ABSTRACT
Background
Periplocin is a monomeric compound that exhibits anti‐tumor activities. It is extracted from Cortex Periplocae.
Objective
This study aimed at determining the effect of periplocin treatment on the apoptosis and proliferation of human pancreatic cancer cells, and to elucidate on its mechanisms of action.
Methods
PANC1 and cfpac1 cells were treated with periplocin. Cell proliferation was detected by RTCA, Ki67 immunofluorescence, and a clonogenic assay. The transwell assay was used to examine cell migration and invasion functions. The expression of apoptosis‐associated proteins was detected by flow cytometry and western blotting. Total RNA was extracted from the treated and untreated group of PANC1 cells for RNA‐seq detection and analysis. Differentially expressed genes were screened for GO biological process and KEGG pathway analysis. Finally, CFPAC1 cells were subcutaneously inoculated into BALB / c nude mice to assess tumor growth.
Results
Periplocin inhibited the proliferation of PANC1 and CFPAC1 cells and induced their apoptosis by activating the AMPK/mTOR pathway and inhibiting p70 S6K. It also attenuated the cell migration, invasion, and inhibited the growth of cfpac1 xenografts in nude mice.
Conclusions
Periplocin inhibits human pancreatic cancer cell proliferation and induces their apoptosis by activating the AMPK / mTOR pathway.
Keywords: apoptosis, pancreatic cancer, Periplocin, proliferation
In this work, we evaluated the effect of Periplocin on pancreatic cancer growth and tried to clarify its molecular mechanism.
1. INTRODUCTION
Cortex periplocae is a traditional herbal medicine with antiradiation, antitumor, anti‐inflammatory, and immune function enhancement effects. 1 Periplocin is a monomer compound with antitumor activities that is isolated and purified from Cortex periplocae. Periplocin activates the β‐catenin/TCF signaling pathway. In human colon cancer SW480 cells and nude mouse intraperitoneal tumor models, intraperitoneal administration of periplocin (30 mg/kg bw/day) for 12 days inhibited colon cancer growth and induced their apoptosis. 2 It inhibits lung cancer growth and induces apoptosis by blocking the AKT/ERK signaling pathway. 3 In normal cells, it exhibits low toxicity. Periplocin inhibits cell viability of human gastric cancer cells through the ERK1 / 2‐EGR1 pathway, thereby inducing cell apoptosis. 4 In addition, it was shown to inhibit growth while inducing apoptosis by altering the expression of death receptors in gastric cancer SW‐872 cells and initiating DNA double‐strand breaks. 5 In two mucinous fibroblastic sarcoma (MFS) cell lines (MUG‐Myx2a and MUG‐Myx2b), periplocin mediated apoptosis and cell cycle arrest through the ERK/p38/JNK pathway. 6 However, the effects of Periplocin in pancreatic cancer have not been established. Therefore, we aimed at determining the effects of periplocin on pancreatic cancer cells as well as its molecular mechanisms of action.
Globally, pancreatic cancer is one of the leading causes of cancer mortalities. 7 In 2012, approximately 338 000 new pancreatic cancer cases were globally diagnosed. In addition, the mortality rate was almost equal to the incidence rate. 8 Pancreatic cancer incidences and mortality rates in China are exhibiting an annual upward trajectory. 9 The prognosis is low, and the 5‐year survival rate is about 5%. Gemcitabine is the primary therapeutic option for pancreatic cancer. It also improves the overall survival rate of very few patients. 10 Reduced chemotherapeutic efficacies could be attributed to pancreatic cancer drug resistance. It is, therefore, important to develop new medicines for effective targeted therapy.
Many signaling pathways, such as AMPK and mTOR signaling pathways, play an essential role in the progression of pancreatic cancer cells. Studies have shown that AMPK is also important in regulating cell survival and death. 11 AMPK activation promotes cancer cell death by regulating downstream target proteins. 12 , 13 , 14 , 15 The mTOR pathway is also involved in various cellular processes, such as protein synthesis, 16 cell proliferation, and apoptosis. 17 Many traditional cytotoxic chemicals and natural compounds stimulate AMPK‐dependent death pathways in cancer cells. 18 , 19 , 20 This study aimed at determining the effect of periplocin on pancreatic cancer cell growth and apoptosis. We also explored the molecular mechanisms of its antipancreatic cancer effects.
2. MATERIALS AND METHODS
2.1. Reagents and antibodies
Periplocin was purchased from Shanghai Yuanye Biotechnology Co. Ltd. DAPI solution was purchased from Solarbio. Cleaved caspase 3, cleaved caspase 8, Bax, Bcl‐2, Ki67, GAPDH antibody, p‐AMPK (Thr172), AMPK, p‐mTOR (Ser2448), mTOR, P‐70S6K (Thr389), and S6K were purchased from Cell Signaling Company. Compound C was purchased from Selleck Chemicals. The BCA protein assay reagent kit and enhanced chemiluminescent (ECL) plus reagent kit were obtained from Pierce (Pierce Biotech).
2.2. Cell lines and cell culture
Human PANC1 and CFPAC1 pancreatic cancer cell lines were purchased from the American Type Culture Collection (ATCC). The cell lines were tested for mycoplasma contamination. They were then cultured in a DMEM medium that was supplemented by 10% fetal bovine serum (FBS), streptomycin (100 U/ml), and penicillin (100 U/ml). They were incubated at 37°C in 5% carbon dioxide.
2.3. Cytotoxicity assays
The RTCA analysis method was used to determine the viability of pancreatic cancer cells treated with periplocin. The xCELLigence RTCA analyzer has a workstation in the CO2 incubator while the control unit has RTCA software (ACEA Biosciences) outside the carbon dioxide incubator. To measure the cytotoxic response of cells in real‐time, they were seeded on embedded 16‐well microplates (E‐plates; Roche Diagnostics). For PANC1 and CFPAC1 cells, the seeding density was 5.0 × 103 cells/well. Impedance was recorded every 15 min. After 20 h of inoculation, 0, 125, and 250 nm periplocin were added to the PANC1 and CFPAC1 cultures, respectively. Between 0 and 72 h at 200 µl is used for all incubations. Cell viability was evaluated by the RTCA‐DP software (Roche Diagnostics GmbH). 18
2.4. Clone formation assay
PANC1 and CFPAC1 cells were inoculated into a six‐well plate at a concentration of 1000 cells/well. They were then treated with periplocin at 0, 125, and 250 nm, respectively, for 24 h. CFPAC1 cells were treated with 250 nm Periplocin and Compound C (5 μm) for 24 h. After 14 days of culture, cell clone formation was checked by staining with crystal violet at room temperature for 15 min.
2.5. Immunofluorescence staining analysis
Coverslip cells were washed twice using PBS, fixed with 4% PFA for 15 min at 37°C, and permeabilized with 0.25% Triton X‐100 for 15 min at room temperature. Then, cells were washed twice using PBS and blocked with 3% BSA for 30 min. They were then stained with a primary antibody (ki67) (1: 200) at 4°C overnight. After staining, they were cultured with goat anti‐rabbit secondary antibodies coupled with Alexa 488 (Jackson ImmunoResearch), and incubated at 37°C for 1 h. DAPI nuclear staining was then done for 3 min and the slides observed using a fluorescence microscope (Olympus).
2.6. Transwell migration and invasion assay
The cells in a medium containing 1% fetal bovine serum were inoculated onto a matrigel coating (8 μm pore size; HTS ™ FluoroBlok invasion system, BD Biosciences) and an uncoated polycarbonate membrane filter (8 μm pore size; Corning). The lower chamber was filled with a medium containing 20% fetal bovine serum. After 24 and 48 h, the cells that migrated through the membrane and attached to the membrane's bottom were fixed and stained with crystal violet for 15 min. Five random field images (magnification; ×200) were captured from each film. The degree of migration and invasion was expressed as the average number of cells in each microscopic field. All experiments were done in triplicate.
2.7. low cytometric analysis of apoptosis
Annexin V‐FITC Apoptosis Detection Kit (BD) was used to evaluate the percentage of apoptotic cells. The PANC1 and CFPAC1 cells were treated with periplocin at 0, 125, and 250 nm for 24 h, respectively. The 5 × 105‐treated cells were centrifuged and resuspended in 1 × cold binding buffer. Five μl Annexin V‐FITC and 5 μl propidium iodide (PI) were added and incubated at room temperature in the dark for 15 min. The Annexin V / PI staining method was performed according to the kit manufacturer's protocol (BD Biosciences). Data collection and analysis were performed on the FC500 MPL system (Beckman Coulter).
2.8. RNA‐seq and pathway enrichment analysis
Total RNA was extracted from the 0 and 125 nm Periplocin 24‐h treatment groups of PANC1 cells using TRIzol reagent (Invitrogen). The extracted RNA samples were transported to BGI (Shenzhen, China) for further RNA‐seq detection and analysis using the BGISEQ‐500 sequencer. Pathway analysis of differentially expressed genes (DEG) was performed based on the KEGG database. And Gene Ontology function enrichment. The obtained data was analyzed through the BGI network platform (http://report.bgi.com).
2.9. Western blot analysis
The treated cells were obtained and resuspended in cell lysate (Thermo) on ice for at least 30 min. The cell lysate was centrifuged at 12 000 × g for 10 min at 4°C. The supernatant was obtained, mixed with 5 × loading buffer, and boiled for 10 min. After boiling, the resulting supernatant containing total protein was quantified using the BCA protein evaluation kit (Beyotime P0012). An 8%, 10%, or 15% polyacrylamide gel (depending on the protein's molecular size to be analyzed) was used to separate a 50 µg total protein sample. The separated sample was transferred to a PVDF membrane (Millipore). The membrane was blocked using 5% skimmed milk and incubated with primary antibodies (1: 1000 in TBST) of cleaved caspase 3, cleaved caspase 8,Bax, Bcl‐2, P‐AMPK, P‐mTOR, p‐p70s6k, and GAPDH. Secondary antibodies coupled with horseradish peroxidase were then introduced into the membrane. The membrane was then incubated. Enhanced chemiluminescence (ECL) reagent (Pierce, Thermo Fisher Scientific) was used to detect protein expression.
2.10. Xenograft mouse models
All animal care, use, and experimental protocols were approved by the animal experiment ethics inspection label of the Experimental Animal Center of Wenzhou Medical University. To induce subcutaneous tumor growths, 2 × 106 CFPAC1 cells were subcutaneously injected into the right dorsal midline of BALB / c nude mice (4–6 weeks old). Once the tumor reached ~50 mm3 on day 7, nude mice were randomly distributed into four groups (n = 5 nude mice/group). Every 2 days, mice were intraperitoneally injected with PBS (200 ul) and periplocin (15 mg/kg). The length and width of the tumors were measured after every 3 days and tumor volumes calculated using the equation: V = (length × width2) /2.
2.11. Immunohistochemistry
Immunohistochemistry (IHC) staining was performed to detect ki67 protein expression in tumor xenografts. The formalin‐fixed paraffin‐embedded tissue was cut into 5 µm pieces, dewaxed in xylene, and rehydrated in graded ethanol. Antigenic treatment was performed by placing the slides in a microwave oven for 5 min at high temperature and repeating it two to three times. After treatment with 3% H2O2 for 10 min, the tissue sections were blocked with 5% normal goat serum. They were first incubated with a primary antibody (ki67) (1: 200) at 4°C overnight, and then, with EnVision Polymer‐HRP secondary antibody at room temperature (Dako, Glostrup, Denmark) for 1 h. After applying the DAB chromogen, tissue sections were stained with hematoxylin, dehydrated, and fixed.
2.12. Statistical analysis
Data analysis was performed by the GraphPad Prism 8 software (Graph Pad Software). The cell index (CI) of the real‐time dynamic cytotoxicity assessment (N = 3) was automatically calculated by the RTCA software package 1.2 of the RTCA system. Quantitative data were calculated on the stable line N = 3 ± SD (dashed line) and averaged. The results are expressed as mean ±SD. The Student's t‐test and one‐way analysis of variance were used to determine statistical significance. p ≤ 0.05 was set as the threshold for statistical significance.
3. RESULTS
3.1. Periplocin inhibited growth in PDAC cell lines
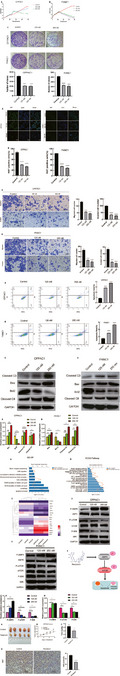
After treatment with periplocin, the cell survival index decreased in a concentration‐dependent manner (Figure 1A,B). In addition, periplocin‐treated PANC1 and CFPAC1 cells exhibited fewer colonies than control cells (Figure 1C).
FIGURE 1.
Proliferation inhibition by Periplocin in PDAC cells. A and B, CFPAC1 and PANC1 cells were treated with 0,125, and 250 nm periplocin for 0–72 h, respectively. Cell viability was determined using an RTCA assay. C, CFPAC1 and PANC1 cells were seeded in six‐well plates and treated with 0, 125, and 250 nm periplocin, respectively. Colony formation was assessed by crystal violet staining after 14 days of culture. The results are the mean ±SD of independent experiments performed in triplicate. p ≤ 0.05 was considered to be statistically significant, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 versus the control
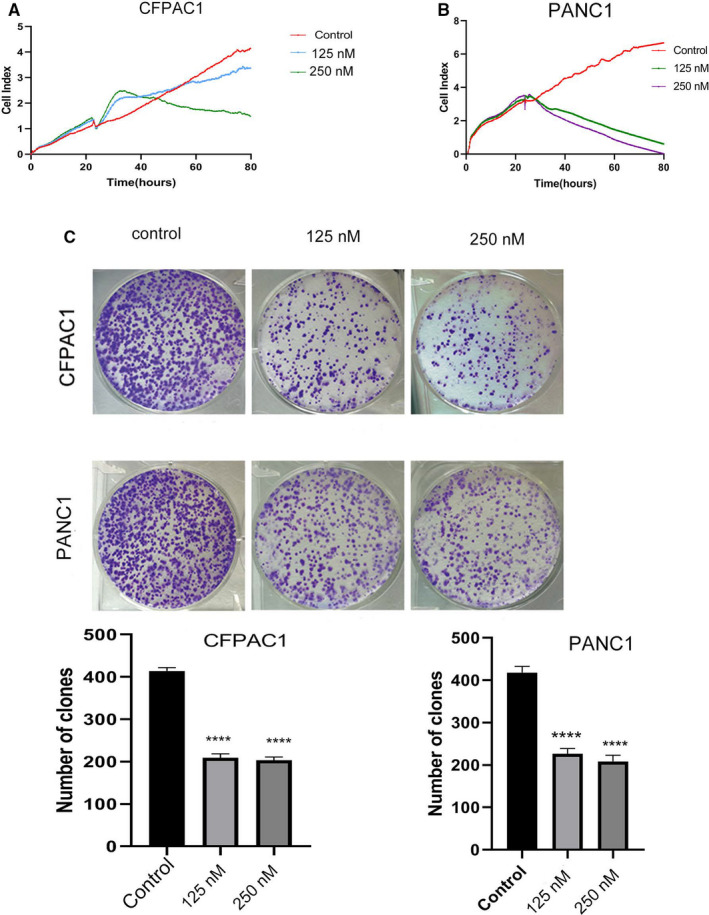
We also determined whether periplocin decreased the proliferation markers of human pancreatic cancer cell (ki67). Correspondingly, Ki67 Immunofluorescence and analysis showed that the ki67 green fluorescence in the tumor cell‐treated group was significantly reduced compared to the untreated group (Figure 2A,B ).
FIGURE 2.
Periplocin reduces the expression of Ki67 Immunofluorescence. A, After respectively treating CFPAC1 and PANC1 cells with 0,125, and 250 nm periplocin for 24 h, Ki67 Immunofluorescence showed cell proliferation at different periplocin concentrations. B, Ki67 Immunofluorescence and quantitative analysis. Scale bar, 50 μm. The results are expressed as the mean ±SD of independent experiments performed in triplicate. p ≤ 0.05 was considered to be statistically significant, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 versus the control
3.2. Periplocin inhibits pancreatic cancer cell migration and invasion
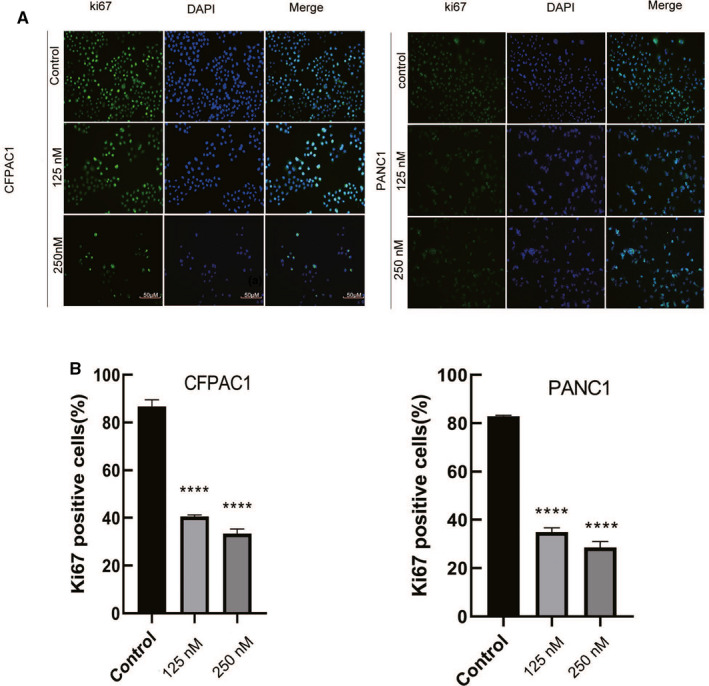
There was a significant reduction in cell migration and invasion in periplocin‐treated CFPAC1 and PANC1 cells, respectively (Figure 3A,B).
FIGURE 3.
Periplocin attenuated the migration and invasive ability of human pancreatic cancer cells. A, CFPAC1 cells were treated with 0,125, and 250 nm periplocin for 24 h for transwell migration experiments and for 48 h for transwell invasion experiments. In the transwell invasion experiment, the cells in the medium containing 5% fetal bovine serum were seeded into the upper cavity of a Matrigel‐coated polycarbonate membrane filter (8 μm pore size). B, PANC1 cells were treated with 0,125, and 250 nm periplocin for 24 h for transwell migration experiments, and 48 h for transwell invasion experiments. Scale bar, 200 μm. The results are the mean ±SD of independent experiments performed in triplicate. p ≤ 0.05 was considered to be statistically significant, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 versus the control
3.3. Periplocin induces apoptosis in pancreatic cancer cells
As shown in Figure 4A,B, the apoptotic rate was significantly high in human pancreatic cancer cells treated with periplocin. In addition, periplocin treatment elevated the expression of apoptosis‐associated proteins (Bax,cleaved Caspase‐8 and cleaved Caspase‐3) and decreased the expression of Bcl‐2 (Figure 4C,D). The data analysis of Figure 4C,D is shown in Figure 5A,B. In summary, periplocin induced apoptosis in PANC1 and CFPAC1 cells.
FIGURE 4.
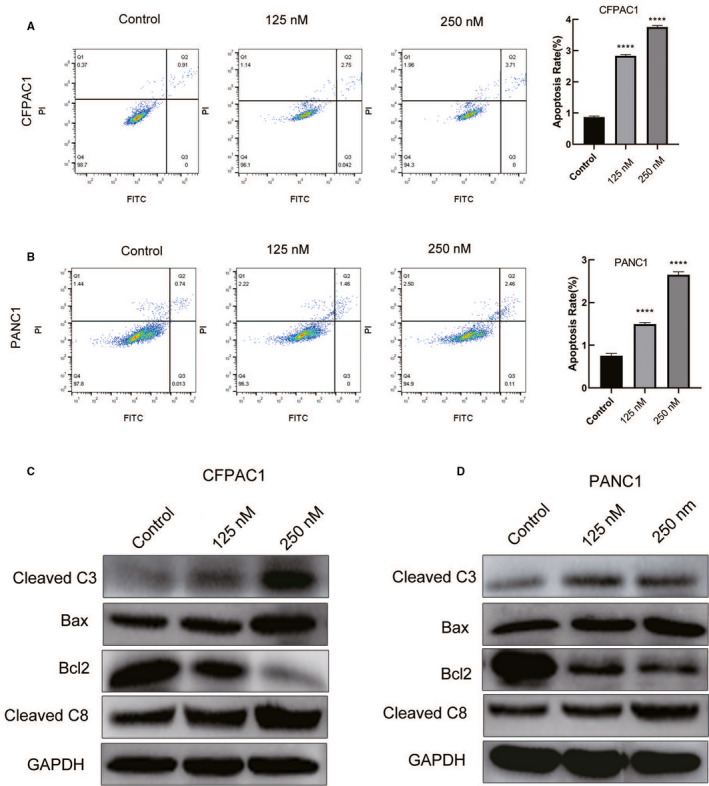
Periplocin induces apoptosis of human pancreatic cancer cells. A and B, CFPAC1 and PANC1 cells were treated with periplocin at 0, 125 nm, and 250 nm for 24 h. The annexin V‐FITC assay was performed to determine the apoptotic rate of cells treated with periplocin at different concentrations. The results were analyzed by flow cytometry. C and D, The proteins expressed by the treated human pancreatic cancer cells were examined by Western blotting. Apoptosis‐associated proteins (cleaved caspase 8, cleaved caspase 3, Bax, and Bcl2) were detected using the corresponding antibodies with GAPDH as the control. The results are presented as the mean ±SD of independent experiments performed in triplicates. p ≤ 0.05 was considered to be statistically significant, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 versus the control
FIGURE 5.
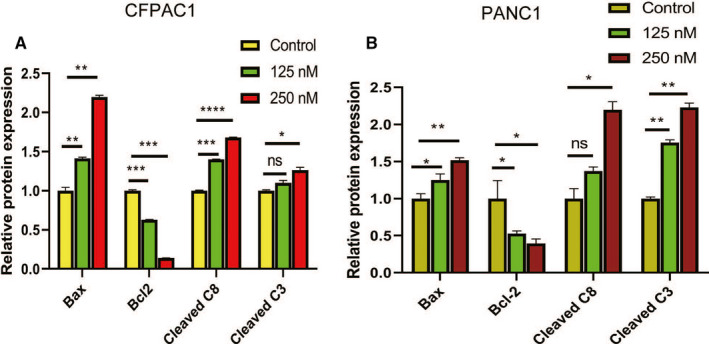
Quantitative analysis of Western blot apoptotic proteins. Data analysis of Figure 4C‐D. Data are presented as the mean ±standard deviation. *p < 0.05, **p < 0.01, n ≥ 3.
3.4. Periplocin regulates the AMPK/mTOR signaling pathways in human pancreatic cancer
By performing a t‐test with a critical (q) value of <0.05 and FC>0.5, DEG was selected. Functional enrichment analysis were then performed on the co‐expressed DEG. In the GO biological process, representative GO terms included apoptosis process, autophagy, cell proliferation, Notch receptor processing, among others (Figure 6A). The KEGG pathway analysis clustered these genes into several pathways (Figure 6B). The AMPK/mTOR signaling pathway exhibited a strong correlation with tumor growth and proliferation. The heat map (Figure 6C) highlights 16 representative genes differentially expressed in the AMPK/mTOR signaling pathway and apoptosis. As shown in Figure 6D,E Western blot analysis confirmed the heat map results in the AMPK/mTOR signaling pathway, which upregulated P‐AMPK and downregulated the expression of P‐mTOR and P‐S6K proteins. Periplocin was found to exert its antitumor effects by inhibiting mTOR signaling through the activation of the AMPK signaling pathway. These effects inhibit S6K and, therefore, exerting antitumor effects (Figures 6F and 7A,B). The data analysis of Figure 6D,E are presented in Figure 7A,B.
FIGURE 6.
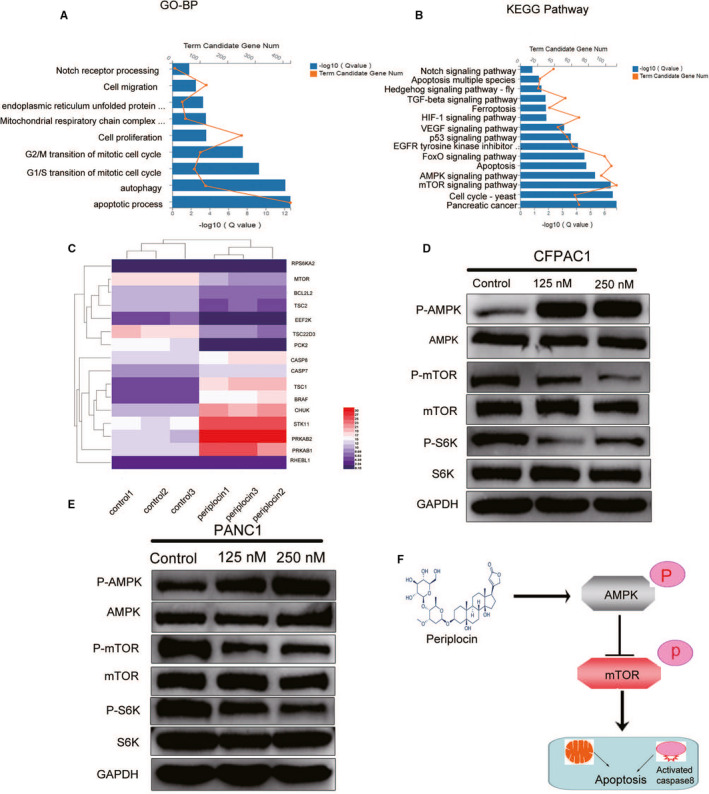
Periplocin regulated the AMPK/mTOR signaling pathways in pancreatic cancer. A and B, Gene ontology (GO) biological process and KEGG pathways analysis based on the RNA‐seq results for control versus Periplocin in PANC1 cells. p‐value was corrected by FDR, and the FDR <0.01 was considered significantly enriched. C, The hierarchical clustering of targets in the AMPK/mTOR signaling pathway depicted as a heat map. Downregulated genes are shown in blue and upregulated genes in red. D and E, Western blot analysis showed the expression changes of critical molecules in the AMPK/mTOR signaling pathway in PANC1 and CFPAC1 cells. F, Proposed mechanism of periplocin‐induced apoptosis in pancreatic cancer cells. Periplocin inhibits mTOR signaling by activating the AMPK signaling pathway, thus, inhibiting s6 k and exerting anti‐tumor effects
FIGURE 7.
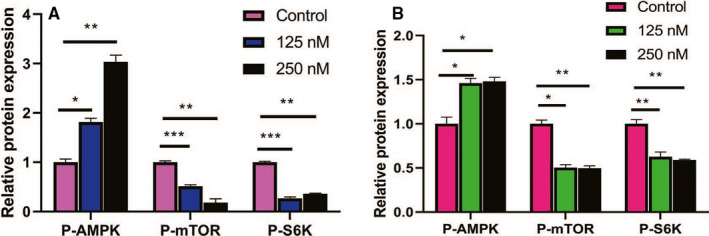
Quantitative analysis of Western blot pathway proteins. Data analysis of Figure 6D–E. Data are presented as the mean ±standard deviation. *p < 0.05, **p < 0.01, n ≥ 3
3.5. Periplocin inhibits the growth of CFPAC1 xenograft mouse models
Figure 8A–C shows the tumor volumes as measured after every three days, and the tumor weights as measured at the end of the study. It is shown that periplocin inhibited the growth of CFPAC1 xenograft tumors in nude mice. In addition, periplocin was associated with reduced expression of Ki67 (Figure 8D). These findings were consistent with the in vitro results.
FIGURE 8.
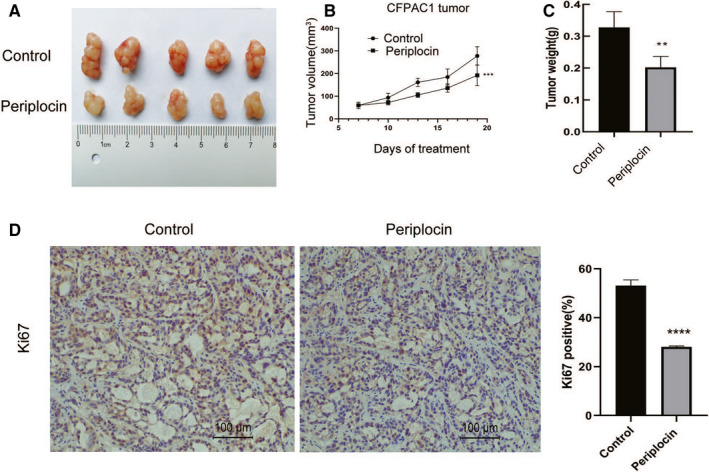
Periplocin inhibits the growth of CFPAC1 xenografts in nude mice. CFPAC1 cells were inoculated into nude mice to establish subcutaneous xenograft tumors. The dissected tumors were photographed. A, Representative macroscopic views showed tumors in indicated groups. B, Tumor volumes were monitored every 3 days after indicated treatments. C, Dissected tumor weights were measured. D, Representative images of IHC staining. Scale bar, 100 μm. Data are presented as means ±SD (* p < 0.05,** p < 0.01,*** p < 0.001), n ≥ 3
4. DISCUSSION
In this study, RNA‐seq differential gene Kegg pathway analysis and Immunoblotting of PANC1 cells treated with periplocin showed that the AMPK/mTOR pathway was activated while p70 S6 K was inhibited. The growth of pancreatic cancer cells was also inhibited. Furthermore, periplocin activated AMPK/mTOR to attenuate the proliferation of human pancreatic cancer cells. MTOR and AMPK were used as low energy and high energy sensors, respectively. They can be activated by glucose starvation and sufficient nutrients to promote cell growth and inhibition. 21 , 22 Forced AMPK activation inhibits the mammalian target of rapamycin (mTOR) complex 1 (mTORC1) 23 and the downstream S6K protein synthesis. These effects suppress tumor growth. This finding is consistent with those reported by previous studies. For example, berberine has been shown to inhibit proliferation, migration, and invasion by targeting the AMPK/HNF4α/WNT5A pathway in gastric cancer cells. 24 It has also been established that AMPK and death receptor five are involved in the apoptosis of hepatocellular carcinoma cells (HCC) and are induced by TRAIL and Berberine combined therapy. 25 Yuanhuacine (YC) was found to significantly enhance the AMP‐activated protein kinase (AMPK) signaling pathway in H1993 human NSCLC cells while inhibiting the downstream signaling pathway mediated by mTORC2, thereby suppressing cell proliferation. 26 The natural compound (Tanshinone IIA) also induces autophagic cell death by activating AMPK and ERK in leukemia KBM‐5 cells and inhibiting mTOR and p70 S6K. 27 Contrastingly, Panduratin A was shown to induce protective autophagy in melanoma A375 cells by activating AMPK and inhibiting mTOR signal transduction, thereby, conferring an antiapoptotic effect. 28 It has also been found that RA‐XII inhibits AMPK phosphorylation in HepG2 cells and activates the mTOR/P70S6K pathway. These effects induce HepG2 cell apoptosis and inhibits protective autophagy. 29 The possible explanation is that the AMPK‐mTOR signaling pathway is involved in the regulation of autophagy. Inhibition or activation of mTOR, therefore, inevitably causes the activation or inhibition of autophagy. Autophagy in cancer cells is conducive for cancer cell proliferation as it exerts antiapoptotic effects. However, excessive autophagy also lead to autophagy‐dependent cell death. The finding that excessive autophagy leads to cell growth inhibition should be further verified. In summary, periplocin inhibits mTOR through AMPK activation, thereby, suppressing the proliferation of pancreatic cancer cells, their migration, and invasion.
We also revealed that periplocin induces pancreatic cancer cell apoptosis through the AMPK/mTOR/S6K pathway. In addition, proapoptotic molecules (Bax and caspase3/8) were activated while the antiapoptotic Bcl‐2 was inhibited. This implies that the apoptosis induced by periplocin is a combination of caspase‐dependent internal and external apoptotic pathways. Programed cell death (PCD) is an orderly process. Apoptosis is a form of programed cell death. Three pathways mediate apoptosis: the mitochondrial pathway, the endoplasmic reticulum pathway, and the death receptor pathway. It has been established that quercetin inhibits S6K by activating the AMPK signaling pathway, thereby inducing bladder cancer cell apoptosis. 30 Periplocin upregulates death receptors DR4 and DR5 by activating the ERK1 / 2‐EGR1 pathway that induces the apoptosis of gastric cancer cells. 18 In TRAIL‐resistant human hepatocellular carcinoma cells, periplocin downregulates IAP to promote apoptosis. 31 It has been shown that S6K is involved in cell growth, cell cycle‐related protein regulation, and translation. 32 , 33 , 34 In addition, S6k is involved in cell apoptosis and is closely related to AKT. 35 It has not been established whether periplocin induces cell cycle arrest, leads to cell apoptosis, or inhibits phosphorylation targets indirectly by regulating S6K and downstream kinases. Proteomics analysis revealed different interactions between p70‐S6K1 and p54‐S6K2 and found evidence of new targets or regulators of the S6K protein family, such as NCL, NPM1, eIF2α, XRCC6, PARP1, and ILF2/ILF3. 36 This study elucidates on the S6K interaction network, and may help in understanding the mTOR/S6K pathway. In conclusion, periplocin induces pancreatic cancer cell apoptosis through the S6k pathway.
The rising pancreatic cancer incidences and mortality have necessitated the development of new treatment strategies. Analogs of natural compounds can be used to treat pancreatic cancer. 37 Periplocin has been shown to inhibit cell proliferation while inducing apoptosis. In addition, it inhibited the growth of the xenograft mouse model of CFPAC1 cells. Therefore, periplocin is a potential alternative for the treatment of human pancreatic cancer.
4.1. Conclusion
This study confirmed the inhibitory effects of periplocin on pancreatic cancer cells. By detecting the protein expression levels of important signaling pathways, periplocin was shown to induce caspase dependent apoptosis through the AMPK / mTOR apoptosis signal.
CONFLICTS OF INTEREST
There are no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
All authors read and approved the manuscript. B.C. and C.W. designed the experiment and analyzed the results. G.X. and L.S. performed the experiment and were responsible for writing the manuscript. Y.L. participated in the experimental procedures and analyzed the data.
ACKNOWLEDGMENTS
There are no special acknowledgments.
Gangyin Xie and Linxiao Sun contributed equally to this work.
Funding information
This study was supported by grants from the Wenzhou Municipal Science and Technology Bureau (Y20190073) and Natural Science Foundation of Zhejiang Province (LQ20H030004)
Contributor Information
Bicheng Chen, Email: bichengchen@hotmail.com.
Cheng Wang, Email: bichengchen@hotmail.com.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Guo H, Mao H, Pan G, et al. Antagonism of Cortex Periplocae extract‐induced catecholamines secretion by Panax notoginseng saponins in cultured bovine adrenal medullary cells by drug combinations. J Ethnopharmacol. 2013;147(2):447‐455. [DOI] [PubMed] [Google Scholar]
- 2. Zhao L, Shan B, Du Y, et al. Periplocin from Cortex periplocae inhibits cell growth and down‐regulates survivin and c‐myc expression in colon cancer in vitro and in vivo via beta‐catenin/TCF signaling. Oncol Rep. 2010;24(2):375‐383. [PubMed] [Google Scholar]
- 3. Lu ZJ, Zhou Y, Song Q, et al. Periplocin inhibits growth of lung cancer in vitro and in vivo by blocking AKT/ERK signaling pathways. Cell Physiol Biochem. 2010;26(4–5):609‐618. [DOI] [PubMed] [Google Scholar]
- 4. Li L, Zhao L‐M, Dai S‐L, et al. Periplocin extracted from cortex periplocae induced apoptosis of gastric cancer cells via the ERK1/2‐EGR1 pathway. Cell Physiol Biochem. 2016;38(5):1939‐1951. [DOI] [PubMed] [Google Scholar]
- 5. Lohberger B, Wagner S, Wohlmuther J, et al. Periplocin, the most anti‐proliferative constituent of Periploca sepium, specifically kills liposarcoma cells by death receptor mediated apoptosis. Phytomedicine. 2018;51:162‐170. [DOI] [PubMed] [Google Scholar]
- 6. Lohberger B, Bernhart E, Stuendl N, et al. Periplocin mediates TRAIL‐induced apoptosis and cell cycle arrest in human myxofibrosarcoma cells via the ERK/p38/JNK pathway. Phytomedicine. 2020;76:153262. [DOI] [PubMed] [Google Scholar]
- 7. Howes N, Lerch MM, Greenhalf W, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004;2(3):252‐261. [DOI] [PubMed] [Google Scholar]
- 8. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 9. He XY, Yuan YZ. Advances in pancreatic cancer research: moving towards early detection. World J Gastroenterol. 2014;20(32):11241‐11248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang ZK, Yang YM. Current research status and progress in comprehensive diagnosis and treatment of pancreatic cancer in the era of targeted therapy. Zhonghua Wai Ke Za Zhi. 2020;58(1):22‐26. [DOI] [PubMed] [Google Scholar]
- 11. Liu J, Long S, Wang H, et al. Blocking AMPK/ULK1‐dependent autophagy promoted apoptosis and suppressed colon cancer growth. Cancer Cell Int. 2019;19:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang F, Cao M, Fan M, et al. AMPK‐mTOR‐ULK1 axis activation‐dependent autophagy promotes hydroxycamptothecin‐induced apoptosis in human bladder cancer cells. J Cell Physiol. 2020;235(5):4302‐4315. [DOI] [PubMed] [Google Scholar]
- 13. Chen M‐B, Zhang Y, Wei M‐X, et al. Activation of AMP‐activated protein kinase (AMPK) mediates plumbagin‐induced apoptosis and growth inhibition in cultured human colon cancer cells. Cell Signal. 2013;25(10):1993‐2002. [DOI] [PubMed] [Google Scholar]
- 14. Zeng P, Zhang P, Zhou L‐N, et al. TMPRSS4 as an emerging potential poor prognostic factor for solid tumors: a systematic review and meta‐analysis. Oncotarget. 2016;7(46):76327‐76336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen MB, Jiang Q, Liu YY, et al. C6 ceramide dramatically increases vincristine sensitivity both in vivo and in vitro, involving AMP‐activated protein kinase‐p53 signaling. Carcinogenesis. 2015;36(9):1061‐1070. [DOI] [PubMed] [Google Scholar]
- 16. Barroso‐Chinea P, Luis‐Ravelo D, Fumagallo‐Reading F, et al. DRD3 (dopamine receptor D3) but not DRD2 activates autophagy through MTORC1 inhibition preserving protein synthesis. Autophagy. 2020;16(7):1279‐1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tian Y, Shen L, Li F, et al. Silencing of RHEB inhibits cell proliferation and promotes apoptosis in colorectal cancer cells via inhibition of the mTOR signaling pathway. J Cell Physiol. 2020;235(1):442‐453. [DOI] [PubMed] [Google Scholar]
- 18. Law BYK, Mok SWF, Chan WK, et al. Hernandezine, a novel AMPK activator induces autophagic cell death in drug‐resistant cancers. Oncotarget. 2016;7(7):8090‐8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi YH. ROS‐mediated activation of AMPK plays a critical role in sulforaphane‐induced apoptosis and mitotic arrest in AGS human gastric cancer cells. Gen Physiol Biophys. 2018;37(2):129‐140. [DOI] [PubMed] [Google Scholar]
- 20. Lee J, Sul JI, Park J, et al. Honokiol induces apoptosis and suppresses migration and invasion of ovarian carcinoma cells via AMPK/mTOR signaling pathway. Int J Mol Med. 2019;43(5):1969‐1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016‐1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577‐590. [DOI] [PubMed] [Google Scholar]
- 24. Hu Q, Li L, Zou X, et al. Berberine attenuated proliferation, invasion and migration by targeting the AMPK/HNF4alpha/WNT5A pathway in gastric carcinoma. Front Pharmacol. 2018;9:1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ke R, Vishnoi K, Viswakarma N, et al. Involvement of AMP‐activated protein kinase and Death Receptor 5 in TRAIL‐Berberine‐induced apoptosis of cancer cells. Sci Rep. 2018;8(1):5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kang JI, Hong J‐Y, Lee H‐J, et al. Anti‐tumor activity of yuanhuacine by regulating AMPK/mTOR signaling pathway and actin cytoskeleton organization in non‐small cell lung cancer cells. PLoS One. 2015;10(12):e0144368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yun S‐M, Jung JH, Jeong S‐J, et al. Tanshinone IIA induces autophagic cell death via activation of AMPK and ERK and inhibition of mTOR and p70 S6K in KBM‐5 leukemia cells. Phytother Res. 2014;28(3):458‐464. [DOI] [PubMed] [Google Scholar]
- 28. Lai SL, Mustafa MR, Wong PF. Panduratin A induces protective autophagy in melanoma via the AMPK and mTOR pathway. Phytomedicine. 2018;42:144‐151. [DOI] [PubMed] [Google Scholar]
- 29. Song L, Wang Z, Wang Y, et al. Natural cyclopeptide RA‐XII, a new autophagy inhibitor, suppresses protective autophagy for enhancing apoptosis through AMPK/mTOR/P70S6K pathways in HepG2 Cells. Molecules. 2017;22(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Su Q, Peng M, Zhang Y, et al. Quercetin induces bladder cancer cells apoptosis by activation of AMPK signaling pathway. Am J Cancer Res. 2016;6(2):498‐508. [PMC free article] [PubMed] [Google Scholar]
- 31. Cheng C‐F, Lu I‐H, Tseng H‐W, et al. Antitumor effect of periplocin in TRAIL‐resistant human hepatocellular carcinoma cells through downregulation of IAPs. Evid Based Complement Alternat Med. 2013;2013:958025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tetsuo F, Arioka M, Miura K, et al. Differentiation‐inducing factor‐1 suppresses cyclin D1‐induced cell proliferation of MCF‐7 breast cancer cells by inhibiting S6K‐mediated signal transducer and activator of transcription 3 synthesis. Cancer Sci. 2019;110(12):3761‐3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kurgan N, Tsakiridis E, Kouvelioti R, et al. Inhibition of human lung cancer cell proliferation and survival by post‐exercise serum is associated with the inhibition of Akt, mTOR, p70 S6K, and Erk1/2. Cancers (Basel). 2017;9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsoi H, Lam KC, Dong Y, et al. Pre‐45s rRNA promotes colon cancer and is associated with poor survival of CRC patients. Oncogene. 2017;36(44):6109‐6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu X, Song M, Wang P, et al. Targeted therapy of the AKT kinase inhibits esophageal squamous cell carcinoma growth in vitro and in vivo. Int J Cancer. 2019;145(4):1007‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pavan ICB, Yokoo S, Granato DC, et al. Different interactomes for p70–S6K1 and p54–S6K2 revealed by proteomic analysis. Proteomics. 2016;16(20):2650‐2666. [DOI] [PubMed] [Google Scholar]
- 37. Xue R, Meng Q, Lu DI, et al. Mitofusin2 induces cell autophagy of pancreatic cancer through inhibiting the PI3K/Akt/mTOR signaling pathway. Oxid Med Cell Longev. 2018;2018:2798070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


