Abstract
Neuroblastoma (NB) is the second most common solid cancer in childhood, accounting for 15% of cancer-related deaths in children. In high-risk NB patients, the majority suffers from metastasis. Despite intensive multimodal treatment, long-term survival remains <40%. The bone marrow (BM) is among the most common sites of distant metastasis in patients with high-risk NB. In this environment, small populations of tumor cells can persist after treatment (minimal residual disease) and induce relapse. Therapy resistance of these residual tumor cells in BM remains a major obstacle for the cure of NB. A detailed understanding of the microenvironment and its role in tumor progression is of utmost importance for improving the treatment efficiency of NB. In BM, mesenchymal stromal cells (MSCs) constitute an important part of the microenvironment, where they support hematopoiesis and modulate immune responses. Their role in tumor progression is not completely understood, especially for NB. Although MSCs have been found to promote epithelial–mesenchymal transition, tumor growth, and metastasis and to induce chemoresistance, some reports point toward a tumor-suppressive effect of MSCs. In this review, we aim to compile current knowledge about the role of MSCs in NB development and progression. We evaluate arguments that depict tumor-supportive versus -suppressive properties of MSCs in the context of NB and give an overview of factors involved in MSC-NB crosstalk. A focus lies on the BM as a metastatic niche, since that is the predominant site for NB metastasis and relapse. Finally, we will present opportunities and challenges for therapeutic targeting of MSCs in the BM microenvironment.
Keywords: neuroblastoma, mesenchymal stromal cells, metastasis, bone marrow, chemoresistance, targeted therapy
Introduction
Constituting 7%–10% of all childhood malignancies, neuroblastoma (NB) is the second most common solid childhood tumor [1,2]. The tumors arise from neuroepithelial cells that migrate from the neural crest to form the sympathetic nervous system in embryonic development [3]. This origin explains some of the most prominent features of the disease: both localization and genetic features are highly heterogeneous, with primary tumors located in various locations of the sympathetic nervous system, most frequently in the adrenal medulla and paraspinal ganglia. Furthermore, similar to sympathetic neurons, NB tumors secrete catecholamines [4,5].
At the time of diagnosis, about 50% of the patients present with disseminated disease [6]. With an incidence rate of >90% in high-risk patients, the bone marrow (BM) is the most frequent site of metastasis [7,8]. To tailor treatment according to the severity of disease, an International Neuroblastoma Risk Group (INRG) classification system has been established and updated throughout the years [9]. Today, patients are classified into very low-, low-, intermediate-, and high-risk groups. Key factors that classify patients into the high-risk group are dissemination status, age >18 months at diagnosis, MYCN amplification, rearrangements of the TERT locus, inactivating mutations in ATRX and chromosome 11q aberration [10–12].
Although nonhigh-risk groups have an excellent prognosis with survival rates of >90% without intensive treatment, the standard-of-care treatment strategy for high-risk patients is much more complex. It includes induction therapy, surgical resection of the primary tumor, high-dose myeloablative chemotherapy with autologous hematopoietic stem cell (HSC) transplantation, radiation therapy, and postconsolidation immunotherapy consisting of antidisialoganglioside (GD2)- and isotretinoin treatment [13]. Despite this intense treatment, >30% of high-risk patients experience relapse [1] and their 5-year overall survival rate remains <40% [14].
Relapse mainly emerges from those tumor cells that survive therapy and remain undetected [minimal residual disease (MRD)]. In the context of various cancer types, these residual cells have been described to adopt a nonproliferative and highly chemoresistant dormant state [15,16]. The cellular and molecular foundation of dormancy, however, as well as its role in NB metastasis are poorly understood. Interestingly, similar to the quiescence of HSCs, the BM might provide favorable conditions for the development of tumor cell dormancy [17].
The Microenvironment in the BM
The BM is the primary site of hematopoiesis and comprises a multitude of cell types, mainly of the hematopoietic and mesenchymal lineage. The hematopoietic stem and progenitor cells (HSPCs) found in these niches, giving rise to immune cells and osteoclasts, maintain a balance of self-renewal and differentiation, which is regulated primarily by signals from the stromal microenvironment [18]. The term “stroma” comprises all nonhematopoietic cells, ie., cells of the mesenchymal lineage, deriving from mesenchymal stromal cells (MSCs), endothelial cells, and nerve cells. Among the BM stromal cell types that are relevant within the tumor microenvironment (TME) are MSCs and their descendants (adipocytes and osteoblasts), fibroblasts and endothelial cells (recently reviewed by Shiozawa [19]). This review focuses on the role of MSCs within the TME.
In the past the acronym MSC has been used for “mesenchymal stem cells,” but is nowadays used in a wider context to include cells whose biologic characteristics do not meet the definition of stem cells [20]. In this review, we use the term MSC to describe multipotent mesenchymal stromal cells. The latter are characterized in vitro by the International Society for Cellular Therapy (ISCT) as cells that (i) express CD105, CD73, and CD90, and lack expression of CD45, CD34, CD14 or CD11b, CD79a, or CD19, and HLA-DR surface molecules, (ii) have the potential to differentiate into osteoblasts, adipocytes, and chondroblasts, and (iii) adhere to plastic in standard culture conditions [21].
In the human body they can be found in various organs and tissues, including the umbilical cord, adipose tissue, placenta, and dental pulp. In fact, MSCs have been described to be present in nearly all postnatal organs and vascularized tissues [22,23]. Within the BM, their main functions are hematopoietic support, immunomodulation, and bone remodeling, which they achieve through physical contact and secretion of soluble factors [24–27].
Important to note when interpreting data from MSC studies is that essential differences exist between primary MSCs directly derived from human BM (BM-MSCs) and (i) culture-expanded MSCs, (ii) MSCs from other human tissues, and (iii) MSCs from other species, for example mouse. (i) Cultured MSCs do not perfectly reflect the properties and physiological functions of MSCs in vivo as they are known to alter the expression of cell surface markers such as CD146, CD271, CD106, and CD44 (I. Timmerman, personal observation, [28–30]) and to impair their capacity for BM-homing [31], hematopoietic support [30], and multilineage differentiation [29]. (ii) MSCs from various human tissues differ from BM-MSCs in their expression of cell surface markers (Rojewski et al. [32] compiled a comprehensive summary of marker expression on MSCs from various tissues), and furthermore in their protein expression profile, and differentiation potency [33,34]. (iii) Characterization of MSCs in other species and translating findings to the human setting is difficult due to the heterogeneity of surface markers expressed in each species (comprehensively reviewed by Boxall and Jones [35]). Mouse models are especially frequently used for in vivo studies of MSCs in the BM niche. Various markers are shared by human and mouse MSCs (eg, CD105, CD73, CD51, platelet-derived growth factor receptor alpha and beta [PDGFRα,β/CD140a,b] [36]), whereas others are predominantly studied in mouse models (Nestin [37], neuron-glial antigen 2 [NG2] [38], Leptin receptor [LepR] [39]). Although the latter have also been shown to be expressed in human MSCs [28,40–42], the concrete function of these cells in the human BM, especially in the metastatic setting of NB, has not yet been addressed.
Overall, insight obtained from studies with mouse MSCs cannot necessarily translate to the human context and require further validation. An interesting approach for avoiding these interspecies differences and studying a human-like environment in a mouse model is the xenotransplantation of a “humanized bone-marrow-ossicle niche,” derived from BM-MSCs [43].
The experimental details and important findings of key studies investigating MSCs in the NB context are summarized in Table 1 to facilitate comprehensive understanding of the studies' content.
Table 1.
Overview of Important Findings and Experimental Procedures of Key Studies Investigating the Contribution of Mesenchymal Stromal Cells to Neuroblastoma
| Topic | Key findings | Component involved | Mesenchymal stromal cells | Tumor cells | Experimental system | Reference |
|---|---|---|---|---|---|---|
| Tumor-suppressive | MSCs reduced primary tumor growth and prolonged survival of mice, decreased proliferation, and increased apoptosis of tumor cells (ex vivo) | Caspase-3 | hBM-MSCs* from healthy donors | hNB cell line ACN | In vivo (mouse), ex vivo (FFPE tumor specimens) | Bianchi et al. [68] |
| Tumor-supportive | ||||||
| MSC homing to tumor site | i.p.-injected MSCs migrated to tumor (i.v.-injected MSCs did not) | — | hAT-MSC* from healthy donors | None (TH-MYCN transgenic mice) | In vivo (mouse), ex vivo (FFPE tumor specimens) | Kimura et al. [51] |
| MSCs given to responders expressed higher CXCR1, CCR1, (CXCR4) levels/MSCs migrated toward CCL5, CXCL12 and tumor cells | CXCR1, CCR1 (CCL5), CXCR4 (CXCL12) | hBM-MSCs* from NB patients | hNB cell line NB1691 | In vivo (clinical trial), in vitro | Melen et al. [52] | |
| General tumor-supportive effects | Increased tumor cell proliferation and survival in vitro, tumor growth in vivo | IL6, IL8, CCL2, CXCL12, JAK2/STAT3 MEK/ERK1,2 | Primary CAF-like MSCs and BM-MSCs from NB patients | hNB cell lines CHLA-255, SK-N-SH, SK-N-BE2, CHLA-90 | In vitro, ex vivo (FFPE tumor specimens), in vivo (mouse) | Borriello et al. [73] |
| Secretion of protumorigenic cytokines and chemokines from BM-MSCs | Exosomes, IL6, IL8, VEGF, CCL2, ERK1/2 | hBM-MSCs from NB patients | 9 hNB cell lines | In vitro | Nakata et al. [129] | |
| Increased proliferation of NB cells, tumor growth in vivo, increased IL6 in serum and BM of patients | IL6, STAT3/ERK | hBM stromal cells | 11 hNB cell lines | In vitro, ex vivo (patient serum samples), in vivo (mouse) | Ara et al. [135] | |
| Gal3BP induced IL6 secretion from BM stromal cells | Gal3BP, IL6, ERK1/2 | hBM stromal cells | hNB cell lines CHLA-255, SK-N-BE(2), NB19 | In vitro | Fukaya et al. [133] | |
| Transcriptional upregulation of IL-6 in BM-MSC, Gal3BP present in tumor cells and ECM of 96% of tumor specimen | Gal-3BP/Ras/MEK/ERK signaling, Gal3BP | hBM-MSCs | 9 hNB cell lines | In vitro, ex vivo (FFPE tumor specimens) | Silverman et al. [134] | |
| Stimulation of metastasis, BM invasion | BM-MSC secretome promoted invasiveness in 4 of 5 cell lines studied | CXCR4, MMP-9 | hBM-MSC-TERT | In total 20 hNB cell lines (5 for invasion assay) | In vitro | Shankar et al. [100] |
| MSC secretome increased migration and invasiveness of NB cells | CXCL12, CXCR4, CXCR7 | hBM-MSCs, hBM-MSC-TERT | hNB cell lines BE(2)-M17, BE(2)-C, IMR32, SK-N-LP, SH-SY5Y | In vitro | Ma et al. [109] | |
| MSC secretome enhanced migratory capacity in 2 of 3 cell lines | CXCR4, CXCL12 | hBM-MSCs* from healthy donors | hNB cell lines SH-SY5Y, GI-LI-N and Htla-230 | In vitro | Bianchi et al. [68] | |
| Chemoresistance/dormancy | Protection from etoposide-induced apoptosis | IL6, STAT3 | hBM-MSCs from healthy donors | hNB cell lines | In vitro, ex vivo (FFPE tumor specimens) | Ara et al. [180] |
| hMSCs and monocytes impair anti-NB activity of aNK/anti-GD2-immunotherapy | TGF-β1 | hBM-MSCs from NB patients | hNB cell lines CHLA-255, CHLA-136 | In vitro, in vivo (mouse) | Wu et al. [182] | |
| protection from etoposide-induced apoptosis | S1PR1, JAK-STAT3 signaling | hBM-MSCs from NB patients | hNB cell lines CHLA-171, CHLA-255 | In vitro, in vivo (mouse) | Lifshitz et al. [179] | |
| Altered bone homeostasis | BM-MSCs drove bone lesions through osteoclast activation | IL6 | hBM-MSCs* from healthy donors | hNB line CHLA-255, rat osteoclasts | In vitro | Sohara et al. [151] |
| Increased osteogenic differentiation of MSCs | BMP4, VEGFa | Primary murine BM-MSCs | hNB cell lines CHLA-255 and SK-N-BE | In vitro | HaDuong et al. [153] | |
| Increased number of MSCs, osteogenic differentiation of MSCs, presence of a MSC subtype | — | hBM-MSCs from NB patients | — | In vitro, ex vivo (BM biopsies) | Hochheuser et al. [113] | |
| NB cells decreased osteogenic differentiation capacity of MSCs | Dkk1 | hMSCs* from healthy pediatric donors | hNB cell lines SH-SY5Y, LAN1, CHP212, NB100 | In vitro | Granchi et al. [161] | |
Italic, cancer-derived.
Bold, MSC-derived.
aNK, activated human natural killer cells; BM, bone marrow; CAF, cancer-associated fibroblast; CCL5, CC chemokine ligand 5; CCR1, CC chemokine receptor 1; CXCR1, C-X-C motif chemokine receptor-1; Dkk1, Dickkopf-related protein-1; ECM, extracellular matrix; FFPE, formalin-fixed paraffin-embedded; Gal-3BP, Galectin-3 binding protein; hAT, human adipose tissue-derived; hBM, human bone marrow-derived; hBM-MSC-TERT, human MSCs immortalized by enforcing the expression of TERT in primary bone marrow MSCs; hNB, human neuroblastoma; i.p., intraperitoneally; i.v., intravenously; MMP-9, matrix metalloproteinase-9; MSC, mesenchymal stromal cell; MSC*, mesenchymal stem cells; NB, neuroblastoma; TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor.
Contribution of MSCs to NB Development and Progression
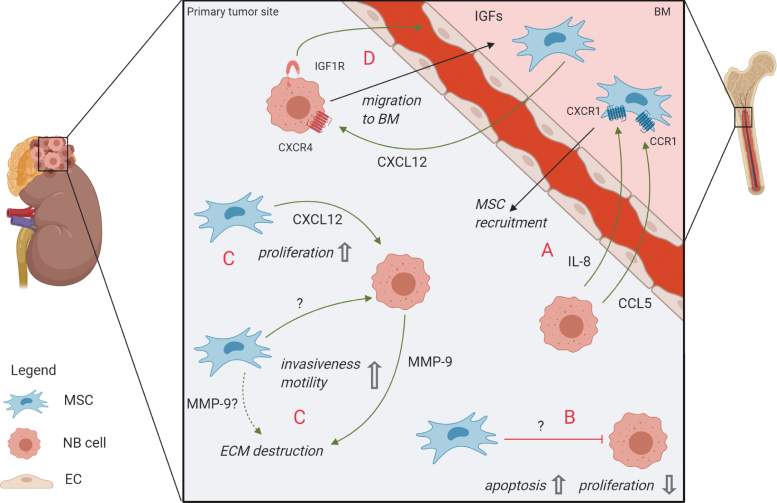
Various forms of interaction between NB cells and the TME at the primary tumor site have been described (Fig. 1). The inflammatory environment of tumors is known to recruit MSCs to the TME in many cancer types [44,45]. Numerous signaling molecules, including stromal derived factor-1 (SDF-1/CXCL12), transforming growth factor-β (TGF-β), interleukin-8 (IL-8), matrix metalloproteinase-1 (MMP-1), and monocyte chemoattractant protein 1 (MCP-1/CCL2) were shown to be involved in MSC recruitment to the primary tumor site [46–49]. A detailed overview of MSC migration to tumors and healthy organs, including chemotactic stimuli, is given by Cornelissen et al. [50].
FIG. 1.
Crosstalk between MSCs and NB cells at the primary tumor site and migration to/from the BM. (A) MSCs are attracted from the BM to the primary site (among others through CXCR1/IL-8 and CCR1/CCL5 signaling) [52]. (B) Unknown MSC-derived mediators can exert a tumor-suppressive effect [68]. (C) The CXCR4/CXCL12 axis plays a role in proliferation and survival of tumor cells and decreased apoptosis rates [74]. MMP-9 [99,100] might play a role in promoting EMT and metastasis: unknown signaling events from MSCs induce MMP-9 expression in NB cells [100], whereas MSCs potentially also secrete MMP-9 themselves (dashed line). (D) NB cells are attracted to the BM metastatic niche through the CXCR4/CXCL12 axis [100,109] and can dock to the BM endothelial cells (ECs) through IGF-1R, subsequently migrating toward IGF-1 in the BM stroma [115]. BM, bone marrow; CCR1/CCL5, CC chemokine receptor 1/CC chemokine ligand 5; CXCR1, C-X-C motif chemokine receptor-1; ECM, extracellular matrix; EMT, epithelial-to-mesenchymal transition; IGF-1, insulin-like growth factor 1; IL-8, interleukin-8; MMP-9, matrix metalloproteinase-9; MSC, mesenchymal stromal cell; NB, neuroblastoma. Color images are available online.
In NB, adipose tissue-derived MSCs were demonstrated to successfully migrate to primary NB tumors in mice when injected intraperitoneally [51]. An in vitro evaluation of a clinical trial for oncolytic virotherapy with 12 patients revealed that receptor/ligand pairs C-X-C motif chemokine receptor-1 (CXCR1)/IL-8 and CC chemokine receptor 1/CC chemokine ligand 5 (CCR1/CCL5) were involved in successful migration of MSCs to the tumor [52] (Fig. 1A).
Once MSCs are part of the microenvironment, they directly or indirectly interact with tumor cells [53]. These interactions can either have phenotypic and functional effects on MSCs themselves, or induce signaling from MSCs to other cell types in the stroma through chemokines or extracellular vesicles (EVs) [54–56]. Both supportive and inhibitory effects on the tumor resulting from these interactions have been described, depending on the cancer type, localization of the tumor, investigation method (in vitro vs. in vivo), and number and origin of MSCs [57].
MSCs exhibiting tumor-suppressive effects
Early evidence of tumor-suppressive effects by the tumor stroma originates from studies from the 1990s and 2000s before a clear concept of MSCs had been developed: “(adherent) BM stromal cells” were described to inhibit the growth of leukemia [58], lung carcinoma [59], and colon carcinoma [60]. Later, MSCs have been demonstrated to inhibit glioma cell proliferation in vitro [61] and to have inhibitory effects on the in vivo growth and metastasis of Kaposi-sarcoma [62], breast cancer [63], and various hematological malignancies (reviewed extensively by Lee et al. [64]).
Some mechanistic insights into the tumor-suppressive effect of MSCs implicate a role of Wnt signaling [65]. Both activation of (noncanonical) Wnt signaling by MSC-derived Wnt5a as well as inhibition of (canonical) Wnt-signaling by MSC-derived Dickkopf-related protein-1 (Dkk1) have been shown to decrease proliferation rates in two leukemia cell lines [66,67]. Concrete mechanistic evidence for tumor-suppressive functions of MSCs in NB is sparse. One study revealed that intratumoral injection of MSCs into primary NB tumors in mice significantly reduced tumor growth and prolonged survival of tumor-bearing mice. These effects were mediated by decreased proliferation and higher apoptosis rates of tumor cells [68] (Fig. 1B). However, assessment of proliferation in an in vitro setting within the same study revealed that MSCs could not only inhibit but also promote proliferation of NB cells, depending on the cell line used. The effect of MSCs on NB tumors is, therefore, not clearly defined and is instead—in this context—dependent on the NB cell line used.
MSCs exhibiting tumor-supportive effects
In contrast to these tumor-suppressive effects of MSCs, multiple studies describe a tumor-supportive role of MSCs instead. Studies in breast cancer (in vitro and in vivo) [69], prostate cancer (PC; in vitro) [70], adenocarcinoma and Lewis lung carcinoma (in vitro and in vivo) [71] demonstrated a beneficial effect of MSCs on tumor growth, cell survival, drug resistance, and angiogenesis. According to studies on several tumor types, it is believed that upon arrival at the primary tumor site, BM-MSCs adapt a cancer-associated fibroblast (CAF)-like phenotype, while still retaining surface marker expression and differentiation potential that is characteristic for MSCs [49,72,73]. In NB, it was shown that these CAF-like MSCs as well as normal BM-MSCs enhance tumor cell proliferation and survival in vitro and stimulate tumor engraftment and growth in vivo through the JAK2/STAT3 and MEK/ERK1/2 pathways in NB cells [73]. The connection between MSCs and CAFs is described in more detail in Box 1.
Furthermore, the CXCL12/CXCR4 axis has been implicated in local tumor-supporting effects: experiments with NB cell lines and an orthotopic NB mouse model revealed a CXCL12-dependent beneficial effect of CXCR4 on tumor growth and -survival [74] (Fig. 1C). In the healthy BM setting, expression of CXCL12 in human and murine MSCs has been shown, for example, in studies by Kortesidis et al. [75] and Méndez-Ferrer et al. [76], who had characterized MSCs by expression of Stro1 and Nestin, respectively, as well as their clonogenicity and trilineage differentiation potential. An additional source of CXCL12 in the BM is likely to be constituted by MSC's progeny like osteoblasts and/or other stromal cells like endothelial and perivascular cells [25,77–79]. Interestingly, in a recent study our group has also detected CXCL12 expression in primary MSCs from metastatic BM samples of NB patients (I. Timmerman, C. Hochheuser, personal observation). Other prominent functions of CXCL12/CXCR4 signaling regarding metastasis are discussed below.
Box 1. MSCs and CAFs
MSCs were first associated with CAFs after BM-derived myofibroblasts were reported to accumulate in tumor stroma and to constitute up to 25% of stromal fibroblasts [80–83]. Subsequently, the question arose whether MSCs differentiate into CAFs or only share certain characteristics with CAFs. It is, therefore, important to define this term: CAFs are cells in the TME defined by (a subset of) the following characteristics: increased proliferation and migration, a “CAF gene expression signature,” activation of TGF-β-, mitogen-activated protein kinase (MAPK)- and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling, and expression of for example α-fibroblast activation protein (αFAP), fibroblast-specific protein-1 (FSP-1), and alpha-smooth muscle actin (α-SMA) [72,73,84–87]. A definition based on genomic landscape, distinct surface markers or cell of origin, however, is lacking.
Madar et al. [88] suggested to define CAF “as a ‘state’ rather than a cell type,” meaning that several different cell types, such as MSCs, fibroblasts, epithelial cells, and tumor cells that have undergone EMT can adapt CAF traits (ie, mesenchymal appearance and tumor-supportive effects). This perception is in line with the finding that (only) up to 20% of CAFs derive from MSCs, implying that the other 80% must derive from other sources [89]. CAF is, therefore, merely to be understood as a “label” that a cell gets once it becomes part of the TME and supports tumorigenesis.
Stimulation of Metastasis
MSCs do not only exert a local tumor-supportive effect at the primary tumor site, but also contribute to metastasis of tumor cells. Two major processes leading to metastasis are EMT, which allows tumor cells to detach from the primary tumor site, and subsequent metastatic migration to distant sites facilitated by adhesion molecules [90,91].
Epithelial-to-mesenchymal transition
During EMT, tumor cells undergo a change in cellular structure and expression of surface molecules until their morphological phenotype resembles that of mesenchymal rather than epithelial cells [91]. Interestingly, this event also happens during embryonic development of the sympathetic nervous system as neuroepithelial cells detach from the neural crest. Researchers, therefore, propose that in special cases of NB, a natural BM dissemination can originate from an early mutation event during the migration of neural crest cells [92].
Although a few factors involved in NB EMT have been discovered [93–95], it is poorly understood to what extent MSCs promote this process. TGF-β, for example, has been described to cause functional changes in NB cells that are characteristic for EMT: upon treatment with recombinant human TGF-β1, NB cells showed a lower expression of adhesion molecule and epithelial marker E-cadherin, a higher expression of fibroblast marker a-SMA, and were generally more motile [93]. MSCs from healthy adult BM were shown to express TGF-β1 [96]. Whether the same holds true for the metastatic pediatric BM environment remains to be elucidated.
Furthermore, matrix metalloproteinase-9 (MMP-9) contributes to EMT by remodeling the extracellular matrix (ECM) and thereby facilitates invasion [97]. In head and neck squamous cell carcinoma, tumor cells have been found to instruct BM-MSCs to secrete MMP-9 in a three-dimensional spheroid system [98]. In NB, however, MMP-9 has only been shown to be present in the tumor-surrounding stroma, consisting of fibroblasts and (peri-)vascular cells [99], but not specifically to be derived from MSCs. Interestingly, MSCs might nevertheless contribute to the MMP-9 pool in the TME by inducing its expression in NB cells, as shown by stimulation of NB cell lines with conditioned medium from cultured MSCs [100]. Interestingly, MMP-9 was also found to be upregulated in high-risk NB tumors [99,101], indicating that this enzyme might play an important role in the dissemination process in NB (Fig. 1C).
Moreover, the reprogramming of adrenergic to mesenchymal NB cells was found to be mediated by a Notch feedforward loop [102,103]. Although the factors inducing this Notch signaling in NB remain to be unraveled, in vitro studies on acute myeloid leukemia (AML) suggest an involvement of MSCs: MSCs from AML patients expressed higher levels of Notch ligands and -receptors than MSCs from healthy donors and induced Notch signaling in AML cells in a coculture system [104].
BM invasion
NB metastasizes to distinct secondary organs, preferentially the BM, which suggests that this invasion depends on interaction with resident cells and signaling factors. One prominent signaling axis involves CXCR4 and its ligand CXCL12: Early research showed that NB cells express CXCR4, which seems to play a critical role in metastasis to the BM [105,106] and that the level of CXCR4 expression is correlated with BM metastasis and poor clinical outcome [107]. Later, in vitro studies suggested that NB cells use the same CXCR4/CXCL12 axis for metastasis as HSPCs do for homing after stem cell transplantation [108] and that this process is supported by MSCs (Fig. 1D): Upon incubation with MSC-conditioned medium, NB cells showed increased migration and invasiveness, which was dependent on the CXCR4/CXCL12 axis [68,100,109].
Similarly, PC cells are also known to make use of the CXCR4/CXCL12 axis for BM metastasis [110,111]. Furthermore, circulating melanoma cells have been described to interact with perivascular MSCs through CXCR4/CXCL12 signaling and melanoma cell adhesion molecule (MCAM, CD146) in vivo, an interaction shown to be required for BM invasion [112]. Interestingly, recent study from our group with primary patient samples has determined CD146 to be one of the surface molecules that identifies an MSC subtype, which is specifically present in the NB metastatic BM and might have tumor-related functions [113].
Other studies proposed a role of CXCR5 and CXCR6 in migration of NB cells to the BM [114]. Invasion into the BM could furthermore be mediated by insulin-like growth factor 1 (IGF-1) receptors on NB cells and the high expression of IGF ligands in the bone, allowing NB cells to bind to BM-endothelial cells and migrate through the endothelium toward the IGF-1 pool in the BM environment [115] (Fig. 1D).
Premetastatic niche
Since NB dissemination has a clear affinity for certain organs, including the BM, the idea of a favorable premetastatic niche (PMN) in the BM microenvironment comes to mind. The PMN concept is based on the idea that circulating tumor cells require a supportive niche at the secondary organ to establish metastases [116]. Although Paget described his “seed and soil” hypothesis about an interaction between tumor cells and their future sites of metastasis already in 1889 [117], the principle of a PMN was only confirmed many years later upon the discovery that melanoma-conditioned medium causes Lewis lung carcinoma cells to metastasize into typical melanoma metastatic sites instead of the lung [118]. The tumor cell secretome and EVs have been proposed as the cause for this distant effect [116], with organotropism being determined by the characteristic secretion profile of individual tumors [119].
PMN formation has been extensively studied in common tumors such as breast cancer, PC, and melanoma, and typical metastasis sites include lymph nodes, liver, bone, and brain [116]. As the majority of NB patients already present with BM metastasis at diagnosis, determining the role of the PMN in NB is difficult and thus not well understood. Nevertheless, understanding the potential role of factors and EVs secreted by NB primary tumors could be of key importance to prevent further metastasis and relapse. Since MSCs represent an important interaction partner of NB cells at the primary tumor site, BM-MSCs could also play a role as a distant messenger preparing the BM niche for metastatic invasion.
MSCs at the BM Metastatic Niche of NB
As the primary site of some hematological malignancies and the main metastatic site of several solid tumors [120–122], the BM microenvironment is subject to intensive investigations in tumors such as multiple myeloma (MM), breast cancer, and PC. Yet, the interactions between NB cells and BM-MSCs are only starting to be investigated, with a few studies indicating a crosstalk of NB cells with BM-MSCs. Interestingly, our group recently demonstrated in primary NB patient samples that the number of MSCs is significantly increased in metastatic BM compared with NB-free BM, pointing toward a direct or indirect effect of NB cells on MSCs [113].
Crosstalk between tumor cells and their environment can occur in a direct manner through membrane protein interaction and integrin signaling or indirectly through cytokines, chemokines, growth factors, and EVs. Apart from their potential role in creating a PMN at the BM, tumor-derived EVs may also have tumor-supportive effects after the invasion of tumor cells into the BM [116].
A proteomic analysis of EVs derived from NB cell lines demonstrated the presence of proteins such as prominin-1, B7H3, basigin, and fibronectin on EVs, which are associated with cell survival and proliferation as well as chemoresistance, immune evasion, and ECM destruction [123–127]. Interestingly, the NB EV signature is suggested to be site- and stage-specific, as EVs secreted by BM-resident NB cells differ from those derived from primary and brain-metastasized NB cells [128], suggesting that they might fulfil distinct functions at their respective location.
EVs derived from NB cell lines have been demonstrated to affect BM-MSCs: they stimulated the secretion of tumor-supportive cytokines and chemokines from BM-MSCs in vitro, most notably IL-6, IL-8/CXCL8, vascular endothelial growth factor (VEGF), and CCL2/MCP-1 [129] (Fig. 2A). While IL-8 and VEGF are known stimulators of angiogenesis [130,131], CCL2/MCP-1 has been demonstrated to promote the recruitment of anti-inflammatory tumor-associated macrophages [132]. Interestingly, IL-6 is a component frequently implicated in tumor-supporting pathways. In NB, its effect is believed to be controlled by a positive feedback loop: NB-derived Galectin-3 binding protein (Gal-3BP) activated the Ras/MEK/ERK pathway in MSCs in vitro, which in turn produced IL-6 [133,134] (Fig. 2A). In a STAT3/ERK1,2-dependent manner, IL-6 promoted proliferation and survival of tumor cells, protected them from drug-induced apoptosis in vitro and stimulated tumor growth in vivo [135]. Of note, these studies [133,135] used “BM stromal cells” that have not been confirmed to be MSCs based on surface marker expression or differentiation capacity. Treatment of NB cell lines with the chemotherapeutic agent sorafenib corroborates the aforementioned findings, as it blocked the IL-6-induced STAT3 phosphorylation and downstream signaling, inducing apoptosis and cell growth arrest of NB cells [136].
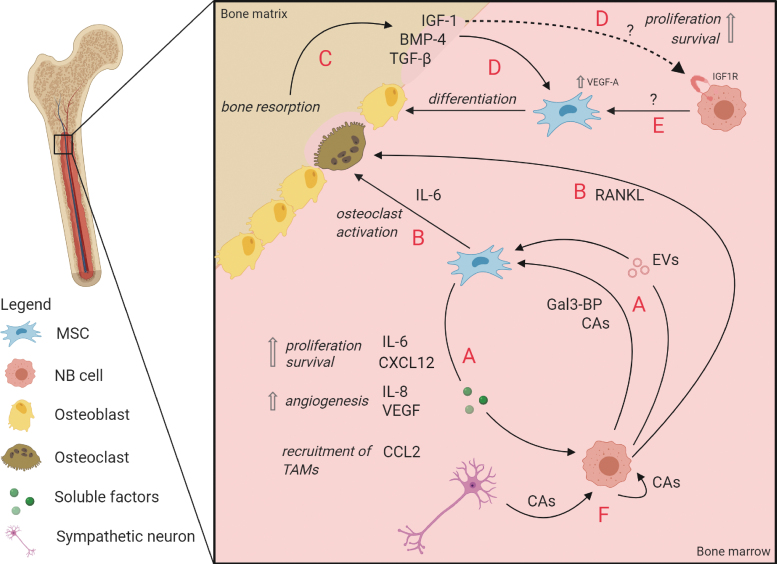
FIG. 2.
Interactions between MSCs and NB cells in the BM metastatic niche. (A) Several mediators, such as EVs [129], Gal-3BP [133,134], and potentially CAs [169,170] can influence MSCs to secrete tumor-supportive factors that increase NB cell proliferation and survival [109,135,151], promote angiogenesis [130,131] and recruit TAMs to the TME [132]. (B) MSCs, stimulated by Gal-3BP and other unknown NB-derived mediators, secrete IL-6, thereby increasing osteoclast differentiation and driving osteolysis [151]. NB cells secrete RANKL with a similar effect [150]. (C) Increased osteoclastic activity leads to additional bone resorption, releasing bone-derived growth factors (TGF-β, BMP-4, and IGF-1) into the marrow [152–154]. (D) BMP-4, IGF-1, and TGF-β increase osteoblastic differentiation of MSCs [152,153,155]. In addition, IGF-1 could potentially support NB cell survival and proliferation through interaction with IGF-1R (dashed line) [157]. (E) Unknown NB-derived factors drive differentiation of MSCs into osteoblasts through intrinsic VEGF-A signaling [153]. (F) It is hypothesized that CAs create a tumor-supportive environment [167]. NB cells might use this mechanism in both para- and autocrine ways to promote tumor progression. CAs, catecholamines; EVs, extracellular vesicles; Gal-3BP, Galectin-3 binding protein; RANKL, receptor activator of nuclear factor kappa-B ligand; TAMs, tumor-associated macrophages; TGF-β, transforming growth factor-β; TME, tumor microenvironment; VEGF, vascular endothelial growth factor. Color images are available online.
Similarly, IL-6/STAT3 signaling has been described in other tumors such as osteosarcoma [137], and targeting this axis has been proposed for ablating tumor-stroma crosstalk [138]. However, targeting IL-6 with receptor blocking antibodies alone seems inefficient, as several alternative pathways lead to STAT3 activation (an overview is given in Wendt et al. [139]).
In conclusion, secreted factors and the content of NB-derived EVs can stimulate the secretion of tumor-supportive factors from BM-MSCs and thereby contribute to communication with the TME to increase NB growth in the BM. Other components of NB-TME crosstalk and their molecular mechanisms remain to be elucidated before we can understand the complex interactions that sustain NB BM metastases.
Bone homeostasis in the metastatic BM niche
The bone is a dynamic tissue subjected to constant remodeling by osteoclasts and osteoblasts, which resorb bone matrix and form new bone material, respectively [140]. Their activity is tightly regulated, resulting in a well-balanced equilibrium of bone homeostasis [141]. However, when tumor cells proliferate in the BM, this homeostasis is disturbed and can lead to osteolytic or osteoblastic lesions. While breast cancer and MM metastases are predominantly osteolytic, characterized by increased osteoclast activity and bone resorption, PC lesions are predominantly osteoblastic [142–146].
In these osteolytic tumors, osteoclastogenesis is activated by PTH-related protein (PTHrP) secretion by the tumor cell or by receptor activator of nuclear factor kappa-B ligand (RANKL) secreted by the tumor cell and/or BM-MSCs [147,148]. Clinically, NB BM metastases have been described to be predominantly of osteolytic nature, which has been confirmed by an increase of osteoclasts in histological examinations of NB bone lesions in a xenograft mouse model [149] and osteoclast activation through upregulation of PTHrP and RANKL in NB cells that were implanted into the femur of mice [150]. In another study, however, it was shown that various NB cell lines that induce osteolytic lesions in mice, did not secrete the osteoclast-activating factors themselves [151]. An alternative way of osteoclast activation through BM-MSC-derived IL6 was demonstrated in vitro in a coculture system of rat osteoclasts, BM-MSCs, and NB cell line CHLA-55. Only in the presence of BM-MSCs, an increased osteoclast activation was observed, which was dependent on IL6, secreted by BM-MSC solely upon contact with NB cells [151] (Fig. 2B).
The subsequent bone resorption does not only create space for tumor growth, but also leads to the release of growth factors such as TGF-β, bone morphogenetic factors (BMPs), and IGFs from the bone matrix (Fig. 2C), which in turn can increase osteoblastic differentiation [152–155] (Fig. 2D). Furthermore, IGFs have been shown to increase survival and proliferation in NB cells, PC cells, and MM cells in vitro, suggesting that IGF-1 released from the bone matrix in the proximity of metastatic tumor cells could also directly benefit tumor progression [156–158] (Fig. 2D).
In contrast to osteoclastic lesions, PC bone metastases are predominantly osteoblastic [159]. Furthermore, AML cells have been demonstrated to induce osteogenic differentiation of MSCs in vitro through BMP-Smad1/5 signaling [160]. Interestingly, several studies also investigated the involvement of osteoblasts in NB, but the results are contradicting. On the one hand, NB cells seemed to impede MSC differentiation into osteoblasts by secretion of Wnt-inhibitor Dkk1 in an in vitro model [161], a process that has likewise been described for osteolytic bone metastases of MM and breast cancer [162,163]. On the other hand, a study with murine BM-MSCs demonstrated that NB cells increased the in vitro differentiation of MSCs into osteoblasts by increasing the expression of intracellular VEGF-A [153] (Fig. 2E). This enhanced the effects of BMP-4, which is—next to Wnt- and Notch signaling—part of one of three pathways that control osteoblastogenesis [164]. Importantly, recent study from our group with ex vivo analyses of NB patient-derived material demonstrated BM-MSCs from metastatic NB patients to be more prone to differentiate toward osteoblasts compared to MSCs from patients without BM metastases [113].
In conclusion, the regulation of bone homeostasis in NB and the involvement of the BM stroma are complex and seem to implicate both osteolytic as well as osteoblastic processes. The latter represent “two extremes of a continuum” [165] and are thus coinciding events. Although bone metastases of most tumors present lesions that display both processes, they are termed “osteolytic” or “osteoblastic” based on the predominantly occurring process [159,166]. The benefit for the tumor is in both cases an increased availability of growth factors, either when being released from the bone matrix (in osteolytic lesions) or produced by an increased number of bone cells (in osteoblastic lesions) [158].
Catecholamines in NB
Other important players in the metastatic BM environment are catecholamines, such as dopamine, epinephrine, norepinephrine, and their metabolites. In normal situations, catecholamines are primarily secreted in a circadian rhythm by sympathetic neurons and are involved in regulating activity and homing of HSPCs to the BM [167]. More specifically, secretion of norepinephrine by sympathetic neurons in the BM downregulates CXCL12 expression by stromal cells, resulting in HSPC release into the blood [168]. As a tumor originating from the neural crest, nearly all NB tumors secrete catecholamines and their metabolites, some of which are utilized as diagnostic markers [5]. Although their function in NB is unknown, they were found to promote tumor proliferation and metastasis in several other tumors [167]. This makes the NB metastatic niche particularly interesting and unique, as NB cells contribute to catecholamine production. Interestingly, MSCs from adipose tissue express various adrenergic receptors [169], and catecholamines were suggested to regulate MSC differentiation and migration (as reviewed by Hajifathali et al. [170]). Considering these findings, one could speculate that NB cells may utilize catecholamines to create a proliferative environment in an autocrine or paracrine (to MSCs) manner (Fig. 2F), or to assist in the creation of space for the tumor within the BM niche by expelling HSPCs [171].
Therapy Resistance and Dormancy
Since >30% of NB patients relapse after complete remission [1], it is essential to understand therapy resistance and MRD in the BM. Whereas most macroscopic tumor lesions respond to therapy, are resected, and become undetectable, some cells may evade therapy, persist, and remain undetected [6]. Although the majority of studies focuses specifically on resistance to chemotherapy, there are also efforts to elucidate resistance to other therapeutic approaches such as immunotherapy (discussed hereunder).
Chemoresistance can arise intrinsically (acquired chemoresistance) or be mediated by cells in the TME [environment-mediated drug resistance (EMDR)] [172]. The latter can be facilitated by soluble factors and EVs from the TME as well as by cell adhesion to the ECM or stromal cells [17]. Furthermore, dormancy of tumor cells enables them to escape treatment, since chemotherapeutic agents often target fast-dividing cells in a nonspecific way [173]. Dormancy on the cellular level is defined by mechanisms that induce cellular quiescence, that is, a reversible nonproliferative state [15,174].
A contribution of MSCs to chemoresistance and dormancy has been demonstrated in several cancer types. In breast cancer, for example, MSCs have been described to promote chemoresistance and induce tumor dormancy by secreting cell cycle-inhibitory miRNAs and creating a tumor-protective niche [175,176]. Breast cancer cells were also shown to enter a dormant state in vitro after cannibalizing BM-MSCs, after which they acquired a senescence-associated secretome [177]. In bone metastatic PC, BMP-7, which normally regulates HSC dormancy, was secreted by BM-MSCs and induced a reversible senescence-like state in the tumor cells by inhibiting EMT [178].
In the BM metastatic setting of NB little is known about the processes leading to dormancy and therapy resistance. However, in in vitro settings and in the in vivo environment of the primary tumor the contribution of MSCs to therapy resistance has been investigated. Chemoresistance in NB was shown to involve MSC-mediated STAT3 signaling in in vitro experiments: NB cells cocultured with patient-derived BM-MSCs were protected from etoposide-induced apoptosis [73,179,180]. The results suggested Sphingosine-1-phosphate receptor 1 (S1PR1) to play a role in the activation of STAT3 signaling in NB cells and showed that antiapoptotic proteins Bcl2 and survivin are involved in the STAT3-related chemoresistance mechanism [179,180]. Consistently, knockdown or inhibition of S1PR1 abrogated the STAT3-mediated chemoresistance [179]. These results are corroborated by in vivo studies: inhibition of STAT3 with AZD9150 increased sensitivity of NB to cisplatin, as seen by decreased tumor growth (64%) and significantly prolonged survival of mice [181]. Furthermore, combined inhibition of STAT3 (by ruxolitinib) and ERK1/2 (by trametinib) sensitized NB cells to etoposide and led to decreased tumor size and prolonged survival of mice [73].
Resistance to anti-GD2-immunotherapy was mediated by BM-MSCs in an in vivo study: BM-MSCs isolated from NB patients, co-injected with monocytes into the renal capsule of mice, protected NB cells from toxicity induced by dinutuximab (an anti-GD2 antibody) and activated natural killer cells (aNKC) [182]. Whether these BM-MSCs were isolated from BM with metastases, where they might have been manipulated by tumor cells to become protective, was not addressed in this study. Addition of an anti-CD105 (Endoglin) antibody restored the efficiency of the aNKC/dinutuximab treatment. Since the anti-CD105 antibody eliminates not only MSCs but also monocytes and endothelial cells, the protective effect cannot be attributed solely to MSCs here. Based on analyses of conditioned medium from cocultures of MSCs, monocytes and NB cells, TGF-β1 was proposed to be a major contributor to MSC-/monocyte-induced protection from aNKC/dinutuximab treatment [182]. Corroborating this hypothesis, another study reported inhibition of TGF-βR1 with galunisertib to restore antitumor activity of the aNKC/dinutuximab combination treatment in vitro and in vivo [183].
These studies provide intriguing evidence for the contribution of MSCs and some molecular mechanisms of therapy resistance. Further research into factors and signaling pathways involved in MSC-mediated therapy resistance in the BM is needed to advance our understanding of the mechanisms that underlie NB relapse.
Clinical Perspective
MSCs as cellular therapy
Because of their multipotent nature, MSCs are often used in regenerative medicine and in addition to treatment for a variety of nonmalignant diseases [184,185]. Although a range of studies show tumor-supportive properties of MSCs, a potential clinical use of MSCs in tumor therapy is being investigated. The safety of such application must, therefore, be taken into account and be treated with caution [186].
One property of interest is their hematopoietic supportive function to promote recovery of the hematopoietic system after myeloablative cancer therapy and stem cell transplantation. MSC co-transplantation can be used to support the nesting of HSPCs in the BM hematopoietic niche, to reduce the inflammation of damaged tissue and thus to sustain an overall functional BM niche [187]. Although the benefits of MSC co-transplantation to enhance engraftment in allogeneic HSC transplantation and to prevent graft-versus-host disease have been studied extensively [188], its use in the autologous context, as common in NB, remains largely unexplored [189,190].
The second MSC property with a potential clinical benefit is their tumor-tropism to selectively deliver anticancer agents to tumors. In NB tumors, a few possible agents have been tested in vitro and in vivo, including TNF-related apoptosis-inducing ligand (TRAIL) [191], interferon-gamma (IFN-γ) [192], IFN-β [193], and the neuronal differentiation-associated microRNA miR-124 [194]. Furthermore, the use of oncolytic virus-infected MSC products has been tested in vivo [195] and showed only small side effects in NB therapy in a phase I/II clinical trial [196].
However, there is evidence that indicates rapid clearance of ex vivo expanded MSCs after systemic administration [197], questioning the ability of MSCs to migrate to their target tissue in NB therapy. Utilizing EVs as a delivery vehicle instead could present a remedy for this limitation: The successful targeting to tumors and effectiveness of EVs loaded with oncolytic virus or chemotherapeutic agents was demonstrated in a mouse model of lung cancer [198] and human BM-MSC-derived EVs resulted in a therapeutic effect in a graft-versus-host disease model [199].
In addition, MSCs might exert an adverse effect on tumor progression, which could diminish the intended benefit of these therapies. The multitude of possible applications of MSCs in cellular therapy stress the need to further investigate the role of MSC-NB crosstalk to ensure their safe use in the clinical setting.
Therapy targeting MSC-NB crosstalk
In addition to the aforementioned approaches that exploit the beneficiary functions of MSCs, there are other endeavors that try to directly target MSCs and the TME they sustain to ablate their tumor-supportive effect, especially their therapy-protective functions. Therapy resistance of tumor cells in BM remains a major obstacle for curing NB [13,17]. Treatment should, therefore, aim to address EMDR effectively and increase chemotherapy efficiency, for example, by mobilizing NBCs from their protective environment in the BM niche. Secondly, targeting the BM more specifically would aid in reducing the chemotherapeutic load for patients. Strategies for achieving the latter have extensively been reviewed by Mu et al. [200]. The following section summarizes existing knowledge about intriguing new ways of targeting NB cells and MSCs and their interaction with the TME to overcome chemoresistance and eliminate MRD.
Targeting NB–MSC interactions in the TME
The CXCR4/CXCL12 axis is an interesting candidate for targeting the BM TME because of its important role in NB metastasis and progression and the contribution of MSCs to this signaling axis, as mentioned earlier in this review. The feasibility is supported by two studies that show reduced primary NB growth in vivo, one using virally delivered and the other systemically injected CXCR4 antagonists [201,202]. In addition, an inhibitory effect on NB proliferation and metastasis, partly due to reduced CXCR4 expression, is observed upon use of isatin, an endogenous indole found in plants and humans [203]. Finally, enhanced CXCR4 expression was found in cisplatin-resistant tumors, and inhibition of CXCR4 expression on NB cells with the VEGFR-inhibitor vandetanib restored cisplatin sensitivity in mice [204].
Directly eliminating BM-MSCs is another approach to abolish their tumor-supportive effects. One of the established targets on MSCs is the transmembrane receptor CD105 (also targeting monocyte and endothelial cells), to which antibody-dependent cellular cytotoxicity by anti-CD105 antibodies can be directed. An in vivo study in mice showed that resistance to anti-GD2 immunotherapy of NB conferred by MSCs and/or monocytes can be overcome by eliminating these cells with anti-CD105 antibodies [182].
Another approach targeting the BM niche and reducing the burden of osteolytic lesions in metastatic NB is to interfere with RANK/RANKL signaling. Endogenously, the RANKL decoy receptor osteoprotegerin (OPG) inhibits osteoclast activation [140,166]. A phase III clinical trial for treatment of osteolytic lesions in MM patients, showed the efficacy and safety of RANKL inhibitor denosumab, which mimics the endogenous OPG effects [205]. Its application in NB has not been investigated yet, but could prove beneficial to prevent osteolysis and the concomitant effects on tumor progression and to provide supportive care for bone disease in NB.
Mobilizing NB cells out of the protective BM niche
Although not directly MSC related, the mobilization of sequestered NB cells out of the protective environment of the BM niche is a valuable approach for improving therapy success. Tumor cells thereby lose their (indirect) contact with MSCs and other cells in the TME and become more accessible for tumor-targeting drugs. Since mobilization could introduce the risk of new metastases, this should be done with great caution and accompanied by a consecutive chemotherapy course. Nevertheless, there are some studies that support this idea, for example, by targeting CXCR4 or adhesion molecules such as integrins.
In a breast cancer xenograft model, the CXCR4-specific inhibitor AMD3100 [206] successfully mobilized dormant tumor cells out of the perisinusoidal niche of the BM, as demonstrated with real-time in vivo imaging [207]. Similarly, AMD3100 diminished adhesion of MM cells to BM stromal cells (identity not further clarified) in vitro and promoted mobilization of tumor cells into the circulation in vivo, subsequently sensitizing them to bortezomib [208]. However, AMD3100 presents a nonspecific way of targeting tumor cells in the BM, as it is also used for mobilizing HSPCs from the BM before stem cell transplantation [209]. All AMD3100-effects must, therefore, be considered during its application, but it might be beneficial in sensitization of NB cells to therapy and specific targeting of dormant NB cells to lower the risk for relapse.
A more precise target for blocking NB cell adhesion could be tumor-specific integrins, which regulate tumor migration, invasion, and adhesion to the ECM, and whose high expression is associated with increased metastasis [210]. For instance, integrin subunits α3β1were previously reported to be upregulated in NB cells exposed to conditioned medium from BM-MSCs, concomitant with increased invasiveness, which implies a functional role for NB metastasis and attachment [100]. Furthermore, combined inhibition of αVβ3 and αVβ5 integrins reduced NB cell attachment to the culturing surface and thereby increased the cytotoxic effects of an anti-GD2 antibody in vitro [211]. Overall, targeting adhesion by blocking integrins and/or CXCR4 might pose an exciting new way to promote mobilization of NB cells out of the BM and sensitize them to treatment.
Open Questions and Outlook
Although an increasing body of evidence suggests a tumor-supportive role of MSCs in various tumor types, more in/ex vivo research is necessary in the context of NB to confirm previous findings and extend our knowledge regarding the role of MSCs in EMT, chemoresistance, and dormancy.
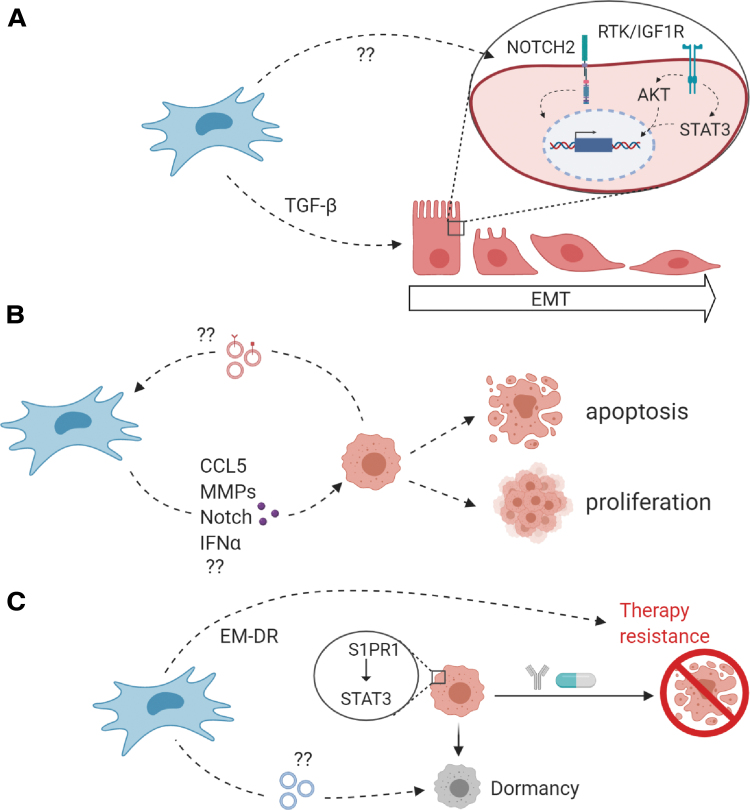
It remains unclear if and to what extent MSCs promote EMT in NB cells, for example. Are they involved in the Notch signaling that induces the switch between adrenergic and mesenchymal NB cells? Could MSCs in the pediatric BM be a source of TGF-β that evokes EMT characteristics in NB cells (Fig. 3A)? What role do EVs and other pro- or antitumorigenic components (such as CCL5 [69], MMPs [98], IFNα [212], and Notch signaling [213]) play in NB progression in the BM (Fig. 3B)? And by what mechanisms do MSCs contribute to EMDR and dormancy in NB cells (Fig. 3C)? These and more questions need to be answered to find out how to harness the MSC-NB crosstalk to our advantage. The complexity of signaling and crosstalk in the TME, which can differ depending on tumor localization and experimental design as well as on the well-known NB heterogeneity, have to be considered when interpreting results.
FIG. 3.
Open questions regarding NB–MSC interactions. (A) Metastasis is dependent on the cell's capacity to migrate to distant sites. Whether MSCs are a source of TGF-β and MMP-9 or activate Notch signaling in NB cells, all of which are known to be involved in EMT and invasion in NB [93,100,102], remains to be elucidated in NB. Furthermore, the PI3K/AKT pathway and STAT3 signaling have been implicated to contribute to EMT [94,95]. (B) Additional signaling between NB cells and MSCs through cytokines, chemokines, and growth factors (purple) might contribute to tumor proliferation and -survival. CCL5 [69], MMPs [98], and Notch signaling [213] have been described to contribute to cancer cell motility, invasion, and differentiation into CAFs. MSC-derived IFNα, in contrast, was suggested to inhibit proliferation of cancer cells [212]. Furthermore, the cargo of exosomes derived from metastatic NB cells (red) and the signaling it induces in MSCs is an interesting field of research [123]. (C) To prevent EMDR and induction of dormancy through MSCs, the signaling components from MSCs contributing to these processes need to be studied in detail. It has been described that MSCs induce expression of S1PR1 in NB cells, which protected NB cells from drug-induced apoptosis through the JAK-STAT3 signaling pathway [179]. In breast cancer, miRNA-loaded exosomes promoted quiescence in tumor cells [175,176]. CAF, cancer-associated fibroblast; EMDR, environment-mediated drug resistance; IFNα, interferon α; RTK, receptor tyrosine kinases. Color images are available online.
The fundamental knowledge of molecular mechanisms is imperative for designing new treatment options that target the tumor and its microenvironment in a more effective and specific way and thereby avoid unfavorable side effects. Furthermore, the development of targeted drug delivery to the BM is crucial for advancing the progress in curing NB.
Acknowledgment
Figures were created with BioRender software.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study is funded by KiKa grant 303, PPOC grant 19-27 (I.T.), the Landsteiner Foundation for Blood Transfusion Research, LSBR grant F1101 (I.T. and C.V.), and Prinses Maxima Center grant P0104 (G.A.M.T. and C.H.)
References
- 1. Park JR, Bagatell R, London WB, Maris JM, Cohn SL, Mattay KM and Hogarty M (2013). Children's Oncology Group's 2013 blueprint for research: neuroblastoma. Pediatr Blood Cancer 60:985–993 [DOI] [PubMed] [Google Scholar]
- 2. Borriello L, Seeger RC, Asgharzadeh S and DeClerck YA (2016). More than the genes, the tumor microenvironment in neuroblastoma. Cancer Lett 380:304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheung NKV and Dyer MA (2013). Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer 13:397–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Le Douarin NM and Teillet M-AM (1974). Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev Biol 41:162–184 [DOI] [PubMed] [Google Scholar]
- 5. Verly IRN, van Kuilenburg ABP, NAbeling GGM, Goorden SMI, Fiocco M, Vaz FM, van Noesel MM, Zwaan CM, Kaspers GJL, et al. (2017). Catecholamines profiles at diagnosis: increased diagnostic sensitivity and correlation with biological and clinical features in neuroblastoma patients. Eur J Cancer 72:235–243 [DOI] [PubMed] [Google Scholar]
- 6. Maris JM, Hogarty MD, Bagatell R and Cohn SL (2007). Neuroblastoma. Lancet 369:2106–2120 [DOI] [PubMed] [Google Scholar]
- 7. Berthold F, Spix C, Kaatsch P and Lampert F (2017). Incidence, survival, and treatment of localized and metastatic neuroblastoma in Germany 1979–2015. Pediatr Drugs 19:577–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stutterheim J, Zappeij-Kannegieter L, Versteeg R, Caron HN, Van Der Schoot CE and Tytgat GAM (2011). The prognostic value of fast molecular response of marrow disease in patients aged over 1 year with stage 4 neuroblastoma. Eur J Cancer 47:1193–1202 [DOI] [PubMed] [Google Scholar]
- 9. Monclair T, Brodeur GM, Ambros PF, Brisse HJ, Cecchetto G, Holmes K, Kaneko M, London WB, Matthay KK, et al. (2009). The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force Report. J Clin Oncol 27:298–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohn SL, Pearson ADJ, London WB, Monclair T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, et al. (2009). The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force Report. J Clin Oncol 27:289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peifer M, Hertwig F, Roels F, Dreidax D, Gartlgruber M, Menon R, Krämer A, Roncaioli JL, Sand F, et al. (2015). Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature 526:700–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Valentijn LJ, Koster J, Zwijnenburg DA, Hasselt NE, van Sluis P, Volckmann R, van Noesel MM, George RE, Tytgat GAM, Molenaar JJ and Versteeg R (2015). TERT rearrangements are frequent in neuroblastoma and identify aggressive tumors. Nat Genet 47:1411–1414 [DOI] [PubMed] [Google Scholar]
- 13. Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, Nakagawara A, Berthold F, Schleiermacher G, et al. (2015). Advances in risk classification and treatment strategies for neuroblastoma. J Clin Oncol 33:3008–3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, Gerbing RB, London WB and Villablanca JG (2009). Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children's oncology group study. J Clin Oncol 27:1007–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sosa MS, Bragado P and Aguirre-Ghiso JA (2014). Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 14:611–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yeh AC and Ramaswamy S (2015). Mechanisms of cancer cell dormancy-another hallmark of cancer? Cancer Res 75:5014–5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meads MB, Hazlehurst LA and Dalton WS (2008). The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res 14:2519–2526 [DOI] [PubMed] [Google Scholar]
- 18. Seita J and Weissman IL (2010). Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med 2:640–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shiozawa Y. (2020). The roles of bone marrow-resident cells as a microenvironment for bone metastasis. Adv Exp Med Biol 1226:57–72 [DOI] [PubMed] [Google Scholar]
- 20. Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS and Keating A (2005). Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy 7:393–395 [DOI] [PubMed] [Google Scholar]
- 21. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D and Horwitz E (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317 [DOI] [PubMed] [Google Scholar]
- 22. da Silva Meirelles L, Chagastelles PC and Nardi NB (2006). Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119:2204–2213 [DOI] [PubMed] [Google Scholar]
- 23. Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, et al. (2008). A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313 [DOI] [PubMed] [Google Scholar]
- 24. Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP and Gerson SL (2000). Cutting edge communication: human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res 9:841–848 [DOI] [PubMed] [Google Scholar]
- 25. Greenbaum A, Hsu YMS, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T and Link DC (2013). CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495:227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bielby R, Jones E and McGonagle D (2007). The role of mesenchymal stem cells in maintenance and repair of bone. Injury 38:S26–S32 [DOI] [PubMed] [Google Scholar]
- 27. Uccelli A, Moretta L and Pistoia V (2008). Mesenchymal stem cells in health and disease. Nat Rev Immunol 8:726–736 [DOI] [PubMed] [Google Scholar]
- 28. Tormin A, Li O, Brune JC, Walsh S, Schütz B, Ehinger M, Ditzel N, Kassem M and Scheding S (2011). CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood 117:5067–5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang Y-HK, Ogando CR, Wang See C, Chang T-Y and Barabino GA (2018). Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther 9:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qian H, Le Blanc K and Sigvardsson M (2012). Primary mesenchymal stem and progenitor cells from bone marrow lack expression of CD44 protein. J Biol Chem 287:25795–25807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rombouts WJC and Ploemacher RE (2003). Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 17:160–170 [DOI] [PubMed] [Google Scholar]
- 32. Rojewski MT, Weber BM and Schrezenmeier H (2008). Phenotypic characterization of mesenchymal stem cells from various tissues. Transfus Med Hemother 35:168–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lv F, Lu M, Cheung K MC, Leung VYL and Zhou G (2012). Intrinsic properties of mesemchymal stem cells from human bone marrow, umbilical cord and umbilical cord blood comparing the different sources of MSC. Curr Stem Cell Res Ther 7:389–399 [DOI] [PubMed] [Google Scholar]
- 34. Heo JS, Choi Y, Kim H-S and Kim HO (2016). Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med 37:115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boxall SA and Jones E (2012). Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int 2012:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jones E and Schäfer R (2015). Where is the common ground between bone marrow mesenchymal stem/stromal cells from different donors and species? Stem Cell Res Ther 6:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, MacArthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN and Frenette PS (2010). Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466:829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, et al. (2013). Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502:637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou BO, Yue R, Murphy MM, Peyer JG and Morrison SJ (2014). Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15:154–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maijenburg MW, Kleijer M, Vermeul K, Mul EPJ, van Alphen FPJ, van der Schoot CE and Voermans C (2012). The composition of the mesenchymal stromal cell compartment in human bone marrow changes during development and aging. Haematologica 97:179–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y and Frenette PS (2013). PDGFRα and CD51 mark human Nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med 210:1351–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Churchman SM, Ponchel F, Boxall SA, Cuthbert R, Kouroupis D, Roshdy T, Giannoudis P V, Emery P, McGonagle D and Jones EA (2012). Transcriptional profile of native CD271+ multipotential stromal cells: evidence for multiple fates, with prominent osteogenic and Wnt pathway signaling activity. Arthritis Rheum 64:2632–2643 [DOI] [PubMed] [Google Scholar]
- 43. Reinisch A, Hernandez DC, Schallmoser K and Majeti R (2017). Generation and use of a humanized bone-marrow-ossicle niche for hematopoietic xenotransplantation into mice. Nat Protoc 12:2169–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Klopp AH, Spaeth EL, Dembinski JL, Woodward WA, Munshi A, Meyn RE, Cox JD, Andreeff M and Marini FC (2007). Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res 67:11687–11695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lourenco S, Teixeira VH, Kalber T, Jose RJ, Floto RA and Janes SM (2015). Macrophage migration inhibitory factor–CXCR4 is the dominant chemotactic axis in human mesenchymal stem cell recruitment to tumors. J Immunol 194:3463–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM, Barry FP, O'Brien T and Kerin MJ (2007). Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res 13:5020–5027 [DOI] [PubMed] [Google Scholar]
- 47. Kim SM, Kim DS, Jeong CH, Kim DH, Kim JH, Jeon HB, Kwon SJ, Jeun SS, Yang YS, Oh W and Chang JW (2011). CXC chemokine receptor 1 enhances the ability of human umbilical cord blood-derived mesenchymal stem cells to migrate toward gliomas. Biochem Biophys Res Commun 407:741–746 [DOI] [PubMed] [Google Scholar]
- 48. Ho IAW, Yulyana Y, Sia KC, Newman JP, Guo CM, Hui KM and Lam PYP (2014). Matrix metalloproteinase-1-mediated mesenchymal stem cell tumor tropism is dependent on crosstalk with stromal derived growth factor 1/C-X-C chemokine receptor 4 axis. FASEB J 28:4359–4368 [DOI] [PubMed] [Google Scholar]
- 49. Barcellos-de-Souza P, Comito G, Pons-Segura C, Taddei ML, Gori V, Becherucci V, Bambi F, Margheri F, Laurenzana A, et al. (2016). Mesenchymal stem cells are recruited and activated into carcinoma-associated fibroblasts by prostate cancer microenvironment-derived TGF-β1. Stem Cells 34:2536–2547 [DOI] [PubMed] [Google Scholar]
- 50. Cornelissen AS, Maijenburg MW, Nolte MA and Voermans C (2015). Organ-specific migration of mesenchymal stromal cells: who, when, where and why? Immunol Lett 168:159–169 [DOI] [PubMed] [Google Scholar]
- 51. Kimura K, Kishida T, Wakao J, Tanaka T, Higashi M, Fumino S, Aoi S, Furukawa T, Mazda O and Tajiri T (2016). Tumor-homing effect of human mesenchymal stem cells in a TH-MYCN mouse model of neuroblastoma. J Pediatr Surg 51:2068–2073 [DOI] [PubMed] [Google Scholar]
- 52. Melen GJ, Franco-Luzón L, Ruano D, Á González-Murillo, Alfranca A, Casco F, Á Lassaletta, Alonso M, Madero L, et al. (2016). Influence of carrier cells on the clinical outcome of children with neuroblastoma treated with high dose of oncolytic adenovirus delivered in mesenchymal stem cells. Cancer Lett 371:161–170 [DOI] [PubMed] [Google Scholar]
- 53. Melzer C, von der Ohe J and Hass R (2018). Concise review: crosstalk of mesenchymal stroma/stem-like cells with cancer cells provides therapeutic potential. Stem Cells 36:951–968 [DOI] [PubMed] [Google Scholar]
- 54. Haga H, Yan IK, Takahashi K, Wood J, Zubair A and Patel T (2015). Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J Extracell Vesicles 4:24900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Whiteside TL. (2018). Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin Immunol 35:69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C, Yuan ZR, Roberts AI, Zhang L, et al. (2012). CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα. Cell Stem Cell 11:812–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Christodoulou I, Goulielmaki M, Devetzi M, Panagiotidis M, Koliakos G and Zoumpourlis V (2018). Mesenchymal stem cells in preclinical cancer cytotherapy: a systematic review. Stem Cell Res Ther 9:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aoyagi A, Aoyagi M, Waga K, Enokihara H and Furusawa S (1996). Preferential inhibitory effect of soluble factor(s) in human bone marrow stromal cells on proliferation of K562 leukemia cells versus normal myeloid progenitor cells. Int J Hematol 63:205–213 [DOI] [PubMed] [Google Scholar]
- 59. Maestroni GJM, Hertens E and Galli P (1999). Factor(s) from nonmacrophage bone marrow stromal cells inhibit Lewis lung carcinoma and B16 melanoma growth in mice. Cell Mol Life Sci 55:663–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ohlsson LB, Varas L, Kjellman C, Edvardsen K and Lindvall M (2003). Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp Mol Pathol 75:248–255 [DOI] [PubMed] [Google Scholar]
- 61. Lu L, Chen G, Yang J, Ma Z, Yang Y, Hu Y, Lu Y, Cao Z, Wang Y and Wang X (2019). Bone marrow mesenchymal stem cells suppress growth and promote the apoptosis of glioma U251 cells through downregulation of the PI3K/AKT signaling pathway. Biomed Pharmacother 112:108625. [DOI] [PubMed] [Google Scholar]
- 62. Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, et al. (2006). Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med 203:1235–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Meleshina AV, Cherkasova EI, Shirmanova MV, Klementieva NV, Kiseleva EV, Snopova LB, Prodanets NN and Zagaynova EV (2015). Influence of mesenchymal stem cells on metastasis development in mice in vivo. Stem Cell Res Ther 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee MW, Ryu S, Kim DS, Lee JW, Sung KW, Koo HH and Yoo KH (2019). Mesenchymal stem cells in suppression or progression of hematologic malignancy: current status and challenges. Leukemia 33:597–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Qiao L, Xu Z, Zhao T, Ye L and Zhang X (2008). Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett 269:67–77 [DOI] [PubMed] [Google Scholar]
- 66. Shen YL, Luo Q, Guo YX, Zheng GH, Yu J and Xu YH (2014). Bone marrow mesenchymal stem cell-derived Wnt5a inhibits leukemia cell progression in vitro via activation of the non-canonical Wnt signaling pathway. Oncol Lett 8:85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhu Y, Sun Z, Han Q, Liao L, Wang J, Bian C, Li J, Yan X, Liu Y, Shao C and Zhao RC (2009). Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia 23:925–933 [DOI] [PubMed] [Google Scholar]
- 68. Bianchi G, Morandi F, Cilli M, Daga A, Bocelli-Tyndall C, Gambini C, Pistoia V and Raffaghello L (2012). Close interactions between mesenchymal stem cells and neuroblastoma cell lines lead to tumor growth inhibition. PLoS One 7:e48654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg RA (2007). Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449:557–563 [DOI] [PubMed] [Google Scholar]
- 70. Chowdhury R, Webber JP, Gurney M, Mason MD, Tabi Z and Clayton A (2015). Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget 6:715–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bergfeld SA, Blavier L and DeClerck YA (2014). Bone marrow–derived mesenchymal stromal cells promote survival and drug resistance in tumor cells. Mol Cancer Ther 13:962–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW and Banerjee D (2008). Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res 68:4331–4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Borriello L, Nakata R, Sheard MA, Fernandez GE, Sposto R, Malvar J, Blavier L, Shimada H, Asgharzadeh S, et al. (2017). Cancer-associated fibroblasts share characteristics and protumorigenic activity with mesenchymal stromal cells. Cancer Res 77:5142–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Meier R, Mühlethaler-Mottet A, Flahaut M, Coulon A, Fusco C, Louache F, Auderset K, Bourloud KB, Daudigeos E, et al. (2007). The chemokine receptor CXCR4 strongly promotes neuroblastoma primary tumour and metastatic growth, but not invasion. PLoS One 2:e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kortesidis A. (2005). Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood 105:3793–3801 [DOI] [PubMed] [Google Scholar]
- 76. Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, MacArthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN and Frenette PS (2010). Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466:829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ding L and Morrison SJ (2013). Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495:231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jung Y, Wang J, Schneider A, Sun Y-X, Koh-Paige AJ, Osman NI, McCauley LK and Taichman RS (2006). Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone 38:497–508 [DOI] [PubMed] [Google Scholar]
- 79. Goedhart M, Gessel S, der Voort R, Slot E, Lucas B, Gielen E, Hoogenboezem M, Rademakers T, Geerman S, et al. (2019). CXCR4, but not CXCR3, drives CD8 + T-cell entry into and migration through the murine bone marrow. Eur J Immunol 49:576–589 [DOI] [PubMed] [Google Scholar]
- 80. Emura M, Ochiai A, Horino M, Arndt W, Kamino K and Hirohashi S (2000). Development of myofibroblasts from human bone marrow mesenchymal stem cells cocultured with human colon carcinoma cells and TGF beta 1. In Vitro Cell Dev Biol Anim 36:77–80 [DOI] [PubMed] [Google Scholar]
- 81. Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR and Wright NA (2004). Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res 64:8492–8495 [DOI] [PubMed] [Google Scholar]
- 82. Ishii G, Sangai T, Oda T, Aoyagi Y, Hasebe T, Kanomata N, Endoh Y, Okumura C, Okuhara Y, et al. (2003). Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Commun 309:232–240 [DOI] [PubMed] [Google Scholar]
- 83. Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, Andreeff M and Marini F (2009). Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One 4:e4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Navab R, Strumpf D, Bandarchi B, Zhu CQ, Pintilie M, Ramnarine VR, Ibrahimov E, Radulovich N, Leung L, et al. (2011). Prognostic gene-expression signature of carcinoma-associated fibroblasts in non-small cell lung cancer. Proc Natl Acad Sci U S A 108:7160–7165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Buganimy Y, Madary S, Rais Y, Pomeraniec L, Harel E, Solomon H, Kalo E, Goldstein I, Brosh R, et al. (2011). Transcriptional activity of ATF3 in the stromal compartment of tumors promotes cancer progression. Carcinogenesis 32:1749–1757 [DOI] [PubMed] [Google Scholar]
- 86. Lutzny G, Kocher T, Schmidt-Supprian M, Rudelius M, Klein-Hitpass L, Finch AJ, Dürig J, Wagner M, Haferlach C, et al. (2013). Protein kinase C-β-dependent activation of NF-κB in stromal cells is indispensable for the survival of chronic lymphocytic leukemia B cells in vivo. Cancer Cell 23:77–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Paggetti J, Haderk F, Seiffert M, Janji B, Distler U, Ammerlaan W, Kim YJ, Adam J, Lichter P, et al. (2015). Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood 126:1106–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Madar S, Goldstein I and Rotter V (2013). “Cancer associated fibroblasts”—more than meets the eye. Trends Mol Med 19:447–453 [DOI] [PubMed] [Google Scholar]
- 89. Quante M, Tu SP, Tomita H, Gonda T, Wang SSW, Takashi S, Baik GH, Shibata W, DiPrete B, et al. (2011). Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 19:257–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ren G, Esposito M and Kang Y (2015). Bone metastasis and the metastatic niche. J Mol Med 93:1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Thiery JP and Sleeman JP (2006). Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol 7:131–142 [DOI] [PubMed] [Google Scholar]
- 92. Delloye-Bourgeois C and Castellani V (2019). Hijacking of embryonic programs by neural crest-derived neuroblastoma: from physiological migration to metastatic dissemination. Front Mol Neurosci 12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shao JB, Gao ZM, Huang WY and Lu ZB (2017). The mechanism of epithelial-mesenchymal transition induced by TGF-β1 in neuroblastoma cells. Int J Oncol 50:1623–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang XH, Wu HY, Gao J, Wang XH, Gao TH and Zhang SF (2019). IGF1R facilitates epithelial-mesenchymal transition and cancer stem cell properties in neuroblastoma via the STAT3/AKT axis. Cancer Manag Res 11:5459–5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tian X, Zhou D, Chen L, Tian Y, Zhong B, Cao Y, Dong Q, Zhou M, Yan J, et al. (2018). Polo-like kinase 4 mediates epithelial-mesenchymal transition in neuroblastoma via PI3K/Akt signaling pathway article. Cell Death Dis 9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Vanneaux V, Farge-Bancel D, Lecourt S, Baraut J, Cras A, Jean-Louis F, Brun C, Verrecchia F, Larghero J and Michel L (2013). Expression of transforming growth factor β receptor II in mesenchymal stem cells from systemic sclerosis patients. BMJ Open 3:e001890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Farina A and Mackay A (2014). Gelatinase B/MMP-9 in tumour pathogenesis and progression. Cancers (Basel) 6:240–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wessely A, Waltera A, Reichert TE, Stöckl S, Grässel S and Bauer RJ (2019). Induction of ALP and MMP9 activity facilitates invasive behavior in heterogeneous human BMSC and HNSCC 3D spheroids. FASEB J 33:11884–11893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sugiura Y, Shimada H, Seeger RC, Laug WE and DeClerck YA (1998). Matrix metalloproteinases-2 and -9 are expressed in human neuroblastoma: contribution of stromal cells to their production and correlation with metastasis. Cancer Res 58:2209–2216 [PubMed] [Google Scholar]
- 100. Shankar V, Hori H, Kihira K, Lei Q, Toyoda H, Iwamoto S and Komada Y (2015). Mesenchymal stromal cell secretome Up-regulates 47 kDa CXCR4 expression, and induce invasiveness in neuroblastoma cell lines. PLoS One 10:e0120069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ribatti D, Marimpietri D, Pastorino F, Brignole C, Nico B, Vacca A and Ponzoni M (2004). Angiogenesis in neuroblastoma. Ann N Y Acad Sci 1028:133–142 [DOI] [PubMed] [Google Scholar]
- 102. Van Nes J, Chan A, Van Groningen T, Van Sluis P, Koster J and Versteeg R (2013). A NOTCH3 transcriptional module induces cell motility in neuroblastoma. Clin Cancer Res 19:3485–3494 [DOI] [PubMed] [Google Scholar]
- 103. van Groningen T, Akogul N, Westerhout EM, Chan A, Hasselt NE, Zwijnenburg DA, Broekmans M, Stroeken P, Haneveld F, et al. (2019). A NOTCH feed-forward loop drives reprogramming from adrenergic to mesenchymal state in neuroblastoma. Nat Commun 10:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kamga PT, Bassi G, Cassaro A, Midolo M, Di Trapani M, Gatti A, Carusone R, Resci F, Perbellini O, et al. (2016). Notch signalling drives bone marrow stromal cell-mediated chemoresistance in acute myeloid leukemia. Oncotarget 7:21713–21727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Geminder H, Sagi-Assif O, Goldberg L, Meshel T, Rechavi G, Witz IP and Ben-Baruch A (2001). A possible role for CXCR4 and its ligand, the CXC chemokine stromal cell-derived factor-1, in the development of bone marrow metastases in neuroblastoma. J Immunol 167:4747–4757 [DOI] [PubMed] [Google Scholar]
- 106. Zhang L, Yeger H, Das B, Irwin MS and Baruchel S (2007). Tissue microenvironment modulates CXCR4 expression and tumor metastasis in neuroblastoma. Neoplasia 9:36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Russell HV, Hicks J, Okcu MF and Nuchtern JG (2004). CXCR4 expression in neuroblastoma primary tumors is associated with clinical presentation of bone and bone marrow metastases. J Pediatr Surg 39:1506–1511 [DOI] [PubMed] [Google Scholar]
- 108. Aiuti A, Webb IJ, Bleul C, Springer T and Gutierrez-Ramos JC (1997). The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med 185:111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ma M, Ye JY, Deng R, Dee CM and Chan GC-FF (2011). Mesenchymal stromal cells may enhance metastasis of neuroblastoma via SDF-1/CXCR4 and SDF-1/CXCR7 signaling. Cancer Lett 312:1–10 [DOI] [PubMed] [Google Scholar]
- 110. Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS and McCauley LK (2002). Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res 62:1832–1837 [PubMed] [Google Scholar]
- 111. Zhu WB, Zhao ZF and Zhou X (2019). AMD3100 inhibits epithelial–mesenchymal transition, cell invasion, and metastasis in the liver and the lung through blocking the SDF-1α/CXCR4 signaling pathway in prostate cancer. J Cell Physiol 234:11746–11759 [DOI] [PubMed] [Google Scholar]
- 112. Correa D, Somoza RA, Lin P, Schiemann WP and Caplan AI (2016). Mesenchymal stem cells regulate melanoma cancer cells extravasation to bone and liver at their perivascular niche. Int J Cancer 138:417–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hochheuser C, van Zogchel LMJ, Kleijer M, Kuijk C, Tol S, van der Schoot CE, Voermans C, Tytgat GAM and Timmerman I (2020). The metastatic bone marrow niche in neuroblastoma: altered phenotype and function of mesenchymal stromal cells. Cancers (Basel) 12:3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Airoldi I, Cocco C, Morandi F, Prigione I and Pistoia V (2008). CXCR5 may be involved in the attraction of human metastatic neuroblastoma cells to the bone marrow. Cancer Immunol Immunother 57:541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. van Golen CM, Schwab TS, Kim B, Soules ME, Su Oh S, Fung K, van Golen KL and Feldman EL (2006). Insulin-like growth factor-I receptor expression regulates neuroblastoma metastasis to bone. Cancer Res 66:6570–6578 [DOI] [PubMed] [Google Scholar]
- 116. Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, et al. (2017). Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer 17:302–317 [DOI] [PubMed] [Google Scholar]
- 117. Paget S. (1889). The distribution of secondary growths in cancer of the breast. Lancet 133:571–573 [PubMed] [Google Scholar]
- 118. Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, et al. (2005). VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438:820–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature 527:329–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Park JR, Eggert A and Caron H (2010). Neuroblastoma: biology, prognosis, and treatment. Hematol Oncol Clin North Am 24:65–86 [DOI] [PubMed] [Google Scholar]
- 121. Rodríguez-Milla MÁ I Mirones, Mariñas-Pardo L, Melen GJ, Cubillo I, Ramírez M and García-Castro J (2012). Enrichment of neural-related genes in human mesenchymal stem cells from neuroblastoma patients. Int J Mol Med 30:365–373 [DOI] [PubMed] [Google Scholar]
- 122. Shiozawa Y, Eber MR, Berry JE and Taichman RS (2015). Bone marrow as a metastatic niche for disseminated tumor cells from solid tumors. Bonekey Rep 4:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Marimpietri D, Petretto A, Raffaghello L, Pezzolo A, Gagliani C, Tacchetti C, Mauri P, Melioli G and Pistoia V (2013). Proteome profiling of neuroblastoma-derived exosomes reveal the expression of proteins potentially involved in tumor progression. PLoS One 8:e75054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bauer N, Fonseca AV, Florek M, Freund D, Jászai J, Bornhäuser M, Fargeas CA and Corbeil D (2008). New insights into the cell biology of hematopoietic progenitors by studying prominin-1 (CD133). Cells Tissues Organs 188:127–138 [DOI] [PubMed] [Google Scholar]
- 125. Sartelet H, Imbriglio T, Nyalendo C, Haddad E, Annabi B, Duval M, Fetni R, Victor K, Alexendrov L, et al. (2012). CD133 expression is associated with poor outcome in neuroblastoma via chemoresistance mediated by the AKT pathway. Histopathology 60:1144–1155 [DOI] [PubMed] [Google Scholar]
- 126. Muramatsu T and Miyauchi T (2003). Basigin (CD147): a multifunctional transmembrane protien involved in reproduction, neural function, inflammation and tumor invasion. Histol Histopathol 18:981–987 [DOI] [PubMed] [Google Scholar]
- 127. Castriconi R, Dondero A, Augugliaro R, Cantoni C, Carnemolla B, Sementa AR, Negri F, Conte R, Corrias MV, et al. (2004). Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci U S A 101:12640–12645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Colletti M, Petretto A, Galardi A, Di Paolo V, Tomao L, Lavarello C, Inglese E, Bruschi M, Lopez AA, et al. (2017). Proteomic analysis of neuroblastoma-derived exosomes: new insights into a metastatic signature. Proteomics 17:1600430. [DOI] [PubMed] [Google Scholar]
- 129. Nakata R, Shimada H, Fernandez GE, Fanter R, Fabbri M, Malvar J, Zimmermann P and DeClerck YA (2017). Contribution of neuroblastoma-derived exosomes to the production of pro-tumorigenic signals by bone marrow mesenchymal stromal cells. J Extracell Vesicles 6:1332941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Shibuya M. (2013). Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 153:13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ferrer FA, Pantschenko AG, Miller LJ, Anderson K, Grunnet M, McKenna PH and Kreutzer D (2000). Angiogenesis and neuroblastomas: interleukin-8 and interleukin-8 receptor expression in human neuroblastoma. J Urol 164(3 Pt 2):1016–1020 [DOI] [PubMed] [Google Scholar]
- 132. Sierra-Filardi E, Nieto C, Á Domínguez-Soto, Barroso R, Sánchez-Mateos P, Puig-Kroger A, López-Bravo M, Joven J, Ardavín C, et al. (2014). CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile. J Immunol 192:3858–3867 [DOI] [PubMed] [Google Scholar]
- 133. Fukaya Y, Shimada H, Wang L-C, Zandi E and DeClerck YA (2008). Identification of galectin-3-binding protein as a factor secreted by tumor cells that stimulates interleukin-6 expression in the bone marrow stroma. J Biol Chem 283:18573–18581 [DOI] [PubMed] [Google Scholar]
- 134. Silverman AM, Nakata R, Shimada H, Sposto R and DeClerck YA (2012). A galectin-3-dependent pathway upregulates interleukin-6 in the microenvironment of human neuroblastoma. Cancer Res 72:2228–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ara T, Song L, Shimada H, Keshelava N, Russell HV, Metelitsa LS, Groshen SG, Seeger RC and DeClerck YA (2009). Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res 69:329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yang F, Jove V, Buettner R, Xin H, Wu J, Wang Y, Nam S, Xu Y, Ara T, et al. (2012). Sorafenib inhibits endogenous and IL-6/S1P induced JAK2-STAT3 signaling in human neuroblastoma, associated with growth suppression and apoptosis. Cancer Biol Ther 13:534–541 [DOI] [PubMed] [Google Scholar]
- 137. Baglio SR, Lagerweij T, Pérez-Lanzón M, Ho XD, Léveillé N, Melo SA, Cleton-Jansen AM, Jordanova ES, Roncuzzi L, et al. (2017). Blocking tumor-educated MSC paracrine activity halts osteosarcoma progression. Clin Cancer Res 23:3721–3733 [DOI] [PubMed] [Google Scholar]
- 138. Kumari N, Dwarakanath BS, Das A and Bhatt AN (2016). Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol 37:11553–11572 [DOI] [PubMed] [Google Scholar]
- 139. Wendt MK, Balanis N, Carlin CR and Schiemann WP (2014). STAT3 and epithelial–mesenchymal transitions in carcinomas. JAK-STAT 3:e28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sohara Y, Shimada H and DeClerck YA (2005). Mechanisms of bone invasion and metastasis in human neuroblastoma. Cancer Lett 228:203–209 [DOI] [PubMed] [Google Scholar]
- 141. Crane JL and Cao X (2014). Bone marrow mesenchymal stem cells and TGF-β signaling in bone remodeling. J Clin Invest 124:466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hall CL, Bafico A, Dai J, Aaronson SA and Keller ET (2005). Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res 65:7554–7560 [DOI] [PubMed] [Google Scholar]
- 143. Michigami T, Ihara-Watanabe M, Yamazaki M and Ozono K (2001). Receptor activator of nuclear factor κB ligand (RANKL) is a key molecule of osteoclast formation for bone metastasis in a newly developed model of human neuroblastoma. Cancer Res 61:1637–1644 [PubMed] [Google Scholar]
- 144. Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, Lin B, Lentzsch S, Davies FE, et al. (2001). Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia 15:1950–1961 [DOI] [PubMed] [Google Scholar]
- 145. Barillé-Nion S, Barlogie B, Bataille R, Bergsagel PL, Epstein J, Fenton RG, Jacobson J, Kuehl WM, Shaughnessy J and Tricot G (2003). Advances in biology and therapy of multiple myeloma. Hematology Am Soc Hematol Educ Program 1:248–278 [PubMed] [Google Scholar]
- 146. Xu S, De Veirman K, De Becker A, Vanderkerken K and Van Riet I (2018). Mesenchymal stem cells in multiple myeloma: a therapeutical tool or target? Leukemia 32:1500–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, Yoneda T and Mundy GR (1996). Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest 98:1544–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Fernández M, Pino AM, Figueroa P and Rodríguez JP (2010). The increased expression of receptor activator of nuclear-κB ligand (RANKL) of multiple myeloma bone marrow stromal cells is inhibited by the bisphosphonate ibandronate. J Cell Biochem 111:130–137 [DOI] [PubMed] [Google Scholar]
- 149. Sohara Y, Shimada H, Scadeng M, Pollack H, Yamada S, Ye W, Reynolds CP and DeClerck YA (2003). Lytic bone lesions in human neuroblastoma xenograft involve osteoclast recruitment and are inhibited by bisphosphonate. Cancer Res 63:3026–3031 [PubMed] [Google Scholar]
- 150. Zhao H, Cai W, Li S, Da Z, Sun H, Ma L, Lin Y and Zhi D (2013). Characterization of neuroblastoma bone invasion/metastasis in established bone metastatic model of SY5Y and KCNR cell lines. Childs Nerv Syst 29:1097–1105 [DOI] [PubMed] [Google Scholar]
- 151. Sohara Y, Shimada H, Minkin C, Erdreich-Epstein A, Nolta JA and DeClerck YA (2005). Bone marrow mesenchymal stem cells provide an alternate pathway of osteoclast activation and bone destruction by cancer cells. Cancer Res 65:1129–1135 [DOI] [PubMed] [Google Scholar]
- 152. Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T, Crane J, Frassica F, Zhang L, et al. (2012). Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med 18:1095–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. HaDuong JH, Blavier L, Baniwal SK, Frenkel B, Malvar J, Punj V, Sposto R and DeClerck YA (2015). Interaction between bone marrow stromal cells and neuroblastoma cells leads to a VEGFA-mediated osteoblastogenesis. Int J Cancer 137:797–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Oreffo ROC, Mundy GR, Seyedin SM and Bonewald LF (1989). Activation of the bone-derived latent TGF beta complex by isolated osteoclasts. Biochem Biophys Res Commun 158:817–823 [DOI] [PubMed] [Google Scholar]
- 155. de Gorter DJ, van Dinther M, Korchynskyi O and ten Dijke P (2011). Biphasic effects of transforming growth factor β on bone morphogenetic protein-induced osteoblast differentiation. J Bone Miner Res 26:1178–1187 [DOI] [PubMed] [Google Scholar]
- 156. Ferlin M, Noraz N, Hertogh C, Brochier J, Taylor N and Klein B (2000). Insulin-like growth factor induces the survival and proliferation of myeloma cells through an interleukin-6-independent transduction pathway. Br J Haematol 111:626–634 [DOI] [PubMed] [Google Scholar]
- 157. van Golen CM, Castle VP and Feldman EL (2000). IGF-I receptor activation and BCL-2 overexpression prevent early apoptotic events in human neuroblastoma. Cell Death Differ 7:654–665 [DOI] [PubMed] [Google Scholar]
- 158. Ritchie CK, Andrews LR, Thomas KG, Tindall DJ and Fitzpatrick LA (1997). The effects of growth factors associated with osteoblasts on prostate carcinoma proliferation and chemotaxis: implications for the development of metastatic disease. Endocrinology 138:1145–1150 [DOI] [PubMed] [Google Scholar]
- 159. Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L and Chirgwin JM (2006). Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res 12:6213s–6216s [DOI] [PubMed] [Google Scholar]
- 160. Battula VL, Le PM, Sun JC, Nguyen K, Yuan B, Zhou X, Sonnylal S, McQueen T, Ruvolo V, et al. (2017). AML-induced osteogenic differentiation in mesenchymal stromal cells supports leukemia growth. JCI Insight 2:e90036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Granchi D, Baglìo SR, Amato I, Giunti A and Baldini N (2008). Paracrine inhibition of osteoblast differentiation induced by neuroblastoma cells. Int J Cancer 123:1526–1535 [DOI] [PubMed] [Google Scholar]
- 162. Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B and Shaughnessy JD (2003). The role of the wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349:2483–2494 [DOI] [PubMed] [Google Scholar]
- 163. Bu G, Lu W, Liu CC, Selander K, Yoneda T, Hall C, Keller ET and Li Y (2008). Breast cancer-derived Dickkopf1 inhibits osteoblast differentiation and osteoprotegerin expression: implication for breast cancer osteolytic bone metastases. Int J Cancer 123:1034–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Phimphilai M, Zhao Z, Boules H, Roca H and Franceschi RT (2006). BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res 21:637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Roodman GD. (2004). Mechanisms of bone metastasis. N Engl J Med 350:1655–1664 [DOI] [PubMed] [Google Scholar]
- 166. Ara T and DeClerck YA (2007). Mechanisms of invasion and metastasis in human neuroblastoma. Cancer Metastasis Rev 25:645–657 [DOI] [PubMed] [Google Scholar]
- 167. Hanns P, Paczulla AM, Medinger M, Konantz M and Lengerke C (2019). Stress and catecholamines modulate the bone marrow microenvironment to promote tumorigenesis. Cell Stress 3:221–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Méndez-Ferrer S, Lucas D, Battista M and Frenette PS (2008). Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452:442–447 [DOI] [PubMed] [Google Scholar]
- 169. Tyurin-Kuzmin PA, Fadeeva JI, Kanareikina MA, Kalinina NI, Sysoeva VY, Dyikanov DT, Stambolsky D V and Tkachuk VA (2016). Activation of β-adrenergic receptors is required for elevated α1A-adrenoreceptors expression and signaling in mesenchymal stromal cells. Sci Rep 6:32835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Hajifathali A, Saba F, Atashi A, Soleimani M, Mortaz E and Rasekhi M (2014). The role of catecholamines in mesenchymal stem cell fate. Cell Tissue Res 358:651–665 [DOI] [PubMed] [Google Scholar]
- 171. Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, et al. (2011). Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest 121:1298–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Meads MB, Gatenby RA and Dalton WS (2009). Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer 9:665–674 [DOI] [PubMed] [Google Scholar]
- 173. Schirrmacher V. (2019). From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int J Oncol 54:407–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Rossari F, Zucchinetti C, Buda G and Orciuolo E (2020). Tumor dormancy as an alternative step in the development of chemoresistance and metastasis—clinical implications. Cell Oncol (Dordr) 43:155–176 [DOI] [PubMed] [Google Scholar]
- 175. Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi R, Yoshida M, Tsuda H, Tamura K and Ochiya T (2014). Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal 7:ra63. [DOI] [PubMed] [Google Scholar]
- 176. Bliss SA, Sinha G, Sandiford OA, Williams LM, Engelberth DJ, Guiro K, Isenalumhe LL, Greco SJ, Ayer S, et al. (2016). Mesenchymal stem cell-derived exosomes stimulate cycling quiescence and early breast cancer dormancy in bone marrow. Cancer Res 76:5832–5844 [DOI] [PubMed] [Google Scholar]
- 177. Bartosh TJ, Ullah M, Zeitouni S, Beaver J and Prockop DJ (2016). Cancer cells enter dormancy after cannibalizing mesenchymal stem/stromal cells (MSCs). Proc Natl Acad Sci U S A 113:E6447–E6456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, et al. (2011). Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med 208:2641–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Lifshitz V, Priceman SJ, Li W, Cherryholmes G, Lee H, Makovski-Silverstein A, Borriello L, DeClerck YA and Yu H (2017). Sphingosine-1-phosphate receptor-1 promotes environment-mediated and acquired chemoresistance. Mol Cancer Ther 16:2516–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Ara T, Nakata R, Sheard MA, Shimada H, Buettner R, Groshen SG, Ji L, Yu H, Jove R, Seeger RC and DeClerck YA (2013). Critical role of STAT3 in IL-6-mediated drug resistance in human neuroblastoma. Cancer Res 73:3852–3864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Odate S, Veschi V, Yan S, Lam N, Woessner R and Thiele CJ (2017). Inhibition of STAT3 with the generation 2.5 antisense oligonucleotide, AZD9150, decreases neuroblastoma tumorigenicity and increases chemosensitivity. Clin Cancer Res 23:1771–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Wu H-W, Sheard MA, Malvar J, Fernandez GE, DeClerck YA, Blavier L, Shimada H, Theuer CP, Sposto R and Seeger RC (2019). Anti-CD105 antibody eliminates tumor microenvironment cells and enhances anti-GD2 antibody immunotherapy of neuroblastoma with activated natural killer cells. Clin Cancer Res 25:4761–4774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Tran HC, Wan Z, Sheard MA, Sun J, Jackson JR, Malvar J, Xu Y, Wang L, Sposto R, Kim ES, Asgharzadeh S and Seeger RC (2017). TGFβR1 Blockade with galunisertib (LY2157299) enhances anti-neuroblastoma activity of the anti-GD2 antibody dinutuximab (ch14.18) with natural killer cells. Clin Cancer Res 23:804–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Bagno L, Hatzistergos KE, Balkan W and Hare JM (2018). Mesenchymal stem cell-based therapy for cardiovascular disease: progress and challenges. Mol Ther 26:1610–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Staff NP, Jones DT and Singer W (2019). Mesenchymal stromal cell therapies for neurodegenerative diseases. Mayo Clin Proc 94:892–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ning H, Yang F, Jiang M, Hu L, Feng K, Zhang J, Yu Z, Li B, Xu C, et al. (2008). The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia 22:593–599 [DOI] [PubMed] [Google Scholar]
- 187. Battiwalla M and Hematti P (2009). Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy 11:503–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Crippa S, Santi L, Bosotti R, Porro G and Bernardo ME (2019). Bone marrow-derived mesenchymal stromal cells: a novel target to optimize hematopoietic stem cell transplantation protocols in hematological malignancies and rare genetic disorders. J Clin Med 9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI and Lazarus HM (2000). Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 18:307–316 [DOI] [PubMed] [Google Scholar]
- 190. Batorov EV, Shevela EY, Tikhonova MA, Batorova DS, Ushakova GY, Sizikova SA, Sergeevicheva VV, Gilevich AV, Kryuchkova IV, Ostanin AA and Chernykh ER (2015). Mesenchymal stromal cells improve early lymphocyte recovery and T cell reconstitution after autologous hematopoietic stem cell transplantation in patients with malignant lymphomas. Cell Immunol 297:80–86 [DOI] [PubMed] [Google Scholar]
- 191. Nieddu V, Piredda R, Bexell D, Barton J, Anderson J, Sebire N, Kolluri K, Janes SM, Karteris E and Sala A (2019). Engineered human mesenchymal stem cells for neuroblastoma therapeutics. Oncol Rep 42:35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Relation T, Yi T, Guess AJ, La Perle K, Otsuru S, Hasgur S, Dominici M, Breuer C and Horwitz EM (2018). Intratumoral delivery of Interferonγ-secreting mesenchymal stromal cells repolarizes tumor-associated macrophages and suppresses neuroblastoma proliferation in vivo. Stem Cells 36:915–924 [DOI] [PubMed] [Google Scholar]
- 193. Maniwa J, Fumino S, Kimura K, Tanaka T, Higashi M, Kishida T, Mazda O and Tajiri T (2019). Novel mesenchymal stem cell delivery system as targeted therapy against neuroblastoma using the TH-MYCN mouse model. J Pediatr Surg 54:2600–2605 [DOI] [PubMed] [Google Scholar]
- 194. Sharif S, Ghahremani MH and Soleimani M (2021). Differentiation induction and proliferation inhibition by a cell-free approach for delivery of exogenous miRNAs to neuroblastoma cells using mesenchymal stem cells. Cell J 22:556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Stoff-Khalili MA, Rivera AA, Mathis JM, Banerjee NS, Moon AS, Hess A, Rocconi RP, Numnum TM, Everts M, et al. (2007). Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res Treat 105:157–167 [DOI] [PubMed] [Google Scholar]
- 196. Ruano D, López-Martín JA, Moreno L, Á Lassaletta, Bautista F, Andión M, Hernández C, Á González-Murillo, Melen G, et al. (2020). First-in-human, first-in-child trial of autologous MSCs carrying the oncolytic virus Icovir-5 in patients with advanced tumors. Mol Ther 28:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA and Cox CS (2009). Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev 18:683–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Garofalo M, Villa A, Rizzi N, Kuryk L, Rinner B, Cerullo V, Yliperttula M, Mazzaferro V and Ciana P (2019). Extracellular vesicles enhance the targeted delivery of immunogenic oncolytic adenovirus and paclitaxel in immunocompetent mice. J Control Release 294:165–175 [DOI] [PubMed] [Google Scholar]
- 199. Fujii S, Miura Y, Fujishiro A, Shindo T, Shimazu Y, Hirai H, Tahara H, Takaori-Kondo A, Ichinohe T and Maekawa T (2018). Graft-versus-host disease amelioration by human bone marrow mesenchymal stromal/stem cell-derived extracellular vesicles is associated with peripheral preservation of naive T cell populations. Stem Cells 36:434–445 [DOI] [PubMed] [Google Scholar]
- 200. Mu CF, Shen J, Liang J, Zheng HS, Xiong Y, Wei YH and Li F (2018). Targeted drug delivery for tumor therapy inside the bone marrow. Biomaterials 155:191–202 [DOI] [PubMed] [Google Scholar]
- 201. Klein S, Abraham M, Bulvik B, Dery E, Weiss ID, Barashi N, Abramovitch R, Wald H, Harel Y, et al. (2018). CXCR4 promotes neuroblastoma growth and therapeutic resistance through miR-15a/16-1-mediated ERK and BCL2/Cyclin D1 pathways. Cancer Res 78:1471–1483 [DOI] [PubMed] [Google Scholar]
- 202. Komorowski M, Tisonczyk J, Kolakowska A, Drozdz R and Kozbor D (2018). Modulation of the tumor microenvironment by CXCR4 antagonist-armed viral oncotherapy enhances the antitumor efficacy of dendritic cell vaccines against neuroblastoma in syngeneic mice. Viruses 10:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Sun W, Zhang L, Hou L, Ju C, Zhao S and Wei Y (2017). Isatin inhibits SH-SY5Y neuroblastoma cell invasion and metastasis through MAO/HIF-1α/CXCR4 signaling. Anticancer Drugs 28:645–653 [DOI] [PubMed] [Google Scholar]
- 204. Li C, Yang C and Wei G (2018). Vandetanib inhibits cisplatin-resistant neuroblastoma tumor growth and invasion. Oncol Rep 39:1757–1764 [DOI] [PubMed] [Google Scholar]
- 205. Raje N, Terpos E, Willenbacher W, Shimizu K, García-Sanz R, Durie B, Legieć W, Krejčí M, Laribi K, et al. (2018). Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol 19:370–381 [DOI] [PubMed] [Google Scholar]
- 206. Hatse S, Princen K, Bridger G, De Clercq E and Schols D (2002). Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett 527:255–262 [DOI] [PubMed] [Google Scholar]
- 207. Price TT, Burness ML, Sivan A, Warner MJ, Cheng R, Lee CH, Olivere L, Comatas K, Magnani J, et al. (2016). Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci Transl Med 8:340ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, Jia X, Wright R, Ospina B, et al. (2009). CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood 113:4341–4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Morland B, Kepak T, Dallorso S, Sevilla J, Murphy D, Luksch R, Yaniv I, Bader P, Rößler J, et al. (2020). Plerixafor combined with standard regimens for hematopoietic stem cell mobilization in pediatric patients with solid tumors eligible for autologous transplants: two-arm phase I/II study (MOZAIC). Bone Marrow Transplant 55:1744–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Desgrosellier JS and Cherech DA (2015). Integrins in cancer: biological implications in therapeutic opportunities. Nat Rev Cancer 10:9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Horwacik I and Rokita H (2017). Modulation of interactions of neuroblastoma cell lines with extracellular matrix proteins affects their sensitivity to treatment with the anti-GD2 ganglioside antibody 14G2a. Int J Oncol 50:1899–1914 [DOI] [PubMed] [Google Scholar]
- 212. Zhang H-M and Zhang L-S (2009). Influence of human bone marrow mesenchymal stem cells on proliferation of chronic myeloid leukemia cells. Ai Zheng 28:29–32 [PubMed] [Google Scholar]
- 213. Peng Y, Li Z, Yang P, Newton IP, Ren H, Zhang L, Wu H and Li Z (2014). Direct contacts with colon cancer cells regulate the differentiation of bone marrow mesenchymal stem cells into tumor associated fibroblasts. Biochem Biophys Res Commun 451:68–73 [DOI] [PubMed] [Google Scholar]