Significance
The radical pair mechanism is the favored hypothesis for explaining biological effects of weak magnetic fields, such as animal magnetoreception and possible adverse health effects. To date, however, there is no direct experimental evidence for magnetic effects on radical pair reactions in cells, the fundamental building blocks of living systems. In this paper, using a custom-built microscope, we demonstrate that flavin-based autofluorescence in native, untreated HeLa cells is magnetic field sensitive, due to the formation and electron spin–selective recombination of spin-correlated radical pairs. This work thus provides a direct link between magnetic field effects on chemical reactions measured in solution and chemical reactions taking place in living cells.
Keywords: magnetic field effect, autofluorescence, flavins, radical pair mechanism, quantum biology
Abstract
We demonstrate, by direct, single-cell imaging kinetic measurements, that endogenous autofluorescence in HeLa cells is sensitive to the application of external magnetic fields of 25 mT and less. We provide spectroscopic and mechanistic evidence that our findings can be explained in terms of magnetic field effects on photoinduced electron transfer reactions to flavins, through the radical pair mechanism. The observed magnetic field dependence is consistent with a triplet-born radical pair and a B1/2 value of 18.0 mT with a saturation value of 3.7%.
Biological effects of magnetic fields have been a major topic of interdisciplinary debate by researchers from a diverse range of research fields for many years, and to date, studies have identified two key phenomena. Epidemiological studies have identified potentially harmful effects on human health (1) [in particular, a nonvanishing association between environmental electromagnetic field exposure and childhood leukemia (2–4)], and behavioral biological studies have examined animal magnetoreception, in which a wide range of animal species are capable of sensing (5, 6) (and, in some cases, extracting directional information from) the geomagnetic field. A strong candidate hypothesis for explaining these phenomena is the radical pair (RP) mechanism (RPM), in which the coherent mixing between singlet (S) and triplet (T) states of spin-correlated RPs is sensitive to the application of weak magnetic fields (7–9) and is coupled to chemical reaction rates and yields through spin-selective reactions. Since the verification of this hypothesis involves electron spin effects, the majority of fundamental research has been conducted by chemists and physicists, and it has recently been positioned as quantum biology (10).
At a fundamental level, establishing the RPM as a viable mechanism for biological responses necessitates the operation of the RPM at the cellular level. To date, although a number of studies have suggested magnetic field effects (MFEs) on the levels of reactive oxygen species (ROS) in cells, different experiments have shown increased, decreased, and no change in these levels (11). A recent review (11) concluded that it is difficult to draw any clear conclusions from these studies due to the variability in observations. Indeed, the study of MFEs on biological processes is extremely challenging, as the effects are typically weak and the assays have a high statistical variability. What is needed to convincingly demonstrate cellular magnetic field responses is a measurement in which the direct influence of the magnetic field on individual cells or subcellular structures can be clearly observed in real time.
It is quite widely believed that blue light photoreceptors called cryptochromes, which contain flavin adenine dinucleotide (FAD) as a noncovalently bound cofactor, are responsible for RPM-based magnetoreception (12). To date, it has been reported in studies at the cellular level, that light activated cryptochromes in live Drosophila motoneurons show an increase of action potential firing recorded using electrophysiological measurements under the application of an external magnetic field of 100 mT (13) and also that cryptochromes in living cells generate FAD radicals, revealed by electron paramagnetic resonance (EPR) measurements (14). However, no studies have been able to directly measure the magnetic field sensitivity of RP reactions (i.e., due to RP spin-state mixing and spin-selective reaction) at the cellular level.
In recent years, to address this problem and to directly investigate MFEs of RPs in biological systems, some research groups, including ours, have developed a number of microscope-based imaging approaches [transient absorption (TA) based (15, 16) and fluorescence based (17)]. Some of our recent experiments have demonstrated that the photochemistry of FAD in aqueous solution is magnetic field sensitive, even at physiological pH, using our TA-based microscope (18). Flavins including FAD are free or bound to biomolecules in many cells and contribute to autofluorescence in cells (19). Other flavins responsible for autofluorescence include riboflavin (RF) and flavin mononucleotide (FMN), both of which have been demonstrated to show MFEs (20) in the presence of suitable electron donors (for example, tryptophan [Trp]) (Scheme 1).
Scheme 1.
Chemical structures of RF, FMN, FAD, and Trp.
Fig. 1A presents a generalized and slightly simplified photochemical reaction scheme for the creation and reaction of RPs generated through electron transfer from electron donors to photoexcited, fully oxidized flavin molecules. The RPs can undergo coherent spin-state mixing, driven by hyperfine coupling to spin-active nuclei in the RP members. This spin-state mixing process is well established to be sensitive to the application of external magnetic fields. Crucial to the RPM is the subsequent spin-selective reaction; RPs are only returned to their original molecular ground states through back electron transfer from the S RP state. For T RPs, the only route back to the ground state is through conversion to the S RP state either through coherent spin-state mixing or (generally more slowly) through incoherent spin relaxation.
Fig. 1.
RP-based magnetic field sensitive photochemistry. (A) Proposed magnetic-sensitive flavin photochemistry based on ref. 26. F = flavin, D = electron donor, Abs = absorption, Fl = fluorescence, ISC = intersystem crossing, ET = electron transfer. (B) Reduction in spin-state mixing due to the electron Zeeman effect for RPs separated sufficiently such that the electron exchange interaction is negligible. hfc = hyperfine coupling constant. (C) MFE on reaction yield (MARY) curve determined by the observed fluorescence intensity. I(B) is the fluorescence intensity in the presence of a magnetic field, I(0) is the fluorescence intensity in the absence of a magnetic field, B is the magnetic field strength, and B1/2 is the magnetic field strength corresponding to half the saturation value of MFE (MFEsat).
Depending on the detailed reaction system and the RP environment, members of the RP may diffuse apart significantly and may undergo further reactions (this is typically observed as photobleaching), but in general, the process tends to return the majority of molecules to the initial ground state (Fig. 1A). Significantly, under continuous photoexcitation, an equilibrium is established between the ground (fully oxidized) state of the flavin and the radical (the partially reduced semiquinone form) state. The application of a magnetic field alters the rate at which flavins are returned to the ground state and thus changes the relative concentrations of ground state flavins and RPs. The change in the concentration of the ground state flavins results in a change of the observed fluorescence signal resulting from their photoexcitation.
Based on this principle, flavin fluorescence can become sensitive to the application of an applied magnetic field if the photoexcitation results in the formation of intermediate RPs. While there are a number of specific mechanisms by which a magnetic field influences the coherent RP spin-state mixing process, the most common is through the electron Zeeman effect, which results in a splitting in the energy levels of the RP T states (labeled T+1, T0, and T−1) proportional to the magnitude of the applied magnetic field. The T+1 and T−1 states increase and decrease in energy, respectively, while the energy of the T0 state remains unchanged. As the T+1 and T−1 states become energetically separated from the S and T0 states by an amount greater than the strength of the hyperfine interactions present in the RP members, coherent mixing between the S and T0 and T+1 and T−1 states becomes increasingly inefficient and eventually ceases (Fig. 1B).
The result of this is that for a RP initially born in the S state, the application of a magnetic field leads to an increase in the ground state flavin concentration and thus an increase in the observed fluorescence. In the case of a T-born RP, the fluorescence will decrease as the magnetic field increases, until saturation is reached. The change in the fluorescence yield with an applied field typically shows a Lorentzian dependence centered at zero field and characterized by the field at which half the maximum effect is observed, the so-called B1/2 value (Fig. 1C). Thus, the direction of the MFE and the B1/2 value are the basic parameters for classifying the nature of the field effect.
In this study, we demonstrate that autofluorescence in HeLa cells (selected as they are the most extensively studied immortal human cell line) shows an MFE consistent with the RPM and present evidence that the source of this effect is the spin-selective reaction of flavin-based RPs generated by electron transfer following blue light photoexcitation of naturally occurring cellular flavin molecules. We then comment on the significance of this finding with respect to biological effects of environmental magnetic fields.
Results
To investigate the magnetic sensitivity of autofluorescence inside HeLa cells, we measured the autofluorescence of a large number of individual HeLa cells for different cell cultures prepared on different days. As a result, there was some variation in the clarity of the observed magnetic response among individual cells, based on the differing levels of autofluorescence.
Fig. 2 shows typical (not best) results for the autofluorescence change of HeLa cells in phosphate-buffered saline (PBS) buffer at pH 7.4 in the presence of a 0.15 Hz triangle wave–modulated magnetic field applied perpendicular to the sample cover glass. Fig. 2A presents bright field, fluorescence, and merged images composed of bright field images captured before irradiation and fluorescence images captured after irradiation, where the blue circle indicates the position of the irradiation region. The figure shows the fluorescence signals recorded from inside individual HeLa cells in PBS buffer. Fig. 2B shows the change of average fluorescence intensity in the region of interest (ROI) under the influence of the modulated magnetic field. The fluorescence decay curves show a small but clear fluorescence change corresponding to the frequency of the applied modulated magnetic field sweep. In order to more clearly examine this change in the average fluorescence intensity, curves were fitted to the data from Fig. 2B using the least squares method, and the residuals (observed value − fitted curve) were normalized using the fitted curve, resulting in a measurement of the fractional MFE (Fig. 2C).
Fig. 2.
MFE on the autofluorescence of HeLa cells. (A) Bright field, fluorescence, and merged images of a representative HeLa cell showing a magnetic field response (Movie S1). (B) Averaged autofluorescence change of the irradiated region of the HeLa cell with the application of a modulated external magnetic field (triangle wave, frequency 0.15 Hz, amplitude 25 mT). The inset figure shows the complete time period of the experiment. (C) Normalized residual intensity calculated by the average autofluorescence intensity divided by the value of the fitted curve representing the fractional MFE.
As a result, a change in the residuals corresponding to the magnetic field frequency was clearly observed, which corresponds precisely (in the time domain) with the change in the magnetic field. The signal-to-noise ratio varies from cell to cell due to variations in the level of autofluorescence, meaning that the effect of the magnetic field is more clearly visible in some cells than others. However, all recorded cells showed a magnetic response with a typical magnitude of 1 to 2.5% (the observed response is frequency dependent for reasons discussed later). SI Appendix, Fig. S1 shows the typical variation per cell on a set of measurements from a single slide. The frequency matching is not coincidental, as demonstrated by the responses at a range of different frequencies (refer to SI Appendix, Fig. S2, which shows the magnetic field dependence at different modulation frequencies at 0.05 Hz and 0.25 Hz) and for the magnetic field applied parallel (rather than perpendicular) to the sample cover glass.
Fig. 3 shows the wavelength dependence of the autofluorescence signals from single HeLa cells, measured by switching the detector of the microscope from the camera to a spectrometer after focusing and ROI selection. The excitation light source utilized the same 450-nm diode laser. The fluorescence spectrum of 10 μM FAD in PBS buffer recorded under identical measurement conditions is shown for comparison. FAD was used as an example of one of the possible flavins responsible for the autofluorescence (RF and FMN have very similar spectra) and is also the same sample presented in SI Appendix. The peak and the shape of the fluorescence spectrum from the individual HeLa cells are almost entirely consistent with the fluorescence spectrum of FAD in solution, although a slightly stronger intensity in the shorter wavelength region between 480 nm and 500 nm is observed in the HeLa cells [this has also been observed in previous multicell autofluorescence measurements and is considered to be due to bound flavins (21)].
Fig. 3.
Fluorescence spectra of single HeLa cells under 450 nm excitation in our microscope. Solid lines show the fluorescence spectra of autofluorescence from each individual HeLa cell. The dotted line shows the fluorescence spectrum of 10 µM FAD in PBS buffer at pH 7.4. The intensity is normalized to the maximum intensity. Scan range = 480 to 800 nm; scan interval = 5 nm.
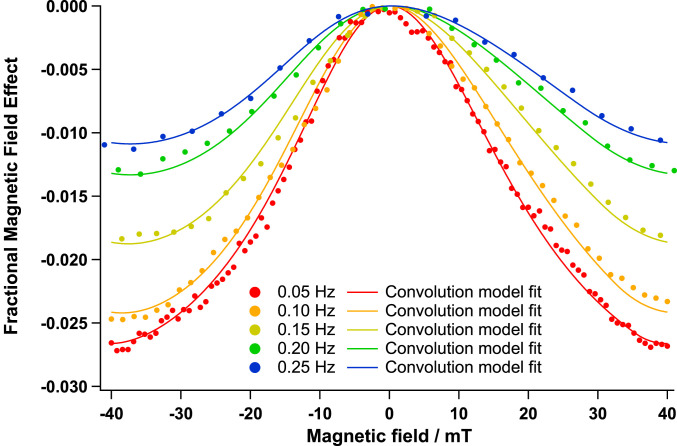
The oscillating magnetic field was typically administered as a triangle wave (either as a linear field sweep from −25 mT to +25 mT or from −40 mT to +40 mT). The purpose of this approach is to allow the change in fluorescence response to be plotted against magnetic field strength in Fig. 4 (referred to as a magnetically affected reaction yield [MARY] curve). Obtaining a MARY spectrum by applying square-wave magnetic fields of increasing magnitude is not possible because of the rapid kinetic decay due to photobleaching. In addition, repeated experiments at different magnetic field strengths are not possible, due to the variability in the response of each cell. Therefore, in order to attempt to demonstrate that the magnetic field response saturates in a manner consistent with the electron Zeeman effect, the triangular magnetic field was determined to be the best approach. Magnetic field responses due to the RPM show saturation at fields greater than the hyperfine couplings present in the members of the RP, and this is clearly observed in this case.
Fig. 4.
MARY curves obtained by averaging the magnetic field response of multiple HeLa cells to a triangular magnetic field swept from −40 to 40 mT over a number of sweep cycles (dependent on the sweep frequency) for five different sweep frequencies (indicated in the legend). The curves are displayed alongside calculated curves obtained by convoluting a MARY curve of B1/2 = 18.0 mT with an exponential decay with rate coefficient 1.49 s−1 for the different sweep rates. For full details of the simulation procedure, see SI Appendix, HeLa cell MARY curve determination.
The application of a triangular magnetic field will only provide an accurate MARY curve in the case that the RP reactions are fast relative to the sweeping rate of the magnetic field. In recent work, Kattnig et al. (22) have demonstrated that in many flavin RP reactions this is not the case and that the RP response to the field can take place on a timescale comparable to the oscillating field frequencies employed here. In order to determine the time response to the magnetic field, we recorded the response to a square-wave magnetic field of 0.20 Hz (SI Appendix, Fig. S3-1a). Indeed, in the cellular measurements, slow kinetics are also observed. The response can be fit very well with a single exponential, with an average rate coefficient of 1.49 s−1 (SI Appendix, Fig. S3-1b).
This should mean that the observed MARY curves show a frequency response, and this is indeed observed (SI Appendix, Fig. S2). The observed B1/2 value, the delay between the application of the magnetic field and the fluorescence response, and the magnitude of the observed field effect all depend on the magnetic field sweep frequency. By using a convolution analysis (described in detail in SI Appendix, HeLa cell MARY curve determination), we are able to decouple the B1/2 value from the slow kinetics and provide an excellent fit to the experimental data at all sweep frequencies for a single true B1/2 of 18.0 ± 0.5 mT and a field effect saturation value of 3.7% (Fig. 4).
Discussion
Our original motivation for investigating the magnetic field sensitivity of cellular autofluorescence came from our measurement of MFEs on FAD photochemistry at physiological pH (18). Thus, our initial hypothesis hinges on the role of flavin RPs in any observed magnetic response. However, could there be an alternative explanation for the observed magnetic field sensitivity? Trp, NADPH, flavins, vitamins, lipofuscin, and lipofuscin-like lipopigments have all been reported as fluorophores contributing to cellular autofluorescence (23). Among them, only flavins, lipofuscin, and lipofuscin-like lipopigments absorb 450 nm light and emit fluorescence at wavelengths over 500 nm, corresponding to the excitation and detection wavelengths used in this study (23). In addition, it has been reported that lipofuscin and lipofuscin-like lipopigments have low autofluorescence contributions in many cultured cells, based on absorption maxima and fluorescence spectral analyses (19, 24, 25).
Thus, it is reasonable to assume that the main fluorophores contributing to the observed autofluorescence are all flavins (primarily RF, FMN, and FAD). All of these molecules have been demonstrated to show magnetic field–dependent RP-based photochemistry in solution in the presence of suitable electron donors (and also alone in the case of FAD, which can generate RPs through intramolecular electron transfer from the adenine moiety) (26). Thus, in the first instance, Occam’s razor leads us to suggest that it is flavin-based RPs that are responsible for the observed magnetic field responses in the cells.
To provide direct evidence that the autofluorescence signal arises from flavins and not other species, we modified the microscope to allow the fluorescence spectrum of the individual cells to be captured (this is technically challenging due to the very weak signals and rapid photobleaching). As Fig. 3 demonstrates, the spectrum of the observed autofluorescence is an excellent match to that of FAD (and other flavins). Therefore, given the rarity of magnetically sensitive RP-based photochemical reactions and the excellent spectral overlap, we can conclude with confidence that flavins play the key role in the observed magnetic field response.
By examining the character of the observed magnetic response, we can learn a little more about the nature of the RPs responsible. In all our measurements, the application of the magnetic field leads to a decrease in the magnitude of the observed fluorescence. This suggests, then, that the RP is initially born in the T spin state (or at least the majority of RPs are). This is consistent with all observed MFE measurements on flavin-based RPs, with the exception of those in cryptochromes (27) and photolyases (28), which represent a very special case. In these specific proteins, the flavin is noncovalently but rigidly bound in the binding pocket of the protein, and the molecular arrangement around the FAD molecule is highly controlled. Electron transfer takes place along a triad or tetrad of Trp residues, the first of which is located very close to the flavin moiety and which can donate an electron to the photoexcited flavin extremely quickly (i.e., at a rate much greater than the intersystem crossing rate), resulting in an S-born RP (27). Flavins responsible for autofluorescence in cells may be free or protein bound but are unlikely to be in such highly optimized functional molecular environments as they are in cryptochromes and photolyases, and so the observed T-born RP, which implies that electron transfer is slower than intersystem crossing in the flavin molecule, is probably to be expected.
Looking at the observed B1/2 value, however, reveals a substantial difference from the field responses observed in free solution and for flavins electrostatically attracted to the surface of water-soluble proteins like lysozyme (29, 30) in free solution. For flavin–Trp RPs in solution, B1/2 values are typically in the range of a few (approximately 4 to 8) millitesla (16), while the observed B1/2 value in the cells is much larger (18.0 mT). The most common reason for the observation of B1/2 values greater than the characteristic average hyperfine couplings in the RP [for flavin–Trp RPs, this is around 3 mT (27)] is due to long-lived RPs in which incoherent electron spin relaxation contributes to the spin dynamics. Such relaxation means that greater magnetic field strengths are required to sufficiently eliminate the mixing between S and T0 and T+1 and T−1 states. Thus, the observed B1/2 values suggest two possible differences between the RPs in the cells and those in free solution. The first is that the RP lifetime is substantially longer, meaning that the effects of electron spin relaxation become competitive with coherent spin-state mixing. This would certainly be expected for a protein-bound RP in which the RP members cannot escape from each other by free diffusion but are maintained in close proximity. The second is that the relaxation rates of the radicals are greater, which again may be consistent with protein-bound RPs, in which the modulation of the local fields experienced by each radical due to molecular tumbling (in this case of a much bigger protein) more effectively matches the transition frequencies. Without additional information, it is not possible to conclude which of these effects is more important or if they both play a role, but both effects are consistent with the hypothesis that (at least some of the) RPs are protein bound.
In the case of cryptochromes and photolyases, the observed B1/2 values lie between those observed here and those for nonbound RPs. These observations come from purified proteins in free (and viscous) solution. In such cases, the relaxation has been explained in terms of electron hopping between adjacent Trp residues in the Trp chain. While we should not speculate too much about the molecular environment of the flavins and the RP dynamics, we can at least infer that the observed B1/2 values in the cells suggest that the RP environment is substantially different from both completely free and cryptochrome-/photolyase-bound flavins. What is clear from the captured fluorescence images and movies (see for example Fig. 2A and Movie S1) is that the fluorescence is not evenly distributed throughout the cell and arises directly from the mitochondria. For example, lipoamide dehydrogenase (LipDH) and electron transfer flavoprotein (ETF) are known FAD-containing mitochondrial flavoprotein contributors to endogenous autofluorescence (31, 32). It has been reported that LipDH in isolated cardiac myocytes and ETF overexpressed in HeLa cells shows a stronger fluorescence intensity (such as shown in Fig. 3) in the shorter wavelength region than free flavin (33, 34).
An important aspect of the way in which these measurements were performed is that we can readily demonstrate that the cells are alive throughout the magnetic field measurement. This is directly observable in Movie S1 and corresponds to the data in Fig. 2. In this video, it is clear that the mitochondria of the cells move normally during the measurement, indicating that the cell is alive. Our technique is readily applicable to a wide variety of different cell types, and we are now investigating the ubiquity of this observation.
What are the consequences of this observation to the question of the effect of weak environmental electromagnetic fields on human health? Here, we must be circumspect in extrapolating too far. Flavins in vivo can be phototoxic (35). This might be directly due to the generation of free radicals (via RPs), which would provide a simple mechanism by which magnetic fields might be able to change the level of phototoxicity (36, 37). For a T-born RP, the RP concentration increases under continuous photoexcitation at fields of a few millitesla or more. However, epidemiological studies suggest that much weaker fields are associated with childhood leukemia (2, 3). The effects of very weak magnetic fields on RP reactions are usually discussed in terms of the low field effect (LFE) which has the opposite direction to the Zeeman effect at higher fields (i.e., the rate of S–T mixing can increase in very weak fields). Thus, if the RPs responsible for the observed effects here show an inverted response at much weaker fields (our future experiments will try to look for such an inverted response), the result would be lower RP concentrations and thus lower apparent phototoxicity.
However, it is also possible that phototoxicity arises from a different mechanism (for example, the reaction of photoexcited flavin with oxygen to generate reactive oxygen species) in competition with RP formation (38). In such a case, a reduction in RP concentration would lead to an increase in excited flavin concentration and thus potentially an increase in the concentration of ROS, leading to increasing phototoxicity in the same sense implied by the epidemiological studies. It is important to emphasize, however, that the magnitude of the magnetic fields in studies correlating environmental magnetic fields and childhood leukemia are extremely weak (∼0.4 μT). To date, there is no experimental evidence for RPM-based MFEs at such weak magnetic fields. However, the observation of RPM-based MFEs on cellular autofluorescence is relevant to the question of magnetic fields and human health in terms of establishing a potential physical interaction mechanism taking place at the cellular level, and a deeper understanding of the precise photochemistry and RP dynamics can provide new insights that may help in addressing this question (particularly, the discrepancy in the positive/negative effects in ROS levels (11) due to magnetic fields). Experiments to provide such information are now underway.
Materials and Methods
Cell Culture.
HeLa cells (ATCC CCL-2) were cultured at 37 °C under 5% CO2 in Minimum Essential Medium Eagle (Sigma-Aldrich) supplemented with 10% Calf Serum (Sigma-Aldrich), 100 unit/mL penicillin and 100 μg/mL streptomycin (Sigma-Aldrich) on a 100-mm culture dish (Eppendorf).
Microscope Principle.
Our self-constructed microscope utilizes a 100× oil objective lens with a numerical aperture of 1.49 (UAPON100XOTIRF, Olympus), a 450-nm laser diode laser (PLT5 450B, OSRAM), a bandpass filter for the laser (AT450/50, Chroma), a dichroic mirror (T470lpxr, Chroma), a reflection mirror (PFR10-P01, Thorlabs), a tube lens (AC254-200-A, Thorlabs), and a longpass filter (ET500lp, Chroma) before an scientific Complementary Metal Oxide Semiconductor (sCMOS) camera (ORCA Flash 4.0 V3, Hamamatsu). The magnetic field is supplied to the sample using a projected vector field electromagnet (GMW 5204), which is capable of generating a magnetic field in any arbitrary direction relative to the sample. The magnet is mounted at a distance of 5 mm or less above the sample slide in order to generate appropriate field strengths and to ensure the uniformity of the magnetic field. The magnitude of the magnetic field of the GMW 5204 was measured with a high resolution (Bz: 0.03 × 0.005 × 0.03 mm3; Bx and By: 0.15 × 0.01 × 0.15 mm3) three-dimensional hall effect sensor (F3A, SENIS) in contact with the objective lens before measurements. Current was applied to three independent coils (A, B, and C) inside the GMW for the generation of the vertical and horizontal magnetic fields. A 25-mT vertical field requires a current of 16 A to be applied to all three coils. A 40-mT horizontal field requires 18.5 A to be applied to Coil A and −9.25 A to be applied to Coils B and C. The GMW was always cooled to18 °C using water circulated at a maximum flow rate of 16 L/min from a cooled water circulator (CA-1112, EYELA) (SI Appendix, Fig. S4).
Careful measurements were conducted to ensure that no artefactual magnetic field responses in the microscope were recorded in the absence of a chemical effect (for example, due to heating by the application of current or mechanical movement affecting light capture or direct influence over the camera or laser). These calibrations were performed using FMN and Alexa Fluor 488 (negative control) and FAD (positive control) solutions under identical irradiation conditions as the cellular autofluorescence magnetic field measurements. Measurements were performed with magnetic fields of up to 40 mT perpendicular and parallel to the sample cover glass (SI Appendix, Fig. S5-1–S5-4). To additionally confirm the absence of any possible heating effects, we performed careful temperature measurements using a digital thermometer (IBS-TH1, Inkbird) on short and long timescales (SI Appendix, Fig. S5-5) and demonstrated no change in the sample temperature with the applied magnetic field.
MFE Measurements in HeLa Cells.
For microscope measurements, the HeLa cells were diluted 150-fold or 200-fold from over 80% confluence in a 10-cm culture dish (Eppendorf), plated in an ethanol-sterilized CultureWell (CW-3S-1.0, Grace Bio-Labs) chambered cover glass (24 × 60 No.1, Matsunami), placed in a 100-mm culture dish with 200 μL of the culture medium and cultured for 42 to 48 h at 37 °C in 5% CO2 until over 80% confluent. The HeLa cells were then measured in 70 μL of PBS buffer (Sigma-Aldrich) after washing twice with PBS buffer, and the sample was enclosed by placing another cover glass on top of the chambered cover glass. The PBS buffer was calcium and magnesium free.
For autofluorescence magnetic field measurements, to avoid photobleaching of flavin autofluorescence, we first used bright-field illumination to identify a HeLa cell for observation and recorded an image of the cell prior to blue light photoexcitation. We then imaged the cell fluorescence under continuous irradiation with 1.0 mW of 450-nm laser light, 100% amplitude modulated at 100 Hz with a 50% duty cycle and an applied triangle-wave–modulated magnetic field varying between either +25 mT and −25 mT or +40 mT and −40 mT at a much lower frequency (between 0.05 and 0.25 Hz). The laser power was monitored with a laser power meter (LP-1, Sanwa). The irradiation intensity was estimated to be 0.5 kW/cm2 at the sample, based on the irradiation spot size. It is important to note that establishing suitable irradiation conditions is important for detecting MFEs from autofluorescence. All images were captured with a camera exposure time of 100 ms.
Fluorescence Spectra Measurements in HeLa Cells.
The fluorescence spectra of single HeLa cells and a FAD solution were measured with a spectrometer (Acton Series SP-2150i, Princeton Instruments) attached to the microscope (SI Appendix, Fig. S6). The excitation was performed with 1.0 mW of 450-nm laser light, 100% amplitude modulated using a pulse generator (Model 555, Berkeley Nucleonics Crop) with the light on for 200 μs of every 30 ms, after predetermining the focus position and the irradiation position (for cells) to avoid the photobleaching. The fluorescence was captured from 480 nm to 800 nm using a 5-nm scan interval and 50 times averaging, using a lens (LA1951-A, Thorlabs) and an optical fiber bundle (LG-456-020-1, Princeton Instruments).
Data Analysis.
Our image analysis employed the open-source imaging processing software ImageJ. The average fluorescence intensity value was measured by calculating the average intensity inside an ROI surrounding the whole fluorescing region of each captured image frame. Curve fitting and residual analysis was performed using Igor Pro. All curve fitting was conducted using an offset double exponential function for (typically) the period between 4 and 21 s after the irradiation commenced. Normalized residuals were calculated as (observed value − fitted curve value)/(fitted curve value). To extract a consistent B1/2 value from the MARY curves recorded at different magnetic field sweep frequencies, we used a convolution analysis based on the time response of the fluorescence to a rapidly switched field. Details of the analysis are provided in the SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (Grant number 17H03005 and 20H02687).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. P.J.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2018043118/-/DCSupplemental.
Data Availability.
All study data are included in the article and supporting information.
References
- 1.IARC, Non-Ionizing Radiation, Part 1: Static and Extremely Low Frequency (ELF) Electric and Magnetic Fields (Lyon, France: International Agency for Research on Cancer, 2002), vol. 80. [PMC free article] [PubMed] [Google Scholar]
- 2.Greenland S., Sheppard A. R., Kaune W. T., Poole C., Kelsh M. A.; Childhood Leukemia-EMF Study Group , A pooled analysis of magnetic fields, wire codes, and childhood leukemia. Epidemiology 11, 624–634 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Ahlbom A., et al. , A pooled analysis of magnetic fields and childhood leukaemia. Br. J. Cancer 83, 692–698 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hore P. J., Upper bound on the biological effects of 50/60 Hz magnetic fields mediated by radical pairs. eLife 8, e44179 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiltschko W., Wiltschko R., Magnetic orientation and magnetoreception in birds and other animals. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 191, 675–693 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Mouritsen H., Long-distance navigation and magnetoreception in migratory animals. Nature 558, 50–59 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Maeda K., et al. , Chemical compass model of avian magnetoreception. Nature 453, 387–390 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Hore P. J., Mouritsen H., The radical-pair mechanism of magnetoreception. Annu. Rev. Biophys. 45, 299–344 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Juutilainen J., Herrala M., Luukkonen J., Naarala J., Hore P. J., Magnetocarcinogenesis: Is there a mechanism for carcinogenic effects of weak magnetic fields? Proc. R. Soc. B 285, 20180590 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert N., et al. , Quantum biology. Nat. Phys. 9, 10–18 (2013). [Google Scholar]
- 11.Wang H., Zhang X., Magnetic fields and reactive oxygen species. Int. J. Mol. Sci. 18, 2175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritz T., Adem S., Schulten K., A model for photoreceptor-based magnetoreception in birds. Biophys. J. 78, 707–718 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giachello C. N., Scrutton N. S., Jones A. R., Baines R. A., Magnetic fields modulate blue-light-dependent regulation of neuronal firing by cryptochrome. J. Neurosci. 36, 10742–10749 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoang N., et al. , Human and Drosophila cryptochromes are light activated by flavin photoreduction in living cells. PLoS Biol. 6, e160 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beardmore J. P., Antill L. M., Woodward J. R., Optical absorption and magnetic field effect based imaging of transient radicals. Angew. Chem. Int. Ed. Engl. 54, 8494–8497 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Antill L. M., Beardmore J. P., Woodward J. R., Time-resolved optical absorption microspectroscopy of magnetic field sensitive flavin photochemistry. Rev. Sci. Instrum. 89, 023707 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Dodson C. A., et al. , Fluorescence-detected magnetic field effects on radical pair reactions from femtolitre volumes. Chem. Commun. (Camb.) 51, 8023–8026 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Antill L. M., Woodward J. R., Flavin adenine dinucleotide photochemistry is magnetic field sensitive at physiological pH. J. Phys. Chem. Lett. 9, 2691–2696 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Benson R. C., Meyer R. A., Zaruba M. E., McKhann G. M., Cellular autofluorescence–Is it due to flavins? J. Histochem. Cytochem. 27, 44–48 (1979). [DOI] [PubMed] [Google Scholar]
- 20.Horiuchi M., Maeda K., Arai T., Magnetic field effect on electron transfer reactions of flavin derivatives associated with micelles. Appl. Magn. Reson. 23, 309 (2003). [Google Scholar]
- 21.Islam M. S., Honma M., Nakabayashi T., Kinjo M., Ohta N., pH dependence of the fluorescence lifetime of FAD in solution and in cells. Int. J. Mol. Sci. 14, 1952–1963 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kattnig D. R., et al. , Chemical amplification of magnetic field effects relevant to avian magnetoreception. Nat. Chem. 8, 384–391 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Croce A. C., Bottiroli G., Autofluorescence spectroscopy and imaging: A tool for biomedical research and diagnosis. Eur. J. Histochem. 58, 2461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaillard E. R., Atherton S. J., Eldred G., Dillon J., Photophysical studies on human retinal lipofuscin. Photochem. Photobiol. 61, 448–453 (1995). [DOI] [PubMed] [Google Scholar]
- 25.Tsai L., Szweda P. A., Vinogradova O., Szweda L. I., Structural characterization and immunochemical detection of a fluorophore derived from 4-hydroxy-2-nonenal and lysine. Proc. Natl. Acad. Sci. U.S.A. 95, 7975–7980 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans E. W., et al. , Magnetic field effects in flavoproteins and related systems. Interface Focus 3, 20130037 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda K., et al. , Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor. Proc. Natl. Acad. Sci. U.S.A. 109, 4774–4779 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henbest K. B., et al. , Magnetic-field effect on the photoactivation reaction of Escherichia coli DNA photolyase. Proc. Natl. Acad. Sci. U.S.A. 105, 14395–14399 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miura T., Maeda K., Arai T., Effect of coulomb interaction on the dynamics of the radical pair in the system of flavin mononucleotide and hen egg‐white lysozyme (HEWL) studied by a magnetic field effect. J. Phys. Chem. B 107, 6474–6478 (2003). [Google Scholar]
- 30.Evans E. W., et al. , Sensitive fluorescence-based detection of magnetic field effects in photoreactions of flavins. Phys. Chem. Chem. Phys. 17, 18456–18463 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Kunz W. S., Gellerich F. N., Quantification of the content of fluorescent flavoproteins in mitochondria from liver, kidney cortex, skeletal muscle, and brain. Biochem. Med. Metab. Biol. 50, 103–110 (1993). [DOI] [PubMed] [Google Scholar]
- 32.Kunz W. S., Kunz W., Contribution of different enzymes to flavoprotein fluorescence of isolated rat liver mitochondria. Biochim. Biophys. Acta 841, 237–246 (1985). [DOI] [PubMed] [Google Scholar]
- 33.Chorvat D. Jr, Chorvatova A., Spectrally resolved time-correlated single photon counting: A novel approach for characterization of endogenous fluorescence in isolated cardiac myocytes. Eur. Biophys. J. 36, 73–83 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Lam A. K., Silva P. N., Altamentova S. M., Rocheleau J. V., Quantitative imaging of electron transfer flavoprotein autofluorescence reveals the dynamics of lipid partitioning in living pancreatic islets. Integr. Biol. 4, 838–846 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Hockberger P. E., et al. , Activation of flavin-containing oxidases underlies light-induced production of H2O2 in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 96, 6255–6260 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Usselman R. J., Hill I., Singel D. J., Martino C. F., Spin biochemistry modulates reactive oxygen species (ROS) production by radio frequency magnetic fields. PLoS One 9, e93065 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Usselman R. J., et al. , The quantum biology of reactive oxygen species partitioning impacts cellular bioenergetics. Sci. Rep. 6, 38543 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baier J., et al. , Singlet oxygen generation by UVA light exposure of endogenous photosensitizers. Biophys. J. 91, 1452–1459 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and supporting information.