Abstract
Simple Summary
The objective of this review is to provide readers with a state-of-the-art description of the main factors affecting farmed fish pathologies and its diagnoses. A special focus is given to the use proteomics technologies as a tool in the evaluation of pathogens and host-pathogen interactions and its impact in disease characterization and control.
Abstract
One of the main constraints in aquaculture production is farmed fish vulnerability to diseases due to husbandry practices or external factors like pollution, climate changes, or even the alterations in the dynamic of product transactions in this industry. It is though important to better understand and characterize the intervenients in the process of a disease outbreak as these lead to huge economical losses in aquaculture industries. High-throughput technologies like proteomics can be an important characterization tool especially in pathogen identification and the virulence mechanisms related to host-pathogen interactions on disease research and diagnostics that will help to control, prevent, and treat diseases in farmed fish. Proteomics important role is also maximized by its holistic approach to understanding pathogenesis processes and fish responses to external factors like stress or temperature making it one of the most promising tools for fish pathology research.
Keywords: proteomics, fish diseases, aquaculture, fish pathology, fish welfare
1. Introduction
The demand for animal protein for human consumption is rising as a result of an exponential increase in the world population. Aquaculture is becoming an increasingly important source of protein available for human consumption since is an industry capable of providing solutions to feed a rapidly growing human population and reduce poverty in many countries [1,2,3]. To achieve that, the scale of aquaculture production and the range of farmed species has increased dramatically over the last two decades [4]. Live production always comprises a risk for loss due to infectious diseases [5], with farmed fish, due to husbandry practices in aquaculture, being more vulnerable than wild fish to diseases from a wide range of bacterial, viral, parasitic and fungal infections [6]. Also, the tendency to higher density production systems, the perturbations in ecological systems balance related to pollution and climatic changes, and the expected increase in international transactions of aquaculture products and their derivatives contributed to alterations on the dynamics of interaction between organisms, infectious agents, and people. This influences pathogen rates of replication and proliferation, leading to a broader geographic distribution of pathogenic agents and an increase in species affected by disease outbreaks [7,8]. This makes disease outbreaks an important constraint to this industry, with a significant impact on the quality, safety and volume of the fish produced throughout the world [9,10,11,12], that can lead to market access exclusion and major economic loss or costs to the producer [8,13,14].
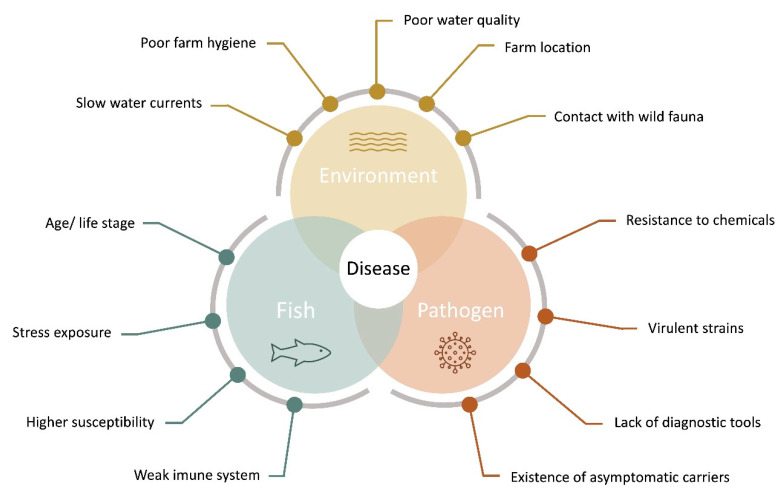
For several authors, disease outbreaks in aquaculture are the result of a complex network of interactions on aquatic systems between the produced organism, several environmental and zootechnical aspects, and possible pathogenic agents, that present a series of unique challenges in aquatic organism’s health [15,16,17,18,19], as represented in Figure 1.
Figure 1.
Aquaculture disease diagram, indicating the main factors for the evaluation of pathogen, and host-pathogen interactions intervening in fish disease outbreaks (adapted from [19,20]).
To address infectious pathologies in farmed fish, approaches like epidemiological studies on main areas of aquatic animal health as transboundary and emerging aquatic animal diseases, animal health surveillance and biosecurity program development should be performed. These are crucial to disease prevalence monitorization, early detection of emerging exotic and new diseases and quality management improvement of aquaculture operations [15,18,19,21].
Nevertheless, to obtain proper epidemiological models, animal health surveillance and biosecurity programs must integrate environmental information and information from different areas like pathogenesis, disease diagnosis, disease resistance, physiological response to pathogens, pathogen characterization, host immune system responses characterization, disease biomarkers and organism response to disease treatment products [22,23].
The amount of data from different origins and an increase in the reported frequency and severity of marine diseases demands that new diagnostic tools should be implemented for a more rapid and effective diagnosis [24,25,26]. Thus, several scientific advances in aquatic health continue to close the gap to veterinary medicine, and new optical, analytical chemistry, molecular biology [27], and Omics techniques are becoming a reality that offers a vast array of benefits to the aquaculture industry [12,28]. Proteomics techniques are one of those new tools, and one of the most interesting approaches for health management, epidemiology, and fish disease research [3,22,23,29,30]. Proteomics refers to the methodology that addresses the study of the entire complement of proteins expressed in a specific state of an organism or a cell population [31,32]. The proteome, or the full protein complement of the genome, is a highly structured entity, where proteins exert their cellular functions with specificity in time and location, in physical or functional association with other proteins or biomolecules [33,34]. High-throughput proteomics methods based on mass spectrometry (MS) allow the measurement of multiple properties for thousands of proteins, including their abundance, tissue distribution, sub-cellular localization, post-translational modifications and protein-protein interactions [34]. Proteomics-based approaches can therefore offer unique insights into fish cellular regulation in response to pathogens and during disease progression, besides enabling fast and sensitive pathogen detection and identification.
In this manuscript, detailed information regarding the use of proteomics in several disease aspects, with a special focus on the role of stress and welfare in disease, and the importance of pathogen identification and host-pathogen interactions on disease diagnostics and characterization, will be provided.
2. Fish Health, Stress and Welfare
Despite being the most consumed animal, fish are seldom afforded the same level of concern regarding their welfare as other vertebrates. The scientific research around fish welfare is at an early stage compared with other land animals produced for human consumption [35]. In part, this lack of consideration is due to the gap between public perception of their intelligence and the scientific evidence [36], along with the absence of a unified definition of the concept [37]. Nevertheless, most definitions consider mainly a feelings-based and a function-based approach [38]. The first gives regard to the emotional-like state of the animal, while good welfare is defined as the absence of negative feelings and the presence of positive feelings [39]. The second definition is more focused on the biological, physiological and health perspective of the animal, while good welfare is defined as the fish’s ability to cope and adapt to its environment while maintaining homeostasis [40]. Although the fish’s health state offers objective criteria as part of a welfare assessment, it does not provide the complete picture. Good health is essential to ensure good welfare, however, it does not necessarily indicate that the fish is in a good welfare state [37]. On the other hand, poor health i.e., the reduced ability of the animal to normal functioning, to cope with stressful conditions and to prevent disease, generally implies/leads to a bad welfare status in a variety of contexts. For example, deceased fish, as a consequence of disease, constitute a source of infection and compromise water quality [41]. Additionally, chemical treatments for specific outbreaks can also trigger some level of disturbance on the fish [42,43]. Importantly, a healthy animal in an optimal welfare environment can also be suddenly struck by an acute infection reducing its welfare. For instance, in the case of fish produced in cages, pathogens are naturally embedded in the environment [44]. In most cases, it is often the lousy welfare status itself, due to poor husbandry conditions, which translates into impaired health. Thus, in summary, health and welfare are intimately linked, and poor welfare can be interpreted both as a cause and a consequence of poor health. This section focuses on health as a cornerstone for fish welfare assessment and the effects of stressors on disease resistance, reviewing the most recent approaches employed to study the relationship between certain diseases/pathologies and welfare.
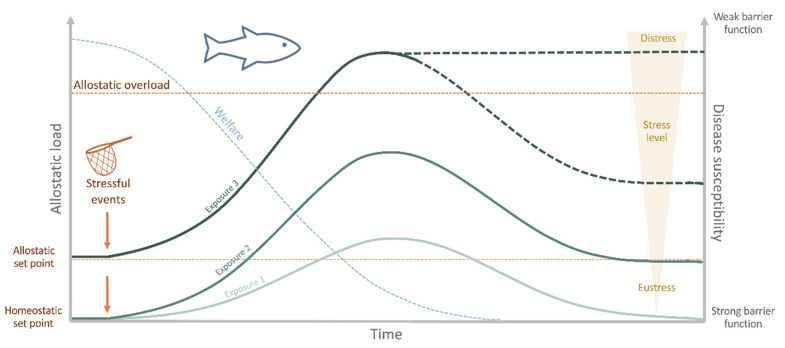
In aquaculture, inappropriate husbandry conditions, or even standard farming practices, are everyday stressors in culture systems [45]. The allostatic load imposed on the animals can reduce functioning immune mechanisms, consequently favoring diseases and threatening fish welfare (Figure 2). For instance, drastic changes in water temperature (from 27 °C to either 19–23 °C or 31–35 °C) decreased the immune response and resistance to pathogens in Mozambique tilapia (Oreochromis mossambicus) [46]. More recently, using a transcriptomics approach, the rearing density in Nile tilapia (Oreochromis niloticus) was shown to significantly impact on the susceptibility to the oomycete Saprolegnia parasitica [47]. However, the association between husbandry-induced stress and disease is not that straightforward. For example, acute stressors have been reported to enhance [48,49,50,51] or decrease [52,53] some innate immune responses in fish. On the contrary, chronic stressors have mainly been indicated as immunosuppressors [54,55,56,57,58]. From a productivity perspective, the health of the fish is often interpreted as “absence of disease”, since from either an ethical or an economic point of view, any disease state is unacceptable for the industry [44]. Therefore, disease prevention and eradication are crucial aspects of a fish farm to ensure the production’s sustainability. Providing optimal welfare conditions, monitoring the health parameters routinely and alleviating stress are necessary steps towards this goal.
Figure 2.
Interaction between welfare, allostatic load, disease susceptibility and the repetitive/chronic stressful experiences appraised by the fish. Stressful stimuli may induce either adaptive (eustress) or maladaptive allostasis (distress). If the stressor persists, recovery to the original homeostatic state (homeostatic set point) may be incomplete. In this case, a newly defined set point for future adaptation is settled (allostatic setpoint). As a result, the welfare status is decreased with time and stress experienced. The cumulative burden of adaptation (allostatic load) is thus constituted by the beneficial stressful events that the fish can cope with, while the allostatic overload represents the state when stress overcomes the organism’s natural regulatory capacity, which may induce a state of no-recovery. At this step, primary barrier function is severely impaired increasing disease susceptibility, which may cause illness and ultimately death.
Stress is considered a state of threatened homeostasis [59], which is re-established by a complex network of changes in the physiological systems (allostasis) [60]. As in all other vertebrates, in the face of a perceived stressor, fish launch a widespread reaction, the so-called physiological stress response, which allows the individual to adjust and cope with the predictable and unpredictable changes in its surroundings (eustress) [61]. As a primary response, cortisol and catecholamines are released into the bloodstream, which will induce a series of downstream reactions [62]. In fact, stress is not necessarily detrimental nor immediately equates compromised welfare. Instead, in the short term, it is an essential adaptation to ensure the best chances of survival [37]. However, when reaching an allostatic overload, usually as a result of a prolonged, repeated and/or unavoidable stressor, maladaptive effects such as impaired growth and/or reproductive and immune functions, arise (Figure 2) [63,64]. In this case, these are largely associated with diminished welfare and may jeopardize fish health and survival (distress) [65]. The questions raised here are the cost of this acclimation and why stress increases diseases’ susceptibility in fish. First, in terms of energetic costs, the adaptive physiological response needed to counteract the disrupted homeostasis requires a significant amount of energy. This means that if part of the fish’s energy is allocated to face the challenge, then fewer resources will be available for other energy-demanding biological functions, such as some mechanisms of the defense repertoire: the epithelial barriers and the immune system [44]. In terms of immune responses, several mechanisms are immediately activated to respond directly to the challenge. These include an increase of inflammatory markers, the release of hormones and the expression of acute-phase proteins [66]. Even if a fish has managed to adapt to the stressor for a certain period, these energy stores will eventually be depleted if the stressor persists. Consequently, the total consumption of energy reserves gives rise to the allostatic overload, and the fish may no longer be able to adapt, which can lead to immunosuppression, disease, and in the case of more severe disturbances, even death (Figure 2) [63]. Moreover, several studies also demonstrated the impact of stressful husbandry conditions on the functioning of the epithelial barriers, i.e., the mucus and the epidermal surfaces of the skin, gills and intestine, which constitute the primary lines of defense against pathogens and harmful substances, showing that injury of these barriers, inevitably leads to impaired disease resistance [67]. Changes in these barriers have been reported in Atlantic salmon (Salmo salar), Atlantic cod (Gadus morhua) and rainbow trout (Oncorhynchus mykiss) subjected to different acute stressors [68,69]. Moreover, in Atlantic salmon reared under low dissolved oxygen levels, impaired intestinal barrier function was also observed [70]. These disturbances have mainly been associated with high cortisol levels, though various other hormones, such as catecholamines, endogenous opioids, pituitary hormones, and serotonin, intervene here [71]. Indeed, it is known that cortisol plays an immunomodulatory role, inhibiting specific constituents of the immune system and enhancing others, such as induction of apoptosis, change of differentiation patterns, inhibition of cytokine release and inhibition of immunocyte migration [72,73,74,75]. Nevertheless, the cortisol response may vary among different species and even among individuals (coping styles) [76] and be affected by several other parameters (e.g., domestication level, age, nutritional state, stressor severity, among others) [53,77,78,79], which may obscure the relationship between stress and immune status. A detailed description of how the endocrine-immune response is mounted and the mechanisms behind these immunoregulatory changes is out of the scope of this review, for this, the authors refer to recent publications [66,80].
Deepening our scientific knowledge on the mechanisms relating to stress, fish health and welfare, is paramount for the sustainable aquaculture industry. In recent years, more advanced high-throughput technologies, as the case of proteomics, started to be successfully employed in aquaculture research, including for the study of fish diseases and welfare, providing a holistic understanding of the molecular events underlying the physiological stress response and valuable insights on the differential proteins involved in inflammatory processes and immune responses [30,58,81]. Proteomic studies on fish target mainly the liver, however, blood plasma and mucus are taking crescent importance, mainly from an immunological point of view, as skin mucus is one of the primary barriers of defense in fish [82,83,84,85,86] and plasma acts as a mirror/reporter of physiological or pathological conditions [87,88]. Important applications of proteomics in this field concern the study of the effects of certain diseases and parasites on the proteins’ abundance and modifications and the investigation of the host-pathogen interactions [88,89,90,91,92,93,94,95]. For example, joint studies evaluating changes in the proteome of fish challenged with a specific pathogen after exposure to a rearing stressor are scarce. However, the existing proteomic studies demonstrating aquaculture and environmental stressors clearly modulating the fish’s immune function [58,96,97] reveal that these technologies are already promising sensitive approaches to study this relationship.
3. Disease Diagnostics
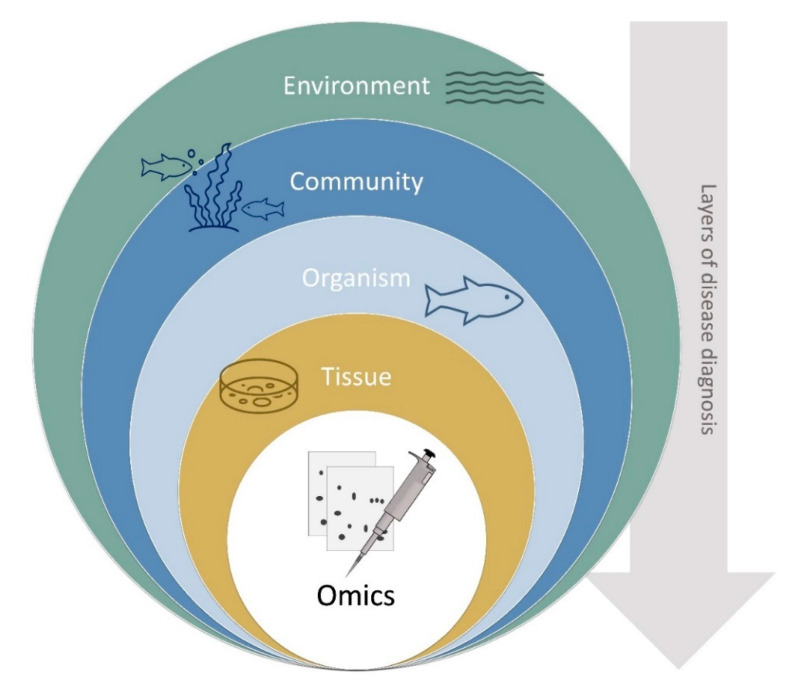
To properly diagnose pathology in aquaculture, we must consider disease as a problem with multiple levels of increasing biological complexity, ranging from environmental to the cell, genome and proteome level (Figure 3) [26,27].
Figure 3.
Disease diagnosis concentric ring, representing layers of disease diagnoses as environment, community, organism, tissue, and omics as a tool to interpret cell/tissue responses (adapted from [26]).
New areas like Proteomics can be an important complement to more classical approaches like pathogen identification, disease symptomatology and histopathological analysis to achieve a good disease diagnosis in aquaculture [22,23,27,29,30]. In Proteomics, regardless the complexity of the analysed protein mixtures that can range from hundreds, to several thousands of proteins, the major goal is the accurate identification of the highest number of proteins as possible in those mixtures [32]. In gel-based approaches, proteins are first separated by one (1-DE)—or two-dimensional gel electrophoresis (2-DE) and then identified by mass spectrometry, whereas in gel-free approaches (or MS-based) protein mixtures remain in solution prior to protein identification. In each case, protein samples may be digested to peptides by a sequence-specific enzyme, typically trypsin, in a so-called peptide-based “bottom-up” proteomics approach, to distinguish it from the analysis of entire proteins in “top-down” proteomics. Peptide samples can then be separated and analysed by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS), usually employing electrospray ionization (ESI) as the method to convert the peptides to gas phase ions for MS analysis. Alternatively, peptide samples can be analysed by matrix-assisted laser desorption/ionization (MALDI) time-of-flight (TOF) mass spectrometry. The method of choice will always depend on the main research objective, costs and expertise, with MALDI-TOF MS based strategies being most suited for microbial identification and diagnosis, as a rapid, sensitive and economical in terms of both labour and costs [98]. On the other hand, LC-MS/MS is most suited for large-scale, systematic characterization of proteomes, e.g., involved in host-pathogen interactions, allowing multiplex sample analysis and quantitation. In the following sections we will discuss in more detail main applications of proteomics in pathogen characterization and in host-pathogen interactions.
3.1. Pathogen Identification
Pathogen identification is a key area in disease diagnosis and management. Classical, immunological and molecular methods have been routinely and extensively used to address this area [26]. However, in the last ten years proteomics has emerged as a powerful tool for pathogen identification, strain typing and epidemiological studies [98], as can be observed in Table 1.
Table 1.
Resume of some of the proteomic techniques applied to pathogen identification, characterization, and virulence.
| Pathogen | Disease | Pathogen ID |
Metabolic Process | Proteomic Method | Reference |
|---|---|---|---|---|---|
| Vírus | |||||
| Singapore grouper iridovirus (SGIV) | Sistemic diseases | Vírus proteín characterization | - | SDS-PAGE MALDI-TOF-MS | [99] |
| Singapore grouper iridovirus (SGIV) | Sistemic diseases | Vírus envelope proteín analysis and characterization | - | 1-DE MALDI-TOF/TOF-MS/MS and LC-MALDI-TOF/TOF-MS/MS | [100] |
| Cyprinid herpesvirus 3 (CyHV-3) | Koi herpesvirus (KHV) disease | Functional analyses of the virion transmembrane proteome | - | SDS-PAGE LC-MS/MS | [101] |
| Bacteria | |||||
| Photobacterium damselae subsp. piscicida | Pasteurellosis | - | Importance of outer membrane proteins in osmotic adaptations and bacterial virulence | 2-DE LC-nano ESI-Q-TOF MS/MS | [102] |
| Photobacterium damselae subsp. damselae | Vibriosis | - | Importance of iron in virulence | 2-DE MALDI-TOF/TOF-MS | [103] |
| Photobacterium damselae | Pasteurellosis | Differentiation of Photobacterium damselae subspecies | - | MALDI-TOF-MS | [104] |
| Edwardsiella tarda | Haemorrhagic septicaemia | Identification of proteins associated with virulent and avirulent strains | - | 2-DE MALDI-TOF MS | [105,106] |
| Edwardsiella tarda | Haemorrhagic septicaemia | - | Virulence determinants | 2-DE ESI MS/MS | [92] |
| Yersinia ruckeri | Enteric redmouth disease | Identification and characterization of biotype 1 and biotype 2 strains | - | Nano LC-MS/MS | [107] |
| Yersinia ruckeri | Enteric redmouth disease | - | Importance of iron in virulence | Nano LC-MS/MS | [108] |
| Yersinia ruckeri | Enteric redmouth disease | - | Importance of outer membrane proteins in bacterial virulence | Gell free and 1-D nLC-ESI-MS/MS | [109] |
| Flavobacterium columnare | Columnaris disease | - | Virulence determinants | 2-D LC ESI MS/MS and 2-DE MALDI TOF/TOF MS | [110] |
| Flavobacterium psychrophilum | Bacterial coldwater disease | Proteomic profiling of strains, the importance of iron in virulence | - | 2-DE LC-MS/MS | [111] |
| Flavobacterium psychrophilum | Bacterial coldwater disease | Differentiation of Flavobacterium psychrophilum from Flavobacterium psychrophilum-like species | - | MALDI-TOF MS | [112] |
| Vibrio parahaemolyticus | Vibriosis | - | Identify proteins regulating antimicrobial peptide resistance | 2-DE LC-ESI-Q-TOF MS/MS | [113] |
| Streptococcus agalactiae | Streptococcosis | - | Temperature effects on bacterial virulence | NanoUPLC-HDMSE | [114] |
| Streptococcus iniae and Streptococcus parauberis | Streptococcosis | Identification and molecular fingerprinting | - | MALDI-TOF-MS | [115] |
| Streptococcus parauberis | Streptococcosis | Identification of bacterial strains | - | MALDI-TOF-MS | [116] |
| Vagococcus salmoninarum | Cold-water streptococcosis | Typing and characterization of bacterial strains | - | MALDI-TOF-MS | [117] |
| Streptococcus iniae | Streptococcosis | Proteomic profile of a pathogenic strain | - | 2-DE MALDI-TOF-MS | [118] |
| Piscirickettsia salmonis | Salmonid rickettsial syndrome | Detection and identification | Virulence determinants | MALDI-TOF-MS | [119] |
| Tenacibaculum sp. | Tenacibaculosis | Identification of Tenacibaculum species | - | MALDI-TOF-MS | [120,121] |
| Mycobacterium marinum | Mycobacteriosis | Identification of Mycobacterium marinum subspecies | - | MALDI-TOF-MS | [122] |
| Several bacterial species | - | Differentiate several gram-negative fish pathogenic bacteria | - | MALDI-TOF-MS | [123] |
| Fungus | |||||
| Saprolegnia parasitica | Saprolegniasis | Identification and characterization of developmental stages | - | iTRAQ SDS-PAGE Nano-LC-MS/MS | [124] |
| Parasites | |||||
| Caligus rogercresseyi | Sea lice | Detection and identification | Virulence determinants | MALDI-TOF-MS | [119] |
Proteomic techniques abbreviations—1-DE: One-dimensional Electrophoresis; 2-DE: Two-dimensional Electrophoresis; SDS-PAGE: Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis; iTRAQ: Isobaric Tag for Relative and Absolute Quantitation; MALDI-TOF-MS: Matrix-Assisted Laser Desorption and Ionization Time-of-Flight Mass Spectrometry; MALDI-TOF/TOF-MS: Matrix-Assisted Laser Desorption and Ionization (Time-of-Flight)2 Mass Spectrometry; MALDI-TOF/TOF-MS/MS: Matrix-Assisted Laser Desorption and Ionization (Time-of-Flight)2 tandem Mass Spectrometry; LC-MALDI-TOF/TOF-MS/MS: Automated Liquid Chromatography Matrix-Assisted Laser Desorption and Ionization (Time-of-Flight)2 tandem Mass Spectrometry; LC-MS/MS: Liquid Chromatography tandem Mass Spectrometry; ESI MS/MS: Electrospray Ionization tandem Mass Spectrometry; LC- ESI-Q-TOF MS/MS: Liquid Chromatography Electrospray Ionization Quadrupole Time-of-Flight tandem Mass Spectrometry; LC-nano ESI-Q-TOF MS/MS: Liquid Chromatography and Nano-Electrospray Ionization Quadrupole Time-of-Flight tandem Mass Spectrometry; LC-ESI-MS/MS: Liquid Chromatography Electrospray Ionization tandem Mass Spectrometry; nLC-ESI-MS/MS: Nano-scale Liquid Chromatography Electrospray Ionization tandem Mass Spectrometry; NanoUPLC-HDMSE: Ultra-Performance Liquid Chromatography with High Definition tandem Mass Spectrometry.
This powerful tool can be used for pathogen identification as a complement to other molecular genetic techniques, being Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) the main technique used for this purpose [98]. Is also very useful for virulence factors characterization and life cycle characterization of pathogens [125,126].
3.2. Symptomatology
Pathogens have different impacts on fish since the severity of infection depends on diverse factors, such as the host species, fish age and physiological state, environmental conditions, and disease stage [127,128].
Generally, diseases can be expressed in different stages and can develop from an acute to a chronic disease or the reverse way. This is the case of the infectious salmon anaemia (ISA) in Atlantic salmon outbreaks, with initial low mortality, causing minor alterations in the fish (e.g., anaemia). This chronic stage can go unnoticed if diagnostic measures are not performed. Acute disease stages with high mortality may occur sporadically, increasing the severity of the disease (e.g., ascitis and haemorrhages). Furthermore, ISA chronic infection develops in the autumn, while the acute stage is observed more in the spring [129].
Besides infections with virus, bacteria, parasites and fungus, fish can be exposed to secondary infections that can aggravate their health status and increase mortality rate [130]. Observation of clinical signs (external or internally) and behaviour alterations can help to detect a pathogen presence in fish. However, the signs exhibited in response to a disease can be non-specific of that disease and very similar between different pathogen infections (Table 2). Moreover, the fish might show few or none of these signs. After these observations, gross and microscopic pathology can be used to confirm some pathogens yet is often necessary the use of more specific types of diagnosis for the identification [19].
Table 2.
Symptomatology of important diseases caused by virus, bacteria, and parasites.
| Disease | Pathogen | Host | Clinical Signs/Pathology | References |
|---|---|---|---|---|
| Virus | ||||
| Epizootic haematopoietic necrosis (EHN) | Epizootic haematopoietic necrosis virus | Redfin perch (Perca fluviatilis), Rainbow trout (Oncorhynchus mykiss) | Erratic swimming, darkened skin, skin ulcers, exophthalmia, swollen spleen and kidney, petechial haemorrhages on fins, ascites, abdominal distension | [131] |
| Infectious haematopoietic necrosis (IHN) | Infectious haematopoietic necrosis virus |
Salmonids | Lethargy, darkened skin, exophthalmia, eye haemorrhage, pale gills, swollen abdomen, opaque faecal casts, petechial haemorrhage on fins, visceral pallor | [132,133] |
| Infectious Pancreatic Necrosis (IPN) | Infectious pancreatic necrosis virus | Salmonids | Irregular swimming, loss of appetite, darkening of the skin, distended abdomen, exophthalmia, pale gills petechial haemorrhages, visceral ascites, intestine with catarrhal exudate and pale liver | [134,135,136] |
| Infectious salmon Anaemia (ISA) | Infectious Salmon Anaemia virus | Atlantic salmon (Salmo salar) | Lethargy, anaemia, exophthalmia, pale gills and internal organs, ascites, oedemas, petechiae in visceral fat, liver and spleen congestion | [137,138] |
| Lymphocystis disease | Lymphocystis disease virus | Broadly infectious | Nodular lesions on the skin, fins and internally | [137] |
| Pancreatic disease (PD) | Salmonid alphaviruses (SAV) | Rainbow trout (Oncorhynchus mykiss), Atlantic salmon (Salmo salar) | Lethargy, hang in the corners of the cage or rest in the bottom, loss of appetite, yellow faecal casts, ascites, petechial haemorrhages in pyloric caecal fat, lesions in pancreas and skeletal and cardiac muscle | [139,140] |
| Viral haemorrhagic septicaemia (VHS) | Viral haemorrhagic septicaemia virus | Broadly infectious | Aberrant swimming (spiral, leaping, flashing), exophthalmia, darkened skin, anaemia, pale gills and liver, internal haemorrhages, ascites leading to a swollen abdomen, swollen and hyperaemic kidney | [137,141] |
| Viral nervous necrosis (VNN) | Betanodaviruses | Broadly infectious | Lethargy, abnormal swimming; anorexia, skin darkening, abdominal distension, hyperinflation of the swim bladder | [142,143] |
| Bacteria | ||||
| Vibriosis | Vibrio anguillarum | Broadly infectious | Lethargy, cease feeding, darkened skin, exophthalmia and corneal opacity, pale gills, petechiae at fin bases and skin, ulcers, generalized septicaemia | [127,144,145] |
| Pasteurellosis |
Photobacterium damselae subsp. piscicida |
White perch (Morone americanus) yellowtail (Seriola quinqueradiata) gilthead seabream (Sparus aurata) | Darkened skin, swollen spleen, white-spotted lesions in spleen and kidney, bacterial accumulations on the tissues of internal organs | [146,147] |
| Furunculosis | Aeromonas salmonicida subsp. salmonicida | Broadly infectious | Lethargy, cease feeding, darkened skin, exophthalmia, haemorrhages at the base of the fins, enlarged spleen, lesions on the skin (furuncles), ulcers, pale liver, general septicaemia | [148,149] |
| Tenacibaculosis | Tenacibaculum maritimum | Broadly infectious | Flashing swimming behaviour, anorexic, erosion on the skin, fins (tail rot), head and gills, petechial haemorrhage on the abdominal peritoneum, ulcers | [150] |
| Bacterial gill disease (BGD) | Flavobacterium branchiophilum | Coldwater fish (mainly salmonids) | Loss of appetite, gill infestation, increased opercular movements, gasping at the water surface, respiratory distress | [151] |
| Rainbow Trout Fry Syndrom (RFTS) | Flavobacterium psychrophilum | Salmonids | Lethargy, anorexia, distended abdomen, darkened skin in caudal peduncle area, skin ulceration, swollen spleen, pale organs | [152] |
| Red spot disease (Winter disease) | Pseudomonas anguilliseptica | Eels, salmonids, gilthead seabream (Sparus aurata), European seabass (Dicentrarchus labrax), European cod (Gadus morhua) | Erratic swimming, petechial haemorrhages in the skin and liver, distended abdomen, ascitic fluid in the peritoneal cavity, pale liver, haemorrhaged kidney, intestine with yellowish exudate | [144,153] |
| Disease | Pathogen | Host | Clinical Signs/Pathology | References |
| Lactococcosis | Lactococcus garvieae | Broadly infectious | Lethargy, anorexia, exophthalmia, distended abdomen, ascitic fluid in the peritoneal cavity, congestion and haemorrhages of liver, intestine, spleen and kidney, haemorrhagic septicaemia | [154] |
| Parasites | ||||
| Amoebic gill disease (AGD) | Neoparamoeba perurans | Atlantic salmon (Salmo salar), rainbow trout (Oncorhynchus mykiss) |
Lethargy, respiratory distress, increased opercular movements, whitish patches on gills and excessive mucus | [155,156] |
| White spot disease/Ichthyophthiriasis | Ichthyophthirius multifiliis | Freshwater fish | Hyperactive (initially), lethargic; pale gills, darkened skin, white spots in the skin, increase of mucus production, skin ulcers, frayed fins, pale liver, enlarged spleen and kidney | [128,157,158] |
| White spot disease/Cryptocaryoniasis | Cryptocaryon irritans | Saltwater fish | Hyperactive (initially), lethargic, numerous small whitish spots on the skin surface, petechial haemorrhages on the skin, excessive mucus production, skin ulcers, corneal clouding and blindness | [128,159] |
| Amyloodiniosis | Amyloodinium ocellatum | Broadly infectious | Cease feeding, scratch against the bottom, infested gills and skin, excessive mucus production, epithelial erosion on attachment sites | [160,161,162] |
| Trichodinosis | Trichodina sp. | Broadly infectious | Lethargy, cease feeding, infested gills, skin and fins, Greyish colour due to excessive mucus production, skin lesions in attachment sites, frayed fins | [163,164] |
| Gyrodactilosis | Gyrodactylus salaris | Salmonids | Infest mainly fins and skin, lethargy, anorexia, emaciated fins, darkened skin, epithelium lesions | [165,166] |
| Sea lice | Lepeophtheirus salmonis | Salmonids | Skin lesions, especially on the head, haemorrhages, scale loss, oedema, hyperplasia and cellular inflammations | [167] |
As shown in Table 2, even if disease symptomatology is extremely used in disease characterization, it is difficult to distinguish between several diseases with similar symptomatology. Taking this into account, several researchers suggested that host-pathogen interaction are more reproducible and more reliable indicators for disease diagnostics [21,81,168].
4. Tools for the Study of Host-Pathogen Interactions
4.1. The “Holobiome” Approach: Metagenomics and Metaproteomics
The host-pathogen interactions are extremely complex and can be established at multiple levels, ranging from molecular, cellular and physiological, to populations and ecosystems levels [169]. The host-pathogen interaction starts when the host organism is challenged by a pathogenic agent e.g., virus, bacteria, prion, fungus, viroid, or parasite, thus triggering a biological response; the pathogen, in turn, develops a back-fighting response [170,171]. This interaction implies induction of gene expression and protein synthesis on both sides, and an infectious process may develop in the host, leading ultimately to death, if the host response or defense system fails to combat the pathogenic challenge [171]. However, a wider perspective on host-pathogen interactions may be undertaken [172], spanning these interactions to the associated microbial populations e.g., the host microbiome, known as the “holobiome” approach [173]. Indeed, it has been demonstrated that the microbiota may play a critical role in the immune response of organisms [174]. The “holobiome” approach on the study of fish host-pathogen interactions i.e., between the fish host, its microbiome, the pathogen, and other environmental microorganisms, has been pointed as a critical aspect for further development of rational strategies aiming at fish disease prevention and resistance [172]. Moreover, this holistic knowledge of fish host-pathogen interactions could contribute to promote sustainability in aquaculture, by reducing the use of antibiotics, responsible for a negative environmental impact of this industry [172].
Metagenomics and metaproteomics are among the most powerful and emerging high-throughput tools in marine/ocean environments to disclose the genome and proteome, of the associated microbial communities [172,175,176,177]. These methodologies are still scarce on aquaculture research, although it might be extremely useful in the study of microbial populations inherent to the farmed fish surrounding environment. Furthermore, metagenomics and metaproteomics approaches enable the characterization of the microbiota associated to fish skin mucous or fish gut, thus unravelling key genes or proteins in the immune function, that may act as the whole biosystem through complex networks during fish host-pathogen interactions. An additional and major benefit of these tools is the possibility of accessing to unculturable species, the vast majority of disease-related microbes in aquaculture, whose identity and function would otherwise remain unknown [172].
4.2. Omics-Based Strategies and Protein-Protein Interaction (PPI) Networks
The knowledge on the genes/proteins and metabolites involved in host-pathogen interactions during infectious events has assisted to considerable advances in the last years, due to the implementation of high-throughput technologies like RNA-sequencing (RNA-Seq) [178] mass-spectrometry based proteomics [126] and metabolomics [179]. On the other hand, the combination of omics-based approaches with in vivo studies, addressing interactions from the single cell to the whole animal level, by using zebrafish (Danio rerio) larvae as infection models [180,181], constituted a step forward in the understanding of the cellular mechanisms that occur during fish-pathogen interactions.
The large-scale proteome characterization from both pathogen(s) and fish host, either in health or disease conditions, allowed to identify proteins with a major role in disease defense mechanisms (recently reviewed by [126]), whose regulatory complexity might be represented by protein-protein interaction (PPI) networks. The integration of proteomics with other omics-based approaches may be used to model networks capable of predicting the interaction dynamics between cellular bio-components involved in fish-pathogen immune responses (e.g., DNA, RNA, protein, metabolite) to foster new therapeutic strategies in aquaculture [27,179]. It can be stated that proteins as the main key players and building blocks across all life forms, since they catalyze and control virtually all cellular processes [33], hence occupy a central role in host-pathogen interactions. PPIs networks, either determined at experimental level e.g., through interactome proteomic approaches [182] or predicted by computational methodologies, are gaining increasing popularity and becoming one of the most useful tools in the understanding of pathogenesis [183]. PPI networks may offer unique insights into host-pathogen and pathogen co-infection interactions, by identifying effective health/disease biomarkers, thus accelerating the implementation of prevention measures, treatment of fish diseases and vaccination development [183]. PPI network analysis will be no doubt, one of the most powerful and cost-effective tools to assist in fish disease management in the aquaculture sector.
In sum, there is a significant number of emerging tools to address fish host-pathogen interactions that can help in the control, prevention, and treatment of diseases in farmed fish, becoming evident that these interactions are extremely complex, requiring integrated, complementary, and holistic approaches to be fully understood.
Proteomics is also highly used to understand the fish immune response, surviving strategies of the pathogen and interactions between fish and pathogen [126]. As this technique can show differential expression of identified proteins in various stages of fish development, and different conditions of feeding, stress and disease [184], it provides a holistic overview of several functions of the fish metabolism [185]. Differential expression of proteins affected by any pathogen might be studied using gel-based (1 or 2-DE) or gel-free applications (LC-MS/MS) [186]. An overview of some proteomic studies with fish pathogens is shown in Table 3. In the case of viruses, several proteins have been modified although differences depend on the type of virus. Spleen tissue of infected zebrafish and turbot (Scophtalmus maximus) with Megalocytivirus showed that cytoskeletal and cellular signal transduction proteins were modified in both species [89,187]. Pancreas disease caused by salmonid alphavirus in Atlantic salmon showed that humoral components of the serum were affected during the first weeks after infection [94]. Proteins involved in the glycolytic pathway and cytoskeleton were modified during viral haemorrhagic septicaemia rhabdovirus in zebrafish [188]. Host defences against spring viremia of carp virus use mainly proteins like vitellogenin and grass carp reovirus induced protein Gig2 [189]. These proteins seem to have a potential antiviral activity. Red blood cells in teleost can respond to pathogens and trigger an immune response against the viral septicaemia haemorrhagic virus [190]. As a defensive mechanism against cyprinid herpesvirus-2 several proteins like herpes simplex infection pathway, p53 signalling pathways and phagosome pathway were induced [191]. Against bacterial infections, the immune system of teleost fish is triggered, as shown by both the induced acute phase and immune responses in the liver or spleen, respectively of rainbow trout against Aeromonas salmonicida [192,193]. Or by the enhanced immune response against Aeromonas hydrophila in common carp (Cyprinus carpio) and zebrafish [91,194]. More specific, proteins involved in the cellular stress response were modified in channel catfish (Ictalurus punctatus) after a challenge with Edwardsiella ictaluri [195]. Enteric redmouth disease in salmonids resulted in several differentially expressed proteins in head kidney and liver samples of rainbow trout like antioxidants, lysozyme, metalloproteinase, cytoskeleton and c-type lectin receptor proteins [95]. Up-regulated proteins involved in peptidase and hydrolase activity, lysosome and metabolic pathways were identified in intestinal mucosal samples [196]. Detected on the first defence barrier of fish, the skin mucus showed differentially expressed proteins of the immune system of Atlantic cod with vibriosis [83]. Also, by proteins like heat-shock proteins, cathepsins and complement components it is shown that the immune response is up-regulated against Streptococcus parauberis in olive flounder (Paralichthys olivaceus) [197]. Mitochondrial enzymes also showed altered expression upon Moraxella sp. infection in kidney tissues of gilthead seabream (Sparus aurata) [198]. Infections with the ciliated parasite Ichthyophthirius multifiliis results in increased mucus secretion in fish. Proteomics of mucus in infested common carp with I. multifiliis showed an up-regulation of immune-related and signal transduction proteins in the first defence barrier of fish [199]. Infestations of Atlantic salmon with the ectoparasite Lepeophtheirus salmonis were studied on fish mucus and detected an increase in proteins involved in proteolysis [82]. When looking into the plasma of infested gilthead seabream with Amyloodinium ocellatum, differences were found in proteins involved in the acute-phase response, inflammation, homeostasis and wound healing but, in this case, the innate immunological system was not activated [88]. Another ectoparasite that affects Atlantic salmon is the amoeba Neoparamoeba perurans, causing amoebic gill disease. Proteomic analysis showed that proteins involved in the cell cycle regulation, inflammation pathway, oxidative metabolism and immunity were affected [200,201].
Table 3.
Summary of some modified proteins identified by proteomics in fish infectious diseases.
| Aetiological Agent | Species | Tissue | Modified Protein Groups | Reference |
|---|---|---|---|---|
| Vírus | ||||
| Infectious spleen and kidney necrosis virus (ISKNV) (Megalocytivirus) | Zebrafish (Danio rerio) | Spleen | Cytoskeletal proteins, stress response, lipoprotein and carbohydrate metabolism, signal transduction, proteolysis, metabolic and catabolic processes | [89] |
| Megalocytivirus | Turbot (Scophtalmus maximus) | Spleen | Cytoskeleton proteins, molecular biosynthesis, cellular signal transduction and chaperone proteins | [187] |
| Salmonid alphavirus subtype 3 (SAV3) | Atlantic salmon (Salmo salar) | Serum | Humoral components of immunity | [94] |
| Rhabdovirus | Zebrafish (Danio rerio) | Fins | Proteins of the glycolytic pathway and cytoskeleton components | [188] |
| Spring viremia of carp virus | Zebrafish (Danio rerio) | Plasma | Vitellogenin and Gig2 | [189] |
| Viral septicemia hemorrhagic virus | Rainbow trout (Oncorhynchus mykiss) | Red blood cells from blood and head kidney | Proteins related to viral transcription | [190] |
| Cyprinid herpesvirus-2 | Crucian carp (Carassius carassius) | Head kidney | Cytoskeleton, transport, immunologic, intracellular and physiologic proteins | [191] |
| Bacteria | ||||
| Aeromonas salmonicida | Rainbow trout (Oncorhynchus mykiss) | Liver | Complement system and acute phase response proteins | [192] |
| Aeromonas salmonicida | Rainbow trout (Oncorhynchus mykiss) | Spleen | Immune system, signaling molecules and interaction | [193] |
| Aeromonas hydrophila | Common carp (Cyprinus carpio) | Intestinal mucosa | Proteins involved in stress and immune response | [194] |
| Aeromonas hydrophila | Zebrafish (Danio rerio) | Gills | Stress and immune response | [91] |
| Edwardsiella ictaluri | Channel catfish (Ictalurus punctatus) | Head kidney | Macrophage function, cellular stress response, cellular energy production and metabolism | [195] |
| Yersinia ruckeri | Rainbow trout (Oncorhynchus mykiss) | Head kidney and spleen | Immune system, cellular, metabolic, developmental, multicellular, adhesion, regulation and response to stimulus | [95] |
| Yersinia ruckeri | Rainbow trout (Oncorhynchus mykiss) | Intestine | Metabolic process biological regulation, cellular processes and component organization | [196] |
| Vibrio anguillarum | Atlantic cod (Gadus morhua) | Mucus | Proteins involved in the immune system | [83] |
| Streptococcus parauberis | Olive flounder (Paralichthys olivaceus) | Kidney | Proteins involved in immune response, cellular recovery and glycoprotein synthesis | [197] |
| Moraxella sp. | Gilthead seabream (Sparus aurata) | Kidney | Mitochondrial proteins, cellular response to oxidative stress, infection and inflammation | [198] |
| Parasites | ||||
| Ichthyophthirius multifiliis | Common carp (Cyprinus carpio) | Mucus | Proteins involved in the immune and inflammatory response | [199] |
| Lepeophtheirus salmonis | Atlantic salmon (Salmo salar) | Mucus | Proteins involved in glycolysis, peptide synthesis, immune and defence response | [82] |
| Amyloodinium ocellatum | Gilthead seabream (Sparus aurata) | Plasma | Proteins involved in the acute-phase response, inflammation, lipid transport, homeostasis and wound healing | [88] |
| Neoparamoeba perurans | Atlantic salmon (Salmo salar) | Gill and skin mucus | Proteins involved in cell to cell signalling and inflammation pathway | [189] |
| Neoparamoeba perurans | Atlantic salmon (Salmo salar) | Gill and skin mucus | Proteins involved in cell cycle regulation, cytoskeletal regulation, oxidative metabolism and immunity | [201] |
Although some examples were given in Table 3, more studies were performed as each tissue/organ in fish represents a specific barrier against pathogens, and several of them have been used in proteomic studies. Like the shotgun proteomic approach of serum proteins from turbot infected by Edwardsiella tarda, showing that immunoglobulins and complement component proteins were important antimicrobial proteins [202]. Or the study on Infections by Mycrocystis aeruginosa infections on medaka (Oryzias latipes) fish, that showed differences in liver proteins such as stress response, lipid metabolism and developmental processes [203].
Proteomics may also be used to analyse the pathogen in vitro, which is shown by the reduced expression of proteins related to the tricarboxylic acid cycle and chemotaxis when chlortetracycline antibiotic was used against A. hydrophila [204]. Virulence mechanisms of bacteria can be studied using proteomics for the visualization of up and down-regulated proteins in virulent and avirulent strains. In the case of E. tarda proteins, like antigenic protein Et 46, bifunctional polymyxin resistance protein and iron-cofactored superoxide dismutase type I were identified [92]. And in the case of Y. ruckeri proteins like anti-sigma regulatory factor, arginine deiminase, and superoxide dismutase Cu-Zu were identified [107]. It is known that different conditions like temperature may affect a facultative pathogen like Pseudomonas plecoglossicida which showed upregulation of the pyoverdine protein at 18 °C, which is important for bacterial multiplication [205]. The iron metal is essential for bacteria [27], as shown in Vibrio spp., which was able to trap iron [125], and by Aeromonas salmonicida [206]. The outer membrane proteins, important for virulence by Y. ruckeri on Atlantic salmon and rainbow trout were identified in different isolates [109]. As parasites go through various life stages different proteins are needed in each one of them. Proteomics was applied to identify these proteins in I. multifiliis and showed proteins involved in biological processes, cellular components, molecular functions, binding and catalytic activity [207]. And in the case of Anisakis simplex proteins like pseudocoelomic globin, endochitinase 1 and paramyosin were identified in L3 developmental stage [208].
To understand the interaction between a pathogen and its host proteomics seems to be a good tool. As mentioned before the outer membrane proteins are important for pathogenicity. The immunity of fish might be reduced as proteins of bacteria are capable of interacting with extracellular proteins [209]. In the case of Gram-negative bacteria, outer membrane proteins seem to be able to survive inside the fish [210] and can present resistance to antimicrobial peptides [211]. Another technique used to identify pathogenic proteins was immunoproteomics. Immunized sera from rohu (Labeo rohita) and grass carp (Ctenopharyngodon idella) was used to identify outer membrane proteins from E. tarda [212] and Flavobacterium columnare [213]. Several outer membrane proteins were identified by immunized sera of Nile Tilapia with Francisella noatunensis subsp. orientalis [211].
5. Conclusions
Overall, we can look at proteomics as a very promising tool for fish pathology research and diagnostic, allowing a more holistic approach to pathogenesis processes, giving important information on pathogen identification and virulence mechanisms characterization and in host-pathogen interactions, enlightening new stress response routes and previously unknown physiological host responses.
However, the use of proteomics in fish aquaculture is still in its early days and limited to some sequenced organisms. Further progress in defining aquacultural proteomes and large-scale datasets from diseased fish and fish pathogens will boost the use of proteomic techniques in aquaculture, that will lead to new and exciting discoveries on this field.
But one of the most promising and interesting areas and one that we believe being the future trend in further understanding the fish response to pathogens, is the study of the interaction holobiome-host-pathogen, with a strong potential for new and more detailed and integrated knowledge of fish pathogenesis.
Author Contributions
M.M.: Conceptualization, Methodology, Formal analysis, Investigation, writing—Original Draft, writing—Review & Editing Visualization. D.S.: Writing—Original Draft, Writing—Review & Editing. A.P.F. and M.C.: Writing—Original Draft. C.R.d.M.: Writing—Original Draft, Visualization. R.C.: Writing—Original Draft, Writing—Review & Editing. P.R.: Project Administration, Funding Acquisition, Conceptualization, Writing—Review & Editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work received national funding through the Foundation for Science and Technology (FCT) through project UID/Multi/04326/2020 and projects WELFISH (Refª 16-02-05-FMP-12, “Establishment of Welfare Biomarkers in farmed fish using a proteomics approach”), ALLYFISH (Refª 16-02-01-FMP-0014, “Development of a farmed fish with reduced allergenic potential”) and project SAÚDE & AQUA (MAR-02.05.01-FEAMP-0009), both financed by Mar2020, in the framework of the program Portugal 2020. Márcio Moreira, Cláudia Raposo de Magalhães, and Denise Schrama acknowledge FCT PhD fellowships SFRH/BD/118601/2016, SFRH/BD/138884/2018, and SFRH/BD/136319/2018, respectively.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bank T.W. Fish to 2030—Prospects for Fisheries and Aquaculture. International Bank for Reconstruction and Development/International Development Association or The World Bank; Washington, DC, USA: 2013. p. 77. [Google Scholar]
- 2.FAO . The State of World Fisheries and Aquaculture 2020. Sustainability in Action. FAO; Rome, Italy: 2020. [Google Scholar]
- 3.Rodrigues P.M., Schrama D., Campos A., Osório H., Freitas M. Applications of Proteomics in Aquaculture. In: Salekdeh G.H., editor. Agricultural Proteomics Volume 1: Crops, Horticulture, Farm Animals, Food, Insect and Microorganisms. Springer International Publishing; Cham, Switzerland: 2016. pp. 165–199. [Google Scholar]
- 4.Murray A.G., Peeler E.J. A framework for understanding the potential for emerging diseases in aquaculture. Prev. Vet. Med. 2005;67:223–235. doi: 10.1016/j.prevetmed.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Waagbø R. Chapter 13 Feeding and disease resistance in fish. In: Mosenthin R., Zentek J., Żebrowska T., editors. Biology of Growing Animals. Volume 4. Elsevier; Amsterdam, The Netherlands: 2006. pp. 387–415. [Google Scholar]
- 6.Barber I. Parasites, behaviour and welfare in fish. Appl. Anim. Behav. Sci. 2007;104:251–264. doi: 10.1016/j.applanim.2006.09.005. [DOI] [Google Scholar]
- 7.Brugere C., Onuigbo D.M., Morgan K.L. People matter in animal disease surveillance: Challenges and opportunities for the aquaculture sector. Aquaculture. 2017;467:158–169. doi: 10.1016/j.aquaculture.2016.04.012. [DOI] [Google Scholar]
- 8.Chintagari S., Hazard N., Edwards G., Jadeja R., Janes M. Risks associated with fish and seafood. Preharvest Food Saf. 2018:123–142. doi: 10.1128/9781555819644.ch7. [DOI] [PubMed] [Google Scholar]
- 9.Hill B.J. The need for effective disease control in international aquaculture. Dev. Biol. 2005;121:3–12. [PubMed] [Google Scholar]
- 10.Shinn A.P., Pratoomyot J., Bron J.E., Paladini G., Brooker E.E., Brooker A.J. Economic costs of protistan and metazoan parasites to global mariculture. Parasitology. 2015;142:196–270. doi: 10.1017/S0031182014001437. [DOI] [PubMed] [Google Scholar]
- 11.Iwama G.K. The welfare of fish. Dis. Aquat. Org. 2007;75:155–158. doi: 10.3354/dao075155. [DOI] [PubMed] [Google Scholar]
- 12.Adams A., Thompson K.D. Biotechnology offers revolution to fish health management. Trends Biotechnol. 2006;24:201–205. doi: 10.1016/j.tibtech.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Aung M.M., Chang Y.S. Traceability in a food supply chain: Safety and quality perspectives. Food Control. 2014;39:172–184. doi: 10.1016/j.foodcont.2013.11.007. [DOI] [Google Scholar]
- 14.Trienekens J., Zuurbier P. Quality and safety standards in the food industry, developments and challenges. Int. J. Prod. Econ. 2008;113:107–122. doi: 10.1016/j.ijpe.2007.02.050. [DOI] [Google Scholar]
- 15.Peeler E.J., Taylor N.G.H. The application of epidemiology in aquatic animal health -opportunities and challenges. Vet. Res. 2011;42:94. doi: 10.1186/1297-9716-42-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedrick R.P. Movement of pathogens with the international trade of live fish: Problems and solutions. Rev. Sci. Tech. 1996;15:523–531. doi: 10.20506/rst.15.2.938. [DOI] [PubMed] [Google Scholar]
- 17.Cameron A. Survey Toolbox for Aquatic Animal Diseases: A Practical Manual and Software Package. Australian Centre for International Agricultural Research; Canberra, Australia: 2002. 375p ACIAR Monograph No. 94. [Google Scholar]
- 18.Oidtmann B.C., Peeler E.J., Thrush M.A., Cameron A.R., Reese R.A., Pearce F.M., Dunn P., Lyngstad T.M., Tavornpanich S., Brun E., et al. Expert consultation on risk factors for introduction of infectious pathogens into fish farms. Prev. Vet. Med. 2014;115:238–254. doi: 10.1016/j.prevetmed.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Sitjà-Bobadilla A., Oidtmann B. Chapter 5—Integrated Pathogen Management Strategies in Fish Farming. In: Jeney G., editor. Fish Diseases. Academic Press; Cambridge, MA, USA: 2017. pp. 119–144. [Google Scholar]
- 20.Freitas J., Vaz-Pires P., Câmara J.S. From aquaculture production to consumption: Freshness, safety, traceability and authentication, the four pillars of quality. Aquaculture. 2020;518:734857. doi: 10.1016/j.aquaculture.2019.734857. [DOI] [Google Scholar]
- 21.Scarfe A.D., Palić D. Aquaculture Health Management. Elsevier; Amsterdam, The Netherlands: 2020. Aquaculture biosecurity: Practical approach to prevent, control, and eradicate diseases; pp. 75–116. [Google Scholar]
- 22.Rodrigues P.M., Martin S.A.M., Silva T.S., Boonanuntanasarn S., Schrama D., Moreira M., Raposo C. Proteomics in Fish and Aquaculture Research. In: de Almeida A.M., Eckersall D., Miller I., editors. Proteomics in Domestic Animals: From Farm to Systems Biology. Springer International Publishing; Cham, Switzerland: 2018. pp. 311–338. [Google Scholar]
- 23.Cash P. Proteomics in the study of the molecular taxonomy and epidemiology of bacterial pathogens. Electrophoresis. 2009;30:S133–S141. doi: 10.1002/elps.200900059. [DOI] [PubMed] [Google Scholar]
- 24.Lafferty K.D. The ecology of climate change and infectious diseases. Ecology. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- 25.Parrington J., Coward K. Use of emerging genomic and proteomic technologies in fish physiology. Aquat. Living Resour. 2002;15:193–196. doi: 10.1016/S0990-7440(02)01172-5. [DOI] [Google Scholar]
- 26.Burge C.A., Friedman C.S., Getchell R., House M., Lafferty K.D., Mydlarz L.D., Prager K.C., Sutherland K.P., Renault T., Kiryu I., et al. Complementary approaches to diagnosing marine diseases: A union of the modern and the classic. Philos. Trans. R. Soc. B Biol. Sci. 2016;371 doi: 10.1098/rstb.2015.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gotesman M., Menanteau-Ledouble S., Saleh M., Bergmann S.M., El-Matbouli M. A new age in AquaMedicine: Unconventional approach in studying aquatic diseases. BMC Vet. Res. 2018;14:178. doi: 10.1186/s12917-018-1501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oskoueian E., Eckersall P.D., Bencurova E., Dandekar T. Application of Proteomic Biomarkers in Livestock Disease Management. In: Salekdeh G.H., editor. Agricultural Proteomics Volume 2: Environmental Stresses. Springer International Publishing; Cham, Switzerland: 2016. pp. 299–310. [Google Scholar]
- 29.Alves R.N., Cordeiro O., Silva T.S., Richard N., de Vareilles M., Marino G., Di Marco P., Rodrigues P.M., Conceição L.E.C. Metabolic molecular indicators of chronic stress in gilthead seabream (Sparus aurata) using comparative proteomics. Aquaculture. 2010;299:57–66. doi: 10.1016/j.aquaculture.2009.11.014. [DOI] [Google Scholar]
- 30.Rodrigues P.M., Silva T.S., Dias J., Jessen F. PROTEOMICS in aquaculture: Applications and trends. J. Proteom. 2012;75:4325–4345. doi: 10.1016/j.jprot.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 31.Wilkins M.R., Pasquali C., Appel R.D., Ou K., Golaz O., Sanchez J.-C., Yan J.X., Gooley A.A., Hughes G., Humphery-Smith I., et al. From Proteins to Proteomes: Large Scale Protein Identification by Two-Dimensional Electrophoresis and Arnino Acid Analysis. Bio/Technology. 1996;14:61–65. doi: 10.1038/nbt0196-61. [DOI] [PubMed] [Google Scholar]
- 32.Cox J., Mann M. Quantitative, high-resolution proteomics for data-driven systems biology. Annu. Rev. Biochem. 2011;80:273–299. doi: 10.1146/annurev-biochem-061308-093216. [DOI] [PubMed] [Google Scholar]
- 33.Aebersold R., Mann M. Mass-spectrometric exploration of proteome structure and function. Nature. 2016;537:347–355. doi: 10.1038/nature19949. [DOI] [PubMed] [Google Scholar]
- 34.Parker C.G., Pratt M.R. Click Chemistry in Proteomic Investigations. Cell. 2020;180:605–632. doi: 10.1016/j.cell.2020.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huntingford F.A., Adams C., Braithwaite V.A., Kadri S., Pottinger T.G., Sandøe P., Turnbull J.F. Current issues in fish welfare. J. Fish Biol. 2006;68:332–372. doi: 10.1111/j.0022-1112.2006.001046.x. [DOI] [Google Scholar]
- 36.Brown C. Fish intelligence, sentience and ethics. Anim. Cogn. 2015;18:1–17. doi: 10.1007/s10071-014-0761-0. [DOI] [PubMed] [Google Scholar]
- 37.Ashley P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007;104:199–235. doi: 10.1016/j.applanim.2006.09.001. [DOI] [Google Scholar]
- 38.Martins C.I.M., Galhardo L., Noble C., Damsgård B., Spedicato M.T., Zupa W., Beauchaud M., Kulczykowska E., Massabuau J.-C., Carter T., et al. Behavioural indicators of welfare in farmed fish. Fish Physiol. Biochem. 2012;38:17–41. doi: 10.1007/s10695-011-9518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dawkins M.S. Evolution and Animal Welfare. Q. Rev. Biol. 1998;73:305–328. doi: 10.1086/420307. [DOI] [PubMed] [Google Scholar]
- 40.Saraiva J.L., Castanheira M.F., Arechavala-López P., Volstorf J., Studer B.H. Animal Domestication. IntechOpen; London, UK: 2018. Domestication and welfare in farmed fish. [Google Scholar]
- 41.Wall T. Fish Welfare. Blackwell Publishing Ltd.; Oxford, Uk: 2008. Disease and Medicines—The Welfare Implications; pp. 195–201. [Google Scholar]
- 42.Sørum U., Damsgård B. Effects of anaesthetisation and vaccination on feed intake and growth in Atlantic salmon (Salmo salar L.) Aquaculture. 2004;232:333–341. doi: 10.1016/S0044-8486(03)00529-5. [DOI] [Google Scholar]
- 43.Huntingford F.A., Kadri S. Defining, assessing and promoting the welfare of farmed fish. Rev. Sci. Tech. 2014;33:233–244. doi: 10.20506/rst.33.1.2286. [DOI] [PubMed] [Google Scholar]
- 44.Segner H., Sundh H., Buchmann K., Douxfils J., Sundell K.S., Mathieu C., Ruane N., Jutfelt F., Toften H., Vaughan L. Health of farmed fish: Its relation to fish welfare and its utility as welfare indicator. Fish Physiol. Biochem. 2012;38:85–105. doi: 10.1007/s10695-011-9517-9. [DOI] [PubMed] [Google Scholar]
- 45.Conte F.S. Stress and the welfare of cultured fish. Appl. Anim. Behav. Sci. 2004;86:205–223. doi: 10.1016/j.applanim.2004.02.003. [DOI] [Google Scholar]
- 46.Ndong D., Chen Y.-Y., Lin Y.-H., Vaseeharan B., Chen J.-C. The immune response of tilapia Oreochromis mossambicus and its susceptibility to Streptococcus iniae under stress in low and high temperatures. Fish Shellfish Immunol. 2007;22:686–694. doi: 10.1016/j.fsi.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 47.Ellison A.R., Uren Webster T.M., Rey O., Garcia de Leaniz C., Consuegra S., Orozco-terWengel P., Cable J. Transcriptomic response to parasite infection in Nile tilapia (Oreochromis niloticus) depends on rearing density. BMC Genom. 2018;19:723. doi: 10.1186/s12864-018-5098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang I.-F., Bharath Kumar V., Lee D.-N., Weng C.-F. Acute osmotic stress affects Tilapia (Oreochromis mossambicus) innate immune responses. Fish Shellfish Immunol. 2008;25:841–846. doi: 10.1016/j.fsi.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Caipang C.M.A., Brinchmann M.F., Berg I., Iversen M., Eliassen R., Kiron V. Changes in selected stress and immune-related genes in Atlantic cod, Gadus morhua, following overcrowding. Aquac. Res. 2008;39:1533–1540. doi: 10.1111/j.1365-2109.2008.02026.x. [DOI] [Google Scholar]
- 50.Korytář T., Nipkow M., Altmann S., Goldammer T., Köllner B., Rebl A. Adverse Husbandry of Maraena Whitefish Directs the Immune System to Increase Mobilization of Myeloid Cells and Proinflammatory Responses. Front. Immunol. 2016;7:631. doi: 10.3389/fimmu.2016.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caipang C.M.A., Berg I., Brinchmann M.F., Kiron V. Short-term crowding stress in Atlantic cod, Gadus morhua L. modulates the humoral immune response. Aquaculture. 2009;295:110–115. doi: 10.1016/j.aquaculture.2009.06.036. [DOI] [Google Scholar]
- 52.Costas B., Conceição L.E.C., Aragão C., Martos J.A., Ruiz-Jarabo I., Mancera J.M., Afonso A. Physiological responses of Senegalese sole (Solea senegalensis Kaup, 1858) after stress challenge: Effects on non-specific immune parameters, plasma free amino acids and energy metabolism. Aquaculture. 2011;316:68–76. doi: 10.1016/j.aquaculture.2011.03.011. [DOI] [Google Scholar]
- 53.Fast M.D., Hosoya S., Johnson S.C., Afonso L.O.B. Cortisol response and immune-related effects of Atlantic salmon (Salmo salar Linnaeus) subjected to short- and long-term stress. Fish Shellfish Immunol. 2008;24:194–204. doi: 10.1016/j.fsi.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Vazzana M., Cammarata M., Cooper E.L., Parrinello N. Confinement stress in sea bass (Dicentrarchus labrax) depresses peritoneal leukocyte cytotoxicity. Aquaculture. 2002;210:231–243. doi: 10.1016/S0044-8486(01)00818-3. [DOI] [Google Scholar]
- 55.Mauri I., Romero A., Acerete L., MacKenzie S., Roher N., Callol A., Cano I., Alvarez M.C., Tort L. Changes in complement responses in Gilthead seabream (Sparus aurata) and European seabass (Dicentrarchus labrax) under crowding stress, plus viral and bacterial challenges. Fish Shellfish Immunol. 2011;30:182–188. doi: 10.1016/j.fsi.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 56.MacKenzie S., Iliev D., Liarte C., Koskinen H., Planas J.V., Goetz F.W., Mölsä H., Krasnov A., Tort L. Transcriptional analysis of LPS-stimulated activation of trout (Oncorhynchus mykiss) monocyte/macrophage cells in primary culture treated with cortisol. Mol. Immunol. 2006;43:1340–1348. doi: 10.1016/j.molimm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 57.Douxfils J., Lambert S., Mathieu C., Milla S., Mandiki S.N.M., Henrotte E., Wang N., Dieu M., Raes M., Rougeot C., et al. Influence of domestication process on immune response to repeated emersion stressors in Eurasian perch (Perca fluviatilis L.) Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014;173:52–60. doi: 10.1016/j.cbpa.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 58.de Magalhães C.R., Schrama D., Farinha A.P., Revets D., Kuehn A., Planchon S., Rodrigues P.M., Cerqueira M. Protein changes as robust signatures of fish chronic stress: A proteomics approach to fish welfare research. BMC Genom. 2020;21:309. doi: 10.1186/s12864-020-6728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moberg G.P. Biological response to stress: Implications for animal welfare. Biol. Anim. Stress Basic Princ. Implic. Anim. Welf. 2000;1:21. [Google Scholar]
- 60.McEwen B.S., Wingfield J.C. What is in a name? Integrating homeostasis, allostasis and stress. Horm. Behav. 2010;57:105–111. doi: 10.1016/j.yhbeh.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wendelaar Bonga S.E. The stress response in fish. Physiol. Rev. 1997;77:591–625. doi: 10.1152/physrev.1997.77.3.591. [DOI] [PubMed] [Google Scholar]
- 62.Pankhurst N.W. The endocrinology of stress in fish: An environmental perspective. Gen. Comp. Endocrinol. 2011;170:265–275. doi: 10.1016/j.ygcen.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 63.Korte S.M., Olivier B., Koolhaas J.M. A new animal welfare concept based on allostasis. Physiol. Behav. 2007;92:422–428. doi: 10.1016/j.physbeh.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 64.Boonstra R. Reality as the leading cause of stress: Rethinking the impact of chronic stress in nature. Funct. Ecol. 2013;27:11–23. doi: 10.1111/1365-2435.12008. [DOI] [Google Scholar]
- 65.Holden C. Researchers Pained by Effort to Define Distress Precisely. Science. 2000;290:1474–1475. doi: 10.1126/science.290.5496.1474. [DOI] [PubMed] [Google Scholar]
- 66.Yada T., Tort L. Interactions. In: Schreck C.B., Tort L., Farrell A.P., Brauner C.J., editors. Fish Physiology. Volume 35. Academic Press; Cambridge, MA, USA: 2016. pp. 365–403. [Google Scholar]
- 67.Gomez D., Sunyer J.O., Salinas I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol. 2013;35:1729–1739. doi: 10.1016/j.fsi.2013.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olsen R.E., Sundell K., Hansen T., Hemre G.-I., Myklebust R., Mayhew T.M., Ringø E. Acute stress alters the intestinal lining of Atlantic salmon, Salmo salar L.: An electron microscopical study. Fish Physiol. Biochem. 2002;26:211–221. doi: 10.1023/A:1026217719534. [DOI] [Google Scholar]
- 69.Olsen R.E., Sundell K., Mayhew T.M., Myklebust R., Ringø E. Acute stress alters intestinal function of rainbow trout, Oncorhynchus mykiss (Walbaum) Aquaculture. 2005;250:480–495. doi: 10.1016/j.aquaculture.2005.03.014. [DOI] [Google Scholar]
- 70.Sundh H., Kvamme B.O., Fridell F., Olsen R.E., Ellis T., Taranger G.L., Sundell K. Intestinal barrier function of Atlantic salmon (Salmo salar L.) post smolts is reduced by common sea cage environments and suggested as a possible physiological welfare indicator. BMC Physiol. 2010;10:22. doi: 10.1186/1472-6793-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Douxfils J., Mathieu C., Mandiki S.N.M., Milla S., Henrotte E., Wang N., Vandecan M., Dieu M., Dauchot N., Pigneur L.M., et al. Physiological and proteomic evidences that domestication process differentially modulates the immune status of juvenile Eurasian perch (Perca fluviatilis) under chronic confinement stress. Fish Shellfish Immunol. 2011;31:1113–1121. doi: 10.1016/j.fsi.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 72.Saeij J.P., Verburg-van Kemenade L.B., van Muiswinkel W.B., Wiegertjes G.F. Daily handling stress reduces resistance of carp to Trypanoplasma borreli: In vitro modulatory effects of cortisol on leukocyte function and apoptosis. Dev. Comp. Immunol. 2003;27:233–245. doi: 10.1016/S0145-305X(02)00093-9. [DOI] [PubMed] [Google Scholar]
- 73.Pruett S.B. Stress and the immune system. Pathophysiology. 2003;9:133–153. doi: 10.1016/S0928-4680(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 74.Esteban M.A., Rodríguez A., Ayala A.G., Meseguer J. Effects of high doses of cortisol on innate cellular immune response of seabream (Sparus aurata L.) Gen. Comp. Endocrinol. 2004;137:89–98. doi: 10.1016/j.ygcen.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 75.Baschant U., Tuckermann J. The role of the glucocorticoid receptor in inflammation and immunity. J. Steroid Biochem. Mol. Biol. 2010;120:69–75. doi: 10.1016/j.jsbmb.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 76.Castanheira M.F., Conceição L.E.C., Millot S., Rey S., Bégout M.-L., Damsgård B., Kristiansen T., Höglund E., Øverli Ø., Martins C.I.M. Coping styles in farmed fish: Consequences for aquaculture. Rev. Aquac. 2017;9:23–41. doi: 10.1111/raq.12100. [DOI] [Google Scholar]
- 77.Koakoski G., Oliveira T.A., da Rosa J.G., Fagundes M., Kreutz L.C., Barcellos L.J. Divergent time course of cortisol response to stress in fish of different ages. Physiol. Behav. 2012;106:129–132. doi: 10.1016/j.physbeh.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 78.Madaro A., Fernö A., Kristiansen T.S., Olsen R.E., Gorissen M., Flik G., Nilsson J. Effect of predictability on the stress response to chasing in Atlantic salmon (Salmo salar L.) parr. Physiol. Behav. 2016;153:1–6. doi: 10.1016/j.physbeh.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 79.Martinez-Porchas M., Martinez-Cordova L.R., Ramos-Enriquez R. Cortisol and glucose: Reliable indicators of fish stress. Pan-Am. J. Aquat. Sci. 2009;4:158–178. [Google Scholar]
- 80.Tort L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011;35:1366–1375. doi: 10.1016/j.dci.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 81.Peng X.-X. Proteomics and its applications to aquaculture in China: Infection, immunity, and interaction of aquaculture hosts with pathogens. Dev. Comp. Immunol. 2013;39:63–71. doi: 10.1016/j.dci.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 82.Provan F., Jensen L.B., Uleberg K.E., Larssen E., Rajalahti T., Mullins J., Obach A. Proteomic analysis of epidermal mucus from sea lice–infected Atlantic salmon, Salmo salar L. J. Fish Dis. 2013;36:311–321. doi: 10.1111/jfd.12064. [DOI] [PubMed] [Google Scholar]
- 83.Rajan B., Lokesh J., Kiron V., Brinchmann M.F. Differentially expressed proteins in the skin mucus of Atlantic cod (Gadus morhua) upon natural infection with Vibrio anguillarum. BMC Vet. Res. 2013;9:1–11. doi: 10.1186/1746-6148-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Easy R.H., Ross N.W. Changes in Atlantic salmon Salmo salar mucus components following short- and long-term handling stress. J. Fish Biol. 2010;77:1616–1631. doi: 10.1111/j.1095-8649.2010.02796.x. [DOI] [PubMed] [Google Scholar]
- 85.Cordero H., Morcillo P., Cuesta A., Brinchmann M.F., Esteban M.A. Differential proteome profile of skin mucus of gilthead seabream (Sparus aurata) after probiotic intake and/or overcrowding stress. J. Proteom. 2016;132:41–50. doi: 10.1016/j.jprot.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 86.Guardiola F.A., Cuartero M., Del Mar Collado-González M., Díaz Baños F.G., Cuesta A., Moriñigo M., Esteban M. Terminal carbohydrates abundance, immune related enzymes, bactericidal activity and physico-chemical parameters of the Senegalese sole (Solea senegalensis, Kaup) skin mucus. Fish Shellfish Immunol. 2017;60:483–491. doi: 10.1016/j.fsi.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 87.Isani G., Andreani G., Carpenè E., Di Molfetta S., Eletto D., Spisni E. Effects of waterborne Cu exposure in gilthead sea bream (Sparus aurata): A proteomic approach. Fish Shellfish Immunol. 2011;31:1051–1058. doi: 10.1016/j.fsi.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 88.Moreira M., Schrama D., Soares F., Wulff T., Pousão-Ferreira P., Rodrigues P. Physiological responses of reared sea bream (Sparus aurata Linnaeus, 1758) to an Amyloodinium ocellatum outbreak. J. Fish Dis. 2017;40:1545–1560. doi: 10.1111/jfd.12623. [DOI] [PubMed] [Google Scholar]
- 89.Xiong X.-P., Dong C.-F., Xu X., Weng S.-P., Liu Z.-Y., He J.-G. Proteomic analysis of zebrafish (Danio rerio) infected with infectious spleen and kidney necrosis virus. Dev. Comp. Immunol. 2011;35:431–440. doi: 10.1016/j.dci.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 90.Ji C., Wu H., Wei L., Zhao J., Wang Q., Lu H. Responses of Mytilus galloprovincialis to bacterial challenges by metabolomics and proteomics. Fish Shellfish Immunol. 2013;35:489–498. doi: 10.1016/j.fsi.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 91.Lü A., Hu X., Wang Y., Shen X., Li X., Zhu A., Tian J., Ming Q., Feng Z. iTRAQ analysis of gill proteins from the zebrafish (Danio rerio) infected with Aeromonas hydrophila. Fish Shellfish Immunol. 2014;36:229–239. doi: 10.1016/j.fsi.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 92.Buján N., Hernández-Haro C., Monteoliva L., Gil C., Magariños B. Comparative proteomic study of Edwardsiella tarda strains with different degrees of virulence. J. Proteom. 2015;127 Part B:310–320. doi: 10.1016/j.jprot.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 93.Xu D., Song L., Wang H., Xu X., Wang T., Lu L. Proteomic analysis of cellular protein expression profiles in response to grass carp reovirus infection. Fish Shellfish Immunol. 2015;44:515–524. doi: 10.1016/j.fsi.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 94.Braceland M., Bickerdike R., Tinsley J., Cockerill D., McLoughlin M.F., Graham D.A., Burchmore R.J., Weir W., Wallace C., Eckersall P.D. The serum proteome of Atlantic salmon, Salmo salar, during pancreas disease (PD) following infection with salmonid alphavirus subtype 3 (SAV3) J. Proteom. 2013;94:423–436. doi: 10.1016/j.jprot.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumar G., Hummel K., Noebauer K., Welch T.J., Razzazi-Fazeli E., El-Matbouli M. Proteome analysis reveals a role of rainbow trout lymphoid organs during Yersinia ruckeri infection process. Sci. Rep. 2018;8:13998. doi: 10.1038/s41598-018-31982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin H.D., Hsu L.S., Chien C.C., Chen S.C. Proteomic analysis of ametryn toxicity in zebrafish embryos. Environ. Toxicol. 2018;33:579–586. doi: 10.1002/tox.22546. [DOI] [PubMed] [Google Scholar]
- 97.Veiseth-Kent E., Grove H., Færgestad E.M., Fjæra S.O. Changes in muscle and blood plasma proteomes of Atlantic salmon (Salmo salar) induced by crowding. Aquaculture. 2010;309:272–279. doi: 10.1016/j.aquaculture.2010.09.028. [DOI] [Google Scholar]
- 98.Singhal N., Kumar M., Kanaujia P., Virdi J. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front Microbiol. 2015;6:791. doi: 10.3389/fmicb.2015.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Song W.J., Qin Q.W., Qiu J., Huang C.H., Wang F., Hew C.L. Functional Genomics Analysis of Singapore Grouper Iridovirus: Complete Sequence Determination and Proteomic Analysis. J. Virol. 2004;78:12576–12590. doi: 10.1128/JVI.78.22.12576-12590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou S., Wan Q., Huang Y., Huang X., Cao J., Ye L., Lim T.K., Lin Q., Qin Q. Proteomic analysis of Singapore grouper iridovirus envelope proteins and characterization of a novel envelope protein VP088. Proteomics. 2011;11:2236–2248. doi: 10.1002/pmic.200900820. [DOI] [PubMed] [Google Scholar]
- 101.Vancsok C., Peñaranda M.M.D., Raj V.S., Leroy B., Jazowiecka-Rakus J., Boutier M., Gao Y., Wilkie G.S., Suárez N.M., Wattiez R., et al. Proteomic and Functional Analyses of the Virion Transmembrane Proteome of Cyprinid Herpesvirus 3. J. Virol. 2017;91:e01209–e01217. doi: 10.1128/JVI.01209-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chuang M.-Y., Tsai W.-C., Kuo T.-Y., Chen H.-M., Chen W.-J. Comparative proteome analysis reveals proteins involved in salt adaptation in Photobacterium damselae subsp. piscicida. J. Basic Microbiol. 2016;56:1234–1243. doi: 10.1002/jobm.201600091. [DOI] [PubMed] [Google Scholar]
- 103.Puentes B., Balado M., Bermúdez-Crespo J., Osorio C.R., Lemos M.L. A proteomic analysis of the iron response of Photobacterium damselae subsp. damselae reveals metabolic adaptations to iron levels changes and novel potential virulence factors. Vet. Microbiol. 2017;201:257–264. doi: 10.1016/j.vetmic.2017.01.040. [DOI] [PubMed] [Google Scholar]
- 104.Pérez-Sancho M., Vela Ana I., Awad M., Kostrzewa M., Domínguez L., Fernández-Garayzábal J.F. Differentiation of Photobacterium damselae subspecies using Matrix-Assisted Laser-Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) in fish isolates. Aquaculture. 2016;464:159–164. doi: 10.1016/j.aquaculture.2016.06.024. [DOI] [Google Scholar]
- 105.Tan Y.P., Lin Q., Wang X.H., Joshi S., Hew C.L., Leung K.Y. Comparative Proteomic Analysis of Extracellular Proteins of Edwardsiella tarda. Infect. Immun. 2002;70:6475–6480. doi: 10.1128/IAI.70.11.6475-6480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Srinivasa Rao P.S., Yamada Y., Tan Y.P., Leung K.Y. Use of proteomics to identify novel virulence determinants that are required for Edwardsiella tarda pathogenesis. Mol. Microbiol. 2004;53:573–586. doi: 10.1111/j.1365-2958.2004.04123.x. [DOI] [PubMed] [Google Scholar]
- 107.Kumar G., Hummel K., Welch T.J., Razzazi-Fazeli E., El-Matbouli M. Global proteomic profiling of Yersinia ruckeri strains. Vet. Res. 2017;48:55. doi: 10.1186/s13567-017-0460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kumar G., Hummel K., Ahrens M., Menanteau-Ledouble S., Welch T.J., Eisenacher M., Razzazi-Fazeli E., El-Matbouli M. Shotgun proteomic analysis of Yersinia ruckeri strains under normal and iron-limited conditions. Vet. Res. 2016;47:100. doi: 10.1186/s13567-016-0384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ormsby M.J., Grahame E., Burchmore R., Davies R.L. Comparative bioinformatic and proteomic approaches to evaluate the outer membrane proteome of the fish pathogen Yersinia ruckeri. J. Proteom. 2019;199:135–147. doi: 10.1016/j.jprot.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dumpala P.R., Gülsoy N., Lawrence M.L., Karsi A. Proteomic analysis of the fish pathogen Flavobacterium columnare. Proteome Sci. 2010;8:26. doi: 10.1186/1477-5956-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.LaFrentz B.R., LaPatra S.E., Call D.R., Wiens G.D., Cain K.D. Proteomic analysis of Flavobacterium psychrophilum cultured in vivo and in iron-limited media. Dis. Aquat. Org. 2009;87:171–182. doi: 10.3354/dao02122. [DOI] [PubMed] [Google Scholar]
- 112.Pérez-Sancho M., Vela A.I., Wiklund T., Kostrzewa M., Domínguez L., Fernández-Garayzábal J.F. Differentiation of Flavobacterium psychrophilum from Flavobacterium psychrophilum-like species by MALDI-TOF mass spectrometry. Res. Vet. Sci. 2017;115:345–352. doi: 10.1016/j.rvsc.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 113.Shen C.-J., Kuo T.-Y., Lin C.-C., Chow L.-P., Chen W.-J. Proteomic identification of membrane proteins regulating antimicrobial peptide resistance in Vibrio parahaemolyticus. J. Appl. Microbiol. 2010;108:1398–1407. doi: 10.1111/j.1365-2672.2009.04544.x. [DOI] [PubMed] [Google Scholar]
- 114.Tavares G.C., Carvalho A.F., Pereira F.L., Rezende C.P., Azevedo V.A.C., Leal C.A.G., Figueiredo H.C.P. Transcriptome and Proteome of Fish-Pathogenic Streptococcus agalactiae Are Modulated by Temperature. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yolanda T.-C., Clara F.-Á., Ysabel S. Proteomic and molecular fingerprinting for identification and tracking of fish pathogenic Streptococcus. Aquaculture. 2019;498:322–334. doi: 10.1016/j.aquaculture.2018.08.041. [DOI] [Google Scholar]
- 116.Kim S.W., Jang H.B., Lee J.S., Im S.P., Lazarte J.M.S., Seo J.P., Lee W.J., Kim J.S., Jung T.S. Comparison of proteome typing and serotyping of Streptococcus parauberis isolates from olive flounder (Paralichthys olivaceus) J. Microbiol. Methods. 2015;118:168–172. doi: 10.1016/j.mimet.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 117.Torres-Corral Y., Santos Y. Identification and typing of Vagococcus salmoninarum using genomic and proteomic techniques. J. Fish Dis. 2019;42:597–612. doi: 10.1111/jfd.12967. [DOI] [PubMed] [Google Scholar]
- 118.Zhang B.-C., Zhang J., Sun L. Streptococcus iniae SF1: Complete Genome Sequence, Proteomic Profile, and Immunoprotective Antigens. PLoS ONE. 2014;9:e91324. doi: 10.1371/journal.pone.0091324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.López-Cortés X.A., Nachtigall F.M., Olate V.R., Araya M., Oyanedel S., Diaz V., Jakob E., Ríos-Momberg M., Santos L.S. Fast detection of pathogens in salmon farming industry. Aquaculture. 2017;470:17–24. doi: 10.1016/j.aquaculture.2016.12.008. [DOI] [Google Scholar]
- 120.Fernández-Álvarez C., Torres-Corral Y., Saltos-Rosero N., Santos Y. MALDI-TOF mass spectrometry for rapid differentiation of Tenacibaculum species pathogenic for fish. Appl. Microbiol. Biotechnol. 2017;101:5377–5390. doi: 10.1007/s00253-017-8324-3. [DOI] [PubMed] [Google Scholar]
- 121.Fernández-Álvarez C., Torres-Corral Y., Santos Y. Use of ribosomal proteins as biomarkers for identification of Flavobacterium psychrophilum by MALDI-TOF mass spectrometry. J. Proteom. 2018;170:59–69. doi: 10.1016/j.jprot.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 122.Kurokawa S., Kabayama J., Fukuyasu T., Hwang S.D., Park C.-I., Park S.-B., del Castillo C.S., Hikima J.-i., Jung T.-S., Kondo H., et al. Bacterial Classification of Fish-Pathogenic Mycobacterium Species by Multigene Phylogenetic Analyses and MALDI Biotyper Identification System. Mar. Biotechnol. 2013;15:340–348. doi: 10.1007/s10126-012-9492-x. [DOI] [PubMed] [Google Scholar]
- 123.Böhme K., Fernández-No I.C., Barros-Velázquez J., Gallardo J.M., Calo-Mata P., Cañas B. Species Differentiation of Seafood Spoilage and Pathogenic Gram-Negative Bacteria by MALDI-TOF Mass Fingerprinting. J. Proteome Res. 2010;9:3169–3183. doi: 10.1021/pr100047q. [DOI] [PubMed] [Google Scholar]
- 124.Srivastava V., Rezinciuc S., Bulone V. Quantitative Proteomic Analysis of Four Developmental Stages of Saprolegnia parasitica. Front. Microbiol. 2018;8 doi: 10.3389/fmicb.2017.02658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tan Y.P., Rao P.S.S., Zhang Y.L., Hew C.L., Leung K.Y. Identification of virulence genes in bacterial fish pathogens: A genomic and proteomic approach. In: Shimizu N., Aoki T., Hirono I., Takashima F., editors. Aquatic Genomics: Steps Toward a Great Future. Springer; Tokyo, Japan: 2003. pp. 340–351. [Google Scholar]
- 126.Ahmed F., Kumar G., Soliman F.M., Adly M.A., Soliman H.A.M., El-Matbouli M., Saleh M. Proteomics for understanding pathogenesis, immune modulation and host pathogen interactions in aquaculture. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019;32:100625. doi: 10.1016/j.cbd.2019.100625. [DOI] [PubMed] [Google Scholar]
- 127.Toranzo A.E., Magariños B., Romalde J.L. A review of the main bacterial fish diseases in mariculture systems. Aquaculture. 2005;246:37–61. doi: 10.1016/j.aquaculture.2005.01.002. [DOI] [Google Scholar]
- 128.Dickerson H.W., Dawe D. Ichthyophthirius multifiliis and Cryptocaryon irritans (phylum Ciliophora) Fish Dis. Disord. 2006;1:116–153. [Google Scholar]
- 129.Rimstad E., Dale O.B., Dannevig B.H., Falk K. Infectious salmon anaemia. Fish Dis. Disord. 2011;3:143–165. [Google Scholar]
- 130.Noga E.J. Fish Disease: Diagnosis and Treatment. John Wiley & Sons; Hoboken, NJ, USA: 2010. [Google Scholar]
- 131.Reddacliff L.A., Whittington R.J. Pathology of epizootic haematopoietic necrosis virus (EHNV) infection in rainbow trout (Oncorhynchus mykiss Walbaum) and redfin perch (Perca fluviatilis L) J. Comp. Pathol. 1996;115:103–115. doi: 10.1016/S0021-9975(96)80033-8. [DOI] [PubMed] [Google Scholar]
- 132.Bootland L.M., Leong J. Infectious haematopoietic necrosis virus. Fish Dis. Disord. 2011;3:66–109. [Google Scholar]
- 133.Ahmadivand S., Soltani M., Mardani K., Shokrpoor S., Hassanzadeh R., Ahmadpoor M., Rahmati-Holasoo H., Meshkini S. Infectious hematopoietic necrosis virus (IHNV) outbreak in farmed rainbow trout in Iran: Viral isolation, pathological findings, molecular confirmation, and genetic analysis. Virus Res. 2017;229:17–23. doi: 10.1016/j.virusres.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 134.Wolf K. Fish Viruses and Fish Viral Diseases. Cornell University Press; Ithaca, NY, USA: 2019. [Google Scholar]
- 135.Munro E.S., Midtlyng P.J. Infectious pancreatic necrosis and associated aquatic birnaviruses. Fish Dis. Disord. 2006;3:1–65. [Google Scholar]
- 136.Roberts R.J., Pearson M.D. Infectious pancreatic necrosis in Atlantic salmon, Salmo salar L. J. Fish Dis. 2005;28:383–390. doi: 10.1111/j.1365-2761.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 137.Smail D.A., Munro E.S. Fish Pathology. Wiley-Blackwell; Oxford, UK: 2012. The Virology of Teleosts; pp. 186–291. [Google Scholar]
- 138.Thorud K., Djupvik H. Infectious anaemia in Atlantic salmon (Salmo salar L.) Bull. Eur. Assoc. Fish Pathol. 1988;8:109–111. [Google Scholar]
- 139.McLoughlin M.F., Nelson R.N., McCormick J.I., Rowley H.M., Bryson D.B. Clinical and histopathological features of naturally occurring pancreas disease in farmed Atlantic salmon, Salmo salar L. J. Fish Dis. 2002;25:33–43. doi: 10.1046/j.1365-2761.2002.00334.x. [DOI] [Google Scholar]
- 140.Taksdal T., Olsen A.B., Bjerkås I., Hjortaas M.J., Dannevig B.H., Graham D.A., McLoughlin M.F. Pancreas disease in farmed Atlantic salmon, Salmo salar L., and rainbow trout, Oncorhynchus mykiss (Walbaum), in Norway. J. Fish Dis. 2007;30:545–558. doi: 10.1111/j.1365-2761.2007.00845.x. [DOI] [PubMed] [Google Scholar]
- 141.Smail D.A., Snow M. Viral haemorrhagic septicaemia. Fish Dis. Disord. 2011;3:110–142. [Google Scholar]
- 142.Volpe E., Gustinelli A., Caffara M., Errani F., Quaglio F., Fioravanti M.L., Ciulli S. Viral nervous necrosis outbreaks caused by the RGNNV/SJNNV reassortant betanodavirus in gilthead sea bream (Sparus aurata) and European sea bass (Dicentrarchus labrax) Aquaculture. 2020;523:735155. doi: 10.1016/j.aquaculture.2020.735155. [DOI] [Google Scholar]
- 143.Qin Y., Liu J., Liu W., Shi H., Jia A., Lu Y., Liu X. First isolation and identification of red-grouper nervous necrosis virus (RGNNV) from adult hybrid Hulong grouper (Epinephelus fuscoguttatus × Epinephelus lanceolatus) in China. Aquaculture. 2020;529:735662. doi: 10.1016/j.aquaculture.2020.735662. [DOI] [Google Scholar]
- 144.Austin B., Austin D.A. Bacterial Fish Pathogens: Disease of Farmed and Wild Fish. Springer; Berlin/Heidelberg, Germany: 2016. [Google Scholar]
- 145.Actis L., Tolmasky M., Crosa J. Fish Diseases and Disorders. Volume 3. CABI; Wallingford, UK: 2011. Vibriosis; pp. 570–605. [Google Scholar]
- 146.Nagano I., Inoue S., Kawai K., Oshima S.-I. Repeatable immersion infection with Photobacterium damselae subsp. piscicida reproducing clinical signs and moderate mortality. Fish. Sci. 2009;75:707–714. doi: 10.1007/s12562-009-0099-8. [DOI] [Google Scholar]
- 147.Toranzo A.E., Barreiro S.N., Casal J.F., Figueras A., Magarinos B., Barja J.L. Pasteurellosis in cultured gilthead seabream (Sparus aurata): First report in Spain. Aquaculture. 1991;99:1–15. doi: 10.1016/0044-8486(91)90284-E. [DOI] [Google Scholar]
- 148.Gudmundsdottir B.K., Bjornsdottir B. Fish Viruses and Bacteria: Pathobiology and Protection. CABI; Boston, MA, USA: 2017. Aeromonas salmonicida and A. hydrophila; pp. 173–189. [Google Scholar]
- 149.Bricknell I.R., Bowden T.J., Bruno D.W., MacLachlan P., Johnstone R., Ellis A.E. Susceptibility of Atlantic halibut, Hippoglossus hippoglossus (L.) to infection with typical and atypical Aeromonas salmonicida. Aquaculture. 1999;175:1–13. doi: 10.1016/S0044-8486(99)00025-3. [DOI] [Google Scholar]
- 150.Handlinger J., Soltani M., Percival S. The pathology of Flexibacter maritimus in aquaculture species in Tasmania, Australia. J. Fish Dis. 1997;20:159–168. doi: 10.1046/j.1365-2761.1997.00288.x. [DOI] [Google Scholar]
- 151.Ferguson H.W., Ostland V.E., Byrne P., Lumsdsen J.S. Experimental Production of Bacterial Gill Disease in Trout by Horizontal Transmission and by Bath Challenge. J. Aquat. Anim. Health. 1991;3:118–123. doi: 10.1577/1548-8667(1991)003<0118:EPOBGD>2.3.CO;2. [DOI] [Google Scholar]
- 152.Nilsen H., Olsen A.B., Vaagnes Ø., Hellberg H., Bottolfsen K., Skjelstad H., Colquhoun D.J. Systemic Flavobacterium psychrophilum infection in rainbow trout, Oncorhynchus mykiss (Walbaum), farmed in fresh and brackish water in Norway. J. Fish Dis. 2011;34:403–408. doi: 10.1111/j.1365-2761.2011.01249.x. [DOI] [PubMed] [Google Scholar]
- 153.Doménech A., Fernández-Garayzábal J.F., García J.A., Cutuli M.T., Blanco M., Gibello A., Moreno M.A., Domínguez L. Association of Pseudomonas anguilliseptica infection with ‘winter disease’ in sea bream, Sparus aurata L. J. Fish Dis. 1999;22:69–71. doi: 10.1046/j.1365-2761.1999.00124.x. [DOI] [Google Scholar]
- 154.Salati F. Enterococcus seriolicida and streptococcus spp. (S. iniae, S. agalactiae and S. dysgalactiae) Fish Dis. Disord. 2011;3:375–396. [Google Scholar]
- 155.Munday B., Foster C., Roubal F., Lester R. Pathology in Marine Science, Proceedings of the Third International Colloquium on Pathology in Marine Aquaculture, Gloucester Point, VA, USA, 2–6 October 1988. Academic Press; Cambridge, MA, USA: 1990. Paramoebic gill infection and associated pathology of Atlantic salmon, Salmo salar and rainbow trout, Salmo gairdneri in Tasmania; pp. 215–222. [Google Scholar]
- 156.Rodger H., McArdle J. An outbreak of amoebic gill disease in Ireland. Vet. Rec. 1996;139:348. doi: 10.1136/vr.139.14.348. [DOI] [PubMed] [Google Scholar]
- 157.Hines R.S., Spira D.T. Ichthyophthirius multifiliis (Fouquet) in the mirror carp, Cyprinus carpio L. I. Course of infection. J. Fish Biol. 1973;5:385–392. [Google Scholar]
- 158.Hines R.S., Spira D.T. Ichthyophthiriasis in the mirror carp Cyprinus carpio (L.) V. Acquired immunity. J. Fish Biol. 1974;6:373–378. doi: 10.1111/j.1095-8649.1974.tb04554.x. [DOI] [Google Scholar]
- 159.Nigrelli R., Ruggieri G. Enzootics in the New York Aquarium caused by Cryptocaryon irritans Brown, 1951, a histophagous ciliate in the skin, eyes and gills of marine fishes. Zoologica. 1966;51:97–102. [Google Scholar]
- 160.Kuperman B.I., Matey V.E. Massive infestation by Amyloodinium ocellatum (Dinoflagellida) of fish in a highly saline lake, Salton Sea, California, USA. Dis. Aquat. Org. 1999;39:65–73. doi: 10.3354/dao039065. [DOI] [PubMed] [Google Scholar]
- 161.Soares F., Quental Ferreira H., Cunha E., Pousão-Ferreira P. Occurrence of Amyloodinium ocellatum in aquaculture fish production: A serious problem in semi-intensive earthen ponds. Aquacult. Eur. 2011;36:13–16. [Google Scholar]
- 162.Marques C.L., Medeiros A., Moreira M., Quental-Ferreira H., Mendes A.C., Pousão-Ferreira P., Soares F. Report and genetic identification of Amyloodinium ocellatum in a sea bass (Dicentrarchus labrax) broodstock in Portugal. Aquac. Rep. 2019;14:100191. doi: 10.1016/j.aqrep.2019.100191. [DOI] [Google Scholar]
- 163.Basson L., Van As J. Trichodinidae and other ciliophorans (Phylum Ciliophora) Fish Dis. Disord. 2006;1:154–182. [Google Scholar]
- 164.Van As J. An experimental elvaluation of the use of formalin to control trichodiniasis and other ectoparasitic protozoans on fry of Cyprinus carpio L. and Oreochromis mossambicus (Peters) S. Afr. J. Wildl. Res. 24 Mon. Delayed Open Access. 1984;14:42–48. [Google Scholar]
- 165.Buchmann K., Bresciani J. Monogenea (Phylum Platyhelminthes) Fish Dis. Disord. 2006;1:297–344. [Google Scholar]
- 166.Buchmann K. Fish Parasites: Pathobiology and Protection. CABI International; London, UK: 2012. 11 Gyrodactylus salaris and Gyrodactylus derjavinoides; p. 193. [Google Scholar]
- 167.Jonsdottir H., Bron J., Wootten R., Turnbull J. The histopathology associated with the pre-adult and adult stages of Lepeophtheirus salmonis on the Atlantic salmon, Salmo salar L. J. Fish Dis. 1992;15:521–527. doi: 10.1111/j.1365-2761.1992.tb00684.x. [DOI] [Google Scholar]
- 168.Carrera M., Piñeiro C., Martinez I. Proteomic Strategies to Evaluate the Impact of Farming Conditions on Food Quality and Safety in Aquaculture Products. Foods. 2020;9:1050. doi: 10.3390/foods9081050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Näpflin K., O’Connor E.A., Becks L., Bensch S., Ellis V.A., Hafer-Hahmann N., Harding K.C., Lindén S.K., Olsen M.T., Roved J., et al. Genomics of host-pathogen interactions: Challenges and opportunities across ecological and spatiotemporal scales. PeerJ. 2019;7:e8013. doi: 10.7717/peerj.8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gallardo-Escárate C., Valenzuela-Muñoz V., Núñez-Acuña G., Carrera C., Gonçalves A.T., Valenzuela-Miranda D., Benavente B.P., Roberts S. Catching the complexity of salmon-louse interactions. Fish Shellfish Immunol. 2019;90:199–209. doi: 10.1016/j.fsi.2019.04.065. [DOI] [PubMed] [Google Scholar]
- 171.Sen R., Nayak L., De R.K. A review on host-pathogen interactions: Classification and prediction. Eur. J. Clin. Microbiol. Infect. Dis. 2016;35:1581–1599. doi: 10.1007/s10096-016-2716-7. [DOI] [PubMed] [Google Scholar]
- 172.Tello M., Valdes N., Vargas R., Rojas J., Parra M., Gajardo G., Gonzalez A. Application of Metagenomics to Chilean Aquaculture. In: Hozzein W.N., editor. Metagenomics—Basics, Methods and Applications. IntechOpen; London, UK: 2019. [Google Scholar]
- 173.Guerrero R., Margulis L., Berlanga M. Symbiogenesis: The holobiont as a unit of evolution. Int. Microbiol. 2013;16:133–143. doi: 10.2436/20.1501.01.188. [DOI] [PubMed] [Google Scholar]
- 174.Thaiss C.A., Zmora N., Levy M., Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 175.Saito M.A., Bertrand E.M., Duffy M.E., Gaylord D.A., Held N.A., Hervey W.J., Hettich R.L., Jagtap P.D., Janech M.G., Kinkade D.B., et al. Progress and Challenges in Ocean Metaproteomics and Proposed Best Practices for Data Sharing. J. Proteome Res. 2019;18:1461–1476. doi: 10.1021/acs.jproteome.8b00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zheng Q., Wang Y., Lu J., Lin W., Chen F., Jiao N. Metagenomic and Metaproteomic Insights into Photoautotrophic and Heterotrophic Interactions in a. mBio. 2020;11 doi: 10.1128/mBio.03261-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Géron A., Werner J., Wattiez R., Lebaron P., Matallana-Surget S. Deciphering the Functioning of Microbial Communities: Shedding Light on the Critical Steps in Metaproteomics. Front. Microbiol. 2019;10:2395. doi: 10.3389/fmicb.2019.02395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sudhagar A., Kumar G., El-Matbouli M. Transcriptome Analysis Based on RNA-Seq in Understanding Pathogenic Mechanisms of Diseases and the Immune System of Fish: A Comprehensive Review. Int. J. Mol. Sci. 2018;19:245. doi: 10.3390/ijms19010245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Low C.F., Rozaini M.Z.H., Musa N., Syarul Nataqain B. Current knowledge of metabolomic approach in infectious fish disease studies. J. Fish Dis. 2017;40:1267–1277. doi: 10.1111/jfd.12610. [DOI] [PubMed] [Google Scholar]
- 180.Díaz-Pascual F., Ortíz-Severín J., Varas M.A., Allende M.L., Chávez F.P. Host-Pathogen Interaction as Revealed by Global Proteomic Profiling of Zebrafish Larvae. Front. Cell Infect. Microbiol. 2017;7:334. doi: 10.3389/fcimb.2017.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Torraca V., Mostowy S. Zebrafish Infection: From Pathogenesis to Cell Biology. Trends Cell Biol. 2018;28:143–156. doi: 10.1016/j.tcb.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li H., Zhu Q.F., Peng X.X., Peng B. Interactome of E. piscicida and grouper liver proteins reveals strategies of bacterial infection and host immune response. Sci. Rep. 2017;7:39824. doi: 10.1038/srep39824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Waiho K., Afiqah-Aleng N., Iryani M.T.M., Fazhan H. Protein–protein interaction network: An emerging tool for understanding fish disease in aquaculture. Rev. Aquac. 2020:1–22. doi: 10.1111/raq.12468. [DOI] [Google Scholar]
- 184.Forné I., Abián J., Cerdà J. Fish proteome analysis: Model organisms and non-sequenced species. Proteomics. 2010;10:858–872. doi: 10.1002/pmic.200900609. [DOI] [PubMed] [Google Scholar]
- 185.Li Y., Xie W., Li Q. Understanding the lipopolysaccharide induced liver proteome changes and identification of immune genes in Lampetra morii. Aquac. Fish. 2016;1:9–14. doi: 10.1016/j.aaf.2016.09.002. [DOI] [Google Scholar]
- 186.Ye H., Lin Q., Luo H. Applications of transcriptomics and proteomics in understanding fish immunity. Fish Shellfish Immunol. 2018;77:319–327. doi: 10.1016/j.fsi.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 187.Zhang J., Hu Y.-H., Xiao Z.-Z., Sun L. Megalocytivirus-induced proteins of turbot (Scophthalmus maximus): Identification and antiviral potential. J. Proteom. 2013;91:430–443. doi: 10.1016/j.jprot.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 188.Encinas P., Rodriguez-Milla M.A., Novoa B., Estepa A., Figueras A., Coll J. Zebrafish fin immune responses during high mortality infections with viral haemorrhagic septicemia rhabdovirus. A proteomic and transcriptomic approach. BMC Genom. 2010;11:518. doi: 10.1186/1471-2164-11-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Medina-Gali R., Belló-Pérez M., Ciordia S., Mena M.C., Coll J., Novoa B., del Mar Ortega-Villaizán M., Perez L. Plasma proteomic analysis of zebrafish following spring viremia of carp virus infection. Fish Shellfish Immunol. 2019;86:892–899. doi: 10.1016/j.fsi.2018.12.035. [DOI] [PubMed] [Google Scholar]
- 190.Nombela I., Lopez-Lorigados M., Salvador-Mira M.E., Puente-Marin S., Chico V., Ciordia S., Mena M.C., Mercado L., Coll J., Perez L., et al. Integrated Transcriptomic and Proteomic Analysis of Red Blood Cells from Rainbow Trout Challenged with VHSV Point Towards Novel Immunomodulant Targets. Vaccines. 2019;7:63. doi: 10.3390/vaccines7030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liu M., Wu T., Li S., Wei P., Yan Y., Gu W., Wang W., Meng Q. Combined transcriptomic/proteomic analysis of crucian carp Carassius auratus gibelio in cyprinid herpesvirus 2 infection. Fish Shellfish Immunol. 2018;82:386–399. doi: 10.1016/j.fsi.2018.07.057. [DOI] [PubMed] [Google Scholar]
- 192.Causey D.R., Pohl M.A.N., Stead D.A., Martin S.A.M., Secombes C.J., Macqueen D.J. High-throughput proteomic profiling of the fish liver following bacterial infection. BMC Genom. 2018;19:719. doi: 10.1186/s12864-018-5092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Long M., Zhao J., Li T., Tafalla C., Zhang Q., Wang X., Gong X., Shen Z., Li A. Transcriptomic and proteomic analyses of splenic immune mechanisms of rainbow trout (Oncorhynchus mykiss) infected by Aeromonas salmonicida subsp. salmonicida. J. Proteom. 2015;122:41–54. doi: 10.1016/j.jprot.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 194.Di G., Li H., Zhang C., Zhao Y., Zhou C., Naeem S., Li L., Kong X. Label-free proteomic analysis of intestinal mucosa proteins in common carp (Cyprinus carpio) infected with Aeromonas hydrophila. Fish Shellfish Immunol. 2017;66:11–25. doi: 10.1016/j.fsi.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 195.Booth N.J., Bilodeau-Bourgeois A.L. Proteomic analysis of head kidney tissue from high and low susceptibility families of channel catfish following challenge with Edwardsiella ictaluri. Fish Shellfish Immunol. 2009;26:193–196. doi: 10.1016/j.fsi.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 196.Kumar G., Hummel K., Razzazi-Fazeli E., El-Matbouli M. Modulation of posterior intestinal mucosal proteome in rainbow trout (Oncorhynchus mykiss) after Yersinia ruckeri infection. Vet. Res. 2019;50:54. doi: 10.1186/s13567-019-0673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cha I.S., Kwon J., Park S.H., Nho S.W., Jang H.B., Park S.B., del Castillo C.S., Hikima J., Aoki T., Jung T.S. Kidney proteome responses in the teleost fish Paralichthys olivaceus indicate a putative immune response against Streptococcus parauberis. J. Proteom. 2012;75:5166–5175. doi: 10.1016/j.jprot.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 198.Addis M., Cappuccinelli R., Tedde V., Pagnozzi D., Viale I., Meloni M., Salati F., Roggio T., Uzzau S. Influence of Moraxella sp. colonization on the kidney proteome of farmed gilthead sea breams (Sparus aurata, L.) Proteome Sci. 2010;8:50. doi: 10.1186/1477-5956-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Saleh M., Kumar G., Abdel-Baki A.-A.S., Dkhil M.A., El-Matbouli M., Al-Quraishy S. Quantitative proteomic profiling of immune responses to Ichthyophthirius multifiliis in common carp skin mucus. Fish Shellfish Immunol. 2019;84:834–842. doi: 10.1016/j.fsi.2018.10.078. [DOI] [PubMed] [Google Scholar]
- 200.Valdenegro-Vega V.A., Crosbie P., Bridle A., Leef M., Wilson R., Nowak B.F. Differentially expressed proteins in gill and skin mucus of Atlantic salmon (Salmo salar) affected by amoebic gill disease. Fish Shellfish Immunol. 2014;40:69–77. doi: 10.1016/j.fsi.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 201.Marcos-López M., Rodger H.D., O’Connor I., Braceland M., Burchmore R.J.S., Eckersall P.D., MacCarthy E. A proteomic approach to assess the host response in gills of farmed Atlantic salmon Salmo salar L. affected by amoebic gill disease. Aquaculture. 2017;470:1–10. doi: 10.1016/j.aquaculture.2016.12.009. [DOI] [Google Scholar]
- 202.Dong M., Liang Y., Ramalingam R., Tang S.W., Shen W., Ye R., Gopalakrishnan S., Au D.W.T., Lam Y.W. Proteomic characterization of the interactions between fish serum proteins and waterborne bacteria reveals the suppression of anti-oxidative defense as a serum-mediated antimicrobial mechanism. Fish Shellfish Immunol. 2017;62:96–106. doi: 10.1016/j.fsi.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 203.Sotton B., Paris A., Le Manach S., Blond A., Lacroix G., Millot A., Duval C., Huet H., Qiao Q., Labrut S., et al. Metabolic changes in Medaka fish induced by cyanobacterial exposures in mesocosms: An integrative approach combining proteomic and metabolomic analyses. Sci. Rep. 2017;7:4051. doi: 10.1038/s41598-017-04423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li W., Ali F., Cai Q., Yao Z., Sun L., Lin W., Lin X. Quantitative proteomic analysis reveals that chemotaxis is involved in chlortetracycline resistance of Aeromonas hydrophila. J. Proteom. 2018;172:143–151. doi: 10.1016/j.jprot.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 205.Huang L., Liu W., Jiang Q., Zuo Y., Su Y., Zhao L., Qin Y., Yan Q. Integration of Transcriptomic and Proteomic Approaches Reveals the Temperature-Dependent Virulence of Pseudomonas plecoglossicida. Front. Cell. Infect. Microbiol. 2018;8:207. doi: 10.3389/fcimb.2018.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Menanteau-Ledouble S., Kattlun J., Nöbauer K., El-Matbouli M. Protein expression and transcription profiles of three strains of Aeromonas salmonicida ssp. salmonicida under normal and iron-limited culture conditions. Proteome Sci. 2014;12:29. doi: 10.1186/1477-5956-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yao J.Y., Xu Y., Yuan X.M., Yin W.L., Yang G.L., Lin L.Y., Pan X.Y., Wang C.F., Shen J.Y. Proteomic analysis of differentially expressed proteins in the two developmental stages of Ichthyophthirius multifiliis. Parasitol. Res. 2017;116:637–646. doi: 10.1007/s00436-016-5328-3. [DOI] [PubMed] [Google Scholar]
- 208.Stryiński R., Mateos J., Pascual S., González Á.F., Gallardo J.M., Łopieńska-Biernat E., Medina I., Carrera M. Proteome profiling of L3 and L4 Anisakis simplex development stages by TMT-based quantitative proteomics. J. Proteom. 2019;201:1–11. doi: 10.1016/j.jprot.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 209.Lin J., Huang S., Zhang Q. Outer membrane proteins: Key players for bacterial adaptation in host niches. Microbes. Infect. 2002;4:325–331. doi: 10.1016/S1286-4579(02)01545-9. [DOI] [PubMed] [Google Scholar]
- 210.Koebnik R., Locher K.P., Van Gelder P. Structure and function of bacterial outer membrane proteins: Barrels in a nutshell. Mol. Microbiol. 2000;37:239–253. doi: 10.1046/j.1365-2958.2000.01983.x. [DOI] [PubMed] [Google Scholar]
- 211.Shahin K., Thompson K.D., Inglis N.F., McLean K., Ramirez-Paredes J.G., Monaghan S.J., Hoare R., Fontaine M., Metselaar M., Adams A. Characterization of the outer membrane proteome of Francisella noatunensis subsp. orientalis. J. Appl. Microbiol. 2018;125:686–699. doi: 10.1111/jam.13918. [DOI] [PubMed] [Google Scholar]
- 212.Kumar G., Rathore G., El-Matbouli M. Outer membrane protein assembly factor YaeT (omp85) and GroEL proteins of Edwardsiella tarda are immunogenic antigens for Labeo rohita (Hamilton) J. Fish Dis. 2014;37:1055–1059. doi: 10.1111/jfd.12205. [DOI] [PubMed] [Google Scholar]
- 213.Luo Z., Fu J., Li N., Liu Z., Qin T., Zhang X., Nie P. Immunogenic proteins and their vaccine development potential evaluation in outer membrane proteins (OMPs) of Flavobacterium columnare. Aquac. Fish. 2016;1:1–8. doi: 10.1016/j.aaf.2016.10.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.