Abstract
Liver fibrosis predicts liver-related and cardiovascular outcomes in chronic liver disease patients. We compared the diagnostic performance of various liver fibrosis biomarkers for identifying histological significant/advanced fibrosis. Additionally, the correlations of such liver fibrosis biomarkers with cardiovascular risk (CVR) scores were evaluated. 173 patients with viral hepatitis (157 HCV and 16 HBV) and 107 with a non-alcoholic fatty liver disease (NAFLD) were consecutively enrolled. Various liver fibrosis biomarkers: aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio (ARR), AST to Platelet Ratio Index (APRI), Fibrosis-4 (FiB-4), Forns index, NAFLD fibrosis score (NFS), BARD (body mass index (BMI), AAR, Diabetes) score, and Hepamet fibrosis score (HFS), were used to identify significant/advanced fibrosis. CVR was assessed by using the SCORE, the Progetto CUORE, or the Framingham risk scoring systems. Liver fibrosis biomarkers performed better in predicting advanced rather than significant liver fibrosis in all patients, regardless of chronic liver disease aetiology. Forns index and HFS performed best in predicting advanced fibrosis in patients with viral chronic liver disease and NAFLD. Lower cut-offs of these liver fibrosis biomarkers had high negative predictive values for advanced fibrosis overall, as well as in patients with NAFLD or viral chronic liver disease. FIB-4, Forns index, NFS, and HFS were positively correlated with SCORE and Framingham risk scores. In conclusion, liver fibrosis biomarkers accurately exclude advanced fibrosis and positively correlate with CVR scores in patients with chronic liver disease.
Keywords: accuracy, aminotransferase, liver biopsy, liver enzymes, major cardiovascular events, mortality, NASH, fibrosis, non-invasive, sensitivity, specificity
1. Introduction
Liver fibrosis defines the final result of repeated bouts of hepatocellular necrosis and liver inflammation, irrespective of the underlying aetiology of chronic liver disease [1]. Combined, metabolic and viral aetiologies account for a large proportion of fibrosing chronic liver disease globally [2]. The prototypic chronic liver disease of metabolic aetiology is Nonalcoholic Fatty Liver Disease (NAFLD). In many areas of the world NAFLD—which spans steatosis through non-alcoholic steatohepatitis (NASH) with/without fibrosis, cirrhosis, and hepatocellular carcinoma (HCC)—is the most common chronic liver disease [3] and its prevalence is escalating in parallel with the surge of diabesity [4,5]. Although the worldwide prevalence of viral chronic liver disease owing to infection with Hepatitis B (HBV) and C (HCV) viruses is declining thanks to effective preventive (HBV) and treatment strategies (HCV), viral chronic liver disease still continues to account for significant morbidity and mortality worldwide [6].
Liver fibrosis is a key determinant of the physiopathology and the natural course of both NAFLD and viral chronic liver disease, in as much as it progressively distorts normal hepatic architecture and, thereby, may eventually be conducive to hepatic insufficiency, portal hypertension, and HCC [1]. Further to these liver-related complications, the severity of liver fibrosis also dictates the risk of extra-hepatic cardio-metabolic complications [7,8,9,10,11,12,13]. Similar to NAFLD, HCV infection is increasingly identified as a systemic disease that may promote metabolic derangements and premature atherosclerosis [14,15].
On these grounds, the identification of subjects with significant/advanced fibrosis is key to implementing adequate treatment schedules and follow-up of patients with chronic liver disease [16,17,18]. Liver biopsy, which is the reference standard for the diagnosis and staging of hepatic fibrosis may cause patient discomfort and major complications [19,20]. Moreover, it may be exposed to sampling errors and diagnostic inaccuracies as a result of fibrosis being unevenly distributed throughout the liver tissue [18]. Therefore, liver biopsy should be reserved only for selected patients at risk of progressive liver disease [19,21]. The notion that they are clinically useful for helping in staging disease progression justifies the research of non-invasive biomarkers of liver fibrosis [19,21,22].
Widely available and inexpensive biomarkers of liver fibrosis include AST-to-ALT ratio (AAR), AST-to platelet ratio index (APRI), Fibrosis-4 (FIB-4), and Forns index, all of which have been applied in patients with chronic liver disease owing to different aetiologies [21,23]. Other non-invasive scores, such as NAFLD fibrosis score (NFS) and BARD (BMI, AAR, Diabetes), have specifically been generated for patients with NAFLD [24]. Generally speaking, these liver fibrosis biomarkers have shown better accuracy in excluding rather than in identifying significant/advanced fibrosis [21,23,25]. A new non-invasive fibrosis scoring system, namely the Hepamet fibrosis score (HFS), recently proposed by Ampuero et al. [26], has shown greater accuracy than the FIB-4 and NFS scores in identifying patients with NAFLD and advanced fibrosis.
In this complex scenario, the present study aims at comparing the diagnostic performance of various liver fibrosis biomarkers for identifying significant/advanced fibrosis on liver histology and their correlations with cardiovascular risk (CVR) scores in a sample of consecutive biopsied patients with either metabolic (NAFLD) or viral chronic liver disease.
2. Results
2.1. Characteristics of Study Cohort
The demographic, anthropometric, metabolic and histological characteristics of the study population are shown in Table 1. Overall, 66% were males, the mean age was 47.6 ± 11.5 years, and the mean BMI was 27.5 ± 4.5 Kg/m2. Among 280 patients with chronic liver disease, 107 had NAFLD (30 of whom had simple steatosis, and 77 had NASH), while 173 had viral hepatitis: 157 of whom had HCV (109 naïve and 48 treatment-experienced) and 16 had HBV (all treatment-naïve). None of the patients with viral chronic liver disease underwent previous treatment with direct acting anti-viral agents. One-hundred patients (36%) had significant fibrosis (42% NAFLD; 32% viral chronic liver disease) while thirty-eight patients (14%) had advanced fibrosis (13% NAFLD; 14% viral chronic liver disease) on liver biopsy. Considering NAFLD severity, the prevalence of significant fibrosis was 7% in simple steatosis and 56% in NASH while that of advanced fibrosis was 0% in simple steatosis and 18% in NASH. Histological fibrosis stages were distributed as follows: F0 33 (30.8%), F1 29 (27.1%), F2 31 (29.0%), F3 9 (8.4%), F4 5 (4.7%) according to Brunt/Kleiner et al. [27,28] in NAFLD (F0 21 (70.0%), F1 7 (23.3%), F2 2 (6.7%), F3 0 (0.0%), F4 0 (0.0%) in simple steatosis; F0 12 (15.6%), F1 22 (28.6%), F2 29 (37.6%), F3 9 (11.7%), F4 5 (6.5%) in NASH); F0 18 (10.4%), F1 57 (32.9%), F2 43 (24.9%), F3 31 (17.9%), F4 4 (2.3%), F5 15 (8.7%), F6 5 (2.9%) according to Ishak et al. [29] in viral chronic liver disease.
Table 1.
Characteristics of the study population.
| All (n = 280) |
Viral CLD * (n = 173) |
NAFLD (n = 107) |
p † | |
|---|---|---|---|---|
| Biometrics | ||||
| Age (years) | 47.6 ± 11.5 | 47.7 ± 11.3 | 47.6 ± 11.9 | 0.983 |
| Male/Female ratio (n, %) | 185/95 (66/34) | 108/65 (62/38) | 77/30 (72/28) | 0.119 |
| BMI (kg/m2) | 27.5 ± 4.5 | 26.0 ± 3.9 | 29.5 ± 4.5 | <0.001 |
| Waist Circumference (cm) | 97.1 ± 12.3 | 91.0 ± 9.9 | 101.8 ± 11.9 | <0.001 |
| Hypertension (n, %) | 82 (29) | 44 (26) | 38 (36) | 0.081 |
| Type 2 diabetes (n, %) | 46 (16) | 13 (8) | 33 (31) | <0.001 |
| Metabolic syndrome (n, %) | 77 (28) | 21 (12) | 56 (52) | <0.001 |
| CVR scores | ||||
| SCORE | 0.5 (0.1 ÷ 1.4) | 0.4 (0.1 ÷ 1.4) | 0.7 (0.2 ÷ 1.7) | 0.052 |
| FRS | 7.3 (3.6 ÷ 14.8) | 6.7 (3.6 ÷ 13.4) | 8.7 (4.0 ÷ 19.9) | 0.026 |
| Progetto CUORE | 1.9 (0.8 ÷ 4.8) | 1.5 (0.6 ÷ 4.1) | 2.5 (1.1 ÷ 6.0) | 0.002 |
| Laboratory | ||||
| Platelet count (×103/mm3) | 212.6 ± 61.4 | 206.2 ± 61.3 | 223.0 ± 60.2 | 0.025 |
| Fasting glucose (mg/dL) | 95.0 (87.0 ÷ 103.0) | 93.5 (85.0 ÷ 100.0) | 98.0 (91.0 ÷ 116.0) | <0.001 |
| Fasting insulin (mIU/L) | 10.7 (7.2 ÷ 15.8) | 9.6 (7.1 ÷ 15.7) | 11.5 (8.0 ÷ 15.9) | 0.244 |
| HOMA-IR score | 2.6 (1.6 ÷ 4.0) | 2.4 (1.5 ÷ 3.8) | 2.9 (1.9 ÷ 4.3) | 0.148 |
| AST (U/L) | 39.7 (28.0 ÷ 58.9) | 43.0 (32.0 ÷ 69.7) | 33.0 (26.0 ÷ 49.0) | <0.001 |
| ALT (U/L) | 41.5 (64.5 ÷ 119.7) | 71.0 (44.0 ÷ 130.2) | 58.0 (40.0 ÷ 91.0) | 0.019 |
| GGT (U/L) | 41.0 (27.4 ÷ 76.8) | 40.0 (24.0 ÷ 62.1) | 49.0 (33.0 ÷ 109.0) | 0.003 |
| Albumin (g/dL) | 4.4 ± 0.5 | 4.4 ± 0.4 | 4.5 ± 0.5 | 0.052 |
| γ-globulin (mg/dL) | 1.3 ± 0.4 | 1.4 ± 0.4 | 1.1 ± 0.3 | <0.001 |
| Total Cholesterol (mg/dL) | 186.7 ± 44.8 | 171.8 ± 37.6 | 210.8 ± 45.1 | <0.001 |
| HDL-Cholesterol (mg/dL) | 46.4 ± 13.9 | 48.3 ± 14.2 | 44.4 ± 13.5 | 0.049 |
| LDL-Cholesterol (mg/dL) | 118.9 ± 39.5 | 102.3 ± 32.5 | 135.2 ± 39.1 | <0.001 |
| Triglycerides (mg/dL) | 69.0 (97.0 ÷ 147.0) | 82.0 (62.0 ÷ 113.0) | 146.0 (92.0 ÷ 228.0) | <0.001 |
| Serum uric acid (mg/dL) | 5.3 ± 1.4 | 4.8 ± 1.2 | 6.0 ± 1.5 | <0.001 |
| Ferritin (mg/dL) | 150.0 (79.0 ÷ 252.5) | 134.0(64.0 ÷ 252.5) | 178.0 (98.5 ÷ 258.3) | 0.036 |
| Liver histology | ||||
| Steatosis ≥ 5% (n, %) | 184 (66) | 75 (43) | 107 (100) | <0.001 |
| Significant fibrosis (n, %) | 100 (36) | 55 (32) | 45 (42) | 0.095 |
| Advanced fibrosis (n, %) | 38 (14) | 24 (14) | 14 (13) | 1.000 |
| Cirrhosis (n, %) | 10 (4) | 5 (3) | 5 (5) | 0.513 |
| Liver fibrosis biomarkers | ||||
| AAR | 0.62 (0.49 ÷ 0.73) | 0.64 (0.52 ÷ 0.74) | 0.56 (0.46 ÷ 0.69) | 0.002 |
| APRI | 0.57 (0.35 ÷ 0.88) | 0.64 (0.40 ÷ 1.08) | 0.43 (0.30 ÷ 0.69) | <0.001 |
| FIB-4 | 1.05 (0.72 ÷ 1.65) | 1.21 (0.82 ÷ 1.92) | 0.84 (0.63 ÷ 1.29) | <0.001 |
| Forns | 4.9 ± 1.8 | 5.1 ± 1.8 | 4.5 ± 1.8 | 0.019 |
* HCV: n = 157; HBV: n = 16. † NAFLD vs. viral CLD. Data are expressed as means (±SD) for continuous variables normally distributed or as medians (25–75th percentile) for those not normally distributed, and as frequencies (percentages) for categorical variables. AAR, AST to ALT ratio; ALT, alanine aminotransferase; APRI, AST to Platelet Ratio Index; AST, aspartate aminotransferase; BMI, Body mass index; CLD, chronic liver disease; CVR, cardiovascular risk; FIB-4, fibrosis 4; FRS, Framingham risk score; GGT, gamma-glutamyltransferase; HBV, hepatitis B virus; HCV, hepatitis C virus; HDL-C, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; NAFLD, non-alcoholic fatty liver disease.
As reported in Table 1, when compared to those with viral chronic liver disease; patients with NAFLD were more likely to be overweight/obese, had a higher prevalence of diabetes and metabolic syndrome, as well as higher values of CVR scores (SCORE, Framingham risk score (FRS), Progetto CUORE), platelets, fasting glucose, fasting insulin, gamma-glutamyltransferase (GGT), total cholesterol, low-density lipoprotein (LDL)-cholesterol, triglycerides, serum uric acid, and ferritin. By contrast, these patients had lower values of aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-globulins, and high-density lipopoprotein (HDL)-cholesterol. Age, sex, prevalence of hypertension, fasting insulin, Homeostasis Model Assessment (HOMA)-estimated insulin resistance (IR), and albumin did not differ between the two groups of patients. Regarding histological features, as shown in Table 1, patients with NAFLD showed a greater prevalence of steatosis ≥5% than those with viral chronic liver disease. Significant/advanced fibrosis and prevalence of cirrhosis did not differ between the two groups. With regard to liver fibrosis biomarkers, patients with NAFLD had significantly lower values of AAR, APRI, FIB-4 score, and Forns when compared to those with viral chronic liver disease.
Clinical and laboratory characteristics of patients with and without significant/advanced fibrosis in the whole population and according to the aetiology of chronic liver disease are shown in Supplementary Tables S1–S4. Clinical and laboratory characteristics of NAFLD patients with and without NASH are shown in Supplementary Table S5.
2.2. Diagnostic Performance of Liver Fibrosis Biomarkers
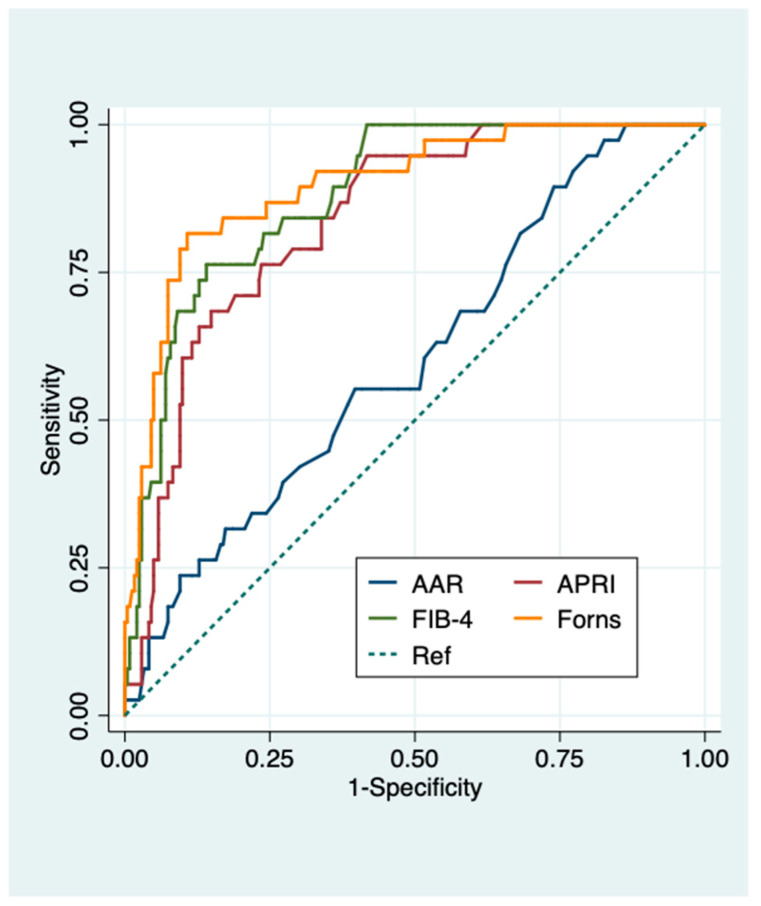
The diagnostic performance of liver fibrosis biomarkers (AAR, APRI, FIB-4, Forns) for predicting significant and advanced fibrosis was assessed in the whole population (Supplementary Table S6 and Table 2). Areas under the receiver operating characteristic (AUROCs) (95% confidence intervals (CI)) showed that liver fibrosis biomarkers were more accurate in predicting advanced rather than significant fibrosis (0.60 (0.51–0.70) vs 0.44 (0.40–0.51) for AAR; 0.84 (0.78–0.90) vs 0.68 (0.62–0.75) for APRI; 0.88 (0.83–0.93) vs 0.66 (0.59–0.73) for Fib-4; 0.90 (0.84–0.95) vs 0.69 (0.63–0.76) for Forns index) (Figure 1 and Supplementary Figure S1). FIB-4 using cut-off >3.25 and Forns index using cut-off <4.2 showed the best positive predictive value (PPV) (81.8%) and negative predictive value (NPV) (79.4%) for significant fibrosis, respectively. APRI, FIB-4 and Forns index using their lower cut-offs performed optimally to exclude (NPVs 98.4, 96.3 and 99.1%, respectively), rather than predict advanced fibrosis (PPVs 48.3, 63.6 and 75.7%, respectively) (Supplementary Table S6 and Table 2).
Table 2.
Diagnostic performance of liver fibrosis biomarkers for predicting advanced liver fibrosis in the whole population (n = 280).
| Cut-Offs | SE | SP | PPV | NPV | ACC | LR+ | LR− | AUROC (95% CI) |
|
|---|---|---|---|---|---|---|---|---|---|
| AAR | ≥0.8 | 26.3 | 84.3 | 20.8 | 87.9 | 76.4 | 1.68 | 0.87 | 0.60 (0.51–0.70) |
| >1 | 13.2 | 93.8 | 25.0 | 87.3 | 82.9 | 2.12 | 0.93 | ||
| APRI | >0.5 | 94.7 | 49.2 | 22.6 | 98.4 | 55.4 | 1.86 | 0.11 | 0.84 (0.78–0.90) |
| >1 | 63.2 | 87.2 | 43.6 | 93.8 | 83.9 | 4.93 | 0.42 | ||
| >1.5 | 36.8 | 93.8 | 48.3 | 90.4 | 86.1 | 5.94 | 0.67 | ||
| FIB-4 | ≥1.45 | 81.6 | 74.4 | 33.3 | 96.3 | 75.4 | 3.18 | 0.25 | 0.88 (0.83–0.93) |
| >3.25 | 36.8 | 96.7 | 63.6 | 90.7 | 88.6 | 11.15 | 0.65 | ||
| Forns | ≥4.2 | 97.4 | 43.8 | 21.4 | 99.1 | 51.1 | 1.73 | 0.06 | 0.90 (0.84–0.95) |
| >6.9 | 40.6 | 95.7 | 75.7 | 83.1 | 82.1 | 9.51 | 0.62 |
Advanced fibrosis was defined as histologic liver fibrosis ≥ F4 according to Ishak et al. [29] for viral chronic liver disease, and ≥F3 according to Brunt/Kleiner et al. for NAFLD [27,28]. AAR, AST to ALT ratio; ACC, accuracy; ALT, alanine aminotransferase; APRI, AST to Platelet Ratio Index; AST, aspartate aminotransferase; AUROC, area under the receiver operating characteristics; BLF, biomarkers of liver fibrosis; Fib-4, fibrosis 4; LR, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value; SE, sensitivity; SP, specificity.
Figure 1.
Diagnostic performance of serum biomarkers for predicting advanced fibrosis in all patients. Receiver operating characteristic (ROC) curves of serum biomarkers for the diagnosis of advanced fibrosis in all patients. AUROC (95% CI): 0.60 (0.51–0.70) for AAR, 0.84 (0.78–0.90) for APRI, 0.88 (0.83–0.93) for FIB-4, 0.90 (0.84–0.95) for Forns index. Advanced fibrosis was defined as histologic liver fibrosis ≥ F4 according to Ishak et al. [29] for viral chronic liver disease, and ≥F3 according to Brunt/Kleiner et al. for NAFLD [27,28]. AAR, AST to ALT ratio; APRI, AST to Platelet Ratio Index; Fib-4, fibrosis 4.
The diagnostic performance of liver fibrosis biomarkers for predicting significant (Supplementary Tables S7 and S8) and advanced fibrosis (Table 3 and Table 4) was then assessed in patients with chronic liver disease owing to different aetiologies, i.e., viral and NAFLD.
Table 3.
Diagnostic performance of liver fibrosis biomarkers for predicting advanced fibrosis in patients with viral chronic liver disease (n = 173).
| Cut-Offs | SE | SP | PPV | NPV | ACC | LR+ | LR− | AUROC (95% CI) |
|
|---|---|---|---|---|---|---|---|---|---|
| AAR | ≥0.8 | 20.8 | 81.9 | 15.6 | 86.5 | 73.4 | 1.15 | 0.97 | 0.56 (0.44–0.68) |
| >1 | 8.3 | 92.6 | 15.4 | 86.3 | 80.9 | 1.13 | 0.99 | ||
| APRI | >0.5 | 95.8 | 38.9 | 20.2 | 98.3 | 46.8 | 1.57 | 0.11 | 0.84 (0.76–0.92) |
| >1 | 79.2 | 82.6 | 42.2 | 96.1 | 82.1 | 4.54 | 0.25 | ||
| >1.5 | 50.0 | 90.6 | 46.2 | 91.8 | 85.0 | 5.32 | 0.55 | ||
| FIB-4 | ≥1.45 | 87.5 | 67.1 | 30.0 | 97.1 | 69.9 | 2.66 | 0.19 | 0.88 (0.82–0.95) |
| >3.25 | 37.5 | 96.0 | 60.0 | 90.5 | 87.9 | 9.31 | 0.65 | ||
| Forns | ≥4.2 | 95.8 | 36.9 | 19.7 | 98.2 | 45.1 | 1.52 | 0.11 | 0.89 (0.82–0.97) |
| >6.9 | 66.7 | 92.6 | 59.3 | 94.5 | 89.0 | 9.03 | 0.36 |
Advanced fibrosis was defined as histologic liver fibrosis ≥ F4 according to Ishak et al. [29]. AAR, AST to ALT ratio; ACC, accuracy; ALT, alanine aminotransferase; APRI, AST to Platelet Ratio Index; AST, aspartate aminotransferase; AUROC, area under the receiver operating characteristics; BLF, biomarkers of liver fibrosis; CI, confidence intervals; FIB-4, fibrosis 4; LR, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value; SE, sensitivity; SP, specificity.
Table 4.
Diagnostic performance of liver fibrosis biomarkers for predicting advanced fibrosis in patients with NAFLD (n = 107).
| Cut-Offs | SE | SP | PPV | NPV | ACC | LR+ | LR− | AUROC (95% CI) |
|
|---|---|---|---|---|---|---|---|---|---|
| AAR | ≥0.8 | 35.7 | 88.2 | 31.3 | 90.1 | 81.3 | 3.02 | 0.73 | 0.66 (0.50–0.82) |
| >1 | 21.4 | 95.7 | 42.9 | 89.0 | 86.0 | 4.98 | 0.82 | ||
| APRI | >0.5 | 92.9 | 65.6 | 28.9 | 98.4 | 69.2 | 2.70 | 0.11 | 0.87 (0.79–0.95) |
| >1 | 35.7 | 94.6 | 50.0 | 90.7 | 86.9 | 6.64 | 0.68 | ||
| >1.5 | 14.3 | 98.9 | 66.7 | 88.5 | 87.9 | 13.29 | 0.87 | ||
| Fib-4 | ≥1.3 | 78.6 | 83.9 | 42.3 | 96.3 | 83.2 | 4.87 | 0.26 | 0.91 (0.84–0.97) |
| ≥1.45 | 71.4 | 86.0 | 43.5 | 95.2 | 84.1 | 5.11 | 0.33 | ||
| >2.67 | 35.7 | 96.8 | 62.5 | 90.9 | 88.8 | 11.07 | 0.66 | ||
| >3.25 | 35.7 | 97.9 | 71.4 | 91.0 | 89.7 | 16.61 | 0.66 | ||
| Forns | ≥4.2 | 100.0 | 54.8 | 25.0 | 100.0 | 60.8 | 2.21 | 0.00 | 0.92 (0.85–0.99) |
| >6.9 | 42.9 | 95.7 | 60.0 | 91.8 | 88.8 | 9.96 | 0.60 | ||
| NFS | ≥−1.455 | 85.7 | 64.5 | 26.7 | 96.8 | 67.3 | 2.42 | 0.22 | 0.89 (0.81–0.97) |
| >0.675 | 42.9 | 96.8 | 66.7 | 91.8 | 89.7 | 13.29 | 0.59 | ||
| BARD | ≥2 | 78.6 | 75.3 | 32.4 | 95.9 | 75.7 | 3.18 | 0.29 | 0.79 (0.66–0.93) |
| HFS | ≥0.12 | 78.6 | 89.3 | 52.4 | 96.5 | 87.9 | 7.31 | 0.24 | 0.94 (0.90–0.99) |
| >0.47 | 50.0 | 96.8 | 70.0 | 92.8 | 90.7 | 15.5 | 0.52 |
Advanced fibrosis was defined as histologic liver fibrosis ≥ F3 according to Brunt/Kleiner et al. [27,28]. Abbreviations: AAR, AST to ALT ratio; ACC, accuracy; ALT, alanine aminotransferase; APRI, AST to Platelet Ratio Index; AST, aspartate aminotransferase; AUROC, area under the receiver operating characteristics; BARD, BMI AST/ALT Ratio Diabetes; BLF, biomarkers of liver fibrosis; CI, confidence intervals; FIB-4, fibrosis 4; HFS, Hepamet fibrosis score; LR, likelihood ratio; NFS, NAFLD fibrosis score; NPV, negative predictive value; PPV, positive predictive value; SE, sensitivity; SP, specificity.
Forns index (0.77 (0.69–0.85)) and APRI (0.69 (0.59–0.80)) had the best AUROCs (95% CI) for predicting significant fibrosis in viral chronic liver disease and NAFLD patients, respectively (Supplementary Figures S2 and S3). AUROCs (95%CI) of all general liver fibrosis biomarkers (AAR, APRI, Fib-4, Forns index) showed a superior accuracy in predicting significant fibrosis among patients with viral chronic liver disease than among those with NAFLD (0.48 vs. 0.39 for AAR; 0.73 vs. 0.69 for APRI; 0.73 vs. 0.61 for FIB-4; 0.77 vs. 0.62 for Forns index). APRI and Forns index using their lower cut-offs showed the best NPV (84.8 and 89.3%) for significant fibrosis in viral chronic liver disease patients while all the scores performed poorly (NPVs < 70%) to exclude significant fibrosis in NAFLD patients (Supplementary Tables S7 and S8). FIB-4 and HFS using higher cut-offs had the best PPV for significant fibrosis in viral chronic liver disease and NAFLD (85.7 and 90.0%, respectively). Forns index showed the best combination of NPV (89.3%) and NPV (81.5%) for significant fibrosis in viral chronic liver disease patients (Supplementary Tables S7 and S8).
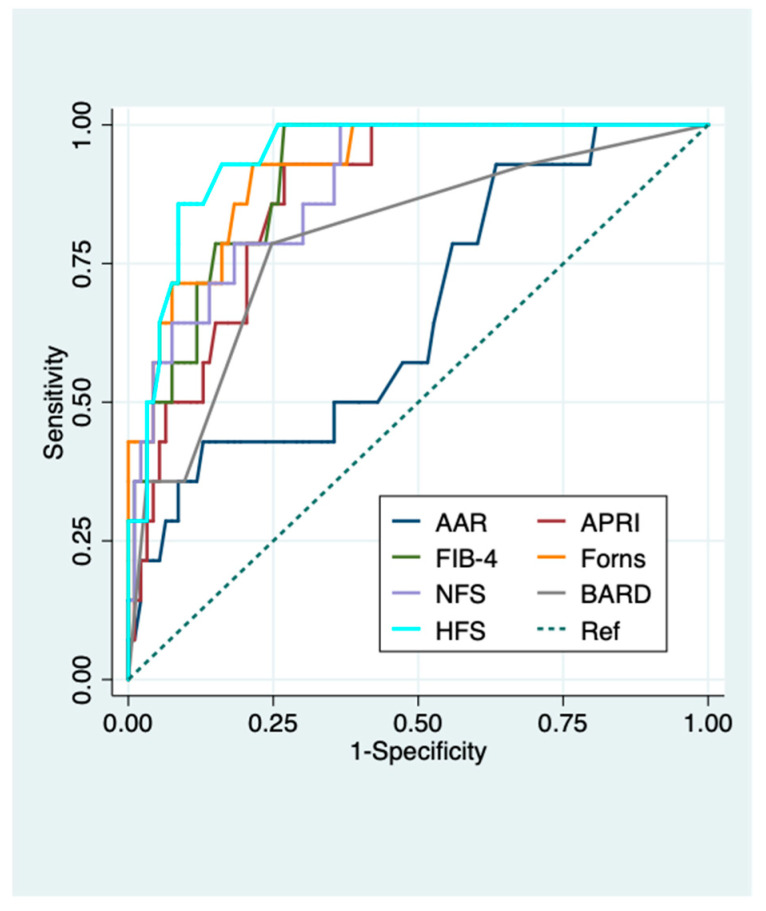
Forns index (0.89(0.82–0.97)) and HFS (0.94 (0.90–0.99)) had the best AUROCs (95% CI) in predicting advanced fibrosis in viral chronic liver disease and NAFLD patients, respectively (Figure 2 and Figure 3).
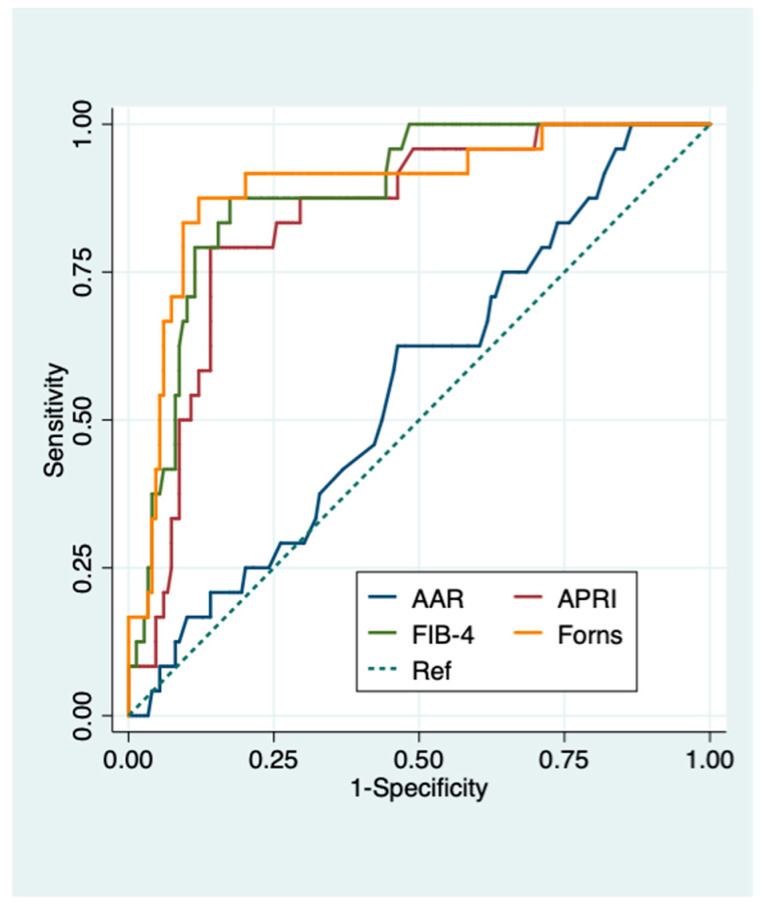
Figure 2.
Diagnostic performance of serum biomarkers for predicting advanced fibrosis in viral chronic liver disease patients. ROC curves of serum biomarkers for the diagnosis of advanced fibrosis in viral chronic liver disease patients. AUROC (95% CI): 0.56 (0.44–0.68) for AAR, 0.84 (0.76–0.92) for APRI, 0.88 (0.82–0.95) for FIB-4, 0.89 (0.82–0.97) for Forns index. Advanced fibrosis was defined as histologic liver fibrosis ≥ F4 according to Ishak et al. [29]. AAR, AST to ALT ratio; APRI, AST to Platelet Ratio Index; Fib-4, fibrosis 4.
Figure 3.
Diagnostic performance of serum biomarkers for predicting advanced fibrosis in NAFLD patients. ROC curves of serum biomarkers for the diagnosis of advanced fibrosis in NAFLD patients. AUROC (95% CI): 0.66 (0.50–0.82) for AAR, 0.87 (0.79–0.95) for APRI, 0.91 (0.84–0.97) for FIB-4, 0.92 (0.85–0.99) for Forns index, 0.89 (0.81–0.97) for NFS, 0.94 (0.90–0.99) for HFS, 0.79 (0.66–0.93) for BARD. Advanced fibrosis was defined as histologic liver fibrosis ≥F3 according to Brunt/Kleiner et al. for NAFLD [27,28]. AAR, AST to ALT ratio; APRI, AST to Platelet Ratio Index; BARD, BMI AST/ALT Ratio Diabetes; Fib-4, fibrosis 4; HFS, Hepamet fibrosis score; NFS, NAFLD fibrosis score.
AUROCs (95 CI%) of APRI, FIB-4 and Forns index all proved highly accurate in predicting advanced fibrosis with slightly better performance in patients with NAFLD compared to those with viral chronic liver disease (0.87 (0.79–0.95) vs. 0.84 (0.76–0.92) for APRI; 0.91 (0.84–0.97) vs. 0.88 (0.82–0.95) for FIB-4; 0.92 (0.85–0.99) vs. 0.89 (0.82–0.97) for Forns index). AAR performed poorly in predicting both significant and advanced fibrosis in the whole population and in patients with different chronic liver diseases (AUROCs (95%CI): 0.44 (0.40–0.51) and 0.60 (0.51–0.70) overall, 0.48 (0.38–0.57) and 0.56 (0.44–0.68) viral chronic liver disease, 0.39 (0.28–0.51) and 0.66 (0.50–0.82) NAFLD for significant and advanced fibrosis, respectively) (Supplementary Figures S1–S3 and Figure 1, Figure 2 and Figure 3). FIB-4, APRI, and Forns index using their lower cut-offs all showed excellent NPVs (95–100%) for advanced fibrosis in both NAFLD and viral chronic liver disease patients (Table 3 and Table 4). NFS, HFS, and BARD also had excellent NPVs (96–97%) for advanced fibrosis in NAFLD patients. Conversely, PPVs of all liver fibrosis biomarkers for advanced fibrosis were modest with FIB-4 showing the best PPV in both NAFLD and viral chronic liver disease patients (71.4% and 60%, respectively) (Table 3 and Table 4).
The finding that all liver fibrosis biomarkers, except for AAR, had NPVs greater than 95% for excluding advanced fibrosis using their lower cut-offs, implies that a significant proportion of liver biopsies ranging from 38% to 67% in our overall series, 32% to 74% in viral chronic liver disease and 48% to 80% in NAFLD patients could have been avoided with a negligible rate of false negatives (0–4%) using APRI, FIB-4, Forns, NFS, BARD, and HFS (Table 5).
Table 5.
Proportion of chronic liver disease patients without advanced fibrosis by liver fibrosis biomarkers who could have potentially avoided liver biopsy.
| All | Viral CLD | NAFLD | |||||
|---|---|---|---|---|---|---|---|
| Cut-Offs | Patients, n (%) |
FN, n (%) |
Patients, n (%) |
FN, n (%) |
Patients, n (%) |
FN, n (%) |
|
| AAR | <0.8 | 232/280 (83) | 28 (12) | 141/173 (82) | 19 (14) | 91/107 (85) | 9 (10) |
| ≤1 | 260/280 (93) | 33 (13) | 160/173 (93) | 22 (14) | 100/107 (94) | 11 (11) | |
| APRI | <0.5 | 121/280 (52) | 2 (2) | 59/173 (34) | 1 (2) | 62/107 (58) | 1 (2) |
| <0.7 | 177/280 (63) | 8 (5) | 93/173 (54) | 3 (3) | 84/107 (79) | 5 (6) | |
| <1 | 225/280 (80) | 14 (6) | 128/173 (74) | 5 (4) | 97/107 (91) | 9 (9) | |
| FIB-4 | <1.3 | 173/280 (62) | 6 (4) | 92/173 (53) | 3 (3) | 81/107 (76) | 3 (4) |
| <1.45 | 187/280 (67) | 7 (4) | 103/173 (60) | 3 (3) | 84/107 (79) | 4 (5) | |
| Forns | <4.2 | 107/280 (38) | 1 (1) | 56/173 (32) | 1 (2) | 51/107 (48) | 0 (0) |
| NFS | <−1.455 | _ | _ | _ | _ | 62/107 (58) | 2 (3) |
| BARD | <2 | _ | _ | _ | _ | 73/107 (68) | 3 (4) |
| HFS | <0.12 | _ | _ | _ | _ | 86/107 (80) | 3 (4) |
AAR, AST to ALT ratio; ALT, alanine aminotransferase; APRI, AST to Platelet Ratio Index; AST, aspartate aminotransferase; BARD, BMI AST/ALT Ratio Diabetes; BLF, biomarkers of liver fibrosis; CLD, chronic liver disease; FIB-4, fibrosis-4; FN, false negative; HFS, Hepamet fibrosis score; NAFLD, nonalcoholic fatty liver disease; NFS, NAFLD fibrosis score.
2.3. Associations of Liver Fibrosis Biomarkers with Cardiovascular Risk Scoring Systems
Correlations between CVR scores (SCORE, Progetto CUORE, and FRS) and liver fibrosis biomarkers are shown in Table 6. All liver fibrosis biomarkers were significantly correlated with CVR scores, except for APRI with FRS, in the whole series, and with Progetto CUORE and FRS in patients with viral chronic liver disease. The best univariate correlations with SCORE were shown by FIB-4 and Forns index in the whole population (r = 0.462 and 0.468, respectively), as well as in patients with viral chronic liver disease (r = 0.476 and 0.454, respectively) and by NFS and Forns in those with NAFLD (r = 0.593 and 0.544, respectively). The best correlations with Progetto CUORE were shown by FIB-4 and Forns index in the whole population (r = 0.402 and 0.437, respectively), as well as in patients with viral chronic liver disease (r = 0.399 and 0.370, respectively) and by NFS and HFS in those with NAFLD (r = 0.658 and 0.643, respectively). FIB-4 and Forns index also had the best correlation with FRS in the whole population (r = 0.343 and 0.454, respectively) and in patients with viral chronic liver disease (r = 0.319 and 0.464, respectively). NFS and HFS showed the best correlation with FRS in NAFLD patients (r = 0.702 and 0.679, respectively). NFS showed the best correlation with all CVR in NAFLD patients. Supplementary Tables S1–S4 show that CVR scores tend to be higher among patients with either significant or advanced fibrosis compared to those individuals with either absent-to-mild or moderate histological fibrosis indirectly supporting the notion that liver fibrosis is associated with CVR.
Table 6.
Correlations between liver fibrosis biomarkers and three different cardiovascular risk (CVR) scores.
| SCORE | Progetto CUORE | FRS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Viral CLD | NAFLD | All | Viral CLD | NAFLD | All | Viral CLD | NAFLD | |
| AAR | 0.302 * | 0.261 † | 0.406 * | 0.302 * | 0.340 * | 0.325 † | 0.252 * | 0.166 § | 0.401 * |
| APRI | 0.208 * | 0.234 † | 0.260 * | 0.145 § | 0.156 | 0.375 * | 0.114 | 0.124 | 0.407 * |
| FIB-4 | 0.462 * | 0.476 * | 0.499 * | 0.402 * | 0.399 * | 0.560 * | 0.342 * | 0.319 * | 0.584 * |
| Forns | 0.468 * | 0.454 * | 0.544 * | 0.437 * | 0.370 * | 0.568 * | 0.445 * | 0.453 * | 0.532 * |
| NFS | _ | _ | 0.593 * | _ | _ | 0.658 * | _ | _ | 0.702 * |
| BARD | _ | _ | 0.449 * | _ | _ | 0.471 * | _ | _ | 0.540 * |
| HFS | _ | _ | 0.509 * | _ | _ | 0.643 * | _ | _ | 0.679 * |
Data are expressed as Pearson’s r correlation coefficients for all correlations except for those with BARD score which are expressed as Spearman’s rho (ρ). * p < 0.001 † p < 0.005 § p < 0.05. The absence of any symbols denotes “p not significant”. Abbreviations: AAR, AST to ALT ratio; ALT, alanine aminotransferase; APRI, AST to Platelet Ratio Index; AST, aspartate aminotransferase; BARD, BMI AST/ALT Ratio Diabetes; CLD, chronic liver disease; CVR, cardiovascular risk; FIB-4, fibrosis-4; FRS, Framingham risk score; HFS, Hepamet fibrosis score; NAFLD, nonalcoholic fatty liver disease; NFS, NAFLD fibrosis score.
3. Discussion
The main findings of our study, involving a large sample of 280 well-characterized patients with biopsy-confirmed chronic liver disease owing to viral and metabolic aetiology with a low prevalence of advanced liver fibrosis, are as follows: (a) liver fibrosis biomarkers accurately exclude advanced fibrosis in both metabolic and viral chronic liver disease; (b) liver fibrosis biomarkers are well correlated with CVR scores assessing 10-year fatal and non-fatal cardiovascular disease (CVD) events.
3.1. Liver Fibrosis Biomarkers Accurately Exclude Advanced Fibrosis in Metabolic and Viral Chronic Liver Disease
In this study, liver fibrosis biomarkers performed better in excluding advanced fibrosis (assessed with NPVs) than previously reported in both NAFLD [30] and viral chronic liver disease patients [31,32,33]. Our data have shown that, overall, NPVs range 96–99%, 96–100% in NAFLD, and 97–98% in viral chronic liver disease compared to previous reported NPVs ranging 89–93% in NAFLD [30], and 73–95% in viral chronic liver disease [31,32,33]. In our study, the Forns index showed the highest NPV in excluding advanced fibrosis in patients with viral chronic liver disease (NPV 98%, as well as APRI) and also in those with NAFLD (NPV 100%) where it was first applied. Moreover, the present investigation, the first external validation of the new HFS, shows that HFS, compared to other liver fibrosis biomarkers, has good accuracy in excluding advanced fibrosis (NPV 96.5%) and the best diagnostic accuracy (AUROC 0.94) to differentiate F3–F4 from F0–F2 in NAFLD patients, in line with the results of Ampuero et al. [26]. Conversely, the Forns index showed the best diagnostic performance in predicting advanced fibrosis in patients with viral chronic liver disease (AUROC 0.89). Nevertheless, all liver fibrosis biomarkers in our study showed sub-optimal PPVs (≤76%) for detecting advanced fibrosis, such as reported by others [16,26,30,33,34,35,36,37,38] indicating that their clinical utility lies in ruling out rather than confirming advanced fibrosis. Recent studies suggest that “FAST” score, i.e., a combination of various indices obtained with Fibroscan (LSM, CAP), and AST may work excellently to this end [39].
Our study confirms that, among the various liver fibrosis biomarkers, HFS demonstrates the best diagnostic performance. This may be explained by the fact that HFS was originally developed on a large multi-centre international cohort [26]; its algorithm—at variance with other scores—is already adjusted for confounding variables such as older age (≥65 years) [40]; and in subjects without diabetes, it includes the calculation of HOMA-IR, a reliable marker of insulin resistance which is intimately associated with the development and progression of the whole NAFLD spectrum, including advanced fibrosis [41]. Data on the diagnostic accuracy of the Forns index in NAFLD patients are scant and limited to the prediction of significant fibrosis [34]. The Forns index is calculated based on cholesterol serum levels, which are negatively associated with the stage of liver fibrosis and may predict the diagnosis of NASH [41,42,43].
3.2. Clinical Implications of Findings: Proportion of Spared Biopsies and NAFLD Screening
Our findings support the notion that liver fibrosis biomarkers may be adopted in clinical practice to accurately exclude advanced liver fibrosis. This would allow avoiding a consistent number of liver biopsies: 40–60% in the whole population of patients using APRI, FIB-4 and Forns index lower cut-offs; 30–70% in those with the viral chronic liver disease using APRI, FIB-4, and Forns lower cut-offs and also APRI intermediate cut-offs; 50–80% in those with NAFLD using FIB-4, Forns index, NFS, BARD, and HFS lower cut-offs. In all groups, the false negative rate was very low (0–4%). Our results outperform those previously reported by McPherson et al. in a European NAFLD patient population featuring a 19% prevalence of advanced fibrosis. McPherson showed that 40–70% of biopsies were avoidable; with a 5–8% false negative rate [44]. Similarly, Treeprasertsuk et al. in Asian patients with NAFLD featuring a 6% prevalence of advanced fibrosis found that the proportion of potentially avoidable liver biopsies was 40–80% with 1–4% false negatives [45]. At variance with other studies, generally reporting a prevalence of advanced fibrosis ranging around 20% to 30% [26,33,35,37,44,46,47], our patient population was characterized by a lower prevalence of advanced fibrosis (14%) suggesting a more liberal use of liver biopsy during the years when our study was conducted.
Our results support the recommendations of current guidelines which suggest screening NAFLD patients with liver fibrosis biomarkers in order to identify those with either suspected advanced fibrosis or indeterminate values. It is these patients who should undergo further non-invasive assessments of liver fibrosis with sono-elastographic techniques [21,48,49] or liver biopsy—the only diagnostic modality remaining to accurately diagnose and stage the severity of NASH [25].
Research on diagnostic tools for non-invasive and accurate identification of NASH and severity of fibrosis is actively progressing and magnetic resonance elastography is the most promising; however, this imaging technique is very expensive and of limited availability [16,21] particularly, but not only, in developing countries. Another emerging technique is the semiquantitative/quantitative liver ultrasonography, which may be combined with other non-invasive tools, such as liver fibrosis biomarkers and sono-elastography [50,51].
3.3. Liver Fibrosis Biomarkers are Correlated with CVR Scores
A consistent body of literature supports the notion that the liver is a key modulator of CVR and that such a risk is associated with the histological features of liver disease both in NAFLD and in HCV infection [10,52,53,54,55,56,57,58]. Based on this robust rationale, our study is the first to report the correlation between the widely used liver fibrosis biomarkers and CVR scores, including the Progetto CUORE, which has specifically been developed for the Italian general population. Moderate liver fibrosis, although to a lesser extent than advanced fibrosis, has also been associated with increased mortality in patients with NAFLD but we cannot accurately rule this out with available non-invasive tests. Therefore, it has been suggested that patients without advanced fibrosis should actively be followed with a focus on metabolic co-morbidities in order to reduce CVR and should be retested after three years to identify those with disease progression [59].
Longitudinal studies have found that liver fibrosis biomarkers may predict the risk of mortality and CVD events in NAFLD [60,61,62,63,64]. Data in viral chronic liver disease, whose epidemiology and natural history has been altered by highly effective, direct-acting antiviral therapies, are eagerly awaited. Conversely, NAFLD is an epidemiologically growing chronic liver disease for which no effective pharmacological treatment has been licensed so far. The NAFLD/NASH pandemic is leading to an increase in CVD, underlining the need to develop non-invasive tools able to assess the progression of liver disease and stratify CVR [12,65,66]. In our study, FIB-4 and NFS scores showed a moderate to strong correlation with FRS in NAFLD patients, which was higher than previously reported [67,68,69]. We are the first to report a correlation between the newly proposed HFS index and CVR scores as well as between different liver fibrosis biomarkers and the SCORE equation estimating 10-year fatal CVD risk in European populations. FIB-4 and Forns index proved moderately correlated with CVR scores in patients with viral chronic liver disease. NFS, followed by HFS, exhibited a moderate to strong correlation with CVR scores in NAFLD patients. At variance with a recent study [69], we found a correlation between liver fibrosis biomarkers and CVR assessed with the Italian scoring system “Progetto CUORE”. This is important given that locally validated scoring systems must be used to obtain an accurate assessment of CVR [70].
3.4. Clinical Implications of Findings: May Liver Fibrosis Biomarkers Be Used as an Adjunct to Traditional CVR Scoring Systems?
Our study indicates that higher scores of estimated CVR are found among those with more advanced liver fibrosis (Table 6; Supplementary Tables S1–S4). This association of biomarkers of liver fibrosis with scores obtained with conventional scoring systems raises the possibility to directly gauge CVR through the use of biomarkers of liver fibrosis in clinical practice. Although this notion has a robust rationale such as discussed above, we believe that, for the time being, this research question should best be addressed with appropriate follow-up studies evaluating the occurrence of major cardiovascular events in chronic liver disease patients owing to either metabolic or viral aetiology and followed-up for adequate periods of time. These studies are eagerly awaited given the need to perform an innovative, non-invasive joint (“one-shot”) assessment of both cardio-metabolic and hepatological risks through non-invasive markers of liver fibrosis. Additionally, we point out that the series of patients labelled in the present study as “viral” mainly consists of individuals with chronic hepatitis C, while those with chronic hepatitis B are only a minority (Table 1). This is important to say, given that HCV and HBV may have a different and potentially opposite impact on the development of hepatic steatosis and cardio-metabolic risk [71,72].
3.5. Strengths and Limitations
The strengths of this study include the large sample of well-characterized patients with biopsy-proven chronic liver disease owing to different aetiologies, which represents the first external validation of HFS. Conversely, the cross-sectional design of the study limits our ability to determine the causality and temporality of the observed associations. Moreover, the mono-centric nature of our study, which was performed at a tertiary liver centre is another limitation of our research. Nevertheless, the proportion of advanced fibrosis in our study was lower than that of other similar studies, which renders our study population more similar to an unselected, general practice setting. Recent studies evaluating the diagnostic performance of liver fibrosis biomarkers for detecting advanced fibrosis in NAFLD patients according to the presence of type 2 diabetes have yielded conflicting results [73,74]. Unfortunately, the proportion of individuals with type 2 diabetes in our series did not allow us to perform separate analyses in patients with and without type 2 diabetes. Clearly, our findings remain to be validated in a prospective confirmation cohort as well as in non-Caucasian patients.
Our study was aimed at describing the performance of non-invasive biomarkers in detecting hepatic fibrosis in a series of patients with chronic liver disease owing to either metabolic or viral aetiology. Even in the direct-acting antivirals era, a proportion of underprivileged patients with HCV infection, usually owing to a limited access to health resources, will follow the natural course of disease [75]. In others, chronic liver disease owing to HCV infection is at an advanced stage of fibrosis when they are first submitted to direct-acting antivirals [76]. Moreover, direct-acting antivirals are expensive and poorly available in many areas of the world [77] and a small proportion of treated patients will fail to clear viral infection [78]. Finally, we believe that the association of liver fibrosis biomarkers with CVR scores fully maintains its proof-of-concept significance even in the direct-acting antivirals era.
4. Materials and Methods
Consecutive patients submitted to liver biopsy at a referral Liver Clinic at the University Hospital of Modena, Italy, between the years 2001 and 2012, with a biopsy-proven diagnosis of either NAFLD or viral (HCV or HBV-related) chronic liver disease were enrolled in a retrospective study. The liver biopsy was performed as part of the diagnostic work-up of abnormal liver tests, suspected liver diseases, or grading/staging of known chronic liver disease. Criteria for exclusion from the study were as follows: (a) diagnosis of alcoholic liver disease or other liver diseases (autoimmune, heredo-metabolic) based on clinical data/appropriate testing and histological criteria; (b) incomplete data to calculate all the non-invasive liver fibrosis biomarkers; (c) the presence of decompensated cirrhosis; (d) either primary or metastatic liver cancer; a history of major cardiovascular events.
The liver biopsies were performed, all subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Modena (Project identification code 0035241/20). All enrolled patients were interviewed regarding their familial and personal medical history, notably including daily alcohol consumption. All patients underwent complete physical examination, assessment of anthropometric indices, recording of blood pressure, and routine blood sampling for laboratory tests. Blood test results were performed either on the day of liver biopsy or within one month. HOMA-IR, body mass index (BMI), waist circumference, impaired fasting glycemia (IFG), diabetes, hypertension, and Metabolic Syndrome were defined based on standard criteria [79,80,81,82]. CVR was assessed based on widely adopted scores validated in European and American populations: the SCORE estimating the total 10-year risk for fatal CVD, the Progetto CUORE, and FRS predicting the total 10-year risk for CVD [83,84,85]. The “Progetto CUORE” CVR scoring system has been specifically developed and validated for the Italian general population [84]. This is important given that commonly used scoring systems have been developed and validated in English-speaking countries and may overestimate true CVR in Southern European populations [86]. The diagnosis of NAFLD was based on ultrasonographic/biopsy-proven fatty liver in the absence of excessive alcohol consumption (defined as daily alcohol intake >20 g) and other competing aetiologies of liver disease [87].
The diagnosis of HCV infection was confirmed by polymerase chain reaction testing in HCV-Ab positive patients, and after excluding competing aetiologies of liver disease [88]. The diagnosis of HBV infection was based on a typical serological pattern determined by a standard commercially available enzyme-linked immunosorbent assay [89].
4.1. Histological Evaluation
Liver biopsies were evaluated by a single experienced liver pathologist (L.L.); only biopsy samples at least 15 mm long with at least 10 portal tracts were considered eligible for analysis.
Biopsy specimens of patients with NAFLD were scored according to the Brunt criteria [27]. Fibrosis was staged (F0 = none; F1 = perisinusoidal/pericellular or portal/periportal; F2 = perisinusoidal/pericellular plus portal/periportal; F3 = bridging; F4 = cirrhosis) according to the Brunt and Kleiner criteria [27,28]. Biopsy specimens of patients with chronic viral hepatitis (HCV and HBV) were scored according to Ishak et al. [29]. Fibrosis was staged as follows: F0 = none; F1 = fibrous expansion of some portal areas with or without short fibrous septa; F2 = fibrous expansion of most portal areas with or without short fibrous septa; F3 = fibrous expansion of most portal areas with an occasional portal to portal bridging; F4 = fibrous expansion of portal areas with marked portal-portal and portal-central bridging; F5 = marked bridging with occasional nodules (incomplete cirrhosis); F6 = cirrhosis, probable or definite [29].
Liver fibrosis was considered: (a) significant in the presence of fibrosis stage ≥ F2 for NAFLD patients and ≥F3 for those with viral chronic liver disease; and (b) advanced (i.e., bridging fibrosis) in the presence of fibrosis stage ≥F3 for NAFLD patients and ≥F4 for those with viral chronic liver disease [30,34,35].
4.2. Liver Fibrosis Biomarkers
The general (AST/ALT, APRI, Fib-4 and Forns) and NAFLD-specific (NFS, BARD and Hepamet) non-invasive liver fibrosis biomarkers were determined according to published formulas. AAR was calculated as: AST(IU/l)/ALT(IU/l) [90]. APRI score was calculated as: AST (IU/l)/platelet count (× 109/l) × 100 [91]; FIB-4 score as: age × AST (IU/l)/platelet count (×109/l) × √ALT (IU/l) [43]; Forns index as: 7.811–3.131 ln (platelet count (×109/l)) + 0.781 ln (GGT (IU/L)) + 3.467 ln (age (years))–0.014 (cholesterol (mg/dl)) [92]; NFS as: –1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × IFG or diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio – 0.013 × platelet (×109/l) – 0.66 × albumin (g/dl) [47]; BARD score (ranging 0–4) as: BMI (>28 = 1; <28 = 0) + AST/ALT ratio (>0.8 = 2; <0.8 = 0) + diabetes (yes = 1, no = 0) [93]; HFS as: 1/(1 + e (5.390–0.986 (if Age 45–64 years) – 1.719 (if Age ≥ 65 years) + 0.875 (if Male sex) – 0.896 (if AST 35–69 IU/L) – 2.126 (if AST ≥ 70 IU/L) – 0.027 (if Albumin 4–4.49 g/dL) – 0.897 (if Albumin <4 g/dL) – 0.899 (if HOMA 2–3.99 with no Diabetes Mellitus) –1.497 (if HOMA ≥ 4 with no Diabetes Mellitus) – 2.184 (if Diabetes Mellitus) – 0.882 (if Platelets 155–219 × 1000/µL) – 2.233 (if Platelets < 155 × 1000/µL))) [26].
Cut-offs for predicting significant and advanced liver fibrosis were applied based on the available literature, including original reports, validation studies and meta-analytic reviews [30,34,35,36,44,46,47,90,91,92,93]. The cut-offs adopted in the above cited studies were as follows: ≤0.5 for APRI, <1.45 (NAFLD/viral chronic liver disease) or <1.3 (NAFLD) for Fib-4, <4.2 for Forns index, <−1.455 for NFS, <0.12 for HFS to exclude significant/advanced fibrosis; >0.7, >1.0, >1.5 for APRI, >3.25 (NAFLD/viral chronic liver disease) or >2.67 (NAFLD) for Fib-4, >6.9 for Forns index, >0.675 for NFS, >0.47 for HFS to predict significant/advanced fibrosis.
4.3. Statistical Analysis
The Kolmogorov–Smirnov test was used to assess the normality of variables. The results are shown as means ± SD for continuous variables normally distributed and medians (25–75th percentile) for variables not normally distributed. Categorical variables are shown as relative or absolute proportions. Comparisons between the means of continuous variables were performed using the unpaired 2-tailed Student’s t-test for normally distributed variables, whereas the Mann–Whitney test was performed for non-normally distributed or ordinal variables. Chi-square test or Fisher’s exact test were used to compare nominal variables.
The diagnostic performance of different liver fibrosis biomarkers for predicting significant and advanced fibrosis according to the above-reported cut-offs was evaluated by calculating the sensitivity, specificity, PPV, NPV, accuracy, positive and negative likelihood ratio with standard formulas. Moreover, the AUROC curves and their corresponding 95% CI were also calculated. Spearman’s rho (ρ) or Pearson’s r were used where appropriate for the analysis of correlations between liver fibrosis biomarkers and CVR scoring systems (SCORE, Progetto CUORE, and Framingham risk scores).
A p-value <0.05 was considered to be statistically significant. Statistical analyses were performed by using the statistical software package SPSS, version 24.0 for Windows (SPSS Inc., Chicago, Illinois, USA) and STATA Stata version 14 for Windows (StataCorp, College Station, Texas).
5. Conclusions
We have shown that liver fibrosis biomarkers may accurately exclude (rather than confirm) advanced liver fibrosis in patients with NAFLD or viral chronic liver disease and correlate with CVD risk scores in a population with a low prevalence of advanced fibrosis. The new HFS had the best diagnostic performance for diagnosing advanced fibrosis among NAFLD patients.
The combination of various non-invasive tools (liver fibrosis biomarkers, sono-elastographic techniques and, if available, magnetic resonance elastography) may allow an accurate and “one-shot” simultaneous non-invasive assessment/stratification of the risks of both liver fibrosis and cardio-metabolic events in patients with chronic liver disease owing to the either metabolic or viral origin. This hypothesis deserves specific investigation through appropriate follow-up studies of well-characterized cohorts of patients with chronic liver disease.
Acknowledgments
We are greatly indebted to Luisa Losi, for her histological assessment of liver biopsies. Editing of English kindly performed by Jacqueline Mole is gratefully acknowledged.
Abbreviations
| AAR | AST-to-ALT ratio |
| ALT | alanine aminotransferase |
| APRI | AST-to platelet ratio index |
| AST | aspartate aminotransferase |
| AUROC | area under the receiver operating characteristic |
| BMI | body mass index |
| CI | confidence intervals |
| CVD | cardiovascular disease |
| CVR | cardiovascular risk |
| FIB-4 | fibrosis-4 |
| FRS | Framingham risk score |
| GGT | gamma-glutamyltransferase |
| HBV | hepatitis B virus |
| HCC | hepatocellular carcinoma |
| HCV | hepatitis C virus |
| HDL | high-density lipopoprotein |
| HFS | hepamet fibrosis score |
| HOMA-IR | homeostasis model assessment of insulin resistance |
| IFG | impaired fasting glycemia |
| LDL | low-density lipopoprotein |
| NAFLD | nonalcoholic fatty liver disease |
| NASH | nonalcoholic steatohepatitis |
| NFS | NAFLD fibrosis score |
| NPV | negative predictive value |
| PPV | positive predictive value |
| ROC | receiver operating characteristic |
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4418/11/1/98/s1, Tables S1: Characteristics of patients with absent-mild vs. significant liver fibrosis in the whole population, Table S2: Characteristics of patients with absent-mild vs. significant liver fibrosis according to CLD aetiology, Table S3: Characteristics of patients with absent-to-moderate vs. advanced liver fibrosis in the whole population, Table S4: Characteristics of patients with absent-to-moderate vs. advanced liver fibrosis according to CLD etiology, Table S5: Clinical and laboratory characteristics of NAFLD patients with and without NASH, Table S6: Diagnostic performance of liver fibrosis biomarkers for predicting significant liver fibrosis in the whole population (n = 280), Table S7: Diagnostic performance of liver fibrosis biomarkers for predicting significant fibrosis in patients with viral CLD (n = 173), Table S8: Diagnostic performance of liver fibrosis biomarkers for predicting significant fibrosis in patients with NAFLD (n = 107), Figure S1: Diagnostic performance of serum biomarkers for predicting significant fibrosis in all patients, Figure S2: Diagnostic performance of serum biomarkers for predicting significant fibrosis in viral CLD patients, Figure S3: Diagnostic performance of serum biomarkers for predicting significant fibrosis in NAFLD patients.
Author Contributions
S.B. conceived the study design. S.B. analysed the data. S.B., F.N. and D.R. collected the data. S.B. and A.L. wrote the first draft of the manuscript, and all the authors (S.B., A.M. (Alessandro Mantovani), E.B., S.L., M.M., F.N., A.M. (Alessandra Marrazzo), D.R., G.T. and A.L.) contributed in the analysis of data and preparation of the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Modena (Project identification code 0035241/20).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study whenever possible owing to the retrospective study design (General Data Protection Regulation 679/2016).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Conflicts of Interest
All authors do not have anything to declare.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bataller R., Brenner D.A. Liver fibrosis. J. Clin. Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Udompap P., Kim D., Kim W.R. Current and Future Burden of Chronic Nonmalignant Liver Disease. Clin. Gastroenterol. Hepatol. 2015;13:2031–2041. doi: 10.1016/j.cgh.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finelli C., Tarantino G. What is the role of adiponectin in obesity related non-alcoholic fatty liver disease? World J. Gastroenterol. 2013;19:802–812. doi: 10.3748/wjg.v19.i6.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 5.Italian Association for the Study of the Liver (AISF) AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig. Liver Dis. 2017;49:471–483. doi: 10.1016/j.dld.2017.01.147. [DOI] [PubMed] [Google Scholar]
- 6.Duffell E.F., Hedrich D., Mardh O., Mozalevskis A. Towards elimination of hepatitis B and C in European Union and European Economic Area countries: Monitoring the World Health Organization’s global health sector strategy core indicators and scaling up key interventions. Euro Surveill. 2017;22:30476. doi: 10.2807/1560-7917.ES.2017.22.9.30476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekstedt M., Hagström H., Nasr P., Fredrikson M., Stål P., Kechagias S., Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 8.Angulo P., Kleiner D.E., Dam-Larsen S., Adams L.A., Bjornsson E.S., Charatcharoenwitthaya P., Mills P.R., Keach J.C., Lafferty H.D., Stahler A., et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballestri S., Zona S., Targher G., Romagnoli D., Baldelli E., Nascimbeni F., Roverato A., Guaraldi G., Lonardo A. Nonalcoholic fatty liver disease is associated with an almost two-fold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016;31:936–944. doi: 10.1111/jgh.13264. [DOI] [PubMed] [Google Scholar]
- 10.Targher G., Byrne C.D., Lonardo A., Zoppini G., Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016;65:589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Hagström H., Nasr P., Ekstedt M., Hammar U., Stål P., Hultcrantz R., Kechagias S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 2017;67:1265–1273. doi: 10.1016/j.jhep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 12.Cai J., Zhang X.J., Ji Y.X., Zhang P., She Z.G., Li H. Nonalcoholic Fatty Liver Disease Pandemic Fuels the Upsurge in Cardiovascular Diseases. Circ. Res. 2020;126:679–704. doi: 10.1161/CIRCRESAHA.119.316337. [DOI] [PubMed] [Google Scholar]
- 13.Taylor R.S., Taylor R.J., Bayliss S., Hagström H., Nasr P., Schattenberg J.M., Ishigami M., Toyoda H., Wai-Sun Wong V., Peleg N., et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;158:1611–1625. doi: 10.1053/j.gastro.2020.01.043. [DOI] [PubMed] [Google Scholar]
- 14.Petta S., Valenti L., Bugianesi E., Targher G., Bellentani S., Bonino F., Special Interest Group on Personalised Hepatology of the Italian Association for the Study of the Liver (AISF) Special Interest Group on Personalised Hepatology of Italian Association for Study of Liver AISF A “systems medicine” approach to the study of non-alcoholic fatty liver disease. Dig. Liver Dis. 2016;48:333–342. doi: 10.1016/j.dld.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Ballestri S., Nascimbeni F., Romagnoli D., Baldelli E., Targher G., Lonardo A. Type 2 Diabetes in Non-Alcoholic Fatty Liver Disease and Hepatitis C Virus Infection—Liver: The “Musketeer” in the Spotlight. Int. J. Mol. Sci. 2016;17:355. doi: 10.3390/ijms17030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotter T.G., Rinella M. Nonalcoholic fatty liver disease 2020: The state of the disease. Gastroenterology. 2020;158:1851–1864. doi: 10.1053/j.gastro.2020.01.052. [DOI] [PubMed] [Google Scholar]
- 17.European Association for the Study of the Liver EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 18.European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Tapper E.B., Lok A.S. Use of Liver Imaging and Biopsy in Clinical Practice. N. Engl. J. Med. 2017;377:756–768. doi: 10.1056/NEJMra1610570. [DOI] [PubMed] [Google Scholar]
- 20.Eguchi Y., Wong G., Akhtar O., Sumida Y. Non-invasive diagnosis of non-alcoholic steatohepatitis and advanced fibrosis in Japan: A targeted literature review. Hepatol. Res. 2020;50:645–655. doi: 10.1111/hepr.13502. [DOI] [PubMed] [Google Scholar]
- 21.Castera L., Friedrich-Rust M., Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1264–1281. doi: 10.1053/j.gastro.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou W., Janech M.G., Sobolesky P.M., Bland A.M., Samsuddin S., Alazawi W., Syn W.K. Proteomic screening of plasma identifies potential noninvasive biomarkers associated with significant/advanced fibrosis in patients with nonalcoholic fatty liver disease. Biosci. Rep. 2020;40:BSR20190395. doi: 10.1042/BSR20190395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Association for Study of Liver. Asociacion Latinoamericana para el Estudio del Higado EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 2015;63:237–264. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 24.De Carli M.A., De Carli L.A., Correa M.B., Junqueira G., Jr., Tovo C.V., Coral G.P. Performance of noninvasive scores for the diagnosis of advanced liver fibrosis in morbidly obese with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2020;32:420–425. doi: 10.1097/MEG.0000000000001519. [DOI] [PubMed] [Google Scholar]
- 25.Nascimbeni F., Ballestri S., Machado M.V., Mantovani A., Cortez-Pinto H., Targher G., Lonardo A. Clinical relevance of liver histopathology and different histological classifications of NASH in adults. Expert Rev. Gastroenterol. Hepatol. 2018;12:351–367. doi: 10.1080/17474124.2018.1415756. [DOI] [PubMed] [Google Scholar]
- 26.Ampuero J., Pais R., Aller R., Gallego-Durán R., Crespo J., García-Monzón C., Boursier J., Vilar E., Petta S., Zheng M.H., et al. Development and Validation of Hepamet Fibrosis Scoring System-A Simple, Noninvasive Test to Identify Patients With Nonalcoholic Fatty Liver Disease With Advanced Fibrosis. Clin. Gastroenterol. Hepatol. 2020;18:216–225. doi: 10.1016/j.cgh.2019.05.051. [DOI] [PubMed] [Google Scholar]
- 27.Brunt E.M., Janney C.G., Di Bisceglie A.M., Neuschwander-Tetri B.A., Bacon B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 28.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., Ferrell L.D., Liu Y.C., Torbenson M.S., Unalp-Arida A., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 29.Ishak K., Baptista A., Bianchi L., Callea F., De Groote J., Gudat F., Denk H., Desmet V., Korb G., MacSween R.N., et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 30.Xiao G., Zhu S., Xiao X., Yan L., Yang J., Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66:1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 31.Vallet-Pichard A., Mallet V., Nalpas B., Verkarre V., Nalpas A., Dhalluin-Venier V., Fontaine H., Pol S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection: Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 32.Li J., Gordon S.C., Rupp L.B., Zhang T., Boscarino J.A., Vijayadeva V., Schmidt M.A., Lu M., Chronic Hepatitis Cohort Study (CHeCS) Investigators The validity of serum markers for fibrosis staging in chronic hepatitis B and C. J. Viral Hepat. 2014;21:930–937. doi: 10.1111/jvh.12224. [DOI] [PubMed] [Google Scholar]
- 33.Wang H.W., Peng C.Y., Lai H.C., Su W.P., Lin C.H., Chuang P.H., Chen S.H., Chen C.H., Hsu W.F., Huang G.T. New noninvasive index for predicting liver fibrosis in Asian patients with chronic viral hepatitis. Sci. Rep. 2017;7:3259. doi: 10.1038/s41598-017-03589-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sebastiani G., Castera L., Halfon P., Pol S., Mangia A., Di Marco V., Pirisi M., Voiculescu M., Bourliere M., Alberti A. The impact of liver disease aetiology and the stages of hepatic fibrosis on the performance of non-invasive fibrosis biomarkers: An international study of 2411 cases. Aliment. Pharmacol. Ther. 2011;34:1202–1216. doi: 10.1111/j.1365-2036.2011.04861.x. [DOI] [PubMed] [Google Scholar]
- 35.Lin Z.H., Xin Y.N., Dong Q.J., Wang Q., Jiang X.J., Zhan S.H., Sun Y., Xuan S.Y. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: An updated meta-analysis. Hepatology. 2011;53:726–736. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 36.Chou R., Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: A systematic review. Ann. Intern. Med. 2013;158:807–820. doi: 10.7326/0003-4819-158-11-201306040-00005. [DOI] [PubMed] [Google Scholar]
- 37.Adler M., Gulbis B., Moreno C., Evrard S., Verset G., Golstein P., Frotscher B., Nagy N., Thiry P. The predictive value of FIB-4 versus FibroTest, APRI, FibroIndex and Forns index to noninvasively estimate fibrosis in hepatitis C and nonhepatitis C liver diseases. Hepatology. 2008;47:762–763. doi: 10.1002/hep.22085. [DOI] [PubMed] [Google Scholar]
- 38.Siddiqui M.S., Yamada G., Vuppalanchi R., Van Natta M., Loomba R., Guy C., Brandman D., Tonascia J., Chalasani N., Neuschwander-Tetri B., et al. Diagnostic Accuracy of Noninvasive Fibrosis Models to Detect Change in Fibrosis Stage. Clin. Gastroenterol. Hepatol. 2019;17:1877–1885. doi: 10.1016/j.cgh.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newsome P.N., Sasso M., Deeks J.J., Paredes A., Boursier J., Chan W.K., Yilmaz Y., Czernichow S., Zheng M.H., Wong V.W., et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020;5:362–373. doi: 10.1016/S2468-1253(19)30383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McPherson S., Hardy T., Dufour J.F., Petta S., Romero-Gomez M., Allison M., Oliveira C.P., Francque S., Van Gaal L., Schattenberg J.M., et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am. J. Gastroenterol. 2017;112:740–751. doi: 10.1038/ajg.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballestri S., Nascimbeni F., Romagnoli D., Lonardo A. The independent predictors of non-alcoholic steatohepatitis and its individual histological features. Insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol. Res. 2016;46:1074–1087. doi: 10.1111/hepr.12656. [DOI] [PubMed] [Google Scholar]
- 42.Carulli L., Ballestri S., Lonardo A., Lami F., Violi E., Losi L., Bonilauri L., Verrone A.M., Odoardi M.R., Scaglioni F., et al. Is nonalcoholic steatohepatitis associated with a high-though-normal thyroid stimulating hormone level and lower cholesterol levels? Intern. Emerg. Med. 2013;8:297–305. doi: 10.1007/s11739-011-0609-4. [DOI] [PubMed] [Google Scholar]
- 43.Loria P., Marchesini G., Nascimbeni F., Ballestri S., Maurantonio M., Carubbi F., Ratziu V., Lonardo A. Cardiovascular risk, lipidemic phenotype and steatosis. A comparative analysis of cirrhotic and non-cirrhotic liver disease due to varying etiology. Atherosclerosis. 2014;232:99–109. doi: 10.1016/j.atherosclerosis.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 44.McPherson S., Stewart S.F., Henderson E., Burt A.D., Day C.P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265–1269. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 45.Treeprasertsuk S., Piyachaturawat P., Soontornmanokul T., Wisedopas-Klaikaew N., Komolmit P., Tangkijavanich P. Accuracy of noninvasive scoring systems to assess advanced liver fibrosis in Thai patients with nonalcoholic fatty liver disease. Asian Biomed. 2017;10:s49–s55. doi: 10.5372/1905-7415.1000.521. [DOI] [Google Scholar]
- 46.Sterling R.K., Lissen E., Clumeck N., Sola R., Correa M.C., Montaner J., Sulkowski M.S., Torriani F.J., Dieterich D.T., Thomas D.L., et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 47.Angulo P., Hui J.M., Marchesini G., Bugianesi E., George J., Farrell G.C., Enders F., Saksena S., Burt A.D., Bida J.P., et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 48.Lonardo A., Ballestri S. Perspectives of nonalcoholic fatty liver disease research: A personal point of view. Explor. Med. 2020;1:85–107. doi: 10.37349/emed.2020.00007. [DOI] [Google Scholar]
- 49.Redman J., Sterling R.K. Non-invasive Assessment of Liver Fibrosis. Curr. Treat. Options Gastroenterol. 2020;18:255–269. doi: 10.1007/s11938-020-00285-z. [DOI] [Google Scholar]
- 50.Ballestri S., Nascimbeni F., Lugari S., Lonardo A., Francica G. A critical appraisal of the use of ultrasound in hepatic steatosis. Expert Rev. Gastroenterol. Hepatol. 2019;13:667–681. doi: 10.1080/17474124.2019.1621164. [DOI] [PubMed] [Google Scholar]
- 51.Ballestri S., Tana C., Di Girolamo M., Fontana M.C., Capitelli M., Lonardo A., Cioni G. Semi-quantitative Ultrasonographic Evaluation of NAFLD. Curr. Pharm. Des. 2020;26:3915–3927. doi: 10.2174/1381612826666200417142444. [DOI] [PubMed] [Google Scholar]
- 52.Lonardo A., Nascimbeni F., Mantovani A., Targher G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J. Hepatol. 2018;68:335–352. doi: 10.1016/j.jhep.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 53.Barone M., Viggiani M.T., Amoruso A., Schiraldi S., Zito A., Devito F., Cortese F., Gesualdo M., Brunetti N., Di Leo A., et al. Endothelial dysfunction correlates with liver fibrosis in chronic HCV infection. Gastroenterol. Res. Pr. 2015;2015:682174. doi: 10.1155/2015/682174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee K.K., Stelzle D., Bing R., Anwar M., Strachan F., Bashir S., Newby D.E., Shah J.S., Chung M.H., Bloomfield G.S., et al. Global burden of atherosclerotic cardiovascular disease in people with hepatitis C virus infection: A systematic review, meta-analysis, and modelling study. Lancet Gastroenterol. Hepatol. 2019;4:794–804. doi: 10.1016/S2468-1253(19)30227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adinolfi L.E., Petta S., Fracanzani A.L., Coppola C., Narciso V., Nevola R., Rinaldi L., Calvaruso V., Staiano L., Di Marco V., et al. Impact of hepatitis C virus clearance by direct-acting antiviral treatment on the incidence of major cardiovascular events: A prospective multicentre study. Atherosclerosis. 2020;296:40–47. doi: 10.1016/j.atherosclerosis.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 56.Chen Q., Li Q., Li D., Chen X., Liu Z., Hu G., Wang J., Ling W. Association between liver fibrosis scores and the risk of mortality among patients with coronary artery disease. Atherosclerosis. 2020;299:45–52. doi: 10.1016/j.atherosclerosis.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 57.Lee J., Kim H.S., Cho Y.K., Kim E.H., Lee M.J., Bae I.Y., Jung C.H., Park J.-Y., Kim H.-K., Lee W.J. Association between noninvasive assessment of liver fibrosis and coronary artery calcification progression in patients with nonalcoholic fatty liver disease. Sci. Rep. 2020;10:18323. doi: 10.1038/s41598-020-75266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schonmann Y., Yeshua H., Bentov I., Zelber-Sagi S. Liver fibrosis marker is an independent predictor of cardiovascular morbidity and mortality in the general population. Dig. Liver Dis. 2021;53:79–85. doi: 10.1016/j.dld.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 59.Tsochatzis E.A., Srivastava A., Rosenberg W. Reply to: “FIB-4 cut off of 1.3 may be inappropriate in a primary care referral pathway for patients with non-alcoholic fatty liver disease”. J. Hepatol. 2020;73:217–218. doi: 10.1016/j.jhep.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 60.Salomone F., Micek A., Godos J. Simple Scores of Fibrosis and Mortality in Patients with NAFLD: A Systematic Review with Meta-Analysis. J. Clin. Med. 2018;7:219. doi: 10.3390/jcm7080219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simon T.G., Corey K.E., Cannon C.P., Blazing M., Park J.G., O’Donoghue M.L., Chung R.T., Giugliano R.P. The nonalcoholic fatty liver disease (NAFLD) fibrosis score, cardiovascular risk stratification and a strategy for secondary prevention with ezetimibe. Int. J. Cardiol. 2018;270:245–252. doi: 10.1016/j.ijcard.2018.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hagström H., Nasr P., Ekstedt M., Stål P., Hultcrantz R., Kechagias S. Accuracy of Noninvasive Scoring Systems in Assessing Risk of Death and Liver-Related Endpoints in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019;17:1148–1156.e4. doi: 10.1016/j.cgh.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 63.Baratta F., Pastori D., Angelico F., Balla A., Paganini A.M., Cocomello N., Ferro D., Violi F., Sanyal A.J., Del Ben M. Nonalcoholic Fatty Liver Disease and Fibrosis Associated With Increased Risk of Cardiovascular Events in a Prospective Study. Clin. Gastroenterol. Hepatol. 2020;18:2324–2331.e4. doi: 10.1016/j.cgh.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 64.Henson J.B., Simon T.G., Kaplan A., Osganian S., Masia R., Corey K.E. Advanced fibrosis is associated with incident cardiovascular disease in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2020;51:728–736. doi: 10.1111/apt.15660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spinosa M., Stine J.G. Nonalcoholic Fatty Liver Disease-Evidence for a Thrombophilic State? Curr. Pharm. Des. 2020;26:1036–1044. doi: 10.2174/1381612826666200131101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ballestri S., Capitelli M., Fontana M.C., Arioli D., Romagnoli E., Graziosi C., Lonardo A., Marietta M., Dentali F., Cioni G. Direct Oral Anticoagulants in Patients with Liver Disease in the Era of Non-Alcoholic Fatty Liver Disease Global Epidemic: A Narrative Review. Adv. Ther. 2020;37:1910–1932. doi: 10.1007/s12325-020-01307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dogan S., Celikbilek M., Yilmaz Y.K., Sarikaya S., Zararsiz G., Serin H.I., Borekci E., Akyol L., Pirti I., Davarci S.E. Association between liver fibrosis and coronary heart disease risk in patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2015;27:298–304. doi: 10.1097/MEG.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 68.Niederseer D., Wernly S., Bachmayer S., Wernly B., Bakula A., Huber-Schönauer U., Semmler G., Schmied C., Aigner E., Datz C. Diagnosis of Non-Alcoholic Fatty Liver Disease (NAFLD) Is Independently Associated with Cardiovascular Risk in a Large Austrian Screening Cohort. J. Clin. Med. 2020;9:1065. doi: 10.3390/jcm9041065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pisetta C., Chillè C., Pelizzari G., Pigozzi M.G., Salvetti M., Paini A., Muiesan M.L., De Ciuceis C., Ricci C., Rizzoni D. Evaluation of Cardiovascular Risk in Patient with Primary Non-alcoholic Fatty Liver Disease. High Blood Press. Cardiovasc. Prev. 2020;27:321–330. doi: 10.1007/s40292-020-00389-8. [DOI] [PubMed] [Google Scholar]
- 70.Lonardo A., Ballestri S., Targher G., Loria P. Diagnosis and management of cardiovascular risk in nonalcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2015;9:629–650. doi: 10.1586/17474124.2015.965143. [DOI] [PubMed] [Google Scholar]
- 71.Lonardo A., Nascimbeni F., Ballestri S. NAFLD, Hepatotropic Viruses, and Cardiometabolic Risk. Hepatology. 2017;65:2122–2123. doi: 10.1002/hep.29052. [DOI] [PubMed] [Google Scholar]
- 72.Wang C.C., Cheng P.N., Kao J.H. Systematic review: Chronic viral hepatitis and metabolic derangement. Aliment. Pharmacol. Ther. 2020;51:216–230. doi: 10.1111/apt.15575. [DOI] [PubMed] [Google Scholar]
- 73.Poynard T., Peta V., Deckmyn O., Pais R., Ngo Y., Charlotte F., Ngo A., Munteanu M., Imbert-Bismut F., Monneret D., et al. Performance of liver biomarkers, in patients at risk of nonalcoholic steato-hepatitis, according to presence of type-2 diabetes. Eur. J. Gastroenterol. Hepatol. 2020;32:998–1007. doi: 10.1097/MEG.0000000000001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh A., Gosai F., Siddiqui M.T., Gupta M., Lopez R., Lawitz E., Poordad F., Carey W., McCullough A., Alkhouri N. Accuracy of Noninvasive Fibrosis Scores to Detect Advanced Fibrosis in Patients With Type-2 Diabetes With Biopsy-proven Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. 2020;54:891–897. doi: 10.1097/MCG.0000000000001339. [DOI] [PubMed] [Google Scholar]
- 75.Lemoine M., Nayagam S., Thursz M. Viral hepatitis in resource-limited countries and access to antiviral therapies: Current and future challenges. Future Virol. 2013;8:371–380. doi: 10.2217/fvl.13.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gayam V., Gill A., Garlapati P., Mohanty S. Direct-Acting Antivirals in Chronic Hepatitis C Infection with Liver Cirrhosis, Hepatitis B and C. IntechOpen. 2019;6:83–98. doi: 10.5772/intechopen.83609. [DOI] [Google Scholar]
- 77.Edwards D.J., Coppens D.G., Prasad T.L., Rook L.A., Iyer J.K. Access to hepatitis C medicines. Bull. World Health Organ. 2015;93:799–805. doi: 10.2471/BLT.15.157784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kondili L.A., Gaeta G.B., Brunetto M.R., Di Leo A., Iannone A., Santantonio T.A., Giammario A., Raimondo G., Filomia R., Coppola C., et al. Incidence of DAA failure and the clinical impact of retreatment in real-life patients treated in the advanced stage of liver disease: Interim evaluations from the PITER network. PLoS ONE. 2017;12:e0185728. doi: 10.1371/journal.pone.0185728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 80.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27:S5–S10. doi: 10.2337/diacare.27.2007.S5. [DOI] [PubMed] [Google Scholar]
- 81.Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M., Clement D.L., Coca A., de Simone G., Dominiczak A., et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 82.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P., Loria C.M., Smith S.C., Jr., et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 83.Conroy R.M., Pyörälä K., Fitzgerald A.P., Sans S., Menotti A., De Backer G., De Bacquer D., Ducimetière P., Jousilahti P., Keil U., et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003;24:987–1003. doi: 10.1016/S0195-668X(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 84.Palmieri L., Panico S., Vanuzzo D., Ferrario M., Pilotto L., Sega R., Cesana G., Giampaoli S., Gruppo di Ricerca del Progetto CUORE Evaluation of the global cardiovascular absolute risk: The Progetto CUORE individual score. Ann. Ist. Super. Sanita. 2004;40:393–399. [PubMed] [Google Scholar]
- 85.D’Agostino R.B., Vasan R.S., Pencina M.J., Wolf P.A., Cobain M., Massaro J.M., Kannel W.B. General cardiovascular risk profile for use in primary care. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 86.Eichler K., Puhan M.A., Steurer J., Bachmann L.M. Prediction of first coronary events with the Framingham score: A systematic review. Am. Heart J. 2007;153:722–731.e7318. doi: 10.1016/j.ahj.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 87.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 88.Gupta E., Bajpai M., Choudhary A. Hepatitis C virus: Screening, diagnosis, and interpretation of laboratory assays. Asian J. Transfus. Sci. 2014;8:19–25. doi: 10.4103/0973-6247.126683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krajden M., McNabb G., Petric M. The laboratory diagnosis of hepatitis B virus. Can. J. Infect. Dis. Med. Microbiol. 2005;16:65–72. doi: 10.1155/2005/450574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Williams A.L., Hoofnagle J.H. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology. 1988;95:734–739. doi: 10.1016/S0016-5085(88)80022-2. [DOI] [PubMed] [Google Scholar]
- 91.Wai C.T., Greenson J.K., Fontana R.J., Kalbfleisch J.D., Marrero J.A., Conjeevaram H.S., Lok A.S. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 92.Forns X., Ampurdanès S., Llovet J.M., Aponte J., Quintó L., Martínez-Bauer E., Bruguera M., Sánchez-Tapias J.M., Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–992. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 93.Harrison S.A., Oliver D., Arnold H.L., Gogia S., Neuschwander-Tetri B.A. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.