Abstract
Background:
Traumatic injury to spine and spinal cord represents a devastating condition, with a huge risk for permanent severe disabilities. Predicting the long-term outcome in this type of trauma is a very difficult task being under the influence of a wide spectrum of biomechanical and pathophysiological factors. The advent of magnetic resonance imaging (MRI) structural evaluation of the spinal cord brought critical supplementary data in the initial evaluation of these cases. Although edema and hemorrhage proved to be valuable in predicting the outcome, there is a well-documented discrepancy between MRI findings and clinical status.
Methods:
We performed diffusion tensor imaging (DTI) MR in 22 symptomatic patients with traumatic cervical spine injuries (mean age 49.6 ± 16, range from 17 to 74 years, 20 males and 2 females). DTI parameters were computed in 15 patients. Regional apparent diffusion coefficient, fractional anisotropy (FA), and fiber length (FL) were calculated in the region of interest defined as the region of maximum structural MR alterations and in the normal cord (above or below the level of the injury). The values for normal and pathological cord were compared. The clinical deficit was assessed with ASIA and subaxial cervical spine injury classification (SLIC) scores. We looked at the correlation between the DTI measures and clinical scores.
Results:
There is a highly significant difference between normal and pathological spinal cord for all DTI properties measured. There is also a strong correlation between DTI measures and SLIC clinical score, especially for FA. Significant results were obtained for CDA and FL as well although with lesser statistical power.
Conclusion:
Our results suggest that DTI measures, especially FA, represent a strong indicator of the severity of the traumatic cervical cord injury. It correlates very well with SLCI score and can be used as an additional confirmation of the real degree of level lesioning and as a prognostic factor for the neurological outcome regardless of the choice of treatment.
Keywords: Cervical, Diffusion tensor imaging, Magnetic resonance imaging, Spine, Subaxial cervical spine injury classification, Trauma

INTRODUCTION
Traumatic injuries of the cervical spine carry a huge risk for serious neurological deficit and other debilitating conditions. Neurological damage in these cases results in devastating medical, social, emotional, and financial consequences. Assessing the degree of structural destruction within the spinal cord and making a reliable and accurate prediction of the neurological outcome continues to be a very difficult task. Conventional magnetic resonance imaging (MRI) has become a standard in the routine exploration of the soft-tissue and spinal cord damages in spinal trauma.[6,11,15] Posttraumatic hemorrhage and edema of the spinal cord, in particular, have been shown to be picked up by MRI with high sensitivity and to have a certain value in predicting the neurologic outcome.[7,12] However, the MRI aspects often are in strong antithesis with the clinical picture and fail to offer significant data to explain the severity of the neurological deficits. Recent studies have demonstrated that diffusion tensor imaging (DTI) is a sensitive measure for in-depth alteration in the structure of the spinal cord and correlates with the severity of the injury when compared to normal subjects.[14] However, its correlation to the clinical status and its potential as a prognostic factor for this category of injuries have yet to be fully demonstrated. In our study, we looked at the changes in the apparent diffusion coefficient (ADC), fractional anisotropy (FA), and fiber length (FL) after traumatic cervical cord injury. We compared these against corresponding data from the same subjects in areas distant to the site of injury and analyzed the correlation of these values with the subaxial cervical spine injury classification (SLIC) and severity scale scores.[16]
MATERIALS AND METHODS
Our institutional ethics review Board approved this study
Twenty-eight patients with traumatic cervical spine injuries (mean, 50.1 ± 19.7 years of age; 3 women and 25 men) have been prospectively included in a MRI protocol that included conventional MRI and DTI. MRI studies were performed 10 h–6 days (mean, 36 ± 24 h) following the traumatic incident. Indications for MRI included neurologic deficit on clinical examination localized to the cervical spine (21 cases), spine fracture demonstrated on the X-ray or CT at admission – for the evaluation of the extent of ligamentous injury (3 cases), and cervical pain unexplained by the cervical spine radiographs or CT at admission (4 cases). Mechanisms of injury included motor vehicle accidents (6 cases), animal-powered vehicle accidents (7 cases), motorcycle accidents (3 cases), fall (8 cases), sports accidents (2 cases), and assault (2 cases). DTI data from 13 patients were not used for further analysis because of their poor quality and/or present artifacts. The 15 remaining patients formed the study group.
MRI technique
All MRI studies were performed on a 1.5T Siemens Avanto scanner (Siemens, Erlangen, Germany) with parallel imaging capability. Conventional MRI included axial and sagittal T1, sagittal and axial T2, fluid-attenuated inversion recovery (TE/ TR/echo train), and axial T2* images. DTIs were obtained using an echo-planar imaging (EPI) sequence at a resolution of 128/128 over a 20 cm FOV. Sections had a 3 mm thickness and on average 80 (between 76 and 84) of them were produced to image the whole spine from cervical-medullar junction to the cervical-thoracic junction. A 12-channel head-neck array coil was used on all patients. Parallel imaging was carried on with the phase encoding in the anterior-to-posterior direction. For anatomic reference, we used sagittal T2-weighted images and b = 0 s/mm2 images from the DTIs.
Image analysis
In echo-planar-based diffusion-weighted imaging (DWI) and DTI, the evaluation of diffusion parameters such as ADCs and anisotropy indices is affected by image distortions that arise from residual eddy currents produced by the diffusion-sensitizing gradients. We used FMRIB’s Diffusion Toolbox from FMRIB Software Library v5.0 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) to correct these currents. Maps of ADC and FA were generated using MedInria (https://med.inria.fr/). We selected two ROIs from the spinal cord. The first one was the region that was placed within the area of the spinal cord injury identified using conventional MRI, from which the DTI parameters were measured. The second was a healthy region of the spinal cord, used as a control [Figure 1]. Regions of interest were drawn using the T2-weighted and/or the b=0 portion of the DTI images; and the ADC, FA, RA, and VR were measured, as well as FL. For each of these parameters, four different values were calculated: the minimum, maximum, the average, and the variance. The regions of interest were carefully placed so that they included both the central gray and white matter. Region-of-interest locations were confirmed using the optimal conventional MRI sequence, and care was taken to avoid inclusion of CSF. Data from the two regions were compared against each other for significant differences in all measured parameters. As ADC has been previously shown to be significantly different between upper, middle, and lower cervical cord, we continued the rest of the analysis with FA and FL.
Figure 1:
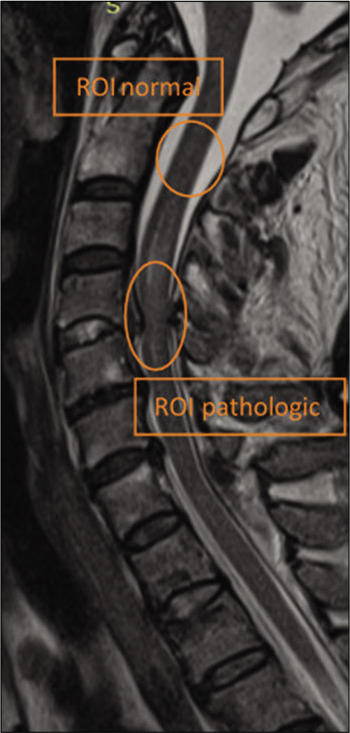
The localization of regions of interest in the sagittal T2-weighted image.
Clinical evaluation
Each patient underwent a full neurological examination at admission. The SLIC score as described by Vaccaro et al.[16] was computed taking into account the CT and MRI findings, as well as the neurological status. ASIA charts were completed as dictated by our institution protocol. Besides being the most important factor in therapeutic decision-making, the SLIC score was used in our study as the morphofunctional parameter against which we analyzed the DTI data.
RESULTS
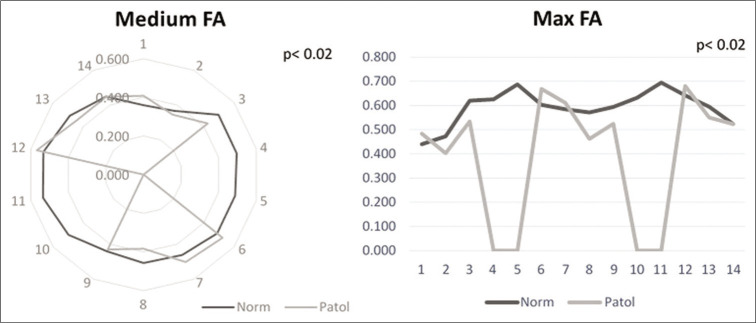
Analyzing the differences in FA between the level of maximal injury and the distant, apparently intact spinal cord we found that both the maximum and the medium values were significantly different between the two ROIs (P < 0.02, Student’s t-test). The minimum did not reach the significance level (P > 0.05). The two significant measures were used in the clinical correspondence test [Figure 2].
Figure 2:
Comparison of fractional anisotropy (FA) values in normal and pathological regions of interest for medium FA (left) and maximal FA (right).
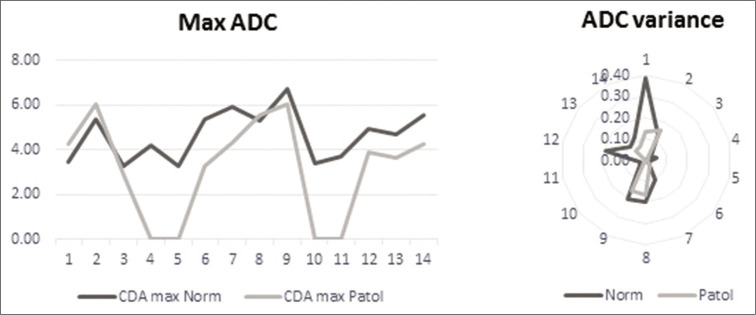
For ADC, we computed, besides the maximal, medial, and minimal values, the variance. When we tested the values between the two ROIs, the minimal and medial values did not reach the level of significance (P > 0.05). The maximal value and the variance, however, showed a highly significant difference (P < 0.003 and P < 0.006) [Figure 3] and were further tested in conjunction with the clinical data.
Figure 3:
Comparison of apparent diffusion coefficient (ADC) values in normal and pathological regions of interest for maximal ADC (left) and ADC variance (right).
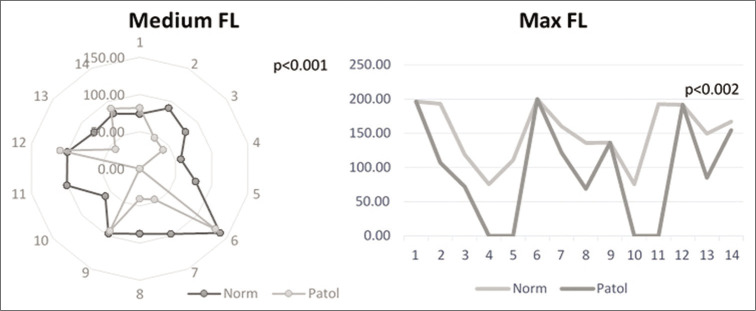
Finally, for FL, the same for values were computed (maximal, minimal, medial, and variance). Again, the minimal values and the variance did not reach the level of significance (P > 0.05). For the maximal and medial values, however, the differences between the two regions were highly significant and were used further in the study [Figure 4].
Figure 4:
Comparison of fiber length (FL) values in normal and pathological regions of interest for medium FL (left) and maximal FL (right).
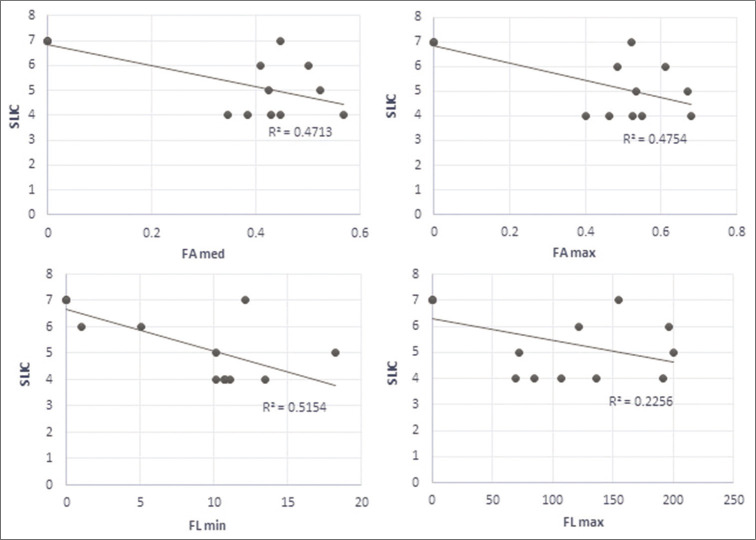
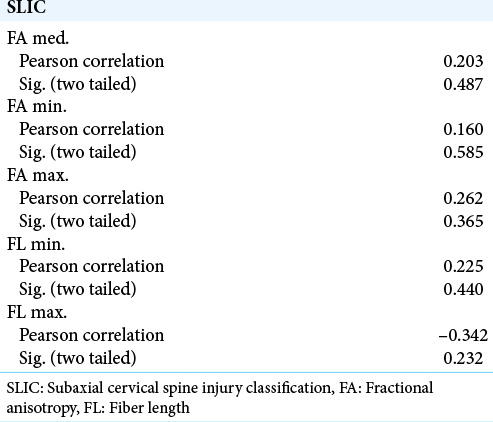
We found that for measures that we decided to use in the context of clinical data, the measured values of DTI parameters and the SLIC score had a strong linear regression, except for the maximal FL [Figure 5]. We then computed the correlation between the four parameters and the SLIC score using Pearson’s correlation method. The results are summarized in [Table 1]. All values tested showed a strong correlation with SLIC except for maximal FL which came close but did not reach the 0.05 level of significance. As a control, we ran the same test for the data from the region of apparently healthy spinal cord. These data failed to correlate with the clinical score at all levels. These results are summarized in [Table 2].
Figure 5:
Regression behavior of fractional anisotropy and fiber length values against subaxial cervical spine injury classification values.
Table 1:
Correlations of FA and FL values in pathological ROIs with SLIC values.
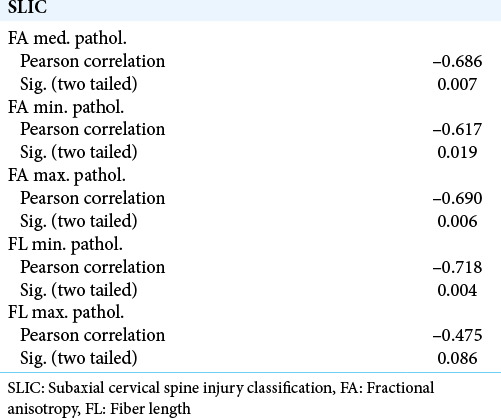
Table 2:
Correlations of FA and FL values in normal ROIs with SLIC values.
DISCUSSION
Traumatic cervical spine injuries may lead to significant morbidity and generate a number of disastrous consequences culminating in severe neurological deficit and death. Two major issues are commanded by these patients. First is the question of the best treatment for each particular case. Second is communicating to the patient and his family an accurate evaluation of the present status and of the prognostic based as much as possible on real data. A multitude of evaluation and classification systems have been introduced overtime. However, none of these showed a real practical validation for a reliable and suggestive subaxial cervical trauma assessment due to a number of factors (vague terminology, retrospective injury mechanism devise, failure in taking into account other essential variables related to spine stability, and insufficient data about spinal cord structural and functional integrity).
Imaging of the spinal cord through conventional MRI is routinely used to document the location of cord injury and the amount of cord compression from extramedullary pathologic factors. Extending the investigation with DTI proved to be of benefit in further defining the morphology changes in white matter tracts within the spinal cord. The measurable parameters of DWI showed a high sensitivity in discovering changes in patients with apparently normal conventional MR images.[4,13]
DTI parameters offer valuable information that evaluate axonal injuries across different axonal tracts, therefore, the diffusion technique is one of the most widely used in evaluating the effects of different pathologies on the nervous tissue. Decreased values of FA were found in studies regarding brain tumor (low- vs. high-grade glioma, glioblastoma, and white matter tracts around glioblastoma), epidermoid cyst, or brain edema.[3,5] Increased values of FA were significant in defining neurocysticercosis (vesicular to calcified stage), brain abscess, and brain tumor (atypical vs. benign meningioma).[1,9,10]
Progress has also been made in the early detection of neurodegenerative diseases such as multiple sclerosis or amyotrophic lateral sclerosis. In his study, Gatto et al.[8] found clear differences in microstructural white matter tract information in a murine model of ALS. As it progresses, the neurodegenerative disease manifests through a combination of elements of neuroinflammation and microstructural remodeling that can be measured with DTI parameters. Wolanczy et al.[17] showed significant differences in FA values between the normal-appearing spinal cords of patients with MS and healthy controls. Lower FA values (P < 0.05) were found at levels C2 and C3 in patients with MS than in the control subjects.
The single direction of the cord white matter tracts (craniocaudal) and their symmetry within the cord makes them potential very good candidates for diffusion measurements.[2] Existing data suggest that there is a variation in the mean ADC and FA values between the upper and lower cervical cord. However, other studies failed to find significant differences in mean diffusivity along the length of the normal cord.[16] Nonetheless, they noted a significant increase in the FA in the mid and lower section of the cord compared with the upper cord. Yet, other studies demonstrated significantly higher mean ADC values in the mid (C3-C5) and lower (C6-T1) cervical cord when compared with the upper cervical (C1-C2) cord in normal subjects. Furthermore, the same study found FA values to be significantly lower in the mid and lower cervical cord. These important differences among reported findings may be attributed to a number of factors (measurements made at different anatomic locations and variations in the choice of pulse sequences among others).[13]
Our results support the hypothesis that DTI parameters, including ADC values for cervical spine contusions, are significantly lower than comparative values in the apparently intact spinal cord. These data suggest that the three DTI parameters we measured (FA, ADC, and FL) represent sensitive markers of spinal cord injury. If we look at the maximal values for all parameters, they are the most sensitive markers of cord injury, especially the ADC and FL. They showed a similar regression with a strong r2 when run against clinical status scale and also showed strong correlations with the clinical status. The apparently normal cord failed to produce the same strong correlations. Our findings are in agreement with previously reported results.
Furthermore, our data suggest that the abnormalities in the DTI parameters in areas of the spinal cord that appears normal on conventional MRI could be used to document the true extent of injury and correlate with the neurologic deficit with a high degree of statistical significance. Our results also indicate that DTI parameters used in conjunction with the clinical evaluation could be an independent predictor of the severity of spinal cord injury.
However, we could not produce histological evidence of the injured cord in the studied patient group. Future studies that can correlate imagistic findings with histologic data are due to confirm these findings. There are also certain limitations inherent in this study that needs further addressing. In addition to its small sample size, the study did not correlate DTI parameters with histologic observations, as mentioned above. Future studies with a large sample size are necessary to further validate our results. Last but not least, further investigation of the dynamics of correlation from the supra-acute to chronic phases of cervical trauma (based on the findings from the above-mentioned studies looking at different pathologies) would be of interest and further added value.
CONCLUSION
Our study comes to strengthen the value of DTI parameters as an indicator of the extent of spinal cord injury and they are apparent even in the absence of changes on the conventional MR images. The DTI changes show a strong correlation with the clinical status and could be used as a supplementary, more in-depth predictor of the neurological outcome in these cases.
Footnotes
How to cite this article: Iliescu BF, Gutu P, Dabija MG. Traumatic subaxial cervical spine injury – Improving initial evaluation through correlation of diffusion tensor imaging and subaxial cervical spine injury classification score. Surg Neurol Int 2021;12:10.
Contributor Information
Bogdan Florin Iliescu, Email: bogdan.iliescu@gmail.com.
Pavel Gutu, Email: levap1506@gmail.com.
Marius Gabriel Dabija, Email: mariusdabija.md@gmail.com.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Agarwal V, Kumar M, Singh JK, Rathore RK, Misra R, Gupta RK. Diffusion tensor anisotropy magnetic resonance imaging: A new tool to assess synovial inflammation. Rheumatology (Oxford) 2009;48:378–82. doi: 10.1093/rheumatology/ken499. [DOI] [PubMed] [Google Scholar]
- 2.Biton IE, Duncan ID, Cohen Y. High b-value q-space diffusion MRI in myelin-deficient rat spinal cords. Magn Reson Imaging. 2006;24:161–6. doi: 10.1016/j.mri.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Cortez-Conradis D, Rios C, Moreno-Jimenez S, RoldanValadez E. Partial correlation analyses of global diffusion tensor imaging-derived metrics in glioblastoma multiforme: Pilot study. World J Radiol. 2015;7:405–14. doi: 10.4329/wjr.v7.i11.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demir A, Ries M, Moonen CT, Vital JM, Dehais J, Arne P, et al. Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Neuroradiology. 2003;229:37–43. doi: 10.1148/radiol.2291020658. [DOI] [PubMed] [Google Scholar]
- 5.El-Serougy L, Razek AA, Ezzat A, Eldawoody H, El-Morsy A. Assessment of diffusion tensor imaging metrics in differentiating low-grade from high-grade gliomas. Neuroradiol J. 2016;29:400–7. doi: 10.1177/1971400916665382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flanders AE, Schaefer DM, Doan HT, Mishkin MM, Gonzalez CF, Northrup BE. Acute cervical spine trauma: Correlation of MR imaging findings with degree of neurologic deficit. Radiology. 1990;177:25–33. doi: 10.1148/radiology.177.1.2399326. [DOI] [PubMed] [Google Scholar]
- 7.Flanders AE, Spettell CM, Tartaglino LM, Friedman DP, Herbison GJ. Forecasting motor recovery after cervical spinal cord injury: Value of MR imaging. Radiology. 1996;201:649–55. doi: 10.1148/radiology.201.3.8939210. [DOI] [PubMed] [Google Scholar]
- 8.Gatto RG, Weissmann C, Amin M, Finkielsztein A, Sumagin R, Mareci TH, et al. Assessing neuraxial microstructural changes in a transgenic mouse model of early stage amyotrophic lateral sclerosis by ultra-high field MRI and diffusion tensor metrics. Animal Model Exp Med. 2020;3:117–29. doi: 10.1002/ame2.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta RK, Srivastava S, Saksena S, Rathore RK, Awasthi R, Prasad KN, et al. Correlation of DTI metrics in the wall and cavity of brain abscess with histology and immunohistochemistry. NMR Biomed. 2010;23:262–9. doi: 10.1002/nbm.1448. [DOI] [PubMed] [Google Scholar]
- 10.Jolapara M, Kesavadas C, Radhakrishnan VV, Saini J, Patro SN, Gupta AK, et al. Diffusion tensor mode in imaging of intracranial epidermoid cysts: One step ahead of fractional anisotropy. Neuroradiology. 2009;51:123–9. doi: 10.1007/s00234-008-0464-9. [DOI] [PubMed] [Google Scholar]
- 11.Katzberg RW, Benedetti PT, Drake CM, Ivanovic M, Levine RA, Beatty CS, et al. Acute cervical spine injuries: Prospective MR imaging assessment at a level 1 trauma center. Radiology. 1999;213:203–12. doi: 10.1148/radiology.213.1.r99oc40203. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni MV, McArdle CB, Kopanicky D, Miner M, Cotler HB, Lee KF, et al. Acute spinal cord injury: MR imaging at 1.5 T. Radiology. 1987;164:837–43. doi: 10.1148/radiology.164.3.3615885. [DOI] [PubMed] [Google Scholar]
- 13.Mamata H, Jolesz FA, Maier SE. Apparent diffusion coefficient and fractional anisotropy in spinal cord: Age and cervical spondylosis-related changes. J Magn Reson Imaging. 2005;22:38–43. doi: 10.1002/jmri.20357. [DOI] [PubMed] [Google Scholar]
- 14.Shanmuganathan K, Gullapalli RP, Zhuo J, Mirvis SE. Diffusion tensor MR imaging in cervical spine trauma. AJNR Am J Neuroradiol. 2008;29:655–9. doi: 10.3174/ajnr.A0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silberstein M, Tress BM, Hennessy O. Prediction of neurologic outcome in acute spinal cord injury: The role of CT and MR. AJNR Am J Neuroradiol. 1992;13:1597–608. [PMC free article] [PubMed] [Google Scholar]
- 16.Vaccaro AR, Hulbert RJ, Patel AA, Fisher C, Dvorak M, Lehman RA, Jr, et al. The subaxial cervical spine injury classification system: A novel approach to recognize the importance of morphology, neurology, and integrity of the disco-ligamentous complex. Spine (Phila Pa 1976) 2007;32:2365–74. doi: 10.1097/BRS.0b013e3181557b92. [DOI] [PubMed] [Google Scholar]
- 17.Wolanczyk M, Bladowska J, Kołtowska A, PokryszkoDragan A, Podgórski P, Budrewicz S, et al. Diffusion tensor imaging of normal-appearing cervical spinal cords in patients with multiple sclerosis: Correlations with clinical evaluation and cerebral diffusion tensor imaging changes, Preliminary experience. Adv Clin Exp Med. 2020;29:441–8. doi: 10.17219/acem/116754. [DOI] [PubMed] [Google Scholar]