Significance
As a strategy to stabilize soil Pb, we evaluated the effect of PLJ formation on the bioavailability of soil Pb. Our technology for PLJ formation resulted in >90% incorporation of soil Pb into the mineral structure of jarosite. This conversion decreased soil Pb bioavailability greater than 90%, even after pH neutralization to revitalize the soil to agronomic health. PLJ formation may be a more effective remediation method than other soil treatment technologies and may reduce removal clean-up efforts and costs. Because jarosite can sequester both cationic and anionic contaminants, it may be effective for both Pb and arsenic remediation. Furthermore, this treatment is not influenced by initial soil pH or organic matter content and uses an environmentally safe chemical.
Keywords: lead, plumbojarosite, bioavailability, speciation, remediation
Abstract
Exposure to lead (Pb) during early life has persistent adverse health effects. During childhood, ingestion of bioavailable Pb in contaminated soils can be a major route of Pb absorption. Remediation to alter physiochemical properties of soil-borne Pb can reduce Pb bioavailability. Our laboratory-based approach for soil Pb remediation uses addition of iron (Fe) sulfate and application of heat to promote formation of plumbojarosite (PLJ), a sparingly soluble Pb-Fe hydroxysulfate mineral. We treated two soils with anthropogenic Pb contamination and samples of clean topsoil spiked with various Pb compounds (i.e., carbonate, chloride, phosphate [P], or sulfate) to convert native Pb species to PLJ and used a mouse assay to assess relative bioavailability (RBA) of Pb in untreated (U) and remediated soils. Bone and blood Pb levels were significantly lower (P < 0.001, Student's t test) in mice that consumed diets amended with remediated soils than with U soils. Estimated RBA for Pb in both remediated natural soils and Pb-mineral spiked soils were reduced by >90% relative to Pb RBA for U soils, which is substantially more effective than other soil amendments, including P. X-ray absorption spectroscopy showed that >90% of all Pb species in remediated soils were converted to PLJ, and ingested PLJ was not chemically transformed during gastrointestinal tract transit. Post treatment neutralization of soil pH did not affect PLJ stability, indicating the feasibility in field conditions. These results suggest that formation of PLJ in contaminated soils can reduce the RBA of Pb and minimize this medium’s role as a source of Pb exposure for young children.
A recent United Nations Children's Fund (1) report highlights the global epidemic of lead (Pb) poisoning where one in three children (∼800 million globally) have blood lead levels above the US Centers for Disease Control and Prevention reference value for elevated blood Pb (5 µg/dL) and over 900,000 premature deaths per year are attributed to lead exposure. Exposure of children to Pb has profound and long-lasting health effects (2, 3). Historical release of Pb into the environment has resulted in widespread and persistent contamination of urban soil and dust with this toxic metal (4–6). Although Pb levels in some media (e.g., air and food) have declined in recent decades (7, 8) soil and dust Pb levels remain elevated (3). As a consequence, Pb in soil and dust is a significant source of Pb exposure in 1–6-y-old children (9). Therefore, reducing exposure of children to Pb through soil or dust ingestion is an important public health goal (10). Ingestion of Pb in soil and surface dust contributes to elevated blood Pb levels in children residing in Pb-contaminated environments (11–16). Thus, exposure of children to Pb occurs, in part, from frequent contact with surface dust, hand-to-mouth activity, and exploratory mouthing behavior which result in relatively high rates of soil ingestion per unit of body mass (17–19). Accordingly, strategies to lower the risk of elevated Pb intake in children who are exposed to impacted soils have emphasized disrupting the soil ingestion pathway. Common approaches involve excavation and removal of soil, capping or covering with vegetation, and institutional controls, such as fencing to restrict access (20). However, soil removal and replacement are expensive and complicated procedures that can be difficult to implement. If removal and replacement of contaminated soil are not feasible, an alternative approach is to reduce the bioavailability of Pb present in soil and dust. Operationally, bioavailability is defined as the amount of a contaminant absorbed into the body following skin contact, oral ingestion, or inhalation (21). Oral bioavailability of Pb is strongly influenced by its solubility in the gastrointestinal (GI) tract (22–24). Lead from ingested soil particles is dissolved in GI-tract fluids and absorbed by physiological transport mechanisms (25, 26). Reducing the bioavailability of ingested Pb in soil or dust can have a salutary effect by reducing the internal dose of this toxic metal (21, 27).
The bioavailability of Pb is a function, in part, of physical and chemical properties of the matrix in which it is ingested and can vary from 0 to 100% (or 0 to 1 as a fractional ratio). One approach to reducing the bioavailability of Pb is in situ solidification and stabilization to reduce the solubility of Pb in soil (28, 29). Some methods include increasing soil pH to induce lead carbonate formation or use of adsorptive materials, such as organic matter, clays, or metal (iron, manganese, and aluminum) oxides (20, 30). One prominent Pb sequestration technology involves addition of phosphorus as phosphate (P) to Pb-contaminated soil to promote formation of the stable and relatively insoluble Pb-P mineral, pyromorphite (Pyr). Although Pb-P interactions can reduce soil Pb bioavailability (31–33), the limitations of this approach and its applicability to a variety of soil types have not been systematically evaluated. In some cases, P treatment may have a minimal effect on soil Pb bioavailability. A bench scale study (34) with a soil used in the current study evaluated Pb bioaccessibility as a function of soil suspension pH relative to the soil’s point of zero charge and three P amendment levels. The best-case scenario found that P treatment produced a modest 35% reduction in Pb bioaccessibility relative to the untreated (U) sample. Similarly, P treatment of a Pb-contaminated soil under field conditions resulted in less than a 50% reduction in the bioavailability of soil Pb (33).
Although in situ solidification and stabilization is appealing due to its relative lower costs than removal of soil, the treatment must show substantial and persistent reduction in Pb bioavailability in order to be an effective alternate to removal. We have evaluated in situ formation of plumbojarosite (PLJ), an insoluble Pb/iron (Fe)-hydroxysulfate mineral, in soils as an option for stabilization of soil Pb. PLJ is one of the end members of the jarosite-group minerals within the alunite supergroup. The alunite supergroup consists of more than 40 pure mineral species possessing a common general formula of
| [1] |
where alkali (A) = sites occupied by monovalent (e.g., K, Na, NH4, Ag, Tl, and H3O), divalent (e.g., Ca, Sr, Ba, and Pb), trivalent (e.g., Bi), or, more rarely, quadrivalent (e.g., Th) ions; B = sites occupied by Al3+ or Fe3+ or, more infrequently, Ga or V; and X = sites occupied by S6+> As5+ or P5+ and may include subordinate amounts of Cr6+, Sb5+, or Si4+. For end-member formulas, charge excesses within the mineral are typically balanced with monovalent cations occupying A sites. However, charge excesses can be balanced by substituting divalent or trivalent cations—something typically achieved by leaving a portion of the A sites unoccupied. Conversely, charge excesses can be balanced by substitutions with divalent anions, such as sulfate, or with trivalent anions, such as P or arsenate (35–38). These features that allow jarosite formation to scavenge various toxic metals and metalloids into a highly stable mineral structure make it a candidate material for sequestering soil contaminants (39–41). Overall, PLJ has a very low solubility (the solubility product or Ksp = 10−26.2, ref. 42, making it similar in stability to galena and chloroPyr, which exhibit extremely low solubility and, presumably, bioavailability.
Jarosite-group minerals tend to form under low-pH (1.5–3.0) and high-sulfate (>3,000 mg/kg) conditions (43). Over the past three decades, jarosite has been used in hydrometallurgical processes to remove unwanted impurities (Fe, Zn, Se, Ni, Co, Cu, U, and sulfate ions) (44–46). In the general scheme for jarosite formation, material containing cationic and/or anionic contaminants is reacted with Fe sulfate-based salts or salt solutions under excess heat. In this process, the contaminants are incorporated directly into the structure of jarosite. Based on this scheme, we hypothesize that promoting jarosite formation in a Pb-contaminated soil will promote PLJ formation and that formation of this species will reduce the solubility and bioavailability of Pb in the remediated soil.
Results
Physicochemical Characterization of Soils.
SI Appendix, Table S1 summarizes total concentrations of Pb, As, Zn, Cd, Fe, Al, Mn, P, S, Mg, Ca, Cu, and Cr in the test soils determined by US Environmental Protection Agency (USEPA) Method 3051a (47). The clean topsoil collected from a farm in Ohio contained a negligible concentration of Pb was used as clean soil that was spiked with Pb mineral compounds. This soil is classified as Clermont series consisting of very deep poorly drained soils formed in loess and the underlying till on till plains. The contaminated soils, Soil 1 (Pb arsenate pesticide contamination) and Soil 2 (mining influenced contamination) contained 2,065 and 6,262 mg of Pb kg−1 (n = 4), respectively. Soil 1 is classified as Saunook series consisting of very deep well-drained moderately permeable soils on benches, fans, and toe slopes. Soil 2 is classified as McCaffery series soil consisting of very deep well-drained soils formed in sandy sediments on lacustrine benches or river terraces in intermountain valleys. The soil series for the tested soils were identified at the county level using the Natural Resources Conservation Service-US Department of Agriculture soil series classification database available online.
Effect of PLJ Treatment Method on Soil Pb.
Our synthesis procedure was a modification of the protocol of ref. 48 in which we added the Fe solution to the Pb suspension to be more similar to how the treatment technology would be implemented at a contaminated site. The experimental setup used for PLJ treatment and the X-ray absorption spectroscopy (XAS) spectra collected for the conversion of Pb nitrate to PLJ are presented in SI Appendix, Figs. S1 and S2. We applied the synthesis procedure to pure Pb compounds (Pb chloride [PbCl2], litharge [PbO], cerussite [PbCO3], anglesite [PbSO4], and lead orthophosphate [Pb3(PO4)2]); these same Pb compounds spiked into the clean topsoil at 2,000 mg Pb kg−1; and two contaminated soils. The PLJ synthesis procedure was able to convert the pure Pb compounds and topsoil spiked with Pb compounds to PLJ (SI Appendix, Table S2).
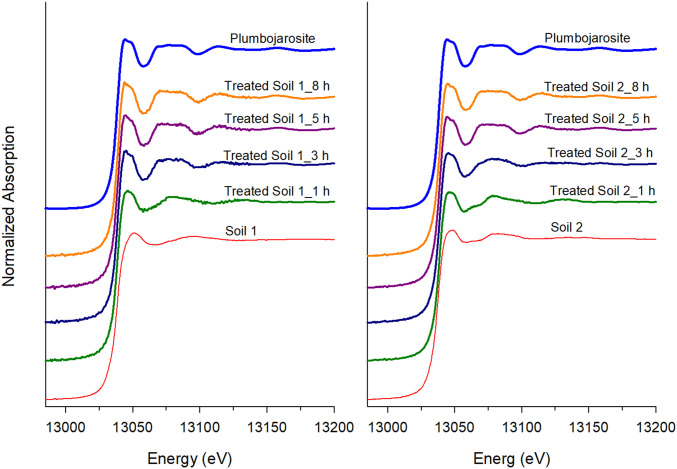
Overall, XRS data demonstrated a change in Pb speciation as a result of the treatment with conversion to PLJ in treated (T) samples. Statistical modeling of XAS data via linear combination fitting to compare the Pb species before the jarosite formation treatment varied widely among the two soils tested, reflecting differences in soil type and contamination sources. For example, Pb speciation was dominated by surface-adsorbed forms in U Soil 1, whereas Pb speciation in Soil 2 was composed largely of minerals, such as PbSO4, Pyr, and Pb sorbed to oxides (Ads) or clay minerals. After treatment, approximately 93–99% of the soil Pb was converted to PLJ within the treatment times of 8 and 67 h (SI Appendix, Table S3). Kinetic observations over an 8-h reaction period shows increasing transformation of original Pb phases in Soil 1 and Soil 2 to PLJ with increasing reaction time as clearly shown in Fig. 1 and SI Appendix, Table S4. In conjunction with PLJ formation in the T samples, in vitro bioaccessibility (IVBA) results showed dramatic reduction in Pb bioaccessibility in these samples (SI Appendix, Table S5). Pb IVBA for Soil 1 and Soil 2 decreased from >70% to less than 1%, an ∼99% reduction in Pb bioaccessibility, in samples T for 8- to 67-h treatment.
Fig. 1.
XAS data showing Pb speciation spectra for Soil 1 (Left) and Soil 2 (Right) at different reaction times of 1, 3, 5, and 8 h. The top spectrum in blue shows a pure PLJ reference compound.
Successful field use of a method for PLJ formation requires that soil pH can be restored to neutrality for healthy agronomic plant growth after treatment without destabilizing PLJ mineral. Preliminary experiments showed that PLJ formation in T soils was not destabilized by pH neutralization. For example, Soil 2 was utilized for conducting pH neutralization experiments where the PLJ T Soil 2 suspension pH was raised from pH 1–6 using potassium hydroxide and maintained for a 24-h time period. The soil suspension was left aerated on a stir plate for a week during which the suspension pH changed nominally (from 6.00 to 5.83). Suspension samples from the pH neutralization experiments were consolidated and utilized for IVBA extraction and Pb speciation by XAS. Negligible Pb concentrations were released into solution during the neutralization experiments, suggesting that PLJ remained stable as the pH was adjusted to pH 6. Pb-XAS analysis confirmed this, indicating no change in Pb speciation (SI Appendix, Table S6) or in vitro bioaccessibility results (SI Appendix, Table S7). However, further studies are needed to verify long-term stability of PLJ near circumneutral soil pH.
Pb Bioavailability in T Soils.
Effect of PLJ treatment on soil Pb relative bioavailability (RBA).
In this assay, tissue Pb concentrations (blood and bone) from mice consuming diets (Ds) containing test materials were used to determine tissue dose ratios and to estimate RBAs for Pb in test materials. Here, treatment of anthropogenic Pb-contaminated soils or soils spiked with various Pb minerals to promote PLJ formation uniformly reduced blood and bone Pb concentrations by, at least, 90% compared to tissue Pb levels in mice consuming Ds amended with the corresponding U soil. Table 1 shows RBA estimates for the contaminated soils (Soil 1 and Soil 2) based on blood or bone data and a point estimate that was the mean of the tissue-derived estimates. RBA estimates for either soil following 8- or 67-h treatment to promote PLJ formation were markedly lower than corresponding estimates for U soils. For both soils, treatment decreased RBA point estimates by >90%. Notably, the magnitude of reduction in the RBA for T soils was not strongly affected by the length of time used for soil treatment.
Table 1.
Effect of treatment on estimated RBA for Pb in two soils*
| Soil | Treatment | Bone | Blood | Point | ||||||
| Mean | LCL | UCL | Mean | LCL | UCL | Mean | LCL | UCL | ||
| 1 | U | 0.545 | 0.461 | 0.655 | 0.750 | 0.374 | 1.190 | 0.648 | 0.513 | 0.781 |
| T 8 h | 0.056 | 0.007 | 0.111 | 0.027 | 0.014 | 0.041 | 0.042 | 0.018 | 0.066 | |
| % Decrease | 89.7 | 96.4 | 93.5 | |||||||
| T 67 h | 0.034 | −0.008 | 0.081 | 0.012 | 0.009 | 0.016 | 0.023 | 0.003 | 0.043 | |
| % Decrease | 93.8 | 98.4 | 96.5 | |||||||
| 2 | U | 0.456 | 0.341 | 0.602 | 0.524 | 0.456 | 0.609 | 0.490 | 0.422 | 0.560 |
| T 8 h | 0.032 | 0.028 | 0.039 | 0.058 | 0.041 | 0.079 | 0.045 | 0.036 | 0.054 | |
| % Decrease | 93.0 | 88.9 | 90.8 | |||||||
| T 67 h | 0.041 | 0.002 | 0.084 | 0.017 | 0.011 | 0.024 | 0.029 | 0.010 | 0.048 | |
| % Decrease | 91.0 | 96.8 | 94.1 | |||||||
Mean RBA estimates with lower (LCL) and upper (UCL) 95% confidence intervals based on bone or blood tissue data. RBA point estimates are means of tissue-derived RBA estimates.
For the soil spiked with a variety of Pb minerals, treatment to promote PLJ formation reduced estimated RBAs for Pb (Table 2). In U soils, RBAs for Pb derived from added minerals varied widely (0.5–0.9). However, after treatment Pb RBAs were consistently reduced to <0.1 despite differences in the physical and chemical properties of Pb minerals used to spike the soil. For comparison, the RBA for Pb in these T soils approximated estimates obtained for authentic PLJ.
Table 2.
Effect of treatment on estimated RBA for Pb in soils spiked with Pb minerals*
| Mineral | Treatment | Bone | Blood | Point | ||||||
| Mean | LCL | UCL | Mean | LCL | UCL | Mean | LCL | UCL | ||
| PbCO3 | U | 0.929 | (0.763 | 1.141 | 0.869 | 0.743 | 1.023 | 0.899 | 0.792 | 1.005 |
| T | 0.024 | 0.003 | 0.047 | 0.038 | 0.017 | 0.060 | 0.031 | 0.017 | 0.044 | |
| % Decrease | 97.4 | 95.6 | 96.6 | |||||||
| PbCl2 | U | 0.632 | 0.328 | 0.987 | 0.727 | 0.458 | 1.035 | 0.680 | 0.512 | 0.848 |
| T | 0.033 | 0.018 | 0.050 | 0.064 | 0.041 | 0.090 | 0.049 | 0.035 | 0.061 | |
| % Decrease | 94.8 | 91.2 | 92.8 | |||||||
| Pb3(PO4)2 | U | 0.423 | 0.323 | 0.547 | 0.575 | 0.482 | 0.689 | 0.499 | 0.429 | 0.568 |
| T | 0.030 | −0.004 | 0.067 | 0.033 | 0.015 | 0.054 | 0.032 | 0.013 | 0.050 | |
| % Decrease | 92.9 | 94.3 | 93.6 | |||||||
| PbSO4 | U | 0.947 | 0.498 | 1.472 | 0.904 | 0.757 | 1.083 | 0.926 | 0.741 | 1.114 |
| T | 0.019 | 0.008 | 0.031 | 0.029 | 0.007 | 0.053 | 0.024 | 0.012 | 0.036 | |
| % Decrease | 98.0 | 96.8 | 97.4 | |||||||
| PLJ | U | 0.068 | 0.051 | 0.089 | 0.013 | 0.010 | 0.016 | 0.041 | 0.032 | 0.049 |
Mean RBA estimates with LCL and UCL 95% confidence intervals based on bone or blood tissue data. RBA point estimates are means of tissue-derived RBA estimates.
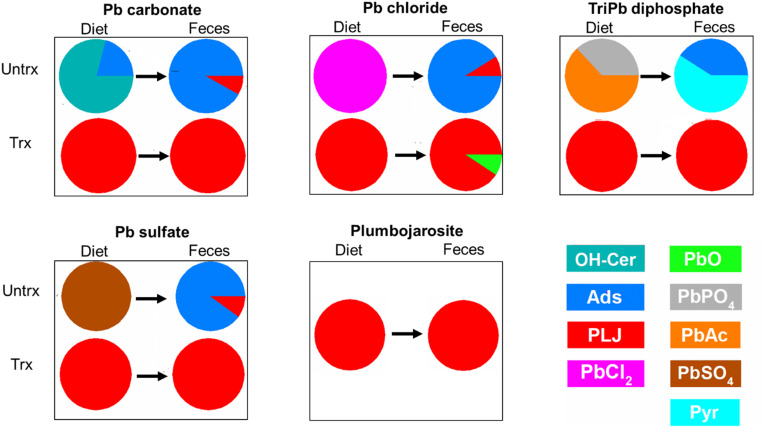
Analysis of spectra from D and feces (F) provided information on Pb speciation in these materials. Comparing Pb species in Ds amended with U or T soils (Fig. 2) or with soils spiked with Pb minerals (Fig. 3) evaluated the effectiveness of treatment in promoting PLJ formation. Similarly, comparing Pb species in Ds and F provided information about stability of Pb species during transit of the GI tract. Fig. 2 shows data on Pb speciation in Ds amended with U and T Pb-contaminated soils. Differences in the Pb species profiles in U Soils 1 and 2 probably reflected differences in the sources of Pb contamination or differences in physicochemical properties of the two soils. For these U soils, the fractional contribution of Pb species differed in D and F. Similar observations were found for U soils spiked with Pb minerals (Fig. 3). Changes in Pb speciation may reflect abiotic or biotic transformations that occur during GI-tract transit. Changes in Pb speciation during GI-tract transit have been reported in other studies using the mouse assay (33, 49). In contrast, T soil amended Ds resulted in quantitative conversion of all Pb species to PLJ. Speciation of Pb in F from mice that consumed Ds amended with T soils identified PLJ as the sole Pb species. These findings demonstrate that the method used for PLJ production in soil was fully successful, that ingested PLJ transited the GI tract without transformation, and that soil Pb incorporated into PLJ demonstrated extremely low Pb bioavailability.
Fig. 2.
Pb species in D and F of mice that consumed Ds amended with Soil 1 or Soil 2. Soils tested in U or T forms with data for both 8 and 67 h of treatment. Fraction of total Pb accounted for by each Pb species present shown. PLJ; PbSO4; Ads; Humic, Pb bound to organic matter; PbO.
Fig. 3.
Pb species in D and F of mice that consumed Ds amended with Pb minerals. Minerals tested in U (Untrx) or T (Trx) forms. Fraction of total Pb accounted for by each Pb species present shown. PLJ; OH-Cer, PbO3; PbSO4; Pyr; Ads; Humic; PbO; PbPO4; PbAc, Pb acetate.
Discussion
The high efficiency of conversion of soil Pb species to PLJ coupled with evidence of low relative bioavailability for Pb ingested in this form suggests that remediation of soils by PLJ formation in situ may be a valuable tool to reduce exposure of children to this toxic metal.
Although the benchtop method for PLJ synthesis reported here examined some aspects of its utility under field conditions, optimization of the soil treatment method and studies of long-term efficacy of treatment under field conditions are needed to validate this approach. Of particular interest is the development of methods to form PLJ with minimal soil disturbance and without application of heat to facilitate the reaction or alternative heating mechanisms, such as microwave (50), reported an addition of small quantities of jarosite seed in a Pb suspension under poor agitation conditions formed PLJ, which contained more than 12% Pb. The efficacy of seeding of soils with jarosite in promoting PLJ formation should be examined using the methods described here. Additional studies should evaluate jarosite seeding as an alternative to soil heating to promote PLJ formation.
Materials and Methods
PLJ Synthesis Method and Its Optimization.
Our initial proof of concept study followed methodology of ref. 48 for PLJ synthesis with a minor modification of switching the additive and receiving solutions in which 120 mL of 0.1-M Fe3+(SO4)–0.01-M H2SO4 was pumped to 1 L of solution containing 2.0-g Pb(NO3)2 at the rate of 1.8 mL h−1 into a well-stirred 2-L reaction vessel at 95–100 °C for 67 h. The modification was adopted to be representative of how the procedure would be applied in the field. The next step was substituting pure Pb compounds for Pb(NO3)2 in the procedure above as a means to demonstrate conversion of various Pb species to PLJ. After successful PLJ formation using pure Pb compounds (PbCl2, PbO, PbCO3, PbSO4 and Pb3[PO4]2) in nonsoil suspensions, the technology was adapted for treatment of the Pb compounds spiked into clean topsoil (2,000 mg kg−1 Pb) or contaminated soils using 8- or 67-h reaction times. The topsoil with negligible Pb content was collected from a farm in Clermont County, Ohio for spiking with pure Pb compounds to evaluate PLJ formation and subsequent bioavailability studies. Two contaminated soils were utilized as substrates. Soil 1 was collected from the Barber Orchard Superfund site in North Carolina where Pb arsenate had been used as a pesticide and the site has since undergone an extensive removal action. Soil 2 is a new US Geological Survey reference soil collected from a mining impacted site in Montana and developed in collaboration with the USEPA as a bioavailability reference soil for Pb and arsenic (As). It is to be noted that all test soils utilized in this study were sieved to produce a <250-µm fraction for testing. Soil treatment conditions were adjusted to optimize the method for use under field conditions. Concentrations of Fe2(SO4)3 and H2SO4 used in PLJ formation were reduced from 0.1 to 0.05 M and 0.01 to 0.0015 M, respectively, by increasing the rate of Fe2(SO4)3 addition. These changes accelerated the kinetics of PLJ formation, reducing the reaction time from 67 to 8 h.
Pb speciation in T pure Pb compounds, clean topsoil spiked with Pb minerals, and both contaminated soils were determined by XAS at Materials Research Collaborative Access Team (MRCAT) Sector 10-ID (51) at the Advanced Photon Source (APS) of the Argonne National Laboratory. The spectra of T materials were collected using a Lytle detector purged with pure argon to capture the fluorescence and an ion chamber purged with pure nitrogen to capture transmission data. The spectra at 10-ID were collected in quick scan mode where the monochromator is continuously moved through the energy region. The data were rebinned upon import into Athena. More details on XAS sample preparation and data analysis are provided in refs. 34, 52.
Remaining soil samples from the T and U spiked topsoils and contaminated soils experiments were retained for bioaccessibility and bioavailability testing. Further details on the PLJ synthesis method and its optimization and test results are provided in the SI Appendix.
Pb Bioaccessibility in U and Remediated Soils.
USEPA Method 1340, a validated IVBA assay, was used to determine the bioaccessibility of Pb in soils. In this assay, 0.5 g of soil in 50 mL of 0.4-M glycine acidified to pHs of 1.5 and 2.5 using hydrochloric acid was agitated with end-over-end shaking for 1 h on ROTAMIX (Appropriate Technical Resources, Inc., Laurel, MD) at 30 rpm at 37 ± 2 °C in the incubation chamber. While USEPA Method 1340 specifies a pH of 1.5 for the extraction solution, extraction at pH 2.5 was also investigated to explore whether PLJ formation and associated reductions in bioaccessibility were pH dependent since much better in vivo/in vitro correlations on bioaccessible Pb are observed when the Method 1340 extraction pH is 2.5 for P T and Pb-contaminated soils due to an artifact with P chemistry (31).
The extraction and filtration process were completed within 90 min. The pHs of the fluid were also measured just after the sample was withdrawn for filtration and analysis in order to confirm that pH values fell within the range of 0.5 pH units of the starting pH. The extraction fluid was directly removed from the extraction bottle into a disposable syringe and filtered via 0.45-µm cellulose acetate syringe filter. The filtered samples were received in disposable vials and stored at 4 °C in a refrigerator. The extracted fluids were diluted 10 times and then analyzed for Pb on inductively coupled plasma (ICP)-optical emission spectrometry (ICP-6500 trace analyzer) within 1–7 d of extraction.
Pb Bioavailability in U and Remediated Soils.
Preparation of test materials.
All test soils were sieved to produce a <250-µm fraction for testing. Pb (Pb acetate trihydrate, Sigma-Aldrich, St. Louis, MO) was used as the RBA reference compound in all studies.
Test Ds.
Test materials, (U or T soils and Pb acetate) were incorporated by the vendor (Dyets, Bethlehem, PA) into powdered AIN-93G purified rodent D. This D meets the nutritional requirements of rapidly growing immature rodents (53).
Mouse assay.
The use of mice in determination of soil lead bioavailability was reviewed and approved by the Institutional Animal Care and Use Committee of the US EPA’s Research Triangle Park facility. The design of the assay to determine the RBA for Pb in soil has been described (54). In this assay, the bioavailability of Pb in a test material (e.g., soil or mineral) was calculated using data on Pb levels in bone and blood of mice that consumed AIN-93G rodent D which was amended with the test material.
Four-week-old female C57BL/6 mice received from Charles River Laboratory (Raleigh, NC) were acclimatized for 12–14 d in a 12-h light–12-h dark photocycle at 20–22 °C with free access to Prolab RMH 3000 rodent D (Lab Diet, St. Louis, MO) and drinking water. During the 9-d assay, mice were housed in groups of three in metabolic cages (Lab Products, Seaford, DE) with free access to AIN-93G rodent D amended with a test material and drinking water. A standard assay consisted of four metabolic cages with the cage as the unit of observation and analysis. Environmental conditions were identical throughout acclimation and assay stages of studies. For each cage, daily food and water consumption were recorded. Cumulative food intake was the sum of daily food consumption. For each cage, daily urine or F production were combined to create cumulative urine and F samples. Combined body weights of mice in each cage were determined before transfer into metabolic cages and at assay termination. At termination, mice were euthanized with CO2, and a heparinized blood sample was collected. After evisceration and pelt removal, carcasses were defleshed in dermestid beetle cultures. This procedure provided a nearly complete skeleton for Pb determination. Samples from three mice in each metabolic cage were pooled by tissue type before processing for Pb determination.
Lead analysis.
Mouse D, blood, and bone samples were analyzed at RTI International’s Center for Analytical Science Trace Metals Laboratory (Research Triangle Park, NC). Samples were prepared by acid digestion using a graphite heating block (SCP Science, Baie d’Urfe, Quebec), and digests were analyzed for Pb content using a Thermo X-series II ICP-MS (Waltham, MA). High-purity deionized water (DI H2O, 16 MΩ or better, Pure Water Solutions), and high-purity nitric acid (HNO3) distilled from trace metals grade concentrated (∼70%) HNO3 (JT Baker, Phillipsburg, NJ) using a DuoPur sub-boiling acid distillation unit (Milestone, Shelton, CT) were used for all sample and standard preparations. Distilled HNO3 was screened for contamination before use in sample preparation and analysis and was used only if Pb concentrations were found to be below the limit of detection (≤ 1.59, 0.195, and 2.83 ng/g for D, blood, and bone, respectively). National Institute of Standards and Technology (NIST)-traceable Pb and internal standard (bismuth with holmium as a secondary internal standard) stock solutions were purchased from High Purity Standards (Charleston, SC) and used for preparation of standards and quality control samples. NIST (Gaithersburg, MD) standard reference material SRM 1486—bone meal (nominal [Pb] = 1.335 mg/kg) was purchased commercially and included during analysis of skeleton samples to verify method performance. All polypropylene labware was soaked in 5% HNO3 solution overnight, rinsed, and dried before use to ensure no Pb contamination from environmental sources. All sample preparations occurred in all-plastic high efficiency particulate air-filtered laboratory hoods to prevent contamination by atmospheric sources.
Pb speciation analysis.
The Pb species in samples of Ds amended with test materials and cumulative F samples were determined at the DuPont-Northwestern-Dow Collaborative Access Team Sector 5, beamline 5BM-D, at the APS of the Argonne National Laboratory, Lemont, IL. The spectra of F and Ds were collected at Sector 5 in step scan mode using two four-element vortex silicon (Si) drift fluorescence detectors mounted perpendicular to the X-ray beam on either side of the sample. The beam was opened to ∼8-mm wide by 1-mm height. The Si drift detectors at 5 BM-D helped to improve data quality in our low Pb-concentration samples, which is not recommended at 10-ID due to the intense flux of the beamline. A reference spectrum of Pb metal was collected simultaneously with each sample spectrum in order to match the data collected from two separate beamlines, Sectors 10-ID and 5 BM-D. The reference spectra were then calibrated to 13,035 eV. The same process was followed for all standard spectra used in the linear combination fitting analysis, thus, making comparable all standards and samples independent of the beamline used. More details on sample preparation and data analysis can be referred to ref. 49.
Data analysis.
All statistical analyses were performed using SAS/STAT software, Version 9.4 of the SAS System for Windows. Tissue-specific RBAs (bone and blood) were estimated for soils and Pb minerals. RBA was estimated as the tissue dose ratio (TDR) for the test material (TM) (e.g., soil or mineral) and reference material ([RM], Pb acetate; Equation 1).
| [1] |
where the TDR is the ratio of the Pb concentration (mg kg−1 total skeleton or mg L−1 blood) to the cumulative Pb dose (mg) for the study. Supporting studies found that RBAs estimated using Eq. 1 yielded RBA estimates not different from estimates based on the ratio of slopes (TM/RM) for simultaneous linear regression models of tissue Pb and cumulative Pb dose (54, 55). Using a single dose level rather than multiple dose levels simplifies the mouse assay and reduces the number of live animals needed to estimate RBA for a single material. Each TDR in Eq. 1 was derived from multiple estimates of TDR for groups of three mice housed together in a single metabolic cage (the unit of measure in the assay is data from a single cage). Confidence limits on each RBA were estimated based on Fieller’s theorem for estimating confidence limits on the ratio of means (56). Normal distributions for estimates of TDRs were assumed with adjustments for unequal variance (SasAS Proc "T Test"). A point estimate for the RBA was calculated as the average of tissue-specific RBA values (55). Confidence intervals on the point estimate were estimated from Monte Carlo simulation, which consisted of averaging repeated random draws from probability distributions of each tissue-specific RBA (normal: mean and SE) with equal probability for each tissue (55).
Supplementary Material
Acknowledgments
We acknowledge the significant scientific contributions and mentorship of former USEPA scientist Dr. James (Jim) A. Ryan. Jim’s career was devoted to practical science to understand paradigm risk analysis to protect human health and the natural environment and who championed USEPA’s soil metal bioavailability research. We are also thankful to Jennifer Goetz for building the accessories required for the experimental setup. The USEPA funded and managed the research described here. It has been subjected to Agency review and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. We acknowledge the support of the MRCAT (Sector 10-ID). MRCAT operations are supported by the Department of Energy (DOE) and the MRCAT member institutions. This research used resources of the APS, a DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Part of this work was also performed at the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) located at Sector 5 of the APS. DND-CAT was supported by Northwestern University, E.I. DuPont de Nemours and Co., and The Dow Chemical Company. This research used resources of the AP under Contract DE-AC02-06CH11357. Portions of this work were funded by USEPA's Office of Superfund Remediation and Technology Innovation (OSRTI) under Contract 68HERH19D022 (Task Order 68HERH19F0313).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2020315117/-/DCSupplemental.
Data Availability.
Research data have been deposited in data.gov.
References
- 1.PureEarth , The toxic truth. https://www.unicef.org/media/73246/file/The-toxic-truth-children%E2%80%99s-exposure-to-lead-pollution-2020.pdf. Accessed 4 August 2020.
- 2.Surkan P. J., et al. , Neuropsychological function in children with blood lead levels <10 microg/dL. Neurotoxicology 28, 1170–1177 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mielke H. W., et al. , The concurrent decline of soil lead and children’s blood lead in New Orleans. Proc. Natl. Acad. Sci. U.S.A. 116, 22058–22064 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Datko-Williams L., Wilkie A., Richmond-Bryant J., Analysis of U.S. soil lead (Pb) studies from 1970 to 2012. Sci. Total Environ. 468-469, 854–863 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Zota A. R., et al. , Metal sources and exposures in the homes of young children living near a mining-impacted Superfund site. J. Expo. Sci. Environ. Epidemiol. 21, 495–505 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longman J., Veres D., Finsinger W., Ersek V., Exceptionally high levels of lead pollution in the Balkans from the Early Bronze Age to the Industrial Revolution. Proc. Natl. Acad. Sci. U.S.A. 115, E5661–E5668 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolger P. M., Yess N. J., Gunderson E. L., Troxell T. C., Carrington C. D., Identification and reduction of sources of dietary lead in the United States. Food Addit. Contam. 13, 53–60 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Richmond-Bryant J., et al. , The influence of declining air lead levels on blood lead-air lead slope factors in children. Environ. Health Perspect. 122, 754–760 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zartarian V., Xue J., Tornero-Velez R., Brown J., Children’s lead exposure: A multimedia modeling analysis to guide public health decision-making. Environ. Health Perspect. 125, 097009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellinger D. C., The protean toxicities of lead: New chapters in a familiar story. Int. J. Environ. Res. Public Health 8, 2593–2628 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bornschein R. L., et al. , The influence of social and environmental factors on dust lead, hand lead, and blood lead levels in young children. Environ. Res. 38, 108–118 (1985). [DOI] [PubMed] [Google Scholar]
- 12.Lanphear B. P., Roghmann K. J., Pathways of lead exposure in urban children. Environ. Res. 74, 67–73 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Lanphear B. P., et al. , The contribution of lead-contaminated house dust and residential soil to children’s blood lead levels. A pooled analysis of 12 epidemiologic studies. Environ. Res. 79, 51–68 (1998). [DOI] [PubMed] [Google Scholar]
- 14.von Lindern I., Spalinger S., Petroysan V., von Braun M., Assessing remedial effectiveness through the blood lead:soil/dust lead relationship at the Bunker Hill Superfund Site in the Silver Valley of Idaho. Sci. Total Environ. 303, 139–170 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Succop P., Bornschein R., Brown K., Tseng C. Y., An empirical comparison of lead exposure pathway models. Environ. Health Perspect. 106 (suppl. 6), 1577–1583 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanphear B. P., et al. , Environmental lead exposure during early childhood. J. Pediatr. 140, 40–47 (2002). [DOI] [PubMed] [Google Scholar]
- 17.USEPA , Regional Screening Levels (RSLs) - User Guide, Section 2.4 Chemcial-specific Parameters. https://www.epa.gov/risk/regional-screening-levels-rsls-users-guide. Accessed 29 November 2020.
- 18.USEPA , Air Quality Criteria for Ozone and Related Photochemical Oxidants (Office of Research and Development, National Center for Environmental Assessment [NCEA], 2006), vol. 1, p. 821. [Google Scholar]
- 19.USEPA , Information Concerning 2012. Clean Water Act Sections 303(d), 305(b), and 314 Integrated Reporting and Listing Decisions. https://www.epa.gov/tmdl/integrated-reporting-guidance-under-cwa-sections-303d-305b-and-314. Accessed 3 December 2020.
- 20.USEPA , “Mid-Atlantic brownfields and land revitalization clean-up fact sheets” (U.S. Environmental Protection Agency, Region 3, Philadelphia, PA, (2012a).
- 21.Traina S. J., Laperche V., Contaminant bioavailability in soils, sediments, and aquatic environments. Proc. Natl. Acad. Sci. U.S.A. 96, 3365–3371 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drexler J. W., Brattin W. J., An in vitro procedure for estimation of lead relative bioavailability: With validation. Hum. Ecol. Risk Assess. 13, 383–401 (2007). [Google Scholar]
- 23.Juhasz A. L., et al. , Evaluation of SBRC-gastric and SBRC-intestinal methods for the prediction of in vivo relative lead bioavailability in contaminated soils. Environ. Sci. Technol. 43, 4503–4509 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Ruby M. V., et al. , Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environ. Sci. Technol. 33, 3697–3705 (1999). [Google Scholar]
- 25.Bannon D. I., Abounader R., Lees P. S., Bressler J. P., Effect of DMT1 knockdown on iron, cadmium, and lead uptake in Caco-2 cells. Am. J. Physiol. Cell Physiol. 284, C44–C50 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Elsenhans B., Janser H., Windisch W., Schümann K., Does lead use the intestinal absorptive pathways of iron? Impact of iron status on murine 210Pb and 59Fe absorption in duodenum and ileum in vivo. Toxicology 284, 7–11 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Chaney R. L., Ryan J. A., Umweltschutz F., Risk Based Standards for Arsenic, Lead and Cadmium in Urban Soils: Summary of Information and Methods Developed to Estimate Standards for Cd, Pb and as in Urban Soils (Dechema Frankfurt, Germany, 1994). [Google Scholar]
- 28.Kumpiene J., Lagerkvist A., Maurice C., Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments–A review. Waste Manag. 28, 215–225 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Council N. R., Innovations in Ground Water and Soil Cleanup: From Concept to Commercialization (The National Academies Press, Washington, DC, 1997). [Google Scholar]
- 30.USEPA , “The use of soil amendments for remediation, revitalization and reuse” (Rep. 542-R-07-013, US Environmental Protection Agency, Washington, DC, 2007), p. 52.
- 31.Scheckel K. G., et al. , Amending soils with phosphate as means to mitigate soil lead hazard: A critical review of the state of the science. J. Toxicol. Environ. Health B Crit. Rev. 16, 337–380 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Hettiarachchi G. M., Pierzynski G. M., Oehme F. W., Sonmez O., Ryan J. A., Treatment of contaminated soil with phosphorus and manganese oxide reduces lead absorption by Sprague-Dawley rats. J. Environ. Qual. 32, 1335–1345 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Bradham K. D., et al. , Long-term in situ reduction in soil lead bioavailability measured in a mouse model. Environ. Sci. Technol. 52, 13908–13913 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karna R. R., Noerpel M. R., Luxton T. P., Scheckel K. G., Point of zero charge: Role in pyromorphite formation and bioaccessibility of lead and arsenic in phosphate amended soils. Soil Syst. 2, 22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jambor J. L., Nomenclature of the alunite supergroup. Can. Mineral. 37, 1323–1341 (1999). [Google Scholar]
- 36.Dutrizac J. E., Jambor J. L., Jarosites and their application in hydrometallurgy. Rev. Mineral. Geochem. 40, 405–452 (2000). [Google Scholar]
- 37.Baron D., Palmer C. D., Solubility of jarosite at 4–35 C. Geochim. Cosmochim. Acta 60, 185–195 (1996). [Google Scholar]
- 38.Smith A. M., Hudson-Edwards K. A., Dubbin W. E., Wright K., Dissolution of jarosite [KFe3 (SO4) 2 (OH) 6] at pH 2 and 8: Insights from batch experiments and computational modelling. Geochim. Cosmochim. Acta 70, 608–621 (2006). [Google Scholar]
- 39.Hudson-Edwards K. A., Schell C., Macklin M. G., Mineralogy and geochemistry of alluvium contaminated by metal mining in the Rio Tinto area, southwest Spain. Appl. Geochem. 14, 1015–1030 (1999). [Google Scholar]
- 40.Hochella M. F. Jr, Moore J. N., Golla U., Putnis A., A TEM study of samples from acid mine drainage systems: Metal-mineral association with implications for transport. Geochim. Cosmochim. Acta 63, 3395–3406 (1999). [Google Scholar]
- 41.Paktunc D., Dutrizac J. E., Characterization of arsenate-for-sulfate substitution in synthetic jarosite using X-ray diffraction and X-ray absorption spectroscopy. Can. Mineral. 41, 905–919 (2003). [Google Scholar]
- 42.Willard L. L., Chemical Equilibria in Soils (John Wiley & Sons, Chichester, UK, 1979). [Google Scholar]
- 43.Bigham J. M., Nordstrom D. K., Iron and aluminum hydroxysulfates from acid sulfate waters. Rev. Mineral. Geochem. 40, 351–403 (2000). [Google Scholar]
- 44.Welch S. A., Christy A. G., Kirste D., Beavis S. G., Beavis F., Jarosite dissolution I—trace cation flux in acid sulfate soils. Chem. Geol. 245, 183–197 (2007). [Google Scholar]
- 45.Franzblau R. E., Loick N., Weisener C. G., Investigating the effects of Se solid phase substitution in jarosite minerals influenced by bacterial reductive dissolution. Minerals (Basel) 4, 17–36 (2014). [Google Scholar]
- 46.Jambor J. L., Nordstrom D. K., Alpers C. N., “Metal-sulfate salts from sulfide mineral oxidation” in Sulfate Minerals: Crystallography, Geochemistry, and Environmental Significance (Mineralogical Society of America, Chantilly, VA, 2018), vol. 40, p. 303. [Google Scholar]
- 47.USEPA , “Method 3051A: Microwave assisted acid dissolution of sediments, sludges, soils, and oils” (US Government Printing Office, Washington, DC, 1997).
- 48.Dutrizac J., Dinardo O., Kaiman S., Factors affecting lead jarosite formation. Hydrometallurgy 5, 305–324 (1980). [Google Scholar]
- 49.Bradham K. D., et al. , Dietary lead and phosphate interactions affect oral bioavailability of soil lead in the mouse. Environ. Sci. Technol. 53, 12556–12564 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dutrizac J., Jarosite Formation in Chloride Media (Canada Centre for Mineral and Energy Technology, 1979). [Google Scholar]
- 51.Segre C. et al., “The MRCAT insertion device beamline at the Advanced Photon Source” in Pianetta, Arthur J., Brennan S., Eds. (American Institute of Physics, Melville, NY, 2000), P, pp. 419–422. [Google Scholar]
- 52.Karna R. R., Hettiarachchi G. M., Newville M., Sun C., Ma Q., Synchrotron-based X-ray spectroscopy studies for redox-based remediation of lead, zinc, and cadmium in mine waste materials. J. Environ. Qual. 45, 1883–1893 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Reeves P. G., Nielsen F. H., Fahey G. C. Jr, AIN-93 purified diets for laboratory rodents: Final report of the American institute of nutrition ad hoc writing committee on the reformulation of the AIN-76a rodent diet. J. Nutr. 123, 1939–1951 (1993). [DOI] [PubMed] [Google Scholar]
- 54.Bradham K. D., et al. , Estimating relative bioavailability of soil lead in the mouse. J. Toxicol. Environ. Health A 79, 1179–1182 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Casteel S. W., Weis C. P., Henningsen G. M., Brattin W. J., Estimation of relative bioavailability of lead in soil and soil-like materials using young Swine. Environ. Health Perspect. 114, 1162–1171 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fieller E. C., Some problems in interval estimation. J. R. Stat. Soc. Series B Stat. Methodol. 16, 175–185 (1954). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data have been deposited in data.gov.