Abstract
Simple Summary
Data on the prognostic and predictive value of germline cancer predisposing pathogenic/likely pathogenic variants (P/LPVs) in patients with pancreatic ductal adenocarcinoma is limited. This research was a study of germline testing of 62 cancer susceptibility genes in 549 unselected patients with pancreatic cancer. We reported a significant proportion of European patients with pancreatic cancer carrying P/LPVs in clinically significant genes, irrespectively of age, family history or disease stage. Importantly, P/LPVs were identified in pancreatic cancer-associated genes and in homologous recombination repair genes in 4.0% and 7.7% of patients, respectively. The presence of any P/LPVs was associated with improved overall survival univariately; however, it did not retain its independent prognostic significance in multivariate analysis. The presence of P/LPVs in homologous recombination repair genes did not predict benefit from platinum-based treatment. These results should be prospectively validated through universal genetic testing of patients with pancreatic cancer, taking into consideration the administration of newer treatments.
Abstract
Our aim was to determine the prevalence, prognostic and predictive role of germline pathogenic/likely pathogenic variants (P/LPVs) in cancer predisposing genes in patients with pancreatic ductal adenocarcinoma (PDAC). Germline testing of 62 cancer susceptibility genes was performed on unselected patients diagnosed from 02/2003 to 01/2020 with PDAC, treated at Hellenic Cooperative Oncology Group (HeCOG)-affiliated Centers. The main endpoints were prevalence of P/LPVs and overall survival (OS). P/LPVs in PDAC-associated and homologous recombination repair (HRR) genes were identified in 22 (4.0%) and 42 (7.7%) of 549 patients, respectively. P/LPVs were identified in 16 genes, including ATM (11, 2.0%) and BRCA2 (6, 1.1%), while 19 patients (3.5%) were heterozygotes for MUTYH P/LPVs and 9 (1.6%) carried the low-risk allele, CHEK2 p.(Ile157Thr). Patients carrying P/LPVs had improved OS compared to non-carriers (22.6 vs. 13.9 months, p = 0.006). In multivariate analysis, there was a trend for improved OS in P/LPV carriers (p = 0.063). The interaction term between platinum exposure and mutational status of HRR genes was not significant (p-value = 0.35). A significant proportion of patients with PDAC carries clinically relevant germline P/LPVs, irrespectively of age, family history or disease stage. The predictive role of these P/LPVs has yet to be defined. ClinicalTrials.gov Identifier: NCT03982446.
Keywords: BRCA2, inherited, overall survival, predictive, prognostic
1. Introduction
Germline pathogenic/likely pathogenic variants (P/LPVs) DNA repair genes, including BRCA1, BRCA2, ATM, PALB2, and mismatch repair (MMR) genes and EPCAM has been associated with increased risk for pancreatic adenocarcinoma (PDAC) and other tumors [1,2,3,4,5,6]. The presence of P/LPVs in these genes has several implications for patients with PDAC and their healthy relatives, who might be carriers of the respective variants. First, the administration of innovative treatments has been shown to be associated with improved clinical outcomes in patients with PDAC with selected molecular alterations [7,8,9,10]. Importantly, patients and healthy individuals who carry cancer predisposing P/LPVs may benefit from intensive screening protocols, risk-reducing surgeries and/or chemoprevention strategies. Therefore, the National Comprehensive Cancer Network (NCCN) currently recommends genetic counseling and genetic testing to all patients with PDAC as well as to first-degree relatives of patients with PDAC [11].
Recent studies have demonstrated that 3.8–20.7% of patients with PDAC carry germline P/LPVs in cancer predisposing genes [6,12,13,14,15,16,17,18,19,20,21,22,23,24]. Through these studies, it has been highlighted that family history, disease stage or age at diagnosis are not always predictors of an underlying genetic factor, while P/LPVs status is often associated with phenotypic heterogeneity. Most studies focused mainly on the prevalence of P/LPVs by using, often limited, gene panels in heterogenic patient populations, while few studies investigated the prognostic significance of PVs [9,12].
The aim of this study was to determine the prevalence of germline P/LPVs in cancer predisposing genes, in unselected patients with PDAC, treated at Departments of Oncology affiliated with the Hellenic Cooperative Oncology Group (HeCOG), with the use of an extensive multigene panel that tests for 62 genes implicated in cancer susceptibility with the concurrent detection single nucleotide variants (SNVs), insertions and deletions (Indels), as well as exon-level copy number variants (CNVs). To our knowledge, this is the first European study of PDAC patients of its size in which the prevalence of germline P/LPV and their prognostic and predictive role are investigated in a large number of genes.
2. Results
2.1. Patient Characteristics and P/LPV Prevalence
In total, 549 patients diagnosed with PDAC were included in the current analysis. Overall, 99.1% of them (n = 544) were treated in Greek medical departments of both public (456 patients; 83.1% of total population) and private hospitals, while 5 patients (0.9%) were recruited in a private oncology center in Cyprus. Median age at diagnosis was 65 years (range 34−86), and 278 (50.6%) patients were men. Of 549 patients, 62 (11.3%) carried P/LPVs in any of the cancer predisposing genes tested and 22 (4.0%) carried P/LPVs in genes known to be associated with pancreatic cancer. Family history of cancer was reported more commonly in patients carrying P/LPVs (n= 26, 44.1%) compared to non-carriers (n = 115, 27.9%) (chi-square p = 0.011). Of 59 patients with P/LPVs and available data for family history, 8 (13.6%) reported a family history of PDAC and 14 (23.7%) of other tumor types (tumor type was not available in 4 patients). Patient detailed clinicopathological characteristics are summarized in Table 1.
Table 1.
Patient and tumor characteristics.
| Total (N = 549) | Pts with P/LPVs (N = 62) | Pts without P/LPVs (N = 487) | p-Value | |
|---|---|---|---|---|
| Age | ||||
| Median (min, max) | 65.0 (33.6, 86.0) | 65.5 (41.8, 82.3) | 65.0 (33.6, 86.0) | 0.57 b |
| Sex | 0.67 c | |||
| Female | 271 (49.4) | 29 (46.8) | 242 (49.7) | |
| Male | 278 (50.6) | 33 (53.2) | 245 (50.3) | |
| Stage | 0.25 c | |||
| Early | 192 (35.0) | 27 (43.5) | 165 (33.9) | |
| Locally advanced | 33 (6.0) | 2 (3.2) | 31 (6.4) | |
| Metastatic | 324 (59.0) | 33 (53.2) | 291 (59.8) | |
| Definitive surgery | 0.071 | |||
| Yes | 175 (31.9) | 26 (41.9) | 149 (30.6) | |
| No | 374 (68.1) | 36 (58.1) | 338 (69.4) | |
| Histological grade * | 0.32 c | |||
| G1 (Well differentiated) | 26 (6.0) | 1 (2.0) | 25 (6.5) | |
| G2 (Moderately differentiated) | 215 (49.7) | 25 (51.0) | 190 (49.5) | |
| G3 (Poorly differentiated) | 172 (39.7) | 21 (42.9) | 151 (39.3) | |
| G4 (Undifferentiated) | 2 (0.46) | 1 (2.0) | 1 (0.26) | |
| GX (Grade cannot be assessed) | 18 (4.2) | 1 (2.0) | 17 (4.4) | |
| Family history of cancer * | 0.011 c | |||
| No | 330 (70.1) | 33 (55.9) | 297 (72.1) | |
| Yes | 141 (29.9) | 26 (44.1) | 115 (27.9) | |
| Chemotherapy * | 0.037 c | |||
| No | 9 (1.7) | 3 (4.9) | 6 (1.3) | |
| Yes | 525 (98.3) | 58 (95.1) | 467 (98.7) | |
| Platinum-based chemotherapy | 0.47 c | |||
| No | 275 (52.4) | 33 (56.9) | 242 (51.8) | |
| Yes | 250 (47.6) | 25 (43.1) | 225 (48.2) |
* Data not available for all subjects. Missing values: Histological grade = 116, Family history of cancer = 78, Chemotherapy = 15. p-values: b = Wilcoxon rank-sum test, c = Pearson’s chi-square/Fisher’s exact test. Abbreviations: Pts: patients, P/LPV: pathogenic/likely pathogenic variant. Bold, italic values denote statistical significance at the 5% level of significance.
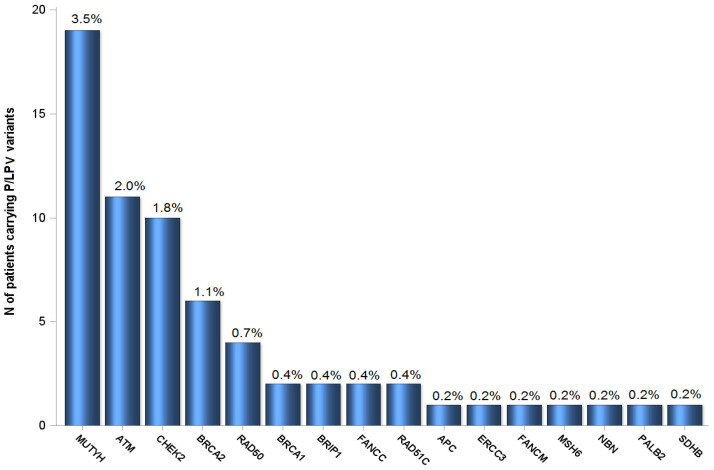
Genes with P/LPVs in ≥2 patients were ATM (n = 11, 2.0%), BRCA2 (n = 6, 1.1%), RAD50 (n = 4, 0.7%), BRCA1 (n = 2, 0.4%), BRIP1 (n = 2, 0.4%), FANCC (n = 2, 0.4%) and RAD51C (n = 2, 0.4%). In addition, 19 patients (3.5%) carried P/LPVs in MUTYH, while 9 (1.6%) carried the low-risk allele, CHEK2 p.(Ile157Thr); none of them had previous history of colorectal or breast cancer, respectively. The distribution of P/LPVs is presented in Figure 1. Notably, P/LPVs in genes participating in the homologous recombination repair (HRR) system (CHEK2 p.(Ile157Thr) excluded) accounted for 7.7% (42 patients). Interestingly, in one patient, a PV was identified in SDHB, a gene with no previous association with PDAC. In addition, 2 CNVs (3-exon deletion in ATM and 2-exon deletion in FANCC) were identified in 2 patients, accounting for 3.2% of the total number of P/LPVs identified. All P/LPVs are reported per patient in Table S1.
Figure 1.
Prevalence of P/LPVs in 549 patients of the study. Single nucleotide variants (SNV), insertions/deletions (Indels) and copy number variant (CNV) detection was performed for the following genes: APC, ATM, BAP1, BARD1, BMPR1A, BRCA1, BRCA2, BRIP1, CDH1, CDK4, CDKN2A, (CDKN2Ap16(INK4A), CDKN2Ap14(ARF)), CHEK2, DDB2, DICER1, EPCAM, ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, FANCA, FANCB, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCL, FANCM, GREM1, HOXB13, MEN1, MLH1, MRE11, MSH2, MSH6, MUTYH, NBN, PALB2, PMS2, POLD1, POLE, POLH, PTEN, RAD50, RAD51C, RAD51D, RB1, RET, SDHAF2, SDHB, SDHC, SDHD, SLX4, SMAD4, SMARCA4, STK11, TP53, VHL, XPA, XPC. Overall, 62 patients (11.3%) carried ≥1 P/LPV in at least 1 of 16 genes. Three patients carried two P/LPVs; two carried P/LPVs in CHEK2 and MUTYH and one in BRCA1 and MUTYH. The rest of the patients (59) had one P/LPV detected. The percentage of patients carrying a P/LPV in each gene is shown on top of each bar.
Of note, most frequent P/LPVs in low-risk genes (MUTYH, RAD50 and CHEK2) were compared to population frequencies (gnomAD database, comparing against the population with the highest frequency for the given P/LPVs, details in Methods). Two P/LPVs were found in higher frequency in our patient population compared to healthy population (control): MUTYH p.Arg245His (0.00820 vs. 0.00117, p = 0.002) and MUTYH p.Glu480del (0.00273 vs. 0.00061, p = 0.049) (Table S2).
2.2. Clinical Outcomes
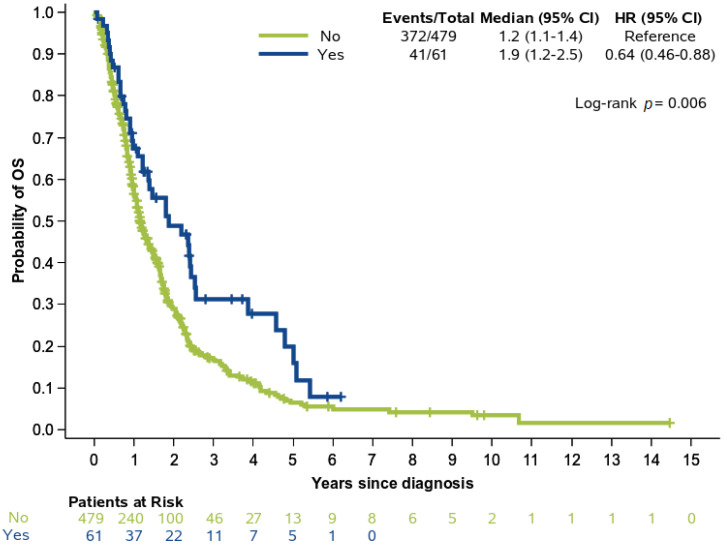
Within a median follow-up of 47.31 months (95% CI 34.75−64.10), a total of 413 (76.5%) deaths were reported. The median OS for the entire population with available survival data (N = 540) was 14.52 months (95% CI 13.08−16.52). Patients with P/LPVs had improved OS compared to non-carriers (22.62 months (95%CI 14.69−30.49) vs. 13.90 months (95% CI 12.82−16.23), log-rank p = 0.006) (Figure 2). The favorable prognostic significance of P/LPVs with respect to OS was underlined in the group of patients with early-stage disease (HR 0.47, 95% CI 0.26−0.84, p = 0.011), while significance was not reached among patients with advanced stage pancreatic cancer (HR 0.91, 95% CI 0.61−1.34, p = 0.62) even though the direction of the hazard ratio was retained. Upon multivariate analysis, there was only a trend for improved OS in P/LPV carriers (Wald’s overall p = 0.063) observed in the group of patients who had not received platinum-based chemotherapy (HR 0.65, 95% CI 0.43−1.05), whereas no difference was detected among platinum-treated patients (HR 0.77, 95% CI 0.47−1.23) (Table 2). The interaction term between the presence of P/LPVs and platinum treatment did not show a significant predictive benefit in the multivariate model (interaction p = 0.60), while, as expected, early stage contributed to significantly longer OS as compared to advanced stage of disease (HR 0.37, 95% CI 0.29−0.46, p < 0.001). The therapeutic effect of platinum exposure was also significant overall in the multivariate analysis (p = 0.002).
Figure 2.
Overall survival (OS) in patients with and without P/LPVs; analysis was performed in 540 patients with available outcome data.
Table 2.
Hazard ratios and 95% Confidence Intervals estimated by multivariate Cox regression analysis in the entire cohort with available data.
| Parameter | Events/Total | HR (95% CI) | p-Value |
|---|---|---|---|
| Stage | |||
| Early | 118/181 (65.2%) | 0.37 (0.29−0.46) | <0.001 |
| Advanced | 284/344 (82.6%) | Reference | -- |
| Interaction term of platinum-based CT with P/LPVs presence | 0.60 | ||
| P/LPVs among patients treated with platinum-based CT (N = 250) | |||
| Yes | 18/25 (72%) | 0.77 (0.47−1.23) | 0.18 |
| No | 167/225 (74.2%) | Reference | -- |
| P/LPVs in non-platinum-based CT (N = 275) | |||
| Yes | 20/33 (60.6%) | 0.65 (0.43−1.05) | 0.077 |
| No | 197/242 (81.4%) | Reference | -- |
Abbreviations: CI: confidence interval, CT: chemotherapy, HR: hazard ratio, N: number, P/LPV: pathogenic variant.
When excluding patients carrying only MUTYH P/LPVs and CHEK2 p.Ile157Thr (N = 25), a trend for improved OS was identified for patients with P/LPVs compared to non-carriers overall (21.61 months, 95%CI 11.34−30.69 vs. 13.90 months, 95% CI 12.82−16.23, respectively, log-rank p = 0.068). In addition, a trend towards longer OS was detected for patients with P/LPVs and early-stage disease (HR 0.52, 95% CI 0.25−1.06, p = 0.073), whereas no prognostic significance was found in patients with advanced disease (HR 1.10, 95% CI 0.65−1.85, p = 0.72). The interaction term between platinum exposure and mutational status of HRR genes (excluding CHEK2 p.Ile157Thr) was not significant either in the entire cohort (interaction p-value = 0.35) or among patients with early (interaction p-value = 0.42) or advanced disease (interaction p-value = 0.50). The prognostic significance of P/LPVs in PDAC-associated and HRR genes are shown in Table 3.
Table 3.
Hazard ratios and 95% Confidence Intervals estimated by univariate Cox regression analysis upon exclusion of patients carrying only MUTYH P/LPVs and CHEK2 p.Ile157Thr.
| Entire Cohort (N = 515) | Treated with Platinum-Based CT (N = 239) | No Platinum-Based CT (N = 262) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Events/Total | HR (95% CI) | p-Value | Events/Total | HR (95% CI) | p-Value | Events/Total | HR (95% CI) | p-Value | |
| Presence of P/LPVs | |||||||||
| No | 372/479 (77.7%) | Reference | -- | 167/225 (74.2%) | Reference | -- | 197/242 (81.4%) | Reference | -- |
| Yes | 23/36 (63.9%) | 0.68 (0.44−1.03) | 0.070 | 9/14 (64.3%) | 0.58 (0.29−1.13) | 0.11 | 12/20 (60%) | 0.69 (0.39−1.24) | 0.21 |
| P/LPVs in PDAC- associated genes | |||||||||
| No | 381/494 (77.1%) | Reference | -- | 172/232 (74.1%) | Reference | -- | 201/250 (80.4%) | Reference | -- |
| Yes | 14/21 (66.7%) | 0.63 (0.37−1.08) | 0.095 | 4/7 (57.1%) | 0.34 (0.12−0.92) | 0.033 | 8/12 (66.7%) | 0.85 (0.42−1.73) | 0.65 |
| P/LPVs in HRR genes | |||||||||
| No | 374/483 (77.4%) | Reference | -- | 168/226 (74.3%) | Reference | -- | 197/244 (80.7%) | Reference | -- |
| Yes | 21/32 (65.63%) | 0.76 (0.49−1.18) | 0.22 | 8/13 (61.5%) | 0.52 (0.25−1.06) | 0.074 | 12/18 (66.7%) | 0.86 (0.48−1.55) | 0.62 |
Abbreviations: CI: confidence interval, CT: chemotherapy, HR: hazard ratio, HRR: homologous recombination repair, N: number, PDAC: pancreatic ductal adenocarcinoma, P/LPV: pathogenic variant.
Among patients who received platinum-based therapy at any line of treatment, only a trend towards improved OS was identified for those who carried P/LPVs in HRR genes compared to non-carriers (HR 0.52, 95% CI 0.25−1.06, p = 0.074) (Table 3). OS was similar in patients who did not receive platinum agents, regardless of HRR P/LPV status (HR 0.86, 95% CI 0.48−1.55, p = 0.62). P/LPVs in PDAC-associated genes showed favorable prognostic significance univariately only among patients treated with platinum-based therapy (HR 0.34, 95%CI 0.12−0.92, p = 0.033). The presence of P/LPVs in HRR associated genes was not found to be prognostic for OS either among patients with early (HR 0.61, 95% CI 0.28−1.30, p = 0.20) or those with advanced stage (HR 1.09, 95% CI 0.63−1.86, p = 0.77).
3. Discussion
In this observational study, germline testing of 62 cancer predisposing genes was performed in a cohort of 549 patients with PDAC. To our knowledge, this is the only European study that tests the presence of both sequence variants (SNVs and Indels) as well as CNVs in a large number of cancer-predisposing genes, providing a comprehensive understanding of the prevalence of P/LPVs in patients with PDAC. Our study revealed P/LPVs in 4.0% and 7.7% of patients, in PDAC-associated genes and HRR genes, respectively. Importantly, patients were not preselected for age, stage or family history of cancer, highlighting the unbiased character of our study, as these factors are not always predictors of an underlying genetic factor. Moreover, association of germline testing results, clinicopathologic parameters and outcome data was performed.
Similar to previous studies, our study has identified P/LPVs in 11.29% of patients [12,20,25]. Investigators have reported significantly different proportions of patients with PDAC carrying P/LPVs in cancer predisposing genes, ranging from 3.8% to 21%. This difference might be attributed to the different number of genes interrogated in each study [15,16], inclusion of high-risk carriers (Ashkenazi ancestry) [19,20], preselection of patients for positive family history [13] and focus on specific patient populations [16]. P/LPVs might also be random findings that are not associated with increased risk for PDAC. For instance, the number of patients carrying MUTYH P/LPVs or the CHEK2 p.(Ile157Thr) might reflect the expected population frequency. Nevertheless, other investigators have previously reported the presence of P/LPVs in these genes in patients with PDAC [13,19,23,26] and, therefore, further investigation of their role is warranted. While the investigation of the presence of polyps in these patients would be clinically relevant, no data was available. Of note, in this study, a PV in SDHB was identified in a patient with PDAC with no prior history. However, detailed family history was not recorded for this patient to assess consistency with the features of the respective syndrome. The clinical significance of SDHB, a gene that has not previously been associated with PDAC predisposition, remains to be shown in future studies. Of note, our study has not identified any patient harboring TP53 P/LPV variants. Recent studies have estimated the prevalence of TP53 P/LPVs between 0.2% and 1.34% [6,16,27,28]. The heterogeneity observed regarding TP53 P/LPV prevalence might be attributed to the different study design (i.e patient cohort size, selection bias, ethnicity). SMAD4 P/LPVs have not been identified in our study, in accordance with the findings in recent studies [6,16,27,28]. Although molecular studies in sporadic pancreatic cancer have identified somatic mutations in TP53 and SMAD4 in high frequency, our study as well as other germline pancreatic cancer predisposition studies outlined here show that the hereditary contribution of these genes is rare. All P/LPVs identified in our study are heterozygous variants. This is in accordance with the mode of inheritance for these genes regarding their association with pancreatic cancer susceptibility [29].
While the presence of any P/LPV was associated with improved OS, in multivariate analysis it did not retain its prognostic significance, showing only a trend for improved OS in P/LPV carriers. In line with our findings, a previous study of 615 prospectively consented patients who underwent germline testing for 410–468 genes showed no difference in OS between patients with and without germline P/LPVs, either in the total population or in subgroup analysis (metastatic patients, carriers vs noncarriers of BRCA PVs or patients with and without tumor loss of heterozygosity of BRCA PVs) [19]. Another study similarly demonstrated that there was no difference in prognosis among carriers of P/LPVs and noncarriers [16]. However, the median OS of patients carrying P/LPVs in DNA damage repair genes was significantly longer compared to patients without such P/LPVs. On the contrary, a different study has shown that the presence of P/LPVs conferred a better prognosis to patients with PDAC [23]. Improved OS appeared to be associated primarily with P/LPVs in DNA damage repair genes.
In our study, P/LPVs in HRR genes, excluding CHEK2 p.(Ile157Thr), were identified in 7.7% patients. This proportion is significant, since these patients might benefit from innovative treatments, such as PARP inhibitors or from platinum agents. Indeed, the addition of olaparib as maintenance treatment for patients with PDAC carrying germline P/LPVs BRCA1/2 led to longer PFS compared to placebo [7]. However, a recent study showed that in pretreated patients with advanced breast cancer, responses to olaparib were gene specific [30]. No patient with CHEK2 or ATM P/LPVs responded to treatment with PARP inhibitor. Therefore, the role of PARP inhibitors or platinum agents needs to be prospectively validated in patients with PDAC carrying HRR P/LPVs in different genes.
The predictive role of P/LPVs in DNA damage repair genes is being evaluated in several tumor types. In our study, we did not observe any statistically significant benefit from the administration of platinum-based therapy in patients carrying P/LPVs in HRR genes. This lack of benefit in our study might be attributed to inclusion of platinum agents administered at any line of treatment and high heterogeneity of treatment regimens. Importantly, to our knowledge, none of our patients received treatment with poly ADP-ribose polymerase (PARP) inhibitors or immunotherapeutic agents during the study period. We did, however, observe improved clinical outcomes in patients treated with platinum-based therapy carrying P/LPVs in PDAC-associated genes. Due to the small number of patients with the event of interest noted in this subgroup, however, this result should be interpreted with caution until further validated in larger cohorts. Other investigators who tested a similar list of HRR genes (ATM, BRCA1, BRCA2, BRIP1, CHEK2, NBN, PALB2, RAD50, RAD51C and RAD51D) also reported similar OS between patients with PVs in DNA damage repair genes who received oxaliplatin-based therapy and those who did not [23]. In contrast, other studies have shown clinical benefit from treatment with platinum agents and/or PARP inhibitors in selected patients with diverse tumor types and importantly in patients with pancreatic cancer [7,8,9]. Investigators have reported that patients with PDAC and somatic mutations in DNA damage repair genes who received platinum-based therapy had improved OS compared to patients without such mutations [31]. In another study, patients with germline PVs in BRCA1, BRCA2 or PALB2 had higher objective response rates when treated with platinum regimens compared to patients who did not carry germline PVs, although this study tested fewer HRR genes compared to our study [9]. The different outcomes amongst all these studies can be attributed to the different number of patients and number of HRR genes tested as well as the different setting of genetic testing (somatic versus germline). These differences additionally highlight the importance of prospective examination of the prognostic and predictive significance of germline P/LPVs in large series of patients with PDAC.
We acknowledge that our study has certain limitations. First is the retrospective nature of the study. Second is the inclusion of only one endpoint. Additionally, this was an observational study and detailed family history was not obtained for some patients. Furthermore, tumor biopsy samples were not subjected to genetic testing. As such, loss of heterozygosity, which could shed some light into possible association causative role of the P/LPVs on PDAC, was not evaluated in the tumors. Finally, the relatively small number of patients with P/LPVs limits analysis in patient subgroups, which would demonstrate critical associations.
4. Materials and Methods
4.1. Study Population
Genomic DNA was retrospectively and prospectively (since May 2019) collected from patients with PDAC, who were unselected for family history, stage of disease, or age at diagnosis and were diagnosed from 02/2003 to 01/2020. Patients were recruited in 24 HeCOG-affiliated Departments of Oncology; 23 located in Greece (16 in Athens) and 1 in Cyprus. Written informed consent was obtained from all patients for the use of their biological material and their participation in research studies. Patient clinicopathologic characteristics, family history and outcome data were retrieved from medical records, following regulations of the Bioethics Committees of participating institutions. The study was approved by the Institutional Review Board of “Agii Anargiri” Cancer Hospital (541/06.05.2019). The trial was registered (NCT03982446).
4.2. Multigene Panel Testing
Next-generation sequencing was performed in a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory, using a panel of 62 cancer susceptibility genes (PreSENTIA™ pan-cancer gene panel, NIPD Genetics™), detecting SNVs, Indels and CNVs (Supplementary methods and Figure S1). Targeted genomic loci were captured using an in-solution hybridization method (NIPD Genetics™).
4.3. Bioinformatics and Data Analysis
Sequence data were de-multiplexed and aligned to the human genome built (hg19) using BWA-MEM to generate alignment (BAM) files. Variant calling were performed following GATK best practices workflow [32]. Custom built bioinformatics tools were used for CNV calling. Classification and interpretation of variants was performed according to established guidelines and were in line with ClinVar database [33,34]. Detailed methodology is described in the Supplement.
4.4. Statistical Analysis
Patient characteristics and P/LPV frequencies were summarized using descriptive statistics and compared with the chi-square/Fisher’s exact (for categorical variables) and Wilcoxon rank-sum test (for continuous variables). Overall survival (OS) was measured from the date of initial diagnosis until the date of death (from any cause) or last contact and was assessed in 540 patients (98.4%) with available survival data. Survival curves were estimated using the Kaplan-Meier method and compared between groups by the two-sided log-rank test. The prognostic significance of P/LPVs was evaluated using Hazard Ratios (HR) and 95% Confidence Intervals (CI) estimated by univariate Cox regression analysis in the entire cohort with available data (N = 540). Additionally, a multivariate Cox model was applied in the entire cohort including the presence of P/LPVs (prognostic term), stage of disease, platinum exposure (treatment term) and the interaction between platinum exposure and the presence of P/LPVs (predictive term).
We further evaluated the prognostic significance of P/LPVs in PDAC- (APC, ATM, BRCA1, BRCA2, MSH6 and PALB2) and HRR- (ATM, BRCA1, BRCA2, BRIP1, FANCC, CHEK2, FANCM, NBN, PALB2, RAD50, RAD51C) associated genes, upon exclusion of patients who carried only MUTYH P/LPVs and CHEK2 p.Ile157Thr, (N = 515) and in the subgroups of patients defined by treatment type (platinum-based chemotherapy yes vs. no). The reference group for comparisons was the group of patients without P/LPVs, including those with no P/LPVs in each of the aforementioned gene categories and patients with no P/LPVs at all. The predictive value of P/LPVs for platinum-based therapy was estimated univariately by interactions tests between each gene category and platinum exposure (yes vs. no). Departures from the proportional hazards assumption were assessed using time dependent covariates. All tests were two-sided and significance was set at 0.05. Analysis was performed using the SAS (version 9.3, SAS Institute Inc., Cary, NC, USA) software.
5. Conclusions
In conclusion, a significant proportion of unselected patients with pancreatic ductal adenocarcinoma carry clinically relevant germline pathogenic/likely pathogenic variants, irrespectively of age, family history or disease stage, while the median overall survival did not differ between pancreatic ductal adenocarcinoma carriers of pathogenic/likely pathogenic variants and non-carriers. In addition, no statistically significant benefit was observed from the administration of platinum-based therapy in patients carrying pathogenic/likely pathogenic variants in homologous recombination repair genes. Prospective studies evaluating the prognostic and predictive role of pathogenic/likely pathogenic variants through universal genetic testing of patients with pancreatic ductal adenocarcinoma, are warranted.
Acknowledgments
The authors are indebted to patients and their families for their trust and participation in the present registry. The authors wish to thank Eneida Jaupaj for the collection of biological material, Maria Moschoni for coordination of data management and Chrysovalanto Marinou, Louisa Constantinou and Charalambos Kkoufou for their laboratory assistance.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/13/2/198/s1, Supplementary methods, Figure S1: CNV-detection risk score plot for an ATM positive patient. Each dot denotes a targeted region with the y-axis representing the normalized read depth ratio (risk score) of the tested sample versus a set of normal samples. Horizontal dashed lines at y = 1.5 and y = 0.5 represent the expected risk score for a duplication and deletion, respectively, Table S1: Pathogenic/Likely pathogenic variants reported per patient, Table S2: P/LPV allele frequency analysis of low risk PVs in comparison with their frequency in the population (comparing against population groups with the highest frequency for each P/LPV).
Author Contributions
Conceptualization, E.F., G.K., G.F. and P.C.P.; methodology, G.K., M.I., K.T. and F.F.; software, C.L. and A.A.; validation, A.E. and E.K.; formal analysis, G.-A.K., C.L. and A.A.; investigation, K.T. and A.E.; resources, E.F., D.P., J.S., P.P., G.R. (Grigorios Rallis), A.P., C.P., G.O., K.F., A.K., G.Z., C.D., G.A., D.B., P.K., G.P., M.T., I.V., N.K., G.R. (Georgios Rigakos), Z.S., A.N., A.C., E.S., E.K. and G.F. data curation, A.E. and A.A.; writing—original draft, E.F., G.-A.K., A.E. and A.A.; writing—review and editing, P.C.P., G.K., C.L., M.I., K.T., A.E., A.A., E.K. and F.F.; visualization, A.A.; supervision, P.C.P., G.K., M.I., and G.F.; project administration, A.E., M.I. and G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NIPD Genetics Limited, Nicosia, Cyprus.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of “Agii Anargiri” Cancer Hospital (541/06.05.2019). The trial was registered (NCT03982446).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to intellectual property reasons.
Conflicts of Interest
E.F.: Stock ownership: GENPREX INC, ARIAD, Deciphera Pharmaceuticals, Inc. Travel grant: Merck, Pfizer, and K.A.M Oncology/Hematology. Advisory: LEO Pharma. Speaker fees: Roche, Pfizer; AE: Employed by NIPD Genetics; has filed a PCT patent application for the Target-enriched multiplexed parallel analysis for assessment of tumor biomarkers (WO2019/008172A1). A.A.: Employed by NIPD Genetics; has filed a PCT patent application for the Target-enriched multiplexed parallel analysis for assessment of tumor biomarkers (WO2019/008172A1); has filed a PCT patent application for the Enrichment of Targeted Genomic Regions for Multiplexed Parallel Analysis (WO2019/008148A9). C.L.: Employed by NIPD Genetics. K.T.: Employed by NIPD Genetics, has filed a PCT patent application for the Target-enriched multiplexed parallel analysis for assessment of tumor biomarkers (WO2019/008172A1), has filed a PCT patent application for the Enrichment of Targeted Genomic Regions for Multiplexed Parallel Analysis (WO2019/008148A9). D.P.: Advisory Role: Roche, MSD, Astellas. Honoraria: Roche, MSD, Astellas. P.P.: Advisory Role: Roche, Merck, Genesis Pharmaceuticals, Honoraria: Roche, Merck. A.P.: Consultation Fees: Amgen, Merck Serono, Roche, BMS, Astra Zeneca, MSD. Honoraria: Amgen, Merck Serono, Roche, BMS, Astra Zeneca, MSD, Research funds: BMS, Kura. C.P.: Speaker honoraria and honoraria for consultancy in advisory boards: Novartis, AstraZeneca, Genesis, MSD, Amgen, Pfizer, Merck, Roche; Research grants: BMS, Roche. A.K.: Advisory Role: Genesis Pharma. Honoraria: Pfizer. Speaker’s bureau: Roche, Research Funding: Merck, Travel: MSD, Educational grants: Novartis, Pfizer, Merck, Roche, BMS, MSD, Genesis, and Ipsen. G.A.: Advisory Boards: Novartis, BMS, Roche Hellas, Astra Zeneca, Sanofi, Amgen, Genesis Pharma, Merck, Pfizer; PK: Honoraria: Novartis, MSD, Pfizer. Travel: Pfizer, MSD, Genesis. A.N.: Advisory Board and Speaker fees: Pfizer, Novartis. E.S.: Advisory Board of Merck, MSD, Asta-Zeneca, Roche, Amgen and Genesis. E.K.: Employed by NIPD Genetics; has filed a PCT patent application for the Target-enriched multiplexed parallel analysis for assessment of tumor biomarkers (WO2019/008172A1); has filed a PCT patent application for the Enrichment of Targeted Genomic Regions for Multiplexed Parallel Analysis (WO2019/008148A9). M.I.: Employed by NIPD Genetics; has filed a PCT patent application for the Target-enriched multiplexed parallel analysis for assessment of tumor biomarkers (WO2019/008172A1), has filed a PCT patent application for the Enrichment of Targeted Genomic Regions for Multiplexed Parallel Analysis (WO2019/008148A9). G.K.: Employed by NIPD Genetics; has filed a PCT patent application for the Target-enriched multiplexed parallel analysis for assessment of tumor biomarkers (WO2019/008172A1); has filed a PCT patent application for the Enrichment of Targeted Genomic Regions for Multiplexed Parallel Analysis (WO2019/008148A9). G.F.: Advisory Board: Pfizer, Sanofi and Roche. Honoraria: Astra Zeneca. Stock ownership: ARIAD and GENPREX. P.C.P.: Employed by NIPD Genetics; has filed a PCT patent application for the Target-enriched multiplexed parallel analysis for assessment of tumor biomarkers (WO2019/008172A1); has filed a PCT patent application for the Enrichment of Targeted Genomic Regions for Multiplexed Parallel Analysis (WO2019/008148A9); The rest of the authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Iqbal J., Ragone A.V., Lubinski J., Lynch H.T., Moller P., Ghadirian P., Foulkes W.D., Armel S., Eisen A.Z., Neuhausen S.L., et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br. J. Cancer. 2012;107:2005–2009. doi: 10.1038/bjc.2012.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhen D.B., Rabe K.G., Gallinger S., Syngal S., Schwartz A.G., Goggins M.G., Hruban R.H., Cote M.L., McWilliams R.R., Roberts N.J., et al. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: A PACGENE study. Genet. Med. 2015;17:569–577. doi: 10.1038/gim.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X., Leslie G., Doroszuk A., Schneider S., Allen J., Decker B., Dunning A.M., Redman J., Scarth J., Plaskocinska I., et al. Cancer Risks Associated With Germline PALB2 Pathogenic Variants: An International Study of 524 Families. J. Clin. Oncol. 2020;38:674–685. doi: 10.1200/JCO.19.01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts N.J., Jiao Y., Yu J., Kopelovich L., Petersen G.M., Bondy M.L., Gallinger S., Schwartz A.G., Syngal S., Cote M.L., et al. ATM Mutations in Patients with Hereditary Pancreatic Cancer. Cancer Discov. 2012;2:41–46. doi: 10.1158/2159-8290.CD-11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kastrinos F. Risk of Pancreatic Cancer in Families with Lynch Syndrome. JAMA. 2009;302:1790–1795. doi: 10.1001/jama.2009.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu C., Hart S.N., Polley E.C., Gnanaolivu R., Shimelis H., Lee K.Y., Lilyquist J., Na J., Moore R.M., Antwi S.O., et al. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA. 2018;319:2401–2409. doi: 10.1001/jama.2018.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golan T., Hammel P., Reni M., Van Cutsem E., Macarulla T., Hall M.J., Park J.-O., Hochhauser D., Arnold D., Oh D.-Y., et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Reilly E.M., Lee J.W., Zalupski M., Capanu M., Park J., Golan T., Tahover E., Lowery M.A., Chou J.F., Sahai V., et al. Randomized, Multicenter, Phase II Trial of Gemcitabine and Cisplatin With or Without Veliparib in Patients With Pancreas Adenocarcinoma and a Germline BRCA/PALB2 Mutation. J. Clin. Oncol. 2020;38:1378–1388. doi: 10.1200/JCO.19.02931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wattenberg M.M., Asch D., Yu S., O’Dwyer P.J., Domchek S.M., Nathanson K.L., Rosen M.A., Beatty G.L., Siegelman E.S., Reiss K.A. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br. J. Cancer. 2020;122:333–339. doi: 10.1038/s41416-019-0582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marabelle A., Le D.T., Ascierto P.A., Di Giacomo A.M., De Jesus-Acosta A., Delord J.-P., Geva R., Gottfried M., Penel N., Hansen A.R., et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NCCN . NCCN; 2020. [(accessed on 7 January 2021)]. NCCN Clinical Practice Giodelines in Oncology, Pancreatic Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. [Google Scholar]
- 12.Brand R.E., Borazanci E., Speare V., Dudley B., Karloski E., Peters M.L.B., Ms L.S., Bahary N., Zeh H., Zureikat A., et al. Prospective study of germline genetic testing in incident cases of pancreatic adenocarcinoma. Cancer. 2018;124:3520–3527. doi: 10.1002/cncr.31628. [DOI] [PubMed] [Google Scholar]
- 13.Chaffee K.G., Oberg A.L., McWilliams R.R., Majithia N., Allen B.A., Kidd J., Singh N., Hartman A.-R., Wenstrup R.J., Petersen G.M. Prevalence of germ-line mutations in cancer genes among pancreatic cancer patients with a positive family history. Genet. Med. 2018;20:119–127. doi: 10.1038/gim.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cremin C., Lee M.K., Hong Q., Hoeschen C., MacKenzie A., Dixon K., McCullum M., Nuk J., Kalloger S., Karasinska J., et al. Burden of hereditary cancer susceptibility in unselected patients with pancreatic ductal adenocarcinoma referred for germline screening. Cancer Med. 2020;9:4004–4013. doi: 10.1002/cam4.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golan T., Kindler H.L., Park J.O., Reni M., Macarulla T., Hammel P., Van Cutsem E., Arnold D., Hochhauser D., McGuinness D., et al. Geographic and Ethnic Heterogeneity of Germline BRCA1 or BRCA2 Mutation Prevalence Among Patients With Metastatic Pancreatic Cancer Screened for Entry Into the POLO Trial. J. Clin. Oncol. 2020;38:1442–1454. doi: 10.1200/JCO.19.01890. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein J.B., Zhao L., Wang X., Ghelman Y., Overman M.J., Javle M.M., Shroff R.T., Varadhachary G.R., Wolff R.A., McAllister F., et al. Germline DNA Sequencing Reveals Novel Mutations Predictive of Overall Survival in a Cohort of Patients with Pancreatic Cancer. Clin. Cancer Res. 2019;26:1385–1394. doi: 10.1158/1078-0432.CCR-19-0224. [DOI] [PubMed] [Google Scholar]
- 17.Grant R.C., Selander I., Connor A.A., Selvarajah S., Borgida A., Briollais L., Petersen G.M., Lerner-Ellis J., Holter S., Gallinger S. Prevalence of Germline Mutations in Cancer Predisposition Genes in Patients With Pancreatic Cancer. Gastroenterology. 2015;148:556–564. doi: 10.1053/j.gastro.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu C., LaDuca H., Shimelis H., Polley E.C., Lilyquist J., Hart S.N., Na J., Thomas A., Lee K.Y., Davis B.T., et al. Multigene Hereditary Cancer Panels Reveal High-Risk Pancreatic Cancer Susceptibility Genes. JCO Precis. Oncol. 2018;2:1–28. doi: 10.1200/PO.17.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowery A.M., Wong W., Jordan E.J., Lee J.W., Kemel Y., Vijai J., Mandelker D., Zehir A., Capanu M., Salo-Mullen E., et al. Prospective Evaluation of Germline Alterations in Patients With Exocrine Pancreatic Neoplasms. J. Natl. Cancer Inst. 2018;110:1067–1074. doi: 10.1093/jnci/djy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salo-Mullen E.E., O’Reilly E.M., Kelsen D.P., Ba A.M.A., Lowery M.A., Yu K.H., Reidy D.L., Epstein A.S., Lincoln A., Bs A.S., et al. Identification of germline genetic mutations in patients with pancreatic cancer. Cancer. 2015;121:4382–4388. doi: 10.1002/cncr.29664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shindo K., Yu J., Suenaga M., Fesharakizadeh S., Cho C., Macgregor-Das A., Siddiqui A., Witmer P.D., Tamura K., Song T.J., et al. Deleterious Germline Mutations in Patients With Apparently Sporadic Pancreatic Adenocarcinoma. J. Clin. Oncol. 2017;35:3382–3390. doi: 10.1200/JCO.2017.72.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holter S., Borgida A., Dodd A., Grant R., Semotiuk K., Hedley D., Dhani N., Narod S., Akbari M., Moore M., et al. Germline BRCA Mutations in a Large Clinic-Based Cohort of Patients With Pancreatic Adenocarcinoma. J. Clin. Oncol. 2015;33:3124–3129. doi: 10.1200/JCO.2014.59.7401. [DOI] [PubMed] [Google Scholar]
- 23.Yurgelun M.B., Chittenden A.B., Morales-Oyarvide V., Rubinson D.A., Dunne R.F., Kozak M.M., Qian Z.R., Ba M.W.W., Brais L.K., Da Silva A., et al. Germline cancer susceptibility gene variants, somatic second hits, and survival outcomes in patients with resected pancreatic cancer. Genet. Med. 2019;21:213–223. doi: 10.1038/s41436-018-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeuchi S., Doi M., Ikari N., Yamamoto M., Furukawa T. Mutations in BRCA1, BRCA2, and PALB2, and a panel of 50 cancer-associated genes in pancreatic ductal adenocarcinoma. Sci. Rep. 2018;8:8105. doi: 10.1038/s41598-018-26526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu C., Hart S.N., Bamlet W.R., Moore R.M., Nandakumar K., Eckloff B.W., Lee Y.K., Petersen G.M., McWilliams R.R., Couch F.J. Prevalence of Pathogenic Mutations in Cancer Predisposition Genes among Pancreatic Cancer Patients. Cancer Epidemiol. Biomark. Prev. 2016;25:207–211. doi: 10.1158/1055-9965.EPI-15-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obazee O., Archibugi L., Andriulli A., Soucek P., Małecka-Panas E., Ivanauskas A., Johnson T., Gazouli M., Pausch T., Lawlor R.T., et al. Germline BRCA2 K3326X and CHEK2 I157T mutations increase risk for sporadic pancreatic ductal adenocarcinoma. Int. J. Cancer. 2019;145:686–693. doi: 10.1002/ijc.32127. [DOI] [PubMed] [Google Scholar]
- 27.Dudley B., Karloski E., Monzon F.A., Singhi A.D., Bs S.E.L., Bahary N., Brand R.E. Germline mutation prevalence in individuals with pancreatic cancer and a history of previous malignancy. Cancer. 2018;124:1691–1700. doi: 10.1002/cncr.31242. [DOI] [PubMed] [Google Scholar]
- 28.Yadav S., Kasi P.M., Bamlet W.R., Ho T.P., Polley E.C., Hu C., Hart S.N., Rabe K.G., Boddicker N.J., Gnanaolivu R.D., et al. Effect of Germline Mutations in Homologous Recombination Repair Genes on Overall Survival of Patients with Pancreatic Adenocarcinoma. Clin. Cancer Res. 2020;26:6505–6512. doi: 10.1158/1078-0432.CCR-20-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrera S., Sancho A., Azkona E., Azkuna J., Lopez-Vivanco G. Hereditary pancreatic cancer: Related syndromes and clinical perspective. Hered. Cancer Clin. Pract. 2017;15:1–9. doi: 10.1186/s13053-017-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tung N.M., Robson M.E., Ventz S., Santa-Maria C.A., Marcom P.K., Nanda R., Shah P.D., Ballinger T.J., Yang E.S.-H., Melisko M.E., et al. TBCRC 048: A phase II study of olaparib monotherapy in metastatic breast cancer patients with germline or somatic mutations in DNA damage response (DDR) pathway genes (Olaparib Expanded) J. Clin. Oncol. 2020;38:1002. doi: 10.1200/JCO.2020.38.15_suppl.1002. [DOI] [PubMed] [Google Scholar]
- 31.Pishvaian M.J., Blais E.M., Brody J.R., Sohal D., Hendifar A.E., Chung V., Mikhail S., Rahib L., Lyons E., Tibbetts L., et al. Outcomes in pancreatic adenocarcinoma (PDA) patients (pts) with genetic alterations in DNA damage repair (DDR) pathways: Results from the Know Your Tumor (KYT) program. J. Clin. Oncol. 2019;37:191. doi: 10.1200/JCO.2019.37.4_suppl.191. [DOI] [PubMed] [Google Scholar]
- 32.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S.B., Daly M.J., et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–423. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landrum M.J., Lee J.M., Riley G.R., Jang W., Rubinstein W.S., Church D.M., Maglott D.R. ClinVar: Public archive of rela-tionships among sequence variation and human phenotype. Nucleic Acids Res. 2014;42:D980–D985. doi: 10.1093/nar/gkt1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to intellectual property reasons.