Abstract
Neurological outcomes following spinal cord injury (SCI) are currently difficult to predict. While the initial American Spinal Injury Association Impairment Scale (AIS) grade can give an estimate of outcome, the high remaining degree of uncertainty has stoked recent interest in biomarkers for SCI. This study aimed to assess the prognostic value of routinely measured blood biomarkers by developing prognostic models of AIS scores at discharge and 12 months post-injury. Routine blood and clinical data were collected from SCI patients (n = 417), and blood measures that had been assessed in less than 50% of patients were excluded. Outcome neurology was obtained from AIS and Spinal Cord Independence Measure III (SCIM-III) scores at discharge and 12 months post-injury, with motor (AIS) and sensory (AIS, touch and prick) abilities being assessed individually. Linear regression models with and without elastic net penalization were created for all outcome measures. Blood measures associated with liver function, such as alanine transaminase, were found to add value to predictions of SCIM-III at discharge and 12 months post-injury. Further, components of a total blood count, including hemoglobin, were found to add value to predictions of AIS motor and sensory scores at discharge and 12 months post-injury. These findings corroborate the results of our previous preliminary study and thus provide further evidence that routine blood measures can add prognostic value in SCI and that markers of liver function are of particular interest.
Keywords: biomarker, blood, modeling, neurology, spinal cord injury
Introduction
Spinal cord injury (SCI) is damage to the spinal cord due to trauma, degeneration, or disease that results in a temporary or permanent change to its neurological function. The global age-standardized incidence of SCI has been estimated to be 13 per 100,000, whereas the age-standardized prevalence was estimated to be 368 per 100,000.1 With respect to the United Kingdom, it has been estimated that over 1000 new SCIs occur each year, and that 40,000 people are living with SCI.2 The majority of SCIs have historically been traumatic in nature, most commonly as a result of vehicular accidents, falls, violence, and sports. But more recently, non-traumatic SCI, usually as a result of infection or cancer, has been increasing in prevalence.3,4
The lifetime cost of SCI in the UK is estimated to be £1.12 million (mean value) per case, with the total cost of SCI in 2016 in the UK being £1.43 billion.5 SCI can lead to secondary conditions that increase morbidity and mortality, including respiratory complications, deep vein thrombosis, muscle spasms, urinary tract infections, osteoporosis, pressure ulcers, risk of fracture, and chronic pain. Further, patients with SCI are often rendered dependent on caregivers and show markedly higher rates of mental illness relative to the general population.6
There is a challenge in the development of novel therapeutic interventions for SCI, with only four large-scale clinical trials having been tested in acute SCI, three of which evaluated methylprednisolone and one evaluated GM-1 ganglioside.7–10 This is due to the SCI population being inherently heterogeneous and experiencing a highly variable degree of “natural” recovery.11 Currently, the best predictor of neurological outcome is the initial measure of neurologic impairment, as assessed with the International Standards for Neurological Classification of SCI (ISNCSCI) examination.12 However, the ISNCSCI examination was not intended to be predictive of functional recovery, and it has been found that changes in American Spinal Injury Association Impairment Scale (AIS) grade do not necessarily indicate meaningful changes to daily living for patients.13 Robust SCI biomarkers could help stratify patients such that their baseline functional recovery could be predicted, allowing any potential novel therapies to be properly assessed, thus accelerating research and clinical trials in particular via covariate adjustment.14
A reliable prognostic model of SCI would also allow healthcare providers to better plan patient care, relieve patients of potentially damaging psychological uncertainty, and could highlight new avenues of research.15 While relatively few studies have sought to identify prognostic biomarkers for SCI, recent years have seen some early/discovery phase publications.16–19 These preliminary studies have largely focused on biomarkers in cerebral spinal fluid during the acute phase of injury, with little information regarding the chronic or recovery phase. Even among these studies, however, there has been little investigation as to the value of blood biomarkers in SCI at any injury phase, despite success in other fields, including cancer, traumatic brain injury, and Alzheimer's disease.20-22
We previously published a preliminary study that highlighted the value of routinely measured blood analytes in prognostic models of SCI, and demonstrated that some blood measures, particularly markers of liver function, added modest but statistically significant value to predictions of 3- and 12-month ISNSCI AIS motor and sensory scores.23 In this study, we have validated our findings in another, independent and larger SCI cohort. We have further developed alternative, more robust methods of modeling and have demonstrated that similar markers, including alanine transaminase (ALT) and gamma-glutamyl transferase (GGT) add value not only when predicting AIS scores at discharge and 12 months, but also with regard to Spinal Cord Independence Measure (SCIM) outcomes.
Methods
Patient and model feature summary
We retrospectively studied the electronic health records of 500 patients who had been admitted to the Midlands Centre for Spinal Injuries in the last 10 years (Table 1). Access to these records was ethically approved by the National Research Ethics Service (NRES) Committee North West Liverpool East (11/NW/0876) and NRES Committee West Midlands, Staffordshire (13/WM/0158). Following the exclusion of patients who had been admitted over 6 months post-injury, 73 individuals were removed from further analysis.
Table 1.
Patient Demographics
| Number of SCI patients (n out of 417) | Percent | ||
|---|---|---|---|
| Age at injury (median years) | 56 ± 28 | ||
| Length of stay (median days) | 100 ± 66 | ||
| Fracture | 225 | 53 | |
| Surgery | 217 | 51 | |
| Traumatic injury | 319 | 75 | |
| Type 1 diabetes | 5 | 1 | |
| Smoker | Type 2 diabetes | 44 | 10 |
| No | 281 | 66 | |
| Yes | 52 | 12 | |
| Alcohol consumption | Unknown | 84 | 20 |
| No | 181 | 42 | |
| Yes | 152 | 36 | |
| Gender | Unknown | 84 | 20 |
| Male | 283 | 66 | |
| Time from injury (median days) | Female | 134 | 31 |
| First blood test | 22 ± 35 | ||
| Admission | 20 ± 34 | ||
| Discharge | 128 ± 82 | ||
| Neurological level of injury | Month-12 assessment | 390 ± 103 | |
| Cervical | 244 | 57 | |
| Lumbar | 30 | 7 | |
| Sacral | 1 | 0 | |
| Admission AIS grade | Thoracic | 142 | 33 |
| A | 108 | 25 | |
| B | 48 | 11 | |
| C | 151 | 35 | |
| D | 110 | 26 | |
| AIS conversion from admission to 12 months | A-B | 4 | 0.9 |
| A-C | 4 | 0.9 | |
| A-D | 1 | 0.2 | |
| B-C | 11 | 2.6 | |
| B-D | 4 | 0.9 | |
| C-D | 47 | 11 | |
| C-E | 1 | 0.2 | |
| D-E | 1 | 0.2 | |
| AIS conversion from admission to discharge | A-B | 4 | 0.9 |
| A-C | 4 | 0.9 | |
| B-C | 13 | 3 | |
| B-D | 4 | 0.9 | |
| C-D | 47 | 11 | |
| D-E | 3 | 0.7 |
Time periods are ± interquartile range.
SCI, spinal cord injury; AIS, American Spinal Injury Association Impairment Scale.
The remaining 417 patients had their initial blood sample taken at a mean of 31 ± 30 (standard deviation) days post-injury. Blood measures that had been assessed in less than 50% of the patient cohort were excluded. The remaining blood measures included adjusted calcium estimate, alkaline phosphatase, C-reactive protein (CRP), hematocrit, hemoglobin, mean cell hemoglobin, mean cell volume, mononucleocytes, platelets, potassium, red blood count, red blood distance width, and white blood count (WBC). Routine blood analyses were conducted in the Hematology and Biochemistry department located at the Robert Jones and Agnes Hunt Orthopedic Hospital. Hematology analyses were performed on either a Beckman Coulter LH-500 (Beckman Coulter, High Wycombe) or a Sysmex XN-1000 (Sysmex America, IL). Biochemical analyses used VITROS slides (dry multi-layered chemistry slides) in conjunction with the VITROS 5,1 FS Chemistry System (Ortho Clinical Diagnostics, NJ) to measure albumin, ALT, calcium, creatinine, GGT, potassium, magnesium, sodium, total bilirubin, total protein, and urea.
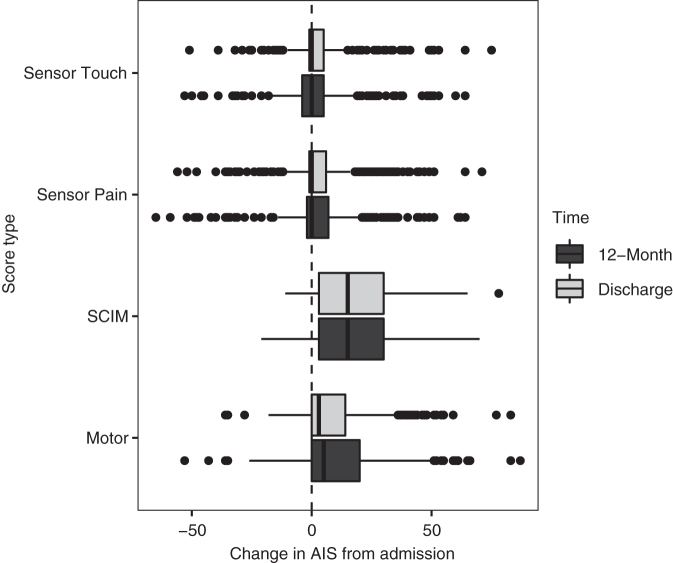
In addition to AIS overall grade, AIS motor, sensory touch, and sensory pin prick scores were recorded at admission, discharge (mean 136 days post-injury ±72), and approximately 12 months post-injury (mean 424 days post-injury ±147). SCIM-III assessments also were recorded at these same time-points.24 The SCIM assessment is a disability scale developed to quantify the ability of SCI patients to perform basic activities of independent daily living, including self-care (feeding, bathing, and dressing), respiration and sphincter management, and mobility (Fig. 1).25,26
FIG. 1.
Boxplots of American Spinal Injury Association Impairment Scale (AIS) score change from admission. SCIM, Spinal Cord Independence Measure.
Additional information that may impact neurological recovery and/or the assessed blood measures were included. The incidence of diabetes (types I and II), smoking, and alcohol drinking status were recorded as binary. The neurological level of the injury was recorded as being cervical, thoracic, lumbar, or sacral. Details were recorded as to whether the injury was traumatic, and whether there were any fractures at the injury site. Age at injury in years, gender, and the time between injury and the first blood tests in days also were included. Medications that patients were prescribed also were collected; however, after filtering to drugs at least 50% of patients were given, the remaining drugs were either painkillers or anti-spasm medication. As the inclusion of these drug data would have added a large number of variables to the model and they correlated strongly with initial injury severity, these data were not included in the modeling process.
Statistical analysis
Data analyses were performed with the statistical programming language R version 3.6.3 (2020-02-29).27–40 Missing blood measures were median imputed, then scaled and centered. Less than 21% of the initial and discharge AIS/SCIM scores were missing, whereas 50-60% of the 12-month scores were missing (Supplementary Table S1). These missing AIS grades or scores were imputed with either last observation carried forwards (LOCF) or next observation carried backwards (NOCB) where relevant. LOCF and NOCB were used as it is unusual for AIS or SCIM scores to have decreased over time in SCI patients. These scores typically only either remain largely unchanged, or improve with time.41 Therefore, the use of this imputation effectively assumes that in cases of missing score data, the patients' score did not change. This assumption can only worsen model performance, as opposed to giving rise to the overly optimistic models that could be generated by more complex multiple imputation techniques. Additionally, we have been advised that most cases where neurological assessment was missing at admission or discharge is due to a transition from Frankel scoring to AIS. In the case of missing 12-month assessments, this is most commonly due to a given patient not attending their appointment or having received follow up from a different hospital (Supplementary Table S1).
As the number of model features was relatively high compared with the number of observations (45 features and 417 observations), linear regression with elastic net penalization was performed in addition to linear regression without any penalization. Elastic net penalization is a hybrid of ridge regression (whereby the penalty term shrinks predictor effect equally and never to 0), and least absolute shrinkage and selection operator, whereby the penalty term shrinks each predictor differently and allows variables to be removed entirely by shrinking coefficients to 0.42,43 Put simply, elastic net reduces the impact of less important model features and can effectively eliminate features entirely, thus performing variable selection during the model building process, as opposed to other methods such as backward variable selection, which are conducted before model building and eliminate features based on co-linearity. Elastic net penalization has been previously found to perform well in models with numerous predictors and in the presence of correlated predictors.44
Eight independent models were generated, with and without elastic net penalization, to determine if the features could predict four outcome measures AIS motor, AIS sensor touch, AIS sensor prick, and SCIM, at two time-points: discharge and 12 months post-injury. The data were randomly split 80-20%, whereupon 80% was used for training the model and the remaining 20% was used to test the model's performance. To reduce model overfitting, internal validation was performed by 10-fold cross-validation.45
Results
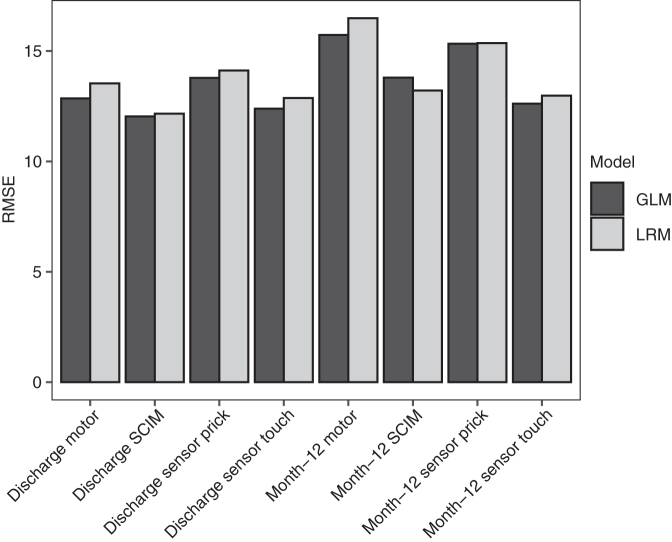
Multiple regression models of the AIS motor and sensory scores and of SCIM at discharge (mean 136 ± 72 days post-injury) and approximately 12 months post injury (mean 424 ± 147 days post-injury) were built (Supplementary Tables S2, S3, and S4). In addition to standard linear regression models (LRMs), generalized linear models (GLMs) with elastic net penalization also were performed. The modeling techniques performed similarly (GLM R2 range 0.56-0.79 and root mean square error (RMSE) range 11-18; LRM R2 range 0.53-0.76 and RMSE range 12-19; Fig. 2 and Fig. 3)
FIG. 2.
R2 for models of neurological outcome at discharge and 12 months post-injury. GLM, generalized linear model; LRM, linear regression model; SCIM, Spinal Cord Independence Measure.
FIG. 3.
Root mean square error (RMSE) for linear regression models with and without elastic net penalization (GLM and LRM, respectively) of neurological outcome at discharge and 12 months post-injury. GLM, generalized linear model; LRM, linear regression model; SCIM, Spinal Cord Independence Measure.
Model features
With respect to model features, AIS measures of initial neurological function were the most consistently conserved features and the most powerful predictors of outcome measures for the generalized models. Initial SCIM was also included for all the models of outcome, except those relating to discharge sensory prick, touch and 12-month sensory touch. The blood markers, ALT, albumin, alkaline phosphatase, CRP, creatinine, GGT, hematocrit, hemoglobin, mean cell hemoglobin, mean cell volume, monocytes, platelets, potassium, total bilirubin, total protein, urea, and WBC were significant (p < 0.05) or included in one or more models (Table 2).
Table 2.
Counts of Model Feature Occurrence
| Model feature | GLM | LRM |
|---|---|---|
| (Intercept) | 8 | 8 |
| Admission AIS grade B | 2 | 2 |
| Admission AIS grade C | 6 | 6 |
| Admission AIS grade D | 6 | 6 |
| Age at injury | 2 | 2 |
| Alanine transaminase (μ/L) | 2 | 0 |
| Albumin (g/L) | 1 | 0 |
| Alkaline phosphatase (μ/L) | 1 | 0 |
| C-reactive protein (mg/L) | 1 | 0 |
| Creatinine (μmol/L) | 4 | 2 |
| Drinking yes | 5 | 1 |
| Fracture | 1 | 1 |
| Gamma GT (μ/L) | 1 | 0 |
| Hematocrit (L/L) | 4 | 0 |
| Hemoglobin (g/L) | 5 | 0 |
| Initial motor | 8 | 6 |
| Initial SCIM | 4 | 2 |
| Initial sensor prick | 8 | 2 |
| Initial sensor touch | 5 | 3 |
| Lumbar injury | 2 | 0 |
| Mean cell Hb (pg) | 4 | 0 |
| Mean cell volume (fL) | 6 | 0 |
| Monocytes (10*9/L) | 7 | 0 |
| Neurological level T | 1 | 0 |
| Platelets (10*9/L) | 1 | 0 |
| Potassium (mmol/L) | 1 | 0 |
| Sex | 2 | 1 |
| Smoker status known | 1 | 0 |
| Smoker status unknown | 0 | 1 |
| Surgery | 1 | 0 |
| Time to first blood test (days) | 0 | 2 |
| Total bilirubin (μmol/L) | 5 | 3 |
| Total protein (g/L) | 1 | 0 |
| Type 1 diabetes | 2 | 0 |
| Type 2 diabetes | 3 | 1 |
| Urea (mmol/L) | 1 | 1 |
| White blood count (10*9/L) | 1 | 0 |
For unpenalized LRM, statistically significant (p < 0.05) features are included. For penalized models (GLM), features that were not penalized to 0 are induced.
GLM, generalized linear model; LRM, linear regression models; AIS, American Spinal Injury Association Impairment Scale; GT, glutamyl transferase; SCIM, Spinal Cord Independence Measure; Hb, hemoglobin.
For the linear regression models, the AIS grade on admission was the only feature that was statistically significant (p < 0.05) in all models except 12-month SCIM. The initial measure of the model target—the initial AIS motor score for the models of discharge and 12-month AIS motor for example—also was significant in all models. Other significant features that were not blood measures included diabetes and smoker status, age at injury, time until first blood test from injury, the neurological level of injury, gender, and the presence of fracture at the injury site. With regard to blood measures, urea, monocytes, mean cell hemoglobin, mean cell volume, hematocrit, and hemoglobin were all significant in one or more of the models (Table 2).
Model performance
With respect to model predictions, both modeling techniques performed similarly when predicting against the test data (Figs. 4 and S1 S2 S3 S4 S5 S6 S7).
FIG. 4.
Predicted Spinal Cord Independence Measure (SCIM) score at discharge compared with the observed SCIM scores in the test data.
Discussion
Penalized GLM was compared with linear regression in the study due to the sample size. While there has long been a dogma that 10 events per variable is sufficient, more recent studies have argued that there is no rational for this.46,47 As there were 417 patients and 45 variables, we also investigated the impact of modeling with and without variable selection in the form of elastic net penalization.
In this study, a standard linear regression model with no variable selection performed very similarly to GLM with elastic net penalization with respect to R2 and RMSE, although the R2 of GLM was slightly higher and RMSE slightly lower for all model targets (Fig. 1 and Fig. 2). This suggests that elastic net penalization does not provide a substantial boost to overall model performance at this sample size relative to linear regression. However, there was a difference in the variables each model utilized.
With regard to blood measures in the linear regression models, urea, total bilirubin and creatinine were significant predictors for one or more outcomes. Creatinine was predictive of discharge SCIM and sensor touch. Total bilirubin was predictive motor, sensor prick, and sensor touch at Month 12, suggesting it is predictive of longer-term outcomes. Urea, which is typically used as an indicator of kidney function but may also be altered due to hydration status, was predictive of discharge SCIM in the standard linear regression model, but was predictive of Month 12 sensor touch in the penalized models.
With the exception of time to first blood test from injury, all of the same features were included in the penalized models and the linear regression models, but other related bloods were also included, such as mean cell hemoglobin, mean cell volume, hematocrit, hemoglobin, platelets, and WBC, which are the components of a complete blood count. The complete blood count is likely related to the initial injury severity via blood loss due to bony soft tissue or visceral injury, gastrointestinal bleeding, and/or surgery.48 Monocytes were included in all GLM models at both time-points except Month 12 SCIM. Similar to the components of the complete blood count, monocytes levels may be indicative of anemia (if low), but have also been associated with hepatitis and inflammatory diseases (if high).49,50 Estimated serum creatinine, based on glomerular filtration rates, are typically used in the evaluation of renal function.51,52 SCI patients also have been found to have an increased risk of renal deterioration and are recommended to receive lifelong, regular renal and upper urinary tract examinations after injury.53,54 SCI has been found to lead to systemic inflammation which can in turn cause secondary organ complications, including in the liver, kidneys, and lungs, which may explain why these blood measures are useful in predicting outcome.55–58
Some studies have found SCI to induce hepatic lipid deposition and inflammation within 3 months of injury in rats, which is symptomatic of non-alcoholic steatohepatitis, the hepatic presentation of metabolic syndrome.59,60 Importantly, the blood measures associated with liver function (alanine transaminase, alkaline phosphatase, CRP, GGT, and total bilirubin) highlighted in this study also were found to be significantly predictive of AIS scores in our preliminary study. Two factors—“liver function,” consisting of alanine transaminase, alkaline phosphatase and GGT, and “liver function and inflammation,” consisting of CRP and total bilirubin—added statistically significant value to models of AIS touch and pain scores at 3 months post-injury, and AIS motor and pain scores at 12 months.23,61,62 Total bilirubin in particular was included in five of eight penalized models and was significant in three of the non-penalized models. This provides further evidence that liver function is relevant to neurological recovery in SCI.
Interestingly, alanine transaminase, alkaline phosphatase, GGT, and albumin were only retained in the models of SCIM. This could be because these markers indicate liver status, which in turn typically reflects general metabolic health. Therefore, aberrant ALT and GGT values may be a proxy measure of poor metabolic health or systemic inflammation. Diabetes status was also significant in six of the 16 models built in this study, which may also reflect the relevance of general metabolic health in recovery. Metabolic syndrome is also more common in SCI patients than the general population and, SCI patients consequently have an increased risk of diabetes, stroke, and heart disease.60,63-65
Serum albumin also has been previously found to be significantly predictive of AIS grade improvement up to 52 weeks.66 Platelets and gender also were only retained in models of SCIM. Previous studies contradict this result and have suggested that gender does not significantly correlate with functional neurology or independence.67,68 However, it may be that some elements of the SCIM questionnaire are easier for males, such as self-catheterization, and so they are able to obtain slightly higher scores than females, even at a similar level of neurological function (as determined by AIS scores). Interestingly, surgery was only found to be a significant predictor of SCIM at both time-points in the GLM models. This suggests surgery does not have a substantial influence on AIS outcomes. It should be stressed that this hospital favors a conservative approach to care of SCI patients, only choosing to operate in the most extreme cases and so both the rate and type of surgery given to this cohort likely differ from other spinal centers.69 Therefore, external validation with data from centers with the more common surgical approach to SCI care is needed to more fully establish the role of surgery in predicting outcomes.70,71
Whether the injury was traumatic or not was not retained in any model. Despite the distinct pathophysiology of non-traumatic injuries, this data suggests trauma status is not a strong predictor of AIS motor or sensor score outcomes.72 Prior studies have also observed similar functional outcomes between traumatic and non-traumatic injuries.73 Further research is needed to establish the role of the liver in SCI, particularly whether the liver is causally implicated in functional recovery, or if it is merely a proxy indicator of systemic inflammation inhibiting healing. Once this association is established, clinicians could consider monitoring the liver function of SCI patients more closely, perhaps attempting to restore/maintain healthy parameters in the interim by minimizing the use of hepatotoxic drugs where possible.
An important limitation of this study is the volume and completeness of the data used in model building. A larger sample size will always lead to a more robust and widely applicable model, and while there was enough to build linear regression models, a larger dataset (> 5000) could allow for robust logistic regression models to predict a change in AIS grade. Further, the data used here contained missing values, and while these were imputed to have minimal effect on model performance, it is still preferable to have a complete dataset. Models of 12-month outcomes were built using discharge and admission scores with the same methodology, and while these models performed better overall, the proportion of missing values at the 12-month time-point, sample size, and more modest difference in average AIS score between discharge and 12 months may cause overfitting, therefore this data was not included. Finally, an independent external validation of these models on separate data, potentially with a cohort with more typical surgical based care, would be desirable, particularly for the GLMs as it is difficult to obtain robust estimates of bias in penalized regression, making standard errors and confidence intervals inappropriate.74
Conclusion
The results from this study suggest that routinely measured blood analytes can provide useful prognostic information for AIS scores and SCIM assessments up to 12 months post-injury, reinforcing the findings of our preliminary study.23 Markers of liver function are of particular interest, and rehabilitation clinicians should consider the maintenance of liver health as a priority as it may be relevant to neurologic functional recovery. More research is needed to establish whether or not the relationship between SCI recovery and liver function is causal. Ultimately these finding need to be validated on a larger independent cohort before any firm clinical recommendations can be made.
Supplementary Material
Acknowledgments
We thank the clinical care team for taking blood samples, and the hematology laboratory for processing the samples and assisting with data collection.
Funding Information
Funding for this study was provided by the Engineering and Physical Sciences Research Council, the Institute of Orthopedics, and the Midlands Centre for Spinal Injuries.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Badhiwala J.H., Wilson J.R., and Fehlings M.G. (2019). Global burden of traumatic brain and spinal cord injury. Lancet Neurol. 18, 24–25 [DOI] [PubMed] [Google Scholar]
- 2. Lee B.B., Cripps R.A., Fitzharris M., and Wing P.C. (2014). The global map for traumatic spinal cord injury epidemiology: Update 2011, global incidence rate. Spinal Cord 52, 110–116 [DOI] [PubMed] [Google Scholar]
- 3. Ge L., Arul K., Ikpeze T., Baldwin A., Nickels J.L., and Mesfin A. (2017). Traumatic and nontraumatic spinal cord injuries. World Neurosurg. 1–7 [DOI] [PubMed] [Google Scholar]
- 4. Sekhon L.H.S., Fehlings M.G., and Frcs C. (2001). Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 26, 2–12 [DOI] [PubMed] [Google Scholar]
- 5. McDaid D., Park A.L., Gall A., Purcell M., and Bacon M. (2019). Understanding and modelling the economic impact of spinal cord injuries in the United Kingdom. Spinal Cord 57, 778–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Furlan J.C., Gulasingam S., and Craven B.C. (2017). The health economics of the spinal cord injury or disease among veterans of war: a systematic review. J. Spinal Cord Med. 40, 649–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bracken M.B., Shepard M.J., Holford T.R., Leo-summers L., Aldrich E.F., Fazl M., Fehlings M., Herr D.L., Hitchon P.W., Marshall L.F., Nockels R.P., Pascale V., Perot P.L., Piepmeier J., Richard H., and Wilberger J.E. (1997). Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. JAMA 277, 1597–1604 [PubMed] [Google Scholar]
- 8. Bracken M.B. (1984). Efficacy of methylprednisolone in acute spinal cord injury. JAMA 251, 45. [PubMed] [Google Scholar]
- 9. Evaniew N., Noonan V.K., Fallah N., Kwon B.K., Rivers C.S., Ahn H., Bailey C.S., Christie S.D., Fourney D.R., Hurlbert R.J., Linassi A.G., Fehlings M.G., and Dvorak M.F. (2015). Methylprednisolone for the treatment of patients with acute spinal cord injuries: a propensity score-matched cohort study from a Canadian multi-center spinal cord injury registry. J. Neurotrauma 32, 1674–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geisler F., Coleman W., Grieco G., and Poonian D. (2001). The Sygen multicenter acute spinal cord injury study. Spine 26. [DOI] [PubMed] [Google Scholar]
- 11. Fawcett J.W., Curt A., Steeves J.D., Coleman W.P., Tuszynski M.H., Lammertse D., Bartlett P.F., Blight A.R., Dietz V., Ditunno J., Dobkin B.H., Havton L.A., Ellaway P.H., Fehlings M.G., Privat A., Grossman R., Guest J.D., Kleitman N., Nakamura M., Gaviria M., and Short D. (2007). Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: Spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45, 190–205 [DOI] [PubMed] [Google Scholar]
- 12. Betz R., Biering-Sørensen F., Burns S.P., Donovan W., Graves D.E., Guest J., Jones L., Kirshblum S., Krassioukov A., Mulcahey M.J., Schmidt Read M., Rodriguez G.M., Rupp R., Schuld C., Tansey K., Walden K., and ASIA IS CoS International Standards Committee. (2019). The 2019 revision of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)—what's new? Spinal Cord 57, 815–817 [DOI] [PubMed] [Google Scholar]
- 13. Middendorp J.J. van, Hosman A.J.F., Pouw M.H., and de Meent H.V. (2009). ASIA impairment scale conversion in traumatic SCI: Is it related with the ability to walk? A descriptive comparison with functional ambulation outcome measures in 273 patients. Spinal Cord 47, 555–560 [DOI] [PubMed] [Google Scholar]
- 14. Kahan B.C., Jairath V., Doré C.J., and Morris T.P. (2014). The risks and rewards of covariate adjustment in randomized trials: An assessment of 12 outcomes from 8 studies. Trials 15, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tuszynski M.H., Steeves J.D., Fawcett J.W., Lammertse D., Kalichman M., Rask C., Curt A., Ditunno J.F., Fehlings M.G., Guest J.D., Ellaway P.H., Kleitman N., Bartlett P.F., Blight A.R., Dietz V., Dobkin B.H., Grossman R., and Privat A. (2007). Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP Panel: clinical trial inclusion/exclusion criteria and ethics. Spinal Cord 45, 222–231 [DOI] [PubMed] [Google Scholar]
- 16. Halford J., Shen S., Itamura K., Levine J., Chong A.C., Czerwieniec G., Glenn T.C., Hovda D.A., Vespa P., Bullock R., Dietrich W.D., Mondello S., Loo J.A., and Wanner I.B. (2017). New astroglial injury-defined biomarkers for neurotrauma assessment. J. Cereb. Blood Flow Metab. 37, 3278–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hulme C.H., Brown S.J., Fuller H.R., Riddell J., Osman A., Chowdhury J., Kumar N., Johnson W.E., and Wright K.T. (2017). The developing landscape of diagnostic and prognostic biomarkers for spinal cord injury in cerebrospinal fluid and blood. Spinal Cord 55, 114–125 [DOI] [PubMed] [Google Scholar]
- 18. Kwon B.K., Bloom O., Wanner I.B., Curt A., Schwab J.M., Fawcett J., and Wang K.K. (2019). Neurochemical biomarkers in spinal cord injury. Spinal Cord 57, 819–831 [DOI] [PubMed] [Google Scholar]
- 19. Moghieb A., Bramlett H.M., Das J.H., Yang Z., Selig T., Yost R.A., Wang M.S., Dietrich W.D., and Wang K.K. (2016). Differential neuroproteomic and systems biology analysis of spinal cord injury. Mol. Cell Proteomics 15, 2379–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blennow K. (2017). A review of fluid biomarkers for Alzheimer's Disease: moving from CSF to blood. Neurol. Ther. 6, 15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kunzmann A.T., McMenamin, Ú.C., Spence A.D., Gray R.T., Murray L.J., Turkington R.C., and Coleman H.G. (2018). Blood biomarkers for early diagnosis of oesophageal cancer: a systematic review. Eur. J. Gastroenterol. Hepatol. 30, 263–273 [DOI] [PubMed] [Google Scholar]
- 22. Lugones M., Parkin G., Bjelosevic S., Takagi M., Clarke C., Anderson V., and Ignjatovic V. (2018). Blood biomarkers in paediatric mild traumatic brain injury: a systematic review. Neurosci. Biobehav. Rev. 87, 206–217 [DOI] [PubMed] [Google Scholar]
- 23. Brown S.J., Harrington G.M.B., Hulme C.H., Morris R., Bennett A., Tsang W.H., Osman A., Chowdhury J., Kumar N., and Wright K.T. (2019). A preliminary cohort study assessing routine blood analyte levels and neurological outcome after spinal cord injury. J. Neurotrauma 37, 466–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amiram C., Itzkovich M., Steinberg F., Ring H., Ronen J., Philo O., Spasser R., Gepstein R., and Tamir A. (2001). The Catz-Itzkovich SCIM: a revised version of the spinal cord independence measure. Disabil. Rehabil. 23, 263–268 [DOI] [PubMed] [Google Scholar]
- 25. Ackerman P., Morrison S.A., McDowell S., and Vazquez L. (2010). Using the Spinal Cord Independence Measure III to measure functional recovery in a post-acute spinal cord injury program. Spinal Cord 48, 380–387 [DOI] [PubMed] [Google Scholar]
- 26. Itzkovich M., Gelernter I., Biering-Sorensen F., Weeks C., Laramee M.T., Craven B.C., Tonack M., Hitzig S.L., Glaser E., Zeilig G., Aito S., Scivoletto G., Mecci M., Chadwick R.J., Masry W.S.E., Osman A., Glass C.A., Silva P., Soni B.M., Gardner B.P., Savic G., Bergström E.M., Bluvshtein V., Ronen J., and Catz P.A. (2007). The Spinal Cord Independence Measure (SCIM) version III: reliability and validity in a multi-center international study. Disabil. Rehabil. 29, 1926–1933 [DOI] [PubMed] [Google Scholar]
- 27. Francois R. (2020). Bibtex: Bibtex parser for R. https://github.com/romainfrancois/bibtex (Last accessed August3, 2020)
- 28. Alathea L. (2015). Captioner: Numbers figures and creates simple captions. https://github.com/adletaw/captioner (Last accessed August3. 2020)
- 29. Kuhn M. (2020). Caret: Classification and regression training. https://github.com/topepo/caret (Last accessed August3. 2020)
- 30. Aust F. (2019). Citr: ‘RStudio’ add-in to insert markdown citations. https://github.com/crsh/citr (Last accessed August3. 2020)
- 31. Dowle M., and Srinivasan A. (2019). Data.table: Extension of ‘data.frame.’ https://rdatatable.gitlab.io/data.table (Last accessed August3. 2020)
- 32. Harrell Jr F.E., Charles Dupont, and others. (2020). Hmisc: Harrell miscellaneous. https://github.com/harrelfe/Hmisc (Last accessed August3. 2020)
- 33. Zhu H. (2019). KableExtra: Construct complex table with ‘kable’ and pipe syntax. https://github.com/haozhu233/kableExtra (Last accessed August3. 2020)
- 34. Tierney N., Cook D., McBain M., and Fay C. (2020). Naniar: Data structures, summaries, and visualisations for missing data. http://naniar.njtierney.com (Last accessed August3. 2020)
- 35. Revelle W. (2019). Psych: Procedures for Psychological, Psychometric, and Personality Research. Northwestern University: Evanston, IL [Google Scholar]
- 36. R_Core_Team. (2019). R: A language and environment for statistical computing. https://cran.r-project.org/doc/manuals/fullrefman.pdf (Last accessed August3. 2020)
- 37. Wickham H., Averick M., Bryan J., Chang W., McGowan L.D., François R., Grolemund G., Hayes A., Henry L., Hester J., Kuhn M., Pedersen T.L., Miller E., Bache S.M., Müller K., Ooms J., Robinson D., Seidel D.P., Spinu V., Takahashi K., Vaughan D., Wilke C., Woo K., and Yutani H. (2019). Welcome to the tidyverse. J. Open Source Softw. 4, 1686 [Google Scholar]
- 38. Zeileis A., and Grothendieck G. (2005). Zoo: S3 infrastructure for regular and irregular time series. J. Stat. Softw. 14, 1–27 [Google Scholar]
- 39. Xie Y. (2020). Knitr: a general-purpose package for dynamic report generation in R. www.rdocumentation.org/packages/knitr/versions/1.29 (Last accessed August3. 2020)
- 40. Allaire J., Xie Y., McPherson J., Luraschi J., Ushey K., Atkins A., Wickham H., Cheng J., Chang W., and Iannone R. (2020). Rmarkdown: dynamic documents for R. https://rmarkdown.rstudio.com/docs/index.html (Last accessed August3. 2020)
- 41. Zariffa J., Kramer J.L.K., Fawcett J.W., Lammertse D.P., Blight A.R., Guest J., Jones L., Burns S., Schubert M., Bolliger M., Curt A., and Steeves J.D. (2011). Characterization of neurological recovery following traumatic sensorimotor complete thoracic spinal cord injury. Spinal Cord 49, 463–471 [DOI] [PubMed] [Google Scholar]
- 42. Tibshirani R. (1996). Regression shrinkage and selection via the Lasso. J. R. Stat. Soc. Series B Stat. Methodol. 58, 267–288 [Google Scholar]
- 43. Zou H. and Hastie T. (2005). Regularization and variable selection via the elastic net. J. R. Stat. Soc. Series B Stat. Methodol. 67, 301–320 [Google Scholar]
- 44. Pavlou M., Ambler G., Seaman S., De Iorio M., and Omar R.Z. (2016). Review and evaluation of penalised regression methods for risk prediction in low-dimensional data with few events. Stat. Med. 35, 1159–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Steyerberg E.W., Bleeker S.E., Moll H.A., Grobbee D.E., and Moons K.G.M. (2003). Internal and external validation of predictive models: a simulation study of bias and precision in small samples. J. Clin. Epidemiol. 56, 441–447 [DOI] [PubMed] [Google Scholar]
- 46. Peduzzi P., Concato J., Kemper E., Holford T.R., and Feinstein A.R. (1996). A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 49, 1373–1379 [DOI] [PubMed] [Google Scholar]
- 47. van Smeden M., de Groot J.A.H., Moons K.G.M., Collins G.S., Altman D.G., Eijkemans M.J.C., and Reitsma J.B. (2016). No rationale for 1 variable per 10 events criterion for binary logistic regression analysis. BMC Med. Res. Methodol. 16, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hirsch G.H., Menard M.R., and Anton H.A. (1991). Anemia after traumatic spinal cord injury. Arch. Phys. Med. Rehab. 72, 195–201 [PubMed] [Google Scholar]
- 49. Shi Q. and Thomas L. (2013). Monocytosis correlated with acute alcoholic Hepatitis: A case report and literature review. Blood 122, 4725–4725 [Google Scholar]
- 50. Yang J., Zhang L., Yu C., Yang X.-F., and Wang H. (2014). Monocyte and macrophage differentiation: Circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark. Res. 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perrone R.D., Madias N.E., and Levey A.S. (1992). Serum creatinine as an index of renal function: New insights into old concepts. Clin. Chem. 38, 1933–1953 [PubMed] [Google Scholar]
- 52. Thomas L. and Huber A.R. (2006). Renal function estimation of glomerular filtration rate. Clin. Chem. Lab. Med. 44, 1295–1302 [DOI] [PubMed] [Google Scholar]
- 53. Elmelund M., Oturai P.S., Toson B., and Biering-Sørensen F. (2016). Forty-five-year follow-up on the renal function after spinal cord injury. Spinal Cord 54, 445–451 [DOI] [PubMed] [Google Scholar]
- 54. Stöhrer M., Blok B., Castro-Diaz D., Chartier-Kastler E., Popolo G.D., Kramer G., Pannek J., Radziszewski P., and Wyndaele J.J. (2009). EAU guidelines on neurogenic lower urinary tract dysfunction. Eur. Urol. 56, 81–88 [DOI] [PubMed] [Google Scholar]
- 55. Bao F., Omana V., Brown A., and Weaver L.C. (2012). The systemic inflammatory response after spinal cord injury in the rat is decreased by a4B1 integrin blockade. J. Neurotrauma 29, 1626–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Campbell S.J., Zahid I., Losey P., Law S., Jiang Y., Bilgen M., van Rooijen N., Morsali D., Davis A.E.M., and Anthony D.C. (2008). Liver Kupffer cells control the magnitude of the inflammatory response in the injured brain and spinal cord. Neuropharmacology 55, 780–787 [DOI] [PubMed] [Google Scholar]
- 57. Fleming J.C., Bailey C.S., Hundt H., Gurr K.R., Bailey S.I., Cepinskas G., Lawendy A.r., and Badhwar A. (2012). Remote inflammatory response in liver is dependent on the segmental level of spinal cord injury. J. Trauma Acute Care Surg. 72, 1194–1201 [DOI] [PubMed] [Google Scholar]
- 58. Gris D., Hamilton E.F., and Weaver L.C. (2008). The systemic inflammatory response after spinal cord injury damages lungs and kidneys. Exp. Neurol. 211, 259–270 [DOI] [PubMed] [Google Scholar]
- 59. Sauerbeck A.D., Laws J.L., Bandaru V.V.R., Popovich P.G., Haughey N.J., and McTigue D.M. (2015). Spinal cord injury causes chronic liver pathology in rats. J. Neurotrauma 32, 159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Farrell G.C. and Larter C.Z. (2006). Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology 43, S99–S112 [DOI] [PubMed] [Google Scholar]
- 61. Giovanni T. and Byrne C.D. (2015). Circulating markers of liver function and cardiovascular disease risk. Arterioscler. Thromb. Vasc. Biol. 35, 2290–2296 [DOI] [PubMed] [Google Scholar]
- 62. Edelstein C.L. (2016). Biomarkers of Kidney Disease. Academic Press. Cambridge, MA [Google Scholar]
- 63. Cragg J.J., Noonan V.K., Krassioukov A., and Borisoff J. (2013). Cardiovascular disease and spinal cord injury. Neurology 81, 723–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cragg J.J., Stone J.A., and Krassioukov A.V. (2012). Management of cardiovascular disease risk factors in individuals with chronic spinal cord injury: an evidence-based review. J. Neurotrauma 29, 1999–2012 [DOI] [PubMed] [Google Scholar]
- 65. Manns P.J., McCubbin J.A., and Williams D.P. (2005). Fitness, inflammation, and the metabolic syndrome in men with paraplegia. Arch. Phys. Med. Rehab. 86, 1176–1181 [DOI] [PubMed] [Google Scholar]
- 66. Tong B., Jutzeler C.R., Cragg J.J., Grassner L., Schwab J.M., Casha S., Geisler F., and Kramer J.L.K. (2018). Serum albumin predicts long-term neurological outcomes after acute spinal cord injury. Neurorehab. Neural Repair 32, 7–17 [DOI] [PubMed] [Google Scholar]
- 67. Cowan R.E. and Anderson K.D. (2019). Replication and novel analysis of age and sex effects on the neurologic and functional value of each spinal segment in the US healthcare setting. Spinal Cord 57, 156–164 [DOI] [PubMed] [Google Scholar]
- 68. New P.W. (2016). The influence of age and gender on rehabilitation outcomes in nontraumatic spinal cord injury. J. Spinal Cord Med. 30, 225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. El Masri Y W.S. (2018). Traumatic spinal injury and spinal cord injury: Point for active physiological conservative management as compared to surgical management. Spinal Cord Ser. Cases 4, 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Batchelor P.E., Wills T.E., Skeers P., Battistuzzo C.R., Macleod M.R., Howells D.W., and Sena E.S. (2013). Meta-analysis of pre-clinical studies of early decompression in acute spinal cord Injury: a battle of time and oressure. PLoS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wilson J.R., Singh A., Craven C., Verrier M.C., Drew B., Ahn H., Ford M., and Fehlings M.G. (2012). Early versus late surgery for traumatic spinal cord injury: The results of a prospective Canadian cohort study. Spinal Cord 50, 840–843 [DOI] [PubMed] [Google Scholar]
- 72. David G., Mohammadi S., Martin A.R., Cohen-Adad J., Weiskopf N., Thompson A., and Freund P. (2019). Traumatic and nontraumatic spinal cord injury: pathological insights from neuroimaging. Nat. Rev. Neurol. 15, 718–731 [DOI] [PubMed] [Google Scholar]
- 73. McKinley W.O., Seel R.T., Gadi R.K., and Tewksbury M.A. (2001). Nontraumatic vs. traumatic spinal cord injury: a rehabilitation outcome comparison. Am. J. Phys. Med. Rehab. 80, 693–699 [DOI] [PubMed] [Google Scholar]
- 74. Jewell N.P. (1984). Small-sample bias of point estimators of the odds ratio from matched sets. Biometrics 40, 421–435 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.