Abstract
The synergistic potential of plant essential oils (EOs) with other conventional and non-conventional antimicrobial agents is a promising strategy for increasing antimicrobial efficacy and controlling foodborne pathogens. Spoilage microorganisms are one of main concerns of seafood products, while the prevention of seafood spoilage principally requires exclusion or inactivation of microbial activity. This review provides a comprehensive overview of recent studies on the synergistic antimicrobial effect of EOs combined with other available chemicals (such as antibiotics, organic acids, and plant extracts) or physical methods (such as high hydrostatic pressure, irradiation, and vacuum-packaging) utilized to reduce the growth of foodborne pathogens and/or to extend the shelf-life of seafood products. This review highlights the synergistic ability of EOs when used as a seafood preservative, discovering the possible routes of the combined techniques for the development of a novel seafood preservation strategy.
Keywords: plant extract, sea product, antimicrobial agent, foodborne pathogen, synergistic effect
1. Introduction
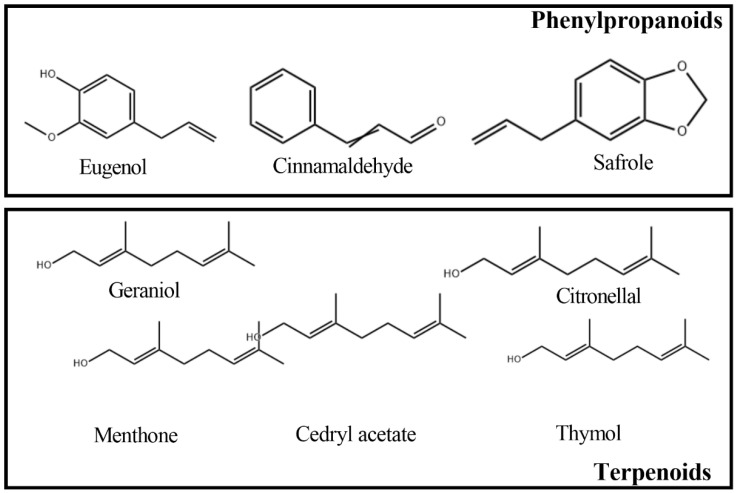
The commonly used antimicrobial agents include two types: Natural and synthetic compounds. With the increasing concerns for food safety and the understanding of the harm of synthetic preservatives to human health on multiple fronts, research of natural preservatives has recently been significantly developed in the field of seafood preservation [1,2], as it is known that seafood is a rich nutrient source for microbial development and very prone to the loss of quality caused by spoilage microorganisms [3]. Essential oils (EOs) are mixtures of volatile and hydrophobic substances from plants, which can be considered ubiquitous in the plant kingdom. While some of them with promising antimicrobial and antioxidative bioactivities extracted from various genera distributed in approximately 60 families, which are known to produce EOs with high application value in the pharmaceutical and cosmetic industries, mainly including Alliaceae, Apiaceae, Asteraceae (Compositae), Lamiaceae (Labiatae), Myrtaceae, Poaceae, Cupressaceae, Lauraceae, Pinaceae, Zingiberaceae, and Rutaceae [4]. EOs are organic volatile compounds, generally the molecular weight is less than 300 Da, and they are composed of two distinct chemical classes including terpenoids and phenylpropanoids, as some examples are shown in Figure 1, and after extensive research, it is known that many compositions of EOs have excellent antimicrobial and antioxidative properties [5]. Among these active compounds, small molecule phenols or aldehydes contributed greatly to the antimicrobial effect, such as carvacrol, cinnamaldehyde, and cuminaldehyde [6]. Additionally, terpene alcohols, ethers, and ketones also have certain antimicrobial activities, such as carvone, geraniol, and γ-tepinyl acetate [7]. Current research has demonstrated that one of the most popular proposed mechanisms is that the action of EOs leads to the breakdown of the microbial membrane, resulting in the leakage of cellular components, loss of ions, and prevention of many cellular life activities, including energy production and cell membrane transport [8].
Figure 1.
Chemical structural formulas of some essential oils.
When two or more compounds are used at the same time, the mixtures with the antagonist result in the chemical substance being antagonized, and if the effectiveness is smaller than that of one of them, it is regarded as antagonism. When the comprehensive effect of the mixture is greater than the sum of the individual actions, it is considered to be a synergistic effect, while an additive effect is defined as the effects of the combinations are equal to the sum of the effect of each chemical substance. Actually, it is possible to combine several antimicrobial compounds to create a synergistic or additive effect. The main objective of the combined techniques in the food industry is to reduce the growth and activity of spoilage microorganisms more efficiently in food products to increase their shelf-life and reduce the losses from decay, with the final aim to protect food safety and quality [9]. The spoilage of seafood could be caused by microbial infection, autolysis, or chemical oxidation, while microbial infection constitutes more spoilage than the others. Microorganisms involved in seafood spoilage mainly include the spoilage bacteria Alteromonas nigrifaciens, Shewanella putrefaciens, Brochothrix thermosphacta, Aeromonas hydrophila, Photobacterium phosphorous, and Pseudomonas spp and foodborne pathogens, such as Listeria monocytogenes, Vibrio spp., and Salmonella spp. [10]. In addition, in seafood products bacterial growth and metabolic activity cause the formation of ammonia, biogenic amines, organic acids, sulfur compounds, and ketones, which are responsible for unpleasant and unacceptable off-flavors [11]. On the other hand, contaminated seafood can cause harm to human health, as seafood is an important reason for the outbreak of foodborne disease. In the EU, seafood accounted for more than 10% of foodborne outbreaks in 2015 [12], and the CDC estimates that approximately 48 million people are affected and 3000 deaths occur each year due to foodborne illness in the United States [13]. Second, pathogens such as Vibrio and norovirus in shellfish are the major causes of seafood-mediated diseases, such as diarrhea and vomiting [14]. Enhanced disinfection technology is therefore needed to improve the safety of seafood for human consumption. In this regard, recently, physical, chemical, and biological methods have been developed and used to reduce or inactivate the growth and survival of seafood spoilage bacteria and foodborne pathogens to extend the shelf-life of seafood products and protect human health. EOs and their bioactive constituents have attracted great attention because of their potential functions and properties for use in the seafood industry, particularly recently, as we have an increased threat of antimicrobial resistance to common preservatives. Fortunately, the synergistic or additive antimicrobial effects of EOs or their components combined with chemical or physical methods have been intensively investigated in the past five years [15,16].
This review paper contains five sections. The Section 1 is the introduction. The Section 2 summarizes the synergistic or additive effects of the combination of EOs with other chemicals, such as antibiotics, organic acids, and plant extracts. The Section 3 reviews the active edible coatings incorporating EOs as synergistic factors. In the Section 4, we discuss the preservation techniques using physical devices, such as high hydrostatic pressure, irradiation, and vacuum-packaging, and their applications in the seafood industry. The Section 5 discusses the further applications of the combined techniques using EOs as one of the main methods in seafood preservation.
2. Synergistic Effect of Essential Oils with Other Compounds on Seafood Preservation
Compared to the use of only an EO, the combination of an EO with other bioactive compounds, including antibiotics, organic acids, and other EOs, may have a stronger antimicrobial activity compared to those of any single compound, which can be attributed to the mixture increasing the diversity of components and resulting in multiple sites of action [17,18]. As mentioned previously, seafood is very perishable, and food-borne pathogens in seafood not only lead to spoilage, but also cause serious illnesses that are harmful to human health. Salmonella bacteria, the most frequently reported cause of foodborne illness, has been linked to contaminated shrimp [19] and other seafood [20]. Nevertheless, EOs combined with other plant extracts showed synergistic antimicrobial effectiveness against food-borne pathogens. For example, Porter et al. (2019) reported MICs (minimum inhibitory concentrations) of 0.5, 0.02, and 0.02% for white mustard essential oil (WMEO), carvacrol, and thymol, respectively, against S. Typhimurium, while WMEO combined with thymol or carvacrol indicated that the concentration of the individual essential oil was reduced in the serovars test for controlling Salmonella [21]. It was also found that the combined effect of Trachyspermum ammi EO and propolis ethanolic extract increases the antimicrobial efficacy against some food-borne pathogenic bacteria, including Escherichia coli, S. Typhimurium, L. monocytogenes, Bacillus cereus, and Staphylococcus aureus [22]. Additionally, mustard EO also exhibited synergistic effects against foodborne pathogenic bacteria when combined with either Mexican oregano or thyme EO [23]. In addition, plant extracts have also been used for food preservation and to extend the shelf-life of seafood products. For example, the combination of turmeric powder (1.0%), galangal powder (1.25%), and lemongrass essential oil (0.5%) created a synergistic effect that could effectively maintain the physico-chemical, microbial, and sensory characteristics of white hard clam muscle for 12 days of storage [24].
The application of antibiotics is a common practice for control food-decaying microorganisms and human pathogens. However, the long-term and extensive use of antibiotics leads to a series of problems, such as residue and environmental pollution, the resistance of bacteria, and human health problems. Moreover, it requires increasing the dosage to treat the antibiotic-resistant disease, which in turn creates a vicious circle. EOs are also alternative compounds to control multidrug-resistant pathogens. Furthermore, synergistic antimicrobial activity against foodborne pathogens was revealed when antibiotics were used in combination with EOs. de Jesus et al. (2020) reported that EOs from Cerrado plants proved to be active against resistant bacteria including S. Typhi and oxacillin-resistant Staphylococcus strains [25]. Clove and thyme essential oils exhibit antimicrobial activities that have been mainly attributed to their ability to inhibit bacterial biofilm formation, and hence, they have been proposed as a natural alternative to be used in combination with antibiotics against multidrug-resistant bacteria [26]. In addition, Fennel essential oil mainly composed of trans-anethole (80%) was found to significantly enhance the inhibition zone against resistant phenotypes of S. aureus in the combined treatment with cefoxitin, mupirocin, cotrimoxazole, and ciprofloxacin [27]. Pereira et al. (2017) reported that Eugenia uniflora essential oil (EuEO) presented a synergistic efficiency associated with amikacin against S. aureus and E. coli, while an exception was observed when EuEO was combined with gentamicin against E. coli, in which an antagonistic effect was observed [28].
Organic acids, such as citric acid (CA), lactic acid (LA), and capric acid (CP), are ‘generally recognized as safe’ (GRAS), as they occur naturally in foods and are particularly suitable for the tread of green consumerism, which encourages food processors to find alternatives to synthetic chemicals for food preservation. Organic acids and their salts were found to have preservative activity in seafood, and the activity can be increased when used in appropriate combination with EOs. Recently, it has been confirmed that mixtures of tartaric acid and garlic oil (or lactic acid and cinnamon oil) are effective in extending the shelf-life of raw shrimp, as indicated by both their microbiological and physicochemical properties [29]. Dogruyol et al. (2020) reported that the thermal sensitivity of L. monocytogenes was increased in sous-vide salmon by the combined treatment of oregano essential oil and citric acid [30].
Spoilage is a major problem in the transportation and selling of fish due to this issue of their limited shelf-life. Thus, methods for fish preservation to prevent quality deterioration and microbial growth are needed. Recently, the comprehensive use of food-grade compounds and EOs to control fish spoilage has achieved a better control effect. As also listed in Table 1, recent studies have described the synergistic antibacterial activity of EOs combined with other food-grade chemicals utilized for the control of seafood spoilage. In addition, it would be interesting to consider the effect of EOs treatment on the gustatory properties of seafood, and this may be widely accepted because people cook seafood often accompanied by the use of plant materials (such as lemon oils). However, the concentrations of EOs should not exceed the acceptability of human taste.
Table 1.
Synergistic or additive effect of essential oils combined with other chemical substances on quality retention of seafood products.
| Plant Sources | Combined Chemical Compounds | Tested Seafood | Effectiveness | Reference |
|---|---|---|---|---|
| Origanum vulgare | Citric acid | Salmon | The lowest D- and z-value in the combined group | [30] |
| Monarda punctate | Sodium alginate | Carp fillet | Reducing the microbial contamination about 1.5 log CFU/g | [31] |
| Cymbopogon citratus | Tumeric and galangal powder | White hard clam | Improving overall acceptability of Meretrix lyrata during 12 days of chilled storage | [24] |
| Curcuma longa | Sodium chloride | Tengra fish | Showing the acceptable fresh condition at the end of 12 months | [32] |
|
Cinnamomum cassia Presl and Allium sativum |
Tartaric acidLactic acid | Shrimp | Improving microbiological and physicochemical qualities of whole shrimps stored at 4 °C | [29] |
| Zataria multiflora | Liposome | Rainbow trout fillet | Showing the most suitable outcomes in term of antioxidant and microbial properties as well as sensory evaluation | [33] |
| Citrus aurantium | Beta-hydroxytoluene | Carp | Reducing chemical deterioration and lipid oxidation in the fillets | [34] |
| Carvacrol | Caprylic acid | Shrimp | Enhancing the efficacy of the chitosan-carvacrol coating on the quality of shrimp during 10 days of iced storage | [35] |
3. Synergistic Effect of EOs with Edible Coatings and Films for Seafood Preservation
EOs are an original pharmaceutical plant resource with promising antibacterial and antioxidant properties. However, their hydrophobic, volatile, strong sensory, UV-sensitive, etc., natural attributes are major impediments in the use of EOs for food preservation. Methods developed to overcome these shortcomings mainly include nanoemulsions or biofilms. Nanoemulsions with a droplet size between 5 and 200 nm are extensively used for the delivery of hydrophobic EOs, which commonly consist of water, oil, and an emulsifier [36]. Meanwhile, the application of EO-based nanoemulsions either as a washing disinfectant or incorporated into edible coatings has been shown to increase the efficacy when combined with other handling techniques [37,38]. On the other hand, active packaging films or antibacterial packaging films have greatly improved the stability of EOs and broadened the path for their application in food preservation. For example, EOs can be used to improve the properties of potato starch-based films and extend the shelf-life of shrimp [39]. Carvacrol incorporated in flaxseed gum films can significantly reduce the TVB-N content as well as the degree of microbial deterioration and improve the quality of Chinese sea bass during cold storage [40].
Edible coatings and films used as a new technology for food packaging are applied directly by forming an edible thin layer on the surface of the food product, and this system has some excellent properties, such as edibility, biocompatibility, and barrier properties. To make better use of edible coatings and films for fishery products, the jointed use of new natural inhibitors and coating carriers for their synergistic or additive effects has been substantially studied (Table 2). Particularly, edible coatings and films consist of biopolymers, such as polysaccharides, protein, and lipids, which is quite promising for their application in seafood preservation, and they are also suitable packaging materials to integrate EOs for use as biodegradable materials. For example, bombacaceae gum incorporated with cinnamon leaf essential oil exhibited antimicrobial activity and antioxidation of lipids in fresh salmon fillets during refrigerated storage [41]. Pectin coatings enriched with clove essential oil can extend the shelf-life of bream fillets by at least 15 days [42].
Table 2.
Synergistic or additive effect of essential oils combined with active packing films on quality retention of seafood products.
| Plant Sources | Combined with Edible Coatings | Tested Sea Food | Effectiveness | Reference |
|---|---|---|---|---|
| Origanum majorana | Alginate/clay | Trout slices | Significantly delayed the growth of Listeria monocytogenes during the 15-day storage | [48] |
| Artemisia dracunculus | Chitosan/whey protein edible coating | Scomberoides commersonnianus fillet | Promising antimicrobial inhibition activity against TMC and PTC bacteria during storage at refrigerator for 16 days | [45] |
| Syzygium aromaticum | Chitosan films/high-pressure (HPP) | Trout fillets | Significant additive effect on the growth of aerobic mesophilic and coliform counts when stored at 4 °C for 22 days | [49] |
| Syzygium aromaticum | Pectin coating | Bream fillets | Prolonging the shelf life to 15 days during refrigeration | [42] |
| Cinnamomum cassia Presl | Chitosan coating/vacuum packaging(VP) | Eel fillets | >18 days for the E-CH and E-CH-OR fillets stored in VP at 4 °C | [45] |
| Citrus | R(+) limonene/vacuum packaging | Bream | Maintain shelf life bream fillets until 15 days of storage at 2 °C without any significant loss of overall acceptability | [50] |
| Thymus mongolicus | Chitosan/vacuum packaging | Smoked eel fillets | The shelf-life over 49 days in chitosan-thyme treated samples | [47] |
|
Syzygium aromaticum and Cinnamomum cassia Presl |
Starch edible film | Shrimp | Extending the shelf life to 14 and 12 days for storage at 10 and 4 °C, respectively | [51] |
| Artemisia dracunculus | Chitosan-whey protein coatings | Scomberoides commersonnianus fillets | Extending the shelf life of the fillet to 12 days at refrigerated condition | [45] |
| Syzygium aromaticum | Chitosan coating | Colossoma macropomum fillets | Significantly delayed lipid oxidation and inhibited the growth of culturable psychrotrophic bacteria | [46] |
| Ferulago angulata | Chitosan coating | Rainbow trout fillets | The shelf life of rainbow trout fillets was extended to 16 days at 4 °C | [52] |
| Zingiber officinale Rosc . and Cinnamomum cassia Presl | Alginate | Oreochromis niloticus | Showing higher sensory acceptability regarding to odor, color, and weight in treated samples | [53] |
Recently, biopolymer-based biodegradable functional films have been found to have great potential for use in food packaging applications. Chitosan, known as a polysaccharide, is derived from chitin, which is rich in the exoskeleton of crustaceans. Chitosan-based films have excellent characteristics, such as antimicrobial activity, barrier properties, and antioxidant activity. Recent advances in the applications of EO-chitosan-based films have been largely reported in the field of seafood preservation [43]. For example, chitosan, in combination with oregano, can significantly reduce the counts of mesophilic bacteria, Pseudomonas, Shewanella, and yeast in eel fillets stored in vacuum packaging at 4 °C for 18 days [44]. It is reported that a chitosan-whey protein composite edible coating containing tarragon Artemisia dracunculus essential oil can improve the deterioration of Scomberoides commersonnianus fillets in refrigerated conditions (4 ± 1 °C) and control the growth of mesophilic bacteria and psychrotrophic bacteria [45]. In addition, the combination of chitosan and clove EOs can inhibit the chemical and microbial deterioration of frozen tambaqui fillets for 120 days [46]. It has also been reported that chitosan, in combination with thyme oil, has the highest bioactivity for inhibiting the growth of mesophilic bacteria and extending the shelf-life of smoked eel fillets over 49 days under VP conditions and refrigeration (4 °C) [47].
In addition to chitosan, other polysaccharide, protein, and lipid-based coatings have also shown promising results. Marjoram essential oil (MEO) was incorporated into alginate/clay nanocomposite films, which were applied to control L. monocytogenes on trout fillets in refrigerated storage (4 °C) for 15 days, and the results showed that the nanocomposite film containing MEO more efficiently inhibited the growth of L. monocytogenes during the 15 day storage period, with final counts reaching 6.23 log CFU/g, while the counts in control samples were significantly higher, reaching 7.38 log CFU/g [48]. The application of pectin coatings with clove essential oil significantly improved the weight loss, water evaporation, and textural and color deterioration of the bream samples compared to the untreated sample, and the composite film showed synergistic action on bacterial growth, especially for gram-negative bacteria during refrigeration over a period of 15 days [42].
Most biopolymers used in edible coatings and films are edible biopolymers or food-grade materials. It can be considered to be eco-friendly and a food safety measure to preserve fishery products, which has the great benefit of providing “green labeling” for the final fishery product to meet the consumer requirements of a system free of chemical-synthesized preservatives.
4. Synergistic Effect of Essential Oils with Physical Methods for Seafood Preservation
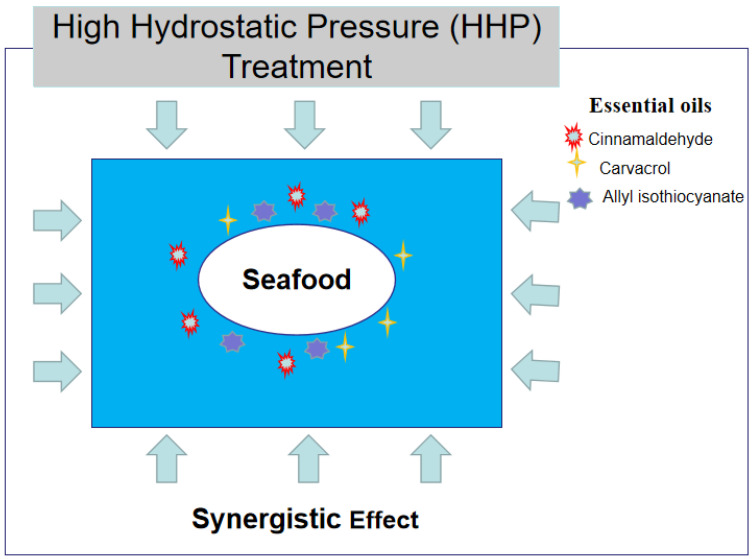
Consumers have raised the requirements for seafood not only with regards to the quality and nutrients, but also with regards to food security, which urges the development of green processing techniques for seafood. It is, therefore, necessary to control seafood spoilage using innoxious and no residue methods. Physical disinfection technologies have been developed during the 20th century, and by their mechanism of action, they can be divided into direct or indirect effects on the inactivation of pathogenic microorganisms. Treatments such as heating, ultraviolet (UV) light, or some thermal or nonthermal processes can directly kill or inhibit the growth of spoilage microorganisms, and these methods also show synergistic antimicrobial effectiveness against food pathogens in the combined treatments with EOs. For example, citrus lemon (CLEO) and C. reticulata oils (CREO) combined with heat treatment affected almost the entire cell population (up to 99%) of Levilactobacillus brevis and Leuconostoc mesenteroides, while with exposure to CLEO and CREO alone, the cell populations with physiological damage were in a range of 19.6–66.8% and 23.8–75.9%, respectively [54]. Synergistic antimicrobial effects generated by UV light and EOs were also reported, and it has been demonstrated that UV-C light enhanced the antibacterial activity of clove oil against S. Typhimurium by inactivating the biofilms on stainless steel [55]. High hydrostatic processing (HPP) is one of the extensively explored nonthermal food processing technologies, which has great potential to preserve the freshness of food without affecting the food quality characteristics, such as the color, natural flavor, and nutrients, in addition to directly killing fish-spoiling bacteria, such as L. monocytogenes, E. coli, and V. parahaemolyticus. Furthermore, the synergistic effect of HPP with EOs has also been demonstrated. Kung et al. (2020) reported that HHP in combination with 0.2% lemon essential oil was more effective under the same pressure to inactivate Morganella morganii, a histamine-forming bacteria [56]. The combined treatment of HPP and carvacrol has also shown synergistic inactivation effects on Salmonella and L. monocytogenes, while only HPP-induced bacterial injury can be recovered [57]. In another interesting study, chitosan films with 20 g kg−1 clove essential oil were combined with HPP processing as preservative treatments for trout fillets and showed a significant additive effect on the inhibition of growth of aerobic mesophilic and coliform counts when stored at 4 °C for 22 days [49].
Another technique has the indirect function of a physical machine to regulate the environment inside the pack in order to maintain seafood quality, such as modified atmosphere packaging (MAP) and vacuum packaging (VP). Both can be considered as packaging techniques, but can be accomplished by coupling with a physical machine. MAP and VP create different gaseous atmospheres surrounding a food product inside a pack. MAP provides alterations of atmospheric gas concentrations in the pack involving the three principal gases, including carbon dioxide, nitrogen, and oxygen, while in VP, the air is completely removed. Both are usually used for seafood preservation in the presence of EOs. Oregano EOs in the vapor phase can enhance the effectiveness under MAP conditions (60:40 CO2/N2) to extend the shelf-life of fish fillets to at least 28 days (4 °C), while the quality declined on day 7 in untreated fillets [58]. In addition, corn zein-based edible coatings incorporating nisin and lemongrass essential oil (8%) have been suggested to control the spoilage bacteria L. monocytogenes on cold-smoked sunshine bass and extend the shelf-life to 14 days and 42 days with polyvinyl chlorine (PVC) and VP treatments, respectively, at 4 °C [59]. The addition, R(+) limonene could extend the shelf-life of gilt-head sea bream fillets packaged in vacuum conditions until 15 days of storage without any significant loss of texture, odor, and color [50]. It has been reported that chitosan in combination with thyme oil has the highest bioactivity to inhibit the growth of mesophilic bacteria and extend the shelf-life of smoked eel fillets over 49 days under VP conditions and refrigeration (4 °C) [47]. Chitosan films mixed with pink pepper residue extract and combined with modified atmosphere packaging (100% CO2) were highly effective in maintaining the quality properties of skinless salmon fillets during refrigerated storage for 28 days [60]. As summarized in Table 3 and Figure 2, MAP, VP, or HHP combined with EOs could be regarded as effective and eco-friendly techniques to preserve the quality and safety of fishery products.
Table 3.
Synergistic or additive effect of essential oils combined with physical methods on quality retention of seafood products.
| Plant Sources | Combined Physical Method | Tested Fish | Effectiveness | Reference |
|---|---|---|---|---|
| Citrus | High hydrostatic pressure | Tuna meat slurry | Lower the D values in the combined treatments for inactivation of Morganella morganii | [51] |
| Cinnamomum cassia Presl | Pilot-plant packaging (high vacuum) | Bream fresh fillets | The lowest growth of Enterobacteria/lactic acid bacteria after 28 days at 4 °C | [58] |
| Citrus or Lavandula angustifolia Mill | Vacuum packing | Anchovy | Reducing the formation of biogenic amine and lowering the risk of histamine in fish | [61] |
| Citrus | Vacuum packing | Bream fillets | Resulting in a shelf-life extension of 6–9 days for treated fish | [50] |
| Thymus mongolicus Ronn | Vacuum packing | Eel fillets | Longer shelf-life to 42 days compared to the control samples of 35 days | [47] |
| Syzygium aromaticum | Vacuum packing | Catfish fillets | More effective in protecting the textural quality and retarding lipid oxidation | [62] |
| Cinnamomum cassia Presl | Vacuum packing | Carp | Decreasing the relative abundance of TVB-N, biogenic amines, and Macrococcus spp. in treated samples | [63] |
Figure 2.
Combined treatment of essential oils and high hydrostatic pressure technique have synergistic inactivation effects on pathogenic and spoilage organisms in seafood.
5. Perspectives
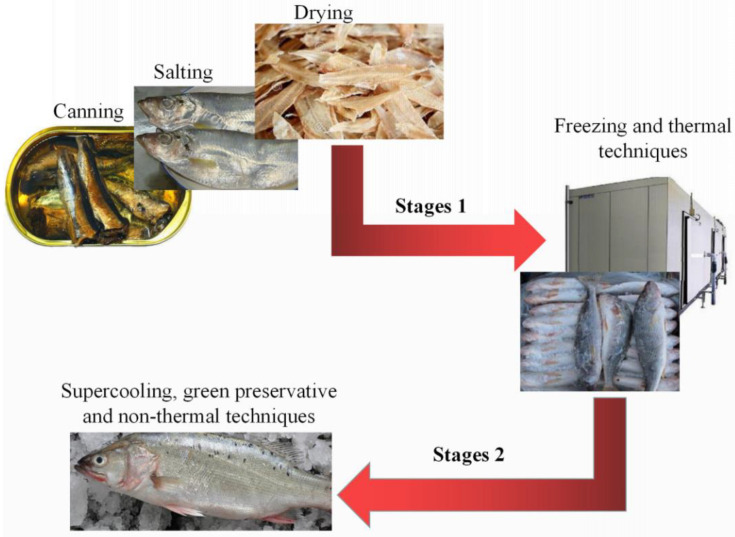
The traditional preservation methods, such as salting, drying, smoking, fermentation, and canning, have been replaced by the modern methods, such as supercooling, freezing, chemical preservation using food-grade compounds, edible films and coatings, HHP, and other nonthermal processes, which are more efficient and more environmentally friendly compared to the historical handling techniques (Figure 3). Moreover, EOs mainly consist of volatile components obtained from special plant materials, which can be added to various preservative approaches to improve the antimicrobial and antioxidant properties. The comprehensive use of these modern preservation techniques can significantly extend the shelf-life of fish and fulfil the consumer’s requirements regarding its texture, appearance, and taste.
Figure 3.
The seafood preservation technology has been developed from the first stage to the second stage, and emerging novel preservation techniques are more effective to extend the shelf-life of fresh seafood products and minimize their nutritional losses.
Fish and other seafood are highly perishable due to the combined action of spoilage caused by bacterial and chemical degradation, as well as enzymatic and mechanical damage. Using edible films/coatings containing EOs combined with cooling and freezing, or further linking them with vacuum packaging seems to be the most effective synergistic method to maintain the quality of seafood and prevent the microbial activity. Future developments in seafood preservation techniques should consider the fish spoilage mechanisms, synergistic antimicrobial mechanisms, optimization of the environmental conditions, minimization of temperature fluctuations, the initial microbial type and load, and their interaction effects in order to optimize the shelf-life of fishery products.
Author Contributions
C.-H.M., designed and supervised the study; X.H., Y.L., H.Z., and N.X., reviewed the references; X.H. and L.G., wrote the manuscript; X.H. and C.-H.M. revised the manuscript; Y.L., Y.P., and Y.C., contributed to the tables and figures; X.H. and C.-H.M., acquired the funding. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) (42030713), the Program of the Guangdong Science and Technology Department (2016B020242005), Provincial Science and Technology Innovation Strategy Special Fund (“Big Special Project + Task List Management Model”) of Shanwei City, Guangdong Province (2018D6001,2018D4002), and the Young Talents Innovation Projects in Ordinary Universities in Guangdong Province (2019GKQNCX117).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baptista R.C., Horita C.N., Sant’Ana A.S. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: A review. Food Res. Int. 2020;127:108762. doi: 10.1016/j.foodres.2019.108762. [DOI] [PubMed] [Google Scholar]
- 2.Silva F., Domingues F.C. Antimicrobial activity of coriander oil and its effectiveness as food preservative. Crit. Rev. Food Sci. Nutr. 2017;57:35–47. doi: 10.1080/10408398.2013.847818. [DOI] [PubMed] [Google Scholar]
- 3.Dehghani S., Hosseini S.V., Regenstein J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018;240:505–513. doi: 10.1016/j.foodchem.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 4.Zhang N., Yao L. Anxiolytic effect of essential oils and their constituents: A review. J. Agric. Food Chem. 2019;67:13790–13808. doi: 10.1021/acs.jafc.9b00433. [DOI] [PubMed] [Google Scholar]
- 5.Dhifi W., Bellili S., Jazi S., Bahloul N., Mnif W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines. 2016;3:25. doi: 10.3390/medicines3040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhakad A.K., Pandey V.V., Beg S., Rawat J.M., Singh A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018;98:833–848. doi: 10.1002/jsfa.8600. [DOI] [PubMed] [Google Scholar]
- 7.Dosoky N.S., Setzer W.N. Chemical composition and biological activities of essential oils of curcuma species. Nutrients. 2018;10:1196. doi: 10.3390/nu10091196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tariq S., Wani S., Rasool W., Shafi K., Bhat M.A., Prabhakar A., Shalla A.H., Rather M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019;134:103580. doi: 10.1016/j.micpath.2019.103580. [DOI] [PubMed] [Google Scholar]
- 9.Gong L., Zhao Z.Y., Yin C.X., Gupta V.K., Zhang X.H., Jiang Y.M. Synergistic interaction of natamycin with carboxymethyl chitosan for controlling Alternata alternara, a cause of black spot rot in postharvest jujube fruit. Postharvest Biol. Tec. 2019;156:110919. [Google Scholar]
- 10.Chintagari S., Hazard N., Edwards G., Jadeja R., Janes M. Risks associated with fish and seafood. Microbiol. Spectr. 2017;5:123–142. doi: 10.1128/microbiolspec.pfs-0013-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odeyemi O.A., Burke C.M., Bolch C.C.J., Stanley R. Seafood spoilage microbiota and associated volatile organic compounds at different storage temperatures and packaging conditions. Int. J. Food Microbiol. 2018;280:87–99. doi: 10.1016/j.ijfoodmicro.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 12.European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2018;16:e05500. doi: 10.2903/j.efsa.2016.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M.A., Roy S.L. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gyawali P., Fletcher G.C., McCoubrey D.J., Hewitt J. Norovirus in shellfish: An overview of post-harvest treatments and their challenges. Food Control. 2019;99:171–179. doi: 10.1016/j.foodcont.2018.12.049. [DOI] [Google Scholar]
- 15.Basavegowda N., Patra J.K., Baek K.H. Essential oils and mono/bi/tri-metallic nanocomposites as alternative sources of antimicrobial agents to combat multidrug-resistant pathogenic microorganisms: An overview. Molecules. 2020;25:1058. doi: 10.3390/molecules25051058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arezoo E., Mohammadreza E., Maryam M., Abdorreza M.N. The synergistic effects of cinnamon essential oil and nano TiO(2) on antimicrobial and functional properties of sago starch films. Int. J. Biol. Macromol. 2020;157:743–751. doi: 10.1016/j.ijbiomac.2019.11.244. [DOI] [PubMed] [Google Scholar]
- 17.Heghes S.C., Vostinaru O., Rus L.M., Mogosan C., Iuga C.A., Filip L. Antispasmodic effect of essential oils and their constituents: A review. Molecules. 2019;24:1675. doi: 10.3390/molecules24091675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dosoky N.S., Setzer W.N. Biological activities and safety of citrus spp. essential oils. Int. J. Mol. Sci. 2018;19:1966. doi: 10.3390/ijms19071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beshiru A., Igbinosa I.H., Igbinosa E.O. Biofilm formation and potential virulence factors of Salmonella strains isolated from ready-to-eat shrimps. PLoS ONE. 2018;13:e0204345. doi: 10.1371/journal.pone.0204345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obaidat M.M., Bani Salman A.E. Antimicrobial resistance percentages of salmonella and shigella in seafood imported to jordan: Higher percentages and more diverse profiles in shigella. J. Food Prot. 2017;80:414–419. doi: 10.4315/0362-028X.JFP-16-322. [DOI] [PubMed] [Google Scholar]
- 21.Porter J.A., Monu E.A. Evaluating the antimicrobial efficacy of white mustard essential oil alone and in combination with thymol and carvacrol against salmonella. J. Food Prot. 2019;82:2038–2043. doi: 10.4315/0362-028X.JFP-19-029. [DOI] [PubMed] [Google Scholar]
- 22.Jebelli Javan A., Salimiraad S., Khorshidpour B. Combined effect of Trachyspermum ammi essential oil and propolis ethanolic extract on some foodborne pathogenic bacteria. Vet. Res. Forum. 2019;10:235–240. doi: 10.30466/vrf.2019.72986.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reyes-Jurado F., López-Malo A., Palou E. Antimicrobial activity of individual and combined essential oils against foodborne pathogenic bacteria. J. Food Prot. 2016;79:309–315. doi: 10.4315/0362-028X.JFP-15-392. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen M.P. Synergistic effect of turmeric (Curcuma longa), galanga (Alpinia galanga) powder and lemongrass (Cymbopogon citratus) essential oil as natural preservative in chilled storage of white hard clam (Meretrix lyrata) Orient. J. Chem. 2020;36:195–200. [Google Scholar]
- 25.De Jesus G.S., Micheletti A.C., Padilha R.G., de Souza de Paula J., Alves F.M., Leal C.R.B., Garcez F.R., Garcez W.S., Yoshida N.C. Antimicrobial potential of essential oils from cerrado plants against multidrug-resistant foodborne microorganisms. Molecules. 2020;25:3296. doi: 10.3390/molecules25143296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajkowska K., Nowicka-Krawczyk P., Kunicka-Styczyńska A. Effect of clove and thyme essential oils on candida biofilm formation and the oil distribution in yeast cells. Molecules. 2019;24:1954. doi: 10.3390/molecules24101954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwiatkowski P., Mnichowska-Polanowska M., Pruss A., Masiuk H., Dzięcioł M., Giedrys-Kalemba S., Sienkiewicz M. The effect of fennel essential oil in combination with antibiotics on Staphylococcus aureus strains isolated from carriers. Burns. 2017;43:1544–1551. doi: 10.1016/j.burns.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Pereira N.L.F., Aquino P.E.A., Júnior J.G.A.S., Cristo J.S., Vieira Filho M.A., Moura F.F., Ferreira N.M.N., Silva M.K.N., Nascimento E.M., Correia F.M.A., et al. Antibacterial activity and antibiotic modulating potential of the essential oil obtained from Eugenia jambolana in association with led lights. J. Photochem. Photobiol. B. 2017;174:144–149. doi: 10.1016/j.jphotobiol.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 29.Noordin W.N.M., Shunmugam N., Huda N., Adzitey F. The effects of essential oils and organic acids on microbiological and physicochemical properties of whole shrimps at refrigerated storage. Curr. Res. Nutr. Food Sci. 2018;6:273. doi: 10.12944/CRNFSJ.6.2.03. [DOI] [Google Scholar]
- 30.Dogruyol H., Mol S., Cosansu S. Increased thermal sensitivity of Listeria monocytogenes in sous-vide salmon by oregano essential oil and citric acid. Food Microbiol. 2020;90:103496. doi: 10.1016/j.fm.2020.103496. [DOI] [PubMed] [Google Scholar]
- 31.Heydari R., Bavandi S., Javadian S.R. Effect of sodium alginate coating enriched with horsemint (Mentha longifolia) essential oil on the quality of bighead carp fillets during storage at 4 °C. Food Sci. Nutr. 2015;3:188–194. doi: 10.1002/fsn3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farid F.B., Latifa G.A., Chakraborty S.C., Nahid M.N., Begum M. Protective effect of salt (sodium chloride) and turmeric (Curcuma longa) on physicochemical attributes of sun-dried Tengra fish (Mystus tengra; Hamilton-Buchanan, 1822) at Laboratory condition. Int. J. Biol. Sci. 2015;4:33–40. [Google Scholar]
- 33.Bahramian G., Golestan L., Khosravi-Darani K. Antimicrobial and antioxidant effect of nanoliposomes containing Zataria multiflora boiss essential oil on the rainbow trout fillets during refrigeration. Biointerface Res. Appl. Chem. 2018;8:3505–3513. [Google Scholar]
- 34.Hasani O., Javadian S.R. Effect of encapsulated bitter orange peel extract and BHT on the quality of common carp fillet during refrigerated storage. Int. J. Food Eng. 2016;12:303–310. doi: 10.1515/ijfe-2015-0185. [DOI] [Google Scholar]
- 35.Wang Q.Y., Lei J., Ma J.J., Yuan G.F., Sun H.Y. Effect of chitosan-carvacrol coating on the quality of Pacific white shrimp during iced storage as affected by caprylic acid. Int. J. Biol. Macromol. 2018;106:123–129. doi: 10.1016/j.ijbiomac.2017.07.180. [DOI] [PubMed] [Google Scholar]
- 36.Prakash A., Baskaran R., Paramasivam N., Vadivel V. Essential oil based nanoemulsions to improve the microbial quality of minimally processed fruits and vegetables: A review. Food Res. Int. 2018;111:509–523. doi: 10.1016/j.foodres.2018.05.066. [DOI] [PubMed] [Google Scholar]
- 37.Liu Q., Huang H., Chen H., Lin J., Wang Q. Food-grade nanoemulsions: Preparation, stability and application in encapsulation of bioactive compounds. Molecules. 2019;24:4242. doi: 10.3390/molecules24234242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pavoni L., Perinelli D.R., Bonacucina G., Cespi M., Palmieri G.F. An overview of micro- and nanoemulsions as vehicles for essential oils: Formulation, preparation and stability. Nanomaterials. 2020;10:135. doi: 10.3390/nano10010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alotaibi S., Tahergorabi R. Development of a sweet potato starch-based coating and its effect on quality attributes of shrimp during refrigerated storage. LWT-Food Sci. Technol. 2018;88:203–209. doi: 10.1016/j.lwt.2017.10.022. [DOI] [Google Scholar]
- 40.Fang S.Y., Zhou Q.Q., Hu Y., Liu F., Mei J., Xie J. Antimicrobial carvacrol incorporated in flaxseed gum-sodium alginate active films to improve the quality attributes of chinese sea bass (Lateolabrax maculatus) during cold storage. Molecules. 2019;24:3292. doi: 10.3390/molecules24183292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao T.L., Song K.B. Development of bioactive Bombacaceae gum films containing cinnamon leaf essential oil and their application in packaging of fresh salmon fillets. LWT-Food Sci. Technol. 2020;131:109647. doi: 10.1016/j.lwt.2020.109647. [DOI] [Google Scholar]
- 42.Nisar T., Yang X., Alim A., Iqbal M., Wang Z.C., Guo Y. Physicochemical responses and microbiological changes of bream (Megalobrama ambycephala) to pectin based coatings enriched with clove essential oil during refrigeration. Int. J. Biol. Macromol. 2019;124:1156–1166. doi: 10.1016/j.ijbiomac.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Mujtaba M., Morsi R.E., Kerch G., Elsabee M.Z., Kaya M., Labidi J., Khawar K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019;121:889–904. doi: 10.1016/j.ijbiomac.2018.10.109. [DOI] [PubMed] [Google Scholar]
- 44.Lambrianidi L., Savvaidis I.N., Tsiraki M.I., El-Obeid T. Chitosan and oregano oil treatments, individually or in combination, used to increase the shelf life of vacuum-packaged, refrigerated European eel (Anguilla anguilla) fillets. J. Food Prot. 2019;82:1369–1376. doi: 10.4315/0362-028X.JFP-19-050. [DOI] [PubMed] [Google Scholar]
- 45.Farsanipour A., Khodanazary A., Hosseini S.M. Effect of chitosan-whey protein isolated coatings incorporated with tarragon Artemisia dracunculus essential oil on the quality of Scomberoides commersonnianus fillets at refrigerated conditionInt. J. Biol. Macromol. 2020;155:766–771. doi: 10.1016/j.ijbiomac.2020.03.228. [DOI] [PubMed] [Google Scholar]
- 46.Vieira B.B., Mafra J.F., Bispo A.S.D., Ferreira M.A., Silva F.D., Rodrigues A.V.N., Evangelista-Barreto N.S. Combination of chitosan coating and clove essential oil reduces lipid oxidation and microbial growth in frozen stored tambaqui (Colossoma macropomum) fillets. LWT-Food Sci. Technol. 2019;116:108546. doi: 10.1016/j.lwt.2019.108546. [DOI] [Google Scholar]
- 47.El-Obeid T., Yehia H.M., Sakkas H., Lambrianidi L., Tsiraki M.I., Savvaidis I.N. Shelf-life of smoked eel fillets treated with chitosan or thyme oil. Int. J. Biol. Macromol. 2018;114:578–583. doi: 10.1016/j.ijbiomac.2018.03.125. [DOI] [PubMed] [Google Scholar]
- 48.Alboofetileh M., Rezaei M., Hosseini H., Abdollahi M. Efficacy of activated alginate-based nanocomposite films to control Listeria monocytogenes and spoilage flora in rainbow trout slice. J. Food Sci. Technol. 2016;53:521–530. doi: 10.1007/s13197-015-2015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Albertos I., Rico D., Diez A.M., González-Arnáiz L., García-Casas M.J., Jaime I. Effect of edible chitosan/clove oil films and high-pressure processing on the microbiological shelf life of trout fillets. J. Sci. Food Agric. 2015;95:2858–2865. doi: 10.1002/jsfa.7026. [DOI] [PubMed] [Google Scholar]
- 50.Giarratana F., Muscolino D., Beninati C., Ziino G., Giuffrida A., Panebianco A. Activity of R(+) limonene on the maximum growth rate of fish spoilage organisms and related effects on shelf-life prolongation of fresh gilthead sea bream fillets. Int. J. Food Microbiol. 2016;237:109–113. doi: 10.1016/j.ijfoodmicro.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 51.Meenatchisundaram S., Chandrasekar C.M., Udayasoorian L.P., Kavindapadi Rajasekaran R., Kesavan R.K., Srinivasan B., Muthusamy S. Effect of spice-incorporated starch edible film wrapping on shelf life of white shrimps stored at different temperatures. J. Sci. Food Agric. 2016;96:4268–4275. doi: 10.1002/jsfa.7638. [DOI] [PubMed] [Google Scholar]
- 52.Shokri S., Parastouei K., Taghdir M., Abbaszadeh S. Application an edible active coating based on chitosan- Ferulago angulata essential oil nanoemulsion to shelf life extension of Rainbow trout fillets stored at 4 degrees C. Int. J. Biol. Macromol. 2020;153:846–854. doi: 10.1016/j.ijbiomac.2020.03.080. [DOI] [PubMed] [Google Scholar]
- 53.Vital A.C.P., Guerrero A., Ornaghi M.G., Kempinski E.M.B.C., Sary C., de Oliveira Monteschio J., Matumoto-Pintro P.T., Ribeiro R.P., do Prado I.V. Quality and sensory acceptability of fish fillet (Oreochromis niloticus) with alginate-based coating containing essential oils. J. Food Sci. Technol. 2018;55:4945–4955. doi: 10.1007/s13197-018-3429-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Souza Pedrosa G.T., de Souza E.L., de Melo A.N.F., da Cruz Almeida E.T., de Sousa Guedes J.P., de Carvalho R.J., Pagán R., Magnani M. Physiological alterations involved in inactivation of autochthonous spoilage bacteria in orange juice caused by Citrus essential oils and mild heat. Int. J. Food Microbiol. 2020;334:108837. doi: 10.1016/j.ijfoodmicro.2020.108837. [DOI] [PubMed] [Google Scholar]
- 55.Silva-Espinoza B.A., Palomares-Navarro J.J., Tapia-Rodriguez M.R., Cruz-Valenzuela M.R., Gonzalez-Aguilar G.A., Silva-Campa E., Pedroza-Montero M., Almeida-Lopes M., Miranda R., Ayala-Zavala J.F. Combination of ultraviolet light-C and clove essential oil to inactivate Salmonella Typhimurium biofilms on stainless steel. J. Food Saf. 2020;40:e12788. doi: 10.1111/jfs.12788. [DOI] [Google Scholar]
- 56.Kung H.F., Lee Y., Hwang C.C., Wu Y.C., Hsieh C.Y., Tsai Y.H. Inactivation of Morganella morganii by high hydrostatic pressure combined with lemon essential oil. Food Sci. Nutr. 2020;8:3435–3441. doi: 10.1002/fsn3.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chuang S.Y., Sheen S., Sommers C.H., Zhou S.Y., Sheen L.Y. Survival Evaluation of Salmonella and Listeria monocytogenes on selective and nonselective media in ground chicken meat subjected to high hydrostatic pressure and carvacrol. J. Food Protect. 2020;83:37–44. doi: 10.4315/0362-028X.JFP-19-075. [DOI] [PubMed] [Google Scholar]
- 58.Navarro-Segura L., Ros-Chumillas M., Martinez-Hernandez G.B., Lopez-Gomez A. A new advanced packaging system for extending the shelf life of refrigerated farmed fish fillets. J. Sci. Food Agric. 2020;100:4601–4611. doi: 10.1002/jsfa.10520. [DOI] [PubMed] [Google Scholar]
- 59.Hager J.V., Rawles S.D., Xiong Y.L.L., Newman M.C., Thompson K.R., Webster C.D. Listeria monocytogenes is inhibited on fillets of cold-smoked sunshine bass, Morone chrysops x Morone saxatilis, with an edible corn zein-based coating incorporated with lemongrass essential oil or nisin. J. World Aquacult. Soc. 2019;50:575–592. doi: 10.1111/jwas.12573. [DOI] [Google Scholar]
- 60.Merlo T.C., Contreras-Castillo C.J., Saldaña E., Barancelli G.V., Dargelio M.D.B., Yoshida C.M.P., Ribeiro Junior E.E., Massarioli A., Venturini A.C. Incorporation of pink pepper residue extract into chitosan film combined with a modified atmosphere packaging: Effects on the shelf life of salmon fillets. Food Res. Int. 2019;125:108633. doi: 10.1016/j.foodres.2019.108633. [DOI] [PubMed] [Google Scholar]
- 61.Ozogul F., Oztekin R., Kulawik P. Biogenic amine formation and microbiological quality of anchovy (Engraulis encrasicolus) treated with lavender and lemon balm ethanol extracts. J. Food Sci. 2017;82:1278–1284. doi: 10.1111/1750-3841.13704. [DOI] [PubMed] [Google Scholar]
- 62.Binsi P.K., Ninan G., Ravishankar C.N. Effect of curry leaf and clove bud essential oils on textural and oxidative stability of chill stored sutchi catfish fillets. J. Texture Stud. 2017;48:258–266. doi: 10.1111/jtxs.12237. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y.M., Li D.P., Lv J., Li Q.Z., Kong C.L., Luo Y.K. Effect of cinnamon essential oil on bacterial diversity and shelf-life in vacuum-packaged common carp (Cyprinus carpio) during refrigerated storage. Int. J. Food Microbiol. 2016;249:1–8. doi: 10.1016/j.ijfoodmicro.2016.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.