | Pterin-6-carboxylic acid | Citrus reticulata | − 7.8 | − 7.6 | − 6.5 |
| 105 | Pyrimidin-2-one, 4-[N-methylureido]-1-[4- methylaminocarbonyloxymethyl | Citrus reticulata | − 9.3 | − 8.4 | − 8.0 |
| 106 | Pyrrolizidine-3-one-5-ol, ethyl ether | Citrus reticulata | − 6.7 | − 6.1 | − 5.9 |
| 107 | R-Limonene | Citrus reticulata | − 7.6 | − 6.8 | − 6.6 |
| 108 | Shihulimonin A | Citrus reticulata | − 10.5 | − 12.9 | − 11.4 |
| 109 | Sinensetin | Citrus reticulata | − 10.9 | − 9.8 | − 10.0 |
| 110 | Styrene | Citrus reticulata | − 6.5 | − 5.5 | − 5.2 |
| 111 | Tangeretin | Citrus reticulata | − 10.8 | − 10.0 | − 10.3 |
| 112 | Tau cadinol | Citrus reticulata | − 8.2 | − 7.9 | − 7.6 |
| 113 | Terpinen-4-ol | Citrus reticulata | − 7.2 | − 6.2 | − 5.9 |
| 114 | Thymol | Citrus reticulata | − 7.8 | − 6.7 | − 6.2 |
| 115 | Trans Limonene oxide | Citrus reticulata | − 7.0 | − 6.2 | − 6.3 |
| 116 | Trans Calamenene | Citrus reticulata | − 10.2 | − 8.3 | − 7.7 |
| 117 | Trans_Carveol | Citrus reticulata | − 7.5 | − 6.4 | − 6.1 |
| 118 | Trans-p-Mentha-2,8-dienol | Citrus reticulata | − 7.2 | − 6.4 | − 6.0 |
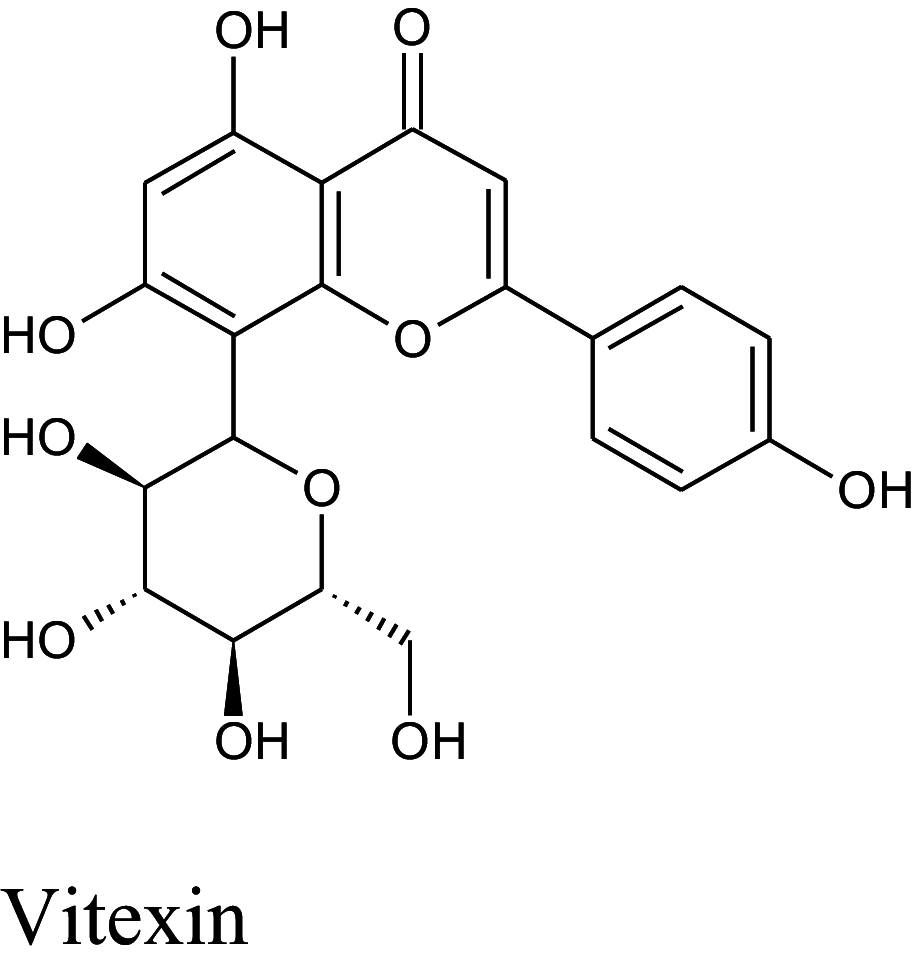
| 119 | Vitexin | Cajanus cajan | − 12.4 | − 10.6 | − 12.1 |
Donepezil, genistin (76), naphthalen-2-yl-acetic acid, 6-hydroxy-6- methyl-cyclodecyl ester (94), naringin (95), orientin (99) and vitexin (119) bind to a similar region in AChE with all visualized in a narrow gorge along the catalytic site in AChE (Figs. 1f–2a). Donepezil, genistin (76), naphthalen-2-yl-acetic acid, 6-hydroxy-6- methyl-cyclodecyl ester (94), naringin (95) bind to a similar region in BChE (Figs. 2d–3a) while orientin (99) and vitexin (119) bind to the same hydrophobic groove in BChE (Fig. 3e, f). All ligands including elenbecestat binds to the same region in BACE1 interacting with key residues in the binding site of the protein (Fig. 4a−f).
Fig. 2.
3D cartoon and stick view of interaction between amino acid in acetylcholinesterase binding sites and a donepezil b genistin c naphthalen-2-yl-acetic acid, 6-hydroxy-6- methyl-cyclodecyl ester d naringin e orientin f vitexin
Fig. 3.
3D cartoon and stick view of interaction between amino acid in butyrylcholinesterase binding sites anda donepezil b genistin c naphthalen-2-yl-acetic acid, 6-hydroxy-6- methyl-cyclodecyl esterd naringine orientinf vitexin
Fig. 4.
3D cartoon and stick view of interaction between amino acid in binding site of β-site amyloid precursor protein cleaving enzyme 1 and a elenbecestat b genistin c naphthalen-2-yl-acetic acid-6, hydroxy-6- methyl-cyclodecyl ester d naringin e orientin f vitexin
As seen in the interaction profile of donepezil, there was an extra π-π stacking with Trp279 and a π-alkyl interaction with Tyr334 (Fig. 5a), genistin (76) covers the active gorge site of AChE showing a π-π stacking with Phe330 and Trp84 as well as π-hydrogen bond with Tyr121 and Trp84 (Fig. 5b). A π-sigma interaction was formed between the heterocyclic ring of naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester (94) and two additional π-alkyl interactions with Phe300 and Phe331 (Fig. 5c). Naringin (95) has 3 hydrogen bond interactions with Tyr70, Phe284 and Ser286 as well as multiple interactions with Trp279 via π-π stacking, π-alkyl interaction and a carbon-hydrogen bond (Fig. 5d). Hydrogen bond with Tyr70, Asp72, Ser286, Phe288, and Arg289 was the main mode of interaction between orientin (99) and vitexin (119) with AChE, with additional π-π stacking seen between the protein and Trp279 and Phe330 in the active site of AChE (Fig. 5e, f).
Fig. 5.
Interaction between amino acids in the binding site of acetylcholinesterase and a donepezil b genistin c naphthalen-2-yl-acetic acid, 6-hydroxy-6- methyl-cyclodecyl ester d naringin e orientin f vitexin
Donepezil binds to Trp82 in the anionic site of BChE via π-π stacking, while π-anion interaction with Asp70 and carbon-hydrogen bond with Ser72 was also observed (Fig. 6a). Hydrogen bond was formed between the catalytic residue His438 of BChE and genistin (76) in addition to two other hydrogen bonds with Asn68 and Tyr332 (Fig. 6b). A single π-alkyl bond was the only mode of interaction observed between naphthalen-2-yl-acetic acid, 6-hydroxy-6- methyl-cyclodecyl ester (94) and, Ala328 of BChE (Fig. 6c). As observed for donepezil, π-anion interaction was also visible between genistin (76) and Asp70 of BChE. In addition to 3 π-π stacking with Ile69, Tyr332 and Phe329, naringin (95) binds to Asn68 and Asp70 through hydrogen bond and π-anion interaction respectively (Fig. 6d). Vitexin (119) and orientin (99) each has a donor-donor interaction with Asn228, π-sigma interaction with Pro527, and π-alkyl interaction with Pro401 of BChE (Fig. 6e, f).
Fig. 6.
Interaction between amino acids in the binding site of butyrylcholinesterase and a donepezil b genistin c naphthalen-2-yl-acetic acid-6, hydroxy-6-methyl-cyclodecyl ester d naringin e orientin f vitexin
Elenbecestat, a standard inhibitor of BACE1 exhibited a remarkable binding profile with BACE1 forming halogen bond with Phe108, Ile126, and with the aid of the three-fluoride residue found in the compound (Fig. 7a) and a carbon-hydrogen bond with Tyr71, Gln73. Multiple hydrogen bond formation was observed in the interaction profile of genistin (76) and BChE in conjunction with carbon-hydrogen bond formation with Gly34, Ser35, and Tyr71 (Fig. 7b). The aromatic groups in naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester (94) have multiple interactions with Val69, Ile118, and Arg128 via π-alkyl interaction together with a π-anionic interaction with catalytic Asp32 in the active site of BACE1 (Fig. 7c). A combination of hydrogen bond with Thr72, π-alkyl interaction with Tyr71, and donor-donor interaction with Arg128 was the only means of interaction between naringin (95) and BChE (Fig. 7d). Due to similarity in structure, orientin (99) and vitexin (119) exhibited similar binding patterns, forming a hydrogen bond with Trp76, Ile126, and Tyr198 (Fig. 7e, f). In addition to the hydrogen bonds, a π-π stacking with Tyr71 as well as donor-donor interaction with Arg235 was also observed.
Fig. 7.
Interaction between amino acids in the binding site of β-site amyloid precursor protein cleaving enzyme 1 and a elenbecestat b genistin c naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester d naringin e orientin f vitexin
Pharmacokinetic properties of notable compounds
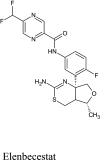
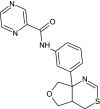
Table 2 shows the results obtained for the ADME study on the 5 compounds with remarkable binding energy for the selected proteins; these compounds include genistin (76), naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester (94), naringin (95), orientin (99) and vitexin (119). Of these 5 compounds, only 3, including, genistin (76), naphthalen-2-yl-acetic acid, 6-hydroxy-6- methyl-cyclodecyl ester (94), and vitexin (119) passed the oral drugability sieve, based on Lipinski’s rule of 5, indicating that only three of the compounds, are potential oral drug candidates. Naringin (95) and orientin (99) have 14 and 11 hydrogen bond acceptors which is higher than the 10-hydrogen bond threshold allowed for oral drug candidates (Table 2). Both also have 8 hydrogen bond donors (which is > 5) as stipulated by the Lipinski rule of 5 (Lipinski 2000) while Naringin (95) had an additional violation of 580.53 g/mol molecular weight (> 500 g/mol) making a total of three violations for naringin (95) and two for orientin (99). Genistin (76) and vitexin (119) have a single violation with 6 and 7 hydrogen bond donors respectively (which are > 5) as stipulated by Lipinski. Also, naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester (94) scaled through the Lipinski’s rule of 5 without any violation. According to Lipinski’s rule, an orally active drug has no more than one violation of the following criteria: (1) not > 5 hydrogen bond donors (nitrogen or oxygen atoms with one or more hydrogen atoms). (2) not > 10 hydrogen bond acceptors (nitrogen or oxygen atoms) (3) a molecular mass < 500 Daltons and (4) an octanol-water partition coefficient log P not greater than 5. Moderately polar (TPSA < 79 Å2) and relatively lipophilic (WLOGP from 0.4 to 6.0) compounds have a high probability to access the central nervous system (CNS) (Daina and Zoete 2016). Thus, only naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester (94) (TPSA = 46.53Å2 and WLOGP = 5.18) fell within this category, while the other 4 violated the threshold (Table 3). This was shown in an egg-yolk-like model where only donepezil and naphthalen-2-yl-acetic acid, 6-hydroxy-6- methyl-cyclodecyl ester (94) fell within the sphere, while elenbecestat and the other 4 compounds, genistin (76), naringin (95), orientin (99) and vitexin (119) fell outside the sphere (Fig. 8), indicating they are non-blood brain barrier (BBB) permeant. Naringin (95), orientin (99), vitexin (119) had identical ring system, with similar scaffold with genistin (76), thus the distinct naphthalene backbone of naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester (94) (Table 3) may be a significant feature responsible for the difference in its pharmacokinetic properties.
Table 2.
Physicochemical properties of alkaloids with remarkable binding energy with acetylcholinesterase, butyrylcholinesterase, and β-site amyloid precursor protein cleaving enzyme-1
| S/N | Compounds | Molecular weight | H-bond acceptor | H-bond donor | MLogP | Lipinski’s violations |
|---|---|---|---|---|---|---|
| S | Donepezil | 379.49 | 4 | 0 | 3.06 | 0 |
| S | Elenbecestat | 437.44 | 8 | 2 | 1.54 | 0 |
| 1 | Genistin | 432.38 | 10 | 6 | − 1.61 | 1 |
| 2 | Naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester | 354.48 | 3 | 1 | 4.02 | 0 |
| 3 | Naringin | 580.53 | 14 | 8 | − 2.77 | 3 |
| 4 | Orientin | 448.38 | 11 | 8 | − 2.51 | 2 |
| 5 | Vitexin | 432.38 | 10 | 7 | − 2.02 | 1 |
MLOGP cctanol/water partition coefficient
Table 3.
Structure and BBB Permeation properties of, donepezil, elenbecestat genistin, naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester, narigin, orientin and vitexin
| Compounds | Murcko scaffold | WLOGP | TPSA (Å2) | BBB permeation |
|---|---|---|---|---|

|

|
3.83 | 38.77 | Yes |
|
|
3.35 | 127.79 | No |
|

|
0.05 | 170.05 | No |

|
|
5.18 | 46.53 | Yes |
|
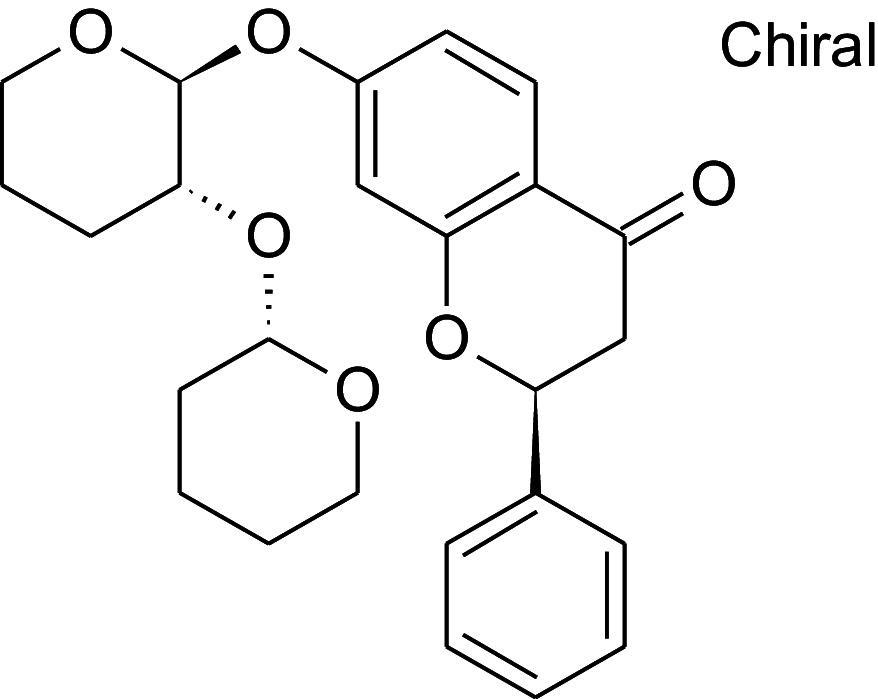
|
− 1.49 | 225.06 | No |

|

|
− 0.53 | 201.28 | No |
|
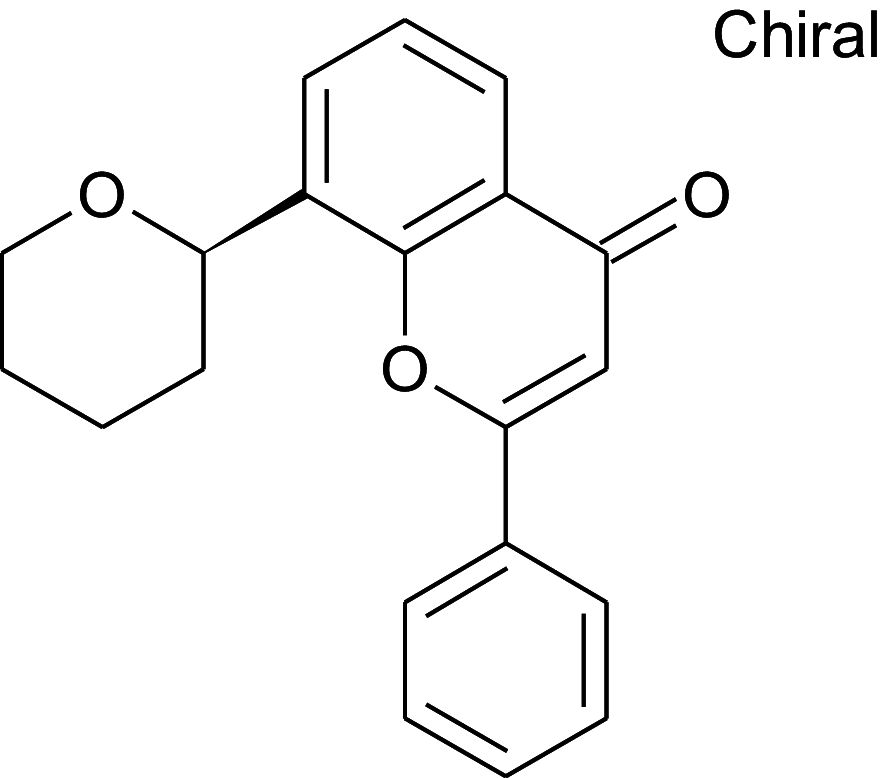
|
− 0.23 | 181.05 | No |
TPSA topological polar surface area , WLOGP lipophilicity
Fig. 8.

Blood brain barrier properties of donepezil, elenbecestat, and 5 compounds with remarkable binding affinity to acetylcholinesterase, butyrylcholinesterase and β-site amyloid precursor protein cleaving enzyme-1. Two permeants (BBB+, yellow dot inside black sphere), 5 non permeants (BBB-, orange dots)
Disucssion
Recently, the need for disease-refining drugs for AD is being addressed via new approaches in designing structures with different targets involved in the pathogenesis of the disease, including multi-target directed ligands (MTDLs), in line with the multifactorial and complex etiology of AD to produce suitable therapeutic efficacy (Morphy and Rankovic 2005). Thus, MTDLs that can engage multiple targets simultaneously represent a promising strategy for the treatment of AD (Jannat et al. 2019). Five compounds, genistin (76), naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester (94), naringin (95), orientin (99) and vitexin (119), out of the one hundred and nineteen screened in this study appear to be potential multi-target inhibitors of AChE, BChE and BACE1, since they displayed remarkable binding affinity relative to standard drugs currently used in the treatment of AD. The ability of these compounds to inhibit the cholinesterases may prevent the breakdown of acetylcholine. Consequently, acetylcholine, which is in short supply in Alzheimer’s patients, is not metabolized so quickly, thereby increasing their chances of them being passed on to the next nerve cell, leading to increased communication between nerve cells, which in turn, may temporarily improve/stabilize the symptoms of dementia. The binding affinities (docking scores) of donepezil for AChE and BChE (− 12.2 Kcal/mol and − 10.5 Kcal/mol respectively) are also in agreement with the IC50 of donepezil for AChE and BChE (6.7 nM and 7400 nM respectively) as reported in earlier studies (Ogura et al. 2000; Sugimoto et al. 2002). However, from the results obtained from the ADME study, only genistin (76), naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester (94) and vitexin (119) fulfilled the criteria of oral drugability. Genistin (76) is one of the popular soybean isoflavones, which have been credited with a battery of health benefits, including relief of menopausal symptoms, management of breast cancer, prostate cancer, incidence of cardiovascular disease, osteoporosis, obesity and diabetes and improvements of cognitive functions (Zaheer and Akhtar 2017). Earlier studies have suggested that isoflavones can be used for preventing AD because of their inhibitory effects on cholinesterases, amyloid-β, and BACE-1 (Ahmad et al. 2015; Kumar et al. 2017).
Vitexin (119) is an apigenin flavone glycoside found in many plant species including Passiflora spp. Vitex spp. Crataegus spp. (Malar et al. 2018) Nelumbo nucifera (Jung et al. 2015), Grewia tiliaefolia (Malar et al. 2017) and C. cajan (Schuster et al. 2016). Various studies have suggested the application of vitexin (119) or vitexin-containing plant extracts in the treatment of AD because of their cholinesterase and BACE1 inhibitory effects, anti-amyloidogenic, and neuroprotective effects (Jung et al. 2015; Malar et al. 2017, 2018). This present in silico study suggests that the AChE-, BChE-, and BACE1- inhibitory effects of genistin (76) and vitexin (119) partly support the reports that C. cajan-derived compound and extracts are relevant in the management of AD (Ruan et al. 2009; Ahmad et al. 2019).
Also, the ability of these compounds to inhibit BACE1 may be beneficial in the treatment of AD since this will result in the reduction of amyloid plaques. Abnormal accumulation of amyloid plaques has been reported in AD patients through the initiation of series of self-assembly steps that ultimately result in the formation of insoluble Aβ aggregates, which are known to impair neuronal functions (Arbel-Ornath et al. 2017; Willén et al. 2017). These insoluble Aβ aggregates deposit extracellularly between neurons or near synapses, forming amyloid plaques. However, practical BACE1 inhibitors should be small in size and cross the BBB easily as BACE1 is richly expressed in the brain, predominantly in neurons, and is easily detected in presynaptic hippocampal mossy fiber terminals (Kandalepas et al. 2013). The results obtained indicate that only naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester (94) fulfills this criterion of crossing the BBB and as such could be an ideal drug candidate with its potential multiple inhibitory activity on AChE, BChE and BACE1. Naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester (94) was identified in the volatile oil of dried, ripe fruit peel of C. reticulata Blanco, a plant commonly used as herbal medicine in China, Japan and Korea (Qin et al. 2013), and studies on its pharmacological abilities are still scanty. More so, the pharmacological effects of this plant, C. reticulata, have long been attributed to its inherent popular bioactive compounds, such as flavonoids, limonoids and phenolic acids (Gorinstein et al. 2001; Qin et al. 2013). The results from the present study indicate that naphthalen-2-yl-acetic acid, 6-hydroxy-6- methyl-cyclodecyl ester (94) from the volatile oil of this plant may possibly be among the compounds contributing, at least in part, to the neuroprotective medicinal relevance of this plant. However, it remains to be seen if this compound will elicit relevant effects in pharmacologically relevant experimental models.
Conclusion
This molecular docking study revealed that five compounds, including genistin (76), naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester (94), naringin (95), orientin (99) and vitexin (119), exhibited multiple inhibitory activities on AChE, BChE, and BACE1. However, only three of these compounds; genistin (76), naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester (94) and vitexin (119) exhibited reasonable pharmacokinetic properties observed in orally drugable compounds, while only naphthalen-2-yl-acetic acid, 6-hydroxy-6-methyl-cyclodecyl ester (94) exhibited the ability to permeate the blood-brain barrier. In conclusion, these three compounds can be optimized for utilization as potential multi-target inhibitors of AChE, BChE and BACE1 in the treatment of AD.
Compliance with ethical standards
Conflict of Interest
The authors declared that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ademosun AO, Oboh G. Anticholinesterase and antioxidative properties of water-extractable phytochemicals from some citrus peels. J Basic Clin Physiol Pharmacol. 2014;25:199–204. doi: 10.1515/jbcpp-2013-0027. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Ramasamy K, Bakar A, et al. Enhancement of b-secretase inhibition and antioxidant activities of tempeh, a fermented soybean cake through enrichment of bioactive aglycones. Pharm Biol. 2015;53:758–766. doi: 10.3109/13880209.2014.942791. [DOI] [PubMed] [Google Scholar]
- Ahmad L, Mishra A, Rahman A. Protective role of hydroalcoholic extract of Cajanus cajan Linn leaves against memory impairment in sleep deprived experimental rats. J Ayurveda Integr Med. 2019 doi: 10.1016/j.jaim.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbel-Ornath M, Hudry E, Boivin JR, et al. Soluble oligomeric amyloid-β induces calcium dyshomeostasis that precedes synapse loss in the living mouse brain. Mol Neurodegener. 2017;12:1–14. doi: 10.1186/s13024-017-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolucci C, Perola E, Pilger C, et al. Three-dimensional structure of a complex of galanthamine (Nivalin®) with acetylcholinesterase from Torpedo californica: implications for the design of new anti-Alzheimer drugs. Proteins Struct Funct Genet. 2001;42:182–191. doi: 10.1002/1097-0134(20010201)42:2<182::AID-PROT50>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Bell EW, Zhang Y. DockRMSD: an open-source tool for atom mapping and RMSD calculation of symmetric molecules through graph isomorphism. J Cheminform. 2019;11:1–9. doi: 10.1186/s13321-019-0362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borah K, Sharma S, Silla Y. Structural bioinformatics-based identification of putative plant based lead compounds for Alzheimer Disease Therapy. Comput Biol Chem. 2019;78:359–366. doi: 10.1016/j.compbiolchem.2018.12.012. [DOI] [PubMed] [Google Scholar]
- Daina A, Zoete V. A BOILED-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem. 2016;11:1117–1121. doi: 10.1002/cmdc.201600182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daina A, Michielin O, Zoete V. ILOGP: a simple, robust, and efficient description of n-octanol/water partition coefficient for drug design using the GB/SA approach. J Chem Inf Model. 2014;54:3284–3301. doi: 10.1021/ci500467k. [DOI] [PubMed] [Google Scholar]
- Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganai SA, Shanmugam K, Mahadevan V. Energy-optimised pharmacophore approach to identify potential hotspots during inhibition of class II HDAC isoforms. J Biomol Struct Dyn. 2015;33:374–387. doi: 10.1080/07391102.2013.879073. [DOI] [PubMed] [Google Scholar]
- Gleeson M, Hersey A, Hannongbua S. In-silico ADME models: a general assessment of their utility in drug discovery applications. Curr Top Med Chem. 2011;11:358–381. doi: 10.2174/156802611794480927. [DOI] [PubMed] [Google Scholar]
- Gopinath K, Sudhandiran G. Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neurosci. 2012;227:134–143. doi: 10.1016/j.neuroscience.2012.07.060. [DOI] [PubMed] [Google Scholar]
- Gorinstein S, Mart´ın-Belloso O, Park YS. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001;74:309–315. doi: 10.1016/S0308-8146(01)00157-1. [DOI] [Google Scholar]
- Gro JK, Yada S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81–100. doi: 10.1016/S0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- Henry W, Querfurth HW, LaFerla FM. Mechanisms of disease Alzheimer’s disease. New Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Ijaopo E. Dementia-related agitation: a review of non-pharmacological interventions and analysis of risks and benefits of pharmacotherapy. Transl Psychiatry. 2017;7:e1250–e1250. doi: 10.1038/tp.2017.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishola AA, Adewole KE. In silico screening of anticholinesterase alkaloids for cyclooxygenase-2 (COX-2) and matrix metalloproteinase 8 (MMP-8) inhibitory potentials as multi-target inhibitors of Alzheimer’s disease. Med Chem Res. 2019;28:1704–1717. doi: 10.1007/s00044-019-02407-4. [DOI] [Google Scholar]
- Jannat S, Balupuri A, Ali MY, et al. Inhibition of β-site amyloid precursor protein cleaving enzyme 1 and cholinesterases by pterosins via a specific structure – activity relationship with a strong BBB permeability. Exp Mol Med. 2019;51:1–18. doi: 10.1038/s12276-019-0205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson P, Kaspersson K, Gurrell IK, et al. Toward β-Secretase-1 Inhibitors with Improved Isoform Selectivity. J Med Chem. 2018;61:3491–3502. doi: 10.1021/acs.jmedchem.7b01716. [DOI] [PubMed] [Google Scholar]
- Jung HA, Karki S, Kim JH, Choi JS. BACE1 and cholinesterase inhibitory activities of Nelumbo nucifera embryos. Arch Pharm Res. 2015;38:1178–1187. doi: 10.1007/s12272-014-0492-4. [DOI] [PubMed] [Google Scholar]
- Kandalepas PC, Sadleir KR, Eimer WA, et al. The Alzheimer’s β-secretase BACE1 localizes to normal presynaptic terminals and to dystrophic presynaptic terminals surrounding amyloid plaques. Acta Neuropathol. 2013;126:329–352. doi: 10.1007/s00401-013-1152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Bhatt CP, Shruti P, Panda PB. Attenuation of neurobehavioral and neurochemical abnormalities in animal model of cognitive deficits of Alzheimer ’ s disease by fermented soybean nanonutraceutical. Inflammopharmacology. 2017;26:105–118. doi: 10.1007/s10787-017-0381-9. [DOI] [PubMed] [Google Scholar]
- Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44:235–249. doi: 10.1016/S1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- Luo M, Liu X, Zu Y, et al. Cajanol, a novel anticancer agent from Pigeonpea [ Cajanus cajan (L.) Millsp.] roots, induces apoptosis in human breast cancer cells through a ROS-mediated mitochondrial pathway. Chem Biol Interact. 2010;188:151–160. doi: 10.1016/j.cbi.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Malar DS, Shafreen RB, Pandian SK, Devi KP. Cholinesterase inhibitory, anti-amyloidogenic and neuroprotective effect of the medicinal plant Grewia tiliaefolia -— An in vitro and in silico study. Pharm Biol. 2017;55:381–393. doi: 10.1080/13880209.2016.1241811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malar DS, Suryanarayanan V, Prasanth MI, Singh SK, Krishnaswamy Balamurugan KPD. Vitexin inhibits Aβ25–35 induced toxicity in Neuro-2a cells by augmenting Nrf-2/HO-1 dependent antioxidant pathway and regulating lipid homeostasis by the activation of LXR-α. Toxicol Vitr. 2018;50:160–171. doi: 10.1016/j.tiv.2018.03.003. [DOI] [PubMed] [Google Scholar]
- McConkey BJ, Sobolev V, Edelman M. The performance of current methods in ligand-protein docking. Curr Sci. 2002;83:845–855. [Google Scholar]
- Meng X-Y, Zhang H-X, Mezei M, Meng C. Molecular Docking: a powerful approach for structure-based drug discovery. J Curr Comput Aided Drug Des. 2011;7:146–157. doi: 10.2174/157340911795677602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinuevo JL, Gispert JD, Dubois B. The AD-CSF-index discriminates Alzheimer’s disease patients from healthy controls: a validation study. J Alzheimers Dis. 2013;36:67–77. doi: 10.3233/JAD-130203. [DOI] [PubMed] [Google Scholar]
- Morphy R, Rankovic Z. Designed multiple ligands. An emerging drug discovery paradigm. J Med Chem. 2005;48:6523–6543. doi: 10.1021/jm058225d. [DOI] [PubMed] [Google Scholar]
- Murphy PM, LeVine H. Alzheimer’s disease and the β-Amyloid Peptide. J Alzheimers Dis. 2010;19:311. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolet Y, Lockridge O, Masson P, et al. Crystal Structure of Human Butyrylcholinesterase and of Its Complexes with Substrate and Products. J Biol Chem. 2003;278:41141–41147. doi: 10.1074/jbc.M210241200. [DOI] [PubMed] [Google Scholar]
- Ogura H, Kosasa T, Kuriya Y, Yamanishi Y. Comparison of inhibitory activities of donepezil and other cholinesterase inhi- bitors on acetylcholinesterase and butyrylcholinesterase in vitro. Methods Find Exp Clin Pharmcol. 2000;22:609–613. doi: 10.1358/mf.2000.22.8.701373. [DOI] [PubMed] [Google Scholar]
- Qin K, Zheng L, Cai H, et al. Characterization of Chemical Composition of Pericarpium Citri Reticulatae Volatile Oil by Comprehensive Two-Dimensional Gas Chromatography with High-Resolution Time-of-Flight Mass Spectrometry. Evidence-Based Complement Altern Med. 2013;2013:1–12. doi: 10.1155/2013/237541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AA, Sridhar GR, Das UN. Elevated butyrylcholinesterase and acetylcholinesterase may predict the development of type 2 diabetes mellitus and Alzheimer’s disease. Med Hypotheses. 2007;69:1272–1276. doi: 10.1016/j.mehy.2007.03.032. [DOI] [PubMed] [Google Scholar]
- Ruan CJ, Si JY, Zhang L, et al. Protective effect of stilbenes containing extract-fraction from Cajanus cajan L. on Aβ 25–35 -induced cognitive deficits in mice. Neurosci Lett. 2009;467:159–163. doi: 10.1016/j.neulet.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Schuster R, Holzer W, Doerfler H, et al. Cajanus cajan -a source of PPARγ activators leading to anti-inflammatory and cytotoxic effects. Food Funct. 2016;7:3798–3806. doi: 10.1039/c6fo00689b. [DOI] [PubMed] [Google Scholar]
- Seki T, Kamiya T, Furukawa K, et al. Nobiletin-rich Citrus reticulata peels, a kampo medicine for Alzheimer’s disease: a case series. Geriat Gerontol Int. 2013;13:236–238. doi: 10.1111/j.1447-0594.2012.00892.x. [DOI] [PubMed] [Google Scholar]
- Sugimoto H, Ogura H, Arai Y, et al. Research and development of donepezil hydrochloride, a new type of acetylcholinesterase inhibitor. Jpn J Pharmacol. 2002;89:7–20. doi: 10.1254/jjp.89.7. [DOI] [PubMed] [Google Scholar]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R. β-secretase (BACE) as a drug target for Alzheimer’s disease. Adv Drug Deliv Rev. 2002;54(12):1589–1602. doi: 10.1016/S0169-409X(02)00157-6. [DOI] [PubMed] [Google Scholar]
- Walters W, Stahl MT, Murcko MA. Virtual screening an overview. Drug Discov Today. 1998;3:160–178. doi: 10.1016/S1359-6446(97)01163-X. [DOI] [Google Scholar]
- Wang L, Tao X, Liu X, et al. Cajaninstilbene acid ameliorates cognitive impairment induced by intrahippocampal injection of amyloid-β1–42 oligomers. Front Pharmacol. 2019;10:1–18. doi: 10.3389/fphar.2019.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willén K, Edgar JR, Hasegawa T, et al. Aβ accumulation causes MVB enlargement and is modelled by dominant negative VPS4A. Mol Neurodegener. 2017;12:1–18. doi: 10.1186/s13024-017-0203-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Blenkowski MJ, Shuck ME, et al. Membrane-anchored aspartyl protease with Alzheimer’s disease β- secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- Zaheer K, Akhtar MH. An updated review of dietary isoflavones: nutrition, processing, bioavailability and impacts on human health. Crit Rev Food Sci Nutr. 2017;57:1280–1293. doi: 10.1080/10408398.2014.989958. [DOI] [PubMed] [Google Scholar]








