Abstract
Stem cell-derived, organotypic in vitro models, known as organoids, have emerged as superior alternatives to traditional cell culture models due to their unparalleled ability to recreate complex physiological and pathophysiological processes. For this reason, they are attractive targets of tissue-engineering efforts, as constructs that include organoid technology would be expected to better simulate the many functions of the desired tissue or organ. While the 3D spheroidal architecture that is the default architecture of most organoid models may be preferred for some applications, 2D monolayer arrangements remain the preferred organization for many applications in tissue engineering. Therefore, in this work, we present a method to create monolayer organoid cultures on poly(ethylene glycol) (PEG) hydrogel scaffolds, using intestinal epithelial organoids (IEOs) as a proof-of-concept. Our process involves two steps: the hydrogel is first functionalized with a layer of poly(D-lysine) (PDL), which then allows the adsorption of pristine, unmodified basement membrane proteins. This approach successfully mediates the formation of IEO monolayer unlike conventional approaches that rely on covalent modification of the hydrogel surface with cell-adhesive peptides and basement membrane proteins. We show that these IEO monolayers recreate important physiological functions of the native intestinal epithelium, including multilineage differentiation, apical-basal polarization, and the ability to model infections with human norovirus. We also show coating of a scaffold mimicking intestinal villous topography, resulting in a 3D IEO monolayer. We expect that this protocol will be useful to researchers attempting to leverage the increased physiological relevance of organoid models to elevate the potential of their tissue-engineered constructs.
Impact statement
While organoids are physiologically superior models of biological functions than traditional cell cultures, their 3D spheroidal architecture is an obstacle to their incorporation in many tissue-engineering applications, which often prefer 2D monolayer arrangements of cells. For this reason, we developed a protocol to establish monolayer cultures of organoids on poly(ethylene glycol) hydrogels and demonstrate its utility using intestinal epithelial organoids as a proof-of-concept. We expect that this protocol will be of use to researchers creating engineered tissues for both regenerative medicine applications, as well as advanced in vitro experimental models.
Keywords: protein functionalization, cell-adhesive peptides, norovirus, organoid
Introduction
Organoid cultures, in which stem cells are differentiated into multicellular three-dimensional (3D) aggregates,1 are able to recapitulate many physiological functions of native tissues that are often absent in other in vitro models. Organoids can be created from a wide variety of tissues, including the brain,2 lung,3 kidney,4 intestine,5 and liver6; they can also be derived from individual patients and used for applications in personalized medicine.7 For these reasons, they show considerable promise for regenerative medicine applications8 and as models of developmental and pathological processes.9
For all their benefits, significant opportunities remain to improve the quality of organoid-derived synthetic tissues. Organoids are typically grown as spheroids distributed within 3D matrices composed of collagen type I or Matrigel. While these naturally-derived materials are plentiful, user-friendly, and economical, they present the growing organoids with a non-native environment that may differ significantly in topography,10 material properties,11 and the molecular profile of the cell-binding proteins (e.g., laminin, fibronectin, collagen, and so on), with wide-ranging effects on the phenotype and physiology of the resident cells.12
To provide better control over the organoid environment, investigators have used both synthetic13–15 and protein engineered16 hydrogels to demonstrate that hydrogel stiffness and the type and density of cell-adhesion molecules, among other properties, affect the survival, proliferation, and cellular phenotype of the organoids, confirming the benefits of tailored culture substrates that can provide these types of tissue-specific extracellular matrix (ECM) cues. These advances, however, still require the organoids to be cultured as 3D spheroids embedded within gel-like matrices, which restrict experimental access to the apical surface of the cells. To improve apical access, researchers have developed techniques to convert different types of 3D organoids into 2D monolayers on Transwell and/or standard multiwell plates.17–19 However, these substrates are orders of magnitude stiffer than the native tissues from which the organoids are derived, with potentially detrimental effects on the biological behavior of the organoids.
For this reason, we set out to develop a protocol to culture 2D monolayers of organoids on synthetic hydrogel matrices customized to present native ECM cues. We started with a model organoid system derived from adult stem cells of the human intestinal epithelium. This intestinal epithelial organoid (IEO) model is one of the oldest organoid systems in existence, recapitulates many important functions of the intestinal epithelium, has already been incorporated into several bio- and tissue engineering efforts, and has a well-established protocol for converting 3D organoids into 2D monolayers.
Although several investigators have cultured monolayers of IEOs atop crosslinked collagen hydrogels,20,21 we used poly(ethylene glycol) (PEG) hydrogels. PEG hydrogels can be fabricated with biologically appropriate stiffnesses and can be readily molded into anatomically-relevant geometries. They are also bioinert and resistant to nonspecific protein adsorption and yet can be functionalized with relevant biomolecules to provide specific bioactivity.22 Surprisingly, we discovered that a wide range of cell-adhesive peptides and ECM proteins, when covalently anchored to the hydrogel surface, were unable to support monolayer culture of the IEOs atop the PEG.
Thus, we developed a two-step biofunctionalization protocol, in which poly(D-lysine) (PDL) is first bound to the PEG hydrogel to provide a high-density positively-charged surface that then promoted adsorption of unmodified ECM protein, thus creating a favorable surface for IEO adhesion and monolayer formation. This approach supports the formation of IEO monolayers that retain many important features of the native intestinal epithelium, including their apical-basal polarization, appropriate cellular differentiation, and the ability to support enteric infections. This approach also can be applied to a PEG hydrogel fabricated with a simple intestinal villous topography, enabling the growth of an IEO monolayer on a 3D surface. We expect that it will be useful for researchers seeking to create synthetic tissues from organoids from a variety of tissues for both regenerative medicine and disease modeling applications.
Methods
Hydrogel synthesis
Hydrogel precursor solutions were prepared by dissolving 20 kDa 8-arm PEG-norbornene (PEG-8N, JenKem Technologies) and 10 kDa 8-arm PEG-thiol (PEG-8T; JenKem Technologies) in a visible light photoinitiation solution [29] consisting of HEPES buffered saline (HBS), pH 8.3 with 1% (v/v) triethanolamine (TEOA, Acros Organics), and 10 μM eosin-Y disodium salt (Sigma Aldrich). To fabricate a hydrogel, 4 μL of this solution was pipetted onto a Sigmacote-treated glass slide between 320 μm tall PDMS spacers and covered with a coverslip functionalized with 3-mercaptopropyl(trimethoxy)silane (Sigma Aldrich) as described previously [30]. The gels were crosslinked using 150 kLux of white light (UltraTow LED Flood Light, Northern Tool and Equipment) for 2 min and transferred to a 12-well plate for swelling in phosphate-buffered saline (PBS) overnight.
The next day, the PBS was removed, and 6 mm diameter cloning cylinders were secured to the coverslips with silicone grease to create small wells (similar in size to one well of a 96-well plate) surrounding the hydrogels for use in all subsequent hydrogel modification and organoid culture steps. Unless stated otherwise, the hydrogels were composed of 5 mM PEG-8N and 2.5 mM PEG-8T, giving an excess norbornene concentration of 20 mM for attachment to thiolated ECM molecules.
Mechanical characterization of hydrogels
Hydrogel stiffness was measured using uniaxial unconfined compression as described previously.23,24 Briefly, hydrogels of 6 mm diameter × 1 mm thickness were fabricated and swollen in PBS overnight. The swollen gels were remeasured then compressed to 40% strain using a Bose ELF 3200 mechanical tester with force measured by a 1000 g load cell (Bose ELF). The elastic modulus was calculated from a linear regression of the stress versus strain plot between 5% and 15% strain.
Noncovalent functionalization of hydrogels with ECM proteins
For noncovalent immobilization of full-length ECM proteins, a layer of thiol-functionalized PDL was anchored to pendant norbornene groups on the PEG hydrogel surface and then subsequently used to adsorb the unmodified ECM proteins (Fig. 1). First, 2-iminothiolane was added to a 2 mg mL−1 solution of PDL hydrobromide (MW >300 kDa; Sigma Aldrich) in HBS with 1% TEOA, pH 8.3 at a 1:10 molar ratio (relative to the lysine monomers), and reacted for 1 h at room temperature. Unreacted 2-iminothiolane was then removed using a PD-10 desalting column with G-25 medium Sephadex resin, and the thiolation of the PDL was measured using Ellman's reagent (Thermo Scientific). The thiolated PDL (PDL-T) was then diluted to a concentration of 0.5 mg mL−1 with HBS + TEOA solution containing eosin-Y for a final eosin-Y concentration of 10 μM.
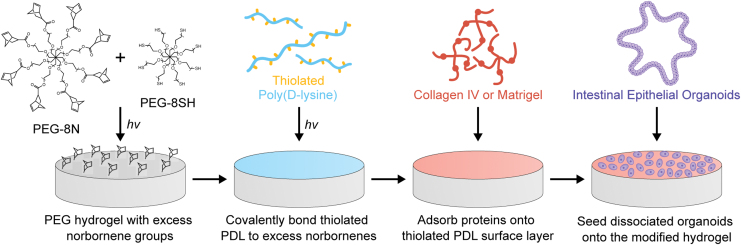
FIG. 1.
Biofunctionalization of PEG hydrogels using PDL-mediated protein adsorption and subsequent seeding with IEOs. PEG hydrogels with pendant norbornene groups are synthesized by reacting off-stoichiometric ratios of 8-arm PEG-Norbornene (PEG-8N) and 8-arm PEG-Thiol (PEG-8SH, also termed PEG-8T in the text). Thiolated PDL (PDL-T) is covalently anchored to the PEG hydrogel surface, and then unmodified ECM proteins are adsorbed before seeding with dissociated IEOs. IEO, intestinal epithelial organoid; PEG, poly(ethylene glycol); PDL, poly(D-lysine); ECM, extracellular matrix. Color images are available online.
After adding 100 μL of this mixture to the hydrogels, the PDL-T was conjugated to the surface with white light for 3 min. The gels were washed twice with PBS and then incubated with a 1 mg mL−1 collagen IV solution in PBS for 1 h at 37°C. For some experiments, growth-factor reduced, phenol-free Matrigel (1 mg mL−1; Corning) was substituted for collagen IV.
Visualization of hydrogel modification with fluorescent collagen IV
For visualization of the noncovalently-immobilized proteins, we generated fluorescently-labeled collagen IV by reacting 5/6-carboxy-tetramethyl-rhodamine succinimidyl ester (Thermo Scientific) with collagen IV (2.0 mg mL−1) in PBS at 4°C overnight following the manufacturer's instructions and then dialyzed extensively against chilled PBS to remove unreacted fluorophore. Hydrogels with excess norbornene concentrations of 0 mM (2.5 mM PEG-8T and 2.5 mM PEG-8N) and 20 mM (2.5 mM PEG-8T and 5 mM PEG-8N) were synthesized and functionalized with the fluorescent protein as described above. The hydrogel surface coverage was imaged using an epifluorescent microscope (Nikon, Eclipse TE300), whereas hydrogel cross-sections (cut into 500 μm thick sections with a razor blade) were imaged with a confocal microscope (Zeiss; LSM 510).
Quantification of protein immobilization on noncovalently modified hydrogels
To measure the efficiency of protein attachment to the hydrogel surface, the concentrations of pendant norbornene groups, PDL, and collagen IV were varied. First, hydrogels were generated with excess norbornene concentrations (0–20 mM) by varying the concentration of PEG-8N between 2.5 and 5 mM, while the concentration of PEG-8T was held constant at 2.5 mM. The hydrogels were functionalized with 0.5 mg mL−1 PDL-T and 1.0 mg mL−1 fluorescent collagen IV, washed overnight in PBS, imaged using epifluorescence microscopy, and analyzed with ImageJ. These fluorescence measurements were repeated for hydrogels functionalized with varied PDL-T concentrations (0–1.0 mg mL−1), while the norbornene and protein concentrations were held constant at 20 mM and 1.0 mg mL−1, respectively. Finally, the protein concentration was varied (0–1.0 mg mL−1), while the norbornene and protein concentrations were maintained at 20 mM and 0.5 mg mL−1. Measurements were performed using three hydrogels per condition.
Experiment
Establishment and maintenance of IEOs
IEOs were established from human jejunal epithelium and cultured as previously described.25,26 Small intestinal biopsies were obtained from adults undergoing routine endoscopy or from surgical specimens following a human subjects protocol approved by the Institutional Review Board at Baylor College of Medicine. Established cultures were grown as multilobular forms termed 3D IEOs in growth factor-reduced, phenol-free Matrigel (Corning). Three different media formulations were used to establish, maintain, and differentiate the organoids. The IEOs were washed and passaged in complete medium without growth factors (CMGF−). Complete medium with growth factors (CMGF+) maintained the IEOs in a predominantly undifferentiated, stem cell-rich state. Differentiation medium (lacking Wnt) allowed the IEOs over the course of 5 days to differentiate into the major cell types found in the intestinal epithelium, including enterocytes, goblet cells, Paneth cells, and enteroendocrine cells. All IEOs were used before passage 20.
Establishment of monolayer IEO cultures
IEO cultures were digested to single cells and seeded atop of the biofunctionalized hydrogels using a previously described method.17,27 Briefly, the IEOs were released from Matrigel by washing each well with 500 μL of cold PBS with 0.5 mM EDTA, pH 7.4, followed by centrifugation (200 rcf for 5 min at 4°C), and then incubation with 0.05% w/v Trypsin-0.48 mM EDTA (Gibco) for 4 min at 37°C. One milliliter of trypsin was used for every eight wells. The trypsin was deactivated with basal media containing 10% serum. The cells were strained (40 μm), and the flow-through was collected and centrifuged at 400 rcf for 5 min at room temperature. Finally, the cell pellet was resuspended in CMGF+ media with 10 μM Y-27632 (EMD Millipore). The cell suspension was pipetted into the cloning cylinder wells around the hydrogels and left to settle overnight on the hydrogel surface at a final density of 2 × 106 cells cm−2. Media was changed to differentiation media the following day and replaced every 48 h afterward. The IEOs were differentiated for a total of 5 days and then used for downstream experiments.
Comparison of IEO attachment on biofunctionalized hydrogels
We compared IEO attachment on the PDL-protein adsorbed (PDL-PA) functionalized PEG hydrogels to hydrogels functionalized with cell-adhesive peptides or covalently modified collagen IV and Matrigel. PEG hydrogels with excess norbornene molecules were synthesized as described previously and then functionalized through covalent linkage of thiolated cell-adhesive peptides or collagen IV.
To add cell-adhesive peptides to the hydrogel surface, a 15 mM stock solution of the cysteine-containing peptide in dimethyl formamide was mixed 1:2 with photoinitiation solution for a final peptide concentration of 5 mM. Ten microliters of this solution was pipetted onto the top of the hydrogels, which were then exposed to white light for 3 min to complete the thiol-ene reaction. The hydrogels were then washed 2 × with PBS to remove unreacted peptide.
To add collagen IV to the hydrogel surface, a 2 mg mL−1 solution of collagen IV (from human placenta, Sigma Aldrich) in PBS was thiolated by reaction with 2-iminothiolane (Thermo Scientific) as per the manufacturer's instructions. Unconjugated 2-iminothiolane was then removed using a PD-10 desalting column with G-25 Sephadex resin (GE Healthcare). The thiolated collagen IV was mixed 1:2 with photoinitiation solution, and 100 μL of this mixture was added to the hydrogels and exposed to white light for 3 min. The gels were washed twice with PBS to remove unbound protein.
IEOs were seeded atop these covalently-functionalized hydrogels, as well as PDL-PA hydrogels prepared as previously described, and differentiated for 5 days. The hydrogels were washed with PBS and imaged using an inverted microscope. The cells were then lysed by a freeze–thaw cycle with 100 μL of ddH2O, and the DNA content of the cell lysate was measured with the Quant-iT PicoGreen dsDNA Reagent Kit (Invitrogen) as per the manufacturer's instructions. Two experimental runs of three hydrogels each were collected per condition.
Immunofluorescent staining of IEOs
Immunofluorescent staining was used to evaluate the morphology of the IEO monolayers. The cells were fixed with 4% w/v paraformaldehyde (Fisher Scientific) in PBS, pH 6.9, permeabilized with 0.125% v/v Triton X-100 (Sigma Aldrich), and blocked with 5% v/v normal donkey serum (Abcam) in PBS with 0.05% v/v Tween-20 (Sigma Aldrich). Primary antibodies were applied overnight at 4°C, followed by secondary antibodies for 1 h at room temperature (Table 1). F-actin was visualized with Alexa Fluor-conjugated phalloidin (Invitrogen), and the nuclei were counterstained with DAPI (Invitrogen). Images were collected with confocal microscopy (Nikon A1-Rsi).
Table 1.
Antibody Specifications for Immunofluorescence Staining
| Protein marker | Dilution | Source |
|---|---|---|
| villin | 1:100 | mouse monoclonal ab3304, Abcam |
| E-cadherin | 1:500 | mouse monoclonal 610181, BD Biosciences |
| sucrase isomaltase | 1:100 | goat polyclonal SC-27603, Santa Cruz |
| mucin 2 | 1:50 | mouse monoclonal SC-7314, Santa Cruz |
| lysozyme | 1:1000 | rabbit polyclonal A0099, Dako |
| secondary | Alexa Fluor-conjugated donkey anti-mouse/rabbit, Invitrogen | |
Phenotypic characterization of IEOs with reverse transcription–quantitative polymerase chain reaction
Phenotypic gene expression was assessed by reverse transcription–quantitative polymerase chain reaction (RT-qPCR). After 5 days of differentiation on either 96 well plates or PEG hydrogels, IEO monolayers were lysed with TRIzol (Thermo Fisher) and homogenized using a TissueLyser II (Qiagen). The cells on hydrogels were flash-frozen before lysis. Total RNA was extracted using qScript XLT One-Step RT-qPCR ToughMix reagent with ROX reference dye (Quanta Biosciences). RT-qPCR was performed using TaqMan primer-probe mixes (Thermo Fisher) in a StepOnePlus system (Applied Biosystems) with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the reference gene. Fold changes were calculated by the 2−ΔΔCt method.
Human norovirus infection of IEO monolayers
IEO monolayers were infected with human norovirus (HuNoV) as previously described.27 Briefly, IEO monolayers were cultured in 96 well plates or on hydrogels, differentiated for 5 days, washed once with CMGF medium, and mock-inoculated or inoculated with 0.9 × 106 genome equivalents of HuNoV GII.P31/GII.4_Sydney_2012 diluted in CMGF- medium containing 500 μM glycochenodeoxycholic acid (GCDCA; Sigma, Cat# G0759) for 1 h at 37°C. The inoculum was removed, and the monolayers were washed twice with CMGF- medium to remove unbound virus. Differentiation medium containing 500 μM GCDCA was then added, and the cultures were incubated at 37°C for 24 h.
At 1 and 24 h postinfection (hpi), 300 μL of TRIzol (Amresco) was added to each mock- and HuNoV-inoculated IEO well, and RNA was extracted as described earlier. To account for virus from the initial infection that remained associated with the cells, the viral load at 24 hpi was normalized to the viral load at 1 hpi.
For RT-qPCR quantification of viral replication, the primer pair COG2R/QNIF2d and probe QNIFS were used for GII28,29 with qScript XLT One-Step RT-qPCR ToughMix reagent with ROX (Quanta Biosciences). Thermocycler reactions were performed in a 15 μL volume using 50°C (15 min) and 95°C (5 min) and then 40 cycles of 95°C (15 s) and 60°C (35 s). A standard curve based on a recombinant HuNoV RNA transcript was used to quantitate viral genome equivalents in RNA samples.30,31
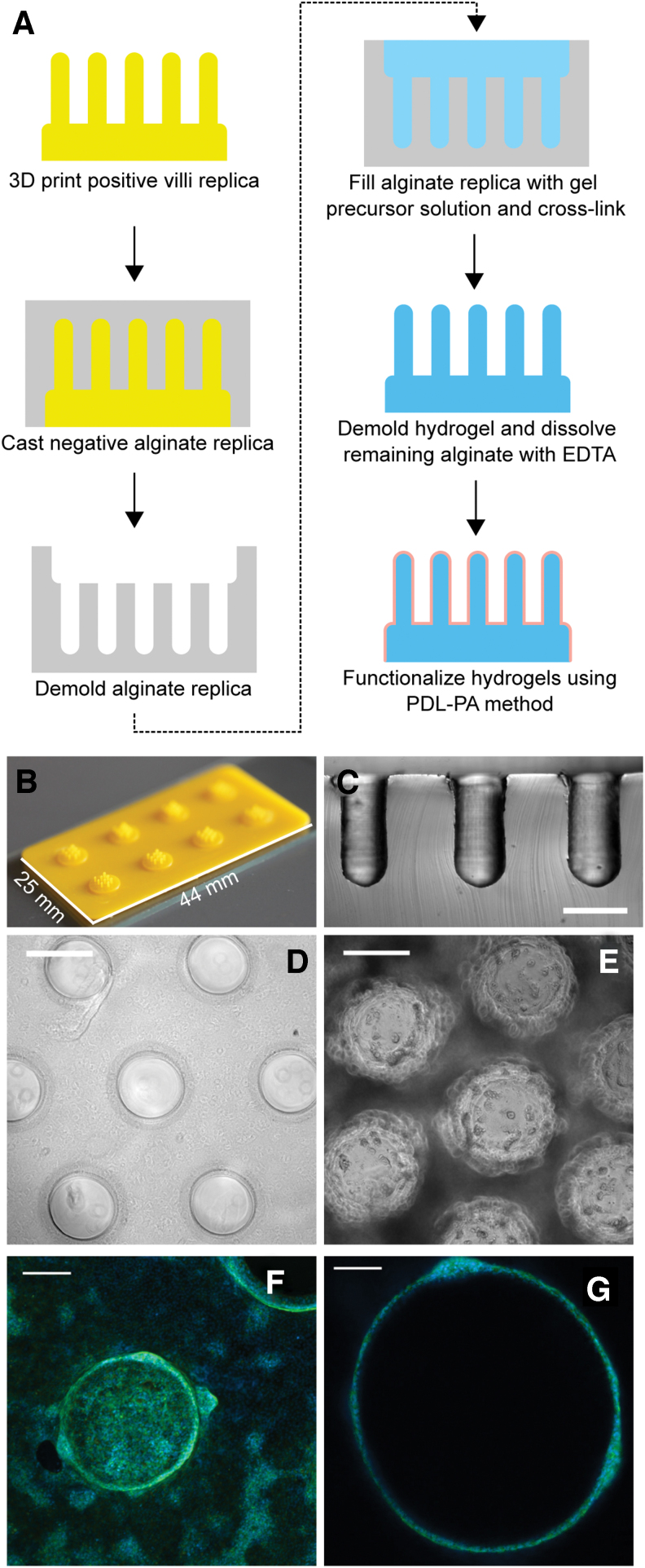
Application of coating method to a 3D surface
Hydrogels with 3D topographical features, resembling villi, were formed with a sacrificial molding process adapted from a previously reported method.32 A positive mold was formed using a photolithographic 3D printer (Kudo 3D) using S-Pro High-Resolution Resin (Spot-A Materials). These models were attached to glass slides using 734 flowable sealant (Dow Corning).
A gasket made from 3 mm thick high-temperature silicone rubber (McMaster-Carr) was placed on top of the glass slide to create a reservoir for a negative alginate replica. This reservoir was filled with autoclave-sterilized solution of 8% w/v sodium alginate (from brown algae, Sigma Aldrich) and covered by a track-etched, porous, polycarbonate Whatman membrane (GE HealthCare). An identical rubber gasket was placed on top of the membrane and filled with 80 mM calcium chloride (Fisher Scientific) solution.
After allowing the alginate to crosslink overnight at room temperature, the gasket holding the negative replica was removed. A gel precursor solution containing 5 mM PEG-8N and 2.5 mM PEG-8T in photoinitiation solution was added to the negative alginate mold. The mold was covered with a thiolated coverslip and crosslinked under white light for 2 min. The hydrogel was removed from the alginate mold and incubated overnight at room temperature in 80 mM EDTA solution to remove residual alginate.
To prepare hydrogels for IEO seeding, the EDTA solution was replaced with PBS for 4 h. The hydrogels were then functionalized with PDL-T and Matrigel, as described for the flat surface monolayers, with special care being taken to apply the protein solutions directly to the center of the villous hydrogels to ensure that the entire hydrogel was modified. IEOs were seeded on the surface of the villous hydrogel in the same manner as the flat hydrogels. After seeding, the cells were maintained in CMGF+ media for 6 days before switching to differentiation media for 5 days.
Statistical methods
For functionalization efficiency and cell attachment experiments, a one-way analysis of variance (ANOVA) was performed using R (The R Foundation), followed by post hoc Tukey's HSD. Significance was defined as p < 0.05.
For the IEO phenotype assessments, the two experimental groups (hydrogel vs. tissue culture plastic 96-well plate) were compared using the nonparametric Mann–Whitney U-test with the Holm-Sidak method for multiple comparisons. Three experiments were performed with at least six technical replicates per run. For norovirus infections, Welch's t-test was used to compare the two groups, which consisted of two runs of at least three technical replicates. All data are reported as mean ± standard deviation.
Results
Noncovalent functionalization of PEG hydrogels generated a thin uniform layer of protein over the hydrogel surface
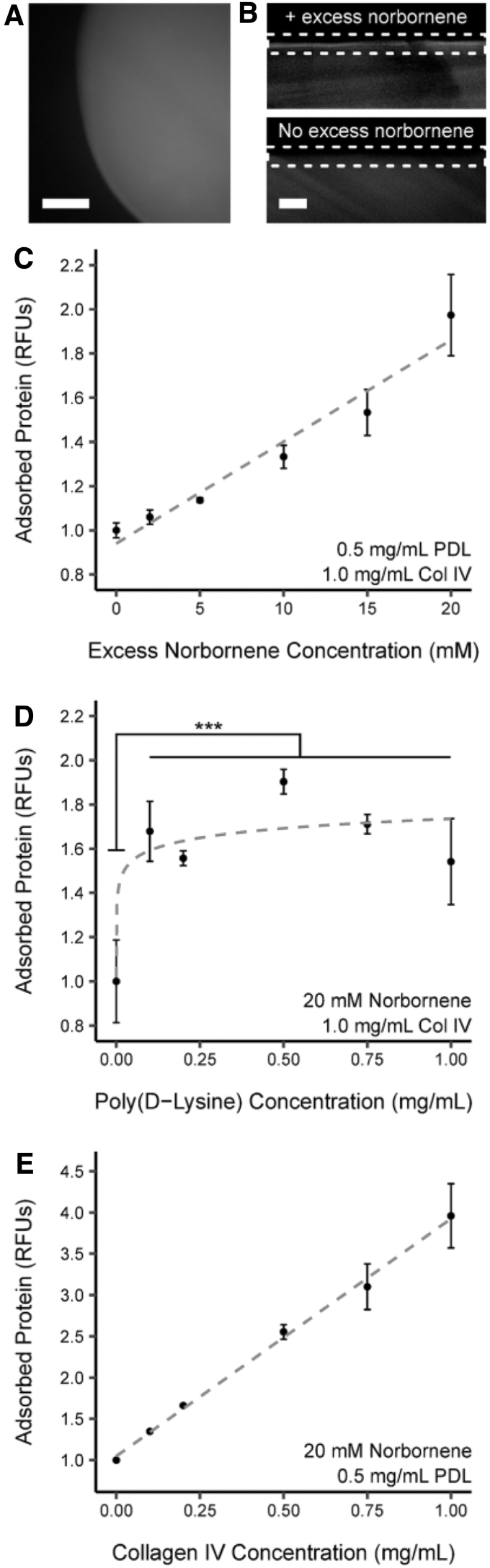
To understand how the PDL-PA functionalization approach distributed ECM proteins atop the hydrogel, we evaluated the attachment pattern of collagen IV labeled with tetramethyl-rhodamine (TMR-collagen IV). A representative top-view (Fig. 2A) of a PDL-PA hydrogel revealed even TMR-collagen IV distribution over the surface. Cross-sectional views (Fig. 2B) indicated that the vast majority of the immobilized ECM protein remained on the gel periphery. The cross-sectional views also demonstrated that enrichment in TMR-collagen IV occurred only when excess norbornene was present on the surface, confirming that in the absence of a target for PDL-T attachment, collagen IV did not adsorb to the PEG.
FIG. 2.
Characterization of the dynamics of PDL-mediated biofunctionalization. Representative top-view (A) and cross-sectional views (B) of PDL-BF hydrogel functionalized with fluorescent collagen IV. Surface enrichment of fluorescence due to immobilized collagen IV is only present in hydrogels that contain excess norbornene moieties. Dashed line boxes indicate the surface region of the hydrogels in (B). (C) Adsorbed collagen IV varies linearly (r2 = 0.909) with the concentration of excess norbornene present in the PEG hydrogel. (D) The relationship between adsorbed collagen IV and PDL concentration reveals a threshold effect: collagen IV levels increase significantly between 0 and 0.1 mg mL−1 (***p < 0.005) but are not significantly different thereafter. (E) The amount of adsorbed collagen IV is positively and linearly correlated with the concentration of collagen IV applied to the hydrogel (r2 = 0.996).
Hydrogel functionalization depended on the amount of excess norbornene in the hydrogel and the concentration of basement membrane proteins applied to the surface
We investigated how the amount of deposited protein was affected by the concentrations of excess norbornene moieties, the thiolated PDL, and the ECM proteins adsorbed onto the PDL layer. Increasing the concentration of excess norbornene from 0 to 20 mM (Fig. 2C) linearly increased the amount of immobilized collagen IV (r2 = 0.909), ultimately doubling the fluorescence intensity. Increasing the concentration of PDL-T (Fig. 2D) increased the immobilized TMR-collagen IV, but only between 0 and 0.1 mg mL−1 PDL-T, with the overall increase in intensity varying between 1.5-fold and 1.9-fold for the nonzero concentrations of PDL-T. Finally, increasing the concentration of TMR-collagen IV from 0 to 1.0 mg mL−1 increased the amount of adsorbed protein linearly (r2 = 0.996), with a roughly four-fold increase in fluorescence intensity (Fig. 2E). Overall, these results indicate that the amount of protein immobilized on the hydrogel surface can be tuned by varying the excess norbornene and protein concentrations, but not the PDL-T concentration.
PDL-PA functionalized hydrogels supported IEO monolayer formation better than covalent biofunctionalized approaches
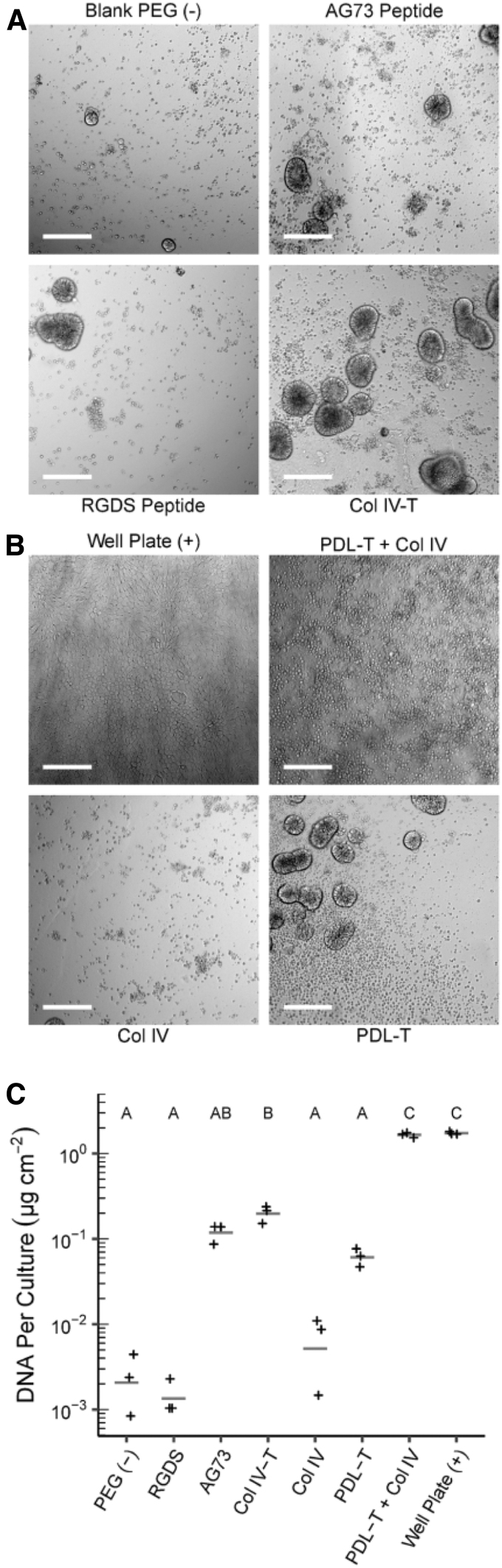
We compared the ability of our two-step PDL-PA biofunctionalization approach to form organoid monolayers to more traditional approaches using covalent attachment of cell-adhesive peptides and full-length ECM proteins. Because intestinal epithelial cells normally adhere to basement membrane, we chose collagen IV and Matrigel as our full-length ECM proteins and a library of laminin-derived sequences33 as our cell-adhesive peptides. A representative sample of these approaches is shown in Figure 3A, with the full list in Table 2.
FIG. 3.
Biofunctionalization of PEG hydrogels and seeding with IEOs. (A) Representative brightfield images of IEOs seeded on covalently biofunctionalized hydrogels; scale = 200 μm. IEOs on CBF hydrogels form scattered three-dimensional cellular aggregates rather than confluent monolayers. (B) Representative brightfield images of IEOs seeded on PDL-PA hydrogels; scale = 200 μm. Neither PDL-T nor unmodified collagen IV alone formed IEO monolayers; however, their combination formed IEO monolayers similar to those found on tissue culture plastic 96-well plates (positive control). (C) Double-stranded DNA content from IEOs seeded on both C-BF and PDL-BF hydrogels shows that only PDL-BF hydrogels contain an equivalent number of cells to tissue culture plastic controls. The letters above each group indicate statistical significance (p < 0.05). Groups that do not share a letter are statistically different from one another, while groups that share a letter are not statistically different.
Table 2.
Covalent Hydrogel Functionalization Approaches Tested and Their Outcomes After Seeding with Intestinal Epithelial Organoids
| Covalent hydrogel modification with peptides |
|
||
|---|---|---|---|
| Hydrogel | Peptide sequence | Outcome | |
| PEGDA | C-RKRLQVQLSIRT (C-AG73) | ** | |
| PEGDA | C-RGDS | ||
| PEGDA | C-RGDS + C-AG73 | * | |
| PEGDA | C-YIGSR | ** | |
| PEGDA | C-IKVAV | * | |
| PEGDA | C-RYVVLPR | − | |
| PEGDA | C-SIYITRF | * | |
| PEGDA | C-IAFQRN | * | |
| PEGDA | C-GEFYF | − | |
| Covalent hydrogel modification with ECM proteins | |||
| Hydrogel | Linking Chemistry | ECM Molecule* | Outcome |
| PEGDA | Sulfo-SANPAH | Collagen IV, Matrigel | ** |
| PEGDA | Acrylic acid-succinimidyl ester | Collagen IV, Matrigel | *** |
| PEGDA | 5-(acryloylamino)hexanoic acid-succinimidyl ester | Collagen IV, Matrigel | *** |
| PEG-norbornene | 2-iminothiolane | Collagen IV, Matrigel | *** |
| Controls | Outcome | ||
| Unmodified PEG hydrogel | − | ||
| Collagen IV-treated 96-well plate | ++++ | ||
| Outcome Key | |||
| − | Scattered dead cells, no cell aggregates | ||
| * | Sparse three-dimensional aggregates, no monolayers | ||
| ** | Scattered three-dimensional aggregates, no monolayers | ||
| *** | Dense three-dimensional aggregates, sparse areas with monolayer | ||
| ++++ | Confluent monolayer over entire area | ||
ECM molecules listed were each tested independently.
PEG hydrogels were synthesized to contain excess, and thus unreacted, norbornene groups for subsequent surface biofunctionalization through reaction with thiolated peptides and proteins. Nonfunctionalized PEG hydrogels and collagen IV-treated polystyrene well plates served as negative and positive controls, respectively. None of the peptides supported the formation of confluent epithelial monolayers. The most common result was a sparse distribution of small spherical aggregates that were firmly attached to the hydrogel, as exemplified by the AG73 and RGDS-functionalized hydrogels (Fig. 3A). On hydrogels with covalently attached collagen IV (Collagen IV-T), the IEOs still predominantly formed 3D aggregates, but they were more densely distributed on the hydrogel surface. IEO monolayers did form in small isolated regions of the hydrogel, but not in a confluent monolayer as seen in 96-well plates. In comparison, IEOs seeded atop PDL-PA functionalized hydrogels (Fig. 3B) formed confluent monolayers that resembled those formed on tissue culture plastic 96-well plates. Although the IEOs formed a small number of 3D aggregates on the PDL-T functionalized hydrogels (without the collagen IV), it was noted that neither individual component of the two-step PDL-PA method (PDL-T or collagen IV) facilitated monolayer formation on its own.
These qualitative observations were confirmed by quantifying the double-stranded DNA extracted from IEOs attached to the surface of the hydrogel (Fig. 3C). Many functionalization approaches (RGDS and AG73 peptide, collagen IV, and PDL-T) failed to adhere to more IEOs than negative controls. Collagen IV-T hydrogels were only able to attach roughly one-tenth of the IEOs found in the positive control. In fact, only the PDL-PA functionalized hydrogels achieved IEO adhesion comparable to that of tissue culture plastic (96-well plates), supporting the conclusion that the PDL-PA treatment facilitated the formation of confluent IEO monolayers on PEG hydrogels.
The stiffness of the hydrogel formulation used for these and all other experiments, as characterized by compression testing, was 23.7 ± 3.3 kPa.
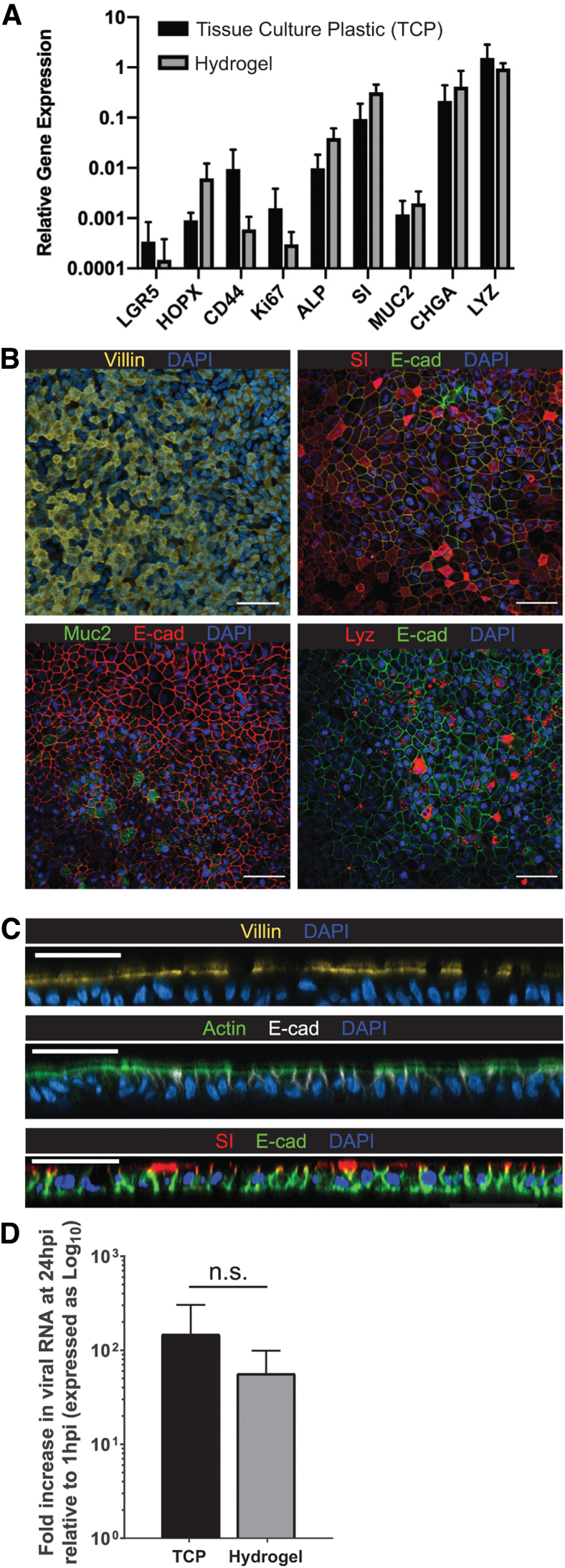
IEO monolayers differentiate into distinct epithelial cell types and retain appropriate apicobasal polarization
Next, we evaluated whether the IEOs retained their ability to transition from a stem-like state into the multiple cell types of the differentiated intestinal epithelium. Growth factor removal from the medium resulted in differentiation of the organoids grown on hydrogels, as well as on tissue culture plastic (96-well plates). As shown in Figure 4A, IEOs grown in the two conditions demonstrated no significant differences in gene expression of markers of enterocytes (SI and ALP), goblet cells (MUC2), enteroendocrine cells (CHGA), Paneth cells (LYZ), or intestinal epithelial stem cells (HOPX, LRG5, CD44, KI67). At the protein level, these markers of differentiated epithelial cells (SI, MUC2, and LYZ), apical microvilli (VIL1, ACT), and basolateral adherens junctions (CHD1) retained their expected subcellular distribution patterns when viewed along the XY plane (Fig. 4B) and along the Z-axis (Fig. 4C). Together, these results demonstrate that IEOs grown on PDL-PA hydrogels retained their diverse cell phenotypes and robust physiological functions.
FIG. 4.
IEO monolayers of PDL-PA hydrogels display appropriate cellular differentiation and polarization and are infectable with norovirus. (A) Analysis of the cellular composition of the IEOs by qRT-PCR reveals multilineage differentiation with no significant differences between cells grown on hydrogels and cells grown on TCP in 96-well plates (p > 0.05). Expression levels of each gene are shown relative to GAPDH. (B) Immunofluorescent staining for markers of differentiated intestinal epithelial cells demonstrates that IEOs cultured on hydrogels contain all the major cell types found in native intestinal epithelium. (C) Cross-sectional views of these monolayers reveal appropriate apicobasal polarization with prominent microvilli (actin and villin) and a robust brush border (sucrase isomaltase) after 5 days of differentiation, scale = 50 μm, vertical slice depth = 0.65 μm. (D) Quantification of noroviral replication following a 24 h infection demonstrates that IEOs grown on hydrogel scaffolds are capable of supporting infection equivalent to IEOs grown on TCP in 96-well plates. TCP, tissue culture plastic. Color images are available online.
IEO monolayers on hydrogels retain their infectivity by HuNoV
To establish that IEOs cultured on the PDL-PA hydrogels can model pathological processes, we inoculated IEOs with HuNoV, GII.4 HuNoV, and then measured viral replication at 24 hpi (Fig. 4D). PDL-PA hydrogels successfully supported the replication of norovirus with no significant difference between replication on PDL-PA hydrogels (70 ± 43-fold increase) and on the established tissue culture plastic 96-well plate model (139 ± 86-fold increase).
Continuous IEO monolayers form on hydrogels with villous topographical patterns
As a proof-of-concept demonstration that the coating method could be applied to generate an IEO monolayer atop a 3D surface, we generated hydrogels with intestinal villous-like protrusions (height 100 μm, width 500 μm) using a sacrificial molding process. The pattern from a 3D printed replica was transferred to the hydrogel by way of a negative mold made of alginate (Fig. 5A–C). We functionalized the villous hydrogels with PDL and adsorbed collagen IV and then seeded them with IEOs. The IEOs initially formed monolayers on the base of the hydrogel and tips of the villi (i.e., the two horizontal surfaces of the gel).
FIG. 5.
Topographical hydrogel synthesis and seeding with IEOs. (A) Schematic for fabrication and PDL-PA modification of high-aspect ratio, topographically patterned hydrogels. (B, C) Examples of positive (B) and negative (C) replicas generated in the molding process, scale = 500 μm. (D, E) Brightfield images of the villous hydrogel before (D) and after (E) seeding with intestinal epithelial organoids; scale = 500 μm. (F, G) Confocal images of villous hydrogels seeded with organoids. Cells are labeled with DAPI (blue) and phalloidin (green). (F) Maximum intensity projection; scale = 200 μm. (G) Representative cross-sectional view through an individual villus; scale = 100 μm. Color images are available online.
Over the course of 6 days, the monolayers proliferated and migrated to cover the vertical sides of the villi, eventually forming a contiguous layer covering the majority of the hydrogel surface. The surface of the gel remained covered throughout the 5-day differentiation period. The coverage was confirmed with both brightfield microscopy (Fig. 5D, E) and confocal microscopy (Fig. 5F, G), indicating that the noncovalent functionalization approach could be adapted to allow cell attachment and migration over a complex three-dimensional surface with high aspect ratio features.
Discussion
Stem cell-derived organoid models show promise as excellent in vitro models of developmental and pathological processes, as well as components of engineered tissues. However, they are predominantly cultured as 3D aggregates within animal-derived matrices, which fail to recreate their native tissue-specific ECM cues. Furthermore, the apical-in orientation of these organoids complicates their experimental utility. Thus, we developed a method to biofunctionalize the surface of PEG hydrogel scaffolds, allowing a model organoid system to be grown in monolayers on a 2D or 3D substrate mimicking aspects of its native microenvironment.
Our two-step method to modify PEG hydrogels with basement membrane proteins adsorbed to a covalently immobilized layer of PDL enabled monolayer formation by our model organoid system, IEOs. In comparison, none of the more traditional hydrogel biofunctionalization methods successfully accomplished this task, possibly due to the low affinity displayed by the cell-adhesive peptides toward nonintegrin cellular adhesion receptors, such as syndecans. Syndecan engagement amplifies downstream integrin signaling34,35 and likely contributes to polarization and formation of cell–cell junctions in epithelial cells.35,36 Although one of the peptides tested, AG73, is reported to interact with syndecans37,38 and has been used together with RGDS peptide to mimic aspects of basement membrane,39 we found AG73 to be no more successful in forming 2D IEO monolayers than the other integrin-engaging peptides, either in isolation or in combination with RGDS.
Despite collagen IV and Matrigel containing ample binding sites for both integrins and syndecans, successful formation of IEO monolayers required immobilization of these proteins on the hydrogel without any chemical modifications of their amino acids. We suspect that these modifications may have involved structurally important side chains of the protein and thus affected how integrins and syndecans could interact with their binding sites. Indeed, conjugation can adversely affect the activity of the modified proteins,40 particularly for basement membrane proteins.41 Nonetheless, direct covalent attachment of basement proteins to PEG hydrogels has successfully mediated attachment of other cell types despite the poor bioactivity of the proteins.42,43 It may be that the adhesion requirements of IEOs and possibly other organoids are especially sensitive.
In addition to facilitating organoid attachment to the hydrogels, the noncovalent protein immobilization approach also promoted polarization and differentiation of the organoids, resulting in a monolayer of IEOs that closely resembled those found in vivo. Furthermore, the model supported the replication of norovirus, an intestinal pathogen that is responsible for severe morbidity and mortality in both the developing and the developed world.44
Given that ECM cues can affect developmental and regenerative processes45,46 and may modulate innate immunity,47 our tunable, organoid-friendly approach for preparing hydrogels will allow for investigation of the roles of microenvironmental factors in essential developmental and pathological processes. In addition, coculture models consisting of stromal cells in the interior of PEG hydrogels and epithelial or endothelial cells on the hydrogel surface48,49 and/or in the interior50 have been used to recapitulate paracrine signaling events, and the incorporation of organoid technology will only improve their ability to model important organotypic processes in vitro.
The amount of ECM protein deposited onto the hydrogel surface depended primarily on the concentrations of unreacted norbornene in the hydrogel and of protein in the solution used to functionalize the hydrogel. Although thiolated-PDL was required for protein adsorption, its concentration was not important, likely because even low concentrations were sufficient to saturate the norbornene functionalities on the hydrogel surface. Although hydrogels with more than 20 mM of unreacted norbornene were fragile and sample dilution during dialysis restricted testing higher fluorescent collagen concentrations, it is likely that protein deposition could be further improved by increasing either of these two factors. However, we observed excellent cell attachment at intermediate concentrations of these reagents, suggesting that it is not necessary to drive protein adsorption to its maximum level.
Importantly, we were able to modify topographically-patterned hydrogels with unmodified ECM proteins just as effectively as the flat hydrogels, allowing the IEOs to completely cover their surface. However, the application of the coating method to this 3D surface was challenging. The array of tall, thin tightly packed villi could tear during demolding, and its tendency to retain liquid slowed the repeated addition and removal of the various protein solutions required to functionalize the 3D surface successfully. Other biologically relevant topographies and architectures, such as tubular, papillary, and acinar structures found in other organs, present many of these same challenges.
Therefore, the successful application of this two-step hydrogel coating method to a simple villous topography serves as proof-of-concept that this approach could be useful for organoids derived from other tissues in the future. Moreover, this work complements previous studies in which monolayers of IEOs were seeded atop villous- or crypt-shaped topographical surfaces and crosslinked collagen I hydrogels.20,21,32,51 Overall, we believe that this hydrogel scaffold that can mimic the stiffness, ECM cues, and high aspect ratio topography of the intestinal environment adds to the range of in vitro strategies to investigate the relationship between intestinal cellular organization and anatomic structure.
Conclusion
In summary, we developed a protocol for depositing unmodified basement membrane proteins onto the surface of PEG hydrogels to support culture of 2D monolayers of organoids. As a proof-of-concept, we demonstrated that IEO monolayers retain many physiological characteristics of the native intestine, including multilineage differentiation, apical-basal polarization, and the ability to sustain infections with HuNoV. This protocol can be applied to both flat and more complex 3D hydrogel surfaces. We believe this technology will be widely applicable to organoids from many tissues and will improve our efforts to create synthetic tissues for improved in vitro experimental models and regenerative medicine applications.
Acknowledgments
The authors thank Dr. Jennifer P. Connell for her critical reading of the article, and Drs. Noah Shroyer, Anthony Maresso, Joe Petrosino, Anubama Rajan, and Tatiana Fofanova, as well as Sarah Hewes, for their insightful and supportive feedback and comments.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by grants from the National Institute of Allergy and Infectious Disease (U19AI11649) and the National Institute of Diabetes and Digestive and Kidney Diseases (F30DK108541).
References
- 1. Schutgens F., and Clevers H.. Human organoids: tools for understanding biology and treating diseases. Annu Rev Pathol Mech Dis 15, 211, 2020 [DOI] [PubMed] [Google Scholar]
- 2. Qian X., Nguyen H.N., Song M.M., et al. . Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dye B.R., Hill D.R., Ferguson M.A., et al. . In vitro generation of human pluripotent stem cell derived lung organoids. Elife 4, 1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takasato M., Er P.X., Chiu H.S., et al. . Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564, 2015 [DOI] [PubMed] [Google Scholar]
- 5. Sato T., Vries R.G.J., Snippert H.J., et al. . Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Huch M., Dorrell C., Boj S.F., et al. . In vitro expansion of single Lgr5 + liver stem cells induced by Wnt-driven regeneration. Nature 494, 247, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dutta D., Heo I., and Clevers H.. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol Med 23, 393, 2017 [DOI] [PubMed] [Google Scholar]
- 8. Nakamura T., and Sato T.. Advancing intestinal organoid technology toward regenerative medicine. CMGH Cell Mol Gastroenterol Hepatol 5, 51, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clevers H. Modeling development and disease with organoids. Cell 165, 1586, 2016 [DOI] [PubMed] [Google Scholar]
- 10. Marti-Figueroa C.R., and Ashton R.S.. The case for applying tissue engineering methodologies to instruct human organoid morphogenesis. Acta Biomater 54, 35, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith L.R., Cho S., and Discher D.E.. Stem cell differentiation is regulated by extracellular matrix mechanics. Physiology 33, 16, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tibbitt M.W., and Anseth K.S.. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng 103, 655, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gjorevski N., Sachs N., Manfrin A., et al. . Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560, 2016 [DOI] [PubMed] [Google Scholar]
- 14. Cruz-Acuña R., Quirós M., Farkas A.E., et al. . Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat Cell Biol 19, 1326, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fong E.L.S., Toh T.B., Lin X., et al. . Generation of matched patient-derived xenograft in vitro-in vivo models using 3D macroporous hydrogels for the study of liver cancer. Biomaterials 159, 229, 2018 [DOI] [PubMed] [Google Scholar]
- 16. DiMarco R.L., Su J., Yan K.S., Dewi R., Kuo C.J., and Heilshorn S.C.. Engineering of three-dimensional microenvironments to promote contractile behavior in primary intestinal organoids. Integr Biol 6, 127, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. VanDussen K.L., Marinshaw J.M., Shaikh N., et al. . Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 64, 911, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou J., Li C., Sachs N., et al. . Differentiated human airway organoids to assess infectivity of emerging influenza virus. Proc Natl Acad Sci U S A 115, 6822, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tong Z., Martyn K., Yang A., et al. . Towards a defined ECM and small molecule based monolayer culture system for the expansion of mouse and human intestinal stem cells. Biomaterials 154, 60, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Costello C.M., Hongpeng J., Shaffiey S., et al. . Synthetic small intestinal scaffolds for improved studies of intestinal differentiation. Biotechnol Bioeng 111, 1222, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y., Gunasekara D.B., Reed M.I., et al. . A microengineered collagen scaffold for generating a polarized crypt-villus architecture of human small intestinal epithelium. Biomaterials 128, 44, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 31, 4639, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Puperi D.S., O'Connell R.W., Punske Z.E., Wu Y., West J.L., and Grande-Allen K.J.. Hyaluronan hydrogels for a biomimetic spongiosa layer of tissue engineered heart valve scaffolds. Biomacromolecules 17, 1766, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mehta S.M., Jin T., Stanciulescu I., and Grande-Allen K.J.. Engineering biologically extensible hydrogels using photolithographic printing. Acta Biomater 75, 52, 2018 [DOI] [PubMed] [Google Scholar]
- 25. Saxena K., Blutt S.E., Ettayebi K., et al. . Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J Virol 90, 43, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zou W.Y., Blutt S.E., Crawford S.E., et al. . Human intestinal enteroids: new models to study gastrointestinal virus infections. Methods Mol Biol 1576, 257, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ettayebi K., Crawford S.E., Murakami K., et al. . Replication of human noroviruses in stem cell-derived human enteroids. Science 353, 1387, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loisy F., Atmar R.L., Guillon P., Le Cann P., Pommepuy M., and Le Guyader F.S.. Real-time RT-PCR for norovirus screening in shellfish. J Virol Methods 123, 1, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Kageyama T., Kojima S., Shinohara M., et al. . Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J Clin Microbiol 41, 1548, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Le Guyader F.S., Le Saux J.-C., Ambert-Balay K., et al. . Aichi virus, norovirus, astrovirus, enterovirus, and rotavirus involved in clinical cases from a French oyster-related gastroenteritis outbreak. J Clin Microbiol 46, 4011, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guix S., Asanaka M., Katayama K., et al. . Norwalk virus RNA is infectious in mammalian cells. J Virol 81, 12238, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sung J.H., Yu J., Luo D., Shuler M.L., and March J.C.. Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip 11, 389, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Suzuki N., Yokoyama F., and Nomizu M.. Functional sites in the laminin alpha chains. Connect Tissue Res 46, 142, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Morgan M.R., Humphries M.J., and Bass M.D.. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol 8, 957, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bass M.D., Roach K.A., Morgan M.R., et al. . Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J Cell Biol 177, 527, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ngok S.P., Lin W., and Anastasiadis P.Z.. Establishment of epithelial polarity – GEF who's minding the GAP? J Cell Sci 127, 3205, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mochizuki M., Philp D., Hozumi K., et al. . Angiogenic activitiy of syndecan-binding laminin peptide AG73 (RKRLQVQLSIRT). Arch Biochem Biophys 459, 249, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Hozumi K., Suzuki N., Nielsen P.K., Nomizu M., and Yamada Y.. Laminin alpha1 chain LG4 module promotes cell attachment through syndecans and cell spreading through integrin alpha2beta1. J Biol Chem 281, 32929, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Yamada Y., Hozumi K., Katagiri F., Kikkawa Y., and Nomizu M.. Laminin-111-derived peptide-hyaluronate hydrogels as a synthetic basement membrane. Biomaterials 34, 6539, 2013 [DOI] [PubMed] [Google Scholar]
- 40. Hermanson G.T. Bioconjugate Techniques. Third Ed. London: Academic Press, 2013 [Google Scholar]
- 41. Beningo K.A., Lo C.-M., and Wang Y.-L.. Flexible polyacrylamide substrata for the analysis of mechanical interactions at cell-substratum adhesions. Methods Cell Biol 69, 325, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Peyton S.R., Raub C.B., Keschrumrus V.P., and Putnam A.J.. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials 27, 4881, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Barney L.E., Dandley E.C., Jansen L.E., Reich N.G., Mercurio A.M., and Peyton S.R.. A cell–ECM screening method to predict breast cancer metastasis. Integr Biol 7, 198, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patel M.M., Widdowson M.A., Glass R.I., Akazawa K., Vinjé J., and Parashar U.D.. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis 14, 1224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gilbert-Honick J., Iyer S.R., Somers S.M., et al. . Engineering 3D skeletal muscle primed for neuromuscular regeneration following volumetric muscle loss. Biomaterials 255, 120154, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miller C.J., and Davidson L.A.. The interplay between cell signalling and mechanics in developmental processes. Nat Rev Genet 14, 733, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pageon S.V., Govendir M.A., Kempe D., and Biro M.. Mechanoimmunology: molecular-scale forces govern immune cell functions. Mol Biol Cell 29, 1919, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Puperi D.S., Balaoing L.R., O'Connell R.W., West J.L., and Grande-Allen K.J.. 3-Dimensional spatially organized PEG-based hydrogels for an aortic valve co-culture model. Biomaterials 67, 354, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cook C.D., Hill A.S., Guo M., et al. . Local remodeling of synthetic extracellular matrix microenvironments by co-cultured endometrial epithelial and stromal cells enables long-term dynamic physiological function. Integr Biol 9, 271, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Valdez J., Cook C.D., Ahrens C.C., et al. . On-demand dissolution of modular, synthetic extracellular matrix reveals local epithelial-stromal communication networks. Biomaterials 130, 90, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang Y., Kim R., Gunasekara D.B., et al. . Formation of human colonic crypt array by application of chemical gradients across a shaped epithelial monolayer. CMGH Cell Mol Gastroenterol Hepatol 5, 113, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]