Abstract
The impaired angiogenic potential of bone substitute materials (BSMs) may limit regenerative processes. Therefore, changes in the angiogenetic properties of different BSMs in combination with platelet-rich fibrin (PRF) in comparison to PRF alone, as well as to native BSMs, were analyzed in vitro and in vivo to evaluate possible clinical application. In vitro, four BSMs of different origins (allogeneic, alloplastic, and xenogeneic) were biofunctionalized with PRF and compared to PRF in terms of platelet interaction and growth factor release (vascular endothelial growth factor (VEGF), tissue growth factor ß (TGFß) and platelet-derived growth factor (PDGF)) after 15 min. To visualize initial cell–cell interactions, SEM was performed. In vivo, all BSMs (±PRF) were analyzed after 24 h for new-formed vessels using a chorioallantoic membrane (CAM) assay. Especially for alloplastic BSMs, the addition of PRF led to a significant consumption of platelets (p = 0.05). PDGF expression significantly decreased in comparison to PRF alone (all BSMs: p < 0.013). SEM showed the close spatial relation of each BSM and PRF. In vivo, PRF had a significant positive pro-angiogenic influence in combination with alloplastic (p = 0.007) and xenogeneic materials (p = 0.015) in comparison to the native BSMs. For bio-activated xenogeneic BSMs, the branching points were also significantly increased (p = 0.005). Finally, vessel formation was increased for BSMs and PRF in comparison to the native control (allogeneic: p = 0.046; alloplastic: p = 0.046; and xenogeneic: p = 0.050). An early enhancement of angiogenetic properties was demonstrated when combining BSMs with PRF in vitro and led to upregulated vessel formation in vivo. Thus, the use of BSMs in combination with PRF may trigger bony regeneration in clinical approaches.
Keywords: angiogenesis, platelet-rich fibrin, tissue engineering, osteogenesis
1. Introduction
Bone substitute materials (BSMs) of allogeneic, xenogeneic, or alloplastic origin represent a valid therapeutic option for regenerative therapy after maxillofacial bone loss [1]. However, due to regulatory reasons, all BSMs are processed non-cellularly and therefore contain only osteoconductive properties, whereas autologous bone (with no antigenic properties) is loaded with cells and growth factors that stimulate, inter alia, new blood vessel formation and trigger osteoinduction [2]. In general, a sufficient blood vessel supply and the formation of new blood vessels from pre-existing ones (angiogenesis) are obligatory prerequisite for bony regeneration [3]. Here, homeostasis, structural pathways, and paracrine functions are some of the main features that couple angiogenesis with osteogenesis, especially in the initial and early regenerative phases [4].
However, the translation of tissue and bone engineering methods that enhances the angiogenic properties of BSMs in clinical workflow is limited, mainly due to regulatory reasons. In contrast, autologous platelet concentrates such as platelet-rich fibrin (PRF) that are now broadly used in dental and craniomaxillofacial regenerative medicine may potentially overcome this limitation [5]. Thus far, the pro-angiogenic effect of the PRF has been demonstrated to mainly impact soft tissue regeneration procedures [6,7], but the complex interplay of different cytokines and growth factors leads to an increased proliferation and differentiation of different cell lines, inter alia, osteoblasts [8,9]. In detail, vascular endothelial growth factor (VEGF), tissue growth factor ß (TGFß), and platelet-derived growth factor (PDGF) have been discussed to trigger vasoformative responses [10]. It was evaluated if the combination of PRF with BSMs in different clinical approaches not only functions as a signaling protein reservoir for osteoinduction but also allows the bone graft particles to stick together for better clinical handling [11]. There are emerging data that suggest that this method seems feasible in maxillary sinus floor lift, graft, and surgical augmentation procedures [12]; for guided bone regeneration methods in dental implantology [13]; alveolar ridge preservation [14]; and the treatment of intrabony periodontal defects [15].
However, there is currently no consistent evidence based on basic research that PRF can support osteogenesis [8,16,17]. The ambivalent data may be explained in the different biophysical properties of BSMs and the variety of investigated time points. More basic research is needed to deliver scientific evidence that can be translated into clinical workflow [12].
Recently, our working group showed a positive effect of PRF in combination with allogeneic and xenogeneic BSMs that enhanced osteoblast activity compared to a native BSM in vitro at later time points [7]. Thus far, no comparative study has analyzed the early angiogenetic interactions of PRF with different BSMs in vitro nor translated these results to in vivo experiments.
Therefore, the aim of this study was to assess the underlying initial cellular mechanism in order to validate possible clinical application of this approach. Here, differences in the interactions and activation of platelets of BSMs biofunctionalized with PRF were analyzed, and the possible implication for angiogenesis and neovascularization in vivo were investigated.
2. Materials and Methods
2.1. Bone Substitute Materials
BSMs of allogeneic (AKM: maxgraft®, botiss biomaterials GmbH, Zossen, Germany, granularity < 2 mm), alloplastic (APKM: maxresob®, botiss biomaterials GmbH, Zossen, Germany, granularity 0.8–1.5 mm), and xenogenic (XKM1: cerabone®, botiss biomaterials GmbH, Zossen, Germany, granularity 1.0–2.0 mm; XKM2: BioOss®, Geistlich Pharma AG, Wolhusen, Switzerland, granularity 1–2 mm) origin were analyzed.
2.2. PRF Protocol
In accordance with the ethical standards of the national research committee (Ärztekammer Rheinland-Pfalz, no. “2019-14705_1”), 10 mL of peripheral venous blood per sample were collected from three healthy donors without severe illnesses after the puncturing of the cephalic or the median cubital vein. A vacutainer system and specific sterile plain vacuum tubes with additional silicone within their coating surface were, respectively, used for solid (A-PRF+, Mectron, Carasco, Italy) and liquid PRF (iPRF, Mectron, Carasco, Italy). PRF was directly manufactured with a fixed angle rotor with a radius of 110 mm at 1200 rpm and a relative centrifugal force of 177 g for 8 min (Duo centrifuge, Mectron, Carasco, Italy) following the manufacturer’s instructions [7].
2.3. Early Interaction of PRF and BSM
To analyze the platelet interaction of BSMs in combination with PRF, 0.5 mL of liquid PRF was transferred into a sterile 5 mL Eppendorf tube (Merck, Darmstadt, Germany), and 100 mg of a native BSM were added (three samples, each in in triplet; total n = 54). Afterwards, the samples were gently mixed using a rotator at 15 rpm at room temperature for 15 min in order to obtain an optimal contact between BSMs and PRF, as previously described for platelet-rich plasma (PRP) [18]. One sample with EDTA-blood and one with PRF without any BSMs served as controls, and 20 μL of each sample were used to count the number of remaining non-aggregated platelets using a hematology analyzer (KX21, Sysmex Europe GmbH, Norderstedt, Germany). Afterwards, the supernatant was pipetted off and snap frozen at −80 °C for further investigation.
2.4. ELISA Quantification of Early Interaction of PRF and BSM
Growth factor release on a protein basis was evaluated after 15 min of incubation of BSM/PRF in comparison to PRF alone (five samples, each in triplet per antibody; total n = 60). Samples were analyzed via an ELISA for VEGF, TGFß, and PDGF (all: R&D Systems, Minneapolis, MN, USA) using the manufacturer’s protocol, as previously described [7]. Measurements were conducted with an ELISA plate reader at 450 nm (Molecular Devices, San Jose, CA, USA) and analyzed using the SoftMax Pro 5.4 (Molecular Devices, San Jose, CA, USA) software.
2.5. Scanning Electron Microscopy
To visualize the direct contact of the BSMs with PRF, one sample of each material was prepared for SEM (n = 4) in a 24-well plate, as previously described [19]. In brief, after 15 min of incubation, cells were fixed with formaldehyde, dehydrated and mounted on conductive stubs before an SCD 040 sputter-coater (BAL-TEC AG, Leica Microsystems, Solms, Germany) was used to coat samples with gold. Next, SEM micrographs were performed (Philips XL30, Eindhoven, The Netherlands), and images were exploratively analyzed with an analysis program (Kontron KS 300, Carl Zeiss Vision, Eching, Germany).
2.6. Quantification of Angiogenesis In Vivo
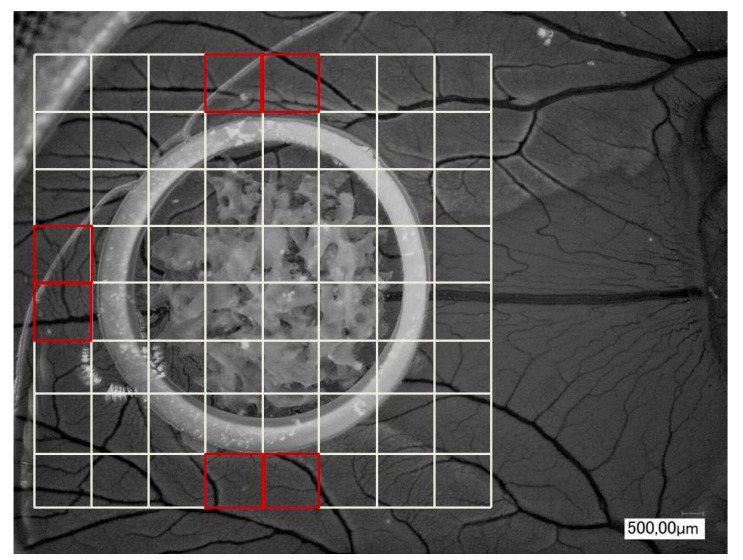
To evaluate the influence of BSM/PRF on angiogenesis in vivo, a chorioallantoic membrane (CAM) assay was used, as previously described [7,20]. The samples, both native (AKM, APKM, XMK1, and XKM2) and with PRF bio-activated BSMs (AKM+, APKM+, XKM1+, and XKM2+), were assessed and compared to the control of the CAM alone (Ctrl.), as well as the pure PRF (triplets per sample; total n = 36). In brief, fertilized white Leghorn chicken eggs (LSL Rhein-Main, Dieburg, Germany) were incubated at 38 °C at constant humidity until the fourth day of embryological development. Then, 8–10 mL of egg white were removed with a sterile syringe, and a 3 × 3 cm2 window was cut into the eggshell under sterile conditions. After another 24 h, we applied a sterile orthodontic elastic rubber ring (Elastics, Dentaurum, Ispringen, Germany), into which the samples were inserted. After 24 h of incubation, analysis in 30-fold and 50-fold magnification by centering the ring was performed using a digital microscope (KEYENCE, Neu-Isenburg, Germany) and its software (KEYENCE, Neu-Isenburg, Germany) after overlaying a grid (with a 500 μm side length) over the micrographs and manually counting all vessels and branching points of the vessels in six defined regions of interest per mm2 around the ring (Figure 1). Afterwards, the embryos were euthanized by cutting the main vessels.
Figure 1.
Exemplary picture (30 × magnification) of an incubated bone substitute materials (BSMs) in the chorioallantoic membrane (CAM) assay with the six defined regions of interest per mm2 around the ring.
2.7. Immune-Histochemically Display of Vessel Formation
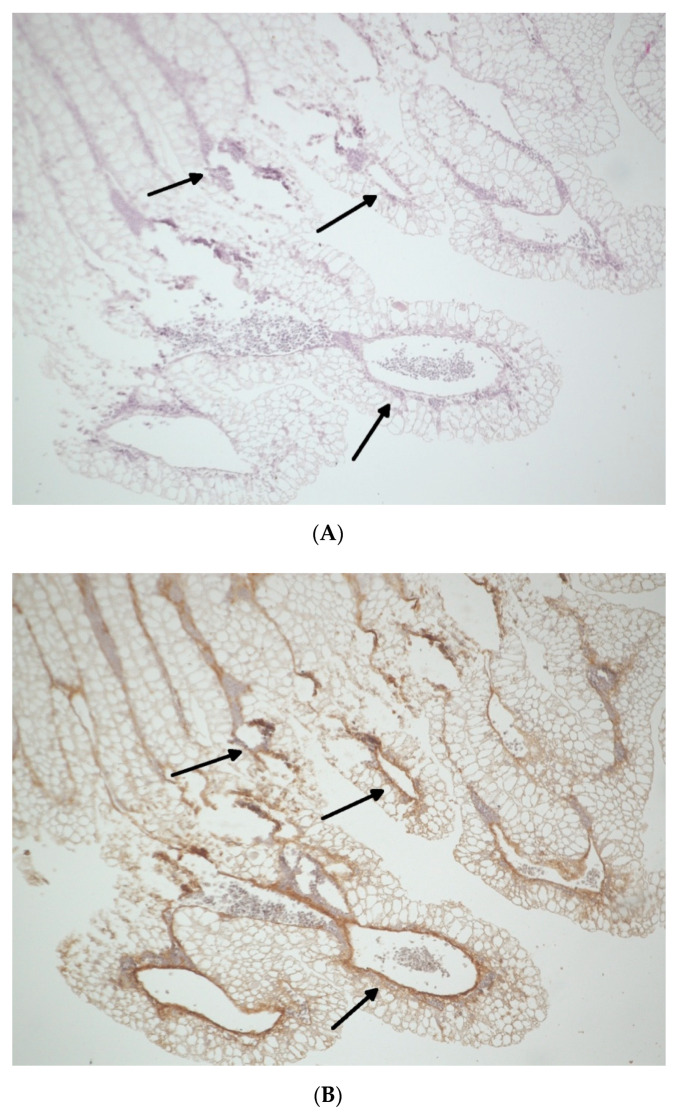
For the descriptive immune-histochemical demonstration of the vessels, the ring and each BSM were removed, and the underlying CAM was fixed in formaldehyde for 24 h, embedded in paraffin, and cut in 5-μm-thick slices (triplets per sample; n = 36). Subsequently, hematoxylin–eosin (HE, Merck, Darmstadt, Germany) and α-smooth muscle actin (αSMA, Sigma-Aldrich, St. Louis, MO, USA) staining was performed in accordance with the manufacturer’s instructions and as previously described [7]. Vessel formation was analyzed via light microscopy (KEYENCE, Neu-Isenburg, Germany) by using a BZ-II Analyzer (KEYENCE, Neu-Isenburg, Germany) software) after overlaying a grid (with a 500 μm side length) over the micrographs and manually counting all vessels in six defined regions of interest per mm2 around the former region of the ring (in Figure 2A,B, arrows mark newly formed vessels).
Figure 2.
Exemplary samples of the CAM (2× magnification) after incubation of allogeneic BSMs with platelet-rich fibrin (PRF) for 24 h displayed immunohistochemically via hematoxylin–eosin (HE) (A) and smooth muscle actin (SMA) (B) antibodies. Arrows indicate vessel formation.
2.8. Statistical Analysis
All results were evaluated in mean values with their standard errors and illustrated as bar charts with error bars. Differences between all groups were analyzed with Kruskal–Wallis rank sum test. After checking on normal distribution with the Shapiro–Wilk test, a Student’s t-test for paired samples (in case of normally distributed values) or a Mann–Whitney test (for non-normal distributions) was used to check for statistically significances (a p-value of ≤0.05 was applied).
3. Results
3.1. Initial Cell–Cell Interaction of BSM in Combination with PRF
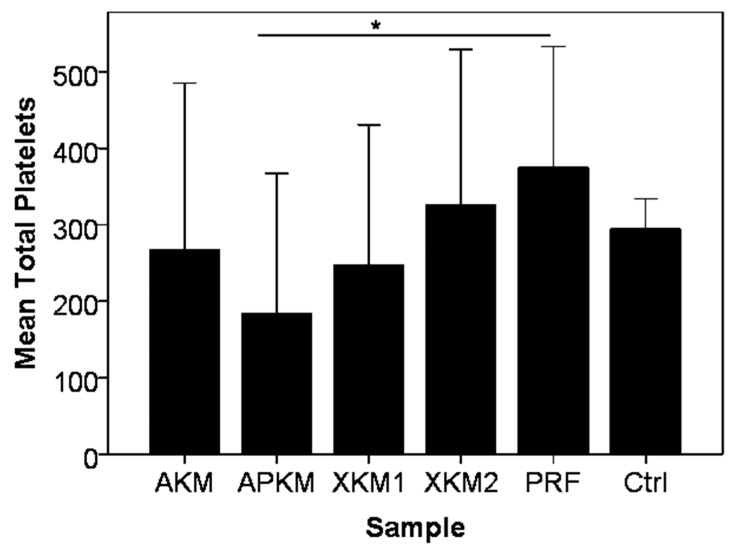
PRF alone showed a higher mean platelet count × 103/µL than whole blood without reaching any reaching statistical significance (p = 0.161). The incubation of PRF with all tested BSMs led to a decrease of platelets (AKM: p = 0.340; XKM1: p = 0.161; and XKM2: p = 0.796), with APKM showing the strongest decrease in comparison to PRF alone (p = 0.05; Table 1 and Figure 3).
Table 1.
Mean total platelet count x 103/µL with standard error after 15 min of incubation of each BSM with PRF in comparison to PRF alone and EDTA blood. p-values are given for the comparison of each sample with PRF alone (Mann–Whitney U-test).
| Sample | Mean Platelet x 103/µL | p-Value (Sample vs. PRF, Mann–Whitney U Test) |
|---|---|---|
| PRF | 374.66 ± 158.16 | - |
| Blood | 294.44 ± 39.83 | 0.161 |
| AKM | 267.22 ± 218.23 | 0.340 |
| APKM | 183.66 ± 183.06 | 0.05 |
| XKM1 | 246.67 ± 184.06 | 0.161 |
| XKM2 | 326.44 ± 202.59 | 0.796 |
Figure 3.
Total platelet count × 103/µL after 15 min of incubation of each BSM with PRF in comparison to PRF alone and EDTA blood (Ctrl: control). In comparison to PRF alone, all BSMs led to an active platelet consumption, with a significant difference for APKM vs. PRF (* p = 0.05).
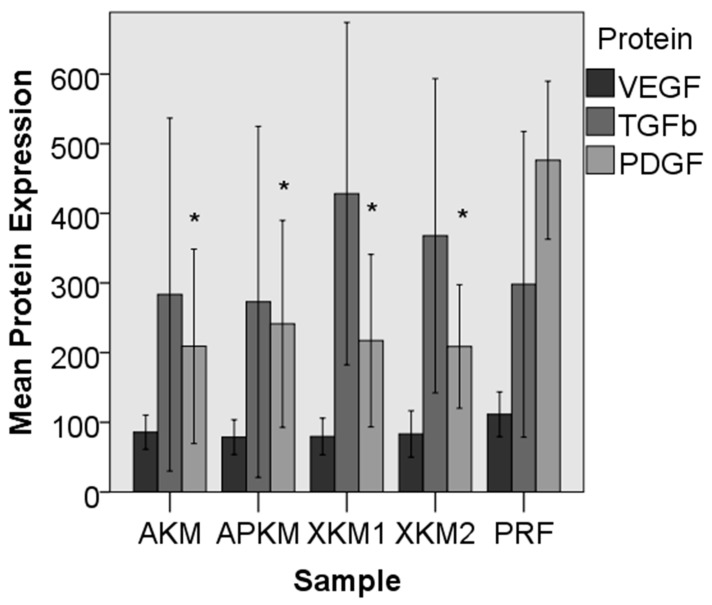
In the ELISA experiment, the combination of BSMs with PRF led to a slightly decreased expression of VEGF when compared to PRF alone (AKM: p = 0.161; APKM: p = 0.089; XKM1: p = 0.098; and XKM2: p = 0.174). Between the groups, no significant differences could be detected (p = 0.35). For TGFß, both xenogeneic BSMs (XKM1 and XKM2) showed an increased expression in comparison to PRF alone but also failed to reach statistical significance (AKM: p = 0.512; APKM: p = 0.775; XKM1: p = 0.285; and XKM2: p = 0.838). Furthermore, no significant differences between the samples could be seen (p = 0.48). For PDGF, a significantly decreased expression in comparison to PRF alone was shown for all BSMs (AKM: p = 0.003; APKM: p = 0.002; XKM1: p = 0.004; and XKM2: p = 0.013). The differences between the groups were statistically significant (p = 0.009; Table 2A–C and Figure 4).
Table 2.
Mean protein expression via ELISA for (A) vascular endothelial growth factor (VEGF), (B) tissue growth factor ß (TGFß) and (C) platelet-derived growth factor (PDGF) in pg/mL with standard error after 15 min of incubation of each BSM with PRF in comparison to PRF alone. p-values are given for the comparison of each sample with PRF alone (Mann–Whitney U test).
| A | ||
| Sample | Mean VEGF Expression (pg/mL) | p-Value (Sample vs. PRF, Mann–Whitney U Test) |
| PRF | 111.47 ± 58.04 | - |
| AKM | 85.90 ± 44.27 | 0.161 |
| APKM | 78.75 ± 45.00 | 0.089 |
| XKM1 | 79.68 ± 47.48 | 0.098 |
| XKM2 | 83.34 ± 59.95 | 0.174 |
| B | ||
| Sample | Mean TGFß Expression (pg/mL) | p-Value (Sample vs. PRF, Mann–Whitney U Test) |
| PRF | 298.16 ± 396.15 | - |
| AKM | 283.44 ± 457.51 | 0.512 |
| APKM | 272.95 ± 455.23 | 0.775 |
| XKM1 | 428.20 ± 444.10 | 0.285 |
| XKM2 | 367.81 ± 407.39 | 0.838 |
| C | ||
| Sample | Mean PDGF Expression (pg/mL) | p-Value (Sample vs. PRF, Mann–Whitney U Test) |
| PRF | 476.29 ± 204.94 | - |
| AKM | 209.08 ± 252.08 | 0.003 |
| APKM | 241.29 ± 268.21 | 0.002 |
| XKM1 | 217.12 ± 223.60 | 0.004 |
| XKM2 | 208.82 ± 160.05 | 0.013 |
Figure 4.
Expression of VEGF, TGF, and PDGF in the BSM samples incubated with PRF in comparison to PRF alone. PDGF was statistically significantly decreased for all tested BSMs in comparison to PRF alone (* p < 0.05; for detailed p-values, please refer to the text).
SEM micrographs showed the BSM surfaces narrowly covered with a thin PRF-layer that is created by closely networked fibrin fibers demonstrated the closest spatial relationship between BSMs and PRF (Figure 5).
Figure 5.
SEM: allogeneic (A), alloplastic (B), xenogenic material 1 (C), and xenogeneic material 2 (D) with PRF-biofunctionalization. The surfaces of the bone substitutes (*) were covered by a thin PRF-layer (white arrows) that was created by closely networked fibrin fibers (red arrows).
3.2. Influence of PRF in Combination with Different BSMs on Angiogenesis In Vivo
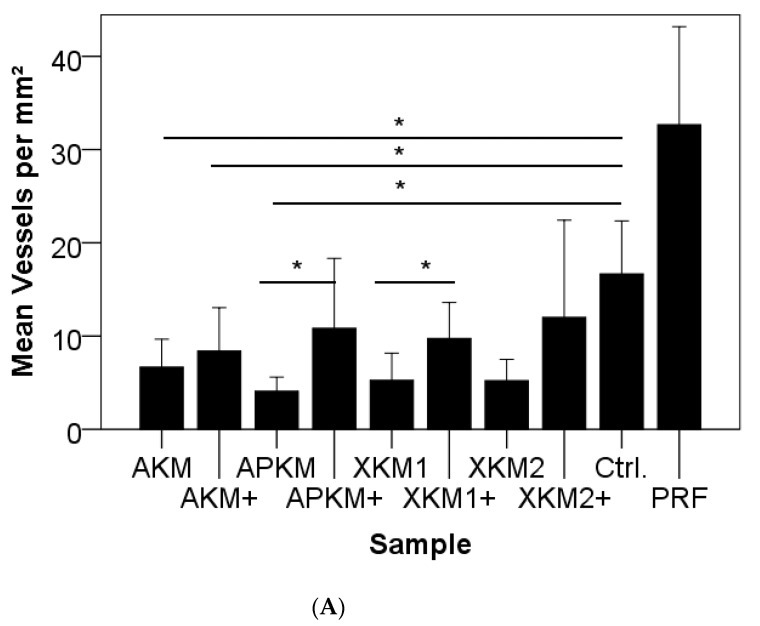
After 24 h of incubation, the total number of vessels did reveal strong differences between the compared groups (p = 0.009). Even though no statistical significance was reached, the total number of vessels was higher for PRF alone in comparison to the negative control group (p = 0.127; Figure 6A and Table 3A). In comparison to the negative control, vessel formation was found to be decreased for all tested materials, to a greater extent for native BSMs (AKM: p = 0.014; AKM + PRF: p = 0.041; APKM: p = 0.009; APKM + PRF: p = 0.241; XKM1: p = 0.018; XKM1 + PRF: p = 0.051; XKM2: p = 0.012; and XKM2 + PRF: p = 0.302). Accordingly, the BSMs in combination with PRF did show significant positive pro-angiogenic effects in comparison to their native correspondents for alloplastic and xenogeneic materials (AKM vs. AKM+: p = 0.406; APKM vs. APKM+: p = 0.007; XKM1 vs. XKM1+: p = 0.015; and XKM2 vs. XKM2+: p = 0.120).
Figure 6.
Newly formed vessels per mm2 (A) and branching points per mm2 (B) of the CAM after 24 h of incubation of BSMs with (+)/without PRF in comparison to the negative control and PRF alone (* p < 0.05).
Table 3.
Mean vessels/mm2 (A) and branching points per mm2 (B) after 24 h of incubation of each sample within the CAM assay in comparison to the negative control and PRF alone, evaluated via light microscopy. p-values are given for a comparison of each sample with the control and with their native BSM (Mann–Whitney U test).
| A | |||
| Sample | Mean Number of Vessels | p -Value (Sample vs. Control, Mann–Whitney U Test) | p -Value (Sample vs. Native BSM, Mann–Whitney U Test) |
| Control | 16.67 ± 5.68 | - | - |
| PRF | 32.66 ± 10.50 | 0.127 | - |
| AKM | 6.66 ± 2.99 | 0.014 | p = 0.406 |
| AKM+ | 8.40 ± 4.64 | 0.041 | |
| APKM | 4.08 ± 1.50 | 0.009 | p = 0.007 |
| APKM+ | 10.83 ± 7.49 | 0.241 | |
| XKM1 | 5.25 ± 2.915 | 0.018 | p = 0.015 |
| XKM1+ | 9.75 ± 3.84 | 0.051 | |
| XKM2 | 5.22 ± 2.27 | 0.012 | p = 0.120 |
| XKM2+ | 12.00 ± 10.43 | 0.302 | |
| B | |||
| Sample | Mean Number of Branching Points/mm2 | p -Value (Sample vs. Control, Mann–Whitney U Test) | p -Value (Sample vs. Native BSM, Mann–Whitney U Test) |
| Control | 7.33 ± 5.68 | - | - |
| PRF | 10.00 ± 7.00 | 0.275 | - |
| AKM | 2.91 ± 2.23 | 0.217 | p = 0.688 |
| AKM+ | 3.50 ± 2.54 | 0.347 | |
| APKM | 1.58 ± 1.16 | 0.138 | p = 0.135 |
| APKM+ | 4.5 ± 4.32 | 0.510 | |
| XKM1 | 1.75 ± 1.48 | 0.133 | p = 0.005 |
| XKM1+ | 4.75 ± 1.66 | 0.412 | |
| XKM2 | 2.33 ± 1.93 | 0.211 | p = 0.103 |
| XKM2+ | 5.66 ± 4.63 | 0.696 | |
For the evaluation of the branching points per mm2, no significant differences between groups was found (p = 0.15). Though PRF alone increased branching points in comparison to the negative control, the difference was not statistically significant (p = 0.275). In comparison to the negative control, vessel formation was found to be decreased for all tested materials, to a greater extent for native BSMs (AKM: p = 0.217; AKM + PRF: p = 0.347; APKM: p = 0.138; APKM + PRF: p = 0.510; XKM1: p = 0.133; XKM1 + PRF: p = 0.412; XKM2: p = 0.211; and XKM2 + PRF: p = 0.696). A tendency for a higher number of branching points for was detected for bio-activated BSMs, but only one xenogeneic material reached statistical significance (AKM vs. AKM + PRF+: p = 0.688; APKM vs. APKM + PRF: p = 0.135; XKM1 vs. XKM1 + PRF: p = 0.005; and XKM2 vs. XKM2 + PRF: p = 0.103; Figure 6B and Table 3B).
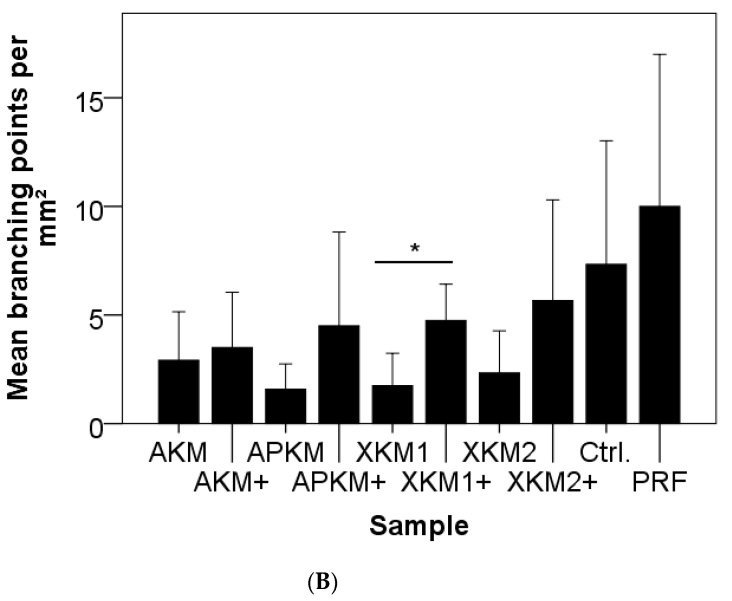
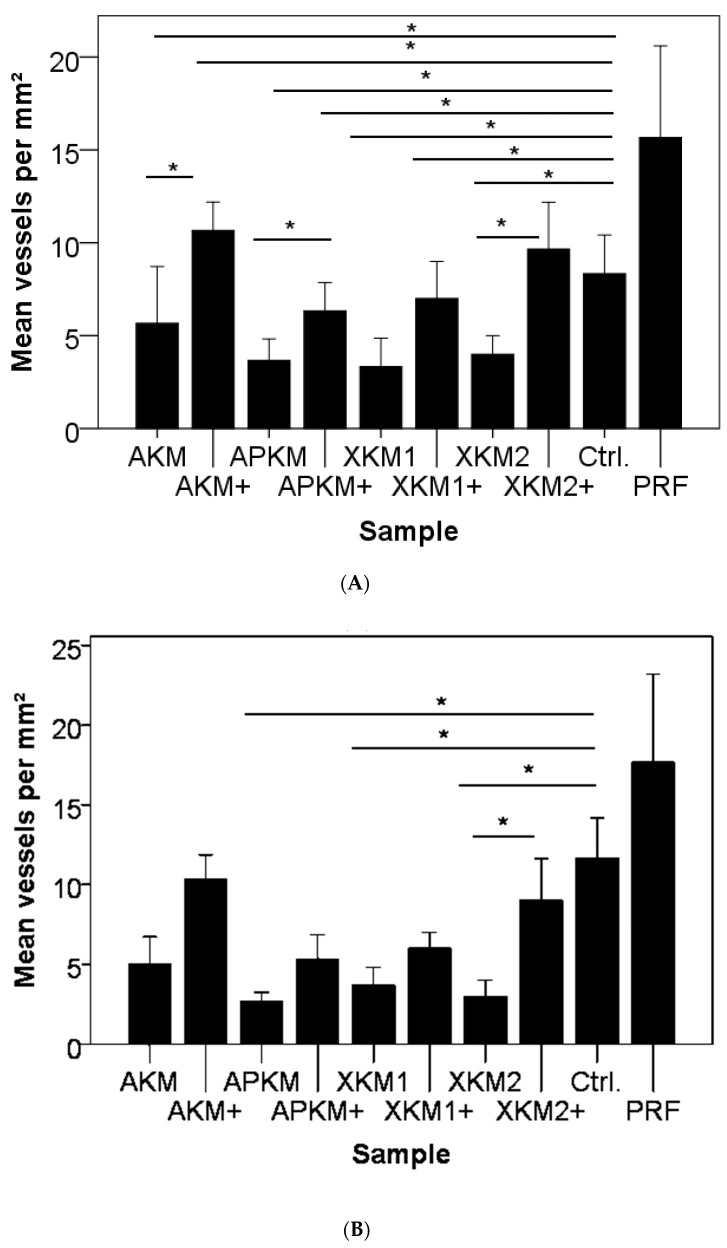
To visualize newly formed vessels, immune-histochemical staining for HE and αSMA was performed, as indicated in representative micrographs (triplets per sample; n = 36). For HE (Figure 7A and Table 4A) and αSMA staining (Figure 7B and Table 4B), no significant differences between the groups were found (p = 0.15). Furthermore, PRF alone did not significantly increase vessel amount in comparison to the negative control (HE: p = 0.184; αSMA: p = 0.077). After HE staining, BSMs with or without PRF had decreases in new vessels in comparison to the negative control (AKM: p = 0.046; AKM + PRF: p = 0.500; APKM: p = 0.046; APKM + PRF: p = 0.050; XKM1: p = 0.046; XKM1 + PRF: p = 0.050; XKM2: p = 0.050; and XKM2 + PRF: p = 0.184). In contrast, vessel formation was significantly increased when the bio-activated allogeneic, alloplastic, and xenogeneic BSMs were compared to the native control except for XKM1 (AKM vs. AKM + PRF: p = 0.046; APKM vs. APKM + PRF: p = 0.046; XKM1 vs. XKM1 + PRF: p = 0.072; and XKM2 vs. XKM2 + PRF: p = 0.05). After αSMA staining, native BSMs displayed less vessels in comparison to the negative control (AKM: p = 0.184; AKM+ PRF: p = 0.184; APKM: p = 0.046; APKM + PRF: p = 0.184; XKM1: p = 0.050; XKM1 + PRF: p = 0.376; XKM2: p = 0.050; and XKM2 + PRF: p = 0.376). In contrast, vessel formation was significantly increased when the bio-activated allogeneic and alloplastic BSMs, as well as XKM2, were compared to the native control (AKM vs. AKM + PRF: p = 0.077; APKM vs. APKM + PRF: p = 0.072; XKM1 vs. XKM1 + PRF: p = 0.077; and XKM2 vs. XKM2 + PRF: p = 0.050).
Figure 7.
Newly formed vessels per mm2 after HE (A) and after αSMA staining (B) after the removal of each BSM with (+)/without PRF in comparison to the negative control and PRF alone (* p < 0.05).
Table 4.
Mean vessels/mm2 after HE staining (A) and aSMA staining (B) after 24 h of incubation of each sample within the CAM assay in comparison to the negative control and PRF alone, evaluated immune-histochemically. p-values are given for a comparison of each sample with the control and with their native BSM (Mann–Whitney U test).
| A | |||
| Sample | Mean Number of Vessels/mm2 (HE Staining) | p-Value (Sample vs. Control, Mann–Whitney U Test) | p-Value (Sample vs. Native BSM, Mann–Whitney U Test) |
| Control | 11.66 ± 2.51 | - | - |
| PRF | 17.66 ± 5.50 | 0.200 | - |
| AKM | 5 ± 1.73 | 0.046 | p = 0.046 |
| AKM+ | 10.33 ± 1.52 | 0.500 | |
| APKM | 2.66 ± 0.57 | 0.046 | p = 0.046 |
| APKM+ | 5.33 ± 1.52 | 0.050 | |
| XKM1 | 3.66 ± 1.15 | 0.046 | p = 0.072 |
| XKM1+ | 6 ± 1.00 | 0.050 | |
| XKM2 | 3.00 ± 1.00 | 0.050 | p = 0.050 |
| XKM2+ | 9.00 ± 2.64 | 0.184 | |
| B | |||
| Sample | Mean Number of Vessels/mm2 (aSMA Staining) | p-Value (Sample vs. Control, Mann–Whitney U Test) | p-Value (Sample vs. Native BSM, Mann–Whitney U Test) |
| Control | 8.33 ± 2.08 | - | - |
| PRF | 15.66 ± 4.93 | 0.077 | - |
| AKM | 5.66 ± 3.05 | 0.184 | p = 0.077 |
| AKM+ | 10.66 ± 1.53 | 0.184 | |
| APKM | 3.66 ± 1.15 | 0.046 | p = 0.072 |
| APKM+ | 6.33 ± 1.52 | 0.184 | |
| XKM1 | 3.33 ± 1.15 | 0.050 | p = 0.077 |
| XKM1+ | 7.00 ± 2.00 | 0.376 | |
| XKM2 | 4.00 ± 1.00 | 0.050 | p = 0.050 |
| XKM2+ | 9.66 ± 2.51 | 0.376 | |
4. Discussion
As a basic research study, this work presents a comparative in vitro analysis of the initial angiogenic interaction and subsequent growth factor expression pattern of BSMs of different origins in combination with PRF. In addition, a CAM assay was used to evaluate possible implications for early angiogenesis and new vessel formation in vivo. In this way, scientific evidence for the clinical and surgical use of PRF as an effective additive to BSMs was analyzed. Among the main findings, the combination of BSMs with PRF led to an initial platelet consumption with a significant decrease of the growth factor expression of PDGF into the supernatant after 15 min. Since platelet count decreases with correlation to platelet consumption [18], it can be hypothesized that PRF initially interacts with its respective BSM via platelet activation. Here, the alloplastic BSM showed the strongest decrease, thus indicating the strongest interaction. To the best of our knowledge, there has not been any other study that has analyzed this possible implication. However, studies with other platelet concentrates such as PRP have revealed similar results [18]. In contrast to the literature [18,21], ELISA quantification has revealed a decrease of VEGF and especially PDGF for all BSMs in this study. Only TGFß, mainly for xenogeneic materials, was slightly increased in comparison to PRF alone. A possible explanation for this observation may be related to a specific cytokine retention by physical interaction with BSMs of different surfaces [18]. It is known that the growth factor release of PRF alone follows certain dynamics with a peak between three and seven days that triggers the migration of different cell lines such as human umbilical vein endothelial cells at these time points and, therefore, directly optimizes vessel formation [22]. However, analyses of these kinetics in combination with BSMs or other biomaterials are sparse. A study by Castro et al. did show a continuous growth factor release from the PRF in combination with a xenogeneic scaffold for 14 days. Interestingly, the authors found a lower extent of growth factor release for a PRF/xenograft combination in comparison to PRF alone, similar to the results presented in this study. They concluded that the PRF became physically entrapped within the xenograft, and release occurred passively as the close fibrin network was degraded via the serin protease plasmin [23].
In this context, the SEM micrographs presented in this study demonstrated a close fibrin network of the PRF, directly in contact with the tested BSMs, that may have contributed to the “storage” of the growth factors within the BSMs. Analogously, a recent study by our working group found that PRF in combination with allogeneic and xenogeneic BSMs enhances osteoblast activity for up to 10 days in vitro [24]. In addition to BSM-induced platelet activation and degranulation, simultaneous cytokine retention with a consecutive and consistent slow release over a physiologic time period in physiologic levels may lead to the these results and, finally, successful tissue regeneration approaches [18].
In general, with recent adjustments in regulatory requirements by the Food and Drug Administration, the application of growth factors related to bone substitute materials, such as recombinant human bone morphogenetic proteins (rhBMPs) [25], platelet-derived growth factor-BB (rhPDGF) [26], and fibroblast growth factor-2 (rhFGF-2) [27], has emerged as a new frontier in the field of reconstructive surgery. Currently, there is an ongoing debate on appropriate concentrations and indications [28]. Interestingly, an analysis of gingival fluid growth factor levels during early the healing period after regeneration of intrabony defects with tricalcium phosphate and PRF vs. a collagen membrane containing rhPDGF showed “similar early wound healing outcomes, although the levels of PDGF were higher in the PRF membrane group for [an] extended period of time“ in a recent clinical trial. The authors stated PRF to be a biodegradable and inexpensive growth factor eluting guided tissue membrane regeneration [29]. Therefore, one may hypothesize that despite the decreased growth factor levels found after the interaction of PRF with each BSM, a significant level could be reached in cervical fluid in clinical applications.
Furthermore, possible implications for angiogenesis were analyzed via the CAM assay in vivo. The assay has been well-described for this indication and widely used in the literature [7,20]. As a major advantage, this approach is in accordance with the concept of the replacement of experimental animals, the reduction of the total number of experimental animals needed, and refined testing protocols (“3R aspects”: replacement, reduction, refinement [30]). In addition, it can respond to osteogenic stimuli and offers significant potential as an in vivo model for xenograft organ culture [31,32]. Evidence has shown that the assay may also be used to evaluate possible inflammatory processes and is therefore of great interest for future studies in the field of tissue engineering, especially with platelet-derived concentrates [20,33]. Here, microscopically and immune-histochemically, all tested BSMs led to a noteworthy decrease in vessel-formation and branching-points in comparison to PRF alone. However, PRF had a significant positive pro-angiogenic effect, especially in combination with alloplastic and xenogeneic materials, thus strengthening the above-mentioned hypothesis of the consecutive release of growth factors through the fibrin network that triggers vasoformative responses. In this context, Ratajczak et al. evaluated the angiogenic capacity of PRF in a CAM assay and found induced blood vessel formation [34]. Thus far, studies that have tested bone substitutes with the CAM assay are sparse [33,35], and no analysis that has evaluated PRF combined with BSMs has been found. Recently, our working group demonstrated that the biofunctionalization of collagen matrices with PRF led to an increased angiogenic potential, as evaluated by the CAM assay. In this study, similar to the presented approach, the matrix was incubated with PRF for only 24 h [7]. This strengthened the hypothesis that PRF may influence the angiogenic potential of different scaffolds at this early time point. The consecutive release of the stored cytokine within the biofunctionalized biomaterial then may improve long-term tissue integration and regeneration. This is in accordance with other in vivo studies. In a murine model, PRF alone did not enhance bone regeneration in non-critical size defects and even had a temporary negative influence on RUNX and VEGF expression [36]. However, combined with an alloplastic hydroxyapatite and ß-tricalcium phosphate, a positive effect on bone formation was found [37]. For other xenogeneic materials, the combination of PRF improved bone repair [38,39,40]. For allografts with PRF, a randomized clinical trial found the combination effective in alveolar ridge preservation [41].
This study suffered from some major limitations. First, the in vitro approach could not describe complex biological interactions occurring in an organism in toto due to the lack of systemic and local factors arising from the complexity of cell and tissue responses [42]. Secondly, only a small sample size was achieved, which led to statistical interpretations of the results that were worth considering. However, the reliability in vitro testing for preclinical biomaterial evaluation is increasingly improving through the continuous expansions of knowledge from basic research, adjustments of in vitro systems, “3R aspects,” and comparative science between humans and animals. Systematic reviews of animal or in vitro research, if they are used to inform the design of clinical trials (particularly with respect to appropriate drug dose, timing, and other crucial aspects of the drug regimen), will further improve the predictability of animal research in human clinical trials.
Within the limitations of this study, no recommendation can be given regarding which BSM may most interact with PRF to optimize bony regeneration. However, it seems that alloplastic and xenogeneic materials may benefit the intended pro-angiogenic effect at an early time point to the greatest extents. This needs to be further addressed to support clinician scientists and surgeons in the field.
5. Conclusions
To conclude, we demonstrated an initial cell–cell interaction of PRF and different BSMs that led to noteworthy changes in growth factor expression in vitro and angiogenic features in vivo. This work provides clinician scientists and surgeons with scientific evidence for the use of PRF as a possible additive to BSMs in surgical reconstruction. Therefore, the presented study may help to translate this practicable method to trigger combined soft and hard tissue defects in the clinical workflow.
Author Contributions
Conceptualization, S.B. and P.W.K.; methodology, A.P. and P.W.K.; validation, D.G.E.T., B.A.-N., and S.B.; formal analysis, B.A.-N.; investigation, S.B.; data curation, P.W.K.; writing—original draft preparation, S.B.; writing—review and editing, D.G.E.T., P.W.K., and B.A.-N.; visualization, A.P.; supervision, B.A.-N.; funding acquisition, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by intramural grant of the BiomaTiCS group for S.B. The data from this study are part of the dissertation work submitted to the Johannes-Gutenberg University, Mainz, as part of medical doctoral thesis of S.B.
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (“Ärztekammer Rheinland Pfalz, reference number: “2019-14705_1”) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data is displayed in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khosropanah H., Lashkarizadeh N., Ayatollahi M., Kaviani M., Mostafavipour Z. The Impact of Calcium Hydroxide on the Osteoinductive Capacity of Demineralized Freeze-Dried Bone Allograft: An In-Vitro Study. J. Dent. 2018;19:19–27. [PMC free article] [PubMed] [Google Scholar]
- 2.Titsinides S., Agrogiannis G., Karatzas T. Bone grafting materials in dentoalveolar reconstruction: A comprehensive review. JPN. Dent. Sci. Rev. 2019;55:26–32. doi: 10.1016/j.jdsr.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rather H.A., Jhala D., Vasita R. Dual functional approaches for osteogenesis coupled angiogenesis in bone tissue engineering. Mater. Sci. Eng. C. 2019;103:109761. doi: 10.1016/j.msec.2019.109761. [DOI] [PubMed] [Google Scholar]
- 4.Grosso A., Burger M.G., Lunger A., Schaefer D.J., Banfi A., Di Maggio N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front. Bioeng. Biotechnol. 2017;5:68. doi: 10.3389/fbioe.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dohan D.M., Choukroun J., Diss A., Dohan S.L., Dohan A.J., Mouhyi J., Gogly B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006;101:e37–e44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Ghanaati S., Herrera-Vizcaino C., Al-Maawi S., Lorenz J., Miron R.J., Nelson K., Schwarz F., Choukroun J., Sader R. Fifteen Years of Platelet Rich Fibrin in Dentistry and Oromaxillofacial Surgery: How High is the Level of Scientific Evidence? J. Oral Implant. 2018;44:471–492. doi: 10.1563/aaid-joi-D-17-00179. [DOI] [PubMed] [Google Scholar]
- 7.Blatt S., Burkhardt V., Kämmerer P.W., Pabst A.M., Sagheb K., Heller M., Al-Nawas B., Schiegnitz E. Biofunctionalization of porcine-derived collagen matrices with platelet rich fibrin: Influence on angiogenesis in vitro and in vivo. Clin. Oral Investig. 2020;24:3425–3436. doi: 10.1007/s00784-020-03213-8. [DOI] [PubMed] [Google Scholar]
- 8.Miron R.J., Zucchelli G., Pikos M.A., Salama M., Lee S., Guillemette V., Fujioka-Kobayashi M., Bishara M., Zhang Y., Wang H.-L., et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017;21:1913–1927. doi: 10.1007/s00784-017-2133-z. [DOI] [PubMed] [Google Scholar]
- 9.Dohle E., El Bagdadi K., Sader R., Choukroun J., Kirkpatrick C.J., Ghanaati S. Platelet-rich fibrin-based matrices to improve angiogenesis in an in vitro co-culture model for bone tissue engineering. J. Tissue Eng. Regen. Med. 2018;12:598–610. doi: 10.1002/term.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicosia R.F., Nicosia S.V., Smith M. Vascular endothelial growth factor, platelet-derived growth factor, and insulin-like growth factor-1 promote rat aortic angiogenesis in vitro. Am. J. Pathol. 1994;145:1023–1029. [PMC free article] [PubMed] [Google Scholar]
- 11.Thanasrisuebwong P., Kiattavorncharoen S., Surarit R., Phruksaniyom C., Ruangsawasdi N. Red and Yellow Injectable Platelet-Rich Fibrin Demonstrated Differential Effects on Periodontal Ligament Stem Cell Proliferation, Migration, and Osteogenic Differentiation. Int. J. Mol. Sci. 2020;21:5153. doi: 10.3390/ijms21145153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damsaz M., Castagnoli C.Z., Eshghpour M., Alamdari D.H., Alamdari A.H., Noujeim Z.E.F., Haidar Z.S. Evidence-Based Clinical Efficacy of Leukocyte and Platelet-Rich Fibrin in Maxillary Sinus Floor Lift, Graft and Surgical Augmentation Procedures. Front. Surg. 2020;7:537138. doi: 10.3389/fsurg.2020.537138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thanasrisuebwong P., Kiattavorncharoen S., Deeb G.R., Bencharit S. Implant site preparation application of injectable platelet-rich fibrin for vertical and horizontal bone regeneration: A clinical report. J. Oral Implant. 2020 doi: 10.1563/aaid-joi-D-20-00031. [DOI] [PubMed] [Google Scholar]
- 14.Kumar N.G., Chaudhary R., Kumar I., Arora S.S., Kumar N., Singh H. To assess the efficacy of socket plug technique using platelet rich fibrin with or without the use of bone substitute in alveolar ridge preservation: A prospective randomised controlled study. Oral Maxillofac. Surg. 2018;22:135–142. doi: 10.1007/s10006-018-0680-3. [DOI] [PubMed] [Google Scholar]
- 15.Lekovic V., Milinkovic I., Aleksic Z., Jankovic S., Stankovic P., Kenney E.B., Camargo P.M. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J. Periodontal Res. 2012;47:409–417. doi: 10.1111/j.1600-0765.2011.01446.x. [DOI] [PubMed] [Google Scholar]
- 16.Pripatnanont P., Nuntanaranont T., Vongvatcharanon S., Phurisat K. The primacy of platelet-rich fibrin on bone regeneration of various grafts in rabbit’s calvarial defects. J. Craniomaxillofac. Surg. 2013;41:e191–e200. doi: 10.1016/j.jcms.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Yoon J.-S., Lee S.-H., Yoon H.-J. The influence of platelet-rich fibrin on angiogenesis in guided bone regeneration using xenogenic bone substitutes: A study of rabbit cranial defects. J. Cranio-Maxillofac. Surg. 2014;42:1071–1077. doi: 10.1016/j.jcms.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 18.Klein M., Kämmerer P.W., Scholz T., Moergel M., Kirchmaier C.M., Al-Nawas B. Modulation of platelet activation and initial cytokine release by alloplastic bone substitute materials. Clin. Oral Implant. Res. 2010;21:336–345. doi: 10.1111/j.1600-0501.2009.01830.x. [DOI] [PubMed] [Google Scholar]
- 19.Pabst A.M., Ackermann M., Wagner W., Haberthür D., Ziebart T., Konerding M.A. Imaging angiogenesis: Perspectives and opportunities in tumour research—a method display. J. Cranio maxillofac. Surg. 2014;42:915–923. doi: 10.1016/j.jcms.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Heimes D., Wiesmann N., Eckrich J., Brieger J., Mattyasovszky S., Proff P., Weber M., Deschner J., Al-Nawas B., Kämmerer P.W. In Vivo Modulation of Angiogenesis and Immune Response on a Collagen Matrix via Extracorporeal Shockwaves. Int. J. Mol. Sci. 2020;21:7574. doi: 10.3390/ijms21207574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kammerer P.W., Schiegnitz E., Alshihri A., Draenert F.G., Wagner W. Modification of xenogenic bone substitute materials—Effects on the early healing cascade in vitro. Clin. Oral. Implants Res. 2014;25:852–858. doi: 10.1111/clr.12153. [DOI] [PubMed] [Google Scholar]
- 22.Schär M.O., Diaz-Romero J., Kohl S., Zumstein M.A., Nesic D. Platelet-rich Concentrates Differentially Release Growth Factors and Induce Cell Migration In Vitro. Clin. Orthop. Relat. Res. 2015;473:1635–1643. doi: 10.1007/s11999-015-4192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castro A., Cortellini S., Temmerman A., Li X., Pinto N., Teughels W., Quirynen M. Characterization of the Leukocyte- and Platelet-Rich Fibrin Block: Release of Growth Factors, Cellular Content, and Structure. Int. J. Oral Maxillofac. Implant. 2019;34:855–864. doi: 10.11607/jomi.7275. [DOI] [PubMed] [Google Scholar]
- 24.Kyyak S., Blatt S., Pabst A., Thiem D., Al-Nawas B., Kämmerer P.W. Combination of an allogenic and a xenogenic bone substitute material with injectable platelet-rich fibrin—A comparative in vitro study. J. Biomater. Appl. 2020;35:83–96. doi: 10.1177/0885328220914407. [DOI] [PubMed] [Google Scholar]
- 25.Cicciù M., Fiorillo L., Cervino G., Habal M.B. BMP Application as Grafting Materials for Bone Regeneration in the Craniofacial Surgery: Current Application and Future Directions by an RCT Analysis. J. Craniofac. Surg. 2020 doi: 10.1097/SCS.0000000000006937. [DOI] [PubMed] [Google Scholar]
- 26.Tavelli L., Ravidà A., Barootchi S., Chambrone L., Giannobile W.V. Recombinant Human Platelet–Derived Growth Factor: A Systematic Review of Clinical Findings in Oral Regenerative Procedures. JDR Clin. Transl. Res. 2020 doi: 10.1177/2380084420921353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoshkam V., Chan H.L., Lin G.H., Mailoa J., Giannobile W.V., Wang H.L., Oh T.J. Outcomes of regenerative treatment with rhPDGF-BB and rhFGF-2 for periodontal intra-bony defects: A systematic review and meta-analysis. J. Clin. Periodontol. 2015;42:272–280. doi: 10.1111/jcpe.12354. [DOI] [PubMed] [Google Scholar]
- 28.Li F., Yu F., Liao X., Wu C., Wang Y., Li C., Lou F., Li B., Yin B., Wang C., et al. Efficacy of Recombinant Human BMP2 and PDGF-BB in Orofacial Bone Regeneration: A Systematic Review and Meta-analysis. Sci. Rep. 2019;9:11. doi: 10.1038/s41598-019-44368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi A.A., Padhye A.M., Gupta H.S. Platelet derived growth factor-BB levels in gingival crevicular fluid of localized intrabony defect sites treated with platelet rich fibrin membrane or collagen membrane containing recombinant human platelet derived growth factor-BB: A randomized clinical and biochemical study. J. Periodontol. 2019;90:701–708. doi: 10.1002/JPER.18-0496. [DOI] [PubMed] [Google Scholar]
- 30.Eder C., Falkner E., Nehrer S., Losert U.M., Schoeffl H. Introducing the concept of the 3Rs into tissue engineering research. ALTEX. 2006;23:17–23. [PubMed] [Google Scholar]
- 31.Eckrich J., Kugler P., Buhr C.R., Ernst B.P., Mendler S., Baumgart J., Brieger J., Wiesmann N. Monitoring of tumor growth and vascularization with repetitive ultrasonography in the chicken chorioallantoic-membrane-assay. Sci. Rep. 2020;10:14. doi: 10.1038/s41598-020-75660-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreno-Jiménez I., Lanham S.A., Kanczler J.M., Hulsart-Billstrom G., Evans N.D., Oreffo R.O.C. Remodelling of human bone on the chorioallantoic membrane of the chicken egg: De novo bone formation and resorption. J. Tissue Eng. Regen. Med. 2018;12:1877–1890. doi: 10.1002/term.2711. [DOI] [PubMed] [Google Scholar]
- 33.Cirligeriu L., Cimpean A., Calniceanu H., Vladau M., Sarb S., Raica M., Nica L. Hyaluronic Acid/Bone Substitute Complex Implanted on Chick Embryo Chorioallantoic Membrane Induces Osteoblastic Differentiation and Angiogenesis, but not Inflammation. Int. J. Mol. Sci. 2018;19:4119. doi: 10.3390/ijms19124119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratajczak J., Vangansewinkel T., Gervois P., Merckx G., Hilkens P., Quirynen M., Lambrichts I., Bronckaers A. Angiogenic Properties of ‘Leukocyte- and Platelet-Rich Fibrin’. Sci. Rep. 2018;8:14632. doi: 10.1038/s41598-018-32936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrovova E., Giretova M., Kvasilova A., Benada O., Danko J., Medvecky L., Sedmera D. Preclinical alternative model for analysis of porous scaffold biocompatibility in bone tissue engineering. Altex. 2019;36:121–130. doi: 10.14573/altex.1807241. [DOI] [PubMed] [Google Scholar]
- 36.Faot F., Deprez S., Vandamme K., Camargos G.V., Pinto N., Wouters J., Oord J.V.D., Quirynen M., Duyck J. The effect of L-PRF membranes on bone healing in rabbit tibiae bone defects: Micro-CT and biomarker results. Sci. Rep. 2017;7:46452. doi: 10.1038/srep46452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acar A.H., Yolcu Ü., Gul M., Keleş A., Erdem N.F., Kahraman S.A. Micro-computed tomography and histomorphometric analysis of the effects of platelet-rich fibrin on bone regeneration in the rabbit calvarium. Arch. Oral Biol. 2015;60:606–614. doi: 10.1016/j.archoralbio.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Lago E.S.D., Ferreira S., Garcia I.R., Okamoto R., Célio-Mariano R. Improvement of bone repair with l-PRF and bovine bone in calvaria of rats. histometric and immunohistochemical study. Clin. Oral Investig. 2019;24:1637–1650. doi: 10.1007/s00784-019-03018-4. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira M., Silva A.D., Ferreira S., Avelino C., Garcia I., Célio-Mariano R. Influence of the association between platelet-rich fibrin and bovine bone on bone regeneration. A histomorphometric study in the calvaria of rats. Int. J. Oral Maxillofac. Surg. 2015;44:649–655. doi: 10.1016/j.ijom.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Baćević M., Brković B., Lambert F., Djukić L., Petrović N., Roganović J. Leukocyte- and platelet-rich fibrin as graft material improves microRNA-21 expression and decreases oxidative stress in the calvarial defects of diabetic rabbits. Arch. Oral Biol. 2019;102:231–237. doi: 10.1016/j.archoralbio.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Clark D., Rajendran Y., Paydar S., Ho S., Cox D., Ryder M., Dollard J., Kao R.T. Advanced platelet-rich fibrin and freeze-dried bone allograft for ridge preservation: A randomized controlled clinical trial. J. Periodontol. 2018;89:379–387. doi: 10.1002/JPER.17-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fini M., Giardino R. In vitro and in vivo tests for the biological evaluation of candidate orthopedic materials: Benefits and limits. J. Appl. Biomater. Biomech. 2010;1:155–163. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is displayed in the manuscript.