Abstract
Co-exposure of nanomaterials and chemicals can cause mixture toxicity effects to living organisms. Predictive models might help to reduce the intensive laboratory experiments required for determining the toxicity of the mixtures. Previously, concentration addition (CA), independent action (IA), and quantitative structure–activity relationship (QSAR)-based models were successfully applied to mixtures of organic chemicals. However, there were few studies concerning predictive models for toxicity of nano-mixtures before June 2020. Previous reviews provided comprehensive knowledge of computational models and mechanisms for chemical mixture toxicity. There is a gap in the reviewing of datasets and predictive models, which might cause obstacles in the toxicity assessment of nano-mixtures by using in silico approach. In this review, we collected 183 studies of nano-mixture toxicity and curated data to investigate the current data and model availability and gap and to derive research challenges to facilitate further experimental studies for data gap filling and the development of predictive models.
Keywords: nano-mixture, toxicity, data curation, predictive models
1. Introduction
The toxicity of single nanomaterials has been intensively tested in recent decades. Combinations of single nanomaterials and chemicals form nano-mixtures, which might cause co-exposure and mixture effects in living organisms. Because nanomaterials present different physicochemical properties in comparison with the properties of bulk chemicals, the mixture effects of nano-mixtures might deviate from the mixture effects of chemical mixtures (e.g., organic mixtures, metal mixtures, etc.). The in silico approach provides predictive models for reducing the experimental costs of in vitro/in vivo toxicity testing [1]. Experimental studies on the toxic effects of mixtures of different compounds have continuously increased, and conventional models, such as concentration addition (CA) and independent action (IA) models, have been frequently used to estimate the toxicity of mixtures based on the additive toxicity of single compounds [2,3,4]. Additionally, some quantitative structure–activity relationship (QSAR)-based models have been applied to mainly predict the toxicity of organic mixtures based on the structures of single compounds [5,6,7,8,9]. However, there have been few studies concerning the predictability of nano-mixture toxicity, and most of these studies employed the CA and IA models to test the applicability of the two conventional models [10,11,12,13,14,15,16,17,18]. The number of studies on QSARs for predicting nano-mixture toxicity is considerably lower, and thus far, there have been only three of them [10,11,12].
For chemical mixtures, previous reviews provided comprehensive knowledge on (1) computational models for chemical mixture toxicity [19,20] and (2) underlying mechanisms of mixture effects [21]. In order to apply in silico methods to nano-mixture toxicity, datasets, models, and toxicity mechanisms would be required. Current reviews for nano-mixture toxicity [22] focused only on a toxicity mechanism known as the Trojan horse phenomenon. There is a gap in the datasets and predictive models, which might cause obstacles in the toxicity assessment of nano-mixtures by using the in silico approach. To provide readers with a picture of the currently available datasets and predictive models of nano-mixture toxicity, in this review, we collected and curated 183 studies [10,11,13,14,15,16,17,18,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197] of nano-mixture toxicity to investigate the current data and model availability and gap, and we derived research challenges to facilitate further experimental studies for data gap filling and the development of predictive models.
2. Literature Collection
Literature was searched from Web of Science (webofknowledge.com) and Google Scholar (scholar.google.com) using the keywords “mixture”, “toxicity”, and “nanomaterials”. Each publication was carefully checked and analyzed to appropriately gather the toxicity test datasets of mixtures of nanomaterials and other substances. Selected publications for the purpose of this review were studies assessing the co-exposure effects of nano-mixtures on in vitro models and environmental species such as crustaceans, fish, bacteria, plants, algae, and mollusks. The last search was conducted in June 2020. A total of 183 publications from 2005 to 2020 were sorted after the searching and checking process. Information on (1) nano-mixture composition, (2) toxicity tests, (3) toxicity endpoints, and (4) mixture toxicity models was extracted from the literature. Nano-mixture composition included the nanomaterials, mixed chemicals, and type of nano-mixture. Toxicity tests included the name and group of tested species and toxicity test guidelines. Toxicity endpoints are the names of the endpoints measured in the toxicity tests. Mixture toxicity models are the available models used to estimate or predict the toxicity of nano-mixtures.
3. Current Available Predictive Models for Nano-Mixture Toxicity
Conventional models for the joint toxicity of organic compounds include CA [2] and IA [4] models. Both models describe the additive joint toxicity of chemicals based on the similarity or dissimilarity of their mode of action (MoA). The models require the toxicity of single substances in a mixture to estimate the toxicity of the mixture as follows:
| (1) |
where ECxmix is the predicted toxic effect of the mixture, pi is the fraction of component i in the mixture, and ECxi is the individual effect concentration when applied singly.
| (2) |
where Y denotes the biological response, Ci is the concentration of chemical i in the mixture, qi(Ci) is the probability of non-response, umax is the control response for endpoints, and ∏ is the multiplication function. Several studies applied CA and IA models to predict the toxicity of nano-mixtures and showed good predictivity (correlation coefficient value of up to 0.91) and explain the synergism and antagonism effects of the mixtures [13,14,15,16,17,18]. For example, Lopes and colleagues [13] utilized CA and IA models for mixtures of ZnO/Ag nanoparticles (NPs) and their metal ion counterparts and showed that both Zn NP and Ag NP mixture showed a deviation from additivity and they showed synergism when the concentration of Ag ions increased and antagonism when the concentration of AgNPs increased in the suspension. The study by Wang and colleagues [15] demonstrated that toxicity predictions of CA/IA models were close to the observed joint toxicities of binary mixtures of graphene and ionic liquids. Although CA/IA models are able to provide hints about the synergistic/antagonistic effects of mixtures, experiments to determine the toxicity of all single components are needed in order to apply CA/IA models. Such a process requires high cost and time, and such experiments might not always be feasible.
QSAR models might be a helpful approach to compensate for the disadvantages of CA and IA models. QSAR models are mathematical relationships between endpoints (e.g., mortality, mitochondrial activity) and descriptors (e.g., concentration of mixtures, physicochemical properties of NPs). The QSAR model inputs do not require the toxicity of all single components in mixtures. Although there were 183 studies that investigated the toxicity of nano-mixtures, only three studies [10,11,12] developed QSAR models for the photocatalytic activity and toxicity of TiO2 NP-based nano-mixtures (Table 1). These studies were conducted to develop models for predicting the photocatalytic activity and cytotoxicity of nano-mixtures consisting of TiO2 NPs and (poly) metallic clusters (Au, Ag, Pd, and Pt). The nano-mixtures were heterogeneous photocatalytic NPs that could be used for ultraviolet and visible (UV–vis) light-induced processes to remove harmful pollutants from gas and aqueous phases. The two endpoints in these studies were the photocatalytic activity under UV–vis light of TiO2 nano-mixtures and the viability of Chinese hamster ovary (CHO-K1) cells exposed to TiO2 nano-mixtures. Descriptors of nano-mixtures (Dmix) were calculated from quantum chemical descriptors of metallic clusters by using the additive equation:
| (3) |
where Dmix is the additive descriptor of nano-mixtures, and and Dn are the mole fraction and quantum chemical descriptors of metal cluster n in the mixtures. The additive equation was based on a simple additive approach for joint toxicity, where the properties of heterogeneous NPs are the result of the additive contribution of each component. Two algorithms were used to develop QSAR models: multiple linear regression (MLR) and decision tree (DT). These QSAR models showed good predictive power and successfully explained the observed photocatalytic activity and cytotoxicity of heterogeneous TiO2 NPs (over 90%). However, the data for model development were limited to 29 data rows of different metal cluster fractions so the applicability domain of these models was limited to only nano-mixtures of TiO2 NPs and four metal clusters (i.e., Ag, Au, Pt, and Pd). Furthermore, nano-toxicology regulation and assessment could adopt additional QSAR models of the toxicity of other NP-based nano-mixtures.
Table 1.
List of previous QSAR models for nano-mixtures.
| No. | Reference | Nano-Mixture | Test System | Descriptors | Endpoint | Algorithm | Equations | No. Data | R2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Mikolajczyk et al., 2016 [12] | TiO2 NPs + Au/Pd | None | 27 structural descriptors | Photocatalytic activity | Multiple linear regression | 17 | 0.80–0.89 | |
| 2 | Mikolajczyk et al., 2018 [11] | TiO2 NPs + Au/Ag/Pt | Chinese hamster ovary cell line | 5 quantum descriptors | Effective concentration (EC50) | Multiple linear regression | 26 | 0.83–0.94 | |
| 3 | Mikolajczyk et al., 2019 [10] | TiO2 NPs + Au/Ag/Pt/Pd | Chinese hamster ovary cell line | 9 quantum descriptors | Effective concentration (EC50) | Multiple linear regression | 29 | 0.80–0.87 | |
| Decision tree | None | 29 | 0.74–0.90 | ||||||
| Photocatalytic activity | Decision tree | None | 29 | 0.80–0.82 |
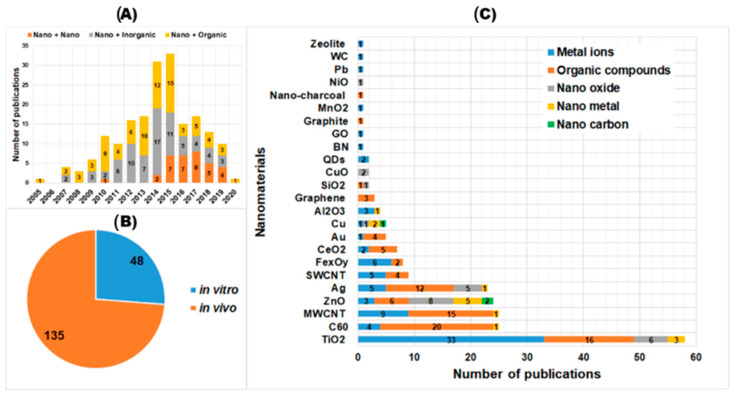
It is feasible to develop QSAR models which have a larger applicability domain for nano-mixture toxicity if larger datasets containing a variety of mixed chemicals are acquired. Our curated literature data contain studies of the in vivo/in vitro toxicity of NP–NP, NP–inorganic chemical, and NP–organic chemical mixtures (Figure 1A,B). TiO2, fullerenes (C60), multiwall carbon nanotubes (MWCNT), ZnO, and Ag are the most popular NPs in the tested nano-mixtures (Figure 1C). The curated data have been successfully exploited to develop QSAR models for single nanomaterial toxicity by using regression and classification algorithms [198,199,200,201,202]. QSAR model development for nano-mixture toxicity might apply similar data curation (e.g., data collection, data gap filling, normalization, etc.) and modeling methods (algorithms, validation) as in the case of single nanomaterials. However, descriptors for nano-mixtures (Dmix) are very important and they are different from the descriptors of single nanomaterials. Because nano-mixtures consist of more than two components, their descriptors should contain information about all components (i.e., concentration, properties). Previous research suggested the calculation of Dmix from the properties of all mixture components by using several methods [5,7,8,10,11,12]. The most used method is based on the assumption that components in a mixture act jointly by simple addition (Equation (3)). In addition to the additive Dmix, other equations such as mean square and mean cubic [5,7,8,10,11,12] were previously applied to calculate Dmix of organic mixtures, and models based on these mixture descriptors showed good prediction performance (R2 = 0.71–0.94). These equations and models have not been applied to nano-mixtures yet. Although there are several available methods for calculating Dmix, they might not cover all nano-mixture toxicity data because the assumption of joint properties (e.g., additive) might not be suitable for all nano-mixtures. New methods for calculating Dmix might be proposed during the development of QSAR models for nano-mixture toxicity. Because NPs and mixed chemicals are often different, in that one component is nano and the other(s) is bulk material, it is difficult to measure their common properties for Dmix calculation. Instead of using experimental properties, theoretical properties are commonly used for nano-mixtures [10,11,12,203,204]. Simulation-based methods for theoretical properties can be classified as quantum mechanics (QM) or molecular dynamics (MD). Mikolajczyk and colleagues [10,11,12] applied QM simulation and density functional theory (DFT) calculation to small metal clusters (0.5 × 0.5 × 0.5 nm3) to obtain QM descriptors for Dmix of heterogeneous TiO2 NPs. The Dmix obtained from this QM approach (electronegativity) worked well to produce QSAR models that explained over 90% the observed photocatalytic activity and cytotoxicity of heterogeneous TiO2 NPs. Although the QM calculations could provide information on electronic energy (e.g., ionization, electronegativity, highest occupied/lowest unoccupied molecular orbital, etc.), calculations of large nano-sized systems are difficult and time-consuming, so this approach is limited by the size of NP clusters. The MD approach was utilized in the work of Burk and colleagues [203,204]. This approach simulated large Fe-Doped ZnO NPs (8–40 nm diameter) and Dmix descriptors were based on a force-field calculation of the potential energies of whole NPs and the core and shell layers. The Dmix descriptors obtained from this approach showed potential for linear regression (i.e., predicting cell viability, membrane damage, and mitochondrial reactive oxygen species (ROS) of HeLa and KLN205 cells with R2 = 0.740–0.877) and classification models (i.e., clustering toxicity data of Fe-Doped ZnO NPs by using principal component analysis with good accordance with the algal growth inhibition data) [204]. The MD method has a disadvantage in that it provides only topological and potential energy descriptors, which are not related to the electronic properties of nano-mixtures. In addition to the QM and MD approaches, the molecular descriptor approach might be applicable to nano-mixture toxicity data. The molecular descriptor method was previously used to obtain Dmix for antibiotics and pesticide mixtures [204]. The QSAR models for antibiotics and pesticide mixture toxicity demonstrated high predictive performance (R2 = 0.9366). Their limitation is similar to the QM approach, namely that calculations for large-sized nano-mixtures are difficult and time-consuming. For QSAR model development of the current curated nano-mixture toxicity data, we suggest considering all three methods for finding the Dmix with the best performance models and the most suitable toxicity mechanisms.
Figure 1.
Number of studies on toxicity of nano-mixtures classified by year and type of nano-mixture (A), by type of toxicity test (B), and by nanomaterials and mixture components (C).
There are several algorithms for QSAR models suggested by the Organization for Economic Co-operation and Development (OECD) [205]: linear and nonlinear algorithms. For conventional organic mixtures, linear algorithms such as MLR are often used for developing models [5,6,7,8,9] because of their transparency and mechanistic interpretation. For nano-mixtures, only MLR and DT algorithms have been used for developing models [10,11,12]. Nonlinear algorithms such as random forest (RF) and artificial neural network (ANN) might be useful for developing models due to their high accuracy and robustness. However, transparency and mechanistic interpretation of these algorithms are lower than MLR and DT algorithms because they do not provide equations or single trees of prediction for other users who could use direct predictions (e.g., equations, etc.). The choice of algorithm is based on the model’s performance, transparency, and mechanistic interpretation. Therefore, it is time-consuming to choose the most suitable algorithms for QSARs of nano-mixture toxicity.
As a part of in silico research, nano-mixture toxicity QSAR models would provide low-cost toxicity screening of nano-mixtures, which would help to reduce animal testing and chemical waste [206]. Additionally, the models would provide warnings about potential nano-mixture toxicity when we design new nanomaterials (e.g., photocatalytic nanomaterials [10]) for safe-by-design approaches and minimal harmful effects. Another application of the models is checking the biocompatibility of new NP-based drug delivery. The NP-based drug might undergo many co-exposures to other organic chemicals and present mixture toxicity (e.g., AuNPs in presence of Polysorbate 20 synergistic toxicity at concentrations where the individual components were benign [52]). Finally, the models might help to control the processes of the release and emission of NPs into the environment, where flows of NP release might interact with dissolved organic matter and provoke mixture toxicity to the environment.
4. Current Available Data of Nano-Mixture Toxicity
Based on the types of mixed chemicals, nano-mixture toxicity data from 183 publications were divided into three groups: (1) NPs and NPs; (2) NPs and inorganic chemicals; and (3) NPs and organic chemicals (Figure 1A). The toxicity data were categorized into two groups: in vitro and in vivo (Figure 1B). The in vitro toxicity group included studies using bacteria, cells from animals, and humans as test systems (48 studies). The in vivo toxicity group included studies using animals as test systems (135 studies) (Figure 1B). The five most popular NPs in the tested nano-mixtures (i.e., TiO2, C60, MWCNT, ZnO, and Ag) occupied around 70% of the curated data (Figure 1C). Their data would be helpful for the meta-analysis of the toxicity of each NP-based nano-mixture.
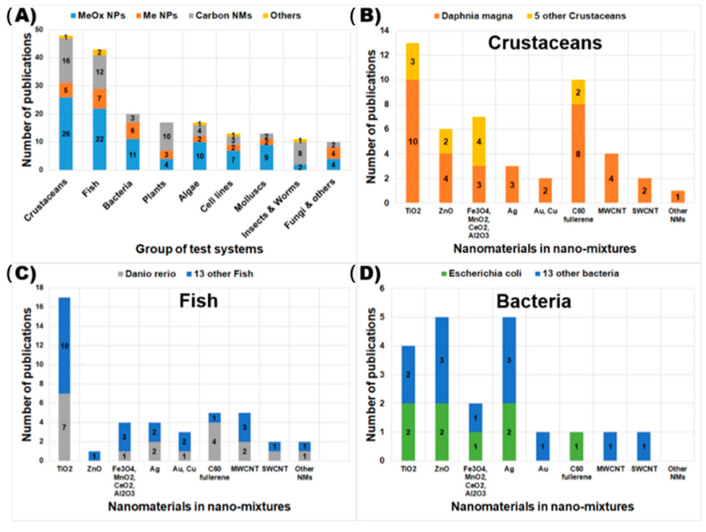
We categorized the tested species into nine groups: crustaceans, fish, bacteria, plants, algae, cultured cell lines, mollusks, insects and worms, and fungi and others (Figure 2A). Among these nine groups, crustaceans, fish, and bacteria were the three most popular tested species, and 111/183 studies used these species to test the toxicity of nano-mixtures (Figure 2A). Metal oxide NPs were most frequently tested on these test systems, and 95/183 studies investigated the toxicity of metal oxide-based nano-mixtures (Figure 2A). Carbonaceous nanomaterial-based nano-mixtures were the second largest group, where 60/183 studies investigated their toxicity (Figure 2A). We further categorized 111 studies of crustaceans, fish, and bacteria into smaller groups of nanomaterials and species (Figure 2B–D). In the crustacean group, Daphnia magna (D. magna) was the most tested species, with 38/48 studies (Figure 2B). In the fish group, Danio rerio (D. rerio) was the most popular species, tested in 17/43 studies (Figure 2C). In the bacterial group, Escherichia coli (E. coli) was the most tested species, with 8/20 studies. TiO2 based nano-mixtures were most commonly tested for toxicity to crustaceans (13/48 studies) and fish (17/43 studies) (Figure 2B,C). C60-based nano-mixtures were the second largest nano-mixture group that was tested for toxicity to crustaceans (10/48 studies) and fish (5/43 studies) (Figure 2B,C). In the bacterial group, Ag NPs and ZnO NP-based nano-mixtures were the largest groups (10/20 studies).
Figure 2.
Number of studies on toxicity of nano-mixtures classified by group of test systems (A). Number of studies on toxicity of nano-mixtures classified by nanomaterials in nano-mixtures for crustaceans (B), fish (C), and bacteria (D). (SWCNT: single-walled carbon nanotube, MWCNT: multiple-walled carbon nanotube, NMs: nanomaterials).
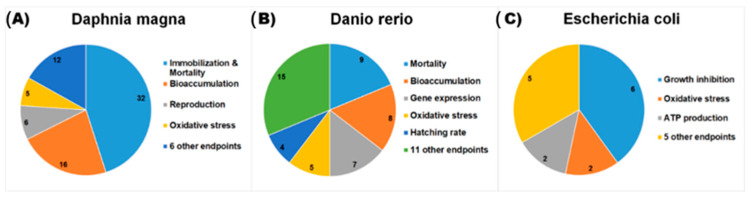
Among the 183 studies, there were 88 different species and 48 different toxicity endpoints for all tested species. The endpoints of the three most popular species (D. magna, D. rerio, and E. coli) are shown in Figure 3. For D. magna, mortality and immobilization were the most abundant endpoints (45%), followed by bioaccumulation (23%), reproduction (8%), and oxidative stress (7%). Other toxicity endpoints (17%) for D. magna were uptake, metal ATPase activity, metallothionein inhibition, retention of dietary, cell damage, and hatching rate. For D. rerio, mortality was the most measured endpoint (19%), followed by bioaccumulation (17%), gene expression (15%), oxidative stress (10%), and hatching rate (8%). Other endpoints (31%) for D. rerio were cell viability, locomotion activity, malformation, abnormality rate, glutathione level, heartbeat, mitochondrial activity, thyroid hormone content, and uptake. For E. coli, growth inhibition was the most measured endpoint (40%). Other endpoints (60%) were oxidative stress, ATP production, photoproduction, indigo degradation, cell wall damage, cell viability, and bioaccumulation.
Figure 3.
Number of studies on toxicity of nano-mixtures classified by endpoints for D. magna (A), D. rerio (B), and E. coli (C).
TiO2 NPs are often mixed with metal ions such as Cd2+, Cu2+, and As5+ and organic compounds such as pesticides and antibiotics for toxicity testing. C60 and MWCNTs are often mixed with organic compounds such as pesticides and antibiotics for toxicity testing. ZnO NPs are often mixed with nanometals/oxides and organic compounds for toxicity testing (Figure 1C).
In the 183 collected studies, nano-mixture properties were described by the properties of NPs and mixture components including core diameter, length (for carbon nanotubes), hydrodynamic diameter, surface charge, surface area, chemical composition, and crystallinity. If the mixture components were nanomaterials, then the core diameter was usually described. For inorganic/organic compounds, chemical names or formulas were provided.
As mentioned in the previous sections, the major test systems are D. magna, D. rerio, and E. coli. We summarize the data of these three species in Table 2, Table 3 and Table 4. Among the various nanomaterials tested for these species, TiO2 NPs, ZnO NPs, and C60 fullerenes were the most tested nanomaterials (Figure 2). According to OECD guidelines [205], to develop reliable QSAR models, defined endpoints should be considered. As shown in Figure 3, there are 34 endpoints for D. magna, D. rerio, and E. coli.
Table 2.
Summary of current nano-mixture toxicity data for D. magna (CA: Concentration Addition, IA: Independent Action).
| No. | Article | Nanomaterials | Mixed Substance | Mixed Substance Type | Toxicity Test Guideline | Toxicity Endpoint | Mixture Toxicity Models |
|---|---|---|---|---|---|---|---|
| 1 | Azevedo et al., 2017 Science of the Total Environment [14] | ZnO NPs | Ag NPs | Nanometal | OECD 202; OECD 211 | Immobilization, Reproduction | CA and IA |
| 2 | Baun et al., 2008 Aquatic Toxicology [98] |
C60 fullerenes | Atrazine, Methylparathione, Pentachlorophenol, Phenanthrene | Organic compounds | OECD 211, ISO 6341 | Reproduction, Immobilization, Bioaccumulation | None |
| 3 | Brausch et al., 2010 Environmental Toxicology and Chemistry [97] | C60 fullerenes | Bifenthrin, Tribufos | Organic compounds | EPA-821-R-02-012 | Mortality, Reproduction, Growth inhibition | None |
| 4 | Cano et al., 2018 Environmental Science and Technology [178] | MWCNT | Cu2+ | Metal ions | None | Metallothionein inhibition, Bioaccumulation | None |
| 5 | Fan et al., 2011 Environmental Pollution [107] |
TiO2 NPs | Cu2+ | Metal ions | None | Metallothionein inhibition, Mortality, Bioaccumulation | None |
| 6 | Fan et al., 2012 Journal of Nanomaterials [106] |
TiO2 NPs | Cu2+ | Metal ions | None | Oxidative stress, Metal-ATPase activity, Mortality | None |
| 7 | Fang et al., 2011 Environmental Toxicology and Chemistry [153] | Nano-charcoal | Tributyltin, Dibutyltin | Organometallic | ISO 6341 | Reproduction, Immobilization, Bioaccumulation | None |
| 8 | Gao et al., 2018 Ecotoxicology and Environmental Safety [181] | MnO2 NPs, Nano-hydroxyapatite | Cd2+ | Metal ions | OECD 202 | Immobilization, Oxidative stress | None |
| 9 | Han et al., 2012 Chinese Journal of Geochemistry [158] |
CeO2 NPs | Atrazine | Organic compounds | US. EPA 2001 | Reproduction, Mortality, Bioaccumulation | None |
| 10 | Hartmann et al., 2012 Aquatic Toxicology [114] |
TiO2 NPs | Cu2+ | Metal ions | OECD 202, ISO 6341 | Bioaccumulation | None |
| 11 | Kim et al., 2009 Environmental Science & Technology [136] | MWCNT | Cu2+ | Metal ions | None | Bioaccumulation, Mortality, Oxidative stress | None |
| 12 | Kim et al., 2010 Environmental Toxicology and Chemistry [73] | Cu NPs | SWCNT | Nanocarbon | None | Mortality, Bioaccumulation | None |
| 13 | Lopes et al., 2016 J. Hazardous Materials [13] |
Ag NPs, ZnO NPs | Ag NPs, ZnO NPs, Ag+, Zn2+ | Nano-oxide, Metal ions | OECD 202 | Immobilization | CA and IA |
| 14 | Martín-de-Lucía et al., 2019 Science of The Total Environment [16] | Graphite-diamond | Fungicide thiabendazole | Organic compounds | OECD 202 | Immobilization | CA and IA |
| 15 | Molins-Delgado et al., 2016 Environmental Research [184] | Ag NPs | Benzophenone, Ethyl-PABA, 4-methylbenzylidene camphor, Ethylhexyl-methoxy cinamate | Organic compounds | ISO 6341 | Immobilization | None |
| 16 | Park et al., 2019 Journal of Nanoparticle Research [117] |
TiO2 NPs, ZnO NPs | Ag+ | Metal ions | OECD 202 | Immobilization | None |
| 17 | Park et al., 2019 Molecular & Cellular Toxicology [81] |
Fe3O4 NPs | Zn2+ | Metal ions | OECD 202 | Immobilization | CA and IA |
| 18 | Rosenfeldt et al., 2014 Environmental Science & Technology [112] | TiO2 NPs | Cu2+, Ag+, As5+ | Metal ions | OECD 202 | Bioaccumulation, Immobilization | None |
| 19 | Rosenfeldt et al., 2015 Environmental Science & Technology [113] | TiO2 NPs | Cu2+ | Metal ions | OECD 202 | Bioaccumulation, Immobilization | None |
| 20 | Sanchis et al., 2016 Environmental Science & Technology [59] | C60 fullerenes | Nonylphenol, Triclosan, Malathion, Diuron, Glyphosate | Organic compounds | OECD 202, ISO 6341 | Immobilization | None |
| 21 | Seitz et al., 2012 Environmental Toxicology and Chemistry [110] | TiO2 NPs | As5+ | Metal ions | OECD 202 | Immobilization | None |
| 22 | Tan and Wang, 2014 Environmental Pollution [109] |
TiO2 NPs | Cd2+, Zn2+ | Metal ions | None | Oxidative stress, Uptake, Retention of dietary, Mortality | None |
| 23 | Tan et al., 2012 Environmental Science & Technology [174] | TiO2 NPs | Cd2+, Zn2+ | Metal ions | None | Uptake, Bioaccumulation, Retention of dietary | None |
| 24 | Tao et al., 2013 Chemosphere [193] | C60 fullerenes | Cu2+ | Metal ions | US EPA 2024 | Metal ATPase activity, Mortality, Bioaccumulation | None |
| 25 | Tian et al., 2014 Advanced Materials Research [108] |
TiO2 NPs | Penta-brominated diphenyl ether | Organic compounds | OECD 202 | Immobilization, Mortality | None |
| 26 | Vega et al., 2019 Ecotoxicology and Environmental Safety [65] | TiO2 NPs | Organic UV filter oxybenzone, Benzylparaben | Organic compounds | ISO 6341 | Immobilization | None |
| 27 | Wang et al., 2014 Environmental Toxicology and Chemistry [148] | MWCNT | Ni2+ | Metal ions | None | Immobilization, Bioaccumulation | None |
| 28 | Wang et al., 2016 Environmental Pollution [34] |
MWCNT, SWCNT | Cd2+ | Metal ions | OECD 202 | Mortality, Bioaccumulation | None |
| 29 | Ye et al., 2018 Nanotoxicology [18] | ZnO NPs | GO NPs | Nanocarbon | OECD 201, 202, 236 | Growth inhibition, Immobilization, Mortality, Oxidative stress | None |
| 30 | Yan et al., 2010 Chinese Science Bulletin [177] |
C60 fullerenes | Atrazine | Organic compounds | None | Reproduction, Deformity rate, Hatching rate | None |
| 31 | Yang et al., 2010 Aquatic Toxicology [189] |
C60 fullerenes | Fluoranthene | Organic compounds | EPA 6004-90027 | Immobilization, Cell damage | None |
| 32 | Ye et al., 2018 Nanotoxicology [18] | ZnO NPs | GO NPs | Nanocarbon | OECD 201, 202, 236 | Growth inhibition, Mortality, Immobilization | None |
| 33 | Yu & Wang, 2013 Water Research [197] | MWCNT, SWCNT | Cd2+, Zn2+ | Metal ions | None | Uptake, Bioaccumulation | None |
| 34 | Yu & Wang, 2014 Environmental Toxicology and Chemistry [26] | C60 fullerenes | Cd2+, Zn2+ | Metal ions | None | Bioaccumulation | None |
| 35 | Zhang et al., 2015 Journal of Environmental Sciences [31] | Ag NPs | Ag+ | Metal ions | OECD 202 | Mortality | None |
Table 3.
Summary of current nano-mixture toxicity data for D. rerio.
| No. | Article | Nanomaterials | Mixed Substance | Mixed Substance Type | Toxicity Test Guideline | Toxicity Endpoint | Mixture Toxicity Models |
|---|---|---|---|---|---|---|---|
| 1 | Azevedo Costa et al., 2012 Comparative Biochemistry and Physiology, Part C [176] | C60 fullerenes | As3+ | Metal ions | None | Cell viability, Mitochondrial dehydrogenase functionality, Oxidative stress, Bioaccumulation | None |
| 2 | Fang et al., 2015 Journal of Hazardous Materials [160] | TiO2 NPs | Pentachlorophenol | Organic compounds | None | Bioaccumulation, Oxidative stress, Gene expression, Glutathione level | None |
| 3 | Ferreira et al., 2014 Aquatic Toxicology [131] | C60 fullerenes | Benzo[a]pyrene | Organic compounds | None | Cell viability, Oxidative stress, Bioaccumulation, Glutathione level | None |
| 4 | Ginzburg et al., 2018 ACS Nano [52] | Au NPs | Polysorbate 20 | Organic compounds | None | Mortality | None |
| 5 | Henry et al., 2007 Environmental Health Perspectives [56] | C60 fullerenes | Tetrahydrofuran | Organic compounds | None | Mortality, Gene expression | None |
| 6 | Henry et al., 2013 Environmental Science & Technology [36] | C60 fullerenes | Hg+ | Metal ions | Plymouth University | Mortality, Bioaccumulation, Gene expression | None |
| 7 | Hu et al., 2011 Environmental Pollution [38] | TiO2 NPs | Cd2+ | Metal ions | None | Bioaccumulation | None |
| 8 | Hua et al., 2016 NanoImpact [74] | TiO2 NPs | ZnO NPs | Nano-oxide | OECD 157 | Mortality | RA and CA |
| 9 | Krysanov & Demidova, 2012 Doklady Biological Sciences [146] | CeO2 NPs | Doxorubicin | Organic compounds | None | Malformations, Hatching rate | None |
| 10 | Miao, 2015 Aquatic Toxicology [119] | TiO2 NPs | Pb2+ | Metal ions | None | Gene expression, Locomotion activity, Thyroid hormone content, Bioaccumulation | None |
| 11 | Park et al., 2011 Nanotoxicolog y [190] | C60 fullerenes | 17α-Ethinyl-estradiol | Organic compounds | None | Bioaccumulation, Gene expression | None |
| 12 | Park et al., 2015 Molecular & Cellular Toxicology [60] | Ag NPs | Ag nanotube | Nanometal | None | Gene expression | None |
| 13 | Pavagadhi et al., 2014 Water Research [77] | Ag NPs, TiO2 NPs | Ni2+, Mg2+, Zn2+, Cu2+, Cd2+, Fe2+, Cr3+, Hg2+, As5+, Al3+, Pb2+, Mn2+ | Metal ions | None | Mortality, Heartbeat, Hatching rate, Uptake | None |
| 14 | Wang et al., 2014 Nanotoxicology [84] | TiO2 NPs | Decabromdiphenyl ether | Organic compounds | None | Bioaccumulation, Locomotion activity, Oxidative stress, Gene expression | None |
| 15 | Ye et al., 2018 Nanotoxicology [18] | ZnO NPs | GO NPs | Nanocarbon | OECD 236 | Mortality, Oxidative stress | None |
| 16 | Yan et al., 2014 Nanoscale Research Letters [103] | TiO2 NPs | Bis-Phenol A | Organic compounds | OECD 212 | Hatching rate, Immobilization, Heart sac edema, Abnormality rate | None |
| 17 | Yan et al., 2018 Environmental Science and Pollution Research [166] | MWCNT | 17 β -estradiol | Organic compounds | None | Mortality, Hatching rate, Abnormality rate | None |
| 18 | Zhang et al., 2012 Environmental Toxicology and Chemistry [140] | QDs (CdSe) | Cu2+ | Metal ions | None | Mortality, Malformations, Hatching rate | None |
Table 4.
Summary of current nano-mixture toxicity data for E. coli.
| No. | Article | Nanomaterials | Mixed Substance | Mixed Substance Type | Toxicity Test Guideline | Toxicity Endpoint | Mixture Toxicity Models |
|---|---|---|---|---|---|---|---|
| 1 | Cuahtecontzi-Delint et al., 2013 International Journal of Chemical Reactor Engineering [138] | CeO2 NPs | Surfactants (Tween 80, Triton X114, and Polyvinyl Pyrrolidone) | Organic compounds | None | Growth inhibition | None |
| 2 | Li et al., 2005 Nanotechnology [134] | Ag NPs | Antibiotics | Organic compounds | None | Growth inhibition | None |
| 3 | Santaella et al., 2014 Environmental Science & Technology [55] | TiO2 NPs | Cd2+ | Metal ions | None | Cell viability, Oxidative stress | None |
| 4 | Shahverdi et al., 2007 Nanomedicine Nanotechnology, Biology and Medicine [129] | Ag NPs | Antibiotics | Organic compounds | None | Growth inhibition | None |
| 5 | Silveira et al., 2015 Journal of Nanoparticle Research [147] | Ag NPs | Hexadecylpyridinium salicylate | Organic compounds | None | Growth inhibition | None |
| 6 | Srivastava et al., 2016 Journal of Environmental Sciences [133] | ZnO NPs | TiO2 NPs | Nano-oxide | None | Cell wall damage, Growth inhibition | None |
| 7 | Tong et al., 2015 Environmental Science & Technology [120] | ZnO NPs | TiO2 NPs | Nano-oxide | None | Photoproduction, ATP production, Oxidative stress, Bioaccumulation | None |
| 8 | Wilke et al., 2016 Environmental Science and Technology [57] | TiO2 NPs | Ag NPs | Nanometal | None | ATP production | None |
| 9 | Zhang et al., 2009 Environmental Science & Technology [37] | C60 fullerenes | Tetrahydrofuran (THF) | Organic compounds | None | Growth inhibition, Indigo degradation | None |
For D. magna, immobilization and mortality endpoints are defined based on two test guidelines, OECD 202 and ISO 6341, where the number of immobilized or dead D. magna is recorded and converted to a percentage over control samples. Because of their popularity in toxicity endpoints of D. magna (32 studies, 45% data), immobilization and mortality datasets might provide a potential dataset with a large applicability domain for QSAR model development. In order to develop nano-mixture QSAR models to predict immobilization/mortality, one might start with a dataset containing only one NP, such as TiO2, ZnO, etc., to test the performance and mechanistic interpretation of models and then extend the nano-mixture dataset to other NPs and widen the applicability domain of the models. The bioaccumulation endpoint is the gradual accumulation of substances such as NPs, metal ions, or other compounds in an organism (e.g., D. magna, D. rerio, etc.). There are sixteen studies investigating the bioaccumulation of D. magna (Figure 3A) of six nanomaterials (TiO2, CeO2, Cu, MWCNT, C60, and SWCNTs), six inorganic compounds (Cu(NO3)2, CuCl2, CdCl2, ZnCl2, AgNO3, and Na2HAsO4), and five organic compounds (atrazine, methylparathione, pentachlorophenol, phenanthrene, and tributyltin). Previous studies suggested a mechanism by which higher bioaccumulation induced by the absorption of metal ions/dissolved organic matter and a low level of agglomeration might cause higher immobilization/mortality of D. magna [16,59,81,117,153,184]. For example, Martin-de-Lucia and colleagues [16] found that at low concentrations, the binary mixtures of graphite–diamond nanoparticles and fungicide thiabendazole expressed synergistic toxic interactions, which could be attributed to the increased bioavailability of fungicide thiabendazole adsorbed on graphite–diamond nanoparticles. At higher concentrations, because of agglomeration, the bioaccumulation decreased and so did the toxicity. Reproduction is another toxicity endpoint for D. magna, which follows the OECD 211 test guideline [207]. In the test guideline, the number of offspring produced by each parent animal is counted for the test and control samples. The reproduction dataset of D. magna might be a candidate for the development of QSAR models. Oxidative stress is the endpoint of measuring the production of reactive oxygen species (ROS). The ROS induction of nano-mixtures might be caused by the photocatalytic activity of Ag [14] or ion exchange and electrostatic adsorption to form surface complexes [181]. Data for each reactive oxygen species should be curated to obtain sufficient data to develop QSAR models.
For D. rerio, mortality is the most abundant endpoint in the current data collection (9 studies, 19% data). Endpoint measurement is based on OECD 236 test guidelines [208]; therefore, it is a well-defined endpoint for QSAR model development. There are only eight studies of D. rerio bioaccumulation (Figure 3B) for TiO2, C60, and eight organic/inorganic compounds. Bioaccumulation of nano-mixtures might be proportional to their toxicity to D. rerio [52,74,190] because of mixed chemicals (e.g., Polysorbate 20, etc.) assembled on the nanoparticle surfaces [52]. The hatching rate of D. rerio is an endpoint based on the OEDC 212 test guideline [209]; thus, it might be a well-defined endpoint for QSAR model development. Gene expression is a complicated endpoint due to various types of genes. Gene expression data are useful for establishing adverse outcome pathways (AOP) [210]. However, with seven studies investigating gene expression (Figure 3B) for D. rerio, well-defined toxicity endpoints relating to gene expression are important in QSAR model development and possible additional data need to be collected/produced in the future.
For E. coli, the current collected data only relate to fifteen studies including eight endpoints such as growth inhibition, ATP production, oxidative stress, and cell wall damage. The main data contain TiO2, ZnO, and Ag NP-based nano-mixtures. The data might be helpful for understanding the antibacterial effects of nano-mixtures and datasets for each endpoint could be exploited in QSAR model development.
5. Conclusions
In this review, we collected the current 183 studies (2005–2020) for nano-mixture toxicity and described current data and predictive models for nano-mixture toxicity. We found that, in the current data on nano-mixture toxicity, the D. magna, D. rerio, and E. coli datasets are the three datasets containing the most studies (38, 17, and 8, respectively). We suggest additional curation of these data for the development of QSAR models with respect to toxicity endpoints. In particular, based on a total of thirty-four toxicity endpoints of these three species, three specific datasets with well-defined endpoints would be potentially useful for QSAR model development: immobilization and mortality of D. magna; mortality of D. rerio, and growth inhibition of E. coli. Data for other endpoints might need further curation and additional experimental data to develop QSAR models. We also suggest potential descriptors for QSAR model development: mixture descriptors (Dmix) could be calculated from descriptors of single components in mixtures obtained by quantum mechanics, molecular dynamics, and molecular descriptor approaches. The available formula of Dmix based on the assumption of joint properties in mixtures might not always be suitable for nano-mixtures so a new formula for Dmix might be required. A variety of linear and nonlinear algorithms, such as MLR, DT, RF, and ANN, might be used for developing QSAR models. The choice of algorithm depends on the nature of the datasets, predictive performance, and possible mechanistic interpretation. Future studies that apply these datasets for QSAR models of nanomaterial toxicity will be conducted to contribute to the risk assessment of nano-mixtures.
Acknowledgments
J.K. acknowledges the support from the European Union’s Horizon 2020 research and innovation programme (SABYDOMA Project under Grant Agreement No. 862296).
Supplementary Materials
The table containing the list of 183 curated literature is available at: https://www.mdpi.com/2079-4991/11/1/124/s1.
Author Contributions
Conceptualization, J.K., T.X.T.; data collection, T.X.T.; writing—original draft preparation, T.X.T.; writing—review and editing, T.X.T. and J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Korea Research Institute of Chemical Technology (KRICT) through the Development of Chemical Safety Platform Technologies (Project No. KK2052-10) and the Korea Environment Industry & Technology Institute (KEITI) through “the Technology Program for Establishing Biocide Safety Management”, funded by Korea Ministry of Environment (MOE) (Grant No. 2018002490006; 1485016918).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sun T.Y., Gottschalk F., Hungerbühler K., Nowack B. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ. Pollut. 2014;185:69–76. doi: 10.1016/j.envpol.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Loewe S., Muischnek H. Über Kombinationswirkungen I. Mitteilung: Hilfsmittel der Fragestellung. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1926;114:313–326. doi: 10.1007/BF01952257. [DOI] [Google Scholar]
- 3.Kim J., Kim S., Schaumann G.E. Reliable predictive computational toxicology methods for mixture toxicity: Toward the development of innovative integrated models for environmental risk assessment. Rev. Environ. Sci. Biotechnol. 2013;12:235–256. doi: 10.1007/s11157-012-9286-7. [DOI] [Google Scholar]
- 4.Bliss C.I. The toxicity of poisons applied jointly 1. Ann. Appl. Biol. 1939;26:585–615. doi: 10.1111/j.1744-7348.1939.tb06990.x. [DOI] [Google Scholar]
- 5.Gaudin T., Rotureau P., Fayet G. Mixture Descriptors toward the Development of Quantitative Structure-Property Relationship Models for the Flash Points of Organic Mixtures. Ind. Eng. Chem. Res. 2015;54:6596–6604. doi: 10.1021/acs.iecr.5b01457. [DOI] [Google Scholar]
- 6.Kar S., Ghosh S., Leszczynski J. Single or mixture halogenated chemicals? Risk assessment and developmental toxicity prediction on zebrafish embryos based on weighted descriptors approach. Chemosphere. 2018;210:588–596. doi: 10.1016/j.chemosphere.2018.07.051. [DOI] [PubMed] [Google Scholar]
- 7.Qin L.T., Chen Y.H., Zhang X., Mo L.Y., Zeng H.H., Liang Y.P. QSAR prediction of additive and non-additive mixture toxicities of antibiotics and pesticide. Chemosphere. 2018;198:122–129. doi: 10.1016/j.chemosphere.2018.01.142. [DOI] [PubMed] [Google Scholar]
- 8.Sobati M.A., Abooali D., Maghbooli B., Najafi H. A new structure-based model for estimation of true critical volume of multi-component mixtures. Chemom. Intell. Lab. Syst. 2016;155:109–119. doi: 10.1016/j.chemolab.2016.04.007. [DOI] [Google Scholar]
- 9.Wang T., Tang L., Luan F., Cordeiro M.N.D.S. Prediction of the toxicity of binary mixtures by qsar approach using the hypothetical descriptors. Int. J. Mol. Sci. 2018;19:3423. doi: 10.3390/ijms19113423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikolajczyk A., Sizochenko N., Mulkiewicz E., Malankowska A., Rasulev B., Puzyn T. A chemoinformatics approach for the characterization of hybrid nanomaterials: Safer and efficient design perspective. Nanoscale. 2019;11:11808–11818. doi: 10.1039/C9NR01162E. [DOI] [PubMed] [Google Scholar]
- 11.Mikolajczyk A., Gajewicz A., Mulkiewicz E., Rasulev B., Marchelek M., Diak M., Hirano S., Zaleska-Medynska A., Puzyn T. Nano-QSAR modeling for ecosafe design of heterogeneous TiO2-based nano-photocatalysts. Environ. Sci. Nano. 2018;5:1150–1160. doi: 10.1039/C8EN00085A. [DOI] [Google Scholar]
- 12.Mikolajczyk A., Malankowska A., Nowaczyk G., Gajewicz A., Hirano S., Jurga S., Zaleska-Medynska A., Puzyn T. Combined experimental and computational approach to developing efficient photocatalysts based on Au/Pd-TiO2 nanoparticles. Environ. Sci. Nano. 2016;3:1425–1435. doi: 10.1039/C6EN00232C. [DOI] [Google Scholar]
- 13.Lopes S., Pinheiro C., Soares A.M.V.M., Loureiro S. Joint toxicity prediction of nanoparticles and ionic counterparts: Simulating toxicity under a fate scenario. J. Hazard. Mater. 2016;320:1–9. doi: 10.1016/j.jhazmat.2016.07.068. [DOI] [PubMed] [Google Scholar]
- 14.Azevedo S.L., Holz T., Rodrigues J., Monteiro T., Costa F.M., Soares A.M.V.M., Loureiro S. A mixture toxicity approach to predict the toxicity of Ag decorated ZnO nanomaterials. Sci. Total Environ. 2017;579:337–344. doi: 10.1016/j.scitotenv.2016.11.095. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z., Zhang F., Wang S., Peijnenburg W.J.G.M. Assessment and prediction of joint algal toxicity of binary mixtures of graphene and ionic liquids. Chemosphere. 2017;185:681–689. doi: 10.1016/j.chemosphere.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Martín-de-Lucía I., Gonçalves S.F., Leganés F., Fernández-Piñas F., Rosal R., Loureiro S. Combined toxicity of graphite-diamond nanoparticles and thiabendazole to Daphnia magna. Sci. Total Environ. 2019;688:1145–1154. doi: 10.1016/j.scitotenv.2019.06.316. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z., Wang S., Peijnenburg W.J.G.M. Prediction of joint algal toxicity of nano-CeO2/nano-TiO2 and florfenicol: Independent action surpasses concentration addition. Chemosphere. 2016;156:8–13. doi: 10.1016/j.chemosphere.2016.04.072. [DOI] [PubMed] [Google Scholar]
- 18.Ye N., Wang Z., Wang S., Peijnenburg W.J.G.M. Toxicity of mixtures of zinc oxide and graphene oxide nanoparticles to aquatic organisms of different trophic level: Particles outperform dissolved ions. Nanotoxicology. 2018;12:423–438. doi: 10.1080/17435390.2018.1458342. [DOI] [PubMed] [Google Scholar]
- 19.Altenburger R., Nendza M., Schuurmann G. Mixture Toxicity and Its Modeling by Quantitative Structure–Activity Relationships. Environ. Toxicol. Chem. 2003;22:1900–1915. doi: 10.1897/01-386. [DOI] [PubMed] [Google Scholar]
- 20.Raies A.B., Bajic V.B. In silico toxicology: Computational methods for the prediction of chemical toxicity. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2016;6:147–172. doi: 10.1002/wcms.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cedergreen N. Quantifying synergy: A systematic review of mixture toxicity studies within environmental toxicology. PLoS ONE. 2014;9:e96580. doi: 10.1371/journal.pone.0096580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naasz S., Altenburger R., Kühnel D. Environmental mixtures of nanomaterials and chemicals: The Trojan-horse phenomenon and its relevance for ecotoxicity. Sci. Total Environ. 2018;635:1170–1181. doi: 10.1016/j.scitotenv.2018.04.180. [DOI] [PubMed] [Google Scholar]
- 23.Qu R., Wang X., Wang Z., Wei Z., Wang L. Metal accumulation and antioxidant defenses in the freshwater fish Carassius auratus in response to single and combined exposure to cadmium and hydroxylated multi-walled carbon nanotubes. J. Hazard. Mater. 2014;275:89–98. doi: 10.1016/j.jhazmat.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 24.Lorenz C.S., Wicht A.J., Guluzada L., Crone B., Karst U., Lee H.J., Triebskorn R., Haderlein S.B., Huhn C., Köhler H.R. Nano-sized zeolites as modulators of thiacloprid toxicity on Chironomus riparius. PeerJ. 2017;2017:1–19. doi: 10.7717/peerj.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu J., Wang D., Forthaus B.E., Wang J. Quantifying the effect of nanoparticles on As(V) ecotoxicity exemplified by nano-Fe2O3 (magnetic) and nano-Al2O3. Environ. Toxicol. Chem. 2012;31:2870–2876. doi: 10.1002/etc.2013. [DOI] [PubMed] [Google Scholar]
- 26.Yu Z.G., Wang W.X. Interaction of functionalized fullerenes and metal accumulation in Daphnia magna. Environ. Toxicol. Chem. 2014;33:1122–1128. doi: 10.1002/etc.2520. [DOI] [PubMed] [Google Scholar]
- 27.Fayaz A.M., Balaji K., Girilal M., Yadav R., Kalaichelvan P.T., Venketesan R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2010;6:103–109. doi: 10.1016/j.nano.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Xia X., Li Y., Zhou Z., Feng C. Bioavailability of adsorbed phenanthrene by black carbon and multi-walled carbon nanotubes to Agrobacterium. Chemosphere. 2010;78:1329–1336. doi: 10.1016/j.chemosphere.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y., Wang S., Wang Z., Ye N., Fang H., Wang D. TiO2, SiO2 and ZrO2 nanoparticles synergistically provoke cellular oxidative damage in freshwater microalgae. Nanomaterials. 2018;8:95. doi: 10.3390/nano8020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freitas R., Coppola F., De Marchi L., Codella V., Pretti C., Chiellini F., Morelli A., Polese G., Soares A.M.V.M., Figueira E. The influence of Arsenic on the toxicity of carbon nanoparticles in bivalves. J. Hazard. Mater. 2018;358:484–493. doi: 10.1016/j.jhazmat.2018.05.056. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z., Yang X., Shen M., Yin Y., Liu J. Sunlight-driven reduction of silver ion to silver nanoparticle by organic matter mitigates the acute toxicity of silver to Daphnia magna. J. Environ. Sci. 2015;35:62–68. doi: 10.1016/j.jes.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Petersen E.J., Pinto R.A., Landrum P.F., Weber W.J. Influence of carbon nanotubes on pyrene bioaccumulation from contaminated soils by earthworms. Environ. Sci. Technol. 2009;43:4181–4187. doi: 10.1021/es803023a. [DOI] [PubMed] [Google Scholar]
- 33.Völker C., Gräf T., Schneider I., Oetken M., Oehlmann J. Combined effects of silver nanoparticles and 17α-ethinylestradiol on the freshwater mudsnail Potamopyrgus antipodarum. Environ. Sci. Pollut. Res. 2014;21:10661–10670. doi: 10.1007/s11356-014-3067-5. [DOI] [PubMed] [Google Scholar]
- 34.Wang X., Qu R., Liu J., Wei Z., Wang L., Yang S., Huang Q., Wang Z. Effect of different carbon nanotubes on cadmium toxicity to Daphnia magna: The role of catalyst impurities and adsorption capacity. Environ. Pollut. 2016;208:732–738. doi: 10.1016/j.envpol.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 35.Iswarya V., Bhuvaneshwari M., Alex S.A., Iyer S., Chaudhuri G., Chandrasekaran P.T., Bhalerao G.M., Chakravarty S., Raichur A.M., Chandrasekaran N., et al. Combined toxicity of two crystalline phases (anatase and rutile) of Titania nanoparticles towards freshwater microalgae: Chlorella sp. Aquat. Toxicol. 2015;161:154–169. doi: 10.1016/j.aquatox.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Henry T.B., Wileman S.J., Boran H., Sutton P. Association of Hg2+ with aqueous (C60)n aggregates facilitates increased bioavailability of Hg2+ in zebrafish (Danio rerio) Environ. Sci. Technol. 2013;47:9997–10004. doi: 10.1021/es4015597. [DOI] [PubMed] [Google Scholar]
- 37.Zhang B., Cho M., Fortner J.D., Lee J., Huang C.H., Hughes J.B., Kim J.H. Delineating oxidative processes of aqueous C60 preparations: Role of THF peroxide. Environ. Sci. Technol. 2009;43:108–113. doi: 10.1021/es8019066. [DOI] [PubMed] [Google Scholar]
- 38.Hu X., Chen Q., Jiang L., Yu Z., Jiang D., Yin D. Combined effects of titanium dioxide and humic acid on the bioaccumulation of cadmium in Zebrafish. Environ. Pollut. 2011;159:1151–1158. doi: 10.1016/j.envpol.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Dalai S., Pakrashi S., Bhuvaneshwari M., Iswarya V., Chandrasekaran N., Mukherjee A. Toxic effect of Cr(VI) in presence of n-TiO2 and n-Al2O3 particles towards freshwater microalgae. Aquat. Toxicol. 2014;146:28–37. doi: 10.1016/j.aquatox.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 40.Yang W.W., Wang Y., Huang B., Wang N.X., Wei Z.B., Luo J., Miao A.J., Yang L.Y. TiO2 nanoparticles act as a carrier of Cd bioaccumulation in the ciliate tetrahymena thermophila. Environ. Sci. Technol. 2014;48:7568–7575. doi: 10.1021/es500694t. [DOI] [PubMed] [Google Scholar]
- 41.Sun H., Ruan Y., Zhu H., Zhang Z., Zhang Y., Yu L. Enhanced bioaccumulation of pentachlorophenol in carp in the presence of multi-walled carbon nanotubes. Environ. Sci. Pollut. Res. 2014;21:2865–2875. doi: 10.1007/s11356-013-2234-4. [DOI] [PubMed] [Google Scholar]
- 42.Srikanth K., Ahmad I., Rao J.V., Trindade T., Duarte A.C., Pereira E. Modulation of glutathione and its dependent enzymes in gill cells of Anguilla anguilla exposed to silica coated iron oxide nanoparticles with or without mercury co-exposure under in vitro condition. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014;162:7–14. doi: 10.1016/j.cbpc.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Yang W.W., Miao A.J., Yang L.Y. Cd2+ toxicity to a green alga Chlamydomonas reinhardtii as influenced by its adsorption on TiO2 engineered nanoparticles. PLoS ONE. 2012;7:e32300. doi: 10.1371/journal.pone.0032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jimeno-Romero A., Oron M., Cajaraville M.P., Soto M., Marigómez I. Nanoparticle size and combined toxicity of TiO2 and DSLS (surfactant) contribute to lysosomal responses in digestive cells of mussels exposed to TiO2 nanoparticles. Nanotoxicology. 2016;10:1168–1176. doi: 10.1080/17435390.2016.1196250. [DOI] [PubMed] [Google Scholar]
- 45.Kodali V.K., Roberts J.R., Shoeb M., Wolfarth M.G., Bishop L., Eye T., Barger M., Roach K.A., Friend S., Schwegler-Berry D., et al. Acute in vitro and in vivo toxicity of a commercial grade boron nitride nanotube mixture. Nanotoxicology. 2017;11:1040–1058. doi: 10.1080/17435390.2017.1390177. [DOI] [PubMed] [Google Scholar]
- 46.Chen C., Unrine J.M., Judy J.D., Lewis R.W., Guo J., McNear D.H., Tsyusko O.V. Toxicogenomic Responses of the Model Legume Medicago truncatula to Aged Biosolids Containing a Mixture of Nanomaterials (TiO2, Ag, and ZnO) from a Pilot Wastewater Treatment Plant. Environ. Sci. Technol. 2015;49:8759–8768. doi: 10.1021/acs.est.5b01211. [DOI] [PubMed] [Google Scholar]
- 47.Martins A.d.C., Azevedo L.F., de Souza Rocha C.C., Carneiro M.F.H., Venancio V.P., de Almeida M.R., Antunes L.M.G., de Carvalho Hott R., Rodrigues J.L., Ogunjimi A.T., et al. Evaluation of distribution, redox parameters, and genotoxicity in Wistar rats co-exposed to silver and titanium dioxide nanoparticles. J. Toxicol. Environ. Health Part A Curr. Issues. 2017;80:1156–1165. doi: 10.1080/15287394.2017.1357376. [DOI] [PubMed] [Google Scholar]
- 48.Mohmood I., Ahmad I., Asim M., Costa L., Lopes C.B., Trindade T., Duarte A.C., Pereira E. Interference of the co-exposure of mercury with silica-coated iron oxide nanoparticles can modulate genotoxicity induced by their individual exposures—A paradox depicted in fish under in vitro conditions. Environ. Sci. Pollut. Res. 2015;22:3687–3696. doi: 10.1007/s11356-014-3591-3. [DOI] [PubMed] [Google Scholar]
- 49.Judy J.D., McNear D.H., Chen C., Lewis R.W., Tsyusko O.V., Bertsch P.M., Rao W., Stegemeier J., Lowry G.V., McGrath S.P., et al. Nanomaterials in Biosolids Inhibit Nodulation, Shift Microbial Community Composition, and Result in Increased Metal Uptake Relative to Bulk/Dissolved Metals. Environ. Sci. Technol. 2015;49:8751–8758. doi: 10.1021/acs.est.5b01208. [DOI] [PubMed] [Google Scholar]
- 50.Hartmann N.B., Von der Kammer F., Hofmann T., Baalousha M., Ottofuelling S., Baun A. Algal testing of titanium dioxide nanoparticles-Testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology. 2010;269:190–197. doi: 10.1016/j.tox.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Alabresm A., Mirshahghassemi S., Chandler G.T., Decho A.W., Lead J. Use of PVP-coated magnetite nanoparticles to ameliorate oil toxicity to an estuarine meiobenthic copepod and stimulate the growth of oil-degrading bacteria. Environ. Sci. Nano. 2017;4:1859–1865. doi: 10.1039/C7EN00257B. [DOI] [Google Scholar]
- 52.Ginzburg A.L., Truong L., Tanguay R.L., Hutchison J.E. Synergistic Toxicity Produced by Mixtures of Biocompatible Gold Nanoparticles and Widely Used Surfactants. ACS Nano. 2018;12:5312–5322. doi: 10.1021/acsnano.8b00036. [DOI] [PubMed] [Google Scholar]
- 53.Yu Y., Duan J., Li Y., Yu Y., Jin M., Li C., Wang Y., Sun Z. Combined toxicity of amorphous silica nanoparticles and methylmercury to human lung epithelial cells. Ecotoxicol. Environ. Saf. 2015;112:144–152. doi: 10.1016/j.ecoenv.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 54.Tourinho P.S., Waalewijn-Kool P.L., Zantkuijl I., Jurkschat K., Svendsen C., Soares A.M.V.M., Loureiro S., van Gestel C.A.M. CeO2 nanoparticles induce no changes in phenanthrene toxicity to the soil organisms Porcellionides pruinosus and Folsomia candida. Ecotoxicol. Environ. Saf. 2015;113:201–206. doi: 10.1016/j.ecoenv.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Santaella C., Allainmat B., Simonet F., Chanéac C., Labille J., Auffan M., Rose J., Achouak W. Aged TiO2-based nanocomposite used in sunscreens produces singlet oxygen under long-wave UV and sensitizes escherichia coli to cadmium. Environ. Sci. Technol. 2014;48:5245–5253. doi: 10.1021/es500216t. [DOI] [PubMed] [Google Scholar]
- 56.Henry T.B., Menn F.M., Fleming J.T., Wilgus J., Compton R.N., Sayler G.S. Attributing effects of aqueous C60 nano-aggregates to tetrahydrofuran decomposition products in larval zebrafish by assessment of gene expression. Environ. Health Perspect. 2007;115:1059–1065. doi: 10.1289/ehp.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilke C.M., Tong T., Gaillard J.F., Gray K.A. Attenuation of Microbial Stress Due to Nano-Ag and Nano-TiO2 Interactions under Dark Conditions. Environ. Sci. Technol. 2016;50:11302–11310. doi: 10.1021/acs.est.6b02271. [DOI] [PubMed] [Google Scholar]
- 58.Huang B., Wei Z.B., Yang L.Y., Pan K., Miao A.J. Combined Toxicity of Silver Nanoparticles with Hematite or Plastic Nanoparticles toward Two Freshwater Algae. Environ. Sci. Technol. 2019;53:3871–3879. doi: 10.1021/acs.est.8b07001. [DOI] [PubMed] [Google Scholar]
- 59.Sanchís J., Olmos M., Vincent P., Farré M., Barceló D. New Insights on the Influence of Organic Co-Contaminants on the Aquatic Toxicology of Carbon Nanomaterials. Environ. Sci. Technol. 2016;50:961–969. doi: 10.1021/acs.est.5b03966. [DOI] [PubMed] [Google Scholar]
- 60.Park H.G., Yeo M.K. Comparison of gene expression patterns from zebrafish embryos between pure silver nanomaterial and mixed silver nanomaterial containing cells of Hydra magnipapillata. Mol. Cell. Toxicol. 2015;11:307–314. doi: 10.1007/s13273-015-0030-6. [DOI] [Google Scholar]
- 61.Hu C.W., Zhang L.J., Wang W.L., Cui Y.B., Li M. Evaluation of the combined toxicity of multi-walled carbon nanotubes and sodium pentachlorophenate on the earthworm Eisenia fetida using avoidance bioassay and comet assay. Soil Biol. Biochem. 2014;70:123–130. doi: 10.1016/j.soilbio.2013.12.018. [DOI] [Google Scholar]
- 62.Nigro M., Bernardeschi M., Costagliola D., Della Torre C., Frenzilli G., Guidi P., Lucchesi P., Mottola F., Santonastaso M., Scarcelli V., et al. n-TiO2 and CdCl2 co-exposure to titanium dioxide nanoparticles and cadmium: Genomic, DNA and chromosomal damage evaluation in the marine fish European sea bass (Dicentrarchus labrax) Aquat. Toxicol. 2015;168:72–77. doi: 10.1016/j.aquatox.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 63.Hernández-Moreno D., Valdehita A., Conde E., Rucandio I., Navas J.M., Fernández-Cruz M.L. Acute toxic effects caused by the co-exposure of nanoparticles of ZnO and Cu in rainbow trout. Sci. Total Environ. 2019;687:24–33. doi: 10.1016/j.scitotenv.2019.06.084. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L., Hu C., Wang W., Ji F., Cui Y., Li M. Acute toxicity of multi-walled carbon nanotubes, sodium pentachlorophenate, and their complex on earthworm Eisenia fetida. Ecotoxicol. Environ. Saf. 2014;103:29–35. doi: 10.1016/j.ecoenv.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 65.Soler de la Vega A.C., Molins-Delgado D., Barceló D., Díaz-Cruz M.S. Nanosized titanium dioxide UV filter increases mixture toxicity when combined with parabens. Ecotoxicol. Environ. Saf. 2019;184:109565. doi: 10.1016/j.ecoenv.2019.109565. [DOI] [PubMed] [Google Scholar]
- 66.Naqvi S.Z.H., Kiran U., Ali M.I., Jamal A., Hameed A., Ahmed S., Ali N. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int. J. Nanomed. 2013;8:3187–3195. doi: 10.2147/IJN.S49284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zindler F., Glomstad B., Altin D., Liu J., Jenssen B.M., Booth A.M. Phenanthrene bioavailability and toxicity to Daphnia magna in the presence of carbon nanotubes with different physicochemical properties. Environ. Sci. Technol. 2016;50:12446–12454. doi: 10.1021/acs.est.6b03228. [DOI] [PubMed] [Google Scholar]
- 68.Zou X.Y., Xu B., Yu C.P., Zhang H.W. Combined toxicity of ferroferric oxide nanoparticles and arsenic to the ciliated protozoa Tetrahymena Pyriformis. Aquat. Toxicol. 2013;134–135:66–73. doi: 10.1016/j.aquatox.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Zhai Y., Hunting E.R., Wouterse M., Peijnenburg W.J.G.M., Vijver M.G. Importance of exposure dynamics of metal-based nano-ZnO, -Cu and -Pb governing the metabolic potential of soil bacterial communities. Ecotoxicol. Environ. Saf. 2017;145:349–358. doi: 10.1016/j.ecoenv.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 70.Svartz G., Papa M., Gosatti M., Jordán M., Soldati A., Samter P., Guraya M.M., Pérez Coll C., Perez Catán S. Monitoring the ecotoxicity of γ-Al2O3 and Ni/γ-Al2O3 nanomaterials by means of a battery of bioassays. Ecotoxicol. Environ. Saf. 2017;144:200–207. doi: 10.1016/j.ecoenv.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 71.Rossi S.C., Mela M., Boschen S.L., da Cunha C., Neto F.F., Ribeiro C.A.O., Neves A.P.P., Silva de Assis H.C. Modulatory effect of nano TiO2 on Pb in Hoplias malabaricus trophically exposed. Environ. Toxicol. Pharmacol. 2014;38:71–78. doi: 10.1016/j.etap.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y., Yuan L., Yao C., Ding L., Li C., Fang J., Sui K., Liu Y., Wu M. A combined toxicity study of zinc oxide nanoparticles and vitamin C in food additives. Nanoscale. 2014;6:15333–15342. doi: 10.1039/C4NR05480F. [DOI] [PubMed] [Google Scholar]
- 73.Kim K.T., Klaine S.J., Lin S., Ke P.C., Kim S.D. Acute toxicity of a mixture of copper and single-walled carbon nanotubes to Daphnia magna. Environ. Toxicol. Chem. 2010;29:122–126. doi: 10.1002/etc.8. [DOI] [PubMed] [Google Scholar]
- 74.Hua J., Peijnenburg W.J.G.M., Vijver M.G. TiO2 nanoparticles reduce the effects of ZnO nanoparticles and Zn ions on zebrafish embryos (Danio rerio) NanoImpact. 2016;2:45–53. doi: 10.1016/j.impact.2016.06.005. [DOI] [Google Scholar]
- 75.Cao Y., Roursgaard M., Kermanizadeh A., Loft S., Møller P. Synergistic effects of zinc oxide nanoparticles and fatty acids on toxicity to caco-2 cells. Int. J. Toxicol. 2015;34:67–76. doi: 10.1177/1091581814560032. [DOI] [PubMed] [Google Scholar]
- 76.Tsugita M., Morimoto N., Nakayama M. SiO2 and TiO2 nanoparticles synergistically trigger macrophage inflammatory responses. Part. Fibre Toxicol. 2017;14:1–9. doi: 10.1186/s12989-017-0192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pavagadhi S., Sathishkumar M., Balasubramanian R. Uptake of Ag and TiO2 nanoparticles by zebrafish embryos in the presence of other contaminants in the aquatic environment. Water Res. 2014;55:280–291. doi: 10.1016/j.watres.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 78.Fan W., Peng R., Li X., Ren J., Liu T., Wang X. Effect of titanium dioxide nanoparticles on copper toxicity to Daphnia magna in water: Role of organic matter. Water Res. 2016;105:129–137. doi: 10.1016/j.watres.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 79.Wang C., Liu H., Chen J., Tian Y., Shi J., Li D., Guo C., Ma Q. Carboxylated multi-walled carbon nanotubes aggravated biochemical and subcellular damages in leaves of broad bean (Vicia faba L.) seedlings under combined stress of lead and cadmium. J. Hazard. Mater. 2014;274:404–412. doi: 10.1016/j.jhazmat.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 80.Jośko I., Oleszczuk P., Pranagal J., Lehmann J., Xing B., Cornelissen G. Effect of biochars, activated carbon and multiwalled carbon nanotubes on phytotoxicity of sediment contaminated by inorganic and organic pollutants. Ecol. Eng. 2013;60:50–59. doi: 10.1016/j.ecoleng.2013.07.064. [DOI] [Google Scholar]
- 81.Park C.B., Jung J.W., Yeom D.H., Jang J., Park J.W., Kim Y.J. Interactive effects between components in binary mixtures of zinc sulfate and iron oxide nanoparticles on Daphnia magna. Mol. Cell. Toxicol. 2019;15:315–323. doi: 10.1007/s13273-019-0035-7. [DOI] [Google Scholar]
- 82.Anjum N.A., Srikanth K., Mohmood I., Sayeed I., Trindade T., Duarte A.C., Pereira E., Ahmad I. Brain glutathione redox system significance for the control of silica-coated magnetite nanoparticles with or without mercury co-exposures mediated oxidative stress in European eel (Anguilla anguilla L.) Environ. Sci. Pollut. Res. 2014;21:7746–7756. doi: 10.1007/s11356-014-2673-6. [DOI] [PubMed] [Google Scholar]
- 83.Davarpanah E., Guilhermino L. Are gold nanoparticles and microplastics mixtures more toxic to the marine microalgae Tetraselmis chuii than the substances individually? Ecotoxicol. Environ. Saf. 2019;181:60–68. doi: 10.1016/j.ecoenv.2019.05.078. [DOI] [PubMed] [Google Scholar]
- 84.Wang Q., Chen Q., Zhou P., Li W., Wang J., Huang C., Wang X., Lin K., Zhou B. Bioconcentration and metabolism of BDE-209 in the presence of titanium dioxide nanoparticles and impact on the thyroid endocrine system and neuronal development in zebrafish larvae. Nanotoxicology. 2014;8:196–207. doi: 10.3109/17435390.2013.875232. [DOI] [PubMed] [Google Scholar]
- 85.Renzi M., Blašković A. Ecotoxicity of nano-metal oxides: A case study on Daphnia magna. Ecotoxicology. 2019;28:878–889. doi: 10.1007/s10646-019-02085-3. [DOI] [PubMed] [Google Scholar]
- 86.Sundukov Y.N. First record of the ground beetle Trechoblemus postilenatus (Coleoptera, Carabidae) in Primorskii krai. Far East. Entomol. 2006;165:16. doi: 10.1002/tox. [DOI] [Google Scholar]
- 87.Qiu T.A., Nguyen T.H.T., Hudson-Smith N.V., Clement P.L., Forester D.C., Frew H., Hang M.N., Murphy C.J., Hamers R.J., Feng Z.V., et al. Growth-Based Bacterial Viability Assay for Interference-Free and High-Throughput Toxicity Screening of Nanomaterials. Anal. Chem. 2017;89:2057–2064. doi: 10.1021/acs.analchem.6b04652. [DOI] [PubMed] [Google Scholar]
- 88.Polak N., Read D.S., Jurkschat K., Matzke M., Kelly F.J., Spurgeon D.J., Stürzenbaum S.R. Metalloproteins and phytochelatin synthase may confer protection against zinc oxide nanoparticle induced toxicity in Caenorhabditis elegans. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014;160:75–85. doi: 10.1016/j.cbpc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 89.Kim I., Lee B.T., Kim H.A., Kim K.W., Kim S.D., Hwang Y.S. Citrate coated silver nanoparticles change heavy metal toxicities and bioaccumulation of Daphnia magna. Chemosphere. 2016;143:99–105. doi: 10.1016/j.chemosphere.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 90.Ogunsuyi O.I., Fadoju O.M., Akanni O.O., Alabi O.A., Alimba C.G., Cambier S., Eswara S., Gutleb A.C., Adaramoye O.A., Bakare A.A. Genetic and systemic toxicity induced by silver and copper oxide nanoparticles, and their mixture in Clarias gariepinus (Burchell, 1822) Environ. Sci. Pollut. Res. 2019;26:27470–27481. doi: 10.1007/s11356-019-05958-6. [DOI] [PubMed] [Google Scholar]
- 91.Fang T., Watson J.L., Goodman J., Dimkpa C.O., Martineau N., Das S., McLean J.E., Britt D.W., Anderson A.J. Does doping with aluminum alter the effects of ZnO nanoparticles on the metabolism of soil pseudomonads? Microbiol. Res. 2013;168:91–98. doi: 10.1016/j.micres.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 92.Canesi L., Frenzilli G., Balbi T., Bernardeschi M., Ciacci C., Corsolini S., Della C., Fabbri R., Faleri C., Focardi S., et al. Interactive effects of n-TiO2 and 2,3,7,8-TCDD on the marine bivalve Mytilus galloprovincialis. Aquat. Toxicol. 2014;153:53–65. doi: 10.1016/j.aquatox.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 93.Deville S., Baré B., Piella J., Tirez K., Hoet P., Monopoli M.P., Dawson K.A., Puntes V.F., Nelissen I. Interaction of gold nanoparticles and nickel(II) sulfate affects dendritic cell maturation. Nanotoxicology. 2016;10:1395–1403. doi: 10.1080/17435390.2016.1221476. [DOI] [PubMed] [Google Scholar]
- 94.Della Torre C., Buonocore F., Frenzilli G., Corsolini S., Brunelli A., Guidi P., Kocan A., Mariottini M., Mottola F., Nigro M., et al. Influence of titanium dioxide nanoparticles on 2,3,7,8-tetrachlorodibenzo-p-dioxin bioconcentration and toxicity in the marine fish European sea bass (Dicentrarchus labrax) Environ. Pollut. 2015;196:185–193. doi: 10.1016/j.envpol.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 95.Della Torre C., Balbi T., Grassi G., Frenzilli G., Bernardeschi M., Smerilli A., Guidi P., Canesi L., Nigro M., Monaci F., et al. Titanium dioxide nanoparticles modulate the toxicological response to cadmium in the gills of Mytilus galloprovincialis. J. Hazard. Mater. 2015;297:92–100. doi: 10.1016/j.jhazmat.2015.04.072. [DOI] [PubMed] [Google Scholar]
- 96.De La Rosa-García S.C., Martínez-Torres P., Gómez-Cornelio S., Corral-Aguado M.A., Quintana P., Gómez-Ortíz N.M. Antifungal activity of ZnO and MgO nanomaterials and their mixtures against colletotrichum gloeosporioides strains from tropical fruit. J. Nanomater. 2018;2018 doi: 10.1155/2018/3498527. [DOI] [Google Scholar]
- 97.Brausch K.A., Anderson T.A., Smith P.N., Maul J.D. Effects of functionalized fullerenes on bifenthrin and tribufos toxicity to Daphnia magna: Survival, reproduction, and growth rate. Environ. Toxicol. Chem. 2010;29:2600–2606. doi: 10.1002/etc.318. [DOI] [PubMed] [Google Scholar]
- 98.Baun A., Sørensen S.N., Rasmussen R.F., Hartmann N.B., Koch C.B. Toxicity and bioaccumulation of xenobiotic organic compounds in the presence of aqueous suspensions of aggregates of nano-C60. Aquat. Toxicol. 2008;86:379–387. doi: 10.1016/j.aquatox.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 99.Balbi T., Smerilli A., Fabbri R., Ciacci C., Montagna M., Grasselli E., Brunelli A., Pojana G., Marcomini A., Gallo G., et al. Co-exposure to n-TiO2 and Cd2+ results in interactive effects on biomarker responses but not in increased toxicity in the marine bivalve M. galloprovincialis. Sci. Total Environ. 2014;493:355–364. doi: 10.1016/j.scitotenv.2014.05.146. [DOI] [PubMed] [Google Scholar]
- 100.Lyon D.Y., Adams L.K., Falkner J.C., Alvarez P.J.J. Antibacterial activity of fullerene water suspensions: Effects of preparation method and particle size. Environ. Sci. Technol. 2006;40:4360–4366. doi: 10.1021/es0603655. [DOI] [PubMed] [Google Scholar]
- 101.Baek M.J., Son J., Park J., Seol Y., Sung B., Kim Y.J. Quantitative prediction of mixture toxicity of AgNO3 and ZnO nanoparticles on Daphnia magna. Sci. Technol. Adv. Mater. 2020;21:333–345. doi: 10.1080/14686996.2020.1766343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Worms I.A.M., Boltzman J., Garcia M., Slaveykova V.I. Cell-wall-dependent effect of carboxyl-CdSe/ZnS quantum dots on lead and copper availability to green microalgae. Environ. Pollut. 2012;167:27–33. doi: 10.1016/j.envpol.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 103.Yan J., Lin B., Hu C., Zhang H., Lin Z., Xi Z. The combined toxicological effects of titanium dioxide nanoparticles and bisphenol A on zebrafish embryos. Nanoscale Res. Lett. 2014;9:1–9. doi: 10.1186/1556-276X-9-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hamdi H., De La Torre-Roche R., Hawthorne J., White J.C. Impact of non-functionalized and amino-functionalized multiwall carbon nanotubes on pesticide uptake by lettuce (Lactuca sativa L.) Nanotoxicology. 2014;9:172–180. doi: 10.3109/17435390.2014.907456. [DOI] [PubMed] [Google Scholar]
- 105.Hu C., Cai Y., Wang W., Cui Y., Li M. Toxicological effects of multi-walled carbon nanotubes adsorbed with nonylphenol on earthworm Eisenia fetida. Environ. Sci. Process. Impacts. 2013;15:2125–2130. doi: 10.1039/c3em00376k. [DOI] [PubMed] [Google Scholar]
- 106.Fan W.H., Cui M.M., Shi Z.W., Tan C., Yang X.P. Enhanced oxidative stress and physiological damage in Daphnia magna by copper in the presence of Nano-TiO2. J. Nanomater. 2012;2012:1–7. doi: 10.1155/2012/398720. [DOI] [Google Scholar]
- 107.Fan W., Cui M., Liu H., Wang C., Shi Z., Tan C., Yang X. Nano-TiO2 enhances the toxicity of copper in natural water to Daphnia magna. Environ. Pollut. 2011;159:729–734. doi: 10.1016/j.envpol.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 108.Tian S.Y., Gao Y.N., Song C.Z. Acute toxicities of penta-BDE in TiO2 nanoparticle suspensions to Daphnia magna. Adv. Mater. Res. 2014;864–867:261–265. doi: 10.4028/www.scientific.net/AMR.864-867.261. [DOI] [Google Scholar]
- 109.Tan C., Wang W.X. Modification of metal bioaccumulation and toxicity in Daphnia magna by titanium dioxide nanoparticles. Environ. Pollut. 2014;186:36–42. doi: 10.1016/j.envpol.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 110.Seitz F., Bundschuh M., Dabrunz A., Bandow N., Schaumann G.E., Schulz R. Titanium dioxide nanoparticles detoxify pirimicarb under UV irradiation at ambient intensities. Environ. Toxicol. Chem. 2012;31:518–523. doi: 10.1002/etc.1715. [DOI] [PubMed] [Google Scholar]
- 111.Liu Y., Yan Z., Xia J., Wang K., Ling X., Yan B. Potential toxicity in crucian carp following exposure to metallic nanoparticles of copper, chromium, and their mixtures: A comparative study. Polish J. Environ. Stud. 2017;26:2085–2094. doi: 10.15244/pjoes/69251. [DOI] [Google Scholar]
- 112.Rosenfeldt R.R., Seitz F., Schulz R., Bundschuh M. Heavy metal uptake and toxicity in the presence of titanium dioxide nanoparticles: A factorial approach using Daphnia magna. Environ. Sci. Technol. 2014;48:6965–6972. doi: 10.1021/es405396a. [DOI] [PubMed] [Google Scholar]
- 113.Rosenfeldt R.R., Seitz F., Senn L., Schilde C., Schulz R., Bundschuh M. Nanosized titanium dioxide reduces copper toxicity-the role of organic material and the crystalline phase. Environ. Sci. Technol. 2015;49:1815–1822. doi: 10.1021/es506243d. [DOI] [PubMed] [Google Scholar]
- 114.Hartmann N.B., Legros S., Von der Kammer F., Hofmann T., Baun A. The potential of TiO2 nanoparticles as carriers for cadmium uptake in Lumbriculus variegatus and Daphnia magna. Aquat. Toxicol. 2012;118–119:1–8. doi: 10.1016/j.aquatox.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 115.Wang D., Hu J., Irons D.R., Wang J. Synergistic toxic effect of nano-TiO2 and As(V) on Ceriodaphnia dubia. Sci. Total Environ. 2011;409:1351–1356. doi: 10.1016/j.scitotenv.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 116.De La Torre-Roche R., Hawthorne J., Musante C., Xing B., Newman L.A., Ma X., White J.C. Impact of Ag nanoparticle exposure on p,p′-DDE bioaccumulation by cucurbita pepo (Zucchini) and glycine max (Soybean) Environ. Sci. Technol. 2013;47:718–725. doi: 10.1021/es3041829. [DOI] [PubMed] [Google Scholar]
- 117.Park C.B., Jung J.W., Baek M., Sung B., Park J.W.J.W., Seol Y., Yeom D.H., Park J.W.J.W., Kim Y.J. Mixture toxicity of metal oxide nanoparticles and silver ions on Daphnia magna. J. Nanoparticle Res. 2019;21:21. doi: 10.1007/s11051-019-4606-2. [DOI] [Google Scholar]
- 118.Zhang X., Sun H., Zhang Z., Niu Q., Chen Y., Crittenden J.C. Enhanced bioaccumulation of cadmium in carp in the presence of titanium dioxide nanoparticles. Chemosphere. 2007;67:160–166. doi: 10.1016/j.chemosphere.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 119.Miao W., Zhu B., Xiao X., Li Y., Dirbaba N.B., Zhou B., Wu H. Effects of titanium dioxide nanoparticles on lead bioconcentration and toxicity on thyroid endocrine system and neuronal development in zebrafish larvae. Aquat. Toxicol. 2015;161:117–126. doi: 10.1016/j.aquatox.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 120.Tong T., Wilke C.M., Wu J., Binh C.T.T., Kelly J.J., Gaillard J.F., Gray K.A. Combined Toxicity of Nano-ZnO and Nano-TiO2: From Single- to Multinanomaterial Systems. Environ. Sci. Technol. 2015;49:8113–8123. doi: 10.1021/acs.est.5b02148. [DOI] [PubMed] [Google Scholar]
- 121.Yi X., Zhang K., Han G., Yu M., Chi T., Jing S., Li Z., Zhan J., Wu M. Toxic effect of triphenyltin in the presence of nano zinc oxide to marine copepod Tigriopus japonicus. Environ. Pollut. 2018;243:687–692. doi: 10.1016/j.envpol.2018.09.038. [DOI] [PubMed] [Google Scholar]
- 122.Tian S., Zhang Y., Song C., Zhu X., Xing B. Titanium dioxide nanoparticles as carrier facilitate bioaccumulation of phenanthrene in marine bivalve, ark shell (Scapharca subcrenata) Environ. Pollut. 2014;192:59–64. doi: 10.1016/j.envpol.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 123.Seo Y., Hwang J., Kim J., Jeong Y., Hwang M.P., Choi J. Antibacterial activity and cytotoxicity of multi-walled carbon nanotubes decorated with silver nanoparticles. Int. J. Nanomed. 2014;9:4621–4629. doi: 10.2147/IJN.S69561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cui X.Y., Jia F., Chen Y.X., Gan J. Influence of single-walled carbon nanotubes on microbial availability of phenanthrene in sediment. Ecotoxicology. 2011;20:1277–1285. doi: 10.1007/s10646-011-0684-3. [DOI] [PubMed] [Google Scholar]
- 125.Rocco L., Santonastaso M., Nigro M., Mottola F., Costagliola D., Bernardeschi M., Guidi P., Lucchesi P., Scarcelli V., Corsi I., et al. Genomic and chromosomal damage in the marine mussel Mytilus galloprovincialis: Effects of the combined exposure to titanium dioxide nanoparticles and cadmium chloride. Mar. Environ. Res. 2015;111:144–148. doi: 10.1016/j.marenvres.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 126.Lammel T., Wassmur B., Mackevica A., Chen C.E.L., Sturve J. Mixture toxicity effects and uptake of titanium dioxide (TiO2) nanoparticles and 3,3″,4,4″-tetrachlorobiphenyl (PCB77) in juvenile brown trout following co-exposure via the diet. Aquat. Toxicol. 2019;213:105195. doi: 10.1016/j.aquatox.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 127.Chen F., Wu L., Xiao X., Rong L., Li M., Zou X. Mixture toxicity of zinc oxide nanoparticle and chemicals with different mode of action upon Vibrio fischeri. Environ. Sci. Eur. 2020;32 doi: 10.1186/s12302-020-00320-x. [DOI] [Google Scholar]
- 128.Vannuccini M.L., Grassi G., Leaver M.J., Corsi I. Combination effects of nano-TiO2 and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on biotransformation gene expression in the liver of European sea bass Dicentrarchus labrax. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016;176–177:71–78. doi: 10.1016/j.cbpc.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 129.Shahverdi A.R., Fakhimi A., Shahverdi H.R., Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007;3:168–171. doi: 10.1016/j.nano.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 130.Gajbhiye M., Kesharwani J., Ingle A., Gade A., Rai M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed. Nanotechnol. Biol. Med. 2009;5:382–386. doi: 10.1016/j.nano.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 131.Ferreira J.L.R., Lonné M.N., França T.A., Maximilla N.R., Lugokenski T.H., Costa P.G., Fillmann G., Antunes Soares F.A., de la Torre F.R., Monserrat J.M. Co-exposure of the organic nanomaterial fullerene C60 with benzo[a]pyrene in Danio rerio (zebrafish) hepatocytes: Evidence of toxicological interactions. Aquat. Toxicol. 2014;147:76–83. doi: 10.1016/j.aquatox.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 132.Mahboob S., Al-Ghanim K.A., Al-Mulhim N.M.A. Fish Exposure to Sub-Lethal Toxicity of Nano-Titanium Oxide and Changes in Muscular Antioxidant Enzymes and Protective Role of Vitamins C and E in Clarias gariepinus. Int. J. Agric. Biol. 2017;19:1505–1510. doi: 10.17957/IJAB/15.0454. [DOI] [Google Scholar]
- 133.Srivastava S., Kumar A. Comparative cytotoxicity of nanoparticles and ions to Escherichia coli in binary mixtures. J. Environ. Sci. 2017;55:11–19. doi: 10.1016/j.jes.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 134.Li P., Li J., Wu C., Wu Q., Li J. Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology. 2005;16:1912–1917. doi: 10.1088/0957-4484/16/9/082. [DOI] [Google Scholar]
- 135.Kühnel D., Busch W., Meißner T., Springer A., Potthoff A., Richter V., Gelinsky M., Scholz S., Schirmer K. Agglomeration of tungsten carbide nanoparticles in exposure medium does not prevent uptake and toxicity toward a rainbow trout gill cell line. Aquat. Toxicol. 2009;93:91–99. doi: 10.1016/j.aquatox.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 136.Kim K.T., Edgington A.J., Klaine S.J., Cho J.W., Kim S.D. Influence of multiwalled carbon nanotubes dispersed in natural organic matter on speciation and bioavailability of copper. Environ. Sci. Technol. 2009;43:8979–8984. doi: 10.1021/es900647f. [DOI] [PubMed] [Google Scholar]
- 137.Chai M., Shi F., Li R., Liu L., Liu Y., Liu F. Interactive effects of cadmium and carbon nanotubes on the growth and metal accumulation in a halophyte Spartina alterniflora (Poaceae) Plant Growth Regul. 2013;71:171–179. doi: 10.1007/s10725-013-9817-4. [DOI] [Google Scholar]
- 138.Cuahtecontzi-Delint R., Mendez-Rojas M.A., Bandala E.R., Quiroz M.A., Recillas S., Sanchez-Salas J.L. Enhanced antibacterial activity of CeO2 nanoparticles by surfactants. Int. J. Chem. React. Eng. 2013;11:781–785. doi: 10.1515/ijcre-2012-0055. [DOI] [Google Scholar]
- 139.Kelsey J.W., White J.C. Effect of C60 fullerenes on the accumulation of weathered p,p′-DDE by plant and earthworm species under single and multispecies conditions. Environ. Toxicol. Chem. 2013;32:1117–1123. doi: 10.1002/etc.2158. [DOI] [PubMed] [Google Scholar]
- 140.Zhang W., Sun X., Chen L., Lin K.F., Dong Q.X., Huang C.J., Fu R.B., Zhu J. Toxicological effect of joint cadmium selenium quantum dots and copper ion exposure on zebrafish. Environ. Toxicol. Chem. 2012;31:2117–2123. doi: 10.1002/etc.1918. [DOI] [PubMed] [Google Scholar]
- 141.Schirinzi G.F., Pérez-Pomeda I., Sanchís J., Rossini C., Farré M., Barceló D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017;159:579–587. doi: 10.1016/j.envres.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 142.Park J.W., Henry T.B., Menn F.M., Compton R.N., Sayler G. No bioavailability of 17α-ethinylestradiol when associated with nC60 aggregates during dietary exposure in adult male zebrafish (Danio rerio) Chemosphere. 2010;81:1227–1232. doi: 10.1016/j.chemosphere.2010.09.036. [DOI] [PubMed] [Google Scholar]
- 143.Vale G., Franco C., Diniz M.S., dos Santos M.M.C., Domingos R.F. Bioavailability of cadmium and biochemical responses on the freshwater bivalve Corbicula fluminea—The role of TiO2 nanoparticles. Ecotoxicol. Environ. Saf. 2014;109:161–168. doi: 10.1016/j.ecoenv.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 144.Sun H., Zhang X., Niu Q., Chen Y., Crittenden J.C. Enhanced accumulation of arsenate in carp in the presence of titanium dioxide nanoparticles. Water. Air. Soil Pollut. 2007;178:245–254. doi: 10.1007/s11270-006-9194-y. [DOI] [Google Scholar]
- 145.Al-Subiai S.N., Arlt V.M., Frickers P.E., Readman J.W., Stolpe B., Lead J.R., Moody A.J., Jha A.N. Merging nano-genotoxicology with eco-genotoxicology: An integrated approach to determine interactive genotoxic and sub-lethal toxic effects of C 60 fullerenes and fluoranthene in marine mussels, Mytilus sp. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012;745:92–103. doi: 10.1016/j.mrgentox.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 146.Krysanov E.Y., Demidova T.B. The effect of low concentrations of nanocrystalline cerium dioxide on the embryotoxicity of doxorubicin for fish. Dokl. Biol. Sci. 2012;443:117–119. doi: 10.1134/S0012496612020135. [DOI] [PubMed] [Google Scholar]
- 147.Silveira L.T., Liberatore A.M.A., Koh I.H.J., Bizeto M.A., Camilo F.F. Combined bactericidal activity of silver nanoparticles and hexadecylpyridinium salicylate ionic liquid. J. Nanoparticle Res. 2015;17:17. doi: 10.1007/s11051-015-2934-4. [DOI] [Google Scholar]
- 148.Wang C., Wei Z., Feng M., Wang L., Wang Z. The effects of hydroxylated multiwalled carbon nanotubes on the toxicity of nickel to Daphnia magna under different pH levels. Environ. Toxicol. Chem. 2014;33:2522–2528. doi: 10.1002/etc.2704. [DOI] [PubMed] [Google Scholar]
- 149.Han Z.X., He G.D., Wang J.H., Lv C.X. Interaction influence of Cd(II) and nano-TiO2 on aggregation and adsorption kinetics toward marine algae. Int. J. Green Nanotechnol. Biomed. 2011;3:229–237. doi: 10.1080/19430892.2011.628590. [DOI] [Google Scholar]
- 150.Glinski A., Liebel S., Pelletier É., Voigt C.L., Randi M.A.F., Campos S.X., Oliveira Ribeiro C.A., Filipak Neto F. Toxicological interactions of silver nanoparticles and organochlorine pesticides in mouse peritoneal macrophages. Toxicol. Mech. Methods. 2016;26:251–259. doi: 10.3109/15376516.2016.1159770. [DOI] [PubMed] [Google Scholar]
- 151.Judy J.D., Kirby J.K., McLaughlin M.J., McNear D., Bertsch P.M. Symbiosis between nitrogen-fixing bacteria and Medicago truncatula is not significantly affected by silver and silver sulfide nanomaterials. Environ. Pollut. 2016;214:731–736. doi: 10.1016/j.envpol.2016.04.078. [DOI] [PubMed] [Google Scholar]
- 152.Farkas J., Bergum S., Nilsen E.W., Olsen A.J., Salaberria I., Ciesielski T.M., Baczek T., Konieczna L., Salvenmoser W., Jenssen B.M. The impact of TiO2 nanoparticles on uptake and toxicity of benzo(a)pyrene in the blue mussel (Mytilus edulis) Sci. Total Environ. 2015;511:469–476. doi: 10.1016/j.scitotenv.2014.12.084. [DOI] [PubMed] [Google Scholar]
- 153.Fang L., Borggaard O.K., Holm P.E., Hansen H.C.B., Cedergreen N. Toxicity and uptake of TRI- and dibutyltin in Daphnia magna in the absence and presence of nano-charcoal. Environ. Toxicol. Chem. 2011;30:2553–2561. doi: 10.1002/etc.649. [DOI] [PubMed] [Google Scholar]
- 154.Su Y., Yan X., Pu Y., Xiao F., Wang D., Yang M. Risks of single-walled carbon nanotubes acting as contaminants-carriers: Potential release of phenanthrene in Japanese medaka (Oryzias latipes) Environ. Sci. Technol. 2013;47:4704–4710. doi: 10.1021/es304479w. [DOI] [PubMed] [Google Scholar]
- 155.Cui X., Wan B., Guo L.H., Yang Y., Ren X. Insight into the mechanisms of combined toxicity of single-walled carbon nanotubes and nickel ions in macrophages: Role of P2X7 receptor. Environ. Sci. Technol. 2016;50:12473–12483. doi: 10.1021/acs.est.6b03842. [DOI] [PubMed] [Google Scholar]
- 156.Wang Y., Peng C., Fang H., Sun L., Zhang H., Feng J., Duan D., Liu T., Shi J. Mitigation of Cu(Ii) Phytotoxicity to Rice (Oryza sativa) in the Presence of TiO2 and CeO2 Nanoparticles Combined with Humic Acid. Environ. Toxicol. Chem. 2015;34:1588–1596. doi: 10.1002/etc.2953. [DOI] [PubMed] [Google Scholar]
- 157.Hu J., Wang D., Wang J., Wang J. Toxicity of lead on Ceriodaphnia dubia in the presence of nano-CeO2 and nano-TiO2. Chemosphere. 2012;89:536–541. doi: 10.1016/j.chemosphere.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 158.Han Z., Li J., Bao W., Wang J. Enhanced toxicity of atrazine to Daphnia magna in the presence of nano-CeO2. Chinese J. Geochemistry. 2012;31:297–302. doi: 10.1007/s11631-012-0578-y. [DOI] [Google Scholar]
- 159.McShan D., Zhang Y., Deng H., Ray P.C., Yu H. Synergistic Antibacterial Effect of Silver Nanoparticles Combined with Ineffective Antibiotics on Drug Resistant Salmonella typhimurium DT104. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2015;33:369–384. doi: 10.1080/10590501.2015.1055165. [DOI] [PubMed] [Google Scholar]
- 160.Fang Q., Shi X., Zhang L., Wang Q., Wang X., Guo Y., Zhou B. Effect of titanium dioxide nanoparticles on the bioavailability, metabolism, and toxicity of pentachlorophenol in zebrafish larvae. J. Hazard. Mater. 2015;283:897–904. doi: 10.1016/j.jhazmat.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 161.Hu X., Liu J., Zhou Q., Lu S., Liu R., Cui L., Yin D., Mayer P., Jiang G. Bioavailability of organochlorine compounds in aqueous suspensions of fullerene: Evaluated with medaka (Oryzias latipes) and negligible depletion solid-phase microextraction. Chemosphere. 2010;80:693–700. doi: 10.1016/j.chemosphere.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 162.Yu R., Wu J., Liu M., Zhu G., Chen L., Chang Y., Lu H. Toxicity of binary mixtures of metal oxide nanoparticles to Nitrosomonas europaea. Chemosphere. 2016;153:187–197. doi: 10.1016/j.chemosphere.2016.03.065. [DOI] [PubMed] [Google Scholar]
- 163.Yang W.W., Li Y., Miao A.J., Yang L.Y. Cd2+ toxicity as affected by bare TiO2 nanoparticles and their bulk counterpart. Ecotoxicol. Environ. Saf. 2012;85:44–51. doi: 10.1016/j.ecoenv.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 164.Magesky A., Pelletier É. Toxicity mechanisms of ionic silver and polymer-coated silver nanoparticles with interactions of functionalized carbon nanotubes on early development stages of sea urchin. Aquat. Toxicol. 2015;167:106–123. doi: 10.1016/j.aquatox.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 165.Shrestha B., Anderson T.A., Acosta-Martinez V., Payton P., Cañas-Carrell J.E. The influence of multiwalled carbon nanotubes on polycyclic aromatic hydrocarbon (PAH) bioavailability and toxicity to soil microbial communities in alfalfa rhizosphere. Ecotoxicol. Environ. Saf. 2015;116:143–149. doi: 10.1016/j.ecoenv.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 166.Yan Z., Liu Y., Sun H., Lu G. Influence of multiwall carbon nanotubes on the toxicity of 17β-estradiol in the early life stages of zebrafish. Environ. Sci. Pollut. Res. 2018;25:7566–7574. doi: 10.1007/s11356-017-1063-2. [DOI] [PubMed] [Google Scholar]
- 167.Hernández-Moreno D., Li L., Connolly M., Conde E., Fernández M., Schuster M., Navas J.M., Fernández-Cruz M.L. Mechanisms underlying the enhancement of toxicity caused by the coincubation of zinc oxide and copper nanoparticles in a fish hepatoma cell line. Environ. Toxicol. Chem. 2016;35:2562–2570. doi: 10.1002/etc.3425. [DOI] [PubMed] [Google Scholar]
- 168.Schwab F., Bucheli T.D., Camenzuli L., Magrez A., Knauer K., Sigg L., Nowack B. Diuron sorbed to carbon nanotubes exhibits enhanced toxicity to chlorella vulgaris. Environ. Sci. Technol. 2013;47:7012–7019. doi: 10.1021/es304016u. [DOI] [PubMed] [Google Scholar]
- 169.Lahive E., Matzke M., Durenkamp M., Lawlor A.J., Thacker S.A., Pereira M.G., Spurgeon D.J., Unrine J.M., Svendsen C., Lofts S. Sewage sludge treated with metal nanomaterials inhibits earthworm reproduction more strongly than sludge treated with metal metals in bulk/salt forms. Environ. Sci. Nano. 2017;4:78–88. doi: 10.1039/C6EN00280C. [DOI] [Google Scholar]
- 170.Tang Y., Li S., Qiao J., Wang H., Li L. Synergistic effects of nano-sized titanium dioxide and zinc on the photosynthetic capacity and survival of Anabaena sp. Int. J. Mol. Sci. 2013;14:14395–14407. doi: 10.3390/ijms140714395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ma X., Wang C. Fullerene nanoparticles affect the fate and uptake of trichloroethylene in phytoremediation systems. Environ. Eng. Sci. 2010;27:989–992. doi: 10.1089/ees.2010.0141. [DOI] [Google Scholar]
- 172.Hu X., Kang J., Lu K., Zhou R., Mu L., Zhou Q. Graphene oxide amplifies the phytotoxicity of arsenic in wheat. Sci. Rep. 2014;4:1–10. doi: 10.1038/srep06122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Farkas J., Salaberria I., Styrishave B., Staňková R., Ciesielski T.M., Olsen A.J., Posch W., Flaten T.P., Krøkje Å., Salvenmoser W., et al. Exposure of juvenile turbot (Scophthalmus maximus) to silver nanoparticles and 17α-ethinylestradiol mixtures: Implications for contaminant uptake and plasma steroid hormone levels. Environ. Pollut. 2017;220:328–336. doi: 10.1016/j.envpol.2016.09.067. [DOI] [PubMed] [Google Scholar]
- 174.Tan C., Fan W.H., Wang W.X. Role of titanium dioxide nanoparticles in the elevated uptake and retention of cadmium and zinc in Daphnia magna. Environ. Sci. Technol. 2012;46:469–476. doi: 10.1021/es202110d. [DOI] [PubMed] [Google Scholar]
- 175.Shen M., Xia X., Zhai Y., Zhang X., Zhao X., Zhang P. Influence of carbon nanotubes with preloaded and coexisting dissolved organic matter on the bioaccumulation of polycyclic aromatic hydrocarbons to Chironomus plumosus larvae in sediment. Environ. Toxicol. Chem. 2014;33:182–189. doi: 10.1002/etc.2414. [DOI] [PubMed] [Google Scholar]
- 176.Azevedo Costa C.L., Chaves I.S., Ventura-Lima J., Ferreira J.L.R., Ferraz L., De Carvalho L.M.H., Monserrat J.M. In vitro evaluation of co-exposure of arsenium and an organic nanomaterial (fullerene, C60) in zebrafish hepatocytes. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2012;155:206–212. doi: 10.1016/j.cbpc.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 177.Yan X.M., Zha J.M., Shi B.Y., Wang D.S., Wang Z.J., Tang H.X. In vivo toxicity of nano-C60 aggregates complex with atrazine to aquatic organisms. Chin. Sci. Bull. 2010;55:339–345. doi: 10.1007/s11434-009-0702-5. [DOI] [Google Scholar]
- 178.Cano A.M., Maul J.D., Saed M., Irin F., Shah S.A., Green M.J., French A.D., Klein D.M., Crago J., Canas-Carrell J.E. Trophic Transfer and Accumulation of Multiwalled Carbon Nanotubes in the Presence of Copper Ions in Daphnia magna and Fathead Minnow (Pimephales promelas) Environ. Sci. Technol. 2018;52:794–800. doi: 10.1021/acs.est.7b03522. [DOI] [PubMed] [Google Scholar]
- 179.Nunes S.M., Josende M.E., Ruas C.P., Gelesky M.A., Júnior F.M.R.d.S., Fattorini D., Regoli F., Monserrat J.M., Ventura-Lima J. Biochemical responses induced by co-exposition to arsenic and titanium dioxide nanoparticles in the estuarine polychaete Laeonereis acuta. Toxicology. 2017;376:51–58. doi: 10.1016/j.tox.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 180.Ko K.S., Koh D.C., Kong I.C. Toxicity evaluation of individual and mixtures of nanoparticles based on algal chlorophyll content and cell count. Materials. 2018;11:121. doi: 10.3390/ma11010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gao M., Zhang Z., Lv M., Song W., Lv Y. Toxic effects of nanomaterial-adsorbed cadmium on Daphnia magna. Ecotoxicol. Environ. Saf. 2018;148:261–268. doi: 10.1016/j.ecoenv.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 182.Yu R., Wu J., Liu M., Chen L., Zhu G., Lu H. Physiological and transcriptional responses of Nitrosomonas europaea to TiO2 and ZnO nanoparticles and their mixtures. Environ. Sci. Pollut. Res. 2016;23:13023–13034. doi: 10.1007/s11356-016-6469-8. [DOI] [PubMed] [Google Scholar]
- 183.Sun H., Zhang X., Zhang Z., Chen Y., Crittenden J.C. Influence of titanium dioxide nanoparticles on speciation and bioavailability of arsenite. Environ. Pollut. 2009;157:1165–1170. doi: 10.1016/j.envpol.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 184.Molins-Delgado D., Gago-Ferrero P., Díaz-Cruz M.S., Barceló D. Single and joint ecotoxicity data estimation of organic UV filters and nanomaterials toward selected aquatic organisms. Urban groundwater risk assessment. Environ. Res. 2016;145:126–134. doi: 10.1016/j.envres.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 185.Ye N., Wang Z., Fang H., Wang S., Zhang F. Combined ecotoxicity of binary zinc oxide and copper oxide nanoparticles to Scenedesmus obliquus. J. Environ. Sci. Health Part A Toxic/Hazardous Subst. Environ. Eng. 2017;52:555–560. doi: 10.1080/10934529.2017.1284434. [DOI] [PubMed] [Google Scholar]
- 186.Liu Y., Baas J., Peijnenburg W.J.G.M., Vijver M.G. Evaluating the Combined Toxicity of Cu and ZnO Nanoparticles: Utility of the Concept of Additivity and a Nested Experimental Design. Environ. Sci. Technol. 2016;50:5328–5337. doi: 10.1021/acs.est.6b00614. [DOI] [PubMed] [Google Scholar]
- 187.Rosenfeldt R.R., Seitz F., Zubrod J.P., Feckler A., Merkel T., Lüderwald S., Bundschuh R., Schulz R., Bundschuh M. Does the presence of titanium dioxide nanoparticles reduce copper toxicity? A factorial approach with the benthic amphipod Gammarus fossarum. Aquat. Toxicol. 2015;165:154–159. doi: 10.1016/j.aquatox.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 188.Jośko I., Oleszczuk P., Skwarek E. Toxicity of combined mixtures of nanoparticles to plants. J. Hazard. Mater. 2017;331:200–209. doi: 10.1016/j.jhazmat.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 189.Yang X.Y., Edelmann R.E., Oris J.T. Suspended C60 nanoparticles protect against short-term UV and fluoranthene photo-induced toxicity, but cause long-term cellular damage in Daphnia magna. Aquat. Toxicol. 2010;100:202–210. doi: 10.1016/j.aquatox.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 190.Park J.W., Henry T.B., Ard S., Menn F.M., Compton R.N., Sayler G.S. The association between nC 60 and 17α-ethinylestradiol (EE2) decreases EE2 bioavailability in zebrafish and alters nanoaggregate characteristics. Nanotoxicology. 2011;5:406–416. doi: 10.3109/17435390.2010.525329. [DOI] [PubMed] [Google Scholar]
- 191.Xia X., Chen X., Zhao X., Chen H., Shen M. Effects of carbon nanotubes, chars, and ash on bioaccumulation of perfluorochemicals by chironomus plumosus larvae in sediment. Environ. Sci. Technol. 2012;46:12467–12475. doi: 10.1021/es303024x. [DOI] [PubMed] [Google Scholar]
- 192.Shen M., Xia X., Wang F., Zhang P., Zhao X. Influences of multiwalled carbon nanotubes and plant residue chars on bioaccumulation of polycyclic aromatic hydrocarbons by Chironomus plumosus larvae in sediment. Environ. Toxicol. Chem. 2012;31:202–209. doi: 10.1002/etc.722. [DOI] [PubMed] [Google Scholar]
- 193.Tao X., He Y., Fortner J.D., Chen Y., Hughes J.B. Effects of aqueous stable fullerene nanocrystal (nC60) on copper (trace necessary nutrient metal): Enhanced toxicity and accumulation of copper in Daphnia magna. Chemosphere. 2013;92:1245–1252. doi: 10.1016/j.chemosphere.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 194.Meng Y., Wang S., Wang Z., Ye N., Fang H. Algal toxicity of binary mixtures of zinc oxide nanoparticles and tetrabromobisphenol A: Roles of dissolved organic matters. Environ. Toxicol. Pharmacol. 2018;64:78–85. doi: 10.1016/j.etap.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 195.Ferguson P.L., Chandler G.T., Templeton R.C., Demarco A., Scrivens W.A., Englehart B.A. Influence of sediment—Amendment with single-walled carbon nanotubes and diesel soot on bioaccumulation of hydrophobic organic contaminants by benthic invertebrates. Environ. Sci. Technol. 2008;42:3879–3885. doi: 10.1021/es702830b. [DOI] [PubMed] [Google Scholar]
- 196.Zhu X., Zhou J., Cai Z. TiO2 nanoparticles in the marine environment: Impact on the toxicity of tributyltin to abalone (Haliotis diversicolor supertexta) embryos. Environ. Sci. Technol. 2011;45:3753–3758. doi: 10.1021/es103779h. [DOI] [PubMed] [Google Scholar]
- 197.Yu Z.G., Wang W.X. Influences of ambient carbon nanotubes on toxic metals accumulation in Daphnia magna. Water Res. 2013;47:4179–4187. doi: 10.1016/j.watres.2013.01.059. [DOI] [PubMed] [Google Scholar]
- 198.Trinh X.T., Choi J.S., Jeon H., Byun H.G., Yoon T.H., Kim J. Quasi-SMILES-Based Nano-Quantitative Structure–Activity Relationship Model to Predict the Cytotoxicity of Multiwalled Carbon Nanotubes to Human Lung Cells. Chem. Res. Toxicol. 2018;31:183–190. doi: 10.1021/acs.chemrestox.7b00303. [DOI] [PubMed] [Google Scholar]
- 199.Choi J.-S., Ha K.M., Trinh X.T., Yoon T.H., Byun H.G. Towards a generalized toxicity prediction model for metal oxide nanomaterials using integrated data from different sources. Sci. Rep. 2018;8:6110. doi: 10.1038/s41598-018-24483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Choi J.S., Trinh T.X., Yoon T.H., Kim J., Byun H.G. Quasi-QSAR for predicting the cell viability of human lung and skin cells exposed to different metal oxide nanomaterials. Chemosphere. 2019;217:243–249. doi: 10.1016/j.chemosphere.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 201.Oh E., Liu R., Nel A., Gemill K.B., Bilal M., Cohen Y., Medintz I.L. Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat. Nanotechnol. 2016;11:479–486. doi: 10.1038/nnano.2015.338. [DOI] [PubMed] [Google Scholar]
- 202.Trinh T.X., Ha M.K., Choi J.S., Byun H.G., Yoon T.H. Curation of datasets, assessment of their quality and completeness, and nanoSAR classification model development for metallic nanoparticles. Environ. Sci. Nano. 2018;5:1902–1910. doi: 10.1039/C8EN00061A. [DOI] [Google Scholar]
- 203.Burk J., Sikk L., Burk P., Manshian B.B., Soenen S.J., Scott-Fordsmand J.J., Tamm T., Tämm K. Fe-Doped ZnO nanoparticle toxicity: Assessment by a new generation of nanodescriptors. Nanoscale. 2018;10:21985–21993. doi: 10.1039/C8NR05220D. [DOI] [PubMed] [Google Scholar]
- 204.Tämm K., Sikk L., Burk J., Rallo R., Pokhrel S., Mädler L., Scott-Fordsmand J.J., Burk P., Tamm T. Parametrization of nanoparticles: Development of full-particle nanodescriptors. Nanoscale. 2016;8:16243–16250. doi: 10.1039/C6NR04376C. [DOI] [PubMed] [Google Scholar]
- 205.Économiques O. De Coopération et de Développement Guidance Document on the Validation of (Quantitative) Structure-Activity Relationship [(Q)Sar] Models. Volume 2. OECD; Paris, France: 2007. pp. 1–154. [DOI] [Google Scholar]
- 206.Raunio H. In silico toxicology non-testing methods. Front. Pharmacol. 2011;2:1–8. doi: 10.3389/fphar.2011.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Économiques O. De Coopération et de Développement Test No. 211: Daphnia magna Reproduction Test. OECD; Paris, France: 2012. [DOI] [Google Scholar]
- 208.Économiques O. De Coopération et de Développement Test No. 236: Fish Embryo Acute Toxicity (FET) Test. OECD; Paris, France: 2013. pp. 1–22. [DOI] [Google Scholar]
- 209.OECD Test Guideline No. 212: Fish, Short-term Toxicity Test on Embryo and Sac-fry Stages. Organisation for Economic Cooperation and Development. Volume 212. OECD; Paris, France: 1998. pp. 1–20. [DOI] [Google Scholar]
- 210.Vinken M. Omics-based input and output in the development and use of adverse outcome pathways. Curr. Opin. Toxicol. 2019;18:8–12. doi: 10.1016/j.cotox.2019.02.006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article or supplementary material.