Abstract
In addition to providing life-giving nutrients and other substances to the breastfed infant, human milk can also represent a vehicle of pathogen transfer. As such, when an infectious disease outbreak, epidemic, or pandemic occurs—particularly when it is associated with a novel pathogen—the question will naturally arise as to whether the pathogen can be transmitted through breastfeeding. Until high-quality data are generated to answer this question, abandonment of breastfeeding due to uncertainty can result. The COVID-19 pandemic, which was in full swing at the time this document was written, is an excellent example of this scenario. During these times of uncertainty, it is critical for investigators conducting research to assess the possible transmission of pathogens through milk, whether by transfer through the mammary gland or contamination from respiratory droplets, skin, breast pumps, and milk containers, and/or close contact between mother and infant. To promote the most rigorous science, it is critical to outline optimal methods for milk collection, handling, storage, and analysis in these situations, and investigators should openly share their methods in published materials. Otherwise, the risks of inconsistent test results from preanalytical and analytical variation, false positives, and false negatives are unacceptably high and the ability to provide public health guidance poor. In this study, we provide “best practices” for collecting human milk samples for COVID-19 research with the intention that this will also be a useful guide for future pandemics.
Keywords: human milk, breast milk, COVID-19, methods, collection, pathogen
Background
Human milk is a complex emulsion consisting of a vast array of constituents providing not only nutrition but also protection from pathogens. Concentrations of these constituents vary within an individual, across the lactation period, and even within a feed. Although we do not understand all the factors driving this variability, we know a substantial amount regarding how some factors influence concentrations of some milk constituents. For instance, total lipid content of milk is affected by time postpartum, time of day, time since last feeding, portion of an individual feed (fore- versus hindmilk), maternal body fat level, and in some cases maternal diet.1–4 To complicate matters, lipids and cells can adhere to some types of collection containers that can impact research results,5 and some milk constituents (e.g., viral particles and RNA) can be entrapped in the lipid fraction or other compartments such as exosomes.6–10
Researchers studying human milk composition should, therefore, consider these factors when designing their protocols for collecting human milk to study its composition. For example, collecting a foremilk sample in the morning using an inappropriate collection container may easily lead to inaccurate quantification of milk's lipophilic compounds. Another example is host RNA, which, although in relatively high concentrations in milk, is quickly degraded by intrinsic RNases3,11; as such, milk must be immediately processed or snap frozen for accurate quantification of host RNA. For other constituents (e.g., iron and lactose), concentrations in milk are less prone to variation12,13; in these situations, sample collection and storage protocols can be less stringent.
Milk composition may even vary between breasts—especially regarding immune factors; this fact has been particularly important in the study of HIV transmission through breastfeeding.14 For other milk components, such as microbiota, there exists very little research characterizing modifiable factors (e.g., time of day and time within feed) related to variation; in these situations, best practices and standardization (although not optimization) are typically employed to ensure that samples are collected in a way that reduces risk of contamination and allows data to be compared across studies.
In summary, because human milk composition is highly variable within and among women and can be influenced by many biological and methodological factors, it is fundamentally important that researchers consider and report core aspects of milk collection, handling, and storage when studying it. These aspects include expression mode (electric pump or manual expression), time of day, time postpartum, complete versus partial expression (and if the latter, whether foremilk or hindmilk was collected), breast preparation (was the breast cleaned and if so with what), collection container material (and whether it was sterile), and storage conditions (e.g., time until refrigeration or freezing, temperature, and duration of storage). In addition, sometimes chemical preservatives are utilized, and these should be carefully evaluated as to whether they might impact the researcher's ability to detect the milk constituent of interest.
The primary purpose of this document is to, using evidence gleaned from the literature and expert opinion, delineate a “best practices” framework related to human milk collection, handling, and storage for COVID-19 research related to breastfeeding. Although we recognize that each microbe is unique, it is our hope that this framework will also be applicable to other pathogenic RNA viruses, DNA viruses, bacteria, and maybe even other organismal taxa. In addition to including information related to the study of presence/absence and viability of these types of pathogens, we provide information on how one might best collect milk for the study of immunoglobulins, cytokines, and other soluble factors, and immune cells as these components are typically studied in this context.
Investigators collecting milk for research during an outbreak, epidemic, or pandemic are urged to consider this framework and best practices both in designing their methods and in reporting their findings. Depending on the research question, not all elements of the framework may be relevant; nor may each element be feasible given the patient population and environmental context. However, it is critical for the interpretation of results and to guarantee comparability of findings across studies that key elements of milk collection, handling, and storage be described in published materials. It is noteworthy that this framework and associated best practices will undoubtedly shift as new data emerge related to the nature of the pathogen and how collection and storage conditions do or do not impact the ability to detect and quantify them. Indeed, “best practices” will need to be periodically revised to reflect the evolving state of the science.
Basic Working Definitions Related to Human Milk Research
To help investigators navigate the somewhat unique vocabulary of human milk and lactation research, selected terms have been briefly defined and are provided in Table 1. Many of these definitions are adapted from those provided in LactaPedia,15 which is an excellent resource in this respect.
Table 1.
Definitions of Selected Terms Relevant to Collection of Human Milk for Analysis of Its Composition
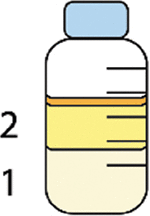 |
Foremilk versus hindmilk | Foremilk: Milk removed from the mammary gland at the beginning of a feed or just before the commencement of a breastfeed or breast expression. This milk often has lower fat and higher cellular contents than milk secreted at the end of a feed. Hindmilk: Milk removed from the mammary gland at the end of a feed or immediately after the completion of a breastfeed or breast expression. This milk often has higher fat and lower cellular contents than milk secreted at the beginning of a feed. |
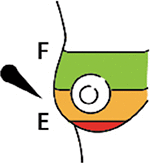 |
Complete breast expression | The process of obtaining nearly all the milk within the mammary gland at a particular pumping or hand expression session. A complete breast expression contains foremilk, hindmilk, and all the milk in between. Obtaining a complete breast expression provides the most representative milk sample possible and is often ideal when there are no data as to whether its composition changes from foremilk to hindmilk for a particular milk component. When complete breast expressions are obtained, researchers should return excess milk to the mother so that she can provide it to her infant. |
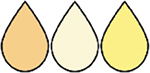 |
Colostrum versus transitional milk versus mature milk | Colostrum: The usually yellowish viscous secretion of the breast synthesized during the first 48 hours postpartum. It is synthesized by the lactocytes of the mammary gland in small volumes (about 30 mL in the first 24 hours after birth). Compared with mature milk, colostrum has high concentrations of sodium, chloride, protein (particularly IgA), and low concentrations of lactose and citrate. Transitional milk: A description of milk as it shifts from colostrum to mature milk after secretory activation (also referred to as “milk coming in”). Transitional milk is yet to be defined objectively, but it is generally considered to extend from about 48 hours after birth to 2–3 weeks postpartum. Mature milk: The secretion produced by the mammary gland after secretory activation (also referred to as “milk coming in”). Human milk is currently considered to be mature after about 2–3 weeks postpartum. |
 |
Exclusive breastfeeding versus partial breastfeeding versus complementary feeding | Exclusive breastfeeding: When the infant receives only human milk through breastfeeding and/or expressed human milk (own mother's or from a donor). This definition often allows the infant to receive drops, syrups (vitamins, minerals, and medicines) but does not allow the infant to receive anything else, including water. Partial breastfeeding: When an infant receives both human milk and any other food or liquid, including water, nonhuman milk, and formula before about 6 months of age. Complementary feeding: Nutrient-containing first foods given during the transition from exclusive breastfeeding to family foods while breastfeeding is maintained. Complementary breastfeeding commences during weaning. |
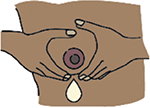 |
Hand expression | The process whereby milk is obtained using manual breast expression by hand alone without the use of a manual or electronic breast pump. |
 |
Subclinical mastitis versus clinical mastitis | Subclinical mastitis: Asymptomatic inflammation of the mammary gland(s) that is not noticeable to the woman or to an observer. Often characterized by a sodium to potassium (Na/K) ratio in milk ≥1. Clinical mastitis: Inflammation of the breast accompanied by pain, swelling, heat/redness, and/or fever. |
 |
Milk fraction | Milk has several collections of components, including cells, lipids, and lactoserum (the aqueous, cell-free phase, whey). These are often referred to as “milk fractions.” |
Framework
In this study, we briefly review the literature describing whether or not selected factors known to impact the concentration and/or stability of some milk components impact a set of components particularly pertinent to research related to potential transmission of pathogens from mother to infant through human milk and/or breastfeeding. These components and attributes include viral DNA and RNA, bacterial DNA, microbial viability, immunoglobulins, cytokines and other soluble components, and immune cells. This information is important because it informs what should be considered, controlled for, or at least reported when human milk is being collected, handled, and stored for this type of research. Table 2 provides a summary of these factors and provides guidance as to whether they should be controlled for and/or reported in studies related to transmission of a pathogen in milk. It is noteworthy that the state of the science for many of these factors is insufficient, and additional research is urgently needed to fill these knowledge gaps.
Table 2.
Milk Components Often Evaluated When Studying Transmission of a Pathogen in Milk, and Whether Various Factors Are Known to Impact Their Concentrations in Milk
| Factor that can impact milk composition | Viral DNA/RNA, bacterial DNA and microbial viability | Antibodies | Cytokines and other soluble factors | Immune cells | |
|---|---|---|---|---|---|
 |
Time of day | Very little known | Very little known | Very little known | May follow diurnal pattern |
 |
Time postpartum | Bacterial profiles likely vary in early lactation | Varies substantially over lactation | Varies substantially over lactation | Varies substantially over lactation |
 |
Hand expression versus pump | Contamination can occur | Very little known | Very little known | Very little known |
 |
Foremilk versus hindmilk versus complete expression | If pathogen is lipophilic it might be more abundant in hindmilk | Very little known | Very little known | Host cells might be higher in hindmilk |
 |
Left versus right breast | May vary with inflammation | May vary with inflammation | May vary with inflammation | May vary with inflammation |
 |
Cleaning breast | DNA and RNA might be on the breast | Not important to control for document | Not important to control for document | Not important to control for document |
 |
Collection container material | Very little known | Very little known | Very little known | Very little known |
Time postpartum
Whereas little is known regarding whether time postpartum per se impacts viral RNA, DNA, and viral viability, some evidence suggests that bacterial profiles and load in milk change over time and particularly between colostrum and mature milk.16–18 In addition, myriad studies have documented an effect of time postpartum on concentrations of immunoglobulins, cytokines soluble factors, and immune cell populations.19–21 For instance, sIgA concentrations in milk decline precipitously from birth to 2 weeks postpartum22; macrophages, lymphocytes, and lactoferrin continue to decrease through 3 months postpartum23; and lysozyme increases.20 Immune cell concentrations also increase during involution, which is generally associated with later times after parturition,23 and during mastitis, which is most common in the first several weeks of lactation.24,25
As such, an attempt should be made to standardize and/or control for time postpartum and breast health (subclinical/clinical mastitis versus none) when comparing data across cohorts (e.g., infected versus noninfected breastfeeding women), and time postpartum when milk was collected should always be reported in publications. In addition, because composition of milk can be affected by premature delivery, whether the infant was born premature or full term should be noted.
Time of day
There is very little published literature rigorously investigating if there is diurnal variation in concentrations of bacterial and viral RNA and DNA, bacterial and viral viability, immunoglobulins, and cytokines and other soluble factors in milk. Limited data, however, suggest that antibody and cytokine concentrations may vary over the course of a day.26,27 A variety of hormones (many of which are known to impact immune function) also vary throughout the day and night.28 Cell content of human milk may also be influenced by the circadian cycle of cortisol, but very little is known about this.29 As such, if collecting repeated milk samples from a woman over time, researchers might consider standardizing the time of day the samples were collected. Alternatively, researchers could employ the “gold standard” approach of collecting complete breast expressions for a 24-hour period and analyzing a representative (composite) sample. If this 24-hour collection methodology is not used, researchers should consider recording time of sample collection in the metadata.
Foremilk versus hindmilk
There is substantial evidence that the lipid content of milk is lower in foremilk than in hindmilk,2,30–32 and that this is likely related to the time since last feeding.33 Whereas some studies have documented lower protein content in foremilk than hindmilk,34–36 others found no difference33 or the opposite.37,38 Limited evidence also suggests that cell content is higher in hindmilk than foremilk.33 To our knowledge, there are no published data using molecular methods relating foremilk versus hindmilk to variation in detectable microbial communities (or their viability), although Rodríguez-Cruz et al. found no difference in microbial profiles between whole milk and skim milk.39 Nonetheless, if the microbe of interest is lipophilic it is possible that it might be found in lower abundance if only foremilk is collected. Because of the potential for differential milk composition within a feed (expression) and the dearth of data related to this factor and microbes, antibodies and other soluble factors, and cells in milk, researchers should report whether and how a complete expression was collected, and if not whether foremilk or hindmilk was primarily obtained, and time since last feed.
Expression mode
Breast pump parts (including tubing) can be contaminated. Consequently, if breast pumps are used, they must be thoroughly disinfected and rinsed to remove all viral/bacterial DNA and RNA and disinfectant. Since foremilk is generally lower in fat than hindmilk, expression mode (hand versus other and complete expression versus partial) might also impact ability to detect a pathogen if it compartmentalizes to the lipid fraction of milk. Nonetheless, Rodríguez-Cruz et al. found no difference in microbial profiles between collected through manual expression and that collected with a pump.39 If hand expression is employed, subjects should thoroughly wash their hands and/or wear clean gloves. Researchers should report if milk was collected using a manual pump, electric pump (and type), or hand expression.
Importantly, both researchers and study participants should follow recommended infection prevention and control measures during the collection and handling of the milk. Depending on the cultural norm, women may be comfortable using breast pumps or using hand expression to express milk; but if not, instruction should be provided by a qualified lactation consultant or personnel with suitable expertise.
Interbreast variation and inflammation
For some milk constituents there can be interbreast variation, and mammary inflammation is known to drive some of these differences. For instance, levels of HIV RNA can differ in milk produced by each breast,40 largely due to differences in mammary inflammatory status.41 Almost nothing is known about intergland difference in other types of pathogens. Pannaraj et al. compared milk microbiomes between healthy human breasts and found no difference42; although studies of dairy cows clearly show that mastitic and healthy glands produce milk with different microbial profiles.43 It is likely that whether there are differences in milk microbiome between mammary glands can depend on mammary health. Because of potential differences in microbial proteins in milk produced by each breast (likely due to inflammation), researchers should ideally collect milk from both breasts; if that is not possible, they should aim to evaluate mammary inflammation either visually (e.g., redness) or chemically in the milk sample (e.g., Na/K ratios, cytokines, and somatic cell count).
Breast preparation
Depending on whether the pathogen is primarily blood-borne, respiratory-borne, or environmental, it is possible that some pathogens may be present on the breast skin. Nonetheless, whether the breast should or should not be cleaned is related to the question at hand. If the question relates to whether the pathogen is incorporated into milk in the mammary gland, then the breast should be cleaned before milk collection. If the question relates to whether the infant might be exposed to the pathogen through breastfeeding or the consumption of pumped milk, then the breast should not be cleaned. If the question relates to antibody or cytokine content of milk, breast cleaning is irrelevant. Researchers should design their collection methods to suit their research question and describe whether/how the breast was cleaned.
Collection containers
Some milk components (e.g., lipids and cells) can adhere to certain materials common to collection containers. Others (e.g., sIgA) have been demonstrated to be stable in both glass and plastic containers.44 Given the inadequate state of the science regarding the importance (or lack thereof) of collection container materials to studying microbes in milk, however, there is no recommendation as to what sort of collection container should be used. This information should be reported, however, in any publication.
Temperature and storage
Refrigeration, freezing, thawing, and application of heat can all affect the stability of many milk components.45–47 In addition, some subpopulations of cells can only be isolated from fresh milk, as they are destroyed or altered by freezing and/or thawing. Conversely, HIV RNA levels have been shown to be remarkably stable in whole milk after three freeze–thaw cycles and for up to 30 hours at room temperature,48 and some data suggest that milk can be stored at 4°C for up to 48 hours or at −20°C or −80°C for at least 6 months without losing its immunological properties.49 For bacterial DNA, Doyle et al. found very little impact of refrigeration temperature (2°C, 4°C, or 6°C) and storage duration (up to 96 hours) on bacterial profiles (through 16S rRNA analysis) in bovine milk.50 There are similar findings for human milk.51,52
Although findings are somewhat mixed,53–55 there is substantial evidence that some soluble factors and characteristics (e.g., antioxidant capacity) in milk can be influenced by refrigeration and freezing, whereas others (e.g., human milk oligosaccharides) are extraordinarily stable.56–58 It has been shown that prolonged storage at 4°C reduces the infectious titer of hepatitis C and Zika virus that has been spiked into milk.59–61
To date, little is known about the stability of SARS-CoV-2 during cold storage, although study from our group suggests that SARS-CoV-2 RNA in milk may be stable for 2 days at 4°C and 7 days at −20°C and can withstand several freeze–thaw cycles.62,63 It is unclear what the impact of freezing and thawing on infectivity would be. However, Walker et al.64 provide evidence that Holder pasteurization (but not cold storage) inactivates SARS-CoV-2; Unger et al.65 and Chambers et al.66 have also shown that Holder pasteurization inactivates SARS-CoV-2 and the former that holding milk at room temperature for 30 minutes also reduces infectious viral titers.
Because they can be destroyed and/or inactivated by temperature changes, care should also be taken to avoid repeat free thaw cycles of milk collected to measure the antibodies to SARS-CoV-2.67,68 In considering the cytopathic effects of viruses (e.g., SARS-CoV-2 and Ebola), researchers need to be aware that the multitude of immune components in human milk with significant antiviral activity may immediately impact cytopathic activity when milk is held at room temperature or 4°C.64,69
In summary, researchers are encouraged to consider whether the milk component of interest is stable under the available storage conditions and, similar to all important factors, report the temperature at which milk was stored before analysis. If in doubt, it is always safest to analyze fresh milk or freeze it at the lowest temperature possible as soon as possible and keep it frozen until it is analyzed. Creating aliquots of the sample is often advised to avoid freeze–thaw cycles of samples.
Milk fraction
When detecting SARS-CoV-2 RNA through quantitative PCR in spiked milk samples, defatted milk yielded better recovery rates than did whole milk.63 Conversely, up to one-third of HIV RNA in milk produced by infected women may be sequestered in the lipid fraction.7 Rodríguez-Cruz et al. found no difference in bacterial profiles between whole milk and skim milk.39
Best Practices for Milk Collection and Storage
Hereunder, we provide “best practices” for research purposes in various settings, recognizing that what is possible, ethical, and desirable depends greatly on the research question, context, and capabilities of each research group as well as maternal and infant factors.
Step 1. Breast cleaning
If the research question is related to exposure of the infant to the pathogen through the complex process of breastfeeding (“breastfeeding transfer”) or consuming pumped milk, it is unnecessary to clean the breast. However, if the research question is related to whether the pathogen is transmitted through the mammary gland into milk (“milk transfer”), the breast should be thoroughly cleaned before milk collection—particularly if the pathogen may be transmitted into milk through respiratory droplets. After donning face covering and a glove on the hand that will clean the breast, research personnel or the mother (depending on cultural acceptability) should clean the “study breast” thoroughly with soap and sterile water or aseptic wipes. The purpose of this step is to physically remove skin pathogens.
Step 2. Milk collection
Using one of the methods detailed hereunder, collect milk from the chosen breast. Depending on which milk constituent is of interest, this milk can be foremilk, hindmilk, or a combination, thereof. If, for some reason, not enough milk can be expressed from the chosen breast, it is generally acceptable to combine milk from both breasts and document how the composite sample was created. If this is needed, the “second” breast should be cleaned (as appropriate to the research question) as described in Step 1 before collecting milk. The following options are equally acceptable.
Option A: Hand expression
With a newly gloved hand, the mother should express the needed volume of milk into the sterile collection container. The goal is to obtain a “clean catch” sample that drips or squirts directly from the nipple into the sterile container.
Option B: Electric or manual pump
Using a sterile or thoroughly cleaned and disinfected pump (including attachments, and tubing) have the mother express the needed amount of milk into the sterile collection container.
Step 3. Milk partitioning and storage
Option A (preferred): To be used when refrigeration/cold box is available at site and freezer is available in nearby laboratory
Place milk immediately in refrigerator, ice, ice box, or cold box. If the research question is related to isolating infectious virus, the sample might need to be snap frozen or analyzed immediately.
If possible, aliquot milk; transfer milk using sterile pipet into sterile storage containers.
As soon as possible, freeze milk at −20°C or (preferable) −80°C.
Option B: To be used when refrigeration/freezing is not available
Within 30 minutes of milk collection, treat milk with appropriate and validated chemical preservative (e.g., Norgen Biotek Corporation's Milk DNA Preservation and Isolation Kit). Preservatives should have been tested and validated for use with human milk to ensure that they do not destroy or destabilize milk components of interest or interfere with assays. These preservatives likely impact viral/bacterial viability.
Store in a cool place, at ambient temperature, or as described in manufacturer's instructions.
When milk supply is limited
Special care should be taken in situations when milk supply is limited and/or when the infant's health is at risk, for example, in the very early postpartum period (colostrum samples) and when the infant is very preterm and/or at risk for developing necrotizing enterocolitis. In these situations, researchers are encouraged to modify the methods described earlier so that the volume of milk available to the infant is not jeopardized. In general, only very small amounts (typically <1 mL) of colostrum should be collected. Sufficient milk may sometimes be obtained from a sterile swab to enable testing of SARS-CoV-2 gene targets through reverse transcription-qPCR testing.70 In many clinical settings, enteral feeds for very preterm or hospitalized infants are prepared in a central milk preparation room in a batch to last 12–24 hours. Often a small volume of milk that would otherwise be discarded can be collected for research purposes and uniquely reflects what an infant would receive the following day.
Ethical considerations
All procedures should be approved by local, regional, and/or national ethics boards (as appropriate) to protect participants' rights and ensure that subjects' identities are not linked to resultant data. For instance, samples and data should always be deidentified from subjects' names. Each mother should provide informed consent for milk collection, understand the purpose of the study, and be reassured that samples will be neither used for other purposes nor sold. Not all the mothers will have the same educational level, so it may be necessary to take extra care when communicating the purpose of the study. Particularly in studies with indigenous populations, researchers must understand how participants may view the collection of human milk and its use for biochemical analysis within the prevailing worldview.
Regarding safety
Researchers working directly with infected breastfeeding women should always follow all infection control and safety recommendations put forth by local, national, and international organizations—including the use of masks, gowns, and gloves. In addition, samples should be processed in an appropriate biosafety cabinet, if available.
Checklist for collecting human milk in light of an infectious disease
A checklist of important factors that should be considered, documented, and reported when collecting human milk to study potential transmission of a pathogen through breastfeeding is provided in Table 3.
Table 3.
Metadata to Consider, Collect, and/or Report When Studying Human Milk in Light of an Infectious Disease
| Related to milk collection and handling methods | ||
|---|---|---|
| √ Type of milk collected |  |
Foremilk, hindmilk, complete breast expression, and composite |
| √ Mode of collection |  |
Hand expression, electric pump, and manual pump |
| √ Breast(s) collected |  |
Right, left, and both |
| √ Collection/storage containers |  |
Glass, polypropylene, sterile, etc. |
| √ Preservative added? |  |
If yes, what kind? |
| √ Storage conditions |
 |
Temperature, duration, and freeze–thaw cycles |
| Additional metadata that should be collected and reported if possible | ||
| √ Time postpartum, and term versus preterm |
 |
Colostrum, transitional milk, mature milk, and gestational age (preterm versus term) |
| √ Time of day |
 |
Morning, afternoon, and evening |
| √ Time since last feed |
 |
Hours since last feeding or pumping session |
| √ Breastfeeding practices |
 |
Exclusively breastfed at the breast, fed pumped milk, and mixed feeding |
| √ Inflammatory state of breast |  |
Na/K ratio, cytokine profile, somatic cell count, breast redness, and breast pain |
Regarding the State of the Science and Urgency
As briefly described in this document, there are myriad gaps in knowledge related to studying the presence/absence of pathogens in human milk; their origin, when they are present; and their ability to be adequately characterized in terms of load and viability. This dearth of knowledge makes quickly assessing risk versus benefit of breastfeeding during a pandemic difficult. In these situations, we encourage researchers to work collaboratively and quickly to develop specialized protocols as needed and openly share information with other researchers so that accurate answers are gleaned in a timely manner. To facilitate this, granting agencies are encouraged to make emergency funding available to engaged and qualified research groups, and to facilitate contact between groups in the interest of collaboration.
Acknowledgments
We greatly appreciate the careful review of this article by Dr. Aleksandra Wesolowska, Dr. Jennifer Yourkavitch, Melinda Boss, Dr. Melanie Martin, and Dr. Janet Williams. We would also like to acknowledge Carron Finnan and her team from the South African Medical Research Council for design and graphics in Tables 1–3.
Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
References
- 1. Bzikowska A, Czerwonogrodzka-Senczyna A, Weker H, et al. . Correlation between human milk composition and maternal nutritional status. Rocz Panstw Zakl Hig 2018;69:363–367 [DOI] [PubMed] [Google Scholar]
- 2. Mitoulas LR, Kent JC, Cox DB, et al. . Variation in fat, lactose and protein in human milk over 24h and throughout the first year of lactation. Br J Nutr 2002;88:29–37 [DOI] [PubMed] [Google Scholar]
- 3. Yahvah KM, Brooker SL, Williams JE, et al. . Elevated dairy fat intake in lactating women alters milk lipid and fatty acids without detectible changes in expression of genes related to lipid uptake or synthesis. Nutr Res 2015;35:221–228 [DOI] [PubMed] [Google Scholar]
- 4. Yang T, Zhang Y, Ning Y, et al. . Breast milk macronutrient composition and the associated factors in urban Chinese mothers. Chin Med J 2014;127:1721–1725 [PubMed] [Google Scholar]
- 5. Chang YC, Chen CH, Lin MC. The macronutrients in human milk change after storage in various containers. Pediatr Neonatol 2012;53:205–209 [DOI] [PubMed] [Google Scholar]
- 6. Admyre C, Johansson SM, Qazi KR, et al. . Exosomes with immune modulatory features are present in human breast milk. J Immunol 2007;179:1969–1978 [DOI] [PubMed] [Google Scholar]
- 7. Becquart P, Foulongne V, Willumsen J, et al. . Quantitation of HIV-1 RNA in breast milk by real time PCR. J Virol Methods 2006;133:109–111 [DOI] [PubMed] [Google Scholar]
- 8. Chahar HS, Bao X, Casola A. Exosomes and their role in the life cycle and pathogenesis of RNA viruses. Viruses 2015;7:3204–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crenshaw BJ, Gu L, Sims B, et al. . Exosome biogenesis and biological function in response to viral infections. Open Virol J 2018;12:134–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manca S, Upadhyaya B, Mutai E, et al. . Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci Rep 2018;8:11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lemay DG, Hovey RC, Hartono SR, et al. . Sequencing the transcriptome of milk production: Milk trumps mammary tissue. BMC Genomics 2013;14:872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allen LH. Maternal micronutrient malnutrition: Effects on breast milk and infant nutrition, and priorities for intervention. SCN News 1994;11:21–24 [PubMed] [Google Scholar]
- 13. Ballard O, Morrow AL. Human milk composition: Nutrients and bioactive factors. Pediatr Clin North Am 2013;60:49–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanosyan A, Rutagwera DG, Molès JP, et al. . Increased Epstein-Barr virus in breast milk occurs with subclinical mastitis and HIV shedding. Medicine (Baltimore) 2016;95:e4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. LactaResearch Group. In: LactaPedia, Boss M, Hartmann P, eds. Frauenfeld (CH): Family Larsson-Rosenquist Foundation, 2018. Available at https://www.lactapedia.com (Accessed December1, 2020)
- 16. Cabrera-Rubio R, Collado MC, Laitinen K, et al. . The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr 2012;96:544–551 [DOI] [PubMed] [Google Scholar]
- 17. Williams JE, Carrothers JM, Lackey KA, et al. . Human milk microbial community structure is relatively stable and related to variations in macronutrient and micronutrient intakes in healthy lactating women. J Nutr 2017;147:1739–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khodayar-Pardo P, Mira-Pascual L, Collado MC, et al. . Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J Perinatol 2014;34:599–605 [DOI] [PubMed] [Google Scholar]
- 19. Chollet-Hinton LS, Stuebe AM, Casbas-Hernandez P, et al. . Temporal trends in the inflammatory cytokine profile of human breastmilk. Breastfeed Med 2014;530–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldman AS, Garza C, Johnson CA, et al. . Immunologic factors in human milk during the first year of lactation. J Pediatr 1982;100:563–567 [DOI] [PubMed] [Google Scholar]
- 21. Van de Perre P, Rubbo PA, Viljoen J, et al. . HIV-1 reservoirs in breast milk and challenges to elimination of breast-feeding transmission of HIV-1. Sci Transl Med 2012;4:143sr3. [DOI] [PubMed] [Google Scholar]
- 22. Ogra SS, Ogra PL. Immunologic aspects of human colostrum and milk. I. Distribution characteristics and concentrations of immunoglobulins at different times after the onset of lactation. J Pediatr 1978;92:546–549 [DOI] [PubMed] [Google Scholar]
- 23. Witkowska-Zimny M, Kaminska-El-Hassan E. Cells of human breast milk. Cell Mol Biol Lett 2017;22:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hughes K, Watson CJ. The Mammary microenvironment in mastitis in humans, dairy ruminants, rabbits and rodents: A one health focus. J Mammary Gland Biol Neoplasia 2018;23:27–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Willumsen JF, Filteau SM, Coutsoudis A, et al. . Breastmilk RNA viral load in HIV infected South African women: effects of subclinical mastitis and infant feeding. AIDS 2003;17:407–414 [DOI] [PubMed] [Google Scholar]
- 26. Morais TC, Honorio-França AC, Silva RR, et al. . Temporal fluctuations of cytokine concentrations in human milk. Biol Rhythm Res 2015;46:811–821 [Google Scholar]
- 27. Peitersen B, Bohn L, Andersen H. Quantitative determination of immunoglobulins, lysozyme, and certain electrolytes in breast milk during the entire period of lactation, during a 24-hour period, and in milk from the individual mammary gland. Acta Paediatr 1975;64:709–717 [DOI] [PubMed] [Google Scholar]
- 28. Hahn-Holbrook J, Saxbe D, Bixby C, et al. . Human milk as “chrononutrition”: Implications for child health and development. Pediatr Res 2019;85:936–942 [DOI] [PubMed] [Google Scholar]
- 29. Alhussien MN, Dang AK. Diurnal rhythm in the counts and types of milk somatic cells, neutrophil phagocytosis and plasma cortisol levels in Karan Fries cows during different seasons and parity. Biol Rhythm Res 2018;49:187–199 [Google Scholar]
- 30. Hall B. Uniformity of human milk. Am J Clin Nutr 1979;32:304–312 [DOI] [PubMed] [Google Scholar]
- 31. Kent J, Mitoulas L, Cregan M, et al. . Volume and frequency of breastfeeds and fat content of breastmilk throughout the day. Pediatrics 2006;117:e387–e395 [DOI] [PubMed] [Google Scholar]
- 32. Khan S, Hepworth AR, Prime DK, et al. Variation in fat, lactose, and protein composition in breast milk over 24 hours: Associations with infant feeding patterns. J Hum Lact 2012. DOI: 10.1177/0890334412448841 [DOI] [PubMed] [Google Scholar]
- 33. Hassiotou F, Hepworth AR, Williams TM, et al. . Breastmilk cell and fat contents respond similarly to removal of breastmilk by the infant. PLoS One 2013;8:e78232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bishara R, Dunn MS, Merko SE, et al. . Nutrient composition of hindmilk produced by mothers of very low birth weight infants born less than 28 weeks' gestation. J Hum Lact 2008;24:159–167 [DOI] [PubMed] [Google Scholar]
- 35. Dorea JG, Horner MR, Bezerra VLV, et al. . Variation in major constituents of fore and hindmilk of Brazilian women. J Trop Pediatr 1982;28:303–305 [DOI] [PubMed] [Google Scholar]
- 36. van Sadelhoff J, Mastorakou D, Weenen H, et al. . Short communication: Differences in levels of free amino acids and total protein in human foremilk and hindmilk. Nutrients 2018;10:1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Neville MC, Keller RP, Seacat J, et al. . Studies on human lactation. I. Within-feed and between-breast variation in selected components of human milk. Am J Clin Nutr 1984;40:635–646 [DOI] [PubMed] [Google Scholar]
- 38. Valentine CJ, Hurst NM, Schanler RJ. Hindmilk improves weight gain in low-birth-weight infants fed human milk. J Pediatr Gastroenterol Nutr 1994;18:474–477 [DOI] [PubMed] [Google Scholar]
- 39. Rodríguez-Cruz M, Alba C, Aparicio M, et al. . Effect of sample collection (manual expression vs. pumping) and skimming on the microbial profile of human milk using culture techniques and metataxonomic analysis. Microorganisms 2020;8:1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neveu D, Viljoen J, Bland RM. Cumulative exposure to cell-free HIV in breast milk rather than feeding pattern per se identifies postnatally infected infants. Clin Infect Dis 2011;52:819–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Willumsen JF, Newell ML, Filteau SM, et al. . Variation in breastmilk HIV-1 viral load in left and right breasts during the first 3 months of lactation. AIDS 2001;15:1896–1898 [DOI] [PubMed] [Google Scholar]
- 42. Pannaraj PS, Li F, Cerini C, Bender JM, et al. . Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr 2017;171:647–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oikonomou G, Bicalho ML, Meira E, et al. . Microbiota of cow's milk; distinguishing healthy, sub-clinically and clinically diseased quarters. PLoS One 2014;9:e85904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goldblum RM, Goldman AS, Garza C, et al. . Human milk banking. II. Relative stability of immunologic factors in stored colostrum. Acta Paediatr 1982;71:143–144 [DOI] [PubMed] [Google Scholar]
- 45. Slutzah M, Codipilly CN, Potak D, et al. . Refrigerator storage of expressed human milk in the neonatal intensive care unit. J Pediatr 2010;156:26–28 [DOI] [PubMed] [Google Scholar]
- 46. Ahrabi AF, Handa D, Codipilly CN, et al. . Effects of extended freezer storage on the integrity of human milk. J Pediatr 2016;177:140–143 [DOI] [PubMed] [Google Scholar]
- 47. Welsh JK, Arsenakis M, Coelen RJ, et al. . Effect of antiviral lipids, heat, and freezing on the activity of viruses in human milk. J Infect Dis 1979;140:322–328 [DOI] [PubMed] [Google Scholar]
- 48. Ghosh MK, Kuhn L, West J, et al. . Quantitation of human immunodeficiency virus type 1 in breast milk. J Clin Microbiol 2003;41:2465–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ramírez-Santana C, Pérez-Cano FJ, Audí C, et al. . Effects of cooling and freezing storage on the stability of bioactive factors in human colostrum. J Dairy Sci 2012;95:2319–2325 [DOI] [PubMed] [Google Scholar]
- 50. Doyle CJ, Gleeson D, O'Toole PW, et al. . High-throughput metataxonomic characterization of the raw milk microbiota identifies changes reflecting lactation stage and storage conditions. Int J Food Microbiol 2017;255:1–6 [DOI] [PubMed] [Google Scholar]
- 51. Schwab C, Voney E, Ramirez Garcia A, et al. . Characterization of the cultivable microbiota in fresh and stored mature human breast milk. Front Microbiol 2019;10:2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marín ML, Arroyo R, Jiménez E, et al. . Cold storage of human milk: Effect on its bacterial composition. J Pediatr Gastroenterol Nutr 2009;49:343–348 [DOI] [PubMed] [Google Scholar]
- 53. Akdag A, Sari FN, Dizdar EA, et al. . Storage at −80°C preserves the antioxidant capacity of preterm human milk. J Clin Lab Anal 2014;28:415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marinković V, Ranković-Janevski M, Spasić S, et al. . Antioxidative activity of colostrum and human milk: Effects of pasteurization and storage. J Pediatr Gastroenterol Nutr 2016;62:901–906 [DOI] [PubMed] [Google Scholar]
- 55. Butts CA, Paturi G, Blatchford P, et al. . Microbiota composition of breast milk from women of different ethnicity from the Manawatu-Wanganui region of New Zealand. Nutrients 2020;12:E1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hanna N, Ahmed K, Anwar M, et al. . Effect of storage on breast milk antioxidant activity. Arch Dis Child Fetal Neonatal Ed 2004;89:F518–F520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hines EP, Rayner JL, Barbee R, et al. . Assays for endogenous components of human milk: Comparison of fresh and frozen samples and corresponding analytes in serum. J Hum Lact 2007;23:144–156 [DOI] [PubMed] [Google Scholar]
- 58. Păduraru L, Dimitriu DC, Avasiloaiei AL, et al. . Total antioxidant status in fresh and stored human milk from mothers of term and preterm neonates. Pediatr Neonatol 2018;59:600–605 [DOI] [PubMed] [Google Scholar]
- 59. Pfaender S, Heyden J, Friesland M, et al. . Inactivation of hepatitis C virus infectivity by human breast milk. J Infect Dis 2013;208:1943–1952 [DOI] [PubMed] [Google Scholar]
- 60. Pfaender S, Vielle NJ, Ebert N, et al. . Inactivation of Zika virus in human breast milk by prolonged storage or pasteurization. Virus Res 2017;228:58–60 [DOI] [PubMed] [Google Scholar]
- 61. Conzelmann C, Zou M, Groß R, et al. . Storage-dependent generation of potent anti-ZIKV activity in human breast milk. Viruses 2019;11:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pace RM, Williams JE, Järvinen KM, et al. . COVID-19 and human milk: SARS-CoV-2, antibodies, and neutralizing capacity. medRxiv 2020. DOI: 10.1101/2020.09.16.20196071 [DOI] [Google Scholar]
- 63. Groß R, Conzelmann C, Müller JA, et al. . Detection of SARS-CoV-2 in human breastmilk. Lancet 2020;6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Walker GJ, Clifford F, Bansal N, et al. SARS-CoV-2 in human milk is inactivated by Holder pasteurization but not cold storage. medRxiv 2020. DOI: 10.1101/2020.06.18.20134395 [DOI] [PMC free article] [PubMed]
- 65. Unger S, Christie-Holmes N, Guvenc F, et al. . Holder pasteurization of donated human milk is effective in inactivating SARS-CoV-2. CMAJ 2020;192:E871–E874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chambers C, Krogstad P, Bertrand K, et al. . Evaluation for SARS-CoV-2 in breast milk from 18 infected women. JAMA 2020;324:1347–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lawrence RA. Storage of human milk and the influence of procedures on immunological components of human milk. Acta Paediatr Suppl 1999;88:14–18 [DOI] [PubMed] [Google Scholar]
- 68. Liebhaber M, Lewiston NJ, Asquith MT, et al. . Alterations of lymphocytes and of antibody content of human milk after processing. J Pediatr 1977;91:897–900 [DOI] [PubMed] [Google Scholar]
- 69. Hamilton Spence E, Huff M, Shattuck K, et al. . Ebola virus and Marburg virus in human milk are inactivated by Holder pasteurization. J Hum Lact 2017;33:351–354 [DOI] [PubMed] [Google Scholar]
- 70. Kirtsman M, Diambomba Y, Poutanen SM, et al. . Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ 2020;192:E647–E650 [DOI] [PMC free article] [PubMed] [Google Scholar]


