Significance
G protein-coupled receptors (GPCRs) are involved in many physiological processes and important drug targets. However, most therapeutic agents targeting GPCRs, such as the coreceptors of HIV-1 CCR5 and CXCR4, also interfere with their signaling function. Here, we used primate lentiviruses as tools to discover novel endogenous ligands of GPR15, a coreceptor for HIV-2 and SIV proposed to play a role in mucosal immunity. We found that C-terminal fragments of cystatin C generated by immune-activated proteases inhibit GPR15-mediated HIV and SIV infection without interfering with GPR15L chemokine signaling. Thus, we identified an endogenous bioactive peptide that specifically prevents the detrimental activity of a GPCR (i.e. virus infection) without compromising its physiological signaling function.
Keywords: G protein-coupled receptors, GPR15, immunodeficiency viruses, chemokines, cystatin C
Abstract
GPR15 is a G protein-coupled receptor (GPCR) proposed to play a role in mucosal immunity that also serves as a major entry cofactor for HIV-2 and simian immunodeficiency virus (SIV). To discover novel endogenous GPR15 ligands, we screened a hemofiltrate (HF)-derived peptide library for inhibitors of GPR15-mediated SIV infection. Our approach identified a C-terminal fragment of cystatin C (CysC95-146) that specifically inhibits GPR15-dependent HIV-1, HIV-2, and SIV infection. In contrast, GPR15L, the chemokine ligand of GPR15, failed to inhibit virus infection. We found that cystatin C fragments preventing GPR15-mediated viral entry do not interfere with GPR15L signaling and are generated by proteases activated at sites of inflammation. The antiretroviral activity of CysC95-146 was confirmed in primary CD4+ T cells and is conserved in simian hosts of SIV infection. Thus, we identified a potent endogenous inhibitor of GPR15-mediated HIV and SIV infection that does not interfere with the physiological function of this GPCR.
G protein-coupled receptors (GPCRs) constitute the largest family of membrane proteins involved in the transduction of signals from the extracellular environment into the cell and play key roles in immune responses, homeostasis, metabolism, and organogenesis (1, 2). Besides their physiological roles, some GPCRs also represent important coreceptors for HIV and/or simian immunodeficiency virus (SIV) entry. HIV-1, the main causative agent of AIDS, utilizes C-C chemokine receptor 5 (CCR5) and CXC chemokine receptor 4 (CXCR4) as major entry cofactors (3–8). The chemokine ligands CCL5 (also known as RANTES) and CXCL12 (also named SDF-1) inhibit CCR5- or CXCR4-mediated HIV-1 infection, respectively. Thus, we have previously taken advantage of HIV-1 entry to examine complex blood-derived peptide libraries for novel naturally occurring ligands of CCR5 and CXCR4 (9, 10). Initially, we identified a truncated form of the chemokine C-C ligand 14 (CCL14) as a CCR5 agonist and potent inhibitor of CCR5-tropic HIV-1 strains (11, 12). More recently, we discovered a small fragment of human serum albumin (EPI-X4) as an effective and highly specific CXCR4 antagonist and inhibitor of CXCR4-tropic HIV-1 strains (13). While HIV-1 coreceptor utilization is mainly restricted to CCR5 and CXCR4, HIV-2 and SIVs are more promiscuous in their entry cofactor usage. For example, many HIV-2 and SIV strains utilize BOB/GPR15 and Bonzo/STRL-33/CXCR6 in addition to CCR5 and/or CXCR4 for viral entry into CD4+ target cells (14–20). GPR15 is a GPCR reported to regulate T cell trafficking to the colon that may play a role in intestinal homeostasis and inflammation (21, 22). Recently, an agonistic C-C chemokine ligand of GPR15, named GPR15L, has been characterized (23, 24). GPR15L is expressed in colon and cervical epithelia and might play a role in mucosal immunity.
To discover novel endogenous GPR15 ligands, we screened a hemofiltrate (HF)-derived peptide library containing essentially all peptides and small proteins circulating in human blood in their final processed and physiologically relevant forms (10) for inhibitors of GPR15-mediated SIV infection. Multiple rounds of peptide separation and antiviral screening identified a C-terminal fragment of cystatin C (named CysC95-146) as a potent and specific inhibitor of GPR15-dependent HIV and SIV infection. Cystatin C is a small (13 kDa) basic protein that is produced by all nucleated cells (25) and represents the most abundant and potent extracellular inhibitor of cysteine proteases (26). It is found in virtually all tissues and body fluids and commonly used as a marker of renal function (27). Accumulating evidence suggests a role of cystatin C in inflammation, neutrophil chemotaxis, and resistance to bacterial as well as viral infections (28–31). We show that cystatin C fragments preventing GPR15-dependent HIV and SIV infection are generated by proteases activated during antiviral immune responses. Unexpectedly, the recently discovered chemokine ligand of GPR15, GPR15L (23, 24), had no significant effect on viral entry. In addition, CysC95-146 prevented SIV and HIV-2 infection without interfering with GPR15L-mediated signaling. Our data support that naturally occurring cystatin C fragments are capable of blocking GPR15-mediated primate lentiviral infection without interfering with the physiological signaling function of this GPCR.
Results
Identification of a GPR15-Specific SIV Inhibitor.
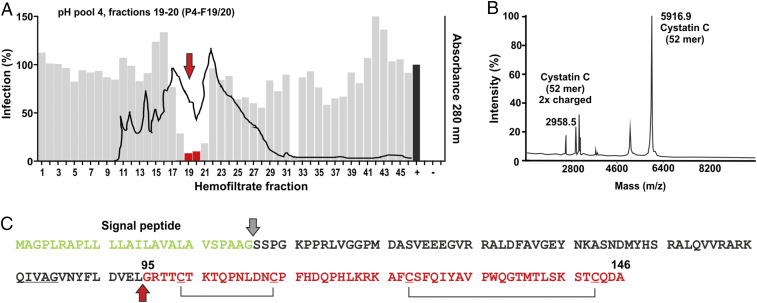
To identify novel ligands of GPR15, peptide libraries were generated from up to 10,000 L of hemofiltrate derived from individuals with chronic renal failure by cation exchange separation followed by reverse-phase (RP) chromatography (10, 32). Initially, the hemofiltrate was applied to a large cation exchange column and eluted using eight buffers with increasing pH ranging from 2.5 to 9.0. Subsequently, the resulting pH pools (P1 to P8) were separated into ∼40 to 50 peptide-containing fractions (F1 to F50) by RP chromatography. The final library comprised about 360 peptide fractions representing essentially the entire blood peptidome in a highly concentrated, salt-free, and bioactive form. Screening of this peptide library using the infectious SIVmac239 molecular clone that efficiently utilizes GPR15 (33) and human osteosarcoma (GHOST) cells stably expressing CD4 and GPR15 (14) identified several neighboring fractions from pH pool 4 that inhibited virus infection. Fractions 19 and 20 were selected for further purification because they blocked GPR15-mediated SIVmac infection by ∼95% (Fig. 1A) without causing cytotoxic effects.
Fig. 1.
Identification of a C-terminal cystatin C fragment inhibiting GPR15-mediated SIVmac infection. (A) The gray bars indicate the efficiency of SIVmac239 infection of GHOST-GPR15 cells in the presence of the hemofiltrate peptide library fractions compared to the absence of peptide (100%), and the black line indicates the peptide/protein elution profile. Fractions used for further peptide purification are indicated in red and highlighted by an arrow. + indicates infection in the absence of peptide; − shows uninfected cells. (B) MALDI-TOF spectrum of the active fraction obtained after the fifth round of purification. Sequence analyses identified the 52 C-terminal residues of cystatin C. (C) Amino acid sequence of human cystatin C. The signal peptide (green), the isolated peptide (red), and putative C-C bridges are indicated. The cleavage site to generate CysC95-146 is indicated by a red arrow.
Matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry analysis of the inhibitory fraction obtained after five rounds of purification revealed a main m/z signal at 5.914 and some minor peaks (Fig. 1B). The search in our (updated version) hemofiltrate peptide database (34) revealed that this signal corresponds to the 52 C-terminal amino acids (aa) of cystatin C (aa 95 to 146) with an oxidation in Met136 (Fig. 1C). Another m/z signal at 2.959 corresponded to the double-charged species of the same peptide. Cystatin C is expressed as a 146-aa precursor with a 26-aa signal peptide (GenBank accession no. AAA52164.1). It represents the most abundant and potent inhibitor of cysteine proteases in the human body and is an early marker of renal failure (26, 35).
A Natural CysC Fragment Inhibits GPR15-Dependent SIV and HIV Infection.
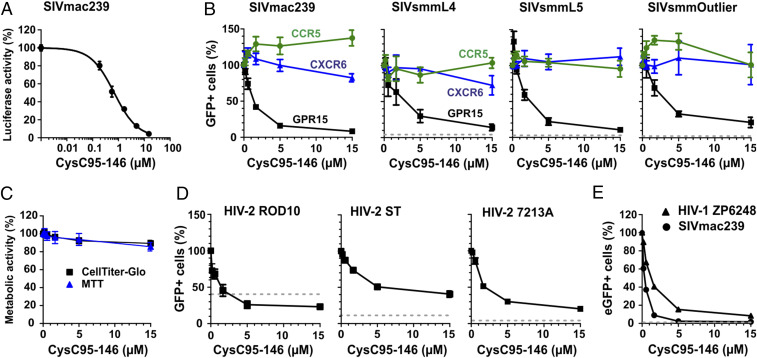
To verify that the identified peptide is responsible for the antiviral activity, we chemically synthesized the 52 C-terminal amino acid residues of cystatin C (indicated in Fig. 1C). The synthetic peptide, referred to as CysC95-146, inhibited GPR15-mediated SIVmac239 infection in a dose-dependent manner with a mean 50% inhibitory concentration (IC50) of ∼0.5 µM (Fig. 2A). Potent inhibition was confirmed for three divergent infectious molecular clones (IMCs) of SIVsmm (Fig. 2B and SI Appendix, Fig. S1), which naturally infects sooty mangabeys (36) and frequently utilizes GPR15 as an entry cofactor (20). CysC95-146 had little if any effect on CCR5- or CXCR6-mediated virus infection, suggesting that it is specific for GPR15 (Fig. 2B) and did not display significant cytotoxic effects (Fig. 2C).
Fig. 2.
CysC95-146 specifically inhibits GPR15-mediated SIV and HIV infection. (A) GHOST-GPR15 cells were infected with a SIVmac239 luciferase reporter construct in the presence of CysC95-146. Experiments shown in all panels were performed at least in triplicates and curves show mean values (±SEM). (B) GHOST cells engineered to express GPR15, CXCR6, or CCR5 were infected with different SIV strains. Values show the percentage of virally infected (GFP+) cells in the presence of increasing concentrations of CysC95-146 compared to the percentage of infected cells obtained in the absence of peptide (100%). The dotted line indicates the percentage of eGFP+ cells obtained after infection of the parental GHOST cell line in the absence of peptide. (C) CysC95-146 is not cytotoxic. GHOST-GPR15 seeded in 96-well F-bottom plates were incubated with increasing amounts of peptide for 3 d at 37 °C. Metabolic activity was analyzed by (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and CellTiter-Glo assay. (D and E) Inhibition of (D) the indicated HIV-2 molecular clones or (E) HIV-1 ZP6248 by CysC95-146. In E, SIVmac239 is shown as positive control for comparison. Experiments were performed as described in B.
SIVsmm is the precursor of HIV-2 (37), which is endemic in West Africa and has infected about 1 to 2 million people (38). To evaluate a possible role of CysC95-146 in HIV-2 infection, we examined its effect on infection by three HIV-2 strains: ROD10, representing a derivative of the first reported infectious HIV-2 clone (39, 40); ST, an attenuated HIV-2 strain with low in vitro cytopathic activity (41, 42); and 7312A, originally isolated from an individual from Côte d’Ivoire with dual HIV-1 and HIV-2 infection (43). HIV-2 ROD10 and ST belong to the group A of HIV-2 that is most widespread in the human population, while 7312A represents a recombinant form of groups A and B (44). CysC95-146 inhibited all HIV-2 strains in a dose-dependent manner with IC50 values ranging from 1.3 to 7.0 µM (Fig. 2D). However, maximal inhibition of HIV-2 entry did not exceed ∼80%. One reason for this might be GPR15-independent infection of GHOST cells. Indeed, HIV-2 ROD10 infected the parental GHOST cell line that expresses low levels of CXCR4 but not GPR15 or other known entry cofactors with significant efficiency (Fig. 2D and SI Appendix, Fig. S1).
HIV-1 is less promiscuous in coreceptor usage than HIV-2. It usually utilizes only CCR5 during chronic infection and frequently evolves the ability to use CXCR4 during or after AIDS progression (45, 46). Some HIV-1 isolates, however, may also utilize GPR15 for productive infection and replication especially at high expression levels (19, 47, 48). Interestingly, it has been reported that the transmitted/founder HIV-1 IMC ZP6248 is severely impaired in CCR5 and CXCR4 coreceptor usage but capable of infecting cell lines expressing alternative coreceptors including GPR15 (48). CysC95-146 inhibited infection by the HIV-1 ZP6248 molecular clone in GPR15-GHOST cells with an IC50 of 1.1 µM (Fig. 2E). Altogether, our results showed that CysC95-146 specifically inhibits GPR15-dependent SIV and HIV infection.
The Inhibitory CysC95-146 Fragment Binds to GPR15-Expressing Cells.
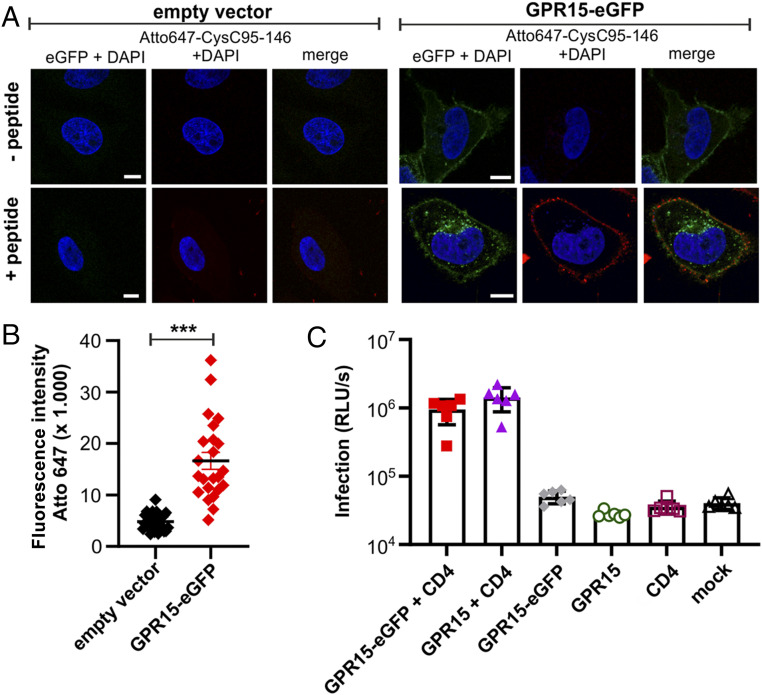
To examine whether CysC95-146 specifically targets GPR15-expressing cells, we generated N- and C-terminal fusions of this GPCR with the enhanced green fluorescent protein (eGFP). N-terminal tagging with eGFP resulted in mislocalization of GPR15 and lack of cell surface expression (SI Appendix, Fig. S2A). In comparison, the C-terminally tagged GPR15-eGFP was efficiently expressed and showed proper localization at the cell surface (SI Appendix, Fig. S2B). For colocalization studies, we transfected HeLa cells with empty control or GPR15-eGFP vector and exposed the cells to Atto647-labeled CysC95-146. The antiviral peptide was readily detectable at the surface of GPR15-expressing HeLa cells but absent from control cells (Fig. 3A). Quantitative analysis confirmed that CysC95-146 accumulates specifically on GPR15-eGFP-expressing cells (Fig. 3B). Consistent with its proper subcellular localization, GPR15-eGFP mediated CD4-dependent entry of SIV with an efficiency similar to the parental GPR15 protein (Fig. 3C). These results are further evidence that CysC95-146 specifically targets GPR15 at the cell surface.
Fig. 3.
CysC95-146 interacts specifically with GPR15-expressing cells. (A) HeLa cells were transfected with an eGFP-tagged GPR15 expression plasmid or a vector control and cultured for 2 d. Subsequently, cells were treated with Atto647-labeled CysC95-146. Excessive peptide was removed in several washing steps before staining the nuclei with Hoechst 33342 (Merck) according to the manufacturer’s instructions. Samples were analyzed by confocal microscopy using a LSM710 (Carl Zeiss). (Scale bar, 10 µm.) (B) Quantification of the Atto647 mean fluorescence intensity at the cell membrane from Atto-647-CysC95-146-treated samples shown in A. n = 23 to 27 cells ± SEM. ***P < 0.001 (Mann–Whitney U test, unpaired t test, nonparametric). (C) HEK293T cells transiently transfected with plasmids expressing the indicated receptors and/or an empty control vector and subsequently infected with a SIVmac239 nano-luciferase reporter virus. Infection rates were quantified as relative light units (RLU) per second. Results are displayed as means ± SEM, n = 6. One representative of two biological repeats is shown; ***P < 0.001 (Mann–Whitney U test, unpaired t test, nonparametric).
A Variety of C-Terminal CysC Fragments Prevent GPR15-Mediated Lentiviral Infection.
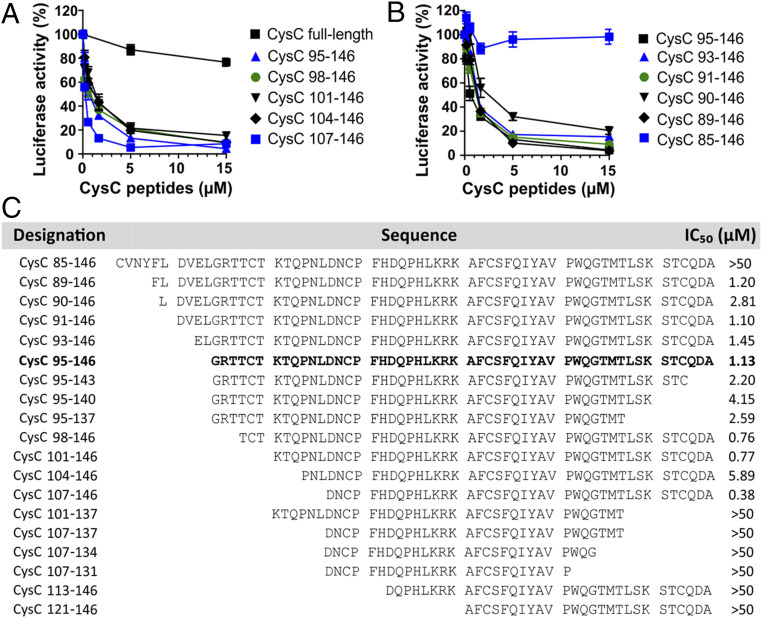
To further examine the specificity of the antiviral activity of CysC95-146, we determined whether full-length cystatin C and other C-terminal fragments also affect primate lentiviral infection. Our results showed that full-length cystatin C displayed little inhibitory effect on SIVmac239 entry (Fig. 4A). In contrast, N-terminal truncations of CysC95-146 by up to 12-aa residues (CysC107-146) (Fig. 4A), as well as expansion by up to 6 residues (89 to 146) (Fig. 4B) did not disrupt its antiviral activity. However, a peptide containing an additional 10 residues at its N terminus (CysC85-146) did not inhibit SIVmac entry into GPR15-GHOST cells (Fig. 4B). Comprehensive analyses of a variety of C-terminal cystatin C fragments revealed that residues 107 to 140 are sufficient for antiviral activity (Fig. 4C). Further truncations at the N or C termini resulted in reduced activity or fully disrupted inhibition of GPR15-mediated SIVmac239 infection. Notably, some of the cystatin C fragments analyzed, such as CysC107-146, were even more potent than CysC95-146 in inhibiting GPR15-mediated SIVmac infection (Fig. 4C). Altogether, the results showed that a large variety of C-terminal cystatin C fragments prevent GPR15-mediated SIVmac infection.
Fig. 4.
Anti-SIV activity of different C-terminal cystatin C fragments. (A and B) Inhibition of SIVmac239 by (A) full-length cystatin C- and N-terminally truncated or (B) extended CysC peptides. Numbers refer to amino acid positions in the full-length cystatin C sequence. GHOST-GPR15 cells were treated with the indicated concentrations of the cystatin peptides for 2 h at 37 °C before infection with a SIVmac239 Firefly luciferase reporter virus. Three days postinfection, Firefly luciferase activities were analyzed. Experiments shown in all panels were performed at least in triplicates and curves show mean values (±SEM). (C) Overview of the amino acid sequences and antiviral activity of chemically synthesized CysC peptides analyzed. IC50 values were determined by infection of GHOST-GPR15 cells with a SIVmac239 luciferase reporter construct in the presence of increasing quantities of the indicated peptides.
Proteolytic Generation of Antiviral CysC Fragments.
CysC95-146 was isolated from a hemofiltrate-derived peptide library, suggesting that it naturally circulates in the blood stream. To further examine this, we developed an MS-based method for the quantification of CysC95-146 in hemofiltrate (SI Appendix, Fig. S3A). These analyses showed that CysC95-146 was present at concentrations of ∼10.7 ng/mL (236 pmol) in the original hemofiltrate fraction (SI Appendix, Fig. S3 B and C). This concentration is lower than the IC50. However, hemofiltrate is significantly diluted compared to blood. In addition, material may have been lost during sample preparation and other C-terminal fragments are also antivirally active (Fig. 4). In addition, cystatin C levels are elevated in HIV-infected patients compared to healthy individuals (49, 50). Thus, our measurements most likely underestimate the concentrations of antiviral C-terminal cystatin C fragments that can be achieved in vivo.
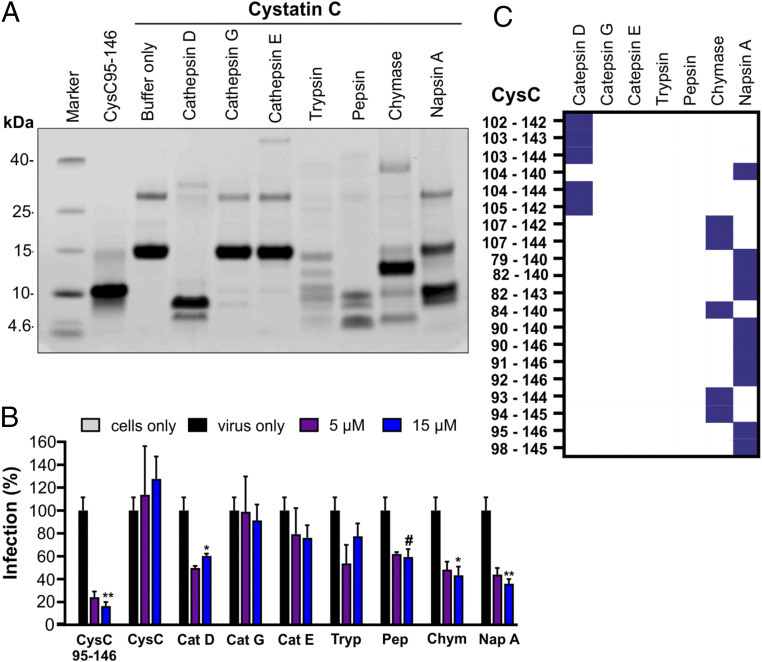
To examine the generation of antiviral peptides from cystatin C, we treated the full-length protein with cathepsins C, D, and G, as well as trypsin, chymase, and napsin A (Fig. 5A). These proteases represent major components of the endo- and lysosomal protein degradation machinery (26, 51) and some of them are efficiently released from immune cells during infectious and inflammatory processes (52, 53). Treatment of cystatin C with cathepsin D, trypsin, pepsin, chymase, and napsin A resulted in the generation of peptides with sizes similar to CysC95-146 (Fig. 5A). Products obtained after digestion with cathepsin D, chymase, and napsin A significantly inhibited GPR15-mediated SIVmac infection (Fig. 5B). In addition, mass spectrometry of the digestion products confirmed the presence of several antivirally active cystatin C fragments in cathepsin D, chymase, and napsin A-digested samples, although only napsin A generated CysC95-146 originally isolated from human serum (Fig. 5C). Cathepsin D is an important component of the lysosomal protein degradation pathway in virtually all cells (54) and released from immune cells during inflammatory processes (52). Chymase is a serine protease that is mainly produced by activated mast cells and elevated in some viral infections (55). Napsin A is an aspartic proteinase that is abundantly expressed in normal lung and kidney tissue and a marker for some neoplasia (56). Altogether, these results show that cystatin C fragments which inhibit GPR15-mediated HIV and SIV infection are detectable in blood-derived human hemofiltrate and can be generated by proteases that are present and activated at sites of infection and inflammation.
Fig. 5.
Treatment with various proteases generates antiviral cystatin C fragments. (A) Human cystatin C protein was digested with the indicated proteases. Digestion products were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by Coomassie Brilliant Blue staining. As controls, nondigested cystatin C as well as the synthesized CysC95-146 were included. (B) Proteases and nondigested protein were removed by ultrafiltration with different kilodalton cutoffs for purification. GHOST-GPR15 cells were then treated with the digestion products or CysC95-146 or full-length cystatin C and subsequently infected with a SIVmac239 luciferase reporter virus. Infection was measured at 3 dpi as described before. Results are displayed as means ± SD of one experiment in triplicate. #P = 0.06; *P < 0.05; **P < 0.01 (Mann–Whitney U test, unpaired t test, nonparametric). (C) Heat map visualization of identified cystatin C fragments in samples digested with the indicated proteases by mass spectrometry.
GPR15L Does Not Prevent SIV Entry.
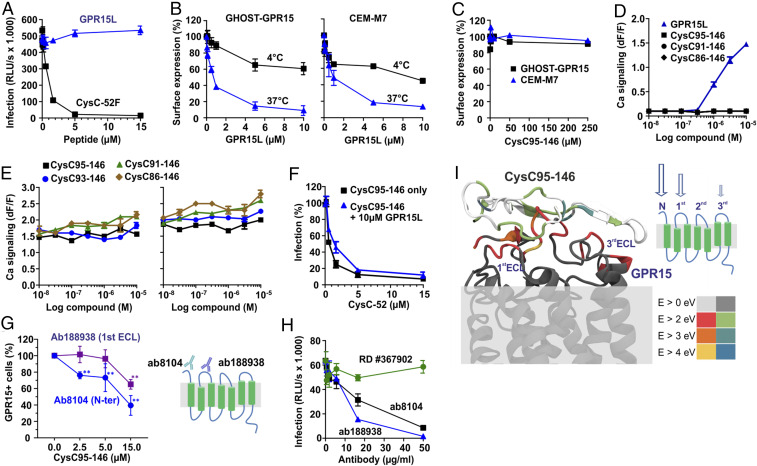
For a long time, GPR15 had remained an orphan receptor but recently an agonistic chemokine ligand (named GPR15L) that modulates lymphocyte recruitment to epithelia has been identified (23, 24). It is well established that the chemokine ligands of the main entry cofactors of HIV-1, CCL5/RANTES and CXCL-12/SDF-1, inhibit CCR5- or CXCR4-mediated HIV-1 infection, respectively (reviewed in ref. 57). Unexpectedly, GPR15L did not display an inhibitory effect on GPR15-mediated SIVmac infection (Fig. 6A), although it induced down-regulation of GPR15 from the surface of GHOST-GPR15 and CEM-M7 cells (Fig. 6B and SI Appendix, Fig. S4). Receptor internalization was strongly reduced when the cells were kept on ice, indicating that it mainly resulted from endocytosis and not from competition with the antibody used for staining. In contrast to GPR15L, CysC95-146 did not affect cell surface expression of GPR15 (Fig. 6C). In addition, CysC95-146 did not induce calcium release in Chinese hamster ovary (CHO) cells engineered to express human GPR15 together with the promiscuous G protein Gα16 (23), while GPR15 signaling was confirmed for GPR15L (Fig. 6D). Altogether, the results showed that CysC95-146 prevents SIV infection without altering GPR15 cell surface expression, while down-modulation of GPR15 by GPR15L was insufficient to cause significant inhibitory effects on SIV entry.
Fig. 6.
Effect of CysC95-146 and GPR15L on GPR15 function. (A) GPR15L does not inhibit SIVmac239 infection. GHOST-GPR15 cells were preincubated with increasing amounts of GPR15L or CysC95-146, infected with an SIVmac239 luciferase reporter virus, and infection rates determined 3 d later. Experiments shown in all panels were performed at least in triplicate and curves show mean values (±SEM). (B and C) GPR15L (B) but not CysC95-146 (C) down-modulate GPR15 from the surface of GHOST-GPR15 and CEM-M7 cells. GHOST-GPR15 or CEM-M7 cells were preincubated with increasing amounts of GPR15L or CysC95-146 at 4 °C. GPR15 expression was analyzed by flow cytometry. (D) Cystatin C fragments do not induce GPR15-mediated calcium signaling. The effect of the indicated CysC fragments and GPR15L on calcium signaling was detected by measuring aequorin fluorescence in GPR15-expressing CHO-K1 cells. Each data point represents the mean relative light units ± SD over background fluorescence of quadruplicate measurements. (E) Cystatin C fragments do not interfere with the signaling function of GPR15L. The effect of CysC fragments on GPR15L signaling was analyzed as described in D. (F) GPR15L does not enhance the antiviral activity of CysC95-146. GHOST-GPR15 cells were treated with CysC95-146 in the presence or absence of GPR15L and infected with a SIVmac239 luciferase reporter virus as described in A. (G) CysC95-146 competes with antibodies targeting the N terminus or ECL1 region for GPR15 binding. GHOST-GPR15 cells were incubated with the indicated concentrations of CysC95-146 and either ab8104 targeting the N terminus of GPR15 or ab188938 targeting the first ECL of GPR15, washed, stained with a PE-conjugated secondary antibody, and analyzed by flow cytometry. The indicated frequencies of GPR15-positive cells were obtained by subtraction of background (secondary antibody only), followed by normalization to the no-peptide samples (100%). Shown are the mean values for n = 3 experiments ± SEM, **P < 0.01 (Welch’s t test, unpaired). (H) Anti-GPR15 antibodies ab8104 targeting the N terminus and ab188938 targeting the first extracellular loop of GPR15 but not RD #367902 (unknown binding site) inhibit SIVmac infection. GHOST-GPR15 cells were preincubated with the indicated anti-GPR15 antibodies prior to infection with a SIVmac239 luciferase reporter virus. (I) Theoretically proposed binding of CysC95-146 with GPR15 based on reactive molecular dynamics (MD) simulations (ReaxFF). In the model, the amino acid-resolved interaction energies are highlighted using the color coding shown in the Lower Right scale.
CysC95-146 Does Not Interfere with GPR15L Signaling.
The results outlined above suggested that CysC95-146 might inhibit GPR15-mediated viral entry without interfering with the physiological signaling activity of this GPCR. Indeed, calcium flux assays performed in the presence of constant quantities of GPR15L and increasing doses of various CysC fragments revealed that C-terminal CysC fragments did not reduce the signaling activity of GPR15L (Fig. 6E). Conversely, GPR15L did not enhance the antiviral activity of CysC95-146 (Fig. 6F). To obtain insights into the region(s) in GPR15 targeted by CysC95-146, we examined the ability of this antiviral peptide to compete with GPR15 antibodies. CysC95-146 competed with Ab8104 targeting the extracellular N terminus as well as (less efficiently) with Ab188938 interacting with the first extracellular loop (ECL1) (Fig. 6G). These two antibodies also significantly inhibited SIVmac entry (Fig. 6H). In contrast, Ab367902 (R&D) that efficiently recognizes GPR15 in FACS-based assays and targets an unknown domain did not show significant effects on SIVmac infection (Fig. 6H). Altogether, these results support that CysC95-146 targets the N-terminal region and ECL1 to inhibit SIV infection.
To further investigate the antiviral mechanism of CysC95-146, we performed molecular modeling on the interaction of CysC95-146 and GPR15 using structures obtained with reactive force field simulations. In agreement with the competition assays, the strongest interactions were predicted between CysC95-146 and the N terminus of GPR15 (Fig. 6I). To gain further insights into the interaction, the contribution of individual amino acids in GPR15 and CysC95-146 was analyzed in detail. We found that most amino acids in GPR15 (SI Appendix, Fig. S5) and CysC95-146 (SI Appendix, Fig. S6) stabilize the interaction by less than 2 eV. For GPR15, the strongest interactions were predicted for amino acid residues D2, E5, Y14, and T16, all located in the N-terminal part of this GPCR. In CysC95-146 only G95 and K120 stabilized the binding with >4 eV (SI Appendix, Fig. S6), suggesting that these residues are important for interaction with GPR15. To test the theoretical predictions, we exchanged residues G95, K120, and Q126 in CysC95-146 to alanine. Indeed, the combination of these three alterations fully disrupted the antiviral activity of CysC95-146 (SI Appendix, Fig. S6). Altogether, our results suggest that the N terminus of GPR15 is important for HIV-2 and SIV entry and the main target site for CysC95-146 interaction. In comparison, the second extracellular loop (ECL2) of GPCRs plays a key role in chemokine binding and signaling (58). Thus, targeting of different surfaces on GPR15 might explain why the antiviral and agonistic functions of CysC95-146 and GPR15L did not interfere with one another.
The Antiviral Activity of CysC95-146 Is Conserved in Simian Hosts of SIV.
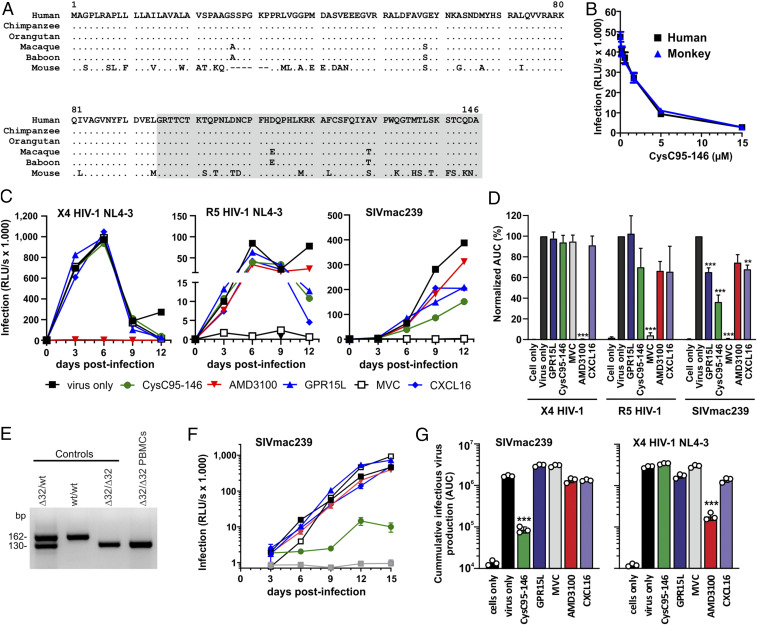
HIV-1 and HIV-2 are the result of at least 13 independent zoonotic transmissions of SIVs from great apes or sooty mangabeys to humans that occurred in the last century (37, 59). In contrast, SIVs have infected nonhuman primate species for many thousands or even millions of years, possibly since primate speciation (60). GPR15 is a major SIV entry cofactor in the two best-studied simian species naturally infected with SIV, i.e., sooty mangabeys and African green monkeys (16, 20). GPR15 also represents a major entry cofactor of SIVmac in macaques, the best established nonhuman primate model for AIDS in humans. To examine whether C-terminal cystatin C fragments may play a role in SIV infection, we examined whether their ability to inhibit GPR15-mediated viral entry is conserved in monkeys. Cystatin C is the most ancestral member of the cystatin family of inhibitors of cysteine peptidases and found in many vertebrates (61). Sequence alignments revealed that the C terminus of human cystatin C is fully conserved in great apes and that the macaque ortholog differs only in two amino acids from its human counterpart (Fig. 7A). We chemically synthesized the macaque CysC95-146 variant and found that it inhibits SIVmac239 infection as efficiently as the human version (Fig. 7B). Thus, the antiviral activity of CysC95-146 is conserved in simian hosts of SIV infection.
Fig. 7.
The antiviral activity of CysC95-146 is conserved in monkeys and affects HIV-2 and SIV infection in human T cells. (A) Alignment of cystatin C amino acid sequences from the indicated species. Dots indicate identity to the human sequence and dashes identify gaps introduced to optimize the alignment. The region corresponding to the CysC95-146 is shaded. (B) Antiviral activity of human and monkey-derived CysC95-146 peptides. GHOST-GPR15 cells were incubated with increasing amounts of human and monkey CysC95-146 for 2 h at 37 °C prior to infection with SIVmac239 Firefly luciferase (F-Luc). At 3 dpi, infection was analyzed via F-Luc reporter assay. The experiment was performed in triplicates. (C) CysC95-146 shows antiviral activity against GPR15-mediated SIVmac239 replication in human primary cells. To examine possible effects on spreading infection, we isolated and stimulated human PBMCs and treated them with the various compounds (CysC95-146, GPR15L, AMD3100, MVC, and CXCL16) prior to virus exposure. Infectious virus production was determined by infection of TZM-bl indicator cells with PBMC culture supernatants obtained at different days post-infection. (D) Calculated area under the curve (AUC) for the virus replication data obtained in C. ***P < 0.001, **P < 0.01 (Welch’s t test, unpaired). (E) Verification of homozygous deletions in the CCR5 gene of a Δ32/Δ32 PBMC donor. (F) Replication kinetics of SIVmac239 in Δ32/Δ32 PBMCs in the presence of various antiviral agents. Experimental details and symbols are provided in C. (G) Calculated AUC for the virus replication of SIVmac239 (see F) and X4 HIV-1 in Δ32/Δ32 PBMCs.
Effect of CysC95-142 and GPR15L on SIV and HIV Infection of Primary T Cells.
To determine whether inhibition of GPR15-mediated HIV or SIV infection by CysC95-146 is relevant in primary viral target cells, we infected phytohaemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMCs) from three human donors in the presence and absence of this peptide. Since PBMCs express various SIV and HIV coreceptors, we performed the experiment in the presence and absence of AMD3100 and Maraviroc, preventing CXCR4- and CCR5-mediated viral entry, respectively. Fluorescence-activated cell sorting (FACS) analyses allowed us to determine the percentages of infected cells by intracellular p24 or p27 antigen staining and showed that PHA stimulation induced CCR5, GPR15, and CXCR6 cell surface expression (SI Appendix, Fig. S7 A and B). CysC95-146 had no inhibitory effect on a CCR5-tropic derivative of HIV-1 NL4-3 and the CXCR4-tropic HIV-2 ROD9 molecular clone (SI Appendix, Fig. S7C). However, the peptide significantly reduced SIVmac239 infection of human PBMCs in both the presence and absence of additional inhibitors (SI Appendix, Fig. S7C). To further examine this, we analyzed the effects on three additional virus strains, i.e., the GPR15-tropic HIV-1 ZP6248 IMC, HIV-2 7312, and SIVsmm L1, which are capable of using CCR5, GPR15, and CXCR6 as entry cofactors (similarly to SIVmac239). CysC95-146 clearly reduced PBMC infection by all three HIV-1, HIV-2, and SIVsmm strains (SI Appendix, Fig. S7D), suggesting that GPR15-mediated entry contributes to primate lentiviral infection of primary CD4+ T cells.
To analyze possible effects on spreading infection, we pretreated PHA-stimulated human PBMCs with the various inhibitors prior to virus exposure. Infectious virus production was determined by infection of TZM-bl indicator cells with PBMC culture supernatants obtained on different days postinfection (dpi). Predictably, AMD3100 blocked CXCR4-tropic HIV-1, while Maraviroc fully prevented CCR5-tropic HIV-1 replication (Fig. 7C). In comparison, GPR15L, CysC95-142, and the CXCR6 chemokine ligand CXCL16 had no significant effect on CXCR4- or CCR5-tropic HIV-1 replication. Exposure of PBMCs to the GPR15-tropic HIV-1 ZP6248 strain did not result in significant replication, precluding meaningful analysis of inhibitors. However, GPR15L, CysC95-146, and CXCL16 all reduced replication of SIVmac239 in human PBMC cultures, albeit less efficiently than Maraviroc (Fig. 7C). CysC95-146 was more effective than GPR15L and CXCL16 and suppressed infectious virus yield on average by ∼60% (Fig. 7D). FACS analyses revealed that CysC95-146 did not affect GPR15 cell surface expression while GPR15L reduced it by ∼40% (SI Appendix, Fig. S8 A and B). Unexpectedly, GPR15L induced significant down-modulation of CXCR4 and competed with the 12G antibody that targets ECL-2 of CXCR4 for binding to this GPCR (SI Appendix, Fig. S8 B and C). However, in contrast to the small molecule inhibitor AMD3100, neither CysC95-146 nor GPR15L inhibited CXCR4-mediated HIV-1 infection (SI Appendix, Fig. S8D). Altogether, these results show that CysC95-146 suppresses GPR15-mediated primate lentiviral infection in primary human target cells and revealed an unexpected effect of GPR15L on CXCR4 cell surface expression.
We hypothesized that CysC95-146 may only reduce SIVmac239 replication by ∼60% because this virus also utilizes CCR5 for entry into primary T cells. To examine this, we screened healthy uninfected individuals for the presence of the Δ32/Δ32 deletion in the CCR5 gene that is present in about ∼1% of Caucasians and disrupts functional CCR5 expression (62). We identified one donor containing homozygous deletions (Fig. 7E) and performed infection experiments in Δ32/Δ32 PBMCs in the presence of various antiviral agents. Predictably, R5-tropic HIV-1 did not replicate in PBMCs lacking CCR5. Replication of SIVmac239 was almost entirely prevented by CysC95-146 but hardly affected by GPR15L, Maraviroc, AMD3100, or CXCL16 (Fig. 7F). On average, CysC95-146 reduced production of infectious SIVmac239 by ∼95% but had no inhibitory effect on X4-tropic HIV-1 (Fig. 7G). Vice versa, AMD3100 reduced infectious yield of X4 HIV-1 by 94% but had no significant effect on SIVmac239. Thus, in the absence of CCR5, CysC95-146 prevents SIV replication in primary T cells almost entirely, suggesting potent inhibition of GPR15-dependent virus entry.
Discussion
In the present study, we identified CysC95-146 and related C-terminal fragments of cystatin C as effective and specific endogenous inhibitors of GPR15-dependent HIV and SIV infection. In contrast to CysC95-146, GPR15L, the recently discovered chemokine ligand of GPR15 (23, 24), displayed little if any inhibitory effect on HIV and SIV entry. This came as a surprise because the chemokine ligands of CCR5 and CXCR4 inhibit R5- or X4-tropic HIV-1 infection, respectively (63, 64). Notably, C-terminal fragments of cystatin C prevent SIV and HIV-2 infection without interfering with GPR15L-mediated signaling activity of GPR15. In contrast, small molecule inhibitors of CCR5- and CXCR4-dependent HIV-1 entry, such as Maraviroc or AMD3100, antagonize chemokine signaling via these GPCRs (65, 66). To our knowledge, CysC95-146 and related peptides are the first agents preventing GPCR-mediated infection by lentiviral pathogens without interfering with the signaling function of the corresponding chemokine receptor. Specific targeting of the detrimental function but not the signaling activity is of significant interest since GPCRs are involved in many physiological and pathological processes and the target of about 30% of all current drugs.
Cystatin C is produced by all nucleated cells, found in all tissues and body fluids, and represents the most abundant cysteine protease inhibitor (27). It is best known as a marker for renal failure. However, accumulating data also support an important role of cystatin C in the immune response against various exogenous or endogenous pathogens (28, 31, 49). The plasma levels in healthy individuals are about 0.1 µM. However, cystatin C is induced in HIV-infected individuals and reaches blood plasma levels up to 0.5 µM under conditions of renal failure, infection, and inflammation (35, 67, 68). This concentration approximates the IC50 of antiviral cystatin C fragments and it is conceivable that the local levels at sites of infection and inflammation might exceed the systemic plasma levels. In fact, we found that peptides blocking GPR15-mediated SIV entry are generated from cystatin C by treatment with cathepsin D, chymase, and napsin A (Fig. 5). These proteases are secreted by lysosomal exocytosis (69) or via specialized secretory granules (53) during immune responses and activated under acidic conditions. Acidification is a hallmark of inflammatory tissues (70) and thought to play a key role in innate immunity (71).
The generation of the active CysC fragments shows notable parallels to the generation of the CXCR4 antagonist and X4 HIV-1 inhibitor EPI-X4, which is generated from albumin by cathepsins D and E under acidic conditions (13). Albumin is more abundant than cystatin C but the IC50 of EPI-X4 is also 10-fold higher than that of CysC95-146. Similarly, it has been reported that proteolytic processing of chemokine (C-C motif) ligand 14 (CCL14), commonly also known as hemofiltrate CC chemokine 1 (HCC-1), by trypsin-like serine proteases generates a potent CCR5 agonist (CCL14[9-74]) that efficiently inhibits R5-tropic HIV-1 strains (11, 12). Similar to cystatin C and albumin, the nonfunctional full-length CCL14 precursor is present at high concentrations in normal plasma. These results suggest that EPI-X4, CCL14[9-74], and active cystatin C fragments are all preferentially generated at sites of infection and inflammation, where they might act locally to cooperatively inhibit CXCR4-, CCR5-, and GPR15-mediated HIV or SIV infection, respectively. It is tempting to speculate that the combination of such endogenous inhibitors may have driven promiscuous coreceptor usage of SIVs that have most likely been infecting primate species for millions of years (72, 73).
We have previously shown that structure–activity relationship (SAR) studies enhance the activity of endogenous peptides by several orders of magnitude and offer perspectives for therapeutic applications (13, 74). For example, an optimized derivative of a natural 20-residue fragment of α(1)-antitrypsin that targets the gp41 fusion peptide of HIV-1 was safe and effective in human individuals (75). In addition, optimized derivatives of the endogenous CXCR4 antagonist Epi-X4 prevent atopic dermatitis and airway inflammation in preclinical mouse models (76). CysC95-146 is relatively large but tolerates truncations without loss of activity (Fig. 4). We will perform structural and molecular modeling studies and SAR analyses to determine the minimal active size of C-terminal cystatin C fragments and to increase their activity by rational design of derivatives predicted to interact more strongly with GPR15.
Our results show that CysC95-146 prevents GPR15-mediated HIV-2 and SIV infection, while GPR15L displayed little if any inhibitory activity although it induced down-modulation of GPR15 from the cell surface. It is known that both receptor removal from the cell surface as well as competitive inhibition by occupation of the interaction site(s) of the HIV envelope glycoprotein by chemokines might contribute to inhibition of CCR5- or CXCR4-dependent HIV-1 infection (77, 78). Our data support that competition by C-terminal cystatin C fragments is more effective than GPR15L-induced down-regulation of GPR15 in inhibiting lentiviral infection in both GHOST indicator and primary CD4+ T cells, suggesting that only a certain threshold is required for viral entry. Further structure–function analyses are required to fully elucidate the antiviral mechanism and the interaction(s) of CysC95-146 and GPR15L with GPR15. Our preliminary results from antibody competition assays and molecular modeling analyses suggest that CysC95-146 interacts most strongly with the N-terminal region of GPR15 (Fig. 6I). The observed antiviral effect agrees with previous results showing that the N-terminal domains of CCR5 and CXCR4 are targeted by the HIV-1 envelope glycoproteins and play a key role in membrane fusion (79, 80). Consistent with published data on CCR5 and HIV-1, we found that antibodies targeting the N terminus or ECL-1 of GPR15 prevented SIVmac239 infection (Fig. 6G). GPR15L differs in GPCR interaction from prototype chemokines (23) and may interact with more C-terminal domains of GPR15. Differential GPR15 interaction sites also explain why CysC95-142 did not affect GPR15L-mediated signaling (Fig. 6E) and, vice versa, the chemokine ligand did not enhance the inhibitory effect of the CysC95-146 fragment (Fig. 6F).
Antiviral cystatin C fragments may have some relevance in humans since GPR15 is a common coreceptor of HIV-2 that infects about 1 to 2 million people mainly in Sub-Saharan Africa and is also used by some HIV-1 strains. We show that CysC95-146 inhibits HIV-2 and the highly unusual HIV-1 ZP6248 strain not only in indicator cell lines but also in primary human cells. Our results also demonstrate that the antiviral activity of CysC95-146 is conserved in monkeys and support that the peptide inhibits SIV infection of primary human cells. HIV entered the human population only about a century ago and was hence clearly not a driving force in the evolution of endogenous peptides blocking GPR15-mediated entry. In comparison, SIVs have infected nonhuman primate species for many thousands if not millions of years (37). GPR15 coreceptor usage is found in diverse groups of primate lentiviruses (81) and most likely represents an ancient function. Thus, it is tempting to speculate that the evolution of inhibitors of GPR15-mediated viral entry by inflammation and infection-associated proteases might have been driven by ancient primate lentiviruses. Finally, our results suggest that GPR15 allows SIV to replicate in Δ32/Δ32 PBMCs cells in the absence of CCR5 (Fig. 7 E–G). In humans, the Δ32/Δ32 genotype is associated with a reduced risk of the acquisition of HIV-1 infection via the sexual route (62, 82). Notably, sooty mangabeys, the original host of SIVsmm/HIV-2, also frequently lack functional CCR5 expression (20). However, the prevalence of natural SIVsmm infection was not significantly reduced in animals lacking functional CCR5 most likely due to efficient coreceptor usage of GPR15 and CXCR6.
Our identification of CysC95-146 provides proof of concept that some ligands of GPCRs can block pathogens without interfering with their physiological signaling function. Notably, this is not the case for inhibitors of CCR5- and CXCR4-mediated HIV infection, and this precludes e.g., usage of AMD3100 for the treatment of chronic diseases since proper CXCR4 signaling is critical for many physiological processes. After the CXCR4 antagonist EPI-X4 (13), CysC95-146 is another example of the proteolytic generation of a peptidic virus inhibitor from an abundant precursor protein by proteases that are activated under acidic conditions. It is conceivable that generation of antimicrobial effects by proteolytic generation of abundant precursors might provide a more effective and rapid means to generate innate immune effectors than de novo synthesis. Further studies to clarify whether generation of antimicrobial molecules by proteolysis of abundant precursor proteins by proteases activated during infection and inflammation represents a common concept of innate immune defense seem warranted.
Materials and Methods
Ethical Statement for Human Samples.
Experiments involving human blood and CD4+ T cells were reviewed and approved by the institutional review board (i.e., the Ethics Committee of Ulm University). Individuals and/or their legal guardians provided written informed consent prior to donating blood. All human-derived samples were anonymized before use. The use of established cell lines did not require the approval of the institutional review board.
Generation and Screening of HF Libraries.
Fractions of a hemofiltrate-derived peptide library generated as described (13) were tested for their ability to suppress SIVmac239 infection in GHOST-GPR15 cells. Cells were seeded in flat-bottomed 96-well dishes, cultured overnight, and incubated with the peptide for 2 h before infection with an SIVmac239 construct containing the Firefly luciferase reporter gene in place of the nef gene. Forty-eight hours later, the cells were lysed with Cell Culture Lysis Reagent (catalog no. E153A; Promega) and relative light units (RLU) were determined using the luciferase assay system (Promega).
Fluorescence-Based Calcium Release Assays.
GPR15 signaling efficiency was determined as described previously (23).
Statistical Methods.
The mean activities were compared using Student’s t test. Similar results were obtained with the Mann–Whitney U test. The software package StatView version 4.0 (Abacus Concepts) was used for all calculations.
Supplementary Material
Acknowledgments
A number of reagents were obtained through the NIH AIDS Reagent Program. N.P., L.S., A.R., S.W., M. Hayn, R.G., C.J., T.J., J.M., and F.K. are supported by CRC 1279 of the Deutsche Forschungsgemeinschaft. K.M.J.S. is supported by a Marie-Sklodowska Curie fellowship and B.H.H. is supported by R01 AI 114266 and UM1 AI 126620. The Alexander von Humboldt Foundation is gratefully acknowledged for financial support to A.R. (postdoctoral fellowship 3.2-KUB/1153731 STP).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2023776118/-/DCSupplemental.
Data Availability.
All data generated in this study are included in the paper and SI Appendix.
References
- 1.Venkatakrishnan A. J., et al. , Molecular signatures of G-protein-coupled receptors. Nature 494, 185–194 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Heng B. C., Aubel D., Fussenegger M., An overview of the diverse roles of G-protein coupled receptors (GPCRs) in the pathophysiology of various human diseases. Biotechnol. Adv. 31, 1676–1694 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Deng H., et al. , Identification of a major co-receptor for primary isolates of HIV-1. Nature 381, 661–666 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Feng Y., Broder C. C., Kennedy P. E., Berger E. A., HIV-1 entry cofactor: Functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272, 872–877 (1996). [DOI] [PubMed] [Google Scholar]
- 5.Dragic T., et al. , HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381, 667–673 (1996). [DOI] [PubMed] [Google Scholar]
- 6.Alkhatib G., et al. , CC CKR5: A RANTES, MIP-1, MIP-1 receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272, 1955–1958 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Choe H., et al. , The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85, 1135–1148 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Doranz B. J., et al. , A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 85, 1149–1158 (1996). [DOI] [PubMed] [Google Scholar]
- 9.Bosso M., Ständker L., Kirchhoff F., Münch J., Exploiting the human peptidome for novel antimicrobial and anticancer agents. Bioorg. Med. Chem. 26, 2719–2726 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Münch J., Ständker L., Forssmann W.-G., Kirchhoff F., Discovery of modulators of HIV-1 infection from the human peptidome. Nat. Rev. Microbiol. 12, 715–722 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Detheux M., et al. , Natural proteolytic processing of hemofiltrate CC chemokine 1 generates a potent CC chemokine receptor (CCR)1 and CCR5 agonist with anti-HIV properties. J. Exp. Med. 192, 1501–1508 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Münch J., et al. , Hemofiltrate CC chemokine 1[9-74] causes effective internalization of CCR5 and is a potent inhibitor of R5-tropic human immunodeficiency virus type 1 strains in primary T cells and macrophages. Antimicrob. Agents Chemother. 46, 982–990 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zirafi O., et al. , Discovery and characterization of an endogenous CXCR4 antagonist. Cell Rep. 11, 737–747 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Mörner A., et al. , Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J. Virol. 73, 2343–2349 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pöhlmann S., et al. , Simian immunodeficiency virus utilizes human and sooty mangabey but not rhesus macaque STRL33 for efficient entry. J. Virol. 74, 5075–5082 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riddick N. E., et al. , Simian immunodeficiency virus SIVagm efficiently utilizes non-CCR5 entry pathways in African green monkey lymphocytes: Potential role for GPR15 and CXCR6 as viral coreceptors. J. Virol. 90, 2316–2331 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farzan M., et al. , Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J. Exp. Med. 186, 405–411 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owen S. M., et al. , Genetically divergent strains of human immunodeficiency virus type 2 use multiple coreceptors for viral entry. J. Virol. 72, 5425–5432 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng H. K., Unutmaz D., KewalRamani V. N., Littman D. R., Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature 388, 296–300 (1997). [DOI] [PubMed] [Google Scholar]
- 20.Riddick N. E., et al. , A novel CCR5 mutation common in sooty mangabeys reveals SIVsmm infection of CCR5-null natural hosts and efficient alternative coreceptor use in vivo. PLoS Pathog. 6, e1001064 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S. V., et al. , GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science 340, 1456–1459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen L. P., et al. , Role and species-specific expression of colon T cell homing receptor GPR15 in colitis. Nat. Immunol. 16, 207–213 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suply T., et al. , A natural ligand for the orphan receptor GPR15 modulates lymphocyte recruitment to epithelia. Sci. Signal. 10, eaal0180 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Ocón B., et al. , A mucosal and cutaneous chemokine ligand for the lymphocyte chemoattractant receptor GPR15. Front. Immunol. 8, 1111 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onopiuk A., Tokarzewicz A., Gorodkiewicz E., Cystatin C: A kidney function biomarker. Adv. Clin. Chem. 68, 57–69 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Turk V., et al. , Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta 1824, 68–88 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villa P., Jiménez M., Soriano M.-C., Manzanares J., Casasnovas P., Serum cystatin C concentration as a marker of acute renal dysfunction in critically ill patients. Crit. Care 9, R139–R143 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magister S., Kos J., Cystatins in immune system. J. Cancer 4, 45–56 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sokol J. P., Schiemann W. P., Cystatin C antagonizes transforming growth factor beta signaling in normal and cancer cells. Mol. Cancer Res. 2, 183–195 (2004). [PubMed] [Google Scholar]
- 30.Xu Y., Ding Y., Li X., Wu X., Cystatin C is a disease-associated protein subject to multiple regulation. Immunol. Cell Biol. 93, 442–451 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zi M., Xu Y., Involvement of cystatin C in immunity and apoptosis. Immunol. Lett. 196, 80–90 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulz-Knappe P., et al. , Peptide bank generated by large-scale preparation of circulating human peptides. J. Chromatogr. A 776, 125–132 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Pöhlmann S., et al. , Co-receptor usage of BOB/GPR15 in addition to CCR5 has no significant effect on replication of simian immunodeficiency virus in vivo. J. Infect. Dis. 180, 1494–1502 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Richter R., et al. , Composition of the peptide fraction in human blood plasma: Database of circulating human peptides. J. Chromatogr. B Biomed. Sci. Appl. 726, 25–35 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Randers E., Kristensen J. H., Erlandsen E. J., Danielsen H., Serum cystatin C as a marker of the renal function. Scand. J. Clin. Lab. Invest. 58, 585–592 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Chahroudi A., Bosinger S. E., Vanderford T. H., Paiardini M., Silvestri G., Natural SIV hosts: Showing AIDS the door. Science 335, 1188–1193 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharp P. M., Hahn B. H., Origins of HIV and the AIDS pandemic. Cold Spring Harb. Perspect. Med. 1, a006841 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visseaux B., Damond F., Matheron S., Descamps D., Charpentier C., Hiv-2 molecular epidemiology. Infect. Genet. Evol. 46, 233–240 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Clavel F., et al. , Molecular cloning and polymorphism of the human immune deficiency virus type 2. Nature 324, 691–695 (1986). [DOI] [PubMed] [Google Scholar]
- 40.Döring M., et al. , A genotypic method for determining HIV-2 coreceptor usage enables epidemiological studies and clinical decision support. Retrovirology 13, 85 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong L. I., et al. , West African HIV-2-related human retrovirus with attenuated cytopathicity. Science 240, 1525–1529 (1988). [DOI] [PubMed] [Google Scholar]
- 42.Kumar P., et al. , Molecular characterization of an attenuated human immunodeficiency virus type 2 isolate. J. Virol. 64, 890–901 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao F., et al. , Genetic diversity of human immunodeficiency virus type 2: Evidence for distinct sequence subtypes with differences in virus biology. J. Virol. 68, 7433–7447 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibe S., et al. , HIV-2 CRF01_AB: First circulating recombinant form of HIV-2. J. Acquir. Immune Defic. Syndr. 54, 241–247 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Connor R. I., Sheridan K. E., Ceradini D., Choe S., Landau N. R., Change in coreceptor use correlates with disease progression in HIV-1–Infected individuals. J. Exp. Med. 185, 621–628 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao L., Rudolph D. L., Owen S. M., Spira T. J., Lal R. B., Adaptation to promiscuous usage of CC and CXC-chemokine coreceptors in vivo correlates with HIV-1 disease progression. AIDS 12, F137–F143 (1998). [DOI] [PubMed] [Google Scholar]
- 47.P Hlmann S., Krumbiegel M., Kirchhoff F., Coreceptor usage of BOB/GPR15 and Bonzo/STRL33 by primary isolates of human immunodeficiency virus type 1. J. Gen. Virol. 80, 1241–1251 (1999). [DOI] [PubMed] [Google Scholar]
- 48.Jiang C., et al. , Primary infection by a human immunodeficiency virus with atypical coreceptor tropism. J. Virol. 85, 10669–10681 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neuhaus J., et al. , Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J. Infect. Dis. 201, 1788–1795 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Odden M. C., et al. , Cystatin C level as a marker of kidney function in human immunodeficiency virus infection: The FRAM study. Arch. Intern. Med. 167, 2213–2219 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaidi N., Kalbacher H., Cathepsin E: A mini review. Biochem. Biophys. Res. Commun. 367, 517–522 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Appelqvist H., Wäster P., Kågedal K., Öllinger K., The lysosome: From waste bag to potential therapeutic target. J. Mol. Cell Biol. 5, 214–226 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto K., Kawakubo T., Yasukochi A., Tsukuba T., Emerging roles of cathepsin E in host defense mechanisms. Biochim. Biophys. Acta 1824, 105–112 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Sun H., et al. , Proteolytic characteristics of cathepsin D related to the recognition and cleavage of its target proteins. PLoS One 8, e65733 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tissera H., et al. , Chymase level is a predictive biomarker of dengue hemorrhagic fever in pediatric and adult patients. J. Infect. Dis. 216, 1112–1121 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bishop J. A., Sharma R., Illei P. B., Napsin A and thyroid transcription factor-1 expression in carcinomas of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesothelioma. Hum. Pathol. 41, 20–25 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Verani A., Lusso P., Chemokines as natural HIV antagonists. Curr. Mol. Med. 2, 691–702 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Woolley M. J., Conner A. C., Understanding the common themes and diverse roles of the second extracellular loop (ECL2) of the GPCR super-family. Mol. Cell. Endocrinol. 449, 3–11 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Sauter D., Kirchhoff F., Key viral adaptations preceding the AIDS pandemic. Cell Host Microbe 25, 27–38 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Pandrea I., Sodora D. L., Silvestri G., Apetrei C., Into the wild: Simian immunodeficiency virus (SIV) infection in natural hosts. Trends Immunol. 29, 419–428 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Sousa-Pereira P., et al. , Evolution of C, D and S-type cystatins in mammals: An extensive gene duplication in primates. PLoS One 9, e109050 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samson M., et al. , Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382, 722–725 (1996). [DOI] [PubMed] [Google Scholar]
- 63.Bleul C. C., et al. , The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382, 829–833 (1996). [DOI] [PubMed] [Google Scholar]
- 64.Oberlin E., et al. , The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature 382, 833–835 (1996). [DOI] [PubMed] [Google Scholar]
- 65.Donzella G. A., et al. , AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat. Med. 4, 72–77 (1998). [DOI] [PubMed] [Google Scholar]
- 66.Dorr P., et al. , Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 49, 4721–4732 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhasin B., et al. , HIV viremia and T-cell activation differentially affect the performance of glomerular filtration rate equations based on creatinine and cystatin C. PLoS One 8, e82028 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Longenecker C. T. et al.; AIDS Clinical Trials Group Study A5224s Team , Reductions in plasma cystatin C after initiation of antiretroviral therapy are associated with reductions in inflammation: ACTG A5224s. J. Acquir. Immune Defic. Syndr. 69, 168–177 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodríguez A., Webster P., Ortego J., Andrews N. W., Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J. Cell Biol. 137, 93–104 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okajima F., Regulation of inflammation by extracellular acidification and proton-sensing GPCRs. Cell. Signal. 25, 2263–2271 (2013). [DOI] [PubMed] [Google Scholar]
- 71.Rajamäki K., et al. , Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J. Biol. Chem. 288, 13410–13419 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Compton A. A., Malik H. S., Emerman M., Host gene evolution traces the evolutionary history of ancient primate lentiviruses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120496 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gifford R. J., et al. , A transitional endogenous lentivirus from the genome of a basal primate and implications for lentivirus evolution. Proc. Natl. Acad. Sci. U.S.A. 105, 20362–20367 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Münch J., et al. , Discovery and optimization of a natural HIV-1 entry inhibitor targeting the gp41 fusion peptide. Cell 129, 263–275 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Forssmann W.-G., et al. , Short-term monotherapy in HIV-infected patients with a virus entry inhibitor against the gp41 fusion peptide. Sci. Transl. Med. 2, 63re3 (2010). [DOI] [PubMed] [Google Scholar]
- 76.Harms M., et al. , An optimized derivative of an endogenous CXCR4 antagonist prevents atopic dermatitis and airway inflammation. bioRxiv: 10.1101/2020.08.28.272781 (29 August 2020). [DOI] [PMC free article] [PubMed]
- 77.Lobritz M. A., et al. , Multifaceted mechanisms of HIV inhibition and resistance to CCR5 inhibitors PSC-RANTES and Maraviroc. Antimicrob. Agents Chemother. 57, 2640–2650 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steen A., Schwartz T. W., Rosenkilde M. M., Targeting CXCR4 in HIV cell-entry inhibition. Mini Rev. Med. Chem. 9, 1605–1621 (2009). [DOI] [PubMed] [Google Scholar]
- 79.Golding H., et al. , CCR5 N-terminal region plays a critical role in HIV-1 inhibition by Toxoplasma gondii-derived cyclophilin-18. J. Biol. Chem. 280, 29570–29577 (2005). [DOI] [PubMed] [Google Scholar]
- 80.Zhou N., et al. , Structural and functional characterization of human CXCR4 as a chemokine receptor and HIV-1 co-receptor by mutagenesis and molecular modeling studies. J. Biol. Chem. 276, 42826–42833 (2001). [DOI] [PubMed] [Google Scholar]
- 81.Unutmaz D., KewalRamani V. N., Littman D. R., G protein-coupled receptors in HIV and SIV entry: New perspectives on lentivirus-host interactions and on the utility of animal models. Semin. Immunol. 10, 225–236 (1998). [DOI] [PubMed] [Google Scholar]
- 82.Liu R., et al. , Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86, 367–377 (1996). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated in this study are included in the paper and SI Appendix.