Significance
The ongoing loss of species around the world is reducing the diversity of ecological roles played by organisms in natural communities, as well as the number of evolutionary lineages that live there. We have limited knowledge about which anthropogenic threats have the strongest influence on functional and evolutionary diversity, and about whether declines in these facets of biodiversity are faster or slower than the corresponding declines in species numbers. Here we show that harvest and habitat loss in the most biodiverse parts of the world disproportionately affect mammal species that have unique roles in their ecosystems. Enhanced conservation, focused particularly on harvest sustainability, is critically needed to avoid deterioration of ecosystem function and impoverishment of our biodiversity heritage.
Keywords: bushmeat hunting, conservation, habitat loss, phylogenetic diversity, harvest
Abstract
Biodiversity is declining worldwide. Because species interact with one another and with their environment, losses of particular organisms alter the function of ecosystems. Our understanding of the global rates and specific causes of functional decline remains limited, however. Species losses also reduce the cumulative amount of extant evolutionary history (“phylogenetic diversity” [PD]) in communities—our biodiversity heritage. Here we provide a global assessment of how each known anthropogenic threat is driving declines in functional diversity (FD) and PD, using terrestrial mammals as a case study. We find that habitat loss and harvest (e.g., legal hunting, poaching, snaring) are by far the biggest drivers of ongoing FD and PD loss. Declines in FD in high-biodiversity countries, particularly in Southeast Asia and South America, are greater than would be expected if species losses were random with respect to ecological function. Among functional guilds, herbivores are disproportionately likely to be declining from harvest, with important implications for plant communities and nutrient cycling. Frugivores are particularly likely to be declining from both harvest and habitat loss, with potential ramifications for seed dispersal and even forest carbon storage. Globally, phylogenetically unique species do not have an elevated risk of decline, but in areas such as Australia and parts of Southeast Asia, both habitat loss and harvest are biased toward phylogenetically unique species. Enhanced conservation efforts, including a renewed focus on harvest sustainability, are urgently needed to prevent the deterioration of ecosystem function, especially in the South American and equatorial Asian tropics.
Earth is likely entering its sixth mass extinction event (1–3), this one attributable to the actions of a single species: humans. The current loss of vertebrate species is estimated to be ∼1,000 times faster than the background rate of extinctions from the fossil record (4). Recent work suggests that we may even be underestimating the scale of the problem because a focus on extinctions (i.e., complete loss of species) ignores the even more dramatic decline and loss of populations (3). For each actual species extinction, there are ∼10 serious declines in abundance in extant populations, leading to “biotic annihilation” across the planet (3). For example, abundance has declined by an average of 60% in ∼17,000 monitored vertebrate populations over the last few decades (2).
These losses can have major ripple effects. Organisms interact with others around them and with their environment, such that each species can affect the function of its ecosystem (5–8). For example, predators might regulate the abundance of herbivores and thereby indirectly influence plant productivity, including human agricultural output (9). Assessments of human-induced extinctions have largely focused on the loss of species (1, 10) and how those losses result in declines in the diversity of taxonomic forms (often measured as “species richness” (SR)— the number of unique species in an area). How these declines in the diversity of species affect the diversity of ecological functions has been much less explored (11), despite the fact that many ecological functions are important to the maintenance of intact ecosystems and also support human economies. For example, ∼15% of humanity depends on protein from wild-caught vertebrates (12).
Moreover, the decline and loss of species reduces the cumulative evolutionary history present in any community (7, 13, 14). This evolutionary history is often represented by phylogenetic diversity (PD), or the diversity of lineages present within an assemblage of species, measured as the cumulative length of the branches on the evolutionary tree (phylogeny) linking the species. In some cases, PD may be a proxy for species interactions or functional diversity (FD) (15). Much more importantly (in our opinion), PD has immense intrinsic value because it is a fundamental measure of biodiversity—arguably the best such measure (16, 17). As such, the protection of PD is a prime conservation objective.
Whether a given level of decline in SR leads to small versus large declines in FD and PD is difficult to predict. SR is often highly correlated with, and thus may be a strong proxy for, both FD and PD (18), but this is by no means the case in all systems or with all taxa (e.g., ref. 16). On the one hand, if many threatened species in a given area are functionally similar or redundant (6), then substantial taxonomic losses could occur with minimal impact on FD. On the other hand, if taxonomic diversity were to decline only slightly but the species that were lost had played unique ecological roles, then declines in FD could be severe (19). Likewise, the loss of many closely related species would have less impact on PD than would the loss of the same number of distantly related species (13, 20). Some simulation analyses have suggested that large numbers of species could be lost with relatively little impact on PD (14) or at least without a proportionally greater loss of PD (21). In contrast, empirical studies suggest that extinction risk is often disproportionately high in evolutionarily unique clades (22, 23).
Research over the last decade has begun to elucidate patterns in regional and global FD and PD (e.g., refs. 24–26). Indeed, mapping standing levels of FD and PD across the globe has provided guidance as to where additional conservation measures are needed to safeguard these facets of biodiversity (27, 28). Because species differ greatly in both their likelihood of near-term extinction and their contributions to FD and PD (20), maps of different aspects of extant biodiversity might not show where diversity is actually at the greatest risk.
Extinction risk across species can be driven by numerous factors (29), but we still lack a general understanding of which of these anthropogenic threats are driving declines in FD and PD. Moreover, we have little knowledge about whether ongoing losses of species and populations are biased toward functionally and phylogenetically unique taxa versus redundant taxa, either globally or in particular regions (30). Such information could help inform global assessments for conservation policy. For example, one of the nine “planetary boundaries” monitored by the Stockholm Resilience Centre (SRC) in its assessment of humanity’s impacts on Earth’s life support systems is biosphere integrity, comprising two components: genetic diversity and FD (11). We have a growing understanding of global changes in genetic diversity (31), but for FD, the SRC displays only a question mark (11).
Here we provide a detailed assessment of how FD and PD are changing across the globe due to ongoing declines of populations from known major anthropogenic threats. For each threat, and for all threats combined, we determined the spatial variation in impacts on FD and PD and assessed whether ongoing declines in these facets of biodiversity are greater or less than what would be expected if declines in SR were random with respect to ecological function and evolutionary relatedness. We focus on mammals (terrestrial and freshwater aquatic species, excluding marine taxa) because many of these species have important ecological roles, trait and phylogenetic data are available for nearly all species (32, 33), and the taxon as a whole (along with other vertebrate groups) is highly threatened by human activities (1). We generated a database categorizing the degree to which each of the terrestrial and freshwater mammal species on the International Union for the Conservation of Nature (IUCN) Red List (34) is affected by anthropogenic threats (SI Appendix, Table S1). This was analyzed in conjunction with a recent phylogeny of mammals (33), a dataset of mammal functional traits (32), and global range maps for mammal species (34). We assessed how ongoing declines in SR associated with different anthropogenic threats influence FD and PD across the world’s landmasses, assuming that currently declining species are lost (3).
The sampling units in our analysis are nations. Using this approach rather than, for example, a grid of points across the Earth was intended to help circumvent some of the fine-scale inaccuracies in the species-level range maps. In other words, it was less likely that a species would be erroneously listed as present or absent in an entire country than at a particular finer-scale grid cell. Moreover, arguably the most important conservation policies are at the national level, so using countries as sampling units means that our analysis is conducted at a scale potentially useful for informing biodiversity policy.
Results
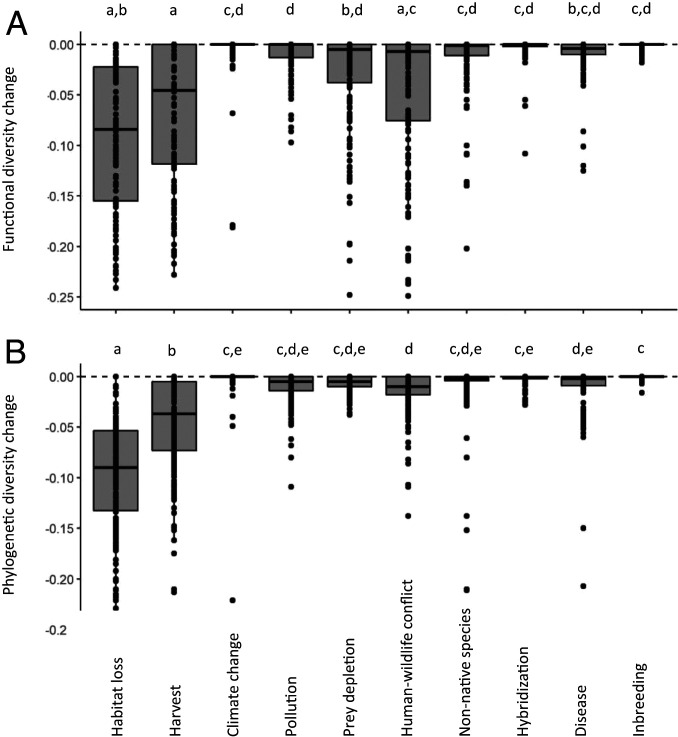
We found that habitat loss and harvest (e.g., hunting, poaching, snaring) are the largest drivers of ongoing declines in FD and PD globally (Fig. 1). (In Southeast Asia, human–wildlife conflict also emerges as an important threat; see SI Appendix, Fig. S2.) Other threats, such as climate change, have received substantial public attention recently. Our analysis shows that, on average, the impacts of habitat loss and harvest on mammal FD exceed those of recent climate change by >25-fold and >28-fold, respectively (Fig. 1A). Likewise, habitat loss had a >32-fold and harvest a >13-fold greater impact than climate change on mammal PD (Fig. 1B). We note, however, that climatic changes are becoming increasingly intense (35) and can interact synergistically with habitat loss (36). Thus, in the near future, climate change may have much stronger impacts on populations and on multiple facets of biodiversity than currently recognized.
Fig. 1.
Anthropogenic threats vary strongly in their impacts on FD (A) and PD (B) diversity (P < < 0.001 in both cases) across countries. Box edges show 25th and 75th quartiles; the thick inner line represents the 50th quartile; and whiskers show the largest value ≤1.5 times the interquartile range. Threats with the same lowercase letter are not significantly different based on Tukey’s honest significant difference test.
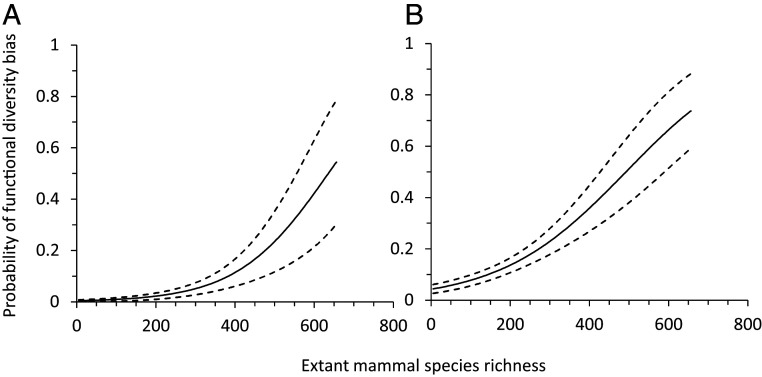
That there is an ongoing extinction event, or rapid loss rate of species and populations, is well known among conservation scientists and even among much of the general public. However, our analysis suggests that some anthropogenic threats are driving even faster losses of FD and PD than would be expected if species loss were random with respect to ecological function or phylogeny. For example, across most of South America, including in Brazil, the country with the world’s highest terrestrial biodiversity, the declines in FD driven by habitat loss are greater than would be expected if species losses were random with respect to ecological function (Fig. 2). For harvest, declines in FD are greater than would be expected based on random species losses across much of South America, Southeast Asia, and tropical Africa, as well as in Australia (Fig. 3). In contrast, harvest-induced declines in FD are less than would be expected in Colombia and several temperate-zone countries, including the United States, Japan, and some Scandinavian nations. The probability that declines in SR were biased toward functionally unique species was positively associated with extant SR across countries for both habitat loss (spatially autoregressive logistic model: β = 0.009, P < 0.001) and harvest (β = 0.006, P < < 0.001) (Fig. 4). Bias toward phylogenetically unique species was not related to extant richness for habitat loss (P = 0.224) or harvest (P = 0.058).
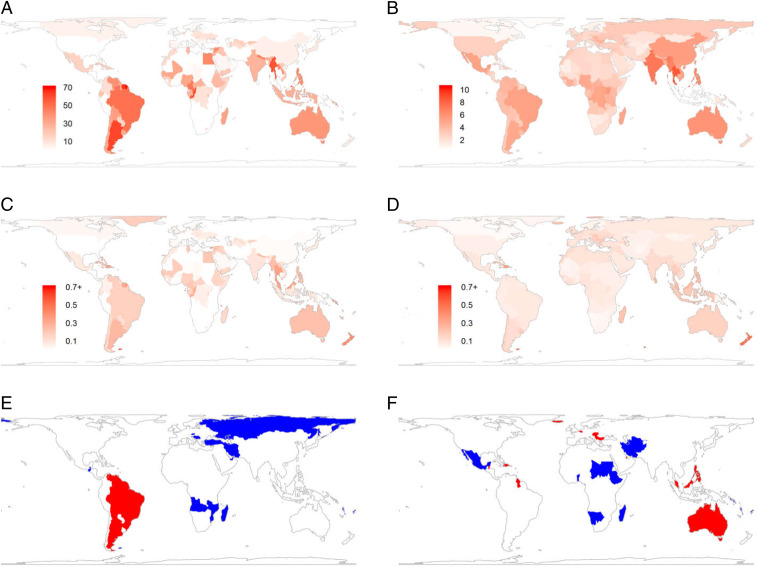
Fig. 2.
(A–D) Effects of habitat loss on absolute declines in FD (A) and PD (B; units are millions of years of cumulative evolutionary history) and proportional declines in FD (C) and PD (D). (E and F) Maps of where declines in SR associated with habitat loss are significantly biased toward functionally (E) and phylogenetically (F) unique species (red) or toward redundant species (blue) or are unbiased with respect to ecological function or phylogeny (white).
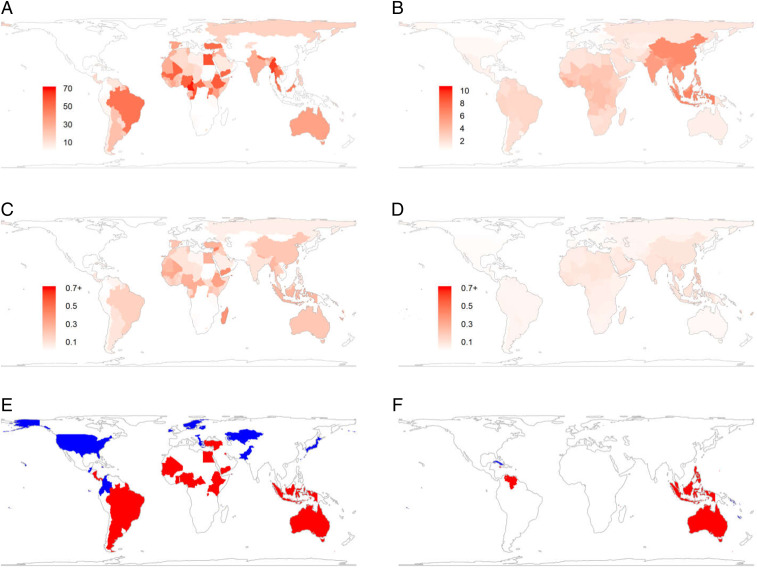
Fig. 3.
(A–D) Effects of harvest on absolute declines in FD (A; units are cumulative dendrogram branch-lengths) and PD (B; units are millions of years of cumulative evolutionary history) and proportional declines in FD (C) and PD (D). (E and F) Maps of where declines in SP associated with harvest are significantly biased toward functionally (E) and phylogenetically (F) unique species (red) or toward redundant species (blue), or are unbiased with respect to ecological function or phylogeny (white).
Fig. 4.
Probability that habitat loss (A) and harvest (B) across countries are biased toward functionally unique mammal species as a function of the current SR of the country. Mean estimate (solid lines) and SEs (dashed lines) are shown; P < 0.001.
Losses of PD driven by both habitat loss (Fig. 2) and harvest (Fig. 3) were greater than would be expected if species losses were random in equatorial Southeast Asia and Australia. Habitat loss-induced declines in PD were also greater than if species losses had been random in Mexico, Madagascar, and parts of Africa and western Asia (Fig. 2). Across most of the world, however, PD declines driven by harvest tended to be statistically indistinguishable from what would be expected if species losses were random with respect to evolutionary history (Fig. 3).
We assessed whether species declines due to habitat loss or harvest were related to functional traits and evolutionary distinctiveness using phylogenetic generalized linear mixed models. Across all species globally, frugivores, but no other feeding categories or other functional traits, were disproportionately likely to be declining due to habitat loss (β = 1.609, 95% CI = 0.844 to 2.405; P < < 0.001, significant at a Bonferroni-corrected, per-variable α = 0.008). Both frugivores (β = 6.047, 95% CI = 4.337 to 7.989; P < < 0.001) and herbivores (β = 4.290, 95% CI = 2.308 to 6.507; P < < 0.001) were also disproportionately likely to be declining due to harvest.
Discussion
Recent studies have identified priority areas where additional conservation measures could help safeguard FD and PD (26–28). Our results demonstrate that these facets of biodiversity are affected differently by different anthropogenic threats, suggesting that conservation efforts focused on protecting FD or PD need to be targeted topically as well as geographically. This contrasts with earlier findings that, averaged across the globe, the number of threats, but not the type of threat, predicts the susceptibility of mammal PD to decline (30). For example, we show that habitat loss is a stronger threat than harvest to mammal FD in Indonesia, Argentina, and Venezuela. This suggests that instead of focusing on harvest management and human diets, conservation actions in these areas might be better directed toward protected areas and land use policy to best conserve this component of biodiversity. Nevertheless, harvest in many of the most biodiverse parts of the world appears to be disproportionately focused on certain mammal guilds, driving declines in FD that are greater than they would be if declines in SR were random with respect to ecological function. The high impacts on frugivores and herbivores that we detected is disconcerting, as these groups play critical roles in their ecosystems. (We note, however, that there was nonindependence among countries in this analysis due to species ranges that cross national borders.) Herbivorous mammals are found in nearly all terrestrial ecosystems, where they can strongly influence plant communities (37) and nutrient cycling (38). Frugivores are particularly common in tropical forests, the world’s most biodiverse ecosystems; the loss of such species from unsustainable hunting can have major repercussions, including altered plant regeneration (39) and even forest carbon storage (40).
We identified harvest as one of the major drivers of declines in mammal FD and PD worldwide. This should not be taken to suggest that all hunting is detrimental to biodiversity. Well-managed harvest systems, such as those in parts of North America and East Africa, can be beneficial for conservation (41). In much of the world, however, hunting may be legal but still unsustainable, and illegal hunting (“poaching”) and unregulated trapping (often by snares) are problems in myriad areas. As such, a renewed focus on harvest sustainability (42) is needed to help stem the losses of FD and PD. Our analysis particularly highlights the critical conservation situation in tropical regions such as Southeast Asia and Brazil, where all facets of mammal diversity are strongly threatened by ongoing loss of habitat and unsustainable harvest, both of which are disproportionately targeted at functionally unique mammal species.
The accelerated rate of extinctions in the Anthropocene, coupled with limited funding and other resources for conservation, have provided impetus for the development of broad-scale planning and prioritization strategies on the global level. Organizational policies and informational warehouses, such as the IUCN Red List, the Convention on Biological Diversity’s Aichi Targets, and the emerging post-2020 targets, exist to assess the loss of global biodiversity while also providing frameworks and benchmarks for future actions. Our results demonstrate that the picture of what we are losing, and where and why it is being lost, can change depending on the component of biodiversity being considered and the local dynamics and threats being applied. Therefore, we suggest that with limited funding and other resource constraints, conservation should be driven by considerations of local threats and varied measures of the components of biodiversity.
Materials and Methods
Data Compilation.
For each of the world’s countries, we compiled a mammal species list from species-level distribution maps and text descriptions in the IUCN Red List (34). We used the UNIGIS International Association map of countries from 2015 (available at the ArcGIS Hub), with 254 jurisdictions.
We assembled a database of how all mammal species on the Red List were affected by known anthropogenic threats, grouped into 10 categories (SI Appendix, Table S1). Using text information in the IUCN Red List species accounts, we quantified whether each threat likely influenced the population trend (i.e., increasing, decreasing, or stable) of each species. Although individual assessments in the Red List are subject to uncertainties when comparing across species (e.g., due to discrepancies in methodology, assessors, or data availability), no other global database is as taxonomically comprehensive in scope, and the Red List is the most commonly used database for global biodiversity threat assessments.
Functional and Phylogenetic Diversity.
For each country, we calculated extant SR, FD, and PD. To measure FD, we used an existing database of mammal traits (32), focusing on those known to influence ecological function: diet (e.g., proportion herbivory, frugivory, granivory, nectarivory, scavenging, carnivory on vertebrates, carnivory on invertebrates, piscivory), body size, and foraging stratum (e.g., ground, climbing, volant). Starting with the 5,674 species in our threats database, we removed marine (n = 91) and then extinct (n = 82) species. Of the remaining 5,501 species, 745 did not have corresponding trait data. For these, we used genus-level averages (533 species) or, when even information on congeners was unavailable, family-level averages (211 species). For a single species in its own family that was not in the trait database, Laonastes aenigmamus, we compiled trait information by literature review. We used the functional richness (FRic) metric of Villéger et al. (43) to measure FD, as this metric does not account for abundance and thus is analogous to taxonomic SR. Trait data were standardized before analysis.
To measure mammal PD in each country, we used a recent phylogeny of mammals from Upham et al. (44). For each of their 10,000 phylogenies, we calculated global mammal FRic, then chose the phylogeny with the corresponding FRic estimate closest to the mean estimate across all phylogenies as our “mean phylogeny” for subsequent analyses. We converted the phylogeny to be ultrametric using the “chronos” function in the ape package (45) in R (46), with the best-fit λ value of 2.0. After reconciling nomenclature differences, seven of the 5,501 species (0.13%) with threat and trait information were not in the phylogenetic tree. Of these, we added five species to the root node of the appropriate genus using the package phytools (47) in R. The remaining two species (Phaiomys leucurus and Pseudoberylmys muongbangensis) lacked corresponding genera in the phylogeny and were excluded from our PD analyses. We estimated PD using the picante package (48) in R.
We then assessed how ongoing species declines are affecting the various facets of mammal diversity by asking how much SR, FD, and PD would be lost in each nation if currently declining species were extirpated, following Ceballos et al. (3). For each anthropogenic threat individually and for all combined, we removed species for which the IUCN-designated population trend was “declining” and where the threats database that we assembled indicated that that particular threat was a major contributor to the decline. The default assumption here was that a declining species was declining throughout its range. However, we then went through the text descriptions in each IUCN species account and added species back to particular countries where they were known to not be in decline. For example, Urus thibetanus is considered to be declining at the species level, but not in certain range countries (Bhutan, Japan, South Korea, and Thailand). We excluded one species, Sylvicapra grimmia, for which the decline information was too vague to be useful. We limited the FD and PD analyses to countries where extant SR was ≥4 species.
We determined whether threat-specific declines in FD and PD in each nation were less than or greater than what would be expected by chance, given the observed amount of SR decline, using randomization tests. In each nation, we determined the number of species that were declining and assessed FD and PD in a suite of 500 iterations in which that same number of species was removed from the assemblage but the identities of the removed species were chosen randomly. If the observed declines in FD or PD were outside the 95% quantiles of the distributions, then diversity loss in that country was considered biased toward unique or redundant species.
To determine whether particular functional traits or evolutionarily unique species were disproportionately impacted by specific anthropogenic threats, we used phylogenetic linear mixed models that are explicitly designed for scenarios in which species occur in multiple sites (49). These models had functional traits and the evolutionary distinctiveness (based on “equal splits”; sensu ref. 50) of each species as explanatory variables and whether the species was declining due to habitat loss or (in a separate model) harvest as binary response variables. The models included “species” (with phylogenetic structure) and “country” as crossed random effects, although there was spatial nonindependence via species that ranged across multiple adjacent countries. To account for the multiple comparisons within each model, we used a Bonferroni correction to achieve a familywise α = 0.05.
Supplementary Material
Acknowledgments
We thank V. Arnold, K. Bentsen, E. Hamant, K. Jacquet, S. Nozawa, S. Pasternak, and B. Stickels for assistance in compiling the database of anthropogenic threats to world mammals, and A. Luis for comments on previous versions of the manuscript. Support was provided by the University of Montana.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1921849118/-/DCSupplemental.
Data Availability.
Data and analysis code are available at https://figshare.com/articles/dataset/2020_PNAS_mammal_FD_PD_decline_zip/13350518 (51).
References
- 1.Tilman D., et al. , Future threats to biodiversity and pathways to their prevention. Nature 546, 73–81 (2017). [DOI] [PubMed] [Google Scholar]
- 2.WWF Ed. , Living Planet Report—2018: Aiming Higher, Grooten M. and Almond R. E. A., Eds. (World Wildlife Fund for Nature, 2018). [Google Scholar]
- 3.Ceballos G., Ehrlich P. R., Dirzo R., Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. U.S.A. 114, E6089–E6096 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pimm S. L., et al. , The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Boast A. P., et al. , Coprolites reveal ecological interactions lost with the extinction of New Zealand birds. Proc. Natl. Acad. Sci. U.S.A. 115, 1546–1551 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brodie J. F., Redford K. H., Doak D. F., Ecological function analysis: Incorporating species roles into conservation. Trends Ecol. Evol. 33, 840–850 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Flynn D. F. B., et al. , Loss of functional diversity under land use intensification across multiple taxa. Ecol. Lett. 12, 22–33 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Petchey O. L., Gaston K. J., Extinction and the loss of functional diversity. Proc. Biol. Sci. 269, 1721–1727 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Bryan C. J., et al. , The contribution of predators and scavengers to human well-being. Nat. Ecol. Evol. 2, 229–236 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Ceballos G., Ehrlich P. R., Global mammal distributions, biodiversity hotspots, and conservation. Proc. Natl. Acad. Sci. U.S.A. 103, 19374–19379 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steffen W., et al. , Sustainability. Planetary boundaries: Guiding human development on a changing planet. Science 347, 1259855 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Brashares J. S., et al. , Conservation policy. Wildlife decline and social conflict. Science 345, 376–378 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Davies T. J., Losing history: How extinctions prune features from the tree of life. Philos. Trans. Roy. Soc. B 370, 20140006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nee S., May R. M., Extinction and the loss of evolutionary history. Science 278, 692–694 (1997). [DOI] [PubMed] [Google Scholar]
- 15.Srivastava D. S., Cadotte M. W., MacDonald A. A. M., Marushia R. G., Mirotchnick N., Phylogenetic diversity and the functioning of ecosystems. Ecol. Lett. 15, 637–648 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Forest F., et al. , Preserving the evolutionary potential of floras in biodiversity hotspots. Nature 445, 757–760 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Lean C., Maclaurin J., “The value of phylogenetic diversity” in Biodiversity Conservation and Phylogenetic Systematics, Pellens R., Grandcolas P., Eds. (Springer International, 2016), pp. 19–38. [Google Scholar]
- 18.Rapacciuolo G., et al. , Species diversity as a surrogate for conservation of phylogenetic and functional diversity in terrestrial vertebrates across the Americas. Nat. Ecol. Evol. 3, 53–61 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Toussaint A., Charpin N., Brosse S., Villéger S., Global functional diversity of freshwater fish is concentrated in the Neotropics while functional vulnerability is widespread. Sci. Rep. 6, 22125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redding D. W., Mooers A. O., Ranking mammal species for conservation and the loss of both phylogenetic and trait diversity. PLoS One 10, e0141435 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parhar R. K., Mooers A. Ø., Phylogenetically clustered extinction risks do not substantially prune the Tree of Life. PLoS One 6, e23528 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purvis A., Agapow P.-M., Gittleman J. L., Mace G. M., Nonrandom extinction and the loss of evolutionary history. Science 288, 328–330 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Vamosi J. C., Wilson J. R., Nonrandom extinction leads to elevated loss of angiosperm evolutionary history. Ecol. Lett. 11, 1047–1053 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Safi K., et al. , Understanding global patterns of mammalian functional and phylogenetic diversity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 2536–2544 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritz S. A., Purvis A., Phylogenetic diversity does not capture body size variation at risk in the world’s mammals. Proc. Roy. Soc. B Biol. Sci. 277, 2435–2441 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang S., Davies T. J., Gittleman J. L., How global extinctions impact regional biodiversity in mammals. Biol. Lett. 8, 222–225 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brum F. T., et al. , Global priorities for conservation across multiple dimensions of mammalian diversity. Proc. Natl. Acad. Sci. U.S.A. 114, 7641–7646 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollock L. J., Thuiller W., Jetz W., Large conservation gains possible for global biodiversity facets. Nature 546, 141–144 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Fritz S. A., Bininda-Emonds O. R., Purvis A., Geographical variation in predictors of mammalian extinction risk: Big is bad, but only in the tropics. Ecol. Lett. 12, 538–549 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Jono C. M., Pavoine S., Threat diversity will erode mammalian phylogenetic diversity in the near future. PLoS One 7, e46235 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miraldo A., et al. , An Anthropocene map of genetic diversity. Science 353, 1532–1535 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Wilman H., et al. , EltonTraits 1.0: Species‐level foraging attributes of the world’s birds and mammals: Ecological Archives E095‐178. Ecology 95, 2027 (2014). [Google Scholar]
- 33.Uyeda J. C., Pennell M. W., Miller E. T., Maia R., McClain C. R., The evolution of energetic scaling across the vertebrate tree of life. Am. Nat. 190, 185–199 (2017). [DOI] [PubMed] [Google Scholar]
- 34.IUCN , The IUCN Red List of Threatened Species version 2018-1 (2018). International Union for the Conservation of Nature. www.iucnredlist.org. Accessed 14 June 2018.
- 35.Brodie J. F., Post E. S., Doak D. F., Wildlife Conservation in a Changing Climate (University of Chicago Press, 2012). [Google Scholar]
- 36.Brodie J. F., Synergistic effects of climate change and agricultural land use on mammals. Front. Ecol. Environ. 14, 20–26 (2016). [Google Scholar]
- 37.Brodie J., Post E., Watson F., Berger J., Climate change intensification of herbivore impacts on tree recruitment. Proc. Roy. Soc. B 279, 1366–1370 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doughty C. E., et al. , Global nutrient transport in a world of giants. Proc. Natl. Acad. Sci. U.S.A. 113, 868–873 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brodie J. F., Aslan C. E., Halting regime shifts in floristically intact tropical forests deprived of their frugivores. Restor. Ecol. 20, 153–157 (2012). [Google Scholar]
- 40.Brodie J. F., How monkeys sequester carbon. Trends Ecol. Evol. 31, 414–416 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Loveridge A. J., Reynolds J. C., Milner-Gulland E., Does sport hunting benefit conservation. Key Topics Conserv. Biol. 1, 238 (2007). [Google Scholar]
- 42.Robinson J., Bennett E. L., Hunting for Sustainability in Tropical Forests (Columbia University Press, 2000). [Google Scholar]
- 43.Villéger S., Mason N. W., Mouillot D., New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89, 2290–2301 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Upham N. S., Esselstyn J. A., Jetz W., Inferring the mammal tree: Species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biol. 17, e3000494 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paradis E., Claude J., Strimmer K., APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (2004). [DOI] [PubMed] [Google Scholar]
- 46.R Core Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2018). [Google Scholar]
- 47.Revell L. J., phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012). [Google Scholar]
- 48.Kembel S. W., et al. , Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Ives A. R., Dinnage R., Nell L. A., Helmus M. R., Daijiang L., Phyr: Model based phylogenetic analysis (2019). R package version 1.0.2. https://CRAN.R-project.org/package=phyr. Accessed 8 September 2020.
- 50.Redding D. W., Mooers A. Ø., Incorporating evolutionary measures into conservation prioritization. Conserv. Biol. 20, 1670–1678 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Brodie J., 2020_PNAS_mammal_FD_PD_decline. Figshare. 10.6084/m9.figshare.13350518.v1. Deposited 8 Dececember 2020. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and analysis code are available at https://figshare.com/articles/dataset/2020_PNAS_mammal_FD_PD_decline_zip/13350518 (51).