Significance
Interactions between pathogens can have substantial negative effects on host health. Therefore, identifying the causes of these interactions is integral for effective disease management and control. Interactions between two pathogens that simultaneously or sequentially infect the same host are common. We show that interactions also arise when genetically based defenses against one pathogen affect responses to another, even when the first pathogen is concurrently absent. This mode of pathogen interaction provides insight for understanding patterns of variation in disease severity in natural populations. It also implies that, under some conditions, control strategies that rely on selecting for resistance to one pathogen, in livestock for example, might jeopardize control of another.
Keywords: African buffalo, coinfection, helminths, resistance, tuberculosis
Abstract
Pathogen interactions arising during coinfection can exacerbate disease severity, for example when the immune response mounted against one pathogen negatively affects defense of another. It is also possible that host immune responses to a pathogen, shaped by historical evolutionary interactions between host and pathogen, may modify host immune defenses in ways that have repercussions for other pathogens. In this case, negative interactions between two pathogens could emerge even in the absence of concurrent infection. Parasitic worms and tuberculosis (TB) are involved in one of the most geographically extensive of pathogen interactions, and during coinfection worms can exacerbate TB disease outcomes. Here, we show that in a wild mammal natural resistance to worms affects bovine tuberculosis (BTB) severity independently of active worm infection. We found that worm-resistant individuals were more likely to die of BTB than were nonresistant individuals, and their disease progressed more quickly. Anthelmintic treatment moderated, but did not eliminate, the resistance effect, and the effects of resistance and treatment were opposite and additive, with untreated, resistant individuals experiencing the highest mortality. Furthermore, resistance and anthelmintic treatment had nonoverlapping effects on BTB pathology. The effects of resistance manifested in the lungs (the primary site of BTB infection), while the effects of treatment manifested almost entirely in the lymph nodes (the site of disseminated disease), suggesting that resistance and active worm infection affect BTB progression via distinct mechanisms. Our findings reveal that interactions between pathogens can occur as a consequence of processes arising on very different timescales.
Interactions between pathogens cooccurring within a single host can have profound effects on infection outcomes, ranging from the severity of clinical disease in individual hosts to the rate of disease spread across populations (1–3). Because most hosts are commonly infected by more than one type of pathogen at a time (4), understanding the consequences of pathogen interactions during concurrent infection (or coinfection) is essential for effective disease management and control. While many studies focus on pathogen interactions that are the result of one pathogen responding to the simultaneous presence of another (5), two pathogens need not overlap in time to interact with one another. For example, heterologous immunity, where prior exposure or infection with one pathogen modifies the immune response to another, can drive both positive and negative interactions between pathogens (6). This phenomenon highlights how modifications of the host immune system by one pathogen that occur during the lifetime of a host (i.e., in ecological time) can shape future responses to secondary pathogens. Likewise, strong selection pressure imposed by pathogens on hosts, particularly on immune function (7), can result in modifications of the host immune system that occur over generations (i.e., in evolutionary time), a process which should also affect responses to secondary infections. In this case, a historical population-level response to selection by one pathogen may generate heritable differences among individuals in contemporary responses to another. Crucially, ecological- vs. evolutionary-scale interactions between pathogens may operate for different reasons, so distinguishing between the two is integral to understanding both the mechanistic basis and consequences of these interactions.
Helminths, or parasitic worms, and tuberculosis (TB) are involved in one of the most geographically extensive of pathogen interactions (2, 8). Both pathogens affect approximately one-third of the world’s human population and are widespread in domestic and wild animals (9–11). Caused by bacteria in the Mycobacterium tuberculosis complex, including M. tuberculosis (Mtb), the causative agent of human TB, and Mycobacterium bovis (Mb), the causative agent of bovine TB, TB is responsible for 2 million human deaths (12) and 25% of all disease-related cattle deaths (13) annually. In humans, about 10% of individuals infected with Mtb progress to active pulmonary disease, but the mechanisms underlying progression to active TB are poorly defined (14). Accumulating evidence suggests that coinfection with worms may be a factor in TB disease progression (2, 15), although some studies do not support this link, highlighting the complex nature of worm–TB interactions (16). Interestingly, research in laboratory animals suggests that enhanced immunity (i.e., resistance) to worms can compromise a host’s ability to control TB even in the absence of active worm infection (17–20), implying that evolved defenses against worms may independently affect the response to TB. Considered in light of widespread worm resistance in human and animal populations (21, 22) and the broad geographic coincidence of worms and TB, worm–TB interactions may represent an illustrative case where variation in evolved resistance to one pathogen (worms) contributes to variable responses to another (TB).
In this study, we tested the hypothesis that resistance to worms modifies the host response to TB. To do this, we monitored gastrointestinal (GI) worm (specifically strongyle nematode) and Mb infections in a cohort of wild African buffalo (Syncerus caffer) to assess the effects of natural variation in worm resistance on the incidence, severity, and progression of bovine TB (BTB). In previous work, we demonstrated the presence of an ecological interaction between worms and BTB in buffalo by showing that clearance of active worm infection via anthelmintic treatment reduces BTB-associated mortality (23). Thus, we took advantage of the fact that half of our study animals were subject to long-term deworming to compare the relative effects of worm coinfection vs. natural worm resistance on BTB outcomes. We found evidence of a genetic basis to worm resistance in buffalo and that buffalo with resistance to worms were more severely affected by BTB in terms of both mortality risk and disease progression. However, the mechanisms by which natural variation in the host response to worms was associated with BTB progression appeared to be distinct from the effects of anthelmintic treatment. Our results suggest that negative effects of worms on BTB outcomes occur as a result of both concurrent worm infection and genetically based differences in host responsiveness to worms. This discovery fundamentally alters our understanding of the timescales over which worms and TB interact in real-world populations.
Results
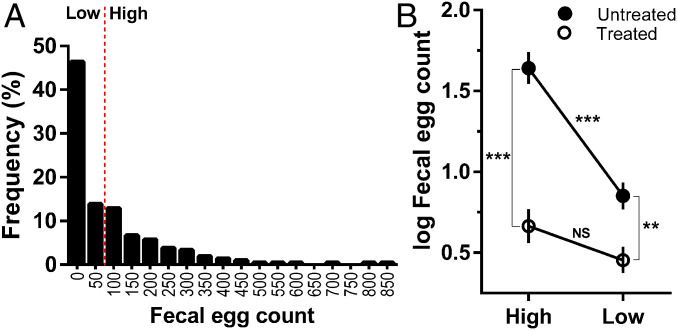
We tracked 209 female African buffalo over 4 y in Kruger National Park (KNP), South Africa. One hundred twelve animals (53%) were infected with worms at initial sampling, and the number of worm eggs shed in the feces of all individuals (hereafter referred to as the fecal egg count [FEC]) varied from 0 to 850 eggs per gram of feces (mean ± SE = 96.9 ± 10). At the first and all subsequent sampling events, approximately half of the study animals, designated as the treatment group, received an anthelmintic drug to control their worm infections. This treatment regime effectively reduced worm egg shedding (23), which is a reliable proxy for adult worm burden in this study population (24). Of the subset of untreated animals that did not receive the anthelmintic, FEC was significantly repeatable within an individual over time (R = 0.41, 95% CI: 0.31 to 0.49; P < 0.0001; n = 103 individuals, 651 observations), revealing a high degree of natural within-individual consistency in the magnitude of worm egg shedding. Since FEC is a widely used proxy of worm resistance in livestock (25) and low vs. high FEC values have been used as a basis for phenotypic selection (26), we investigated the potential source of consistent variation in egg shedding in buffalo by grouping animals into discrete categories based on egg shedding levels at initial sampling. We used a model-based approach to identify statistically distinct clusters in the FEC data (SI Appendix, Fig. S1) and then classified individuals into two groups based on these clusters: “low” FEC (≤50) representing the largest cluster and “high” FEC (≥100) representing all other clusters. We then tested whether this low vs. high FEC phenotype was predictive of variation in the host immune response to worm infection and looked for evidence of a genetic basis to the phenotype. The model-guided approach of discretizing FEC values allowed us to identify patterns that might have been obscured due to a highly overdispersed distribution of the FEC data (Fig. 1A), potential nonlinear relationships between continuous FEC and our focal predictor variables, or both.
Fig. 1.
FECs at initial capture were highly aggregated across individuals, and worm egg shedding phenotype (high or low) at first capture interacted with anthelmintic treatment to determine shedding throughout the study. (A) At the beginning of the study, 60% of buffalo were shedding fewer than 100 worm eggs per gram of feces and were classified as the “low” phenotype, while 40% were shedding more than 100 eggs per gram of feces and were classified as the “high” phenotype (SI Appendix, Fig. S1). (B) Throughout the study, high-FEC individuals in the untreated group (untreated-high) shed significantly more worm eggs than either low-FEC individuals in the untreated group (untreated-low) or high-FEC individuals in the treated group (treated-high). The magnitude of the effect of worm phenotype on worm egg shedding (untreated-high – untreated-low: 0.79; SI Appendix, Table S2) was nearly equivalent to the effect of anthelmintic treatment (untreated-high – treated-high: 0.98; SI Appendix, Table S2). FECs were log (x + 1)-transformed; symbols show means and SEs and asterisks denote significant differences from an LMM (***P < 0.001, **P < 0.01; NS = not significant).
Sixty percent of animals fell into the low FEC category and 40% fell into the high FEC category (Fig. 1A), and the probability of an individual expressing a low vs. high FEC phenotype was repeatable over time (R logit-link approximation = 0.355, 95% CI: 0.22 to 0.44; P < 0.0001), indicating that our discretized measure of FEC phenotype captured the consistent variation in egg shedding apparent in the continuous FEC metric. Furthermore, FEC phenotype at initial capture was associated with differences in worm egg shedding throughout the study nearly equivalent in magnitude to the effect of anthelmintic treatment (SI Appendix, Tables S1 and S2). Accounting for a range of ecological factors associated with worm exposure risk (age, herd, and season), we found that among untreated animals individuals with the high phenotype shed significantly more worm eggs than individuals with the low phenotype, but treatment eliminated the difference between high and low individuals (Fig. 1B and SI Appendix, Table S2). Moreover, the difference in egg shedding between untreated-high and untreated-low individuals was similar to the difference between untreated-high and treated-high individuals (Fig. 1B and SI Appendix, Table S2), indicating that FEC phenotype had effects on worm egg shedding almost as potent as anthelmintic treatment. Crucially, the high–low phenotype was reflective of differences in key immune responses to worms. Among untreated animals, low individuals had more eosinophils, mast cells, and immunoglobulin A (IgA) than high individuals in both the abomasum (Wilcoxon rank sum test, n = 7 high, 7 low, eosinophils: Z = −2.41, P = 0.0159, Fig. 2A; mast cells: Z = −2.13, P = 0.0328, Fig. 2B; IgA: Z = −3.07, P = 0.00006, Fig. 2C) and small intestine (n = 6 high, 6 low, eosinophils: Z = −1.86, P = 0.0632; mast cells: Z = −2.81, P = 0.0049; IgA: Z = −2.80, P = 0.0021), two primary sites of worm infection. The fact that all three of these well-known markers of antihelminth immunity (27) were higher in low FEC individuals supports the presence of a more robust worm resistance response in these animals.
Fig. 2.
The high–low FEC phenotype in buffalo reflects variation in the immune response to worms at the site of infection in the gut and has an underlying genetic basis. Low-FEC individuals had significantly more (A) eosinophils (cells per 2 mm2), (B) mast cells (cells per 2 mm2), and (C) IgA (number IgA+ leukocytes) in the abomasum than high-FEC individuals. Horizontal bars show median values, open circles are raw data points, and asterisks denote significant differences from Wilcoxon rank sum tests (***P < 0.001, *P < 0.05). Furthermore, FEC phenotype was associated with polymorphism at the BL4 locus near the interferon gamma gene. (D) Allele BL4-141 was overrepresented among low-FEC (dark red vs. light red) compared to high-FEC (dark blue vs. light blue) individuals, and individuals carrying allele 141 shed significantly fewer worm eggs (cross represents median and interquartile range, open circles show full range of FEC values, and the P value is from a Wilcoxon rank sum test). (E) In contrast, allele BL4-167 was overrepresented among high-FEC (dark blue vs. light blue) compared to low-FEC individuals (dark red vs. light red), and individuals carrying this allele tended to shed more worm eggs (cross represents median and interquartile range, open circles show full range of FEC values, and the P value is from a Wilcoxon rank sum test).
FEC phenotype was also associated with allelic variation near the interferon gamma gene (IFNG), a gene region that has previously been linked to variation in nematode egg counts in domestic sheep (28), feral Soay sheep (29), and buffalo (30). Focusing on the BL4 locus, which is located 3.6 cM upstream of IFNG (31), we identified nine alleles, two of which, BL4-141 and BL4-167, were associated with FEC phenotype. BL4-141 occurred at a frequency of 29% in the study population (51 out of 177 sampled individuals), and low animals were significantly more likely to carry this allele (high = 12/67, low = 39/110, Pearson’s χ2 test: χ2 = 6.25, P = 0.0124; Fig. 2D). By contrast, BL4-167 occurred at a frequency of only 7%, and in this case high animals were significantly more likely to be carriers of the allele (high = 8/67, low = 4/110, Pearson’s χ2 test: χ2 = 4.54, P = 0.033; Fig. 2E). Further supporting these patterns, the presence of both alleles was correlated with continuous FEC at initial sampling. Individuals carrying BL4-141 were shedding significantly fewer worm eggs (Wilcoxon rank sum test: Z = −2.49, P = 0.0129; Fig. 2D), while those carrying BL4-167 tended to shed more eggs (Z = 1.94, P = 0.0518; Fig. 2E). In combination, both the immunological and genetic patterns suggest that the high–low FEC phenotype in buffalo is a reliable proxy for genetically based differences in natural resistance to worm infection.
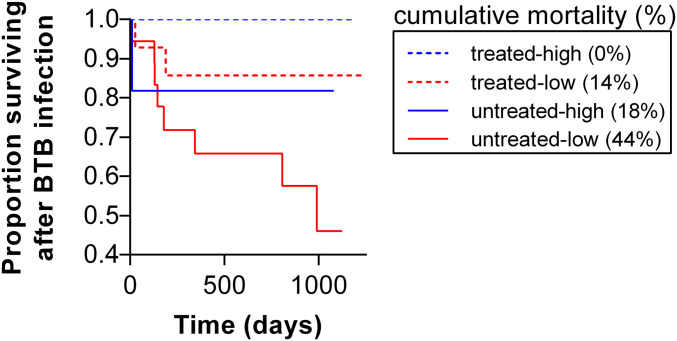
When we examined the consequences of FEC phenotype (herafter called “worm resistance” phenotype) for BTB disease, we found that resistance did not affect the risk of buffalo acquiring BTB (high = 29/77, low = 37/118; hazard ratio [HR] = 0.693, 95% CI: 0.406 to 1.185, P = 0.181; SI Appendix, Table S3) but was a strong predictor of mortality risk after BTB infection. Among BTB-infected individuals, mortality was approximately six times higher in low compared to high individuals (high = 2/24, low = 10/32; HR = 5.68, 95% CI: 1.08 to 29.9, P = 0.04; Fig. 3 and SI Appendix, Table S4). This worm resistance effect was independent of anthelmintic treatment status, but lack of treatment had an additive effect on mortality risk (SI Appendix, Table S4). There was a 26% cumulative mortality difference between untreated-high and untreated-low individuals (Fig. 3). However, cumulative mortality of treated-high individuals was 0% compared to 14% in treated-low individuals (Fig. 3), suggesting that treatment dampened the effect of worm resistance phenotype, although worm resistance still imposed a BTB-associated mortality cost despite significantly reduced worm burdens due to treatment. Treatment reduced BTB-associated mortality in both high and low individuals, as reflected by 18% and 30% reductions in cumulative mortality in untreated vs. treated, high and low individuals, respectively (Fig. 3). Also, the additive negative effect of worm resistance and lack of treatment was most apparent in the substantial cumulative mortality differential between treated-high and untreated-low individuals (0% vs. 44%; Fig. 3). Importantly, there was no effect of worm resistance phenotype on mortality among individuals that were not infected with BTB (SI Appendix, Table S5), implicating an interaction between worm resistance phenotype and BTB as the driver of the resistance-based mortality pattern. Finally, when we examined associations between continous FEC and mortality, we found patterns consistent with results based on worm resistance phenotype. Among BTB-infected individuals, those with lower FECs showed a tendency toward higher mortality (P = 0.07; SI Appendix, Table S6), whereas this trend disappeared among individuals not infected with BTB (P = 0.42; SI Appendix, Table S7). These patterns corroborate that our discretized FEC (i.e., worm resistance) phenotype captures an important underlying biological phenomenon.
Fig. 3.
Worm resistance phenotype affects the survival of buffalo infected with BTB, and anthelmintic treatment reduces, but does not eliminate, this effect. Survival curves showing the proportion of worm-resistant (low worm FEC phenotype) and nonresistant (high FEC phenotype) buffalo by anthelmintic-treated and untreated status surviving BTB infection as a function of time in days. The probability of death given BTB infection was significantly higher for low-FEC individuals compared to high-FEC individuals and worm resistance effects were independent of anthelmintic treatment (SI Appendix, Table S4). Cumulative mortality rates across all groups show that while treatment moderated the mortality effect of worm resistance (treated-high vs. untreated-high and treated-low vs. untreated-low), resistant animals treated for worms (treated-low) still suffered a mortality cost of resistance.
To better understand how worm resistance phenotype and anthelmintic treatment accelerated BTB mortality, we performed gross and histolopathological examination of the lungs and respiratory lymph nodes of a subset of BTB-infected individuals to identify the effect of these traits on BTB disease progression. The lungs are the primary site of BTB infection and the lymph nodes, a common site of extrapulmonary infection, reflect dissemination of the disease (32). In the lungs, we quantified the host’s ability to control bacterial infection (number of lesions, multinucleated giant cell [MNGC] formation), the length and severity of infection (lesion stage), and the degree of tissue damage within lesions (presence of mineralization), including the inability to return to normal tissue architecture (fibrosis). After accounting for the duration of BTB infection, subjects with the low phenotype tended to have more lung lesions (Table 1). Lesions in this group of animals were also significantly more likely to have MNGCs present (Table 1 and SI Appendix, Fig. S2A) and were at a significantly more advanced stage (Table 1), both of which are indicators of less effectively controlled BTB infection. Only one index of disease in the lung, the presence of fibrosis within lesions, was affected by anthelmintic treatment and not worm resistance phenotype. Treated animals had a higher degree of fibrosis within lung lesions (Table 1), which suggests that treatment was associated with an inability to reverse tissue damage in the lung, while worm infection promoted tissue regeneration.
Table 1.
Effects of anthelmintic treatment, worm resistance phenotype, and duration of infection on BTB severity quantified by gross and histolopathological examination of the lungs and respiratory lymph nodes
| Variable | Model type | n | Anthelmintic treatment | Resistance phenotype | Duration of infection | Treatment × resistance phenotype | Treatment/resistance phenotype effect direction |
| Lungs | |||||||
| No. of positive lesions | Negative binomial GLM | 34 | — | 1.079+ | 0.0024* | — | (Low: +) |
| Mineralization | Binomial GLM | 29 | — | — | 0.0022+ | — | |
| Fibrosis | Binomial GLM | 29 | −2.996* | 20.33 | — | — | Untreated: − |
| MNGC | Binomial GLM | 29 | — | 3.00* | 0.0039* | — | Low: + |
| Stage | Ordinal regression | 29 | — | 1.99* | 0.003* | — | Low: + |
| Lymph nodes | |||||||
| No. of positive respiratory nodes | Negative binomial GLM | 34 | 2.12** | 1.84+ | 0.0045* | — | Untreated: + (Low: +) |
| Mineralization | Binomial GLM | 34 | −0.287 | −18.06 | — | 19.85 | |
| Caseous | Binomial GLM | 34 | — | — | — | — | |
| MNGC | Binomial GLM | 34 | 2.602** | — | — | — | Untreated: + |
| Granuloma score | Ordinal regression | 34 | 2.45** | — | — | — | Untreated: + |
| Stage | Ordinal regression | 34 | 2.505** | — | — | — | Untreated: + |
In the lungs, severity was measured as the number of BTB lesions and MNGC formation (indicators of the host’s ability to control bacterial infection), lesion stage (indicator of the length and severity of infection), and the presence of mineralization and fibrosis (indicators of the degree of tissue damage within lesions). In the lymph nodes, severity was measured as the number of respiratory nodes with BTB lesions (indicator of disease dissemination), the RPLN granuloma score and stage (indicators of the distribution and severity of infection), and mineralization, caseous necrosis, and MNGC formation (indicators of the degree of tissue damage within RPLN lesions). Full models of the structure: Y ∼ anthelmintic treatment + resistance phenotype + infection duration + treatment × resistance phenotype were run for each variable and final models were selected using AIC-based model simplification. Values represent estimates from the final models. Significant factors appear in bold (significance codes: **P < 0.01, *P < 0.05, +P < 0.1). Effect description summarizes the direction of each significant treatment and resistance phenotype effect; trends appear in parentheses.
In contrast to the lungs, BTB progression in the lymph nodes was exclusively associated with anthelmintic treatment status and not worm resistance phenotype (Table 1). To evaluate lymph node disease, we quantified the number of respiratory lymph nodes with BTB lesions and also assessed lesion severity within the retropharyngeal lymph node (RPLN). In the RPLN, we quantified the distribution (granuloma score), severity (stage), and degree of tissue damage (mineralization, caseous necrosis, and MNGC formation). Animals treated with an anthelmintic had signficantly fewer positive lymph nodes (Table 1), and lesions in the RPLN were less advanced in terms of disease stage, with less node involvement (i.e., lower granuloma score) and fewer MNGCs (Table 1 and SI Appendix, Fig. S2B).
Discussion
Our study revealed that resistance to worms, like worm coinfection, affects the host response to BTB infection. Specifically, worm-resistant hosts with naturally low worm burdens were more severely affected by BTB, while anthelmintic-treated hosts with artifically low worm burdens were less severely affected by BTB. We also showed that worm resistance and anthelmintic treatment had distinct effects on BTB disease progression. These opposing and distinct effects of treatment vs. resistance phenotype suggest that TB severity is independently affected by the timescale of the host response to worms (i.e., ecological [active infection] vs. evolutionary [genetically determined resistance]). This conclusion is further supported by our finding that worm-resistant individuals infected with BTB suffered from more severe disease progression and higher mortality, even when they were treated for worms. Thus, concurrent interactions between worms and TB were not required for resistance-induced negative TB outcomes to manifest. Rather, genetic variation in worm resistance, likely driven by historical evolutionary interactions between hosts and worms (33, 34), appeared to be sufficient to generate variation in TB disease progression. Counterintuitively, this means that hosts who control worms best and have the lowest rates of worm coinfection may be as compromised in their ability to control TB as hosts with high worm burdens.
In livestock, FECs are widely used as a proxy for worm resistance (25). In sheep, for example, FECs are a heritable trait predictive of variation in antihelminth immunity (35). We showed that FEC phenotype in buffalo was repeatable, linked to differences in antihelminth immunity, and associated with variation at an immune gene known to play a role in worm resistance. In aggregate, this body of evidence supports the use of FEC phenotype as a proxy for worm resistance in our population. Crucially, our proxy for worm resistance was highly predictive of variation in mortality among BTB-infected, but not uninfected, buffalo, suggesting that worm resistance phenotype and BTB interacted to drive mortality outcomes. Furthermore, the effect of worm resistance on BTB mortality was independent of the presence or burden of worms, and the dual effects of worm resistance and treatment status were additive, such that individuals who were both resistant to worms and actively infected with worms experienced the most severe mortality costs. The additive effect implies that worm–TB interactions can arise from distinct processes.
The nonoverlapping effects of resistance and anthelmintic treatment on BTB pathology support the idea that treatment and resistance affect BTB progression via distinct mechanisms. We found that the effects of worm resistance on BTB pathology manifested primarily in the lungs, whereas the effects of anthelmintic treatment manifested primarily in the lymph nodes. The most commonly cited mechanism by which worms affect TB is via modulation of the host immune response. Specifically, activation of T helper cell 2 (Th2) immune responses by worms and concomitant down-regulation of antibacterial T helper cell 1 (Th1) responses has been identified as a central mechanism linking worm infection to increased TB severity (36–39). More recently, induction of alternatively activated macrophages (AAMs) in the lungs during worm coinfection has been associated with compromised TB control (17, 18). Intriguingly, AAMs are also associated with enhanced immunity to worms (19, 20), and genetic variability in arginase-1 activity, the key enzyme expressed by AAMs, affects the outcome of Mtb infection even in the absence of worm infection (17, 40). Taken together, these observations suggest a potential mechanistic pathway (i.e., level of AAM activation) by which resistance to worms may independently affect TB control and disease progression.
The idea that AAM activation may explain the link we found between worm resistance and BTB disease progression is indirectly supported by several of our histopathology observations. First, MNGC formation is typically associated with chronic inflammation in the lung (41), and the more severe lung inflammatory state we described in worm-resistant, BTB-infected animals (SI Appendix, Fig. S2A) mirrors observations that Mtb-infected mice with increased arginase-1 activity show greater inflammatory damage in the lungs (17). Second, an AAM-mediated inflammatory effect would explain why we saw only a weak association between resistance phenotype and the number of lung lesions, since the primary effect of worm resistance should operate via inflammation-induced pathology rather than via bacterial control. Indeed, if this hypothesis is correct, the lack of an effect of worm resistance phenotype on BTB progression in the lymph nodes may be a direct consequence of a less immunoreactive environment in the lymph node (explaining no effect on tissue architecture) and limited impact on bacterial replication (explaining the weak effect on bacterial dissemination to lymph nodes). Third, in direct contrast, the strong effect of anthelmintic treatment on the lymph nodes, particularly the reduction in numbers of Mb-infected nodes, is consistent with a dampening of worm-induced Th1 impairment by anthelmintic treatment and concomitant increase in host control over bacterial replication. Together, these observations suggest an intriguing role for AAM activation in explaining effects of worm resistance on BTB; however, future studies at the mechanistic level are required to test this hypothesis. More broadly, understanding the contribution of host genetic variation to heterogeneities in immune cell populations and how this creates assymmetries in disease outcomes (e.g., ref. 42) is integral to identifying the range of plausible mechanisms that might account for the phenomenon we describe in this study.
Irrespective of the precise mechanisms governing the effects of worm resistance and anthelmintic treatment on BTB disease progression, our results shed important light on the nature and complexity of worm–TB interactions specifically and pathogen interactions more generally. Despite overwhelming laboratory evidence that worms and various microbes interact in ways that can be detrimental to hosts (2, 43), there is very little consensus on the consequences of these interactions for disease outcomes in real-world populations. Our work in wild buffalo reveals that interactions between the same pair of pathogens can originate from processes operating on very different timescales, driven by potentially distinct underlying mechanisms. Crucially, both ecological and evolutionary phenomena may act in tandem to shape the outcome of pathogen interactions. For example, all of the animals in our study had prior exposure to worms. Wild buffalo are exposed to worms soon after weaning (median age of first infection is ∼9 mo), with worm burdens hitting a peak around 3 to 4 y of age, after which they begin to decline, likely as a result of acquired immunity (44, 45). In livestock, worm exposure triggers the development of protective immunity, with the level of protection varying by genotype (27). Likewise, in buffalo, we hypothesize that early life exposure to worms helped shape the degree of phenotypic variation in worm resistance we observed. Moreover, worm exposure (an ecological phenomenon) interacted with host genotype (the product of evolutionary history) to produce significant variation in how buffalo responded to BTB infection. Thus, ecological pressure from worms was critical in unmasking the full negative effect of worm resistance, a result easily obscured in laboratory studies where study animals are typically not subject to natural ecological processes such as prior pathogen exposure or other environmental stressors that can magnify variation among individuals (46).
Finally, our findings may be central to fully understanding the dynamics of coinfection and implications for disease control. For TB, accounting for worm–TB interactions driven by processes operating on an evolutionary timescale might help reconcile inconsistencies in empirical patterns (e.g., ref. 15) currently viewed solely through the lens of ecological time. There are also ramifications for disease control. For example, in humans, accounting for patterns of genetic variation in worm resistance may provide new targets in the search for biomarkers of TB disease progression, while in livestock, where breeding for worm resistance is an increasingly necessary alternative to anthelmintic drug use (25), it may be economically expedient to understand whether breeding for resistance is associated with unanticipated BTB-associated health costs.
Materials and Methods
Animal protocols for this study were approved by the University of Georgia and Oregon State University Institutional Animal Care and Use Committees (UGA AUP no. A2010 10-190-Y3-A5; OSU AUP nos. 3822 and 4325).
Animal Captures.
We captured female African buffalo (S. caffer) in the southern portion of KNP, South Africa between June 2008 and August 2012. Animals were captured by helicopter in 2008, fitted with radio collars, and then recaptured twice a year by vehicle. Animals were captured from two herds in different locations, the Crocodile Bridge (CB) herd and the adjacent Lower Sabie (LS) herd. At initial capture, we randomly assigned animals to an anthelmintic treatment group (fenbendazole bolus; Intervet) or untreated group. Individuals lost due to death or emigration during the study were replaced and replacements were assigned to the same experimental group as the original animal. Animals were captured on average six times (range: two to nine times), and at all captures we collected fecal and blood samples to monitor worm infection and BTB infection status, respectively. We assessed each animal’s age as described in ref. 47. At the outset of the study, the average age of study animals was 4 y (range: 1.4 to 11 y). In total, we followed 209 unique individuals who were BTB-negative at the beginning of the study and for whom data were available on worm infection at initial capture.
Worm Infection, Resistance, and Immunity.
The GI worm community of buffalo in our study was comprised of at least seven species of nematodes: Cooperia fuelleborni, Haemonchus placei, Haemonchus bedfordi, Parabronema sp., Trichostrongylus sp., Africanastrongylus giganticus, and Africanastrongylus buceros, dominated by the strongyle nematodes C. fuelleborni, H. placei, and H. bedfordi (24). The drug fenbendazole is highly effective against strongyle nematodes (48), and lethal sampling of a subset of our study animals indicated that treatment with a slow-release fenbendazole bolus nearly eliminated adult worms in the GI tract of treated animals for up to 200 d (49). In live animals, we monitored strongyle worm infection status using fecal samples collected at capture. Feces were collected directly from the rectum of immobilized animals, stored on ice in the field, and processed the same day. We quantified nematode egg output using a modification of the McMaster fecal egg counting technique with a sensitivity of 50 eggs per gram of feces (50). Strongyle nematode eggs are not distinguishable to the species level, so our FECs reflect the output of all nematode species combined. Nevertheless, egg counts provide an accurate representation of total adult worm counts in our buffalo population (24), and as with adult worms our anthelmintic treatment regime effectively reduced worm egg shedding in experimental subjects throughout the study, such that only 8% of fecal samples collected from treated buffalo within the mean ∼180-d recapture interval contained worm eggs, compared to 55% for untreated buffalo (23).
To maximize our ability to discern signal in buffalo worm shedding patterns across analyses with variable sample sizes, we used an unsupervised machine-learning approach to identify statistically distinguishable groups in the FEC data that allowed us to categorize individuals into two discrete worm egg-shedding phenotypes. We used a Gaussian mixture model implemented in the mclust v5 package (51) in R version 3.4.3 to identify clusters in an FEC dataset that included each study subject’s level of worm egg shedding at initial capture only. Model parameters were estimated using the expectation-maximization algorithm and the optimal model was selected using the Bayesian information criteria (BIC). The model that maximizes BIC is considered optimal since it represents the model with the highest integrated likelihood (52). All possible models, including up to nine clusters with two different covariance structures (E and V), were compared. The optimal model had seven clusters (log-likelihood = −1,221.863, n = 209, degrees of freedom = 14, BIC = −2,518.518), with 126 of 209 individuals placed in the first cluster, representing FECs of 0 to 50, and 83 individuals placed in all remaining clusters, representing FECs of 100 to 850 (SI Appendix, Fig. S1 A–C). Based on these results, we classified animals into two FEC categories (i.e., worm resistance phenotypes), “low” vs. “high,” which separated animals that shed no to very few worm eggs (0 to 50), the lowest statistically distinct FEC cluster, from those that shed higher numbers of worm eggs (≥100). Finally, we verified that these two resistance phenotypes were associated with differences in immunological resistance to worms by testing for an association between phenotype and key mucosal immunological responses to worm infection: numbers of eosinophils, mast cells, and total IgA secretion in the GI tract (27, 53).
We quantified GI tract immune responses to worms focusing on the abomasum and small intenstine (jejunum), the two primary sites of infection for our focal nematode species. GI tract tissue was collected from a subset of study animals killed and necropsied at the conclusion of the study. Killing was performed as part of the KNP BTB surveillance program and implemented according to KNP standard operating procedure. The number of eosinophils was recorded by examining standard formalin-fixed, paraffin-embedded sections of abomasum and proximal jejunum tissue sections (8 mm thick) stained with hematoxylin and eosin. For mast cells, replicates of the same tissue sections were stained with Giemsa stain and then counted. The presence of eosinoophilic granules and a polymorphic nucleus allowed the identification of eosinophils and the presence metachromatic granules facilitated the identification of mast cells (53). Eosinophil and mast cell counts are reported as the number of cells per 10 random high-power fields at the villi lamina propria, which is equivalent to the number of cells per 2 mm2.
IgA production was measured using immunohistochemistry. In this case, formalin-fixed, paraffin-embedded sections of abomasum and proximal jejeunum were deparaffinized, rehydrated, and incubated with a diluted (1:800), rabbit, anti-bovine IgA primary antibody for 1 h (A10-108A; Bethyl Laboratories). After 30 min of incubation with biotinylated universal goat link (Dako), visualization of antigen–antibody complexes was obtained via a 10-min incubation with streptavidin-conjugated horseradish peroxidase (Biocare), followed by a 5-min incubation with diaminobenzidine-peroxidase (Vector Laboratories). For negative controls, we applied the same protocol to abomasum and small intestine sections but replaced the primary antibody with phosphate-buffered saline. For positive controls, we used sections of jejunum and mesenteric lymph node from domestic cattle infected with Haemonchus sp. and Cooperia sp. No staining was detected in negative controls, and positive controls always yielded marked cytoplasmic staining of crypt enterocytes, plasma cells, and other leukocytes. IgA production in the mucosa was estimated based on the number of IgA-positive leukocytes in 10 randomly selected microscope fields (400×) along the intestinal villi and crypts (54). We compared gut immunity between high- and low-FEC animals using data from untreated animals that did not receive anthelmintic treatment. For abomasal immunity, we used data from 14 animals (seven high FEC, seven low FEC), and for intestinal immunity we used data from 12 animals (six high FEC, six low FEC). Since individuals can acquire immuity with age, we verified that the subset of animals used for both analyses did not differ in age at the time of sampling (abomasal immunity mean age [range]: high = 7.3 [5.4 to 9.8] y, low = 7.4 [5.5 to 9.5] y; t test: n = 14, t = −0.12, P = 0.9; intestinal immunity mean age [range]: high = 7.3 [5.4 to 9.8] y, low = 7.4 [5.5 to 9.5] y; t test: n = 12, t = −0.08, P = 0.93).
Genetics of Worm Resistance.
To test for an underlying genetic basis to the worm resistance phenotypes characterized in our study population, we collected tissue samples from buffalo and stored them in silica gel at room temperature for up to 24 mo prior to DNA extraction. DNA was extracted from tissue samples using the Qiagen Blood & Tissue Kit following the manufacturer’s protocol. DNA samples were genotyped at the BL4 microsatellite locus, which is located ∼3 cM upstream of the interferon gamma gene (31). BL4 and other microsatellites in this chromsomal region have been associated with nematode resistance in sheep (28, 29, 55, 56). The BL4 locus has also previously been associated with nematode resistance in African buffalo (30). Genotyping was performed using PCR and fluorescently labeled DNA fragment visualization on an ABI3130xl automated capillary sequencer (Applied Biosystems) as described in ref. 31. Allele sizes were determined using the ABI GS600LIZ ladder (Applied Biosystems). Chromatograms were analyzed by two independent technicians using GeneMapper v3.7. Tests for the presence of null alleles and deviations from Hardy–Weinberg proportions (HWP) showed no null alleles present at BL4 and no deviations from HWP (31).
BTB Infection and Disease Progression.
We tested buffalo for BTB at each capture using a whole-blood interferon gamma (IFNγ) assay (BOVIGAM) implemented according to the manufacturer’s instructions. We used bovine and avian tuberculin as stimulants for in vitro release of IFNγ in buffalo blood samples. BTB tests were considered negative if the optical density reading for bovine tuberculin-stimulated samples was below a cutoff of 0.375 and positive at readings of 0.375 or higher. Tests were also considered negative if the optical density reading for bovine tuberculin-stimulated samples failed to exceed the reading for avian tuberculin-stimulated samples by at least 10%, due to likely cross-reactivity to exposure to Mycobacterium avium. This test protocol achieves a specificity of ∼93.5% and a sensitivity of 85.4% (57). For each buffalo, we used a time series of two to nine test results to classify BTB conversion status. For each animal, each test was interpreted in the context of the full time series of results to increase confidence in our assignment of BTB infection status (see ref. 23 for details).
To quantify BTB progression, we performed gross and histopathological examinations on killed animals. First, animals were necropsied to assess the distribution of macroscopic BTB lesions in the lungs and respiratory lymph nodes (mandibular, parotid, palatine tonsil, retropharyngeal, caudal mediastinal, and tracheobronchial). Necropsies were performed by South African National Parks Veterinary Wildlife Services and South African State Veterinary Services veterinarians following standard protocols for BTB detection in buffalo (58). During necropsies, we serially sectioned the bronchial and RPLNs from every culled buffalo at 1-cm intervals to examine for tuberculous or other lesions and immediately placed a 2- × 2- × 0.5-cm section from each lymph node (with or without lesions) in 10% neutral-buffered formalin (NBF). We thoroughly examined the lungs and diagrammed and measured all BTB lesions. We collected a 2- × 2- × 0.5-cm section from the edge of the largest pulmonary lesion of each animal into 10% NBF. From animals with no TB lesions, we collected a representative section of lung into 10% NBF. All formalin-fixed tissues were routinely processed for histopathology and stained with hematoxylin and eosin stains. Histological sections were examined by a board-certified veterinary pathologist who was blinded to the experimental groups. Sections of all tissues with tuberculous granulomas were examined for approximate percentage of the section affected by granulomatous inflammation and the percentage of the lesion that was necrotic. Lung sections were assessed for granuloma stage (59) and presence or absence of mineralization, fibrosis, and multinucleated giant cells. RPLN sections were assessed for stage (59), granuloma score (1 = focal, 2 = multifocal, 3 = coalescing, 4 = majority of the section), and presence or absence of mineralization, caseous necrosis, and multinucleated giant cells. We performed gross and histopathological examinations on 34 animals (18 high FEC, 16 low FEC; 21 treated, 13 untreated) that tested positive for BTB via BOVIGAM assay. Neither high vs. low nor treated vs. untreated subsets of animals differed in age at the time of examination (t test: high–low, t = −0.99, P = 0.33; treated–untreated, t = −1.37, P = 0.18).
Buffalo Mortality.
All study animals were fitted with radio collars, so we continuously monitored the activity of all individuals to detect natural mortality events. For all possible mortality cases, we searched for the collars associated with these cases to determine the animal’s fate. Mortality was confirmed in 50 out of 67 cases and cause of death was ascertained where possible (e.g., predation). Three deaths that were deemed capture-related (e.g., a lion attack immediately after a captured animal was released) were excluded from the dataset. For each mortality event, the date of last recorded collar activity was assigned as the death date.
Statistical Analyses: Repeatability of FEC and Worm Resistance Phenotype.
To examine the degree to which buffalo showed consistency in their level of worm egg shedding over time, we estimated the repeatability (R) of both continuous FEC [log10 (FEC) + 1] and categorical worm egg shedding phenotype (high FEC or low FEC). We estimated repeatability of FEC and worm phenotype using the linear mixed model (LMM) (Gaussian) and generalized LMM (binary) methods, respectively, implemented with the rptR package (60) in R version 3.4.3. Confidence intervals were estimated using 1,000 parametric bootstraps. For binary repeatability, we present R as a logit link-scale approximation, which provides a better estimate of the underlying propensity for an individual to express a high or low FEC. For both repeatability analyses, we restricted our analyses to the subset of nonanthelmintic treated buffalo (n = 103 individuals, 651 observations) in order to estimate the levels of natural consistency in worm shedding in the absence of anthelmintic treatment, which we expected to disrupt egg shedding patterns.
Effect of Initial Worm Phenotype on Egg Shedding over Time and Interaction with Anthelmintic Treatment.
To evaluate whether worm phenotype (low vs. high) at an animal’s initial capture predicted worm egg shedding over the course of the study and how this related to anthelmintic treatment, we tested for an effect of worm phenotype at capture #1 on FEC at captures #2-n using an LMM. Animal ID was included as a random effect in the model, and treatment status (untreated, treated), age, herd (CB, LS, Other), season (early dry, late dry, early wet, late wet), and capture interval (time since last capture) were included as fixed effects. We accounted for treatment and an interaction between worm phenotype and treatment, since our past work showed that treatment significantly affected egg shedding rates (23). Age, herd, and season were included as predictors since these factors are likely associated with differences in worm exposure and have a known influence on worm egg shedding in buffalo (23). Finally, we included capture interval to account for variable parasite reaccumulation in treated animals captured at different interval lengths. Prior to analysis, we normalized the distribution of the dependent variable (FEC) using log transformation. The model was run using the R packages lme4 and lmerTest. Model validity was assessed by visual inspection of residuals as described in (61). The difflsmeans function in lmerTest was used to test for significant differences between least squared means. P values were adjusted for multiple comparisons using Holm’s method implemented with the p.adjust function in the stats package.
Associations between Worm Resistance Phenotype and GI Immunity and BL4 Genotype.
We tested for an association between worm resistance phenotype (high FEC vs. low FEC) and GI tract immune responses using Wilcoxon rank sum tests. This analysis focused only on non-anthelmintic-treated buffalo and we evaluated the effect of high vs. low status on numbers of eosinophils, mast cells, and IgA production in the abomasum and small intestine. We tested for associations between worm resistance phenotype and allelic variation at the BL4 locus (presence/absence of alleles 141 and 167) using χ2 tests. We also used Wilcoxon rank sum tests to examine whether the presence of the two BL4 alleles were associated with differences in FEC at initial capture. For these genotype-based analyses, we included all sampled individuals (untreated and anthelmintic-treated) because treatment should have had no impact on worm phenotype (which was classified at initial capture prior to first treatment) or genotype.
Effect of Worm Resistance Phenotype on BTB Infection Probability and Mortality.
To examine the effects of worm resistance phenotype on BTB infection probability while accounting for anthelmintic treatment status, we added resistance status (high FEC vs. low FEC) and age at first capture as covariates into a multivariate proportional hazards model previously used to test the effects of anthelmintic treatment on BTB infection risk (see ref. 23). The model included worm resistance phenotype, treatment, herd of origin, and age as predictors. BTB infection probability was estimated as the time from an animal’s initial capture to BTB infection or until the last BTB test. Including an interaction term between treatment status and worm resistance phenotype had no qualitative effect on the model results, so we do not report the interaction model. Our analysis included 195 individuals with known BTB infection histories that were also characterized for worm phenotype at initial capture. Data for individuals that did not test BTB-positive by the end of the study period or that left the study due to death or emigration were right-censored. The Efron method was used to address ties in event times, and the validity of the proportional hazards assumption was examined using Schoenfeld residuals (worm phenotype, low: χ2 = 0.048, P = 0.826, treatment, control: χ2 = 0.241, P = 0.623, herd, LS: χ2 = 0.014, P = 0.906, age: χ2 = 1.59, P = 0.207, global model: χ2 = 2.566, P = 0.633). Cox regression models and residual checks were performed in R with the survival package. HRs, CIs, and likelihood ratio tests for the model are reported in SI Appendix, Table S3. The HR for the worm-resistant phenotype variable (i.e., high FEC vs. low FEC) represents the instantaneous probability that a low-FEC individual is more or less likely than a high-FEC individual to acquire BTB.
We used a similar modeling procedure to test the effects of worm resistance phenotype on mortality risk for BTB-infected individuals. In this case, the multivariate model included worm resistance phenotype, treatment, herd, and age at first capture as predictors. A model including an interaction term between treatment status and worm resistance phenotype was undefinable due to low sample size. For all BTB-positives with known fates that were characterized for worm phenotype at initial capture (n = 56), we used the time from BTB conversion to either death or the end of the study as the response variable. Animals that did not die by the end of the study or those removed from the study for other reasons (e.g., emigration from the study area) were right-censored. Four BTB-positive individuals that converted at their last capture were truncated from the dataset. Checks of the Schoenfeld residuals upheld assumptions of proportional hazards (worm phenotype, low: χ2 = 3.344, P = 0.0675, treatment, control: χ2 = 0.113, P = 0.7363, herd, LS: χ2 = 0.645, P = 0.4217, age: χ2 = 0.267, P = 0.6052, global model: χ2 = 6.307, P = 0.1774). HRs with confidence intervals and likelihood ratio tests are reported in SI Appendix, Table S4.
To verify that the effect of worm resistance phenotype on buffalo mortality we observed was restricted to BTB-positive individuals and was not characteristic of BTB-negative individuals, we also examined the predictors of mortality risk in BTB-negative buffalo. There were 116 BTB-negative animals with known fates and worm phenotype characterizations. For these individuals we used the time from initial capture to death or the end of the study as the relevant response variable. Animals that did not die by the end of the study or those removed from the study for other reasons were right-censored. The multivariate proportional hazards model had the same structure as the model run for BTB-positive individuals (SI Appendix, Table S5). Residual checks upheld the assumptions of proportional hazards for this model (worm phenotype, low: χ2 = 0.085, P = 0.771, treatment, control: χ2 = 0.105, P = 0.706, herd, LS: χ2 = 0.702, P = 0.402, age: χ2 = 0.156, P = 0.693, global model: χ2 = 1.076, P = 0.898).
Finally, to assess whether our use of discretized FEC categories (i.e., worm resistance phenotypes) influenced conclusions about mortality patterns, we reran the mortality analyses described above for both BTB-infected and uninfected buffalo substituting continuous FEC for worm resistance phenotype. All other predictors remained the same and residual checks were performed as described. Model details are reported in SI Appendix, Tables S6 and S7.
Effects of Worm Resistance Phenotype and Treatment on BTB Progression.
We examined the combined effects of worm resistance phenotype (high FEC vs. low FEC) and anthelmintic treatment on the progression of BTB disease using general and generalized linear models (GLMs). Dependent variables were gross and histological measures of lung and lymph node pathology. Nominal variables were modeled using binomial GLM, ordinal variables were modeled using ordinal regression (R package: ordinal), and two continuous variables (number of lung lesions and number of positive lymph nodes) were modeled using negative binomial GLMs. For all models, worm resistance phenotype, treatment, duration of BTB infection, and the interaction between worm phenotype and treatment were included as predictor variables. A forward and backward stepwise Akaike information criterion (AIC)-based model selection approach (implemented with the function “step”) was used to simplify each model and identify the key predictor variables explaining variation in disease progression.
Supplementary Material
Acknowledgments
We thank South African National Parks (SANParks) for permission to conduct this study in Kruger and the entire SANParks Veterinary Wildlife Services Department for invaluable assistance with animal captures and project logistics. We thank R. Spaan, J. Spaan, K. Thompson, B. Beechler, E. Gorsich, P. Snyder, J. Alagappan, S. Amish, L. Austin, L. Megow, K. Raum, N. Rogers, and M. Smith for work on animal captures and sample processing. F. Quinn and P. Rohani provided valuable comments on the manuscript draft. This study was supported by the NSF (NSF DEB-1102493) and NIH (NIH 1R01GM131319) as part of the joint NSF/NIH/US Department of Agriculture Ecology and Evolution of Infectious Diseases Grant Program.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2015080118/-/DCSupplemental.
Data Availability.
Data have been deposited in Dryad, https://doi.org/10.5061/dryad.pk0p2ngmh (62).
References
- 1.Abu-Raddad L. J., Patnaik P., Kublin J. G., Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science 314, 1603–1606 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Salgame P., Yap G. S., Gause W. C., Effect of helminth-induced immunity on infections with microbial pathogens. Nat. Immunol. 14, 1118–1126 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woolhouse M. E., et al. , Co-infections determine patterns of mortality in a population exposed to parasite infection. Sci. Adv. 1, e1400026 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petney T. N., Andrews R. H., Multiparasite communities in animals and humans: Frequency, structure and pathogenic significance. Int. J. Parasitol. 28, 377–393 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Cox F. E., Concomitant infections, parasites and immune responses. Parasitology 122 (suppl.), S23–S38 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Agrawal B., Heterologous immunity: Role in natural and vaccine-induced resistance to infections. Front. Immunol. 10, 2631 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sironi M., Cagliani R., Forni D., Clerici M., Evolutionary insights into host-pathogen interactions from mammalian sequence data. Nat. Rev. Genet. 16, 224–236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotez P. J., et al. , Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS Med. 4, 1553 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pullan R. L., Smith J. L., Jasrasaria R., Brooker S. J., Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit. Vectors 7, 37 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva Miranda M., Breiman A., Allain S., Deknuydt F., Altare F., The tuberculous granuloma: An unsuccessful host defence mechanism providing a safety shelter for the bacteria? Clin. Dev. Immunol. 2012, 139127 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claridge J., et al. , Fasciola hepatica is associated with the failure to detect bovine tuberculosis in dairy cattle. Nat. Commun. 3, 853 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO , Global Tuberculosis Report 2018 (World Health Organization, 2018), https://apps.who.int/iris/handle/10665/274453. [Google Scholar]
- 13.World Bank; TAFS Forum , World Livestock Disease Atlas: A Quantitative Analysis of Global Animal Health Data (2006–2009) (World Bank and TAFS Forum, 2011). [Google Scholar]
- 14.Flynn J. L., Chan J., Lin P. L., Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunol. 4, 271–278 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babu S., Nutman T. B., Helminth-tuberculosis co-infection: An immunologic perspective. Trends Immunol. 37, 597–607 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterjee S., et al. , Incidence of active pulmonary tuberculosis in patients with coincident filarial and/or intestinal helminth infections followed longitudinally in South India. PLoS One 9, e94603 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monin L., et al. , Helminth-induced arginase-1 exacerbates lung inflammation and disease severity in tuberculosis. J. Clin. Invest. 125, 4699–4713 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potian JA, et al. (2011) Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J. Exp. Med. 208, 1863–1874 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anthony R. M., et al. , Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat. Med. 12, 955–960 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreider T., Anthony R. M., Urban J. F. Jr, Gause W. C., Alternatively activated macrophages in helminth infections. Curr. Opin. Immunol. 19, 448–453 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinnell R. J., Genetics of susceptibility to human helminth infection. Int. J. Parasitol. 33, 1219–1231 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Bishop S., Morris C., Genetics of disease resistance in sheep and goats. Small Rumin. Res. 70, 48–59 (2007). [Google Scholar]
- 23.Ezenwa V. O., Jolles A. E., Epidemiology. Opposite effects of anthelmintic treatment on microbial infection at individual versus population scales. Science 347, 175–177 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Budischak S. A., Hoberg E. P., Abrams A., Jolles A. E., Ezenwa V. O., A combined parasitological molecular approach for noninvasive characterization of parasitic nematode communities in wild hosts. Mol. Ecol. Resour. 15, 1112–1119 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Bishop S. C., A consideration of resistance and tolerance for ruminant nematode infections. Front. Genet. 3, 168 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris C. A., Wheeler M., Watson T. G., Hosking B. C., Leathwick D. M., Direct and correlated responses to selection for high or low faecal nematode egg count in Perendale sheep. N. Z. J. Agric. Res. 48, 1–10 (2005). [Google Scholar]
- 27.McRae K. M., Stear M. J., Good B., Keane O. M., The host immune response to gastrointestinal nematode infection in sheep. Parasite Immunol. 37, 605–613 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayers G., Good B., Hanrahan J. P., Ryan M., Sweeney T., Intron 1 of the interferon γ gene: Its role in nematode resistance in Suffolk and Texel sheep breeds. Res. Vet. Sci. 79, 191–196 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Coltman D. W., Wilson K., Pilkington J. G., Stear M. J., Pemberton J. M., A microsatellite polymorphism in the gamma interferon gene is associated with resistance to gastrointestinal nematodes in a naturally-parasitized population of Soay sheep. Parasitology 122, 571–582 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Ezenwa V. O., Etienne R. S., Luikart G., Beja-Pereira A., Jolles A., Hidden consequences of living in a wormy world: Nematode‐induced immune suppression facilitates tuberculosis invasion in African buffalo. Am. Nat. 176, 613–624 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Lane-deGraaf K. E., et al. , Signatures of natural and unnatural selection: Evidence from an immune system gene in African buffalo. Conserv. Genet. 16, 289–300 (2015). [Google Scholar]
- 32.Domingo M., Vidal E., Marco A., Pathology of bovine tuberculosis. Res. Vet. Sci. 97 (suppl.), S20–S29 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Stear M. J., Singleton D., Matthews L., An evolutionary perspective on gastrointestinal nematodes of sheep. J. Helminthol. 85, 113–120 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Fumagalli M., et al. , Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J. Exp. Med. 206, 1395–1408 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stear M. J., Boag B., Cattadori I., Murphy L., Genetic variation in resistance to mixed, predominantly Teladorsagia circumcincta nematode infections of sheep: From heritabilities to gene identification. Parasite Immunol. 31, 274–282 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Elias D., et al. , Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guérin (BCG) vaccination. Clin. Exp. Immunol. 123, 219–225 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elias D., Mengistu G., Akuffo H., Britton S., Are intestinal helminths risk factors for developing active tuberculosis? Trop. Med. Int. Health 11, 551–558 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Obieglo K., et al. , Chronic gastrointestinal nematode infection mutes immune responses to mycobacterial infection distal to the gut. J. Immunol. 196, 2262–2271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Resende Co T., Hirsch C. S., Toossi Z., Dietze R., Ribeiro-Rodrigues R., Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin. Exp. Immunol. 147, 45–52 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El Kasmi K. C., et al. , Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat. Immunol. 9, 1399–1406 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson S., Shires V. L., Wilson R. A., Mountford A. P., Formation of multinucleated giant cells in the mouse lung is promoted in the absence of interleukin-12. Am. J. Respir. Cell Mol. Biol. 20, 371–378 (1999). [DOI] [PubMed] [Google Scholar]
- 42.Orrù V., et al. , Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat. Genet. 52, 1036–1045 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graham A. L., Ecological rules governing helminth-microparasite coinfection. Proc. Natl. Acad. Sci. U.S.A. 105, 566–570 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Combrink L., et al. , Age of first infection across a range of parasite taxa in a wild mammalian population. Biol. Lett. 16, 20190811 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Budischak S. A., O’Neal D., Jolles A. E., Ezenwa V. O., Differential host responses to parasitism shape divergent fitness costs of infection. Funct. Ecol. 32, 324–333 (2018). [Google Scholar]
- 46.Hoffmann A. A., Hercus M. J., Environmental stress as an evolutionary force. Bioscience 50, 217–226 (2000). [Google Scholar]
- 47.Jolles A. E., Ezenwa V. O., Etienne R. S., Turner W. C., Olff H., Interactions between macroparasites and microparasites drive infection patterns in free-ranging African buffalo. Ecology 89, 2239–2250 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Williams J. C., Efficacy of albendazole, levamisole and fenbendazole against gastrointestinal nematodes of cattle, with emphasis on inhibited early fourth stage Ostertagia ostertagi larvae. Vet. Parasitol. 40, 59–71 (1991). [DOI] [PubMed] [Google Scholar]
- 49.Budischak S. A., Hoberg E. P., Abrams A., Jolles A. E., Ezenwa V. O., Experimental insight into the process of parasite community assembly. J. Anim. Ecol. 85, 1222–1233 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Ezenwa V. O., Habitat overlap and gastrointestinal parasitism in sympatric African bovids. Parasitology 126, 379–388 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Scrucca L., Fop M., Murphy T. B., Raftery A. E., mclust 5: Clustering, classification and density estimation using Gaussian finite mixture models. R J. 8, 289–317 (2016). [PMC free article] [PubMed] [Google Scholar]
- 52.Fraley C., Raftery A. E., Model-based clustering, discriminant analysis, and density estimation. J. Am. Stat. Assoc. 97, 611–631 (2002). [Google Scholar]
- 53.Bricarello P. A., et al. , Immunological responses and cytokine gene expression analysis to Cooperia punctata infections in resistant and susceptible Nelore cattle. Vet. Parasitol. 155, 95–103 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Grootjans J., et al. , Epithelial endoplasmic reticulum stress orchestrates a protective IgA response. Science 363, 993–998 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davies G., et al. , Quantitative trait loci associated with parasitic infection in Scottish blackface sheep. Heredity 96, 252–258 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Beh K. J., et al. , A genome scan for quantitative trait loci affecting resistance to Trichostrongylus colubriformis in sheep. Anim. Genet. 33, 97–106 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Michel A. L., Cooper D., Jooste J., de Klerk L. M., Jolles A., Approaches towards optimising the gamma interferon assay for diagnosing Mycobacterium bovis infection in African buffalo (Syncerus caffer). Prev. Vet. Med. 98, 142–151 (2011). [DOI] [PubMed] [Google Scholar]
- 58.de Klerk L. M., Michel A. L., Bengis R. G., Kriek N. P., Godfroid J., BCG vaccination failed to protect yearling African buffaloes (Syncerus caffer) against experimental intratonsilar challenge with Mycobacterium bovis. Vet. Immunol. Immunopathol. 137, 84–92 (2010). [DOI] [PubMed] [Google Scholar]
- 59.Wangoo A., et al. , Advanced granulomatous lesions in Mycobacterium bovis-infected cattle are associated with increased expression of type I procollagen, gammadelta (WC1+) T cells and CD 68+ cells. J. Comp. Pathol. 133, 223–234 (2005). [DOI] [PubMed] [Google Scholar]
- 60.Nakagawa S., Schielzeth H., Repeatability for Gaussian and non-Gaussian data: A practical guide for biologists. Biol. Rev. Camb. Philos. Soc. 85, 935–956 (2010). [DOI] [PubMed] [Google Scholar]
- 61.Zuur A., Ieno E. N., Walker N., Saveliev A. A., Smith G. M., Mixed Effects Models and Extensions in Ecology with R (Springer Science & Business Media, 2009). [Google Scholar]
- 62.Ezenwa V. O., et al. , Data from: Natural resistance to worms exacerbates bovine tuberculosis severity independently of worm coinfection. Dryad . 10.5061/dryad.pk0p2ngmh. Deposited 4 January 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data have been deposited in Dryad, https://doi.org/10.5061/dryad.pk0p2ngmh (62).