Abstract
We developed this study to describe the patterns of distant metastasis (DM) and explore the predictive and prognostic factors of DM in clear cell renal cell carcinoma (ccRCC) patients. We collected the eligible patients from the Surveillance, Epidemiology, and End Result (SEER) database from 2010 to 2015. Then, comparisons of baseline characteristics between patients in different metastatic patterns were made. In addition, proportional mortality ratios (PMRs) and proportion trends of different patterns were calculated. Afterward, survival outcomes were explored by Kaplan–Meier (KM) analyses. Finally, predictive and prognostic factors of DM were investigated. A total of 33,449 ccRCC patients were eventually identified, including 2931 patients with DM and 30,518 patients without DM. 8.76% of patients suffered DM at their initial diagnosis, 35.01% of them had multiple metastases. Generally, lung (6.19%) was the most common metastatic site in patients with DM, and brain (1.20%) was the least frequent metastatic organ. The proportion trends of different metastatic patterns tended to be stable between 2010 and 2015. Moreover, higher tumor grade, T stage, and N stage were identified as risk factors of DM. Finally, age at diagnosis, grade, T stage, N stage, the administration of surgery, the number of metastatic sties, marital status, and household income were found to be significantly associated with prognosis. Lung was the most common metastatic site in ccRCC patients. Different survival outcomes and prognostic factors were identified for different metastatic patterns. Hence, our study would have great value for clinical practice in the future.
Keywords: metastasis, prognosis, renal cancer, SEER
Lung was the most common metastatic site in ccRCC patients. Different survivals and prognostic factors were identified for different metastatic patterns. Hence, our study would have great value for clinical practice in the future.
1. INTRODUCTION
Kidney cancer is one of the most common malignancies of urinary system, second only to bladder cancer. The latest research revealed that the estimated new cases and deaths are 73,750 and 14,830 in 2020 in the United States. 1 Renal cell carcinoma (RCC) accounts for about 90% of all kidney malignancies, and is mainly composed of clear cell RCC (ccRCC), papillary RCC, chromophobe RCC, and so on. 2 , 3 Among them, ccRCC is the most common subtype, responsible for 70% of all RCC cases. 4 , 5
Previous studies have reported that up to 18%–30% of RCC patients were with systemic metastases at the time of initial diagnosis, and another third progressed to metastatic diseases after nephrectomy during the long‐term follow‐up. 6 , 7 Generally, patients with advanced or metastatic RCC (mRCC) have poor prognosis, with a median overall survival (OS) of about 13 months. 8 Recently, the 5‐year OS increased slightly from 7.3% to 12.3%. 9 Despite the distant metastasis (DM), many studies have confirmed that mRCC patients could benefit from cytoreductive nephrectomy (CN), 10 , 11 , 12 and CN has been the standard treatment since 2001. 13 , 14 Guo et al. 15 explored the value of CN among RCC patients with liver metastasis, and they demonstrated that CN prolonged the OS in this population. Lin et al. 16 investigated the role of surgical intervention on RCC patients with lung and bronchus metastasis, they found that these patients could obtain better prognosis after having surgical intervention than those without surgery.
Lung was considered to be the most common metastatic site in patients with ccRCC, followed by bone. 16 , 17 Previous studies concluded that the 5‐year OS rates for patients with lung metastases received pulmonary metastasectomy varied from 36% to 83%. Ljungberg et al. 18 demonstrated that antiangiogenic therapy was strongly recommended in mRCC patients without choice of further surgical treatment, while its actual efficacy was limited, with a median OS of 26.4–32.0 months for those patients.
Considering the high rate of metastatic diseases and the poor prognosis of mRCC patients, it was of great value to investigate the predictive and prognostic factors of DM in ccRCC patients. However, most of previous studies were single‐center, with small sample size, and without long‐term follow‐up. Hence, we developed this study on the basis of the Surveillance, Epidemiology, and End Results (SEER) database to investigate the risk factors of DM and the prognosis of mRCC patients with different metastatic patterns.
2. METHODS
2.1. Database
All data used in this study were downloaded from the SEER database retrospectively. SEER registry is a public database collects the detailed information of all cancer patients, including incidence rates, basic characteristics, treatment, mortality, and long‐term follow‐up outcomes. Initially, there were only nine regions in this project. However, with increasing regions take part in this program, the SEER 18 covers approximately 30% of the whole U.S. population. For this study, we signed the data agreement and utilized the SEER database with the username 15440‐Nov2018. Moreover, the application of SEER database was exempt by Institutional Review Board approval.
2.2. Patient identification
In this study, patients diagnosed with ccRCC from 2010 to 2015 were retrospectively extracted from SEER 18 using the SEER* Stat software (Version 8.3.6; NCI). The inclusion criteria were as follows: (a) patients diagnosed with ccRCC with positive pathology (C74.9, International Classification of Diseases for Oncology: 8310/3), (b) patients with active follow‐up and complete data, (c) ccRCC was the first primary malignancy. Furthermore, patients met any of following criteria should be excluded: (a) tumor laterality was unknown or patients with bilateral tumors; (b) metastatic status was unknown (including brain, bone, liver, and bone); (c) missing/unknown data on the administration of surgery, lymph node removal, median household income, and so on; (d) reporting source was autopsy/death certificate only.
2.3. Data extraction
Baseline characteristics and follow‐up outcomes were collected for each eligible patient utilizing the “Case Listing Session” in the SEER*Stat software, variables including age at diagnosis, gender, race, year of diagnosis, tumor laterality, grade, American Joint Committee on Cancer (AJCC) 7th T stage, N stage, the administration of surgery and lymph node removal, metastatic status, vital status, survival months, cause of death (COD), insurance status, marital status at diagnosis, and median household income. All the enrolled patients were divided into two groups (With DM and Without DM) depending on whether the patients had DM. Additionally, patients with DM were further categorized based on the number and patterns of metastatic sites.
In our study, age at diagnosis was classified into <65 and ≥65 years old. Race was divided into White, Black, and Other (including American Indian/AK Native, Asian/Pacific Islander). Tumor grade was categorized into Grade I (well differentiated), Grade II (moderately differentiated), Grade III (poorly differentiated), and Grade IV (undifferentiated). High and low levels of household income were defined according to the median value. Moreover, in order to investigate the death patterns of died patients, we divided the COD into “RCC,” “cardiovascular disease (CVD),” and “other cause.”
2.4. Incidence of distant metastasis, proportional mortality ratio
To study the trends of DM in recent years, we calculated the specific proportions of different groups in newly diagnosed cases. Besides, further subgroup analyses were performed on the basis of the number and patterns of metastatic sites.
Proportional mortality ratio (PMR) was calculated as the death number due to a specific cause divided by the number of deaths in the whole population. Similarly, we compared the PMRs of RCC, CVD, and other cause between ccRCC patients with or without DM, and further stratified by the number and patterns of metastatic sites.
2.5. Survival outcomes
Kaplan–Meier (KM) analyses were constructed to investigate the long‐term survival outcomes of ccRCC patients. Then, univariate and multivariate logistic/Cox regression analyses were developed to explore the predictive and prognostic factors of DM.
2.6. Statistical analysis
Chi‐square test was used to make comparisons of categorical variables between different groups. Cancer‐specific survival (CSS) or OS curves were presented by utilizing KM plots. The complete analyses were performed via SPSS 23.0 software (SPSS Inc) and R software (Version 3.4.1). All analyses were two‐sided and p < 0.05 was considered to be statistically significant.
3. RESULTS
3.1. Baseline characteristics and survival outcomes
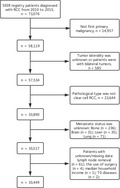
The flowchart of patient selection is shown in Figure 1. As shown in Table 1, a total of 33,449 ccRCC patients were eventually identified in this study, including 2931 patients with DM and 30,518 patients without DM, with an average age of 60.10 years old. In general, most patients were male (62.38%), White (84.59%), with early stage of diseases (T1: 65.02%, N0: 95.06%, localized histology: 72.13%), and cancer‐directed surgery (94.05%). When compared with patients without DM, those with DM had an older age (mean: 62.29 vs. 59.89 years, p < 0.001), higher probability of male (70.62% vs. 61.68%, p < 0.001), and later stage of diseases (T3‐4: 56.23% vs. 19.84%, p < 0.001. N1: 24.26% vs. 1.55%, p < 0.001). Furthermore, the rate of surgery was significantly higher (97.63% vs. 56.67%, p < 0.001) while lymph node removal rate was significantly lower (10.47% vs. 21.22%, p < 0.001) in patients without DM. However, no significant difference was detected in the comparisons in tumor laterality (p = 0.127) and median household income (p = 0.597).
FIGURE 1.
Flowchart of patient selection
TABLE 1.
Baseline characteristics of included patients
| Total | Without DM | With DM | p value | |
|---|---|---|---|---|
| N | 33,449 | 30,518 | 2931 | |
| Age, y, Mean±SD | 60.10±12.30 | 59.89±12.42 | 62.29±10.78 | <0.001 |
| <65 | 20,723 (61.95%) | 18,999 (62.26%) | 1724 (58.82%) | <0.001 |
| ≥65 | 12,726 (38.05%) | 11,519 (37.74%) | 1207 (41.18%) | |
| Sex | ||||
| Male | 20,864 (62.38%) | 18,794 (61.58%) | 2070 (70.62%) | <0.001 |
| Female | 12,585 (37.62%) | 11,724 (38.42%) | 861 (29.38%) | |
| Race | 0.001 | |||
| White | 28,293 (84.59%) | 25,794 (84.52%) | 2499 (85.26%) | |
| Black | 2421 (7.24%) | 2235 (7.32%) | 186 (6.35%) | |
| Other | 2453 (7.33%) | 2217 (7.26%) | 236 (8.05%) | |
| Unknown | 282 (0.84%) | 272 (0.89%) | 10 (0.34%) | |
| Laterality | 0.127 | |||
| Left | 16,406 (49.05%) | 14,929 (48.92%) | 1477 (50.39%) | |
| Right | 17,043 (50.95%) | 15,589 (51.08%) | 1454 (49.61%) | |
| Grade a | <0.001 | |||
| Grade I | 3297 (9.86%) | 3235 (10.60%) | 62 (2.12%) | |
| Grade II | 15,438 (46.15%) | 14,989 (49.12%) | 449 (15.32%) | |
| Grade III | 8314 (24.86%) | 7497 (24.57%) | 817 (27.87%) | |
| Grade IV | 2001 (5.98%) | 1457 (4.77%) | 544 (18.56%) | |
| Unknown | 4399 (13.15%) | 3340 (10.94%) | 1059 (36.13%) | |
| Histology | <0.001 | |||
| Localized | 24,128 (72.13%) | 24,128 (79.06%) | 0 (0.00%) | |
| Regional | 5882 (17.58%) | 5882 (19.27%) | 0 (0.00%) | |
| Distant | 3339 (9.98%) | 408 (1.34%) | 2931 (100.00%) | |
| Unstaged | 100 (0.30%) | 100 (0.33%) | 0 (0.00%) | |
| T stage | <0.001 | |||
| T1 | 21,749 (65.02%) | 21,278 (69.72%) | 471 (16.07%) | |
| T2 | 3493 (10.44%) | 2950 (9.67%) | 543 (18.53%) | |
| T3 | 7128 (21.31%) | 5827 (19.09%) | 1301 (44.39%) | |
| T4 | 576 (1.72%) | 229 (0.75%) | 347 (11.84%) | |
| Tx | 503 (1.50%) | 234 (0.77%) | 269 (9.18%) | |
| N stage | <0.001 | |||
| N0 | 31,797 (95.06%) | 29,802 (97.65%) | 1995 (68.07%) | |
| N1 | 1184 (3.54%) | 473 (1.55%) | 711 (24.26%) | |
| Nx | 468 (1.40%) | 243 (0.80%) | 225 (7.68%) | |
| Median household income b | 0.597 | |||
| Low | 16,848 (50.37%) | 15,358 (50.32%) | 1490 (50.84%) | |
| High | 16,601 (49.63%) | 15,160 (49.68%) | 1441 (49.16%) | |
| Marital status | 0.003 | |||
| Married | 20,482 (61.23%) | 18,669 (61.17%) | 1813 (61.86%) | |
| Previous married | 5877 (17.57%) | 5344 (17.51%) | 533 (18.18%) | |
| Never married | 5293 (15.82%) | 4822 (15.80%) | 471 (16.07%) | |
| Unknown | 1797 (5.37%) | 1683 (5.51%) | 114 (3.89%) | |
| Insurance status | <0.001 | |||
| Insured | 31,971 (95.58%) | 29,173 (95.59%) | 2798 (95.46%) | |
| Uninsured | 1008 (3.01%) | 895 (2.93%) | 113 (3.86%) | |
| Unknown | 470 (1.41%) | 450 (1.47%) | 20 (0.68%) | |
| Lymph node removal | <0.001 | |||
| No | 29,633 (88.59%) | 27,324 (89.53%) | 2309 (78.78%) | |
| Yes | 3816 (11.41%) | 3194 (10.47%) | 622 (21.22%) | |
| Cancer‐directed Surgery | <0.001 | |||
| No c | 1991 (5.95%) | 722 (2.37%) | 1269 (43.30%) | |
| Yes | 31,458 (94.05%) | 29,796 (97.63%) | 1662 (56.70%) | |
| Surgical methods | <0.001 | |||
| No surgery | 1991 (5.95%) | 722 (2.37%) | 1269 (43.30%) | |
| Local tumor excision/destruction | 1293 (3.87) | 1280 (4.21) | 13 (0.44) | |
| Partial nephrectomy | 11,267 (33.68) | 11,207 (36.84) | 60 (2.05) | |
| Radical nephrectomy | 18,471 (55.22) | 16,963 (55.77) | 1508 (51.45) | |
| Nephrectomy, NOS | 380 (1.14) | 305 (0.67) | 75 (2.56) | |
| Surgery, NOS | 47 (0.14) | 41 (0.13) | 6 (0.20) |
Data were n (%), unless otherwise specified.
Abbreviations: DM, distant metastasis; NOS, not otherwise specified; SD, standard deviation; y, years.
Grade I = Well differentiated; Grade II = Moderately differentiated; Grade III = Poorly differentiated; Grade IV = Undifferentiated.
Median household income: defined by earnings above the median of the median household income in this sample.
Including “no surgical procedure,” “needle, or aspiration biopsy,” or “Non‐cancer directed surgery.”
Lung (6.19%) was the most common metastatic site, and brain (1.20%) was the least frequent metastatic organ. Moreover, 3.07% patients had two or more metastatic sites (data were not shown). As exhibited in Table 2, patients with multiple metastatic sites had later tumor stage (T4: 15.69% vs. 9.76%, p < 0.001. N1: 29.92% vs. 21.21%, p < 0.001) when compared with those with single metastatic site. As for therapies, patents with single metastatic organ had higher proportion of surgery (66.25% vs. 38.99%, p < 0.001) and lymph node removal (24.93% vs. 14.33%, p < 0.001). However, no significant difference was found in the comparisons of other variables. Finally, patients with single metastatic site were further divided into four groups (brain alone, bone alone, liver alone, and lung alone), and comparisons between groups are shown in Table S1. Patients with lung metastasis received more often surgery (p < 0.001) and lymph node removal (p < 0.001) when compared with other sites.
TABLE 2.
Baseline characteristics of patients with DM, stratified by the number of metastatic sites
| 1 site | >1 site | p value | |
|---|---|---|---|
| N | 1905 | 1026 | |
| Age, y, Mean ± SD | 62.63 ± 10.80 | 61.66 ± 10.72 | 0.0203 |
| <65 | 1105 (58.01%) | 619 (60.33%) | 0.222 |
| ≥65 | 800 (41.99%) | 407 (39.67%) | |
| Sex | 0.255 | ||
| Male | 1332 (69.92%) | 738 (71.93%) | |
| Female | 573 (30.08%) | 288 (28.07%) | |
| Race | 0.142 | ||
| White | 1642 (86.19%) | 857 (83.53%) | |
| Black | 107 (5.62%) | 79 (7.70%) | |
| Other | 150 (7.87%) | 86 (8.38%) | |
| Unknown | 6 (0.31%) | 4 (0.39%) | |
| Laterality | 0.755 | ||
| Left | 964 (50.60%) | 513 (50.00%) | |
| Right | 941 (49.40%) | 513 (50.00%) | |
| Grade a | <0.001 | ||
| Grade I | 40 (2.10%) | 22 (2.14%) | |
| Grade II | 336 (17.64%) | 113 (11.01%) | |
| Grade III | 583 (30.60%) | 234 (22.81%) | |
| Grade IV | 378 (19.84%) | 166 (16.18%) | |
| Unknown | 568 (29.82%) | 491 (47.86%) | |
| T stage | <0.001 | ||
| T1 | 350 (18.37%) | 121 (11.79%) | |
| T2 | 332 (17.43%) | 211 (20.57%) | |
| T3 | 891 (46.77%) | 410 (39.96%) | |
| T4 | 186 (9.76%) | 161 (15.69%) | |
| Tx | 146 (7.66%) | 123 (11.99%) | |
| N stage | <0.001 | ||
| N0 | 1382 (72.55%) | 613 (59.75%) | |
| N1 | 404 (21.21%) | 307 (29.92%) | |
| Nx | 119 (6.25%) | 106 (10.33%) | |
| Median household income b | 0.413 | ||
| Low | 979 (51.39%) | 511 (49.81%) | |
| High | 926 (48.61%) | 515 (50.19%) | |
| Marital status | 0.487 | ||
| Married | 1182 (62.05%) | 631 (61.50%) | |
| Previous married | 350 (18.37%) | 183 (17.84%) | |
| Never married | 294 (15.43%) | 177 (17.25%) | |
| Unknown | 79 (4.15%) | 35 (3.41%) | |
| Insurance status | 0.359 | ||
| Insured | 1822 (95.64%) | 976 (95.13%) | |
| Uninsured | 68 (3.57%) | 45 (4.39%) | |
| Unknown | 15 (0.79%) | 5 (0.49%) | |
| Lymph node removal | <0.001 | ||
| No | 1430 (75.07%) | 879 (85.67%) | |
| Yes | 475 (24.93%) | 147 (14.33%) | |
| Cancer‐directed Surgery | <0.001 | ||
| No c | 643 (33.75%) | 626 (61.01%) | |
| Yes | 1262 (66.25%) | 400 (38.99%) |
Data were n (%), unless otherwise specified.
Abbreviations: DM, distant metastasis; SD, standard deviation; y, years.
Grade I = Well differentiated; Grade II = Moderately differentiated; Grade III = Poorly differentiated; Grade IV = Undifferentiated.
Median household income: defined by earnings above the median of the median household income in this sample.
Including “no surgical procedure,” “needle, or aspiration biopsy,” or “Non‐cancer directed surgery.”
3.2. Incidence of distant metastasis
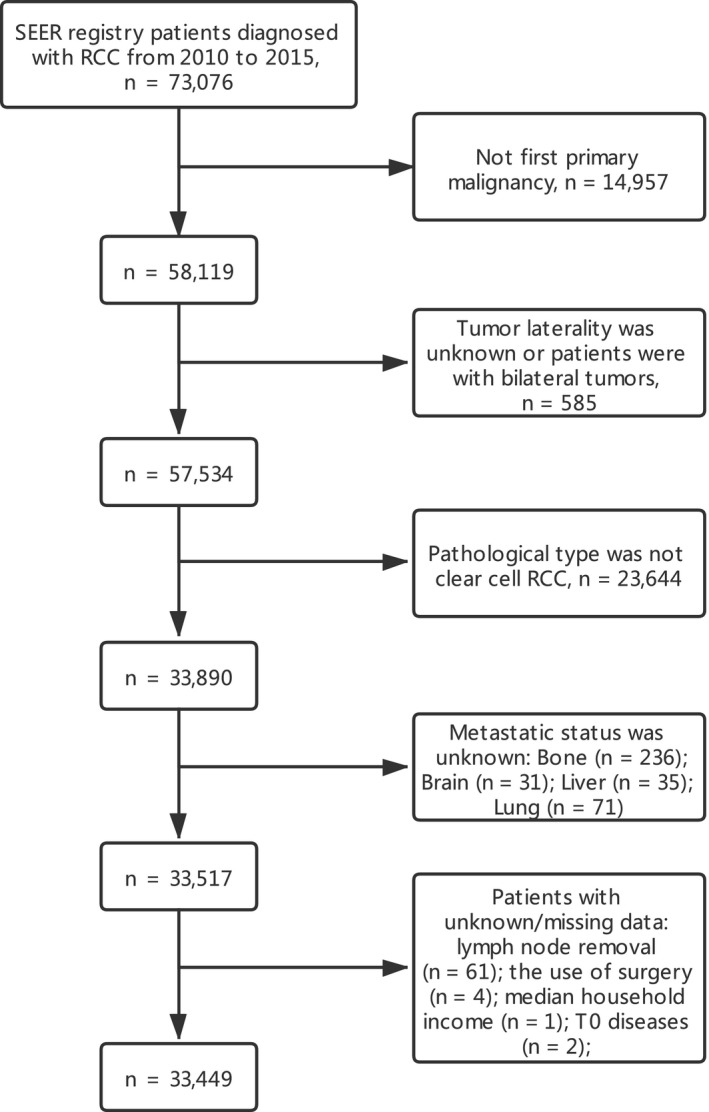
To explore the proportion trends of metastatic patients in the total ccRCC patients, the proportion trends of patients with single or multiple metastatic sites in the metastatic patients, we developed the related time line charts. Proportion trends of metastatic patients in ccRCC patients is shown in Figure 2A. Moreover, proportion trends of different metastatic patterns are shown in Figure 2B‐E: the number of metastatic sites (Figure 2B), patients with two sites (Figure 2C), patients with three sites (Figure 2D), and one site vs. more than one site (Figure 2E). All the line charts tended to be stable from 2010 to 2015, except for those with multiple metastatic sites, especially for three sites. We attributed the large fluctuation of some specific curves to the small population of these groups, and a slight change in the number of patients would lead to a larger change in the rate.
FIGURE 2.
Proportion trends of metastatic patients in ccRCC patients (A). Proportion trends of different metastatic patterns: the number of metastatic sites (B), patients with two sites (C), patients with three sites, (D) one site or more than one site (E)
3.3. Proportional mortality ratio
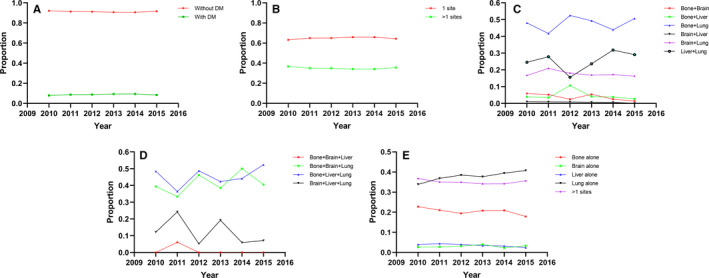
In this study, a total of 5745 patients died up to 31 December 2017, including 3616 nonmetastatic patients and 2129 metastatic patients. PMRs were as follows: RCC 67.4% (3873/5745), CVD 13.5% (776/5745), and other causes 19.1% (1096/5745). Figure 3 showed the outcomes of subgroup analyses. Conclusions could be drawn that in patients with DM, the proportion of death from RCC increased significantly (51.63%–94.22%), while the proportion of death from CVD (20.05%–2.40%) and other causes (28.32%–3.38%) decreased significantly when compared with patients without DM (Figure 3A). That was, once the patient had DM, it was extremely likely to die from the disease itself. With the increase of metastatic sites, this trend became more obvious (Figure 3B). The distribution of PMRs in patients with the same number of metastases did not change significantly (Figure 3C–E). Furthermore, in patients with three or four metastases, the proportion of death from RCC can be as high as 100% (Figure 3E).
FIGURE 3.
PMRs of ccRCC patients (A), and PMRs of patients with DM: the number of metastatic sites (B), patients with one site (C), patients with two sites (D), patients with three sites (E)
3.4. Survival outcomes
As shown in Figure 4A,B, in terms of OS, patients without DM had better OS and CSS than those with DM. Besides, as the number of metastases increased in patients with DM, the long‐term OS, and CSS probabilities decreased significantly (Figure 4C, D). In addition, patients with multiple metastatic sites had worse OS and CSS than those with single metastatic site (Figure 4E, F), and the same in the comparisons between two metastatic sites and more (Figure 4G, H). Among all metastatic sites, those with liver metastasis had the worst OS and CSS (Figure 5A, B). Moreover, survival curves of patients with two or more metastatic sites are shown in Figure 5C, D and Figure 5E, F, respectively. And no significant differences were identified in OS (Figure 5E, p = 0.0508) and CSS (Figure 5F, p = 0.0457) for various metastatic patterns among patients with three or more metastatic sites.
FIGURE 4.
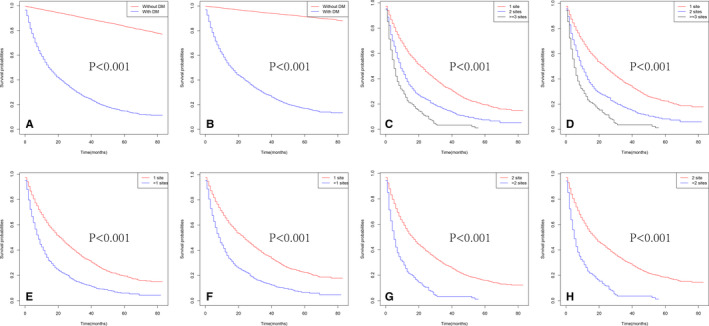
Kaplan–Meier curves of OS in patients according to metastatic status: with or without DM (A), the number of metastatic sites (C), 1 site versus >1 sites (E), 2 sites versus >2 sites (G). Kaplan–Meier curves of CSS in patients according to metastatic status: with or without DM (B), the number of metastatic sites (D), 1 site versus >1 sites (F), 2 sites versus >2 sites (H)
FIGURE 5.
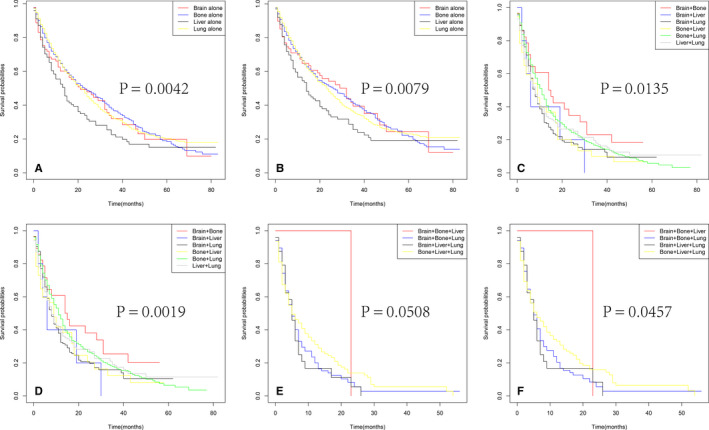
Kaplan–Meier curves of OS in patients according to metastatic status: with single site (A), with two sites (C), with three sites (E). Kaplan–Meier curves of CSS in patients according to metastatic status: with single site (B), with two sites (D), with three sites (F)
As shown in Table 3, ccRCC patients with higher tumor grade (Grade III: odds ratio (OR) = 2.538, Grade IV: OR = 4.694, all p < 0.001), T stage (T2: OR = 7.177, T3: OR = 7.964, T4: OR = 34.118, all p < 0.001), and N stage (N1: OR = 5.873, p < 0.001) had a higher risk of DM. In patients with DM, age at diagnosis (hazard ratio (HR) = 1.206, p = 0.004), grade (Grade IV: HR = 2.256, p < 0.001), T stage (T4: HR = 1.461, p = 0.003), N stage (HR = 1.576, p < 0.001), the administration of surgery (HR = 0.380, p < 0.001), the number of metastatic sties (2 sites: HR = 1.418, p < 0.001. ≥3 sites: HR = 2.552, p < 0.001), and marital status (Previous married: HR = 1.240, p = 0.006) were important factors affecting the OS (Table 4), and grade (Grade III: HR = 1.693, p = 0.019. Grade IV: HR = 2.436, p < 0.001), T stage (T3: HR = 1.248, p = 0.044. T4: HR = 1.544, p = 0.001), N stage (HR = 1.558, p < 0.001), the administration of surgery (HR = 0.370, p < 0.001), the number of metastatic sties (2 sites: HR = 1.479, p < 0.001. ≥3 sites: HR = 2.695, p < 0.001), marital status (previous married: HR = 1.249, p = 0.006), and household income (HR = 0.842, p = 0.001) were significantly related to CSS (Table 5).
TABLE 3.
Univariate and multivariate logistic regression analyses of risk factors for patients with DM
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age | 0.850 | |||||
| <65 | Reference | |||||
| ≥65 | 0.990 | 0.893–1.098 | 0.850 | |||
| Race | 0.027 | 0.151 | ||||
| White | Reference | Reference | ||||
| Black | 0.777 | 0.626–0.965 | 0.023 | 0.861 | 0.675–1.100 | 0.231 |
| Other | 1.222 | 0.936–1.346 | 0.214 | 1.161 | 0.947–1.424 | 0.150 |
| Sex | <0.001 | 0.234 | ||||
| Male | Reference | Reference | ||||
| Female | 0.675 | 0.605–0.752 | <0.001 | 0.928 | 0.821–1.049 | 0.234 |
| Grade a | <0.001 | <0.001 | ||||
| Grade I | Reference | Reference | ||||
| Grade II | 1.862 | 1.360–2.550 | <0.001 | 1.315 | 0.951–1.820 | 0.098 |
| Grade III | 6.908 | 5.079–9.394 | <0.001 | 2.538 | 1.838–3.504 | <0.001 |
| Grade IV | 24.848 | 18.139–34.037 | <0.001 | 4.694 | 3.355–6.566 | <0.001 |
| Laterality | 0.087 | |||||
| Left | Reference | |||||
| Right | 0.917 | 0.830–1.013 | 0.087 | |||
| T stage | <0.001 | <0.001 | ||||
| T1 | Reference | Reference | ||||
| T2 | 9.991 | 8.349–11.955 | <0.001 | 7.177 | 5.965–8.636 | <0.001 |
| T3 | 15.203 | 13.071–17.683 | <0.001 | 7.964 | 6.763–9.379 | <0.001 |
| T4 | 93.577 | 73.210–119.612 | <0.001 | 34.118 | 26.023–44.733 | <0.001 |
| N stage | <0.001 | <0.001 | ||||
| N0 | Reference | Reference | ||||
| N1 | 20.801 | 17.852–24.237 | <0.001 | 5.873 | 4.959–6.957 | <0.001 |
| Insurance status | 0.280 | |||||
| Insured | Reference | |||||
| Uninsured | 1.160 | 0.886–1.519 | 0.280 | |||
| Marital status | 0.347 | |||||
| Married | Reference | |||||
| Previous married | 0.959 | 0.840–1.094 | 0.533 | |||
| Never married | 0.904 | 0.785–1.040 | 0.158 | |||
| Household income b | 0.664 | |||||
| Low | Reference | |||||
| High | 0.978 | 0.885–1.081 | 0.664 | |||
The bold value means that the corresponding p of the variable is less than 0.05, with statistical significance.
Abbreviations: CI, confidence interval; DM, distant metastasis; OR, odds ratio.
Grade I = Well differentiated; Grade II = Moderately differentiated; Grade III = Poorly differentiated; Grade IV = Undifferentiated
Median household income: defined by earnings above the median of the median household income in this sample.
TABLE 4.
Univariate and Multivariate Cox regression analyses of prognostic factors for OS in patients with DM
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age | 0.027 | 0.004 | ||||
| <65 | Reference | Reference | ||||
| ≥65 | 1.147 | 1.016–1.296 | 0.027 | 1.206 | 1.063–1.368 | 0.004 |
| Race | 0.717 | |||||
| White | Reference | |||||
| Black | 1.012 | 0.781–1.312 | 0.926 | |||
| Other | 0.914 | 0.734–1.138 | 0.422 | |||
| Sex | 0.233 | |||||
| Male | Reference | |||||
| Female | 1.082 | 0.951–1.231 | 0.233 | |||
| Grade a | <0.001 | <0.001 | ||||
| Grade I | Reference | Reference | ||||
| Grade II | 1.068 | 0.702–1.625 | 0.758 | 1.259 | 0.823–1.924 | 0.288 |
| Grade III | 1.192 | 0.791–1.797 | 0.401 | 1.610 | 1.055–2.455 | 0.207 |
| Grade IV | 1.651 | 1.092–2.495 | 0.017 | 2.256 | 1.466–3.472 | <0.001 |
| Laterality | 0.661 | |||||
| Left | Reference | |||||
| Right | 0.974 | 0.865–1.096 | 0.661 | |||
| T stage | <0.001 | 0.003 | ||||
| T1 | Reference | Reference | ||||
| T2 | 1.273 | 1.014–1.598 | 0.037 | 0.993 | 0.789–1.251 | 0.955 |
| T3 | 1.396 | 1.150–1.695 | 0.001 | 1.176 | 0.960–1.439 | 0.117 |
| T4 | 2.256 | 1.780–2.859 | <0.001 | 1.461 | 1.138–1.874 | 0.003 |
| N stage | <0.001 | <0.001 | ||||
| N0 | Reference | Reference | ||||
| N1 | 2.015 | 1.767–2.297 | <0.001 | 1.576 | 1.374–1.808 | <0.001 |
| Surgery | <0.001 | <0.001 | ||||
| No b | Reference | Reference | ||||
| Yes | 0.373 | 0.324–0.430 | <0.001 | 0.380 | 0.322–0.447 | <0.001 |
| Lymph node removal | 0.744 | |||||
| No | Reference | |||||
| Yes | 1.021 | 0.901–1.158 | 0.744 | |||
| Number of metastatic sites | <0.001 | <0.001 | ||||
| 1 site | Reference | Reference | ||||
| 2 sites | 1.810 | 1.576–2.079 | <0.001 | 1.418 | 1.284–1.709 | <0.001 |
| ≥3 sites | 3.474 | 2.760–4.372 | <0.001 | 2.552 | 2.014–3.233 | <0.001 |
| Insurance status | 0.737 | |||||
| Insured | Reference | |||||
| Uninsured | 1.056 | 0.770–1.447 | 0.737 | |||
| Marital status | 0.001 | 0.020 | ||||
| Married | Reference | Reference | ||||
| Previous married | 1.332 | 1.147–1.547 | <0.001 | 1.240 | 1.064–1.445 | 0.006 |
| Never married | 1.086 | 0.917–1.286 | 0.341 | 1.005 | 0.846–1.193 | 0.958 |
| Median household income c | 0.113 | |||||
| Low | Reference | |||||
| High | 0.908 | 0.807–1.023 | 0.113 | |||
The bold value means that the corresponding p of the variable is less than 0.05, with statistical significance.
Abbreviations: CI, confidence interval; DM, distant metastasis; HR, hazard ratio; OS, overall survival.
Grade I = Well differentiated; Grade II = Moderately differentiated; Grade III = Poorly differentiated; Grade IV = Undifferentiated.
Including “no surgical procedure,” “needle, or aspiration biopsy,” or “Non‐cancer directed surgery.”
Median household income: defined by earnings above the median of the median household income in this sample.
TABLE 5.
Univariate and Multivariate Cox regression analyses of prognostic factors for CSS in patients with DM
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age | 0.137 | |||||
| <65 | Reference | |||||
| ≥65 | 1.101 | 0.970–1.250 | 0.137 | |||
| Race | 0.567 | |||||
| White | Reference | |||||
| Black | 0.958 | 0.728–1.261 | 0.758 | |||
| Other | 0.885 | 0.702–1.115 | 0.299 | |||
| Sex | 0.323 | |||||
| Male | Reference | |||||
| Female | 1.070 | 0.936–1.224 | 0.323 | |||
| Grade a | <0.001 | <0.001 | ||||
| Grade I | Reference | Reference | ||||
| Grade II | 1.028 | 0.662–1.594 | 0.903 | 1.275 | 0.818–1.987 | 0.283 |
| Grade III | 1.195 | 0.778–1.834 | 0.416 | 1.693 | 1.089–2.632 | 0.019 |
| Grade IV | 1.753 | 1.140–2.697 | 0.011 | 2.436 | 1.553–3.820 | <0.001 |
| Laterality | 0.602 | |||||
| Left | Reference | |||||
| Right | 0.968 | 0.856–1.094 | 0.602 | |||
| T stage | <0.001 | 0.002 | ||||
| T1 | Reference | Reference | ||||
| T2 | 1.349 | 1.060–1.717 | 0.015 | 1.042 | 0.816–1.330 | 0.742 |
| T3 | 1.501 | 1.220–1.847 | <0.001 | 1.248 | 1.006–1.548 | 0.044 |
| T4 | 2.463 | 1.920–3.161 | <0.001 | 1.544 | 1.188–2.005 | 0.001 |
| N stage | <0.001 | <0.001 | ||||
| N0 | Reference | Reference | ||||
| N1 | 2.046 | 1.786–2.343 | <0.001 | 1.558 | 1.352–1.795 | <0.001 |
| Surgery | <0.001 | <0.001 | ||||
| No b | Reference | Reference | ||||
| Yes | 0.379 | 0.327–0.439 | <0.001 | 0.370 | 0.312–0.439 | <0.001 |
| Lymph node removal | 0.406 | |||||
| No | Reference | |||||
| Yes | 1.056 | 0.928–1.203 | 0.406 | |||
| Number of metastatic sites | <0.001 | <0.001 | ||||
| 1 site | Reference | Reference | ||||
| 2 sites | 1.834 | 1.597–2.126 | <0.001 | 1.479 | 1.275–1.714 | <0.001 |
| ≥3 sites | 3.645 | 2.886–4.604 | <0.001 | 2.695 | 2.119–3.429 | <0.001 |
| Insurance status | 0.839 | |||||
| Insured | Reference | |||||
| Uninsured | 1.035 | 0.742–1.444 | 0.839 | |||
| Marital status | 0.003 | 0.012 | ||||
| Married | Reference | Reference | ||||
| Previous married | 1.308 | 1.120–1.528 | 0.001 | 1.249 | 1.066–1.463 | 0.006 |
| Never married | 1.067 | 0.893–1.274 | 0.475 | 0.958 | 0.802–1.146 | 0.641 |
| Median household income c | 0.030 | 0.007 | ||||
| Low | Reference | Reference | ||||
| High | 0.872 | 0.771–0.987 | 0.030 | 0.842 | 0.743–0.954 | 0.007 |
The bold value means that the corresponding p of the variable is less than 0.05, with statistical significance.
Abbreviations: CI, confidence interval; CSS, cancer‐specific survival; DM, distant metastasis; HR, hazard ratio.
Grade I = Well differentiated; Grade II = Moderately differentiated; Grade III = Poorly differentiated; Grade IV = Undifferentiated.
Including “no surgical procedure,” “needle, or aspiration biopsy,” or “Non‐cancer directed surgery”.
Median household income: defined by earnings above the median of the median household income in this sample.
Similarly, in patients with single metastatic site, multivariate Cox analysis showed that: age at diagnosis (HR = 1.212, p = 0.014), grade (Grade III: HR = 2.131, p = 0.015. Grade IV: HR = 3.039, p < 0.001), T stage (T3: HR = 1.313, p = 0.029. T4: HR = 1.814, p < 0.001), N stage (HR = 1.735, p < 0.001), the administration of surgery (HR = 0.367, p < 0.001), and marital status (HR = 1.324, p = 0.003) were significant factors associated with OS (Table S2). Age at diagnosis (HR = 1.185, p = 0.037), Grade (Grade III: HR = 2.157, p = 0.019. Grade IV: HR = 3.228, p < 0.001), T stage (T3: HR = 1.381, p = 0.015. T4: HR = 1.915, p < 0.001), N stage (HR = 1.749, p < 0.001), the administration of surgery (HR = 0.369, p < 0.001), marital status (Previous married: HR = 1.303, p = 0.007), and household income (HR = 0.853, p = 0.045) were significantly related to CSS (Table S3).
4. DISCUSSION
In our study, we found that 8.76% of ccRCC patients had DM at the time of diagnosis, and 3.07% of patients suffered multiple metastases. Lung (6.19%, 2070/33,44) was the most common organ of metastasis, followed by bone (3.74%, 1251/33,449). In addition, we found that the proportion of all metastatic patterns showed stable trends with no obvious changes in recent years. However, the metastatic rate in this study was significantly lower than 18%–30% reported in previous studies. 6 , 7 We attributed this to the fact that we only included patients with distant organs metastases, while regional (did not meet the inclusion criteria) and distant (due to the limitation of the database itself) lymph node metastases (LNM) were excluded. Moreover, only ccRCC was included in this study, while some other pathological types had high potential for malignant metastasis, such as Bellini duct carcinoma and medullary carcinoma. Therefore, it was believed that this data only represented ccRCC, which was more convincing. Last but not least, with the understanding of disease and the rapid improvement of diagnostic technology, increasing early stage RCC or small renal mass were found in clinical work.
By far, there were only few studies focused on the combined metastatic patterns of RCC. However, 35.01% (1026/2931) of the metastatic patients suffered multiple metastases in our study. In these patients, lung plus bone (36.84%) was the most common co‐metastases type, followed by lung plus liver (19.8%) and lung plus brain (13.5%). Furthermore, patients with multiple metastases had worse survival outcomes than those with single metastatic site, and the prognosis was getting worse with the increase of the number of metastatic sites (Tables 4 and 5). Hence, it was of great importance to examine the possibility of combined metastases, by which we can fully grasp the progress of the disease and make individualized treatment plans. However, comparisons of prognosis in patients with three of more metastatic sites were not different significantly, which may due to the fact that the prognosis of all these patients was very poor.
Nowadays, CVDs are the leading causes of mortality worldwide. 19 It was reported that there were 17.7 million deaths because of CVDs and 8.8 million deaths due to cancer worldwide in 2015. 20 Sturgeon et al. 20 investigated CVD mortality risk in cancer patients, and demonstrated that cancer patients with higher risk of dying from CVDs when compared with the general population. Ward et al. 21 proposed that CVD was the leading cause of death among endometrial cancer patients. Mehta 22 discussed that CVD resulted in heavier burden than breast cancer itself in older women. Previous studies have reported that smoking, 23 , 24 obesity, 25 , 26 and hypertension 27 were risk factors of RCC, and thus, we discussed CVD mortality in the analysis of PMRs. In our study, we found that the PMR was 20.05% in patients without DM died due to CVDs, while the PMR was only 2.4% in patients with DM. Moreover, with the increase of the number of metastatic sites, the PMR of CVD decreased accordingly and tended to be zero. Consequently, although patients with RCC should pay special attention to CVD, they should be more committed to the treatment of the disease itself in case of metastasis.
Survival analysis showed that the prognosis of patients with DM was significantly worse. In addition, as mentioned above, the prognosis was worse as the increase of the number of metastatic sites. Therefore, it was extremely urgent to identify the risk factors of DM and the prognostic factors for metastatic patients. Multivariate logistic regression analysis revealed that higher tumor grade, T stage, and N stage were important risk factors for DM. A meta‐analysis carried out by Thompson et al. 28 showed that poor differentiation was a risk factor for metastasis of cutaneous squamous cell carcinoma. Zhang et al. 29 discovered that higher T stage, higher N stage, and poor tumor differentiation grade were positively related to bone metastases in initial bladder cancer. Similar conclusion was drawn in the study conducted by Moon. 30 In patients with DM, multivariate Cox analysis showed that tumor grade, age at diagnosis, T stage, N stage, marital status, and the administration of surgery was a prognostic factor for OS. Many previous studies have found that age at diagnosis, T stage, N stage, and tumor differentiation played important roles in cancer survival outcomes. 31 , 32 , 33 Moreover, CN has been recognized to have survival benefits in mRCC patients. As for CSS, higher median household income was associated with better prognosis. Daniel Lin et al. 34 found that median household income may be an independent predictor for CSS in patients with squamous cell carcinoma of the anus. However, Torbrand et al. 35 considered that socioeconomic status influenced the stage and risk but not survival outcomes in patients with penile cancer.
However, there were some limitations that should not be ignored in our study. First of all, we did not included patients with distant LNM because the related data were available in the database after 2016. Second, the sequence of metastases could not be known for patients with multiple metastases, which may be an important obstacle to carry out further exploration. Furthermore, there were few patients in some specific distant patterns, and the epidemiological tendency and survival outcomes may be influenced. Additionally, several important factors are lacking in the SEER database, including LDH, hemoglobin, neutrophil count platelet count, MSKCC or IMDC risk classification, and so on. Finally, it was a retrospective and database‐based research, further studies with large sample size and detailed related information are needed in the future.
5. CONCLUSION
About 8.76% of ccRCC patients suffered DM at their initial diagnosis, among them 35.01% of the patients with multiple metastases. Patients with DM had poor survival outcomes than those without DM, and decreased survival was identified in patients with increased number of metastatic sites. Furthermore, predictive and prognostic factors of DM were then investigated to provide potential values in clinical guidance.
AUTHORS’ CONTRIBUTIONS
FQ and ZW conceived and designed the study. JX, WC, and WX collected the data. JX, WC, and ZX analyzed the data. JX, WC, and XL provided the resources for the study. ZW supervised the study. JX, WC, and FQ wrote the manuscript. All authors read and approved the final manuscript prior to submission.
ETHICS APPROVAL AND CONSENT PARTICIPATE
As the data used were from SEER data set (public). Ethics approval and consent to participate could be checked in SEER.
Jianxin Xue, Wensun Chen, Wenbo Xu have contributed equally to this work.
Contributor Information
Feng Qi, Email: qf199408@163.com.
Zengjun Wang, Email: wzj196611@163.com.
DATA AVAILABILITY STATEMENT
All data included in this study are available on reasonable request from the corresponding author.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Shuch B, Amin A, Armstrong AJ, et al. Understanding pathologic variants of renal cell carcinoma: distilling therapeutic opportunities from biologic complexity. Eur Urol. 2015;67:85–97. [DOI] [PubMed] [Google Scholar]
- 3. Capitanio U, Montorsi F. Renal cancer. Lancet. 2016;387:894–906. [DOI] [PubMed] [Google Scholar]
- 4. Sanchez‐Gastaldo A, Kempf E, Gonzalez Del Alba A, Duran I. Systemic treatment of renal cell cancer: a comprehensive review. Cancer Treat Rev. 2017;60:77–89. [DOI] [PubMed] [Google Scholar]
- 5. Ljungberg B, Campbell SC, Cho HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615–621. [DOI] [PubMed] [Google Scholar]
- 6. Tadayoni A, Paschall AK, Malayeri AA. Assessing lymph node status in patients with kidney cancer. Transl Androl Urol. 2018;7:766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Procházková K, Vodička J, Fichtl J, et al. Outcomes for patients after resection of pulmonary metastases from clear cell renal cell carcinoma: 18 years of experience. Urol Int. 2019;103:297–302. [DOI] [PubMed] [Google Scholar]
- 8. Coppin C, Porzsolt F, Awa A, Kumpf J, Coldman A, Wilt T. Immunotherapy for advanced renal cell cancer. Cochrane Database Syst Rev. 2005;CD001425. [DOI] [PubMed] [Google Scholar]
- 9. Harada K, Nozawa M, Uemura M, et al. Treatment patterns and outcomes in patients with unresectable or metastatic renal cell carcinoma in Japan. Int J Urol. 2019;26:202–210. [DOI] [PubMed] [Google Scholar]
- 10. Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon‐alfa‐based immunotherapy compared with interferon alfa alone in metastatic renal‐cell carcinoma: a randomised trial. Lancet. 2001;358:966–970. [DOI] [PubMed] [Google Scholar]
- 11. Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa‐2b compared with interferon alfa‐2b alone for metastatic renal‐cell cancer. N Engl J Med. 2001;345:1655–1659. [DOI] [PubMed] [Google Scholar]
- 12. Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171:1071–1076. [DOI] [PubMed] [Google Scholar]
- 13. Jeldres C, Baillargeon‐Gagne S, Liberman D, et al. A population‐based analysis of the rate of cytoreductive nephrectomy for metastatic renal cell carcinoma in the United States. Urology. 2009;74:837–841. [DOI] [PubMed] [Google Scholar]
- 14. Aben K, Heskamp S, Janssen‐Heijnen ML, et al. Better survival in patients with metastasised kidney cancer after nephrectomy: a population‐based study in the Netherlands. Eur J Cancer. 2011;47:2023–2032. [DOI] [PubMed] [Google Scholar]
- 15. Guo B, Liu S, Wang M, Hou H, Liu M. The role of cytoreductive nephrectomy in renal cell carcinoma patients with liver metastasis. Bosn J Basic Med Sci. 2020. Online ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin S, Zheng Y, Qin Z, et al. Surgical intervention in renal cell carcinoma patients with lung and bronchus metastasis is associated with longer survival time: a population‐based analysis. Ann Transl Med. 2019;7:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chandrasekar T, Klaassen Z, Goldberg H, Kulkarni GS, Hamilton RJ, Fleshner NE. Metastatic renal cell carcinoma: patterns and predictors of metastases‐A contemporary population‐based series. Urol Oncol. 2017;35(661):e7–e14. [DOI] [PubMed] [Google Scholar]
- 18. Ljungberg B, Albiges L, Abu‐Ghanem Y, et al. European Association of Urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75:799–810. [DOI] [PubMed] [Google Scholar]
- 19. Mortality GBD , Causes of Death C . Global, regional, and national age‐sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sturgeon KM, Deng L, Bluethmann SM, et al. A population‐based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40:3889–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ward KK, Shah NR, Saenz CC, McHale MT, Alvarez EA, Plaxe SC. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecol Oncol. 2012;126:176–179. [DOI] [PubMed] [Google Scholar]
- 22. Mehta LS, Watson KE, Barac A, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137:e30–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patel NH, Attwood KM, Hanzly M, et al. Comparative analysis of smoking as a risk factor among renal cell carcinoma Histological subtypes. J Urol. 2015;194:640–646. [DOI] [PubMed] [Google Scholar]
- 24. Kroeger N, Li H, De Velasco G, et al. Active smoking is associated with worse prognosis in metastatic renal cell carcinoma patients treated with targeted therapies. Clin Genitourin Cancer. 2019;17:65–71. [DOI] [PubMed] [Google Scholar]
- 25. Callahan CL, Hofmann JN, Corley DA, et al. Obesity and renal cell carcinoma risk by histologic subtype: a nested case‐control study and meta‐analysis. Cancer Epidemiol. 2018;56:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Landberg A, Falt A, Montgomery S, Sundqvist P, Fall K. Overweight and obesity during adolescence increases the risk of renal cell carcinoma. Int J Cancer. 2019;145:1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Colt JS, Schwartz K, Graubard BI, et al. Hypertension and risk of renal cell carcinoma among white and black Americans. Epidemiology. 2011;22:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thompson AK, Kelley BF, Prokop LJ, Murad MH, Baum CL. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease‐specific death: a systematic review and meta‐analysis. JAMA Dermatol. 2016;152:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang C, Liu L, Tao F, et al. Bone metastases pattern in newly diagnosed metastatic bladder cancer: a population‐based study. J Cancer. 2018;9:4706–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moon DH, Jeon JH, Yang HC, et al. Intramural metastasis as a risk factor for recurrence in esophageal squamous cell carcinoma. Ann Thorac Surg. 2018;106:249–256. [DOI] [PubMed] [Google Scholar]
- 31. Li M, Trivedi N, Dai C, et al. Does T stage affect prognosis in patients with stage Iv B differentiated thyroid cancer? Endocr Pract. 2019;25:877–886. [DOI] [PubMed] [Google Scholar]
- 32. Rayess HM, Dezube A, Bawab I, et al. Tumor differentiation as a prognostic factor for major salivary gland malignancies. Otolaryngol Head Neck Surg. 2017;157:454–461. [DOI] [PubMed] [Google Scholar]
- 33. Zhang Y, Guo Y, Zhou X, Wang X, Wang X. Prognosis for different patterns of distant metastases in patients with uterine cervical cancer: a population‐based analysis. J Cancer. 2020;11:1532–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin D, Gold HT, Schreiber D, Leichman LP, Sherman SE, Becker DJ. Impact of socioeconomic status on survival for patients with anal cancer. Cancer. 2018;124:1791–1797. [DOI] [PubMed] [Google Scholar]
- 35. Torbrand C, Wigertz A, Drevin L, et al. Socioeconomic factors and penile cancer risk and mortality; a population‐based study. BJU Int. 2017;119:254–260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in this study are available on reasonable request from the corresponding author.


