When healthy, wild-type individuals from closely related species are crossed to each other, the resulting hybrids often display fundamental problems in development. Problems such as hybrid sterility or hybrid inviability can sometimes act as barriers to gene flow between species and are therefore considered to be the stuff of speciation—the process by which one species splits into two (1). These developmental problems are known to manifest from deleterious genetic interactions, known as hybrid incompatibilities, between the two divergent parental genomes (2). The particular phenotype from hybrid crosses can unmask cryptic evolutionary processes that are otherwise not visible in pure species. Understanding the genetic and molecular nature of these incompatibilities can therefore provide fundamental insights into normal developmental processes within species and the origins of reproductive barriers between them. In PNAS, Lu et al. resolve a long-standing mystery about hybrids between Xiphophorus fish hybrids that suffer lethal melanoma (3).
In the 1920s, three independent researchers, Myron Gordon, George Haeussler, and Kurt Kosswig, discovered that hybrids between two distant Xiphophorus fish species, the southern platyfish (Xiphophorus maculatus) and its sister species the green swordtail (Xiphophorus hellerii), develop spontaneous and lethal tumors on the dorsal fin (4–6). X. maculatus exhibits a spotted pigmentation pattern in its dorsal fin (called spotted dorsal, Sd), which is not seen in X. helleri. In F1 hybrids between these species, the Sd pigmentation pattern becomes expanded, with the melanin pattern covering the entire dorsal fin due to melanocyte hyperplasia. These F1 hybrids are fertile, and crossing them to X. hellerii parents produces backcross hybrids in classic Mendelian ratios of 1:2:1. A quarter of the hybrids show hyperplasia of the pigmentation pattern as in the F1 hybrids, half of the hybrids show normal pigmentation pattern, and the remaining one-quarter of hybrids develop spontaneous and lethal tumors due to invasive modular exophytic melanoma.
In the 1950s, based on the Mendelian ratios of phenotypes seen in these crosses, Anders argued that the spontaneous tumorigenesis resulting from the hybrid cross was due to the segregation of two distinct genetic loci from X. maculatus. The first locus is called Tu (for tumor), and the second locus is called R(diff) (for repressor/differentiation). The Xiphophorus hybrid system, referred to as the Gordon–Kosswig–Anders (GKA) model, and the discovery of the genetic interaction between Tu and R(diff) as the cause of melanoma in these hybrids contributed to the concepts of “oncogenes” and “tumor suppressors.” The GKA model also provides a natural two-hit melanoma model, where the oncogenic effect of Tu (from the X. maculatus allele) is suppressed by the coevolved R(diff) locus (also from X. maculatus). The study of Xiphophorus hybrids has not only been critical in understanding hybrid incompatibilities in vertebrates but has also led to the development of foundational concepts in cancer biology. Understanding the mechanisms of oncogenicity and tumor suppression within the X. maculatus species, and why this system becomes derepressed in hybrids, is therefore an important problem in both cancer and speciation research.
In the 1980s, the Tu gene was shown to encode a duplicated mutant copy of the epidermal growth factor receptor (egfr) and was named Xiphorphus melanoma regulatory kinase (xmrk) (7). EGFR is one of the most commonly mutated oncogenes in human cancers, acting upstream of BRAF and NRAS signaling cascades, which are found to be misregulated in over 50% of all human melanomas. This observation highlights the importance of uncovering the identity of the R(diff) locus, which counteracts the oncogenic effects of the xmrk locus. Here, through a series of genetic mapping experiments, Lu et al. (3) bring to a conclusion this long-standing question by identifying rab3d as the gene underlying the R(Diff) locus: a ras-related small G protein thought to regulate exocytosis.
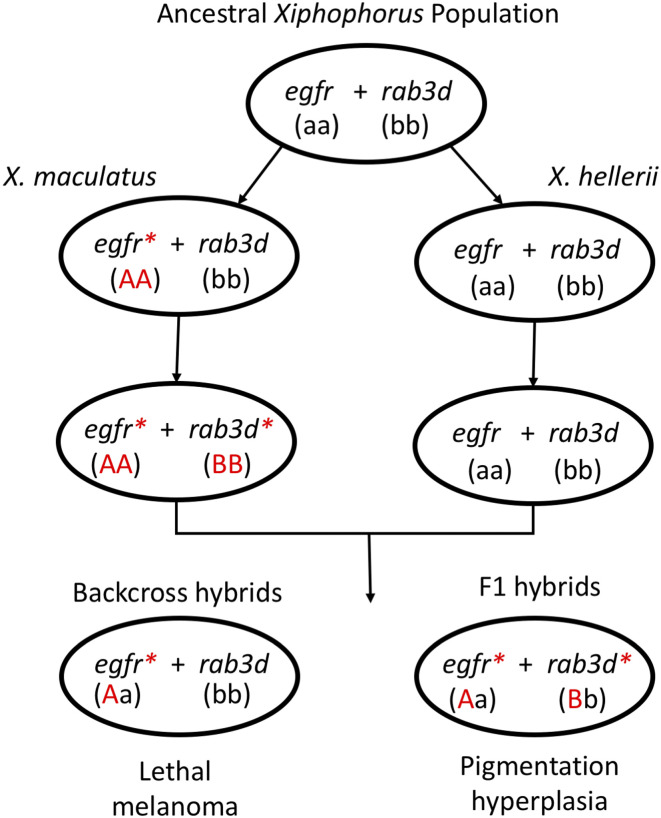
Hybrids between X. maculatus and X. hellerii that are hemizygous for xmrkX. mac/− and heterozygous for R(diff)X. mac/X. hel do not produce tumors but are readily identifiable due to their enhanced pigmentation of the dorsal fin. Lu et al. (3) successively backcrossed these individuals to X. hellerii and selected hybrid individuals with enhanced pigmentation to introgress the R(diff) locus from X. maculatus into an X. helleri genetic background. Using a combination of genome sequencing and association analyses, they mapped this locus to a ∼100-kb interval containing three genes. Expression analyses using RNA sequencing showed that only one of these three genes, rab3d, is expressed in the dorsal fin. Although validation of rab3d to confirm its identity as the causal gene underlying the R(Diff) locus requires transgenic or genome editing experiments, these approaches are currently thwarted by the lack of established techniques in this viviparous fish. The mapping and expression analyses together, however, establish rab3d as a strong candidate gene for the R(Diff) locus. The identification of xmrk as a mutant copy of X. maculatus egfr and R(diff) as the X. maculatus allele of rab3d provides an example in vertebrates of the genic identities of a pair of interacting hybrid incompatibility genes (Fig. 1). Along with transcriptional differences at rab3d, there is only a single amino acid difference in the RAB3D protein between X. hellerii and X. maculatus rab3d. The identification of rab3d as the causal gene underlying R(Diff) also opens the door to understanding how the oncogenic effects of xmrk are suppressed by rab3d at a cellular and organismal level.
Fig. 1.
A model for the evolution of hybrid incompatibility that causes melanoma in Xiphophorus hybrids. X. maculatus has an oncogenic copy of egfr (xmrk) that is suppressed by the X. maculatus alelle of Rab3d. This copy of egfr is unsuppressed in X. maculatus–X. hellerii hybrids and causes melanonoma.
Currently, the role rab3d plays in tumor etiology is not fully understood (8–10). One hypothesis for the mechanism of tumor suppression by rab3d involves the turnover of the membrane-bound EGFR. The egfr gene encodes a receptor tyrosine kinase that dimerizes when presented with a number of different ligands, mediating cell growth, cell proliferation, and survival. Once dimerized, the membrane protein activates downstream proproliferative pathways. Turnover of the receptor protein is generally accelerated when activated by any number of its ligands (11). The xmrk gene originated from the duplication and divergence of the X. maculatus egfr gene, resulting in a mutant EGFR that autodimerizes in a ligand-independent manner. Understanding how the tumor-inducing properties of xmrk are suppressed by rab3d in X. maculatus now provides a fertile avenue for further research to uncover fundamental mechanisms of EGFR regulation and new cancer therapeutics.
The successful identification of the suppressor locus originally hypothesized to exist 70 y ago in this hybrid disease model now allows researchers to molecularly characterize what was previously only understood at a broad genetic level. Another interspecies cross in different Xiphophorus species, a cross between Xiphophorus birchmanni and X. malinche, results in a tail fin melanoma in hybrids in a manner similar to X. maculatus × X. hellerii. Mapping of the hybrid incompatibilities from this cross independently identified xmrk, suggesting that EGFR misregulation may be a common cause of melanoma in these hybrids as well (12). Surprisingly, tumor suppression in these hybrids does not seem to involve rab3d, and instead another locus, myrip, appears to be the interacting hybrid incompatibility locus (13). These results suggest that although EGFR misregulation may be a common mechanism of tumorigenesis in Xiphophorus hybrids, tumor suppression may involve independent genes and mechanisms in different species. Together, these studies of cancers in hybrids between Xiphophorus species hold the promise of not only shedding light on the nature of hybrid incompatibilities and the origins of species but also of providing a unique view into the inner mechanisms and evolution of fundamental cellular mechanisms involving oncogenes and tumor suppressors.
Acknowledgments
Our research is supported by National Institute of General Medical Sciences grant R01 GM115914 and by the Pew Biomedical Scholar Program.
Footnotes
The authors declare no competing interest.
See companion article, “Oncogenic allelic interaction in Xiphophorus highlights hybrid incompatibility,” 10.1073/pnas.2010133117.
References
- 1.Coyne J. A., Orr H. A., Speciation (Sinauer Associates, 2004). [Google Scholar]
- 2.Dobzhansky T., Further data on the variation of the Y chromosome in Drosophila pseudoobscura. Genetics 22, 340–346 (1937). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu Y., et al. , Oncogenic allelic interaction in Xiphophorus highlights hybrid incompatibility. Proc. Natl. Acad. Sci. U.S.A. 117, 29786–29794 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon M., The genetics of a viviparous top-minnow Platypoecilus; the inheritance of two kinds of melanophores. Genetics 12, 253–283 (1927). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haiissler G., Über Melanombildungen bei Bastarden von Xiphophorus Helleri und Platypoecilus Maculatus var. Rubra. Klinische Wochenschrift 7, 1561–1562 (1928). [Google Scholar]
- 6.Kosswig C., Über Bastarde der Teleostier Platypoecilus und Xiphophorus. Z. Indukt. Abstamm. Vererbungsl. 44, 253 (1928). [Google Scholar]
- 7.Schartl A., Dimitrijevic N., Schartl M., Evolutionary origin and molecular biology of the melanoma-inducing oncogene of Xiphophorus. Pigment Cell Res. 7, 428–432 (1994). [DOI] [PubMed] [Google Scholar]
- 8.Martelli A. M., et al. , Rab3A and Rab3D control the total granule number and the fraction of granules docked at the plasma membrane in PC12 cells. Traffic 1, 976–986 (2000). [PubMed] [Google Scholar]
- 9.Millar A. L., Pavios N. J., Xu J., Zheng M. H., Rab3D: A regulator of exocytosis in non-neuronal cells. Histol. Histopathol. 17, 929–936 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Pavlos N. J., et al. , Rab3D regulates a novel vesicular trafficking pathway that is required for osteoclastic bone resorption. Mol. Cell. Biol. 25, 5253–5269 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gómez A., Wellbrock C., Gutbrod H., Dimitrijevic N., Schartl M., Ligand-independent dimerization and activation of the oncogenic Xmrk receptor by two mutations in the extracellular domain. J. Biol. Chem. 276, 3333–3340 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Schumer M., et al. , High-resolution mapping reveals hundreds of genetic incompatibilities in hybridizing fish species. eLife 3, e02535 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powell D. L., et al. , Natural hybridization reveals incompatible alleles that cause melanoma in swordtail fish. Science 368, 731–736 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]