Abstract
Piper betle var. nigra is a tropical plant closely related to the common piper. P. betle has also been dubbed a promising source of natural antioxidants in herbal health products, antibacterial, antifungal, antimalarial, cytotoxic activity against the cancer cell lines K562 and HL-60, and antileishmanial. The aim of this study to observation Antimicrobial activity and isolation of chemical compound. The antimicrobial activity of P. betle extract was performed by well diffusion method against two oral pathogenic bacteria (Streptococcus mutans and Streptococcus sanguinis) and opportunistic pathogenic yeast (Candida albicans). The inoculum (bacterial and yeast suspension) was prepared from a 24-h culture on NB for bacterial suspension and on TSB for yeast suspension. Extraction and isolation using various method of chromatography. Isolated compounds were characterized by spectroscopic means. Our study showed antimicrobial activity from crude ethanol extract of leaves P. betle L. var. nigra against two oral pathogenic bacteria and opportunistic pathogenic yeast with concentration 0.5% and 1%. The first report of two new amides derivatives, piperenamide A (1) and piperenamide B (2) in P. betle L. var. nigra.
Keywords: Piper betle var. nigra, Streptococcus mutans, Streptococcus sanguinis, Candida albicans, piperenamide A, piperenamide B
1. Introduction
Piper betle a piper species have a simple profile contain very diverse suites of secondary metabolites and responsible for their use in traditional medicines to treat several disease [1]. Piper betle L. var. nigra or black betle (in Indonesia known as Sirih Hitam) is a tropical plant closely related to the common piper and belongs to the Piperaceae family and the genus of piper. This genus consists of five subgenera and approximately 1400 species spread throughout tropical and subtropical regions and widely cultivated in Indonesia, India, Sri Lanka, Malaysia, Thailand, Taiwan, and other Southeast Asian countries and has a long history of over 2000 years. This plant use for decoration and medicinal plants. P. betle is one of the most potent medicinal herbs that has been used over the years. In addition to the large number of beneficial properties, P. betle has also been dubbed a promising source of natural antioxidants in herbal health products, antibacterial, antifungal, antimalarial, cytotoxic activity against the cancer cell lines K562 and HL-60, and antileishmanial [2,3,4,5,6,7,8]. Chemical composition from Piper betle L. var. nigra included caryophyllene, cadinene, γ-lactone, allyl catechol, p-cymene, eugenol methyl ether, 4-allyl resorcinol, stigmast-4-en-3,6-dione, and aristololactam A-II, and essential oils such as chavicol, chavibetol, chavabetyl acetate, eugenol, eugenyl acetate, safrole, (E) Isoeugenol, and B-caryophyllen, pipercerebrosides A and B, and amides alkaloid [8,9,10]. Amides as a class of typical constituents. More than 300 members of amide alkaloids are already found in species of the genus Piper. It seems most of them have possible bioactivity, such as antifungal, antiepileptic, antidepressant, hepatoprotective, and antiplatelet aggregation activities [11]. Isobutyl amides are one of the most frequently known classes o the amides in the plants. These amides are primarily found as long chain conjugates in piper genus. Arboreumine, pellitorine, fagaramide, brachystamides-C, D, E, retrofractamide-D, N-isobutyl-4-hexanoyl-hydroxypyrrolidin-1-one, (±)-threo-N-isobutyl-4,5-dihydroxy-2E-octaenamide, scutifoliamide A, B, hoffmannseggiamide A, B, and cyclopipperettine [12,13].
In this study we reported the isolation and elucidation of the chemical structures two novel amide derivatives compound and evaluated the antimicrobial activity effect of the crude ethanol extract of Piper betle var. nigra leaves from Indonesia. Antimicrobial assay to be carried out for the activity of ethanol extract from P. betel leaves against the activities of antimicrobial against two oral pathogenic bacteria (Streptococcus mutans and Streptococcus sanguinis) and opportunistic pathogenic yeast (Candida albicans).
2. Results and Discussion
2.1. Antimicrobial Activity
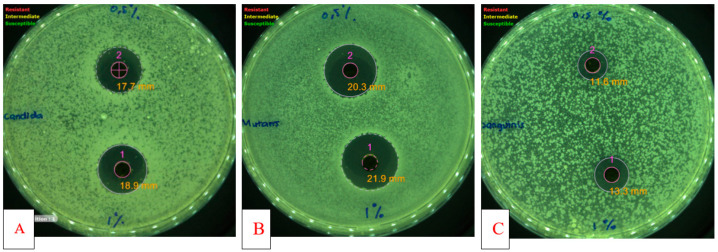
Table 1 shows the antibacterial activity of P. betle extract against two oral pathogenic bacteria and an oral opportunistic fungal. P. betle extract in all concentration from 0.5% to 1% inhibited growth of S. mutans, S. sanguinis, and C. albicans with increasing diameters of inhibition observed with increasing concentration of P. betle extract (Figure 1). S. mutans was the most sensitive strain compared to S. sanguinis and C. albicans against P. betle extract (0.5%) with inhibition diameters of 18.2 mm, 9.9mm, and 16.7 mm, respectively. The difference in sensitivity of these organisms is probably due to different cell types. Candida (yeast) cell types are different from streptococci (bacteria). This causes differences in sensitivity to P. betle extract. Sensitivity of S. mutans are more susceptible than S. sanguinis. The possible causes are many factors, including differences in virulence. In 2015, Azizi et al. assed the sensitivity of S. mutans and S. sanguinis to Zingiber officinale. They showed that S. mutans more susceptible than S. sanguinis with MIC value of 0.02 and 0.3 mg/mL, respectively [14]. In addition, their sensitivity to chlorhexidine were also reported by Medina-Flores et al. (2016), where S. mutans more susceptible than S. sanguinis with growth inhibition 23.97 mm and 19.80 mm, respectively. Alkaloid, terpenoid, flavonoid, polyphenols, tannin, and saponin compounds have been identified from P. betle leaf extract [15]. Flavonoids, polyphenols, and tannins are known to have antibacterial activity with at least five possible mechanisms: damage the cell membrane permeability, inhibit protein synthesis, damage the bacterial cell wall, inhibit ATP synthesis, and interfere with cell [16]. The antibacterial activity that we obtained is thought to be derived from the activity of the isolate compounds of Piperamide A and B was isolated from Piper betle var. nigra. This is as has been reported from other plants of the Piper genus because of the similarity in structure to the compounds we obtained although further testing is needed to ensure the strength of the antibacterial activity of these compounds [17,18,19]. Table 2 shows none of the tested samples of Piperamide A and B showed antimicrobial activity against S. mutans, S. sanguinis, and C. albicans up to 0.02%. These result from amides derivative showed the same results in tests for cyclopipperetine on the Piper nigrum [13]. In recent years, Candida species has demonstrated resistance to many synthetic medications, indicating the need for new antifungal drugs with less side effects to treat candidiasis effectively. Several experiments used natural substances against multiresistant strains and in particular against azole resistant candidiasis have indicated that certain species of plants have promising antimicrobial compounds from natural substances [20,21,22].
Table 1.
Growth inhibition diameters of Candida albicans, Streptococcus mutans, and Streptococcus sanguinis by Piper betle var. nigra extract.
| Microbial | Inhibition Diameters ± SD (mm) | |
|---|---|---|
| Extract Concentration of 0.5% | Extract Concentration of 1% | |
| Candida albicans | 16.7 ± 0.8 | 18.1 ± 0.7 |
| Streptococcus mutans | 18.2 ± 1.8 | 19.6 ± 1.9 |
| Streptococcus sanguinis | 9.9 ± 1.8 | 12.3 ± 1.1 |
Figure 1.
Antimicrobial activity of Piper betle var. nigra extract. (A) Antifungal activity against Candida albicans; (B) Antibacterial activity against Streptococcus mutans; (C) Antibacterial activity against Streptococcus sanguinis. 1 (concentration of 1%); 2 (concentration of 0.5%).
Table 2.
Growth inhibition diameters of Candida albicans, Streptococcus mutans, and Streptococcus sanguinis by Piper betle var. nigra isolate piperamide A and B.
| Microbial | Inhibition Diameters ± SD (mm) | |
|---|---|---|
| Piperamide A | Piperamide B | |
| Concentration of 0.02% | Concentration of 0.02% | |
| Candida albicans | - | - |
| Streptococcus mutans | - | - |
| Streptococcus sanguinis | - | - |
2.2. Isolation of Crude Ethanol Extracf of Piper betle var. nigra
The EtOH extract from the leaf of P. betle L. var. nigra was chromatographed over a vacuum-liquid chromatographed (VLC) column, packed with silica gel 60 by gradient elution. The VLC fractions were repeatedly subjected to normal and reverse phase column chromatography, as well as preparative TLC on silica gel GF254 to accommodate compounds 1–2.
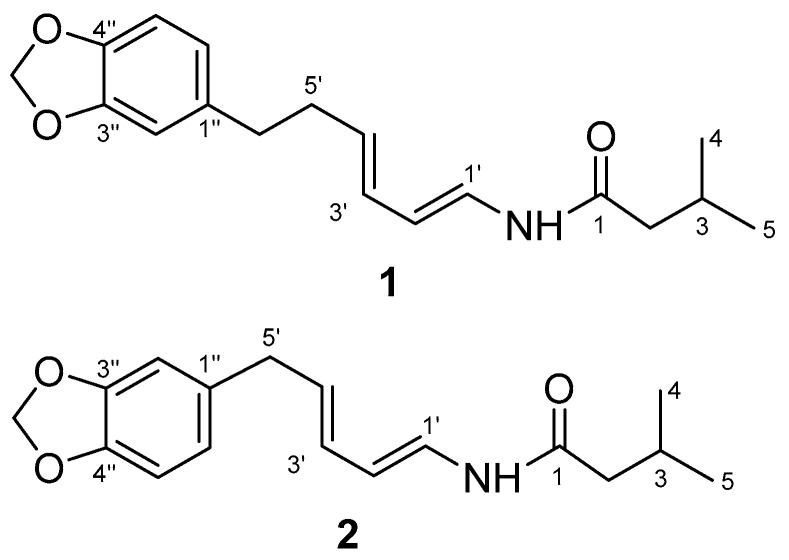
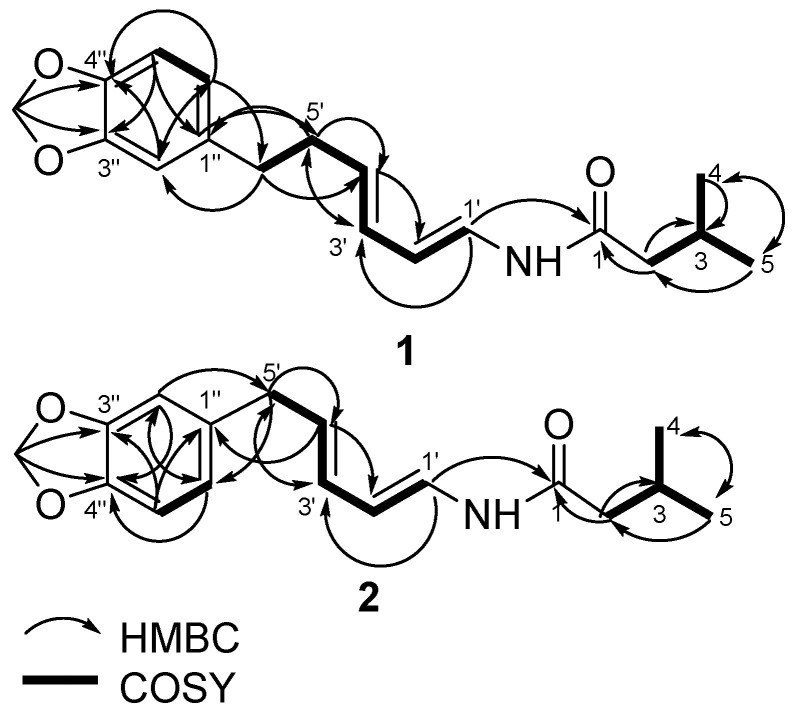
Piperenamide A (1) was observed as a colorless amorphous solid, with its molecular composition established as C18H23NO3, based on HR-TOFMS. This showed a [M + H]+ ion peak at m/z 302.1749 (calcd. C18H24NO3 m/z 302.1756), requiring to eight degrees of unsaturation (Figure 2). The UV spectrum showed maximum absorption at 270 and 241 nm, indicating the presence of a benzene and conjugated alkene. The IR spectrum showed bands which were ascribed to an amine (νmax 3295 cm−1), amide carbonyl (νmax 1656 cm−1), isolated double bond conjugated (νmax 1611 and 1504 cm−1), and ether groups (νmax 1241 cm−1). Furthermore, the 1H-NMR spectrum (Table 3) showed two primary methyls at δH 0.90 (6H, d, 6.7 Hz, Me-4 and 5), four sp2 methine protons at δH 5.92 (1H, dd, J = 4.6; 15.1 Hz, H-1′), 7.02 (1H, dd, J = 10.6; 15.1 Hz, H-2′), 6.09 (1H, dd, 10.6; 15.2 Hz, H-3′), and 6.18 (1H, dd, 7.2; 15.2 Hz, H-4′), suggested the presence of an α,β,γ,δ-unsaturated secondary amide, two trans double bonds (J1′,2′ = 15.1 Hz, J3′,4′ = 15.2 Hz), one cis double bonds (J2′,3′ = 10.6 Hz), tri-substituted benzene at δH 6.63 (1H, dd, J = 1.5; 7.9 Hz, m; o, H-6″), 6.69 (1H, d, 1.5 Hz, m, H-2″), and 6.69 (1H, d, 7.9 Hz, m, H-5″), a methylene dioxyphenyl δH 5.88 (2H, s) and some aliphatic signals in the upfield region (Supplementary Materials). Comparison of the NMR data of 1 with those of guineensine [23] shows the same amide chain but with different N-substituent positions. The N-substituent of 1 position is attached to the olefin proton resonances, this is indicated δH 3.06 (2H, d, 6.7 Hz, H-2) no correlation to δH 4.62 (NH s), δH 5.92 (1H, dd, J = 4.6; 15.1 Hz, H-1′) and guineensine position is attached to the iso-butyl, this is indicated δH 3.16 (2H, t, 6.4; 12.9 Hz, H-1′) correlation to δH 5.60 (NH brs), δH 5.76 (1H, d, 15.0 Hz, H-2). The 13C NMR together with the DEPT spectra revealed eighteen carbons consisting of an amide carbonyl at δC 169.1, α,β,γ,δ-unsaturated secondary amide at δC 123.3, 141.9, 142.8 and 130.2, tri-substituted benzene at δC 136.5, 109.7, 149.0, 147.9, 147.9, 108.8, and 122.3, carbon methylene dioxyphenyl at δC 102.0, two methyls at δc 20.1 (Me-4) and 20.1 (Me-5). The 1H-1H COSY spectrum of compound 1 showed correlations in H2-H3-H4 and H5, H1′-H2′-H3′-H4′-H5′-H6′ and H5″-H6″, supporting the presence of a secondary amide [10]. The HMBC correlations from H-2 to C-1, C-3, C-4 and C-5, H-1′ to C-1 and C-3′, H-2′ to C-4, H-3′ to C-1 and C-5, H-4′ to C-2′ and C-6′, H-5′ to C-3′ and C-1″, H-6′ to C-4′, C-2″ and C-6″, H-2″ to C-4′, C-4″ and C-6″, H-5″ to C-1″ and C-3″, H-6″ to C-6′, C-2″ and C4″, and OCH2O to C-3″ and 4″, which was verified by correlations observed in the 1H-1H COSY and HMBC spectra (Figure 3) Therefore, the structure of compound 1 was elucidated as the new amide and namely as piperenamide A (Figure 2).
Figure 2.
Structures of piperenamide A-B (1–2).
Table 3.
NMR data compound 1–2 (500 MHz for 1H dan 125 MHz for 13C).
| Position Carbon | 1 | 2 | ||
|---|---|---|---|---|
| 13C-NMR | 1H-NMR | 13C-NMR | 1H-NMR | |
| δC (mult.) | δC [(ΣH, mult, J(Hz)] | δC (ppm) | δC [(ΣH, mult, J(Hz)] | |
| 1 | 169.1 (s) | - | 168.4 (s) | - |
| 2 | 48.0 (t) | 3.06 (2H, d, 6.7) | 47.3 (t) | 3.10 (2H, d, 6.7) |
| 3 | 29.7 (d) | 1.78 (1H, qt, 6.7) | 28.9 (d) | 1.82 (1H, qt, 6.7) |
| 4 | 20.1 (q) | 0.90 (3H, d, 6.7) | 20.1 (q) | 0.94 (3H, d, 6.7) |
| 5 | 20.1 (q) | 0.90 (3H, d, 6.7) | 20.1 (q) | 0.94 (3H, d, 6.7) |
| 1′ | 123.3 (d) | 5.92 (1H, dd, 4.6; 15.1) | 122.6(d) | 5.96 (1H, dd, 4.6; 15.1) |
| 2′ | 141.9 (d) | 7.02 (1H, dd, 10.6; 15.1) | 141.2 (d) | 7.12 (1H, dd, 10.6; 15.1) |
| 3′ | 142.8 (d) | 6.09 (1H, dd, 10.6; 15.2) | 142.0 (d) | 6.12 (1H, dd, 10.6; 15.2) |
| 4′ | 130.2 (d) | 6.18 (1H, dd, 7.2; 15.2) | 129.6 (d) | 6.19 (1H, dd, 7.2; 15.2) |
| 5′ | 36.1 (t) | 2.44 (2H, dd, 7.2; 7.5) | 35.4 (t) | 2.39 (2H, d, 7.2) |
| 6′ | 35.9 (t) | 2.66 (2H, t, 7.5) | - | - |
| 1″ | 136.5 (s) | - | 135.8 (s) | - |
| 2″ | 109.7 (d) | 6.69 (1H, d, 1.5) | 109.8 (d) | 6.73 (1H, d, 1.5) |
| 3″ | 149.0 (s) | - | 148.3 (s) | - |
| 4″ | 147.9 (s) | - | 146.4 (s) | - |
| 5″ | 108.8 (t) | 6.69 (1H, d, 7.9) | 108.2 (t) | 6.73 (1H, d, 7.9) |
| 6″ | 122.3 (d) | 6.63 (1H, dd, 1.5, 7.9) | 121.6 (d) | 6.68 (1H, dd, 1.5, 7.9) |
| OCH2O | 102.0 (t) | 5.88 (2H, s) | 101.2 (t) | 5.91 (2H, s) |
| NH | - | 4.62 (1H, br.s) | - | 4.65 (1H, br.s) |
Figure 3.
Selected HMBC and COSY correlations for piperenamide A-B (1–2).
Piperenamide B (2) was observed as a colorless amorphous solid, with its molecular composition established as C17H21NO3, based on HR-TOFMS. This showed a [M + H]+ ion peak at m/z 288.1567 (calcd. C17H22NO3 m/z 288.1521), requiring to eight degrees of unsaturation (Figure 2). The UV spectrum showed maximum absorption at 270 and 241 nm, indicating the presence of a benzene and conjugated alkene. The IR spectrum showed bands which were ascribed to an amine (νmax 3235 cm−1), amide carbonyl (νmax 1690 cm−1), isolated double bond conjugated (νmax 1610 and 1504 cm−1), and ether groups (νmax 1241 cm−1). The NMR spectra of two was very similar to those of one (Table 3), except with lost of methylene at δH 2.66 (2H, t, 7.5 Hz, H-6′); δC 35.9, C-6′) and methylene at δH 2.44 (2H, dd, 7.2; 7.5 Hz, H-5′), while two, methylene at δH 2.39 (2H, d, 7.2 Hz, H-5′), indicating that 2 was a demethylene derivative of one. In the HMBC spectrum, methylene correlations from H-5′ to C-1″, C-2″ and C-6″, suggest methylene δH 2.39; δC 35.4, directly apply to benzene (Supplementary Materials). Therefore, leading to the structure of 2 had been elucidated as the new amide, namely as piperenamide B (Figure 2).
3. Materials and Methods
3.1. General Experimental Procedures
UV spectra was measured using a TECAN Infinite M200 pro, with MeOH. The IR spectra and mass spectra were recorded on a SHIMADZU IR Prestige-21 in KBr and Waters Xevo QTOF MS, respectively. Using an NMR JEOL ECZ-500 and Variant Unity INOVA-500 Spectrometer (Agilent Technologies, Santa Carla, CA, USA), the NMR data was recorded at 500 MHz for 1H and 125 MHz for 13C, using TMS as internal standard. Column chromatography was conducted on the silica gel 60 (70–230 and 230–400 mesh, Merck, Kenilworth, NJ, USA), after which TLC analysis was carried out on 60 GF254 (Merck, 0.25 mm) using various solvent systems, in order to detect spots by irradiating under ultraviolet-visible light (257 and 364 nm) and heating of silica gel plates, sprayed with H2SO4 in EtOH (10%).
3.2. Plant Material
The leaf of P. betle L. var. nigra were collected from Samarinda, East Kalimantan, Indonesia in June 2020. Futhermore, the plant was identified By Dr. Atik Retnowati a staff of the Bogoriense Herbarium, Bogor, Indonesia. Finally, a voucher specimen (No. 749/IPH.1.01/If.07/VII/2020) was deposited at the Herbarium.
3.3. Extraction and Isolation
Method for extraction and isolation was referred to supriatno et al. (2018) [24]. The dried ground leaf (473.21 g) of P. betle L. var nigra was extracted with ethanol 70% (14 L), at room temperature for 7 days. After removal of the solvent under vacuum, the viscous concentrated EtOH extract (14.61 g) was obtained. The EtOH extract (14.61 g) was fractionated by column chromatography on silica gel, using a gradient of n-hexane, EtOAc and MeOH (10% stepwise) resulting into eight fractions (A–H). Fraction D (2.32 g) was subjected to column chromatography on silica gel using n-hexane-CHCl3-EtOAc (5% stepwise), as eluting solvents to afford seven subfractions (D1–D7). Subfraction D3 (882.2 mg) was chromatographed on a column of silica gel, eluted with n-hexane: EtOAc (7:3), to give seven subfractions (D3A–D3G). Similarly, subfraction D3D (120.3 mg) was chromatographed on silica gel eluted with n-hexane: CHCl3: EtOAc (7:2:1), to give 1 (8.2 mg). Subfraction D3E (90.1 mg) was chromatographed on silica gel eluted with petroleum ether: CHCl3 (4.5:5.5), to give 2 (2.8 mg) [24].
3.3.1. Piperenamide A (1)
Colorless amorphous solid; mp 185−190 °C; UV (MeOH) λmax (log ε) 270 (4.01) and 241 (3.91) nm; IR (KBr) vmax 3295, 1656, 1611, 1504, 1490, 1443, 1141 and 1030 cm−1; HR-TOFMS m/z 302.1751 [M + H]+, (calcd. C18H23NO3 m/z 301,1749); 1H-NMR (CDCl3, 500 MHz), see Table 1; 13C-NMR (CDCl3, 125 MHz), see Table 1.
3.3.2. Piperenamide B (2)
Colorless amorphous solid; mp 199−211 °C; UV (MeOH) λmax (log ε) 268 (3.91), and 219 (3.67) nm; IR (KBr) vmax 3235, 1690, 1610, 1504, 1480, 1443, 1141 and 1030 cm−1; HR-TOFMS m/z 288.1567 (calcd. C17H22NO3 m/z 288.1521) 1H-NMR (CDCl3, 500 MHz), see Table 1; 13C-NMR (CDCl3, 125 MHz), see Table 1.
3.4. Antimicrobial Activity
3.4.1. Material
Liquid media for this study were nutrient broth (NB; Oxoid, Hampshire, UK) and tryptic soy broth (TSB; Merck). Solid media were Mueller Hinton Agar (MHA; Oxoid) and Tryptic soy agar (TSA; Merck)
3.4.2. Method
The antimicrobial activity of P. betle extract was performed by well diffusion method against two oral pathogenic bacteria (Streptococcus mutans, Streptococcus sanguinis) and opportunistic pathogenic yeast (Candida albicans). The inoculum (bacterial and yeast suspension) was prepared from a 24-h culture on NB for bacterial suspension and on TSB for yeast suspension. Each of inoculum was diluted with sterile physiological solution (0.9%) to 108 CFU/mL (McFarland standard 0.5). 20 mL of each agar media were melted (NA media for bacterial and TSA media for yeast), cooled to 50 °C and then inoculated with 0.2 mL of the microbial suspension. The inoculated agar was poured into sterile petri dish, and then allowed to cool down on a leveled surface. Once the media had compacted, two wells were cut out of the agar, each 6 mm in diameter. Then, 30 µL of the extract sample (concentration of 0.5 and 1%) were added into each well and incubated for 24 h at 36 °C ± 1°C under aerobic condition. Inhibition of microbial growth was measured in mm using SCAN 500® tools. Tests were performed in duplicate.
4. Conclusions
The presents study showed antimicrobial activity from crude ethanol extract of leaves P. betle L. var. nigra. The first report of two new amides derivatives, piperenamide A (1) and piperenamide B (2) in P. betle L. var. nigra.
Acknowledgments
Our thanks go to the Managing Director of the Educational Fund Management Agency of the Ministry of Finance of the Republic of Indonesia who has provided funding for Productive Innovative Research (RISPRO) according to the funding agreement number: PRJ-52/LPDP/2019 dated 22 August 2019.
Supplementary Materials
The following are available online. Figure S1: 1H-NMR Spectra of (1), Figure S2: 13C-NMR Spectra of (1), Figure S3: DEPT-135° Spectrum of (1), Figure S4: HMQC Spectrum of (1), Figure S5: HMBC Spectrum of (1), Figure S6: 1H-1H-COSY Spectra of (1), Figure S7: TOF MS Spectra of (1), Figure S8: 1H-NMR Spectra of (2), Figure S9: 13C-NMR Spectrum of (2), Figure S10: DEPT-135° Spectrum of (2), Figure S11: HMQC Spectrum of (2), Figure S12: HMBC Spectrum of (2), Figure S13: 1H-1H-COSY Spectra of (2), Figure S14: TOF MS Spectra of (2)
Author Contributions
Conceptualization, H.K. F.P. and L.R.; data curation and formal analysis, S.S., A.R., L.F., K.H., H.A., M.A., V.O.S., N.I., A.I., R.R.; software, A.C.N., H.H., and I.A.; validation, H.K., F.P., and L.R.; formal analysis, S.S.; investigation, H.K.; resources, F.P.; data curation, F.P., R.R.; writing—original draft preparation, H.K.; writing—review and editing, H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the RISPRO-LPDP Research Grant from Ministry of Finance, Republic of Indonesia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Salehi B., Zakaria Z.A., Gyawali R., Ibrahim S.A., Rajkovic J., Shinwari Z.K., Khan T., Sharifi-Rad J., Ozleyen A., Turkdonmez E., et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules. 2019;24:1364. doi: 10.3390/molecules24071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budiman A., Aulifa D.L. A Study Comparing Antibacterial Activity of Ageratum Conyzoides, L. Extract and Piper Betle, L. Extract in Gel Dosage Forms Against Staphylococcus Aureus. Pharmacogn. J. 2020;12:473–477. doi: 10.5530/pj.2020.12.73. [DOI] [Google Scholar]
- 3.Choudhary D., Kale R.K. Antioxidant and Non-Toxic Properties Of Piper Betle Leaf Extract:In Vitro Andin Vivo Studies. Phytother. Res. 2002;16:461–466. doi: 10.1002/ptr.1015. [DOI] [PubMed] [Google Scholar]
- 4.Sarkar A., Sen R., Saha P., Ganguly S., Mandal G., Chatterjee M. An Ethanolic Extract of Leaves of Piper Betle (Paan) Linn Mediates Its Antileishmanial Activity via Apoptosis. Parasitol. Res. 2008;102:1249–1255. doi: 10.1007/s00436-008-0902-y. [DOI] [PubMed] [Google Scholar]
- 5.Al-Adhroey A.H., Nor Z.M., Al-Mekhlafi H.M., Amran A.A., Mahmud R. Antimalarial Activity of Methanolic Leaf Extract of Piper Betle L. Molecules. 2010;16:107–118. doi: 10.3390/molecules16010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali I., Khan F.G., Suri K.A., Gupta B.D., Satti N.K., Dutt P., Afrin F., Qazi G.N., Khan I.A. In Vitro Antifungal Activity of Hydroxychavicol Isolated from Piper Betle L. Ann. Clin. Microbiol. Antimicrob. 2010;9:7. doi: 10.1186/1476-0711-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rijai H.R., Fakhrudin N., Wahyuono S. Isolation and Identification of DPPH Radical (2,2-Diphenyl-1-Pikrylhidrazyl) Scavenging Active Compound in Ethyl Acetat Fraction of Piper Acre Blume. Maj. Obat Tradis. 2019;24:204. doi: 10.22146/mot.48173. [DOI] [Google Scholar]
- 8.Chen D.-Z., Xiong H.-B., Tian K., Guo J.-M., Huang X.-Z., Jiang Z.-Y. Two New Sphingolipids from the Leaves of Piper betle L. Molecules. 2013;18:11241–11249. doi: 10.3390/molecules180911241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh K., Bhattacharya T. Chemical Constituents of Piper Betle Linn. (Piperaceae) Roots. Molecules. 2005;10:798–802. doi: 10.3390/10070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang G.-H., Chen D.-M., Qiu B.-Y., Sheng L., Wang Y.-H., Hu G.-W., Zhao F.-W., Ma L.-J., Wang H., Huang Q.-Q., et al. Cytotoxic Amide Alkaloids from Piper Boehmeriaefolium. J. Nat. Prod. 2011;74:45–49. doi: 10.1021/np100606u. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y.-N., Liu F.-F., Jacob M., Li X.-C., Zhu H.-T., Wang D., Cheng R.-R., Yang C.-R., Xu M., Zhang Y.-J. Antifungal Amide Alkaloids from the Aerial Parts of Piper Flaviflorum and Piper Sarmentosum. Planta Med. 2016;83:143–150. doi: 10.1055/s-0042-109778. [DOI] [PubMed] [Google Scholar]
- 12.Kumar V. Studies in Natural Products Chemistry. Elsevier; Amsterdam, The Netherlands: 2018. Amides from Plants: Structures and Biological Importance; pp. 287–333. [Google Scholar]
- 13.Ren J., Zeng T., Ali Z., Wang M., Bae J., Chittiboyina A.G., Wang W., Li S., Khan I.A. Cyclopiperettine, A New Amide from Piper Nigrum. Nat. Prod. Commun. 2017;12:1934578X1701201210. doi: 10.1177/1934578X1701201210. [DOI] [Google Scholar]
- 14.Azizi A., Aghayan S., Zaker S., Shakeri M., Entezari N., Lawaf S. In Vitro Effect of Zingiber Officinale Extract on Growth of Streptococcus Mutans and Streptococcus Sanguinis. Int. J. Dent. 2015;2015:1–5. doi: 10.1155/2015/489842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junairiah J., Ni’matuzahroh N., Zuraidassanaaz N.I., Sulistyorini L. Isolation and identification of secondary metabolites of black betel (Piper betle L. var Nigra) J. Kim. Ris. 2019;3:131. doi: 10.20473/jkr.v3i2.12064. [DOI] [Google Scholar]
- 16.Tamhid H.A. Chemical Compounds and Antibacterial Activity of Garcinia Dulcis (Roxb)Kurz. J. Kedokt. Dan Kesehat. Indones. 2019;10:71–85. doi: 10.20885/JKKI.Vol10.Iss1.art11. [DOI] [Google Scholar]
- 17.Chanprapai P., Chavasiri W. Antimicrobial Activity from Piper sarmentosum Roxb. against Rice Pathogenic Bacteria and Fungi. J. Integr. Agric. 2017;16:2513–2524. doi: 10.1016/S2095-3119(17)61693-9. [DOI] [Google Scholar]
- 18.Mgbeahuruike E.E., Yrjönen T., Vuorela H., Holm Y. Bioactive Compounds from Medicinal Plants: Focus on Piper Species. South Afr. J. Bot. 2017;112:54–69. doi: 10.1016/j.sajb.2017.05.007. [DOI] [Google Scholar]
- 19.Nascimento S.A., Araújo E.A., Da Silva J.M., Ramos C.S. Chemical study and antimicrobial activities of piper arboreum (piperaceae) J. Chil. Chem. Soc. 2015;60:2837–2839. doi: 10.4067/S0717-97072015000100013. [DOI] [Google Scholar]
- 20.Sivareddy B., Reginald B., Sireesha D., Samatha M., Reddy K., Subrahamanyam G. Antifungal Activity of Solvent Extracts of Piper Betle and Ocimum Sanctum Linn on Candida Albicans: An in Vitro Comparative Study. J. Oral Maxillofac. Pathol. 2019;23:333. doi: 10.4103/jomfp.JOMFP_167_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taweechaisupapong S., Singhara S., Khunkitti W. Antimicrobial effects of boesenbergia pandurata and piper sarmentosum leaf extracts on planktonic cells and biofilm of oral pathogens. Pak. J. Pharm. Sci. 2010;9:224–231. [PubMed] [Google Scholar]
- 22.Donadu M.G., Usai D., Marchetti M., Usai M., Mazzarello V., Molicotti P., Montesu M.A., Delogu G., Zanetti S. Antifungal Activity of Oils Macerates of North Sardinia Plants against Candida Species Isolated from Clinical Patients with Candidiasis. Nat. Prod. Res. 2020;34:3280–3284. doi: 10.1080/14786419.2018.1557175. [DOI] [PubMed] [Google Scholar]
- 23.Park S.-D., Oh J.-H., Lim D. A New Type of Amide Formation from Thiocarboxylic Acid and Alkyl Azide. Tetrahedron Lett. 2002;43:6309–6311. doi: 10.1016/S0040-4039(02)01397-7. [DOI] [Google Scholar]
- 24.Supriatno, Nurlelasari, Herlina T., Harneti D., Maharani R., Hidayat A.T., Mayanti T., Supratman U., Azmi M.N., Shiono Y. A new limonoid from stem bark of Chisocheton pentandrus (Meliaceae) Nat. Prod. Res. 2018;32:2610–2616. doi: 10.1080/14786419.2018.1428600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.