Abstract
Natural killer (NK) cells are lymphocytes that can directly destroy cancer cells. When NK cells are activated, CD56 and CD107a markers are able to recognize cancer cells and release perforin and granzyme B proteins that induce apoptosis in the targeted cells. In this study, we focused on the role of phytoncides in activating NK cells and promoting anticancer effects. We tested the effects of several phytoncide compounds on NK-92mi cells and demonstrated that α-pinene treatment exhibited higher anticancer effects, as observed by the increased levels of perforin, granzyme B, CD56 and CD107a. Furthermore, α-pinene treatment in NK-92mi cells increased NK cell cytotoxicity in two different cell lines, and immunoblot assays revealed that the ERK/AKT pathway is involved in NK cell cytotoxicity in response to phytoncides. Furthermore, CT-26 colon cancer cells were allografted subcutaneously into BALB/c mice, and α-pinene treatment then inhibited allografted tumor growth. Our findings demonstrate that α-pinene activates NK cells and increases NK cell cytotoxicity, suggesting it is a potential compound for cancer immunotherapy.
Keywords: natural killer cell, phytoncide, α-pinene, NK cell cytotoxicity, anticancer effect, ERK/AKT pathway
1. Introduction
Natural killer (NK) cells are lymphocytes with a cytotoxic role, possessing an important function in immunity, as they are able to recognize and eliminate virus-infected (or “stressed”) cells, as well as cancer cells [1]. NK cells comprise 10% of the lymphocytes contained in the blood and peripheral lymphoid organs [2], containing abundant cytoplasmic granules and characteristic cell surface markers [3]. When NK cells are activated, they release proteins contained in their granules, such as perforins and granzymes, to attack invasive cells and induce apoptosis. NK cells and macrophages feed on microorganisms and produce interleukin 12 (IL-12), which, in turn, activates NK cells to secrete interferon-γ (IFN-γ), and IFN-γ subsequently activates macrophages to eliminate target cells [4].
Cancer immunotherapy is a type of strategy for cancer treatment that uses the individual’s natural immune system to combat cancer [5]. Moreover, many cancer patients who receive cancer immunotherapy treatment have shown a good prognosis [6,7,8]. Chimeric antigen receptor (CAR)-engineered T (CAR-T) cells are a known treatment with noticeable efficacy in cancer immunotherapy, allowing tumor selectivity and elimination by separating T-cells from the patient’s white blood cells and adding the specific CAR to target particular cancer cell antigens. Recently, the use of CAR-NK cells has been the subject of growing interest as a suitable candidate for anticancer research since, contrarily to CAR-T therapy, it induces minimal cytokine release and neurotoxicity, possesses multiple mechanisms for activating cytotoxic activity and has a high feasibility [9]. In fact, NK cells have revealed several new principles for recognizing and responding to tumors, demonstrating the potential positive effect on the clinical application of NK cells in cancer immunotherapy [10]. One example is the transfer of expanded and activated NK cells, in which autologous NK cells or the development of the CAR on NK cells by genetic engineering techniques facilitates adoptive transfer to patients. The adoptive transfer of NK cells has the advantage of being highly effective in promoting antitumor activity [11,12,13]. A second example is cytokine-based therapy. IL-2, IL-15 and IL-21 have been used to boost NK cell and T cell activity [14,15,16,17]. A third example is the targeting of the inhibitory receptors of NK cells, such as PD1/PD-L1 blockade [18,19], mAbs to KIRs [20,21], mAbs to NKG2A [22], TIGIT/CD96 blockade [23] and TIM-3 blockade [24,25]. These receptor modulations boost NK cell activity against tumor cells and metastasis [26].
It has been well documented that “forest bathing” enhances human innate immune cell activity, especially in NK cells, by boosting the expression of anticancer proteins [27]. This beneficial effect of “forest bathing” is known to be mainly due to phytoncides, aromatic volatile substances derived from trees. Phytoncides are most commonly released from conifers, especially pine and nut pine trees. Additionally, pine tree essential oil extracted from the leaf was found to contain 50 different terpene components, the majority being α- and β-pinene, myrcene, β-thujene and bornyl acetate. Among them, the content of α-pinene was revealed to be the highest [28].
Several types of terpene anticancer studies have been previously conducted. In particular, it was found that 37 types of monoterpenes present in the essential oils of plants possess anticancer effects [29]. Among several monoterpenes, anticancer studies of α-pinene have been established in various types of cancer and in vivo experiments, such as human hepatocellular carcinoma [30,31] and prostate cancer [32] in xenograft mouse models. Moreover, α-pinene is known to induce apoptosis by G2/M phage cycle arrest in human ovarian cancer and hepatocellular carcinoma [33,34]. In addition, α-pinene can inhibit the expression of matrix metalloproteinase-9 in human breast cancer cells, thereby inhibiting cancer invasion [35].
Phytoncides significantly increase NK cell activity with a concomitant increase in the expression levels of perforin and granzyme B, crucial effector molecules in NK cell-mediated cytotoxicity [36,37,38]. Furthermore, phytoncides have been observed to increase the number of CD56 molecules, which, along with CD107a, mark the activation of NK cells [39]. Lupeol, a triterpene, has been speculated to increase IFN-γ and CD107a via the activation of PI3K/AKT and Wnt/β-catenin signaling pathways [40]. In our previous study, the scent of phytoncide was revealed to have anticancer effects as well [41].
In this work, we studied the effects of phytoncide on NK cell activation. First, as phytoncide contains several compounds, we analyzed the effects of specific substances contained in phytoncides to test their efficacy in NK cell activation. Furthermore, by using α-pinene, known for its high effectiveness in NK cell activation, we analyzed NK cell cytotoxicity in mouse tumor allograft models and human NK-92mi cell lines. Additionally, since several reports demonstrate that NK cell activation occurs through the ERK/AKT signal pathway [42,43], an immunoblot assay was performed to confirm whether this was also the case for NK cells activated by α-pinene. Our results show that α-pinene increases the anticancer effect by accelerating NK cell activation and cytotoxicity via ERK/AKT signal pathways.
2. Results
2.1. Phytoncides Elevate the Expression of Activation Proteins in NK-92mi Cells
Phytoncide is a mixture of volatile components. Human NK-92mi cells were treated with each phytoncide component to determine which components of phytoncide activate NK cells. Perforin is a representative protein marker of the cytotoxicity of NK cells [44]. Therefore, we tested the effect of phytoncide constituents on the expression of perforin in NK-92mi cells after phytoncide treatment (Figure 1a). The results showed that α-pinene, o-cymene and camphor increased perforin mRNA expression in NK-92mi cells compared to the control (phorbol 12-myristate 13-acetate (PMA) and ionomycin treated). The mRNA expression of granzyme B [45], CD56 [46] and CD107a [47] also increased compared to the control (Figure 1b–d).
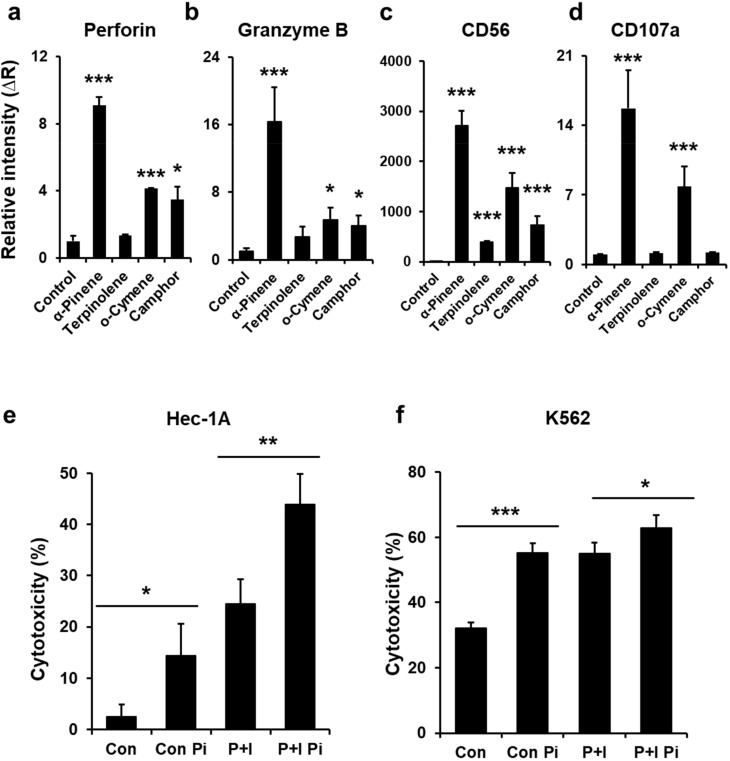
Figure 1.
Phytoncides increase natural killer (NK) cell activation and cytotoxicity in NK-92mi cells. Human NK-92mi cells were activated by incubation with phorbol 12-myristate 13-acetate (PMA) and ionomycin for 6 h after treatment with α-pinene, terpinolene, o-cymene and camphor at 100 μM. The expression levels of (a) perforin, (b) CD56, (c) granzyme B and (d) CD107a mRNA were analyzed with real-time PCR. The data are expressed as the mean ± SD of three independent experiments (* p < 0.05, *** p < 0.005). Human NK-92mi cells were incubated with target K562 cells or HEC-1A cells, which were prelabeled with calcein-AM for 4 h, as described. Calcein-AM released from K562 and HEC-1A target cells was measured by a fluorescence microplate reader. (e) NK-92mi cytotoxicity towards Hec-1A cells. (f) NK-92mi cytotoxicity towards K562 cells. Con: control, Con Pi: control with α-pinene treatment, P+I: PMA and ionomycin activation, P+I Pi: PMA and ionomycin activation with α-pinene treatment. Results are expressed as the mean ± SD of three independent experiments (* p < 0.05, ** p < 0.01, *** p < 0.005).
2.2. α-Pinene Enhances NK-92mi Cell Cytotoxicity
Based on our previous observation that α-pinene stimulates the expression of several NK cell markers (Figure 1a–d), NK cytotoxicity assays were performed to observe whether α-pinene activates NK-92mi cell cytotoxicity. The results indicated that α-pinene elevated NK-92mi cell cytotoxicity when NK-92mi cells were cocultured with target Hec-1A cells. Its cytotoxicity was increased 5.8-fold compared to the control and 1.8-fold compared to the PMA and ionomycin treatment (Figure 1e). In addition, when cocultured with K562 cells, the cytotoxicity of NK-92mi cells was increased further (by 1.7-fold) compared to the control and increased 1.2-fold compared to the PMA and ionomycin treatment (Figure 1f). This result demonstrates that HEC-1A cells are more sensitive to NK cytotoxicity than K562 cells, which is in accordance with a previous report demonstrating that the sensitivity of NK cytotoxicity depends on the target cell or its conditions [48]. Moreover, PMA and ionomycin treatment acting as an NK activator was found to be more cytotoxic than the control in HEC-1A cells. These results show that, even after NK activator treatment, α-pinene further increased NK cell cytotoxicity.
2.3. α-Pinene Induces NK Cell Cytotoxicity via the ERK and AKT Pathways
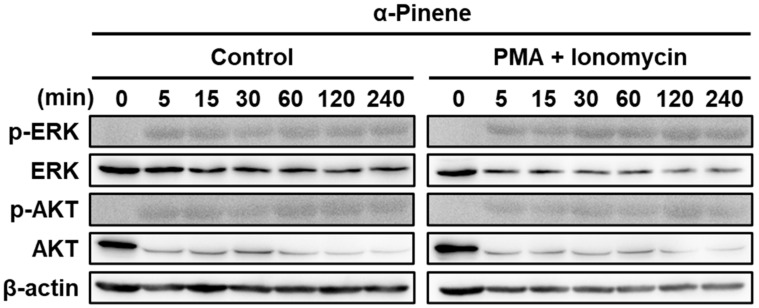
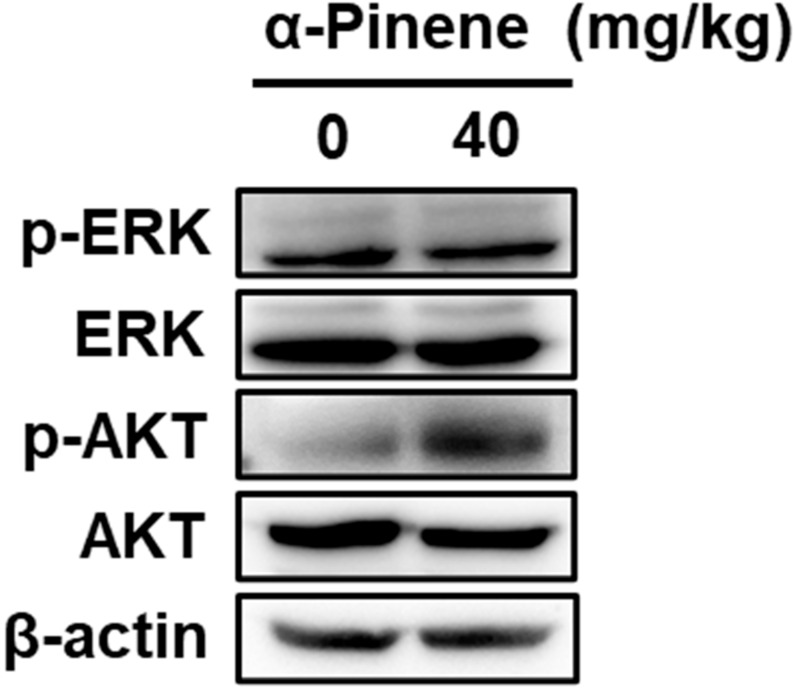
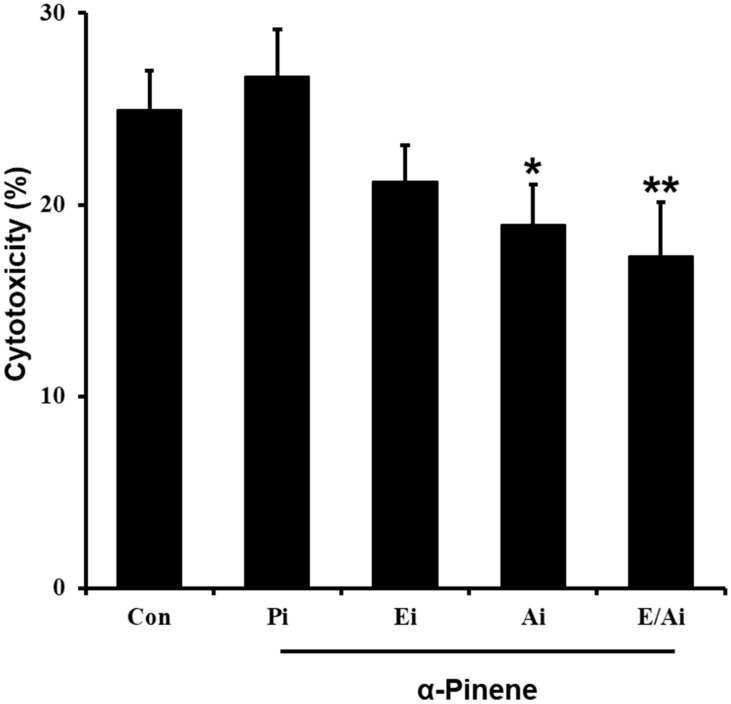
To determine whether the ERK/AKT pathway is involved in NK cell cytotoxicity in response to phytoncides [49], we performed immunoblot assays. α-Pinene treatment induced the activation of several ERK/AKT signaling molecules such as total ERK1/2, phospho-ERK1/2 (Thr202/Tyr204), AKT and phospho-AKT (Y312). The experiment was divided into a PMA and ionomycin-treated group and a control group. Cells were harvested at 5, 15, 30, 60, 120 and 240 min after α-pinene treatment to confirm the ERK and AKT pathway expression patterns over time. The emergence of ERK phosphorylation and AKT phosphorylation began around 5 min after α-pinene treatment (Figure 2). A similar time frame was manifested in PMA and ionomycin-activated NK-92mi cells. In the PMA and ionomycin treatment, the emergence of ERK phosphorylation and AKT phosphorylation occurred 5 min after treatment (Figure 2). Furthermore, to further confirm that α-pinene increases the ERK/AKT signaling pathway in NK cells, we conducted an immunoblot assay using mouse splenic NK cells. As a result, similarly to NK-92mi cells, it was found that α-pinene causes an increase in AKT phosphorylation in mouse splenic cells (Figure A1). However, α-pinene did not induce ERK phosphorylation, implying the presence of a cell- or model-type dependence on the ERK/AKT pathway for the response. α-Pinene enhanced the expression of signaling molecules in ERK and AKT pathway signaling, indicating that α-pinene activates NK cells through ERK and AKT signaling. In addition, we performed NK cytotoxicity toward K562 using α-pinene in combination with ERK and AKT inhibitors. As a result, it was confirmed that the NK cytotoxicity increased by α-pinene was reduced by ERK and AKT inhibitors (Figure A2).
Figure 2.
α-Pinene increases the expression of signaling molecules in the ERK/AKT signaling pathway in NK-92mi cells. Human NK-92mi cells were treated with α-pinene at 100 μM for 48 h. The total cell lysate was obtained and subjected to immunoblotting. Two groups were tested. NK-92mi control: NK-92mi cells without activation. PMA + ionomycin treatment: NK-92mi activated by PMA + ionomycin.
2.4. α-Pinene Inhibited the Growth of CT-26 Colon Cancer Allografts in BALB/c Mice
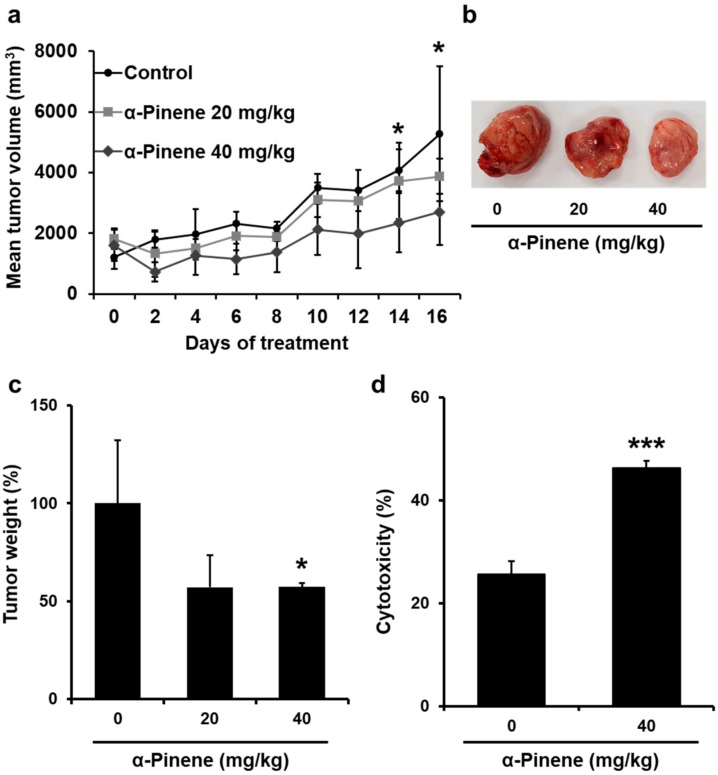
CT-26 colon cancer cells were allografted in BALB/c mice by subcutaneously inoculating cells to the left flank to ensure that phytoncides would prevent cancer growth. The growth of the tumor allografts was inhibited significantly by α-pinene (Figure 3a–c). After α-pinene treatment was completed, the tumor-bearing mice were sacrificed and the tumors were excised to measure their weight. Compared to the control, tumor weight in mice treated with 40 mg/kg α-pinene was significantly reduced (Figure 3c). α-Pinene inhibited the growth of CT-26 allograft in BALB/c mice, and the inhibition ratio (%) compared to the control group was 42.83% in the 40 mg/kg α-pinene treatment group. These data indicate that α-pinene has an inhibitory effect on tumor growth.
Figure 3.
α-Pinene inhibited the growth of CT-26 colon cancer allografts in BALB/c mice by increasing NK cytotoxicity. (a–c) Tumor volume and weight were significantly decreased by α-pinene 40 mg/kg treatment compared to the control (* p < 0.05, *** p < 0.005). (d) The cytotoxicity of mouse splenic NK cells was measured. Splenic NK cells were isolated from those of normal or tumor-transplanted mice and incubated with YAC-1 target cells prelabeled with calcein-AM as described in Section 4. Hank’s Balanced Salt Solution was used for spontaneous fluorescence from YAC-1 cells without effector NK cells, and total fluorescence was acquired from wells where YAC-1 cells were incubated with a lysis buffer. NK cell cytotoxicity was calculated as described in Section 4.
2.5. α-Pinene Enhances Mouse Splenic NK Cell Cytotoxicity
To examine whether the NK cells were activated by phytoncides, we performed an NK cytotoxicity assay. The tumor-bearing mice were treated with 20 or 40 mg/kg α-pinene for 16 days. The mouse spleen was removed to isolate NK cells (effector cells). To measure the cytotoxicity of effector NK cells, the amount of calcein-AM released from target YAC-1 cells was measured. In response to the 40 mg/kg α-pinene treatment of control mice, NK cell activities were increased 1.8-fold (Figure 3d).
3. Discussion
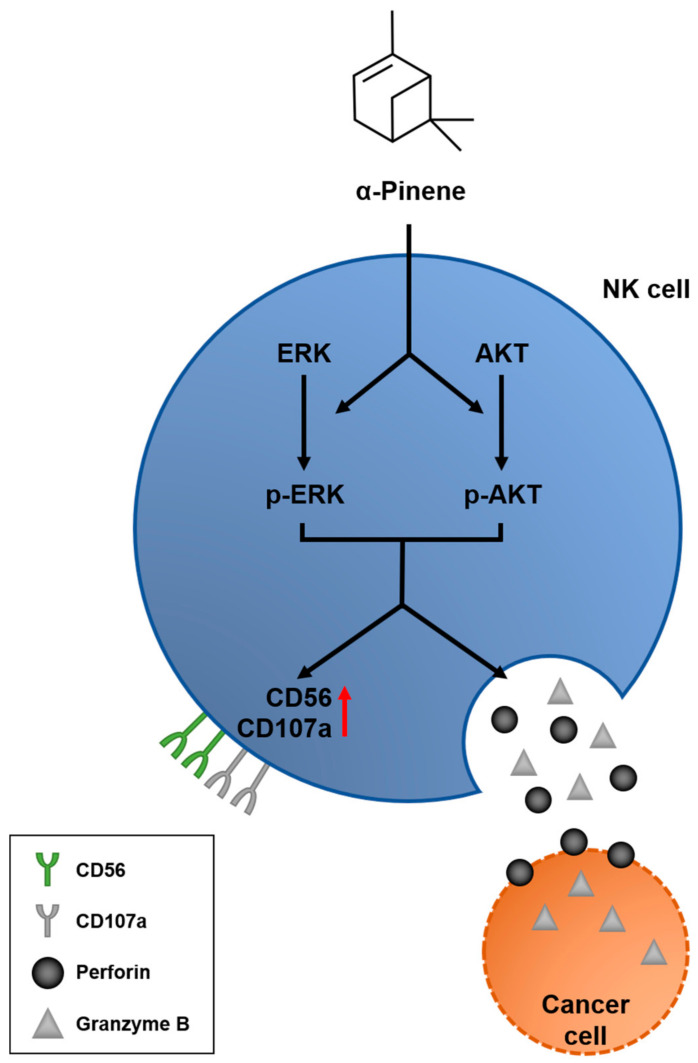
In this study, we demonstrate that α-pinene, a terpene originating from phytoncide, enables the ERK and AKT phosphorylation signaling pathways, leading to NK cell activation—observed by increased expressions of CD56 and CD107a (Figure 4). NK cell activation further induces the release of perforins, which attach to the cancer cell membrane and open pores on its surface, allowing the diffusion of granzyme B proteins and inducing cell apoptosis.
Figure 4.
Schematic model of α-pinene promoting an anticancer effect via NK cell activation. α-Pinene stimulates ERK and AKT phosphorylation signaling pathways and leads to NK cell activation, which is confirmed by the increased expression of receptors CD56 and CD170. Consequently, the NK cell product proteins perforin and granzyme B are induced, leading to cancer cell death.
Terpenes are a significant component of phytoncides and have many beneficial effects on the body’s biological activity. Forest environments are known to be rich in various terpenes, which are widely used for their anti-inflammatory, antitumorigenic or neuroprotective activities [50]. Many types of monoterpenes are known to have anticancer effects [29].
α-Pinene, one type of monoterpene, has been previously studied in various cancer cases. Most studies have been carried out with the monoterpene being directly delivered into cells or tumors transplanted in mice. However, in our experiment, cancer cells and tumor allografts were not directly treated with α-pinene. Instead, we measured immune cell activity and observed that human and mouse splenic NK cell cytotoxicity increased with α-pinene treatment (Figure 1b,c and Figure 3d). These results indicate that α-pinene has an indirect anticancer effect by boosting NK cell cytotoxicity.
CD56 and CD107a are markers associated with the activation and cytotoxicity in NK cells [47,51]. Our study demonstrates that α-pinene enables the ERK and AKT phosphorylation signaling pathways, thus leading to NK cell activation, as verified by the increased expression of receptors CD56 and CD107a. CD56 and CD107a are correlated with various forms of cytokine secretion production, including perforin, granzyme B and IFN-γ [52,53,54]. In a previous study, it was found that NK cells require PI3K-ERK1/2-dependent signaling activation for the killing of Cryptococcus neoformans [55]. Furthermore, Kwon et al. have demonstrated that phosphorylation of NF-κB activates NK cells via synergistic ERK and PI3K-Akt pathways [49]. Similarly, our results also indicate that α-pinene increases phosphorylation of the ERK/AKT pathway, thus inducing NK cell activation (Figure 2).
The stimulation of NK cells is controlled by a balance of inhibitory and activator receptors expressed by NK cells and target cells [56,57]. Interestingly, the results of this study showed different responses to NK cytotoxicity in Hec-1A and K562 cells (Figure 1e,f). Human NK-92mi cell cytotoxicity was 2.5% towards Hec-1A cells and 32% towards K562 cells, suggesting that the immune response was different for different tumor receptor types [58]. However, upon α-pinene treatment, NK cytotoxicity increased regardless of the type of cancer. These results suggest that α-pinene enhances NK cytotoxicity regardless of tumor receptor type.
Furthermore, one important consideration for further research of this topic, which is relevant for anticancer technology, is the mechanism by which phytoncides enter the body and reach NK cells. We hypothesize that phytoncides can dissolve in the blood when inhaled into the lungs. Afterward, dissolved phytoncide components can directly stimulate NK cell activation. According to our results, this hypothesis is plausible since α-pinene directly stimulates NK cells in a similar manner. Consequently, further research must be done to demonstrate this in vivo, particularly by measuring the concentration of phytoncide in the blood. Secondly, we hypothesized that phytoncides are able to stimulate the olfactory epithelium and regulate hormones. Among these hormones, vasoactive intestinal peptide (VIP) is known to be able to control NK cell activation [59,60].
In conclusion, the results of this study show an indirect anticancer effect via NK cell activation by phytoncides, suggesting that immunity increases when humans are frequently exposed to plants that release these compounds. Furthermore, our findings also reveal the potential use of α-pinene as a new approach in cancer immunotherapy by NK cell therapy, or even as a complement for existing cancer treatments.
4. Materials and Methods
4.1. Cell Culture and Reagents
NK-92mi cells (a human IL-2 independent NK cell line) HEC-1A cells (a human endometrial carcinoma cell line), K562 cells (a human lymphoblast cell line), CT-56 cells (a mouse colon carcinoma cell line) and YAC-1 cells (a mouse lymphoma cell line) were obtained from the American Type Culture Collection (Manassas, VA, USA). NK-92mi cells were cultured in α-MEM media supplemented with 100 U/mL penicillin, 100 U/mL streptomycin, 20% FBS (Hyclone, Logan, UT, USA), 1% vitamin solution X100 (Gibco, Waltham, MA, USA) and 2-mercaptoethanol (Gibco). HEC-1A, K562, CT-26 and YAC-1 cells were cultured at 37 °C in DMEM/F12 media supplemented with 100 U/mL penicillin, 100 U/mL streptomycin and 10% FBS in a humidified incubator in the presence of 5% CO2. All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise specified.
4.2. Mice Tumor Allografts
All procedures, including mice handling, were executed at the Department of Meridian and Acupoint of the College of Korean Medicine at Semyung University (smecae 20-07-01, approved on 1 July 2020), according to the guidelines approved by the Institutional Animal Care and Use Committee (IACUC). Female BALB/c mice, 6 weeks of age, were obtained from Joongah Bio (Suwon, Korea). Animals were housed in sterile plastic cages at controlled temperature (26 ± 2 °C), lighting (12 h) and humidity (60 ± 5%). Food and water were available ad libitum. To generate tumor allografts, CT-26 cells were subcutaneously injected at 1 × 107 cells/200 μL in saline into the dorsal flank of female BALB/c mice (n = 10 mice/group), as described previously [61]. After being allowed to grow for 2 weeks to reach the proliferative phase, mice were subdivided into three groups for α-pinene (Sigma-Aldrich) treatments.
4.3. Tumor Volume Estimation
The sizes of the allografted tumors were measured externally every 2 days from the last measured date, and the tumor volumes were obtained according to the equation Volume = (L × S2)/2, where L and S are the lengths of the major and minor axes, respectively. The animals were monitored for 30 days after allograft implantation.
4.4. Mouse Splenic Natural Killer Cell Cytotoxicity
The mouse spleens were isolated, and red blood cells were removed as described previously [62]. The spleens were mashed through a 100-µm cell strainer (Corning Inc., Corning, NY, USA) in complete media consisting of DMEM/F12 media (Gibco) with 10% fetal bovine serum and antibiotics as described above. Spleen mononuclear cells were obtained by centrifuging the mashed spleen cell suspension on 4 mL of Histopaque-1077 (Sigma). The cells in the interface were withdrawn and washed three times with complete media. The resulting mononuclear cells were resuspended in complete media at 1 × 106 cells/mL. YAC-1 cells, used as the target cells of spleen NK cells, were loaded with calcein-AM (Sigma), according to the method of Roden et al. [63]. The loading of YAC-1 cells (1 × 106 cells/mL) was performed at a final calcein-AM concentration of 10 μM for 30 min at 37 °C. Purified mononuclear effector cells and labeled YAC-1 target cells were added at a ratio of 20:1 to a 96-well plate and cocultured for 4 h at 37 °C. Following centrifugation at 2000 rpm for 3 min, 150 μL of the supernatant from each well was collected to measure the fluorescence of the calcein released into the media using a microplate reader. The absorbance of the contents of each well was measured at the excitation and emission wavelengths of 485 and 538 nm, respectively. Spontaneous calcein release was determined by culturing the labeled YAC-1 cells in complete media without effector NK cells. Total fluorescence was acquired from wells where target cells were incubated with a lysis buffer consisting of 50 mM sodium borate and 0.1% Triton X-100 (pH 9.0). Specific lysis was calculated according to the following equation:
| (1) |
4.5. Natural Killer Cell NK-92mi Activation
NK-92mi cells cultured at 1 × 107 cells/mL were activated by PMA at 250 ng/mL and ionomycin at 1 μM for 6 h. Immediately after activation by PMA and ionomycin, the NK-92mi cells were further treated with phytoncide compounds (namely α-pinene, terpinolene, o-cymene and camphor) at 100 μM each in the cell culture media for 48 h before being subjected to reverse transcription PCR, real-time PCR, immunoblot and NK cell cytotoxicity assay.
4.6. Reverse Transcription and Real-Time PCR
Trizol reagent (Life Technologies, Waltham, MA, USA) was used for the extraction of total mRNA, according to the manufacturer’s instructions. Approximately 1 μg of each total RNA was subjected to reverse transcription reaction at 37 °C for 1 h using AccuPower RT premix (Bioneer, Daejeon, Korea) as per the manufacturer’s instructions. PCR was performed using a Mastercycler (Eppendorf, Hamburg, Germany). PCR was carried out for each sample with appropriate PCR primer sets using AccuPower PCR PreMix (Bioneer). The primers for perforin (5′-GTTTCCATGTGGTACACACTC-3′; 5′-GTGCCGTAGTTGGAGATAAG-3′), granzyme B (5′-TTCCTGATACGAGACGACTT-3′; 5′-TTTCACAGGGATAAACTGCT-3′), CD56 (5′-GATTCATAGTCCTGTCCAACA-3′; 5′-GACCTGAATGTCCTTGAAGTT-3′), CD107a (5′-ACACTCACTCTCAATTTCACG-3′; 5′-CTGCCCTGATGTCAGTTATAG-3′) and human GAPDH (5′-AACCTGCCAAATATGATGAC-3′; 5′-TTGAAGTCAGAGGAGACCAC-3′) (as a loading control) were purchased from Bionics (Seoul, Korea). Each primer sequence was designed based on the full-length mRNA sequence using Primer3 software (http://bioinfo.ut.ee/primer3-0.4.0/primer3/). Real-time PCR gene expression analyses were performed using CYBR Q Green 2× qPCR Master Mix (CellSafe, Yongin, Korea). Real-time PCR was performed with an Agilent AriaMx Real-Time PCR System (Agilent, Santa Clara, CA, USA).
4.7. NK Cell Cytotoxicity Assay
Two target cell lines, namely K562 cells and HEC-1A cells, were loaded with calcein-AM at 10 μM in media for 30 min at 37 °C and subsequently washed with phosphate-buffered saline three times. Stained target cells were incubated with NK-92mi cells as effector cells at a ratio of 20:1 for 4 h at 37 °C. After incubation, the cell plate was centrifuged at 2000 rpm for 10 min. The supernatant (150 μL) from each well was collected to measure calcein-AM fluorescence released into the media using a microplate reader (SpectraMAX GeminiXS). The absorbance was measured at excitation and emission wavelengths of 485 and 538 nm, respectively. Based on fluorescence intensity, NK cell cytotoxicity was calculated using Equation (1).
4.8. Immunoblot Assay
NK-92mi cell lines were seeded in 75T cell culture flasks at 1 × 107 cells/mL. Treatment with 100 μM α-pinene was carried out to activate the NK-92mi cells. After treatment with α-pinene for 48 h, immunoblotting was performed. Briefly, NK-92mi cells were harvested by centrifugation at 2000 rpm for 3 min, and the cell pellet was lysed with RIPA buffer (cell lysis buffer). Bradford assay was used to determine the protein concentration. For each sample, 20 μg of protein was loaded onto a 15% polyacrylamide gel and subjected to SDS-PAGE. The proteins on the polyacrylamide gel were transferred to a PVDF membrane (Roche, Basel, Switzerland) and incubated for 2 h at room temperature with primary antibodies against ERK1/2, phospho-ERK1/2 (Thr 202/Tyr204), AKT, phospho-AKT (Y312) or β-actin. The membranes were washed further with TBS-T three times and incubated with secondary antibodies for 1 h at room temperature. All antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Immunoblot bands were detected using an enhanced chemiluminescent substrate (Thermo Scientific, Waltham, MA, USA) and exposed to a luminescent image analyzer LAS-3000 (Fuji, Tokyo, Japan). Las-3000 Image Reader software was used to detect the dots on the membrane.
4.9. Statistical Analysis
A two-tailed Student’s t-test was used for statistical analysis between two groups.
Appendix A
Figure A1.
α-Pinene increases the expression of signaling molecules of the AKT signaling pathway in mouse splenic NK cells. BALB/c mice were treated with α-pinene at 0 or 40 mg/kg for 3 days. After 3 days, splenic NK cells were isolated from the mice. The total cell lysate was obtained and subjected to immunoblotting.
Figure A2.
The ERK/AKT inhibitor decreases NK-92mi cytotoxicity against K562. Con: No treatment, Pi: α-pinene, Ei: ERK inhibitor (PD98059, 50 μM), Ai: AKT inhibitor (LY294002, 20 μM), E/Ai: ERK + AKT inhibitor. (* p < 0.05, ** p < 0.01).
Author Contributions
Conceptualization, H.J., M.-G.L., S.T.K., B.-H.B.; methodology, formal analysis, investigation, resources, data curation and writing—original draft preparation, H.J., B.C., H.K., B.M.K., S.T.K., B.-H.B.; writing—review and editing, H.J., B.C., H.K., S.B., B.-H.B., M.-G.L.; funding acquisition, M.-G.L., B.-H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2019005607) (to BHB). This work was supported by the Ajou University Research Fund (to BHB).
Institutional Review Board Statement
Ethical review and approval were waived for this study due to procedures involving animals not humans. All animal research was conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee at the Semyung University, Republic of Korea (smecae 20-07-01, approved on 1 July 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article text and figures.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prianishnikov V.A. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception. 1978;18:213–223. doi: 10.1016/S0010-7824(78)80015-8. [DOI] [PubMed] [Google Scholar]
- 2.Cooper M.A., Fehniger T.A., Turner S.C., Chen K.S., Ghaheri B.A., Ghayur T., Carson W.E., Caligiuri M.A. Human natural killer cells: A unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.V97.10.3146. [DOI] [PubMed] [Google Scholar]
- 3.Grégoire C., Chasson L., Luci C., Tomasello E., Geissmann F., Vivier E., Walzer T. The trafficking of natural killer cells. Immunol. Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 5.Sharma P., Wagner K., Wolchok J.D., Allison J.P. Novel cancer immunotherapy agents with survival benefit: Recent successes and next steps. Nat. Rev. Cancer. 2011;11:805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Y., Zeng D., Ou Q., Liu S., Li A., Chen Y., Lin D., Gao Q., Zhou H., Liao W., et al. Association of Survival and Immune-Related Biomarkers With Immunotherapy in Patients With Non-Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis. JAMA Netw. Open. 2019;2:e196879. doi: 10.1001/jamanetworkopen.2019.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garon E.B., Hellmann M.D., Rizvi N.A., Carcereny E., Leighl N.B., Ahn M.J., Eder J.P., Balmanoukian A.S., Aggarwal C., Horn L., et al. Five-Year Overall Survival for Patients With Advanced Non‒Small-Cell Lung Cancer Treated with Pembrolizumab: Results from the Phase I KEYNOTE-001 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019;37:2518–2527. doi: 10.1200/JCO.19.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golshani G., Zhang Y. Advances in immunotherapy for colorectal cancer: A review. Adv. Gastroenterol. 2020;13 doi: 10.1177/1756284820917527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie G., Dong H., Liang Y., Ham J.D., Rizwan R., Chen J. CAR-NK cells: A promising cellular immunotherapy for cancer. Ebio. Med. 2020;59:102975. doi: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canter R.J., Murphy W.J. A possible new pathway in natural killer cell activation also reveals the difficulty in determining human NK cell function in cancer. J. Immunother. Cancer. 2018;6:79. doi: 10.1186/s40425-018-0392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porrata L.F., Inwards D.J., Ansell S.M., Micallef I.N., Johnston P.B., Gastineau D.A., Litzow M.R., Winters J.L., Markovic S.N. Early lymphocyte recovery predicts superior survival after autologous stem cell transplantation in non-Hodgkin lymphoma: A prospective study. Biol. Blood Marrow Transpl. J. Am. Soc. Blood Marrow Transpl. 2008;14:807–816. doi: 10.1016/j.bbmt.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parkhurst M.R., Riley J.P., Dudley M.E., Rosenberg S.A. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011;17:6287–6297. doi: 10.1158/1078-0432.CCR-11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill S., June C.H. Going viral: Chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol. Rev. 2015;263:68–89. doi: 10.1111/imr.12243. [DOI] [PubMed] [Google Scholar]
- 14.Jiang T., Zhou C., Ren S. Role of IL-2 in cancer immunotherapy. Oncoimmunology. 2016;5:e1163462. doi: 10.1080/2162402X.2016.1163462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson T.O., Schluns K.S. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol. Lett. 2017;190:159–168. doi: 10.1016/j.imlet.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parrish-Novak J., Dillon S.R., Nelson A., Hammond A., Sprecher C., Gross J.A., Johnston J., Madden K., Xu W., West J., et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 17.Thompson J.A., Curti B.D., Redman B.G., Bhatia S., Weber J.S., Agarwala S.S., Sievers E.L., Hughes S.D., DeVries T.A., Hausman D.F. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008;26:2034–2039. doi: 10.1200/JCO.2007.14.5193. [DOI] [PubMed] [Google Scholar]
- 18.Böttcher J.P., Bonavita E., Chakravarty P., Blees H., Cabeza-Cabrerizo M., Sammicheli S., Rogers N.C., Sahai E., Zelenay S., Reis E.S.C. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell. 2018;172:1022–1037.e14. doi: 10.1016/j.cell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu J., Hodgins J.J., Marathe M., Nicolai C.J., Bourgeois-Daigneault M.C., Trevino T.N., Azimi C.S., Scheer A.K., Randolph H.E., Thompson T.W., et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Investig. 2018;128:4654–4668. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romagné F., André P., Spee P., Zahn S., Anfossi N., Gauthier L., Capanni M., Ruggeri L., Benson D.M., Jr., Blaser B.W., et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–2677. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlsten M., Korde N., Kotecha R., Reger R., Bor S., Kazandjian D., Landgren O., Childs R.W. Checkpoint Inhibition of KIR2D with the Monoclonal Antibody IPH2101 Induces Contraction and Hyporesponsiveness of NK Cells in Patients with Myeloma. Clin. Cancer Res. 2016;22:5211. doi: 10.1158/1078-0432.CCR-16-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McWilliams E.M., Mele J.M., Cheney C., Timmerman E.A., Fiazuddin F., Strattan E.J., Mo X., Byrd J.C., Muthusamy N., Awan F.T. Therapeutic CD94/NKG2A blockade improves natural killer cell dysfunction in chronic lymphocytic leukemia. Oncoimmunology. 2016;5:e1226720. doi: 10.1080/2162402X.2016.1226720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q., Bi J., Zheng X., Chen Y., Wang H., Wu W., Wang Z., Wu Q., Peng H., Wei H., et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018;19:723–732. doi: 10.1038/s41590-018-0132-0. [DOI] [PubMed] [Google Scholar]
- 24.Da Silva I.P., Gallois A., Jimenez-Baranda S., Khan S., Anderson A.C., Kuchroo V.K., Osman I., Bhardwaj N. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol. Res. 2014;2:410–422. doi: 10.1158/2326-6066.CIR-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu L., Huang Y., Tan L., Yu W., Chen D., Lu C., He J., Wu G., Liu X., Zhang Y. Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int. Immunopharmacol. 2015;29:635–641. doi: 10.1016/j.intimp.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Lorenzo-Herrero S., López-Soto A., Sordo-Bahamonde C., Gonzalez-Rodriguez A.P., Vitale M., Gonzalez S. NK Cell-Based Immunotherapy in Cancer Metastasis. Cancers. 2018;11:29. doi: 10.3390/cancers11010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q., Morimoto K., Nakadai A., Inagaki H., Katsumata M., Shimizu T., Hirata Y., Hirata K., Suzuki H., Miyazaki Y., et al. Forest Bathing Enhances Human Natural Killer Activity and Expression of Anti-Cancer Proteins. Int. J. Immunopathol. Pharmacol. 2007;20(Suppl. 2):3–8. doi: 10.1177/03946320070200S202. [DOI] [PubMed] [Google Scholar]
- 28.Kang H. Phytoncide secrets. Historynet. 2003;1:1–200. [Google Scholar]
- 29.Sobral M.V., Xavier A.L., Lima T.C., de Sousa D.P. Antitumor Activity of Monoterpenes Found in Essential Oils. Sci. World J. 2014;2014:953451. doi: 10.1155/2014/953451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye L., Zhang X., Xu Q., Cai Y., Gao W., Chen W. Anti-tumor activities and mechanism study of α-pinene derivative in vivo and in vitro. Cancer Chemother Pharm. 2020;85:367–377. doi: 10.1007/s00280-019-03997-x. [DOI] [PubMed] [Google Scholar]
- 31.Chen W., Liu Y., Li M., Mao J., Zhang L., Huang R., Jin X., Ye L. Anti-tumor effect of α-pinene on human hepatoma cell lines through inducing G2/M cell cycle arrest. J. Pharm. Sci. 2015;127:332–338. doi: 10.1016/j.jphs.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y., Chen R., Wang Y., Yang Y. α-Pinene Inhibits Human Prostate Cancer Growth in a Mouse Xenograft Model. Chemotherapy. 2018;63:1–7. doi: 10.1159/000479863. [DOI] [PubMed] [Google Scholar]
- 33.Hou J., Zhang Y., Zhu Y., Zhou B., Ren C., Liang S., Guo Y. α-Pinene Induces Apoptotic Cell Death via Caspase Activation in Human Ovarian Cancer Cells. Med. Sci. Monit. 2019;25:6631–6638. doi: 10.12659/MSM.916419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Q., Li M., Yang M., Yang J., Xie J., Lu X., Wang F., Chen W. α-pinene regulates miR-221 and induces G(2)/M phase cell cycle arrest in human hepatocellular carcinoma cells. Biosci. Rep. 2018;38:6. doi: 10.1042/BSR20180980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang E., Lee D.H., Jung Y.J., Shin S.Y., Koh D., Lee Y.H. α-Pinene inhibits tumor invasion through downregulation of nuclear factor (NF)-κB-regulated matrix metalloproteinase-9 gene expression in MDA-MB-231 human breast cancer cells. Appl. Biol. Chem. 2016;59:511–516. doi: 10.1007/s13765-016-0175-6. [DOI] [Google Scholar]
- 36.Li Q., Kobayashi M., Wakayama Y., Inagaki H., Katsumata M., Hirata Y., Hirata K., Shimizu T., Kawada T., Park B.J., et al. Effect of phytoncide from trees on human natural killer cell function. Int. J. Immunopathol. Pharm. 2009;22:951–959. doi: 10.1177/039463200902200410. [DOI] [PubMed] [Google Scholar]
- 37.Li Q., Nakadai A., Matsushima H., Miyazaki Y., Krensky A.M., Kawada T., Morimoto K. Phytoncides (wood essential oils) induce human natural killer cell activity. Immunopharmacol. Immunotoxicol. 2006;28:319–333. doi: 10.1080/08923970600809439. [DOI] [PubMed] [Google Scholar]
- 38.Lowin B., Peitsch M.C., Tschopp J. Perforin and granzymes: Crucial effector molecules in cytolytic T lymphocyte and natural killer cell-mediated cytotoxicity. Curr. Top. Microbiol. Immunol. 1995;198:1–24. doi: 10.1007/978-3-642-79414-8_1. [DOI] [PubMed] [Google Scholar]
- 39.Li Q., Kobayashi M., Inagaki H., Hirata Y., Li Y.J., Hirata K., Shimizu T., Suzuki H., Katsumata M., Wakayama Y., et al. A day trip to a forest park increases human natural killer activity and the expression of anti-cancer proteins in male subjects. J. Biol. Regul. Homeost. Agents. 2010;24:157–165. [PubMed] [Google Scholar]
- 40.Wu X.T., Liu J.Q., Lu X.T., Chen F.X., Zhou Z.H., Wang T., Zhu S.P., Fei S.J. The enhanced effect of lupeol on the destruction of gastric cancer cells by NK cells. Int. Immunopharmacol. 2013;16:332–340. doi: 10.1016/j.intimp.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Mo K.H., Kyeong C.J., Min A.K., Yun K.T., Hyun L.J., Hantae J., Min J.D., Churl K.M. Antitumor Effects of Phytoncides: Vitalization of Natural Killer Cells. J. Sci. Educ. Gift. 2013;5:96–104. [Google Scholar]
- 42.Tabellini G., Baronio M., Patrizi O., Benevenuto A., Gazzurelli L., Plebani A., Parolini S., Lougaris V. The RAC2-PI3K axis regulates human NK cell maturation and function. Clin. Immunol. Orlando Fla. 2019;208:108257. doi: 10.1016/j.clim.2019.108257. [DOI] [PubMed] [Google Scholar]
- 43.Yu T.-K., Caudell E.G., Smid C., Grimm E.A. IL-2 Activation of NK Cells: Involvement of MKK1/2/ERK But Not p38 Kinase Pathway. J. Immunol. 2000;164:6244. doi: 10.4049/jimmunol.164.12.6244. [DOI] [PubMed] [Google Scholar]
- 44.Thiery J., Keefe D., Boulant S., Boucrot E., Walch M., Martinvalet D., Goping I.S., Bleackley R.C., Kirchhausen T., Lieberman J. Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nat. Immunol. 2011;12:770–777. doi: 10.1038/ni.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ida H., Utz P.J., Anderson P., Eguchi K. Granzyme B and natural killer (NK) cell death. Mod. Rheumatol. 2005;15:315–322. doi: 10.3109/s10165-005-0426-6. [DOI] [PubMed] [Google Scholar]
- 46.Poli A., Michel T., Thérésine M., Andrès E., Hentges F., Zimmer J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology. 2009;126:458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alter G., Malenfant J.M., Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Dayanc B.E., Bansal S., Gure A.O., Gollnick S.O., Repasky E.A. Enhanced sensitivity of colon tumour cells to natural killer cell cytotoxicity after mild thermal stress is regulated through HSF1-mediated expression of MICA. Int. J. Hyperth. 2013;29:480–490. doi: 10.3109/02656736.2013.821526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon H.J., Choi G.E., Ryu S., Kwon S.J., Kim S.C., Booth C., Nichols K.E., Kim H.S. Stepwise phosphorylation of p65 promotes NF-κB activation and NK cell responses during target cell recognition. Nat. Commun. 2016;7:11686. doi: 10.1038/ncomms11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho K.S., Lim Y.R., Lee K., Lee J., Lee J.H., Lee I.S. Terpenes from Forests and Human Health. Toxicol. Res. 2017;33:97–106. doi: 10.5487/TR.2017.33.2.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Acker H.H., Capsomidis A., Smits E.L., Van Tendeloo V.F. CD56 in the Immune System: More Than a Marker for Cytotoxicity? Front Immunol. 2017;8:892. doi: 10.3389/fimmu.2017.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kubota A., Lian R.H., Lohwasser S., Salcedo M., Takei F. IFN-gamma production and cytotoxicity of IL-2-activated murine NK cells are differentially regulated by MHC class I molecules. J. Immunol. Baltim. Md. 1950. 1999;163:6488–6493. [PubMed] [Google Scholar]
- 53.Wang H., Zhang Y., Wu X., Wang Y., Cui H., Li X., Zhang J., Tun N., Peng Y., Yu J. Regulation of Human Natural Killer Cell IFN-γ Production by MicroRNA-146a via Targeting the NF-κB Signaling Pathway. Front. Immunol. 2018;9:293. doi: 10.3389/fimmu.2018.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez O.D., Mitchell D., Jager G.C., Nolan G.P. LFA-1 signaling through p44/42 is coupled to perforin degranulation in CD56+CD8+ natural killer cells. Blood. 2004;104:1083–1093. doi: 10.1182/blood-2003-08-2652. [DOI] [PubMed] [Google Scholar]
- 55.Wiseman J.C.D., Ma L., Marr K.J., Jones G., Mody C. Perforin-Dependent Cryptococcal Microbicidal Activity in NK Cells Requires PI3K-Dependent ERK1/2 Signaling1. J. Immunol. 2007;178:6456–6464. doi: 10.4049/jimmunol.178.10.6456. [DOI] [PubMed] [Google Scholar]
- 56.Diefenbach A., Raulet D.H. Strategies for target cell recognition by natural killer cells. Immunol. Rev. 2001;181:170–184. doi: 10.1034/j.1600-065X.2001.1810114.x. [DOI] [PubMed] [Google Scholar]
- 57.Zwirner N.W., Ziblat A. Regulation of NK Cell Activation and Effector Functions by the IL-12 Family of Cytokines: The Case of IL-27. Front. Immunol. 2017;8:25. doi: 10.3389/fimmu.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chester C., Fritsch K., Kohrt H.E. Natural Killer Cell Immunomodulation: Targeting Activating, Inhibitory, and Co-stimulatory Receptor Signaling for Cancer Immunotherapy. Front Immunol. 2015;6:601. doi: 10.3389/fimmu.2015.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang C., Zhou X.-J., Li Y.-Y., Wan J., Yang L.-Y., Li G.-H. Effect of Vasoactive Intestinal Peptide (VIP) on NKG2D Signal Pathway and Its Contribution to Immune Escape of MKN45 Cells. Sci. World J. 2013;2013:429545. doi: 10.1155/2013/429545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin B., Maudsley S., White C.M., Egan J.M. Hormones in the naso-oropharynx: Endocrine modulation of taste and smell. Trends Endocrinol. Metab. 2009;20:163–170. doi: 10.1016/j.tem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim S.P., Kang M.Y., Kim J.H., Nam S.H., Friedman M. Composition and Mechanism of Antitumor Effects of Hericium erinaceus Mushroom Extracts in Tumor-Bearing Mice. J. Agric. Food Chem. 2011;59:9861–9869. doi: 10.1021/jf201944n. [DOI] [PubMed] [Google Scholar]
- 62.Trop S., Samsonov D., Gotsman I., Alper R., Diment J., Ilan Y. Liver-associated lymphocytes expressing NK1.1 are essential for oral immune tolerance induction in a murine model. Hepatology. 1999;29:746–755. doi: 10.1002/hep.510290334. [DOI] [PubMed] [Google Scholar]
- 63.Roden M.M., Lee K.H., Panelli M.C., Marincola F.M. A novel cytolysis assay using fluorescent labeling and quantitative fluorescent scanning technology. J. Immunol. Methods. 1999;226:29–41. doi: 10.1016/S0022-1759(99)00039-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article text and figures.