Significance
Despite decades of research, an effective HIV-1 vaccine remains elusive. One potential vaccine target is the N-heptad repeat (NHR) region of gp41, which is the target of the FDA-approved drug enfuvirtide. However, monoclonal antibodies and antisera targeting this region have only been modestly neutralizing to date. Here, we show that the neutralization potency of the well-characterized anti-NHR antibody D5 is increased >5,000-fold by expression of FcγRI (CD64) on cells. Since FcγRI is expressed on macrophages and dendritic cells, which are implicated in the early establishment of HIV-1 infection following sexual transmission, these results may be important to HIV-1 vaccine development.
Keywords: HIV-1, vaccine, prehairpin intermediate, gp41, Fc receptor
Abstract
The HIV-1 gp41 N-heptad repeat (NHR) region of the prehairpin intermediate, which is transiently exposed during HIV-1 viral membrane fusion, is a validated clinical target in humans and is inhibited by the Food and Drug Administration (FDA)-approved drug enfuvirtide. However, vaccine candidates targeting the NHR have yielded only modest neutralization activities in animals; this inhibition has been largely restricted to tier-1 viruses, which are most sensitive to neutralization by sera from HIV-1–infected individuals. Here, we show that the neutralization activity of the well-characterized NHR-targeting antibody D5 is potentiated >5,000-fold in TZM-bl cells expressing FcγRI compared with those without, resulting in neutralization of many tier-2 viruses (which are less susceptible to neutralization by sera from HIV-1–infected individuals and are the target of current antibody-based vaccine efforts). Further, antisera from guinea pigs immunized with the NHR-based vaccine candidate (ccIZN36)3 neutralized tier-2 viruses from multiple clades in an FcγRI-dependent manner. As FcγRI is expressed on macrophages and dendritic cells, which are present at mucosal surfaces and are implicated in the early establishment of HIV-1 infection following sexual transmission, these results may be important in the development of a prophylactic HIV-1 vaccine.
Membrane fusion between HIV-1 and host cells is mediated by the viral envelope glycoprotein (Env), a trimer consisting of the gp120 and gp41 subunits. Upon interaction with cellular receptors, Env undergoes a dramatic conformational change and forms the prehairpin intermediate (PHI) (1–3), in which the fusion peptide region at the amino terminus of gp41 inserts into the cell membrane. In the PHI, the N-heptad repeat (NHR) region of gp41 is exposed and forms a stable, three-stranded α-helical coiled coil. Subsequently, the PHI resolves when the NHR and the C-heptad repeat (CHR) regions of gp41 associate to form a trimer-of-hairpins structure that brings the viral and cell membranes into proximity, facilitating membrane fusion (Fig. 1).
Fig. 1.
HIV-1 membrane fusion. The surface protein of the HIV-1 envelope is composed of the gp120 and gp41 subunits. After Env binds to cell-surface receptors, gp41 inserts into the host cell membrane and undergoes a conformational change to form the prehairpin intermediate. The N-heptad repeat (orange) region of gp41 is exposed in the PHI and forms a three-stranded coiled coil. To complete viral fusion, the PHI resolves to a trimer-of-hairpins structure in which the C-heptad repeat (blue) adopts a helical conformation and binds the NHR region. Fusion inhibitors such as enfuvirtide bind the NHR, preventing viral fusion by inhibiting formation of the trimer of hairpins (1–3). The membrane-proximal external region (red) is located adjacent to the transmembrane (TM) region of gp41.
The NHR region of the PHI is a validated therapeutic target in humans: the Food and Drug Administration (FDA)-approved drug enfuvirtide binds the NHR and inhibits viral entry into cells (4, 5). Various versions of the three-stranded coiled coil formed by the NHR have been created and used as vaccine candidates in animals (6–10). The neutralization potencies of these antisera, as well as those of anti-NHR monoclonal antibodies (mAbs) (11–15), are modest and mostly limited to HIV-1 isolates that are highly sensitive to antibody-mediated neutralization [commonly referred to as tier-1 viruses (16)]. These results have led to skepticism about the PHI as a vaccine target.
Earlier studies showed that the neutralization activities of mAbs that bound another region of gp41, the membrane-proximal external region (MPER) (Fig. 1), were enhanced as much as 5,000-fold in cells expressing FcγRI (CD64) (17, 18), an integral membrane protein that binds the Fc portion of immunoglobulin G (IgG) molecules with high (nanomolar) affinity (19, 20). This effect was not attributed to phagocytosis and occurred when the cells were preincubated with antibody and washed before adding virus (17, 18). Since the MPER is a partially cryptic epitope that is not fully exposed until after Env engages with cellular receptors (21, 22), these results suggest that by binding the Fc region, FcγRI provides a local concentration advantage for MPER mAbs at the cell surface that enhances viral neutralization (17, 18). While not expressed on T cells, FcγRI is expressed on macrophages and dendritic cells (23), which are present at mucosal surfaces and are implicated in sexual HIV-1 transmission and the early establishment of HIV-1 infection (22–34).
Here we investigated whether FcγRI expression also potentiates the neutralizing activity of antibodies targeting the NHR, since that region, like the MPER, is preferentially exposed during viral fusion. We found that D5, a well-characterized anti-NHR mAb (11, 12), inhibits HIV-1 infection ∼5,000-fold more potently in TZM-bl cells expressing FcγRI (TZM-bl/FcγRI cells) than in TZM-bl cells that do not. Further, while antisera from guinea pigs immunized with (ccIZN36)3, an NHR-based vaccine candidate (7), displayed weak neutralizing activity in TZM-bl cells, they exhibited enhanced neutralization in TZM-bl/FcγRI cells, including against some tier-2 HIV-1 isolates that are more resistant to antibody-mediated neutralization (16) and that serve as benchmarks for antibody-based vaccine efforts. These results indicate that FcγRI can play an important role in neutralization by antibodies that target the PHI. Since these receptors are expressed on cells prevalent at mucosal surfaces thought to be important for sexual HIV-1 transmission, our results motivate vaccine strategies that harness this potentiating effect.
Results
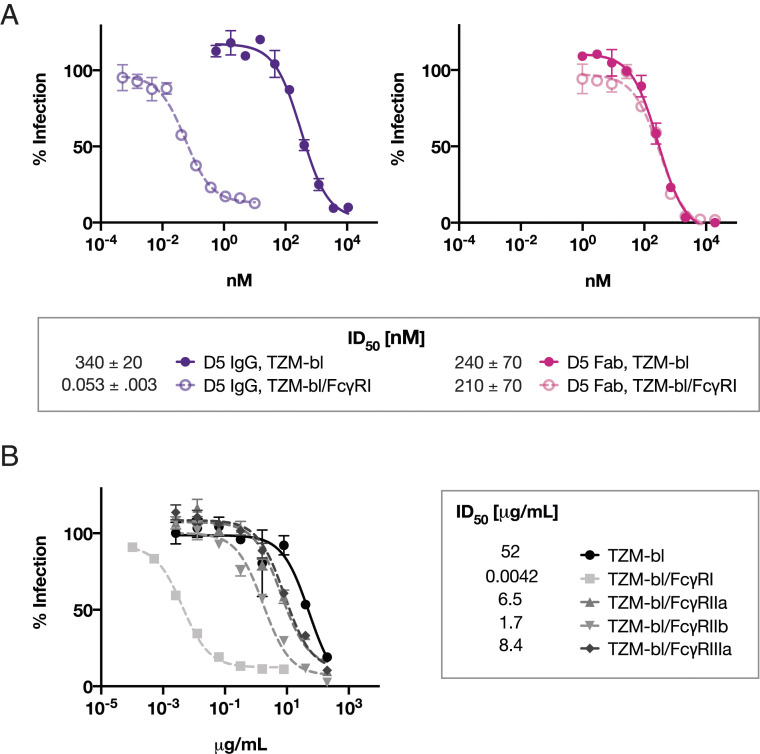
D5, a mAb shown by X-ray crystallography to bind a highly conserved epitope on the NHR (12), has weak but relatively broad neutralizing activity against HIV-1 strains (11). We measured the neutralizing activity of D5 against HXB2 (a clade B tier-1 virus) in both TZM-bl and TZM-bl/FcγRI cells. The presence of FcγRI increased the neutralization potency of D5 IgG by ∼5,000-fold (Fig. 2A). In contrast, this effect was not observed with the Fab fragment of D5 (Fig. 2A), indicating that this phenomenon is Fc-dependent. When tested in TZM-bl cells expressing FcγRIIa, FcγRIIb, or FcγRIIIa, only modest potentiation of D5 IgG neutralizing activity was observed (Fig. 2B). These results were obtained using media containing 10% fetal bovine serum; the interaction of bovine IgG with human FcγRII (35) may have interfered with our ability to detect a larger effect with FcγRIIa and FcγRIIb. With this caveat, the pronounced enhancement of D5 IgG neutralization was specific to FcγRI.
Fig. 2.
Neutralization potency of the anti-NHR antibody D5 is enhanced by FcγRI. (A) Inhibition of infection by viruses pseudotyped with Env from HXB2 (tier-1, clade B) by D5 IgG (Left) and D5 Fab (Right) in TZM-bl cells not expressing (solid) or expressing (open) FcγRI. Potentiation of >5,000-fold occurs in TZM-bl/FcγRI cells for the IgG but not the Fab form of D5. Curves plotted are from a single experiment; error bars are the range of n = 2 measurements. The table shows ID50 mean values and SEM from duplicate experiments. (B) ID50 values (in μg/mL) and neutralization curves of D5 IgG inhibiting infection of Env-pseudotyped lentivirus (HXB2) in TZM-bl cells stably expressing various Fcγ receptors. Each point is the mean value of a triplicate measurement; error bars are the SEM from n = 3 values. Comparable results were obtained with Env from the tier-2 HIV-1 isolate 25710 (67).
D5 weakly inhibits a diverse range of HIV-1 viruses across clades, as expected given the high (>95%) conservation of residues that form the D5 epitope on the NHR (11, 12). Given the increase in potency afforded by FcγRI, we investigated the neutralization by D5 in a wide panel of HIV-1–pseudotyped viruses (36) in TZM-bl/FcγRI cells. At concentrations up to 25 µg/mL, D5 failed to neutralize viruses in the panel when measured in TZM-bl cells not expressing FcγRI (Table 1). However, when measured across the same concentration range in TZM-bl/FcγRI cells, D5 inhibited eight of the nine tier-2 viruses in the panel, spanning five clades (Table 1).
Table 1.
D5 IgG neutralizes tier-1 and tier-2 viruses across clades in TZM-bl/FcγRI cells
| Virus | Tier | Clade | ID50 in TZM-bl cells, µg/mL | ID50 in TZM-bl/FcγRI cells, µg/mL |
| SVA-MLV | Negative control | >25 | >25 | |
| X2278 | Tier 1B | Clade B | >25 | 0.53 |
| 246-F3 | Tier 2 | Clade AC | >25 | >25 |
| CNE55 | Tier 2 | CRF01_AE | >25 | 0.88 |
| TRO.11 | Tier 2 | Clade B | >25 | 4.8 |
| BJOX2000 | Tier 2 | CRF07_BC | >25 | 0.53 |
| CH119 | Tier 2 | CRF07_BC | >25 | 1.8 |
| Ce1176 | Tier 2 | Clade C | >25 | 7.0 |
| 25710 | Tier 2 | Clade C | >25 | 0.36 |
| Ce0217 | Tier 2 | Clade C | >25 | 0.43 |
| X1632 | Tier 2 | Clade G | >25 | 0.71 |
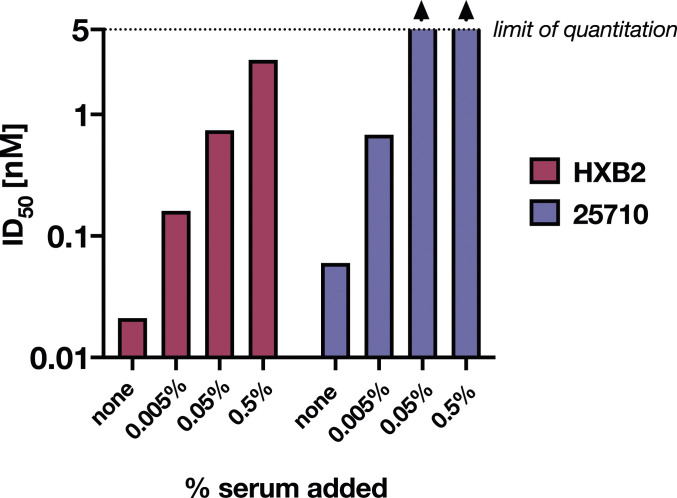
Consistent with earlier studies (17), addition of normal human serum to the neutralization assay diminished the potentiation of D5 neutralizing activity in TZM-bl/FcγRI cells (Fig. 3). This decrease in neutralization was dependent on the concentration of added human serum (Fig. 3), as expected if serum IgG competes for binding to FcγRI and thereby reduces the potentiation of D5 neutralizing activity. Because D5 has such high potency in TZM-bl/FcγRI cells (50% inhibitory dose [ID50] < 0.01 μg/mL; Fig. 2A), it is not surprising that 0.5% human serum (∼50 μg/mL IgG) greatly diminishes the observed potentiation (Fig. 3). However, in a vaccine setting, a substantial fraction (∼1 to ∼10%) of serum immunoglobulin can be antigen-specific (37–40). Indeed, antisera from guinea pigs immunized with the NHR-based vaccine candidate (ccIZN36)3, which had weak or no neutralizing activity in TZM-bl cells, neutralized tier-2 viruses from multiple clades when tested in TZM-bl/FcγRI cells (Fig. 4). These data demonstrate that even in the presence of non–vaccine-elicited serum IgG, vaccine-elicited antibodies against the NHR have enhanced FcγRI-dependent neutralization.
Fig. 3.
Addition of normal human serum to infection assays diminishes the potentiation of D5 neutralization activity by FcγRI. Values of ID50 for D5 IgG (nM) against viruses pseudotyped with Env from two HIV-1 strains (HXB2 and 25710) measured in the presence of 0.005 to 0.5% added human serum. Values above the limit of quantitation for this assay (5 nM) are indicated with arrowheads. ID50 values were obtained from nonconstrained fits of four- and five-point dilution curves, where the neutralization value at each dilution was measured in duplicate.
Fig. 4.
Antisera from guinea pigs immunized with an NHR-based vaccine candidate, (ccIZN36)3, neutralize multiple tier-2 HIV-1 strains in TZM-bl/FcγRI cells. (A) (ccIZN36)3 contains 36 residues from the NHR region of gp41 (HXB2) and is stabilized by disulfide bonds and a coiled-coil domain (7). (B) ID50 titers (serum dilution) were determined for antisera against viruses pseudotyped with Env from various HIV-1 strains measured in TZM-bl cells not expressing (Top; closed) or expressing (Bottom; open) FcγRI using a validated neutralization assay (63–66). V570A is a mutant of the HXB2 strain that is more sensitive to antibodies that target the PHI (7), and P.I. denotes preimmune antisera tested against this strain. Preimmune antiserum from each animal was tested against all viruses and did not have detectable neutralization in any of the strains (ID50 titers were below the limit of quantitation, which was 100 for TZM-bl/FcγRI assays with V570A antisera and 10 for all others). Each data point represents the ID50 value of antiserum from a single guinea pig after a prime and two boosts with (ccIZN36)3.
Discussion
Here we have established that D5, a mAb targeting the NHR region of the PHI, is ∼5,000-fold more potent at preventing HIV-1 infection in FcγRI-expressing cells than in cells that do not express this receptor. This potentiation was found to be specific to FcγRI, and was not observed with equimolar concentrations of D5 Fab (Fig. 2), providing strong evidence that potentiation was the result of Fc-dependent interaction of D5 IgG with FcγRI. We have also shown that antisera against the NHR of the HIV-1 PHI elicited with a vaccine candidate have substantially enhanced neutralization activity in cells expressing FcγRI. In particular, antisera from guinea pigs immunized with (ccIZN36)3 exhibited FcγRI-dependent cross-clade neutralization of a diverse panel of tier-2 viruses (Fig. 4).
Earlier studies reported a similar effect with MPER-binding mAbs, including 2F5 and 4E10 (17, 18), but not with mAbs that target other HIV-1 epitopes. Importantly, these studies also demonstrated that this enhancement occurred when cells were preincubated with antibody and washed before virus was added. As in this work, only slight potentiation was observed for the MPER-binding mAbs in the presence of the other FcγRs tested (IIa, IIb, IIIa).
In contrast to the other, low-affinity FcγRs which generally bind IgG in the form of immune complexes, human FcγRI is able to bind monomeric IgG with KD ∼15 nM (19, 20). As the concentration of IgG in serum is ∼70 μM (∼10 mg/mL), FcγRI receptors will be fully occupied with IgG, and over 1% can be expected to be antigen-specific following vaccination (37–40). Given that human classical monocytes (precursors of most dendritic cells and macrophages) express ∼70,000 FcγRIs per cell (41), each cell would have over 700 FcγRIs occupied with antigen-specific antibodies. Moreover, both FcγRI (42) and CD4 (43, 44) are preferentially localized to lipid rafts, substantially increasing the likelihood that FcγRI is in close proximity to gp41 during viral fusion.
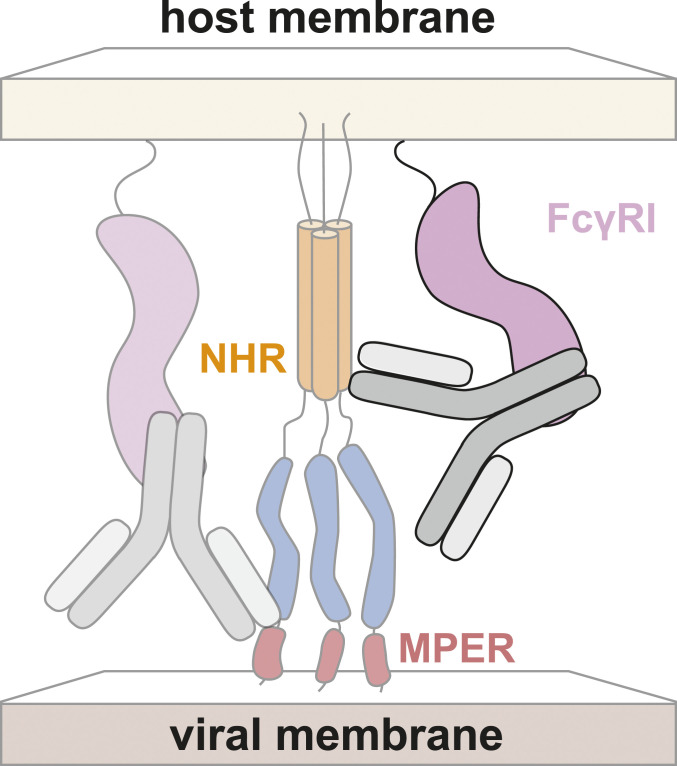
Taken together, these results support a model for potentiation (Fig. 5) in which prepositioning of antibodies by FcγRI at cell surfaces increases the local concentration of antibodies and thereby enhances neutralization (17, 18) (see also ref. 45). Such a mechanism would be expected to impact HIV-1 antibodies that target epitopes on Env that are only exposed after engagement with cellular receptors, such as the MPER or the NHR. Since other viruses that utilize type-I fusion proteins appear to proceed through a PHI during cell entry (2, 3), potentiation of anti-PHI antibodies against other viruses can also be expected.
Fig. 5.
Hypothesized mechanism for FcγRI-mediated potentiation of antibodies targeting the NHR. The Fc domain of the antibody is bound by FcγRI, similar to the previously characterized mechanism of FcγRI-mediated potentiation of antibodies targeting the MPER (17, 18). Both the NHR and the MPER are inaccessible or only partially accessible in the native state of Env.
The relevance of these findings to potential protection from HIV-1 infection is not yet clear. Although FcγRI is not normally expressed on CD4+ T cells, studies of nonhuman primates 24 to 48 h following intravaginal simian immunodeficiency virus (SIV) inoculation demonstrate infection of a substantial number of dendritic cells and macrophages, that often express FcγRI, in addition to T lymphocytes (32–34, 46). Studies using an SIV-based dual-reporter system find that 48 h after vaginal inoculation, while the majority of infected cells are T cells, 25% are dendritic cells or macrophages (47, 48). The relevance of these reports to the question of which cells are first infected following atraumatic vaginal inoculation is complicated by the ability of SIV to enter the vaginal mucosa within 60 min of exposure, such that by 48 h, viral replication will have occurred and infected cells will include many more than those directly infected by the inoculum (33, 48).
While there is not yet consensus in the field regarding which cell types are first infected by HIV-1 in the early minutes to hours of transmission, it is clear that T cells, dendritic cells, and macrophages are all infected in substantial numbers (34). Importantly, HIV-1–infected dendritic cells and macrophages (24–27), both of which express FcγRI, can transmit virus to CD4+ T cells (28–31). In addition, dendritic cells extend dendrites to the luminal surface of the vaginal mucosa where they could be infected directly, and the migration of HIV-1–positive dendritic cells from the initial site of infection to lymph nodes results in dissemination of virus to large numbers of CD4+ T cells (34, 49, 50). Thus, the enhanced protection of FcγRI-expressing cells such as dendritic cells and macrophages by antibodies that target the PHI might decrease the likelihood of HIV-1 transmission, particularly during atraumatic vaginal infection (see also refs. 17, 18, and 51).
Previous studies of nonhuman primates support the notion that FcγRI may have an important role in protection provided by MPER antibodies against simian-HIV (SHIV) challenge. First, in a vaginal challenge with SHIV-BaL in rhesus macaques, dose-dependent protection was observed for an MPER mAb (2F5) when it was administered as an IgG but not when dosed in Fab form, despite higher vaginal Fab levels at the time of challenge (52). This result likely implicates an Fc-dependent mechanism, although the possibility that it is related to the valency difference between the IgG and the Fab cannot be ruled out. Second, in a comprehensive meta-analysis of numerous passive immunization studies showing that serum-neutralization antibody titers associate with protection against SHIV challenge, mAbs targeting the MPER were a highly significant outlier compared with other neutralizing mAbs, with much greater potency than would be predicted from serum-neutralization titers measured in cell culture (53) (see also refs. 54 and 55).
Our finding that anti-PHI antibodies are potentiated by FcγRI in vitro motivates efforts to investigate anti-PHI antibodies in vivo, especially in the context of studies that suggest potential Fc-dependent protection against infection by anti-MPER antibodies in nonhuman primates. In particular, passive transfer experiments using anti-PHI antibodies in nonhuman primate mucosal challenge studies could reveal whether FcγRI-mediated potentiation of anti-PHI antibodies impacts protection from HIV-1 infection. Such results would contribute to our understanding of Fc-mediated correlates of protection against HIV-1 transmission and may have important implications for HIV-1 vaccine development.
Materials and Methods
Antibody Expression and Purification.
D5 IgG and Fab were produced in Expi293F cells. Constructs were cloned using In-Fusion HD Cloning Kit Master Mix (Clontech); the heavy- and light-chain regions were cloned into the CMV/R plasmid backbone for expression under a cytomegalovirus (CMV) promoter. This vector includes the HVM06_Mouse (P01750) Ig heavy-chain V region 102 signal peptide to induce protein secretion and to enable purification from the supernatant. These plasmids were transfected into Expi293F cells at 3 × 106 cells per milliliter with FectoPRO (Polyplus), with the antibody heavy- and light-chain plasmids cotransfected at a 1:1 ratio. Cell cultures were incubated at 37 °C and 8% CO2 with shaking at 120 rpm on a MaxQ 2000 CO2-resistant digital shaker (Thermo Fisher Scientific). Cells were harvested 3 d post transfection by spinning at 4,000 × g for 15 min and filtered through a 0.22-μm filter. IgG-containing supernatants were diluted 1:1 with 1× phosphate-buffered saline (pH 7.4) and batch-bound to Pierce protein A agarose (Thermo Fisher Scientific) overnight at 4 °C. The supernatant/resin slurry was added to a column and the resin was washed with 1× phosphate-buffered saline (pH 7.4) and eluted with 100 mM glycine (pH 2.8) into 1/10 volume of 1 M Tris (pH 8.0). Similarly, Fab-containing supernatants were diluted 1:1 with 50 mM sodium acetate (pH 5.0), batch-bound to Pierce protein G agarose (Thermo Fisher Scientific) overnight at 4 °C, washed with 50 mM sodium acetate (pH 5.0), and eluted with 100 mM glycine (pH 2.8) into 1/10 volume of 1 M Tris (pH 8.0).
Viral Neutralization Assay.
Neutralizing antibody activity of monoclonal antibodies and serum samples was measured in 96-well culture plates using Tat-regulated luciferase reporter gene expression to quantify reductions in virus infection in TZM-bl and TZM-bl/FcγRI cells. TZM-bl cells were obtained from the NIH AIDS Research and Reference Reagent Program, as contributed by John Kappes and Xiaoyun Wu (56–60). TZM-bl cells transduced to stably express FcγRI, TZM-bl/FcγRI (17, 18), were also used as target cells in the neutralization assays. Neutralization assays were performed using well-established Env-pseudotyped lentiviral reference strains (16, 36, 61, 62) in TZM-bl and TZM-bl/FcγRI cells essentially as previously described (63). Serum samples were heat-inactivated at 56 °C for 1 h, and then diluted over a range of 1:20 to 1:43,740 in cell-culture medium and preincubated with virus (∼150,000 relative luminescence units [RLUs]) for 1 h at 37 °C before addition of cells. Experiments with D5 IgG reported in Table 1 included the 1-h 37 °C incubation, while those in Figs. 2 and 3 did not. After incubation for 48 h, cells were lysed and luciferase activity was determined using a microtiter plate luminometer and BriteLite Plus Reagent (PerkinElmer). Neutralization titers were defined as the sample dilution at which RLUs were reduced by 50% compared with RLUs in virus control wells after subtraction of background RLUs in cell control wells. This assay was previously optimized and validated (63, 64) and was conducted in compliance with good clinical laboratory procedures (65), including participation in a formal TZM-bl assay proficiency program for Good Clinical Laboratory Practice-compliant laboratories (66).
(ccIZN36)3 Immunizations.
(ccIZN36)3 was produced as previously described (7). Seven female Hartley guinea pigs from Charles River were immunized intramuscularly with (ccIZN36)3 at 0, 4, and 8 wk. For each immunization, a total volume of 400 μL containing 100 μg (ccIZN36)3, 180 μg aluminum hydroxyphosphate sulfate, and 40 μg Iscomatrix (CSL Biotherapies) was evenly divided between two injection sites. Serum was collected 8 wk before the first immunization and 3 wk after each boost. Animal work was performed in accordance with the Merck Research Laboratories Institutional Animal Care and Use Committee 8119974780067.
Acknowledgments
Research reported in this publication was supported by the NIH under Awards T32GM007276 (to B.N.B.), T32GM007365 (to M.V.F.I.), and DP1DA043893 (to P.S.K.), NIH/National Institute of Allergy and Infectious Diseases Contract HHSN272201800004C (to C.C.L.), the Bill and Melinda Gates Foundation (OPP1113682), Virginia and D. K. Ludwig Fund for Cancer Research, and Chan Zuckerberg Biohub (P.S.K.). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH. B.N.B. is supported by the NSF Graduate Research Fellowship Program. We thank members of the P.S.K. laboratory for valuable discussions, Nielson Weng for consultation on statistics, and Drs. Lillian Cohn, Abigail Powell, and Shaogeng Tang for helpful feedback on this manuscript.
Footnotes
Competing interest statement: D.C.M. runs a central reference laboratory for the HIV vaccine research community and, together with C.C.L., has provided this standardized service to one or both of the reviewers.
Data Availability.
All study data are included in the article.
References
- 1.Chan D. C., Kim P. S., HIV entry and its inhibition. Cell 93, 681–684 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Eckert D. M., Kim P. S., Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70, 777–810 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Harrison S. C., Viral membrane fusion. Virology 479–480, 498–507 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilby J. M., et al. , Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 4, 1302–1307 (1998). [DOI] [PubMed] [Google Scholar]
- 5.LaBonte J., Lebbos J., Kirkpatrick P., Enfuvirtide. Nat. Rev. Drug Discov. 2, 345–346 (2003). [DOI] [PubMed] [Google Scholar]
- 6.de Rosny E., Vassell R., Wingfield P. T., Wild C. T., Weiss C. D., Peptides corresponding to the heptad repeat motifs in the transmembrane protein (gp41) of human immunodeficiency virus type 1 elicit antibodies to receptor-activated conformations of the envelope glycoprotein. J. Virol. 75, 8859–8863 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianchi E., et al. , Vaccination with peptide mimetics of the gp41 prehairpin fusion intermediate yields neutralizing antisera against HIV-1 isolates. Proc. Natl. Acad. Sci. U.S.A. 107, 10655–10660 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi Z., et al. , A recombinant mimetics of the HIV-1 gp41 prehairpin fusion intermediate fused with human IgG Fc fragment elicits neutralizing antibody response in the vaccinated mice. Biochem. Biophys. Res. Commun. 398, 506–512 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Nelson J. D., et al. , Antibody elicited against the gp41 N-heptad repeat (NHR) coiled-coil can neutralize HIV-1 with modest potency but non-neutralizing antibodies also bind to NHR mimetics. Virology 377, 170–183 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louis J. M., Nesheiwat I., Chang L., Clore G. M., Bewley C. A., Covalent trimers of the internal N-terminal trimeric coiled-coil of gp41 and antibodies directed against them are potent inhibitors of HIV envelope-mediated cell fusion. J. Biol. Chem. 278, 20278–20285 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Miller M. D., et al. , A human monoclonal antibody neutralizes diverse HIV-1 isolates by binding a critical gp41 epitope. Proc. Natl. Acad. Sci. U.S.A. 102, 14759–14764 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luftig M. A., et al. , Structural basis for HIV-1 neutralization by a gp41 fusion intermediate-directed antibody. Nat. Struct. Mol. Biol. 13, 740–747 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Gustchina E., et al. , Structural basis of HIV-1 neutralization by affinity matured Fabs directed against the internal trimeric coiled-coil of gp41. PLoS Pathog. 6, e1001182 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gustchina E., et al. , Complexes of neutralizing and non-neutralizing affinity matured Fabs with a mimetic of the internal trimeric coiled-coil of HIV-1 gp41. PLoS One 8, e78187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabin C., et al. , Crystal structure and size-dependent neutralization properties of HK20, a human monoclonal antibody binding to the highly conserved heptad repeat 1 of gp41. PLoS Pathog. 6, e1001195 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seaman M. S., et al. , Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J. Virol. 84, 1439–1452 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez L. G., Costa M. R., Todd C. A., Haynes B. F., Montefiori D. C., Utilization of immunoglobulin G Fc receptors by human immunodeficiency virus type 1: A specific role for antibodies against the membrane-proximal external region of gp41. J. Virol. 83, 7397–7410 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez L. G., Zolla-Pazner S., Montefiori D. C., Antibody-dependent, FcγRI-mediated neutralization of HIV-1 in TZM-bl cells occurs independently of phagocytosis. J. Virol. 87, 5287–5290 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nimmerjahn F., Ravetch J. V., Fcgamma receptors: Old friends and new family members. Immunity 24, 19–28 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Bruhns P., et al. , Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 113, 3716–3725 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Montero M., van Houten N. E., Wang X., Scott J. K., The membrane-proximal external region of the human immunodeficiency virus type 1 envelope: Dominant site of antibody neutralization and target for vaccine design. Microbiol. Mol. Biol. Rev. 72, 54–84 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gach J. S., Leaman D. P., Zwick M. B., Targeting HIV-1 gp41 in close proximity to the membrane using antibody and other molecules. Curr. Top. Med. Chem. 11, 2997–3021 (2011). [DOI] [PubMed] [Google Scholar]
- 23.van der Poel C. E., Spaapen R. M., van de Winkel J. G. J., Leusen J. H. W., Functional characteristics of the high affinity IgG receptor, FcγRI. J. Immunol. 186, 2699–2704 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Patterson S., Rae A., Hockey N., Gilmour J., Gotch F., Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J. Virol. 75, 6710–6713 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smed-Sörensen A., et al. , Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J. Virol. 79, 8861–8869 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen R., et al. , Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J. Virol. 83, 3258–3267 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruize Z., Kootstra N. A., The role of macrophages in HIV-1 persistence and pathogenesis. Front. Microbiol. 10, 2828 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loré K., Smed-Sörensen A., Vasudevan J., Mascola J. R., Koup R. A., Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J. Exp. Med. 201, 2023–2033 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groot F., Welsch S., Sattentau Q. J., Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood 111, 4660–4663 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Waki K., Freed E. O., Macrophages and cell-cell spread of HIV-1. Viruses 2, 1603–1620 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bracq L., Xie M., Benichou S., Bouchet J., Mechanisms for cell-to-cell transmission of HIV-1. Front. Immunol. 9, 260 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spira A. I., et al. , Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183, 215–225 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu J., Gardner M. B., Miller C. J., Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74, 6087–6095 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pope M., Haase A. T., Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat. Med. 9, 847–852 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Jungi T. W., Peterhans E., Pfister H., Fey H., The interaction of ruminant IgG with receptor type II for IgG on human phagocytes. Immunology 66, 143–148 (1989). [PMC free article] [PubMed] [Google Scholar]
- 36.deCamp A., et al. , Global panel of HIV-1 Env reference strains for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 88, 2489–2507 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brocca-Cofano E., et al. , Vaccine-elicited SIV and HIV envelope-specific IgA and IgG memory B cells in rhesus macaque peripheral blood correlate with functional antibody responses and reduced viremia. Vaccine 29, 3310–3319 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez-Quintela A., et al. , Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin. Exp. Immunol. 151, 42–50 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roth G. A., et al. , Injectable hydrogels for sustained codelivery of subunit vaccines enhance humoral immunity. ACS Cent. Sci. 6, 1800–1812 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao Q., et al. , Development of an inactivated vaccine candidate for SARS-CoV-2. Science 369, 77–81 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerntke C., Nimmerjahn F., Biburger M., There is (scientific) strength in numbers: A comprehensive quantitation of Fc gamma receptor numbers on human and murine peripheral blood leukocytes. Front. Immunol. 11, 118 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beekman J. M., van der Linden J. A., van de Winkel J. G. J., Leusen J. H. W., FcgammaRI (CD64) resides constitutively in lipid rafts. Immunol. Lett. 116, 149–155 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Popik W., Alce T. M., Au W.-C., Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4(+) T cells. J. Virol. 76, 4709–4722 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yi L., Fang J., Isik N., Chim J., Jin T., HIV gp120-induced interaction between CD4 and CCR5 requires cholesterol-rich microenvironments revealed by live cell fluorescence resonance energy transfer imaging. J. Biol. Chem. 281, 35446–35453 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Alam S. M., et al. , Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc. Natl. Acad. Sci. U.S.A. 106, 20234–20239 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z., et al. , Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286, 1353–1357 (1999). [DOI] [PubMed] [Google Scholar]
- 47.Stieh D. J., et al. , Vaginal challenge with an SIV-based dual reporter system reveals that infection can occur throughout the upper and lower female reproductive tract. PLoS Pathog. 10, e1004440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stieh D. J., et al. , Th17 cells are preferentially infected very early after vaginal transmission of SIV in macaques. Cell Host Microbe 19, 529–540 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turville S. G., et al. , Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103, 2170–2179 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Martín-Moreno A., Muñoz-Fernández M. A., Dendritic cells, the double agent in the war against HIV-1. Front. Immunol. 10, 2485 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su B., et al. , Update on Fc-mediated antibody functions against HIV-1 beyond neutralization. Front. Immunol. 10, 2968 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klein K., et al. , Neutralizing IgG at the portal of infection mediates protection against vaginal simian/human immunodeficiency virus challenge. J. Virol. 87, 11604–11616 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pegu A., et al. , A meta-analysis of passive immunization studies shows that serum-neutralizing antibody titer associates with protection against SHIV challenge. Cell Host Microbe 26, 336–346.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hessell A. J., et al. , Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J. Virol. 84, 1302–1313 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pegu A., et al. , Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci. Transl. Med. 6, 243ra88 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Platt E. J., Bilska M., Kozak S. L., Kabat D., Montefiori D. C., Evidence that ecotropic murine leukemia virus contamination in TZM-bl cells does not affect the outcome of neutralizing antibody assays with human immunodeficiency virus type 1. J. Virol. 83, 8289–8292 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeuchi Y., McClure M. O., Pizzato M., Identification of gammaretroviruses constitutively released from cell lines used for human immunodeficiency virus research. J. Virol. 82, 12585–12588 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei X., et al. , Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46, 1896–1905 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Derdeyn C. A., et al. , Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J. Virol. 74, 8358–8367 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Platt E. J., Wehrly K., Kuhmann S. E., Chesebro B., Kabat D., Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72, 2855–2864 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li M., et al. , Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in southern Africa. J. Virol. 80, 11776–11790 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hraber P., et al. , Panels of HIV-1 subtype C Env reference strains for standardized neutralization assessments. J. Virol. 91, e00991-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montefiori D. C., Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol. Biol. 485, 395–405 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Sarzotti-Kelsoe M., et al. , Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J. Immunol. Methods 409, 131–146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ozaki D. A., et al. , International technology transfer of a GCLP-compliant HIV-1 neutralizing antibody assay for human clinical trials. PLoS One 7, e30963 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Todd C. A., et al. , Development and implementation of an international proficiency testing program for a neutralizing antibody assay for HIV-1 in TZM-bl cells. J. Immunol. Methods 375, 57–67 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Montefiori D. C., et al. , The high-affinity immunoglobulin receptor FcγRI potentiates HIV-1 neutralization via antibodies against the gp41 N-heptad repeat. bioRxiv:10.1101/2020.08.27.271064 (28 August 2020). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are included in the article.