Abstract
Simple Summary
We identified the sequence encoding galectin-9 from Pagrus major and subsequently investigated the molecular characteristics and changes in gene expression patterns in response to artificial infection with major pathogens. Overall, our data suggest that galectin-9 plays a pivotal role in the immune system of P. major. The findings of this study can potentially serve as a reference for understanding the function of galectin-9 in the P. major immune system; moreover, galectin-9 has been identified as a potential candidate for use as a disease-related molecular marker.
Abstract
Galectin (Gal) is a member of a family of β-galactoside-binding lectin. The members of this family play important roles in the recognition of carbohydrate ligands and in various other biological processes. In this study, we identified the gene encoding Gal-9 in Pagrus major (PmGal-9) and analyzed its expression in various tissues after pathogen challenge. Alignment analysis revealed that the two galactose-binding lectin domains of the deduced protein were highly conserved among all the teleosts. Phylogenetic analysis revealed that PmGal-9 is most closely related to the Gal-9 gene of gilthead sea bream. PmGal-9 was ubiquitously expressed in all tissues analyzed but was predominantly expressed in the spleen, head kidney, and intestine. After challenges with major microbial pathogens (Edwardsiella piscicida, Streptococcus iniae, or red sea bream iridovirus) of red sea bream, PmGal-9 mRNA expression was significantly regulated in most immune-related tissues. These results suggested that PmGal-9 not only plays an important role in the immune system of red sea bream but is also a possible inflammatory marker for pathogenic diseases.
Keywords: aquaculture, gene profiling, Streptococcus iniae, Edwardsiella piscicida, iridovirus
1. Introduction
Galectins (Gals) are a family of β-galactoside-binding lectin. They were formerly known as S-type or S-lac lectins [1,2]. Gals play significant roles during innate immune responses by mediating the recognition of pathogens by host cells and are involved in the elimination of pathogens; they interact with carbohydrates on the surface of invading microorganisms and function as pattern recognition receptors (PRRs) in both vertebrates and invertebrates [3]. In addition, they are involved in many homeostasis and physiological processes such as apoptosis, inflammation, immune responses, cell migration, proliferation, adhesion, autophagy, and signal transduction [4,5,6]. Gals can be classified into three main types: the proto type (Gal-1, -2, -5, -7, -10, -11, -13, -14, -15, and -16), the tandem-repeat type (Gal-4, -6, -8, -9, and -12), and the chimera type (Gal-3), but they all have a highly conserved structure [7].
Gal-9 was first isolated from mouse embryonic kidneys in 1997, demonstrating the family’s characteristically conserved sequence motifs and specific binding activity [8]. Gal-9 is a ligand for the T cell immunoglobulin domain and mucin domain protein 3 and is involved in autoimmune- and allograft-related apoptosis actions [9,10]. In addition, previous studies confirmed the function of Gal-9 in human plasmacytoid dendritic cells and B cells as well as its role in regulating TLR7/TLR9 signaling and its function as an eosinophil chemotactic factor [11]. Interferon-gamma-induced production of Gal-9 has been shown to play an important role in the immune response by regulating the interaction between vascular walls and eosinophils, and researchers have reported the importance of Gal-9 as an inflammatory mediator in acute dengue virus infection [12,13]. Gal-9 induced the expression of apolipoprotein B editing complex 3 protein and mutated human immunodeficiency virus (HIV), weakening its infectivity and triggering the expression of various other anti-HIV factors [14].
Recently, there has been much interest in the correlation between Gal-9 and tumors in humans, and many studies have reported its role as a prognostic factor [12,15,16]. In teleosts, Gal-9 has been subjected to gene expression profiling and evaluations of its immunological function in a variety of species, but this research is still limited and has not been previously reported in red sea bream [17,18,19,20,21,22].
Red sea bream (Pagrus major) has been an important aquaculture species for many years in Korea and Japan. Although its production has increased due to the development of aquaculture technology, the disease is still a problem, especially those caused by dangerous pathogens that can lead to mass mortality, such as edwardsiellosis, streptococcosis, and red sea bream iridovirus (RSIV) disease [23]. Therefore, in order to solve problems related to pathogenic diseases, an understanding of the immune system and related research is necessary.
In this study, we obtained the cDNA sequence of Gal-9 from P. major (PmGal-9) and identified its molecular biological characteristics using its deduced amino acid sequence. In addition, the expression pattern of PmGal-9 mRNA was confirmed in the healthy state and after artificial infection by each pathogen.
2. Materials and Methods
2.1. Identification and Characterization of the Galectin-9 Gene
The coding sequence (CDS) of PmGal-9 was obtained by next-generation sequencing (NGS) analysis in our previous study [24]. Suitability of the designed polymerase chain reaction (PCR) primers (forward: ATGGCTTTTAATCAGCAGTC, reverse: CTACACCACCACAGATGTCA) was confirmed using the primer3 primer-design program of the GENETYX software version 8.0 (GENETYX Corporation, Tokyo, Japan). Amplification of PmGal-9 proceeded using ExPrime™ Tag Premix (GeNet Bio, Daejeon, Korea) according to the manufacturer’s instructions. The PCR product was then cloned into a pGEM-T Easy Vector (Promega, Madison, WI, USA), which was transformed into Escherichia coli (E. coli) JM109 competent cells. Plasmids were extracted using an Exprep Plasmid SV mini kit (GeneAll, Seoul, Korea). We confirmed the integrity of the sequence additional Sanger sequencing and predicted the amino acid sequence by using the GENETYX software version 8.0. Gal-9 related amino acid sequences were retrieved from the National Biotechnology Information Center (http://www.ncbi.nlm.nih.gov/blast) database and used for multiple alignment analysis and phylogenetic analysis. Multiple alignment analysis of PmGal-9 with deduced amino acid sequences of other species was performed with the ClustalX 2.1 program, and the characteristic domains and sequences of Gal-9 were identified with the Expert Protein Analysis System PROSITE Scan tool (http://prosite.expasy.org) and simple modular architecture research tool (http://smart.embl-heidelberg.de/). For phylogenetic analysis, a tree was created using the MEGA 6.0 program (http://www.megasoftware.net) with a neighbor-joining algorithm. Support for each node was derived from 2000 resamplings.
2.2. Experimental Fish and Microorganisms
The red sea breams (weight: 173.2 ± 31.1 g, full length: 22.4 ± 0.9 cm) were provided by the Gyeongsangnam-do Fisheries Resources Research Institute and acclimated for two weeks in the laboratory. A total of 63 fish (21 individuals per group) were used to measure PmGal-9 mRNA expression after pathogen challenge. The fish were randomly sampled prior to use in the experiment to identify major pathogen infections. All animal experiments were performed according to the guidelines of the Animal Protection and Use Committee of Gyeongsang National University (Approval Number: 2020-0002).
Bacteria and virus strain used in the experiments to analyze transcript expression levels after pathogen infection were obtained from the Fish Pathology Division of the National Institute of Fisheries Science (NIFS, Korea). The strains used were Streptococcus iniae (S. iniae) FP5228, Edwardsiella piscicida (E. piscicida) FSW910410, and RSIV.
2.3. Tissue Collection and Processing
For the analysis of PmGal-9 mRNA transcript levels under healthy conditions, tissues from three independent fish were sampled after euthanasia via benzocaine (Sigma-Aldrich, St. Louis, MI, USA) and stored at −80 °C until total RNA extraction. Total RNA was extracted from the prepared tissues (brain, gills, head kidney, heart, intestine, liver, muscle, skin, spleen, stomach, and trunk kidney) according to the manufacturer’s instructions with RNAiso Plus reagent (Takara, Tokyo, Japan), and genomic DNA was removed. The extracted total RNA was measured for concentration and purity using a NanoVue spectrophotometer (GE Healthcare, London, UK) and synthesized into cDNA using a PrimeScript™ 1st strand cDNA Synthesis Kit (Takara, Tokyo, Japan) according to the manufacturer’s instructions. The PCR products were detected by electrophoresis on ethidium-bromide-stained agarose gels and visualized under ultraviolet light.
To measure the expression changes in PmGal-9 mRNA after artificial infection by major pathogens in the red sea bream aquaculture industry, the experimental fish were divided into three groups, and S. iniae (1 × 105 CFU/fish), E. piscicida (1 × 105 CFU/fish) or RSIV (1 × 106 copies/fish) were injected into their abdominal cavity. The control group was intraperitoneally injected with the same volume of phosphate-buffered saline (PBS) buffer. Then, the major tissues were aseptically extracted at various sampling times (1, 12, 24, 72, 120, and 168 h), and total RNA was extracted and synthesized into cDNA as in the method described above.
2.4. Quantitative Real-Time PCR
The expression level of the PmGal-9 transcript was measured by quantitative real-time PCR using the prepared cDNA samples and specific primer sets (forward: CAGGCAGAATGCAGACATTG and reverse: GCTGAAGGTAGAGCCAGCAG). The SYBR green method was used, and real-time PCR was performed with TB Green™ Premix Ex Taq™ (Takara, Tokyo, Japan) and Thermal Cycler Dice Real Time System III (Takara, Tokyo, Japan) according to the manufacturer’s instructions. The measured threshold cycle (Ct) values were calculated using the Ct values of the red sea bream elongation factor 1-alpha gene (forward: CCTTCAAGTACGCCTGGGTG and reverse: CTGTGTCCAGGGGCATCAAT) and the delta-delta Ct method as the fold change relative to the control group. All experiments were repeated three times, and statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. The analysis was conducted using SPSS software version 19 (IBM, Chicago, IL, USA). Significance was indicated by the p-value (* p < 0.05 and ** p < 0.01).
3. Results
3.1. Identification and Characterization of PmGal-9 Sequence
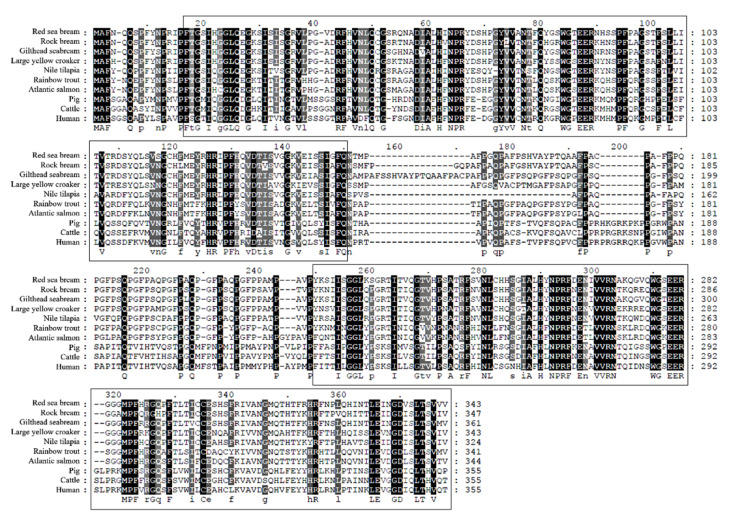
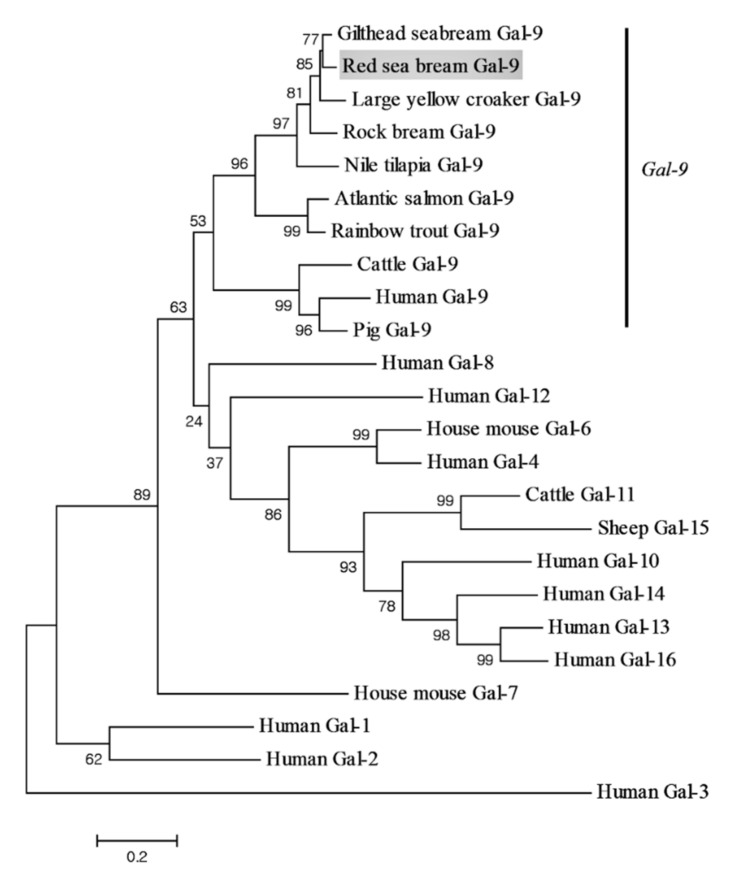
The CDS of PmGal-9 is 1032 bp long (GenBank accession No. QLI33832), which encodes a mature peptide of deduced 343 amino acids (aa) with a calculated molecular weight of 37.43 kDa and a theoretical isoelectric point of 9.11. PmGal-9 has two putative galactose-binding lectin domains located at 16 to 148 and 217 to 343 aa (Figure 1). The deduced amino acid sequence alignment of the PmGal-9 sequence with those from other species indicated their high shared identity (Table 1). Multiple sequence alignment showed that PmGal-9 had the highest similarity to rock bream Gal-9 (85.6%), followed by gilthead sea bream Gal-9 (85.0%), large yellow croaker Gal-9 (79.0%), and Nile tilapia Gal-9 (72.9%). There was a comparatively low level of identity with pig Gal-9 (45.4%), cattle Gal-9 (44.0%), and human Gal-9 (43.4%). Furthermore, a phylogenetic tree was constructed using the deduced amino acid sequences (Figure 2). PmGal-9 formed distinct clusters with the Gal-9 of teleosts, implying a closer relationship between PmGal-9 and that of other teleosts.
Figure 1.
Multiple sequence alignment analysis of the deduced amino acid sequence of PmGal-9 with fish Gal-9 sequences. This analysis is based on the following sequence data: rock bream (ANN46244), gilthead sea bream (XP_030261557), large yellow croaker (XP_010754381), Nile tilapia (XP_003458375), rainbow trout (ACO08221), Atlantic salmon (ACI67584), pig (NP_999097), cattle (NP_001034266), and human (CAB93851). The predicted galactose-binding lectin domains are indicated by the box. Black boxes: identity = 100%; gray boxes: 80% ≤ identity < 100%; light gray boxes: 50% ≤ identity < 80%.
Table 1.
Similarities between deduced amino acid sequences of PmGal-9 and those of homologs in other species.
| Common Name | Species | Protein Sequence Length | GenBank Accession No. | Protein Similarity (%) |
|---|---|---|---|---|
| Rock bream | Oplegnathus fasciatus | 347 | ANN46244 | 85.6 |
| Gilthead sea bream | Sparus aurata | 361 | XP_030261557 | 85.0 |
| Large yellow croaker | Larimichthys crocea | 343 | XP_010754381 | 79.0 |
| Nile tilapia | Oreochromis niloticus | 324 | XP_003458375 | 72.9 |
| Rainbow trout | Oncorhynchus mykiss | 341 | ACO08221 | 65.0 |
| Atlantic salmon | Salmo salar | 344 | ACI67584 | 64.1 |
| Pig | Sus scrofa | 355 | NP_999097 | 45.4 |
| Cattle | Bos taurus | 355 | NP_001034266 | 44.0 |
| Human | Homo sapiens | 355 | CAB93851 | 43.4 |
Figure 2.
A phylogenetic tree of PmGal-9 and other known Gal-9 homologs based on the neighbor-joining (NJ) method. The scale bar indicates a branch length of 0.2. Numbers are bootstrap percentiles from 1000 replicates. This analysis is based on the following sequence data: Human Gal-1 (NP_002296), Human Gal-2 (NP_006489), Human Gal-3 (BAA22164), Human Gal-4 (NP_006140), House mouse Gal-6 (AAI60275), House mouse Gal-7 (NP_032522), Human Gal-8 (AAF19370), Gilthead sea bream Gal-9 (XP_030261557), Large yellow croaker Gal-9 (XP_010754381), Rock bream Gal-9 (ANN46244), Nile tilapia Gal-9 (XP_003458375), Atlantic salmon Gal-9 (ACI67584), Rainbow trout Gal-9 (ACO08221), Cattle Gal-9 (NP_001034266), Human Gal-9 (CAB93851), Pig Gal-9 (NP_999097), Human Gal-10 (NP_001819), Cattle Gal-11 (CBX54571), Human Gal-12 (NP_001136007), Human Gal-13 (ACR09640), Human Gal-14 (ACR09644), Sheep Gal-15 (NP_001009238), and Human Gal-16 (NP_001177370).
3.2. Expression of PmGal-9 mRNA in Various Tissues
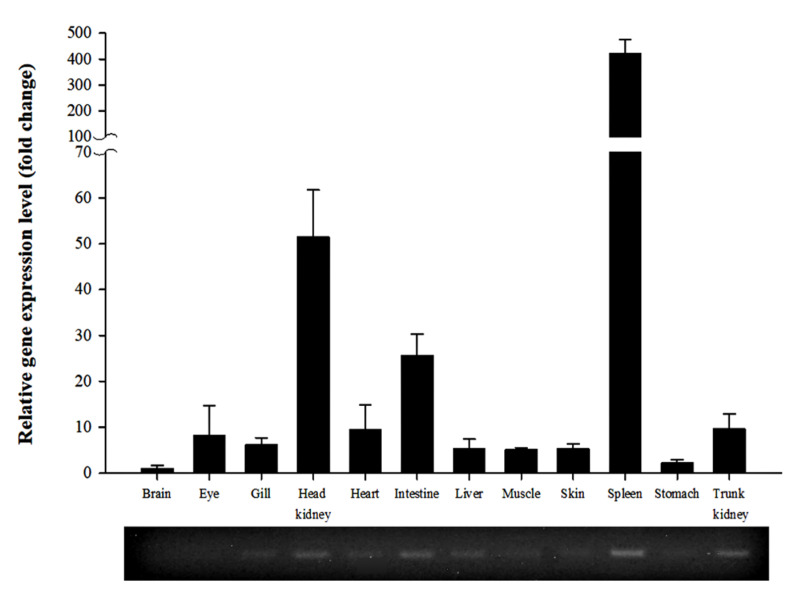
We used RT-qPCR to extract total RNA from three healthy fish to investigate the distribution of PmGal-9 mRNA in various tissues (Figure 3). It was expressed ubiquitously in all tissues (trunk kidney, head kidney, gill, spleen, heart, liver, brain, stomach, intestine, skin, muscle, and peripheral blood lymphocytes) analyzed. Compared with the brain, the spleen exhibited higher expression of PmGal-9 (422.7-fold), followed by the head kidney (51.5-fold) and intestine (25.6-fold). In contrast, relatively low levels of expression were observed in the liver, muscle, skin, and stomach.
Figure 3.
Expression level of PmGal-9 mRNA in various tissues of healthy red sea bream. The PmEF-1α gene was used to normalize the RT-qPCR results. The expression level is expressed as the fold change compared to the expression level of PmGal-9 mRNA in the brain. An agarose gel image of the PCR products is shown at the bottom. All data are presented as the mean ± SD from three independent cDNA samples with three replicates per sample.
3.3. Expression of PmGal-9 mRNA after Pathogen Infection
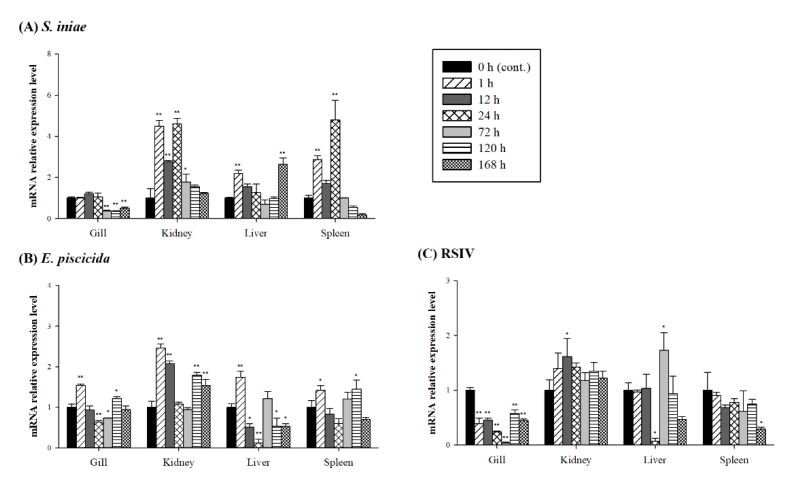
The mRNA expression levels of PmGal-9 in the gill, whole kidney, liver, and spleen at 1, 12, 24, 72, 120, and 168 h after pathogen challenge (S. iniae, E. piscicida or RSIV) were determined using RT-qPCR (Figure 4). The PmGal-9 transcript levels were significantly up- or downregulated in all tested tissues and were increased more in response to bacterial infection than in response to viral infection. After S. iniae inoculation and during the initial stage of this artificial infection (between 1 and 24 h), PmGal-9 mRNA transcript levels were highest in the gills, kidneys, and spleen and gradually declined thereafter. In contrast, although hepatic PmGal-9 mRNA transcript levels were significantly upregulated at 1 h postinfection, the highest levels were achieved at 168 h, after a transient intervening decrease. After artificial E. piscicida infection, all examined tissues exhibited the highest PmGal-9 mRNA transcript levels at 1 h, followed by a transient decline before re-upregulation at 72 and 120 h. After RSIV infection, gill PmGal-9 mRNA transcript levels remained significantly downregulated for the duration of the infection period, and they were maintained in the spleen until 168 h, when significant downregulation became apparent. In the kidney, levels were significantly upregulated at 12 h postinfection, and then declined to baseline, where they remained for the duration of the infection period. Hepatic levels were significantly downregulated at 24 h postinfection, prior to significantly increasing at 72 h and then declining again.
Figure 4.
Expression level of PmGal-9 mRNA in the gill, kidney, liver, and spleen of red sea bream after infection with (A): Streptococcus iniae, (B): Edwardsiella piscicida, or (C): red sea bream iridovirus (RSIV). Gene expression levels and their significance are represented as the mean ± SD. Asterisks indicate significant differences (* p < 0.05, ** p < 0.01) versus the control group.
4. Discussion
Gal-9 forms a dimeric structure with a linker peptide bond, and a specific region at the N-terminus is known to be highly conserved between the Gal family [25,26,27]. Moreover, the structural features of PmGal-9 were consistent with our results, as observed in previous reports where Gal-9 was highly conserved throughout the teleost Gal-9 sequence [19,22]; however, certain sequences in the galactose-binding lectin domains were missing compared to those in mammalian Gal-9, and in particular, the sequence of the linker peptide (151 to 249 aa) connecting the two galactose-binding lectin domains was confirmed to vary distinctly between species (Figure 1). In a study by Nishi et al., the lack of a linker peptide in Gal-9 indicated more stability against proteolysis; however, some sequence mutations occurring in the galactose-binding lectin domain and changes in the sugar-binding activity were not confirmed [28]. Similarly, these were also reported in Gal-7, demonstrating that the mutation was not related to the ability of the protein to regulate apoptosis; instead, the activation of the galactose-binding lectin domain was required to inhibit the invasiveness of cancer cells [29]. On the other hand, compared to the wild type, the mutated Gal-9 protein found in the canine gastrointestinal nematode parasite considerably decreased its affinity for carbohydrates or lost its activity [30]. Although the obtained results differ from those reported for the Gal homolog and are in conflict with the study purpose, they suggest the importance of the conservation of major residues—such as polar amino acids—within the galactose-binding lectin domain.
Gal-9 is highly expressed in many immune organs, including the kidney, bone marrow, lymph nodes, spleen, and thymus. It is known to have a direct role in immune-related cells and systems [31,32,33,34]. In mammals, Gal-9 interacts with the T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), a marker of various immune cells, and is involved in the apoptosis of T cells and the activation of dendritic cells [9,35,36]. In healthy Pelteobagrus fulvidraco and Labeo rohita, PfGal-9 and LrGal-9 mRNAs are most abundantly distributed in the blood, spleen, kidney, or intestine and least abundantly distributed in the muscle, skin, or brain [19,21]. In healthy rainbow trout, abundant expression was also found in lymphoid organs such as the thymus, head kidney, and spleen, all of which contain macrophages and lymphocytes [37]. On the other hand, different results have been reported for the fish species Rhodeus uyekii, Larimichthys crocea, and Oreochromis niloticus, and the differences in expression patterns were confirmed [18,20,22]. However, overall, the results clearly indicate that Gal-9 mRNA expression is relatively abundant in major immune and peripheral lymphoid tissues in fish, which may be related to its previously reported immunological correlation with lymphoid cells. In addition, Gal-9 can promote or inhibit T cell differentiation in the mammalian thymus; however, studies on teleost thymus are limited [9,31,38]. Since the teleost thymus also functions similarly to that of mammals, further studies should evaluate the role of Gal-9 expression in the thymus.
In previous studies on teleosts, Gal-9 mRNA expression levels were significantly upregulated in immune-related tissues after bacterial infection [19,20,21,22]. In addition, Gal-9 mRNA in Labeo rohita was upregulated in muscle and heart, which is thought to be an immunologically important function of Gal-9, requiring it to be expressed in various tissues throughout the body in response to bacterial infection [21]. Gal-9 stimulates neutrophils during bacterial infections and contributes to the removal of bacteria, but in mouse model studies, Gal-9 exacerbates the inflammatory response during sepsis caused by pulmonary Francisella novicida infection [39,40]. These findings suggest the need for Gal-9 in vivo functional analysis during different stages of illness in fish models, especially those of pathogen infection. In rainbow trout, Gal-9 was upregulated in leukocytes during viral hemorrhagic septicemia virus infection [17], and our results showed that it was also upregulated in the kidney and liver. However, the expression level of Gal-9 mRNA was significantly downregulated after artificial infection in the spleen and gills. After viral infection with megalocytivirus and RSIV, a lot of the virus was found to accumulate in the gills and spleen, which could be associated with the survival rate [41,42,43,44]. Downregulation of Gal expression due to viral infection has been reported in previous studies [45,46,47], and hence, it may be considered an indicator of interference between viral infection and immunomodulation. RSIV inclusion bodies are mainly found in the gills and spleens of infected fish [44,48]. Moreover, the gills and spleen of Pagrus major are believed to have decreased PmGal-9 mRNA expression due to the mobilization or activity of PmGal-9 protein in the immune system. The functional studies of Gal-9 against viral diseases in teleosts are still very limited, and further research is needed. These additional studies will be important to properly and effectively control and treat disease by understanding pathogen-derived diseases and the immune system.
5. Conclusions
We confirmed the sequence of PmGal-9 from red sea bream and characterized its deduced amino acid sequence and conserved sequences. Expression profiling of PmGal-9 mRNA confirmed its expression characteristics and significant changes in expression immune-related tissues in response to pathogen infection.
Acknowledgments
This research was a part of the project titled “Development of rapid and sensitive methods for assessing health in farmed fish (2016),” funded by the Ministry of Oceans and Fisheries, Korea (Fishery Commercialization Technology Development Program) and the Marine Biotechnology Program of the Korea Institute of Marine Science and Technology promotion funded by the Ministry of Oceans and Fisheries (No. 20180430).
Author Contributions
Conceptualization, C.-I.P.; methodology, C.-I.P. and D.-H.K.; software, K.-M.C. and M.-S.J.; validation, C.-I.P. and K.-M.C.; formal analysis, M.-S.J. and D.-H.C.; investigation, W.-S.W., G.K. and M.J.H.; resources, C.-I.P.; data curation, K.-M.C.; writing—original draft preparation, K.-M.C.; writing—review and editing, C.-I.P.; visualization, K.-M.C.; supervision, C.-I.P. and D.-H.K.; project administration, C.-I.P.; funding acquisition, C.-I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Oceans and Fisheries (No. 20180430).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Animal Protection and Use Committee of Gyeongsang National University (Approval Number: 2020-0002).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barondes S.H., Cooper D.N., Gitt M.A., Leffler H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994;269:20807–20810. [PubMed] [Google Scholar]
- 2.Mehul B., Bawumia S., Martin S.R., Hughes R.C. Structure of baby hamster kidney carbohy-drate-binding protein CBP30, an S-type animal lectin. J. Biol. Chem. 1994;269:18250–18258. [PubMed] [Google Scholar]
- 3.Vasta G.R. Roles of galectins in infection. Nat. Rev. Genet. 2009;7:424–438. doi: 10.1038/nrmicro2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes R.C. Galectins as modulators of cell adhesion. Biochimie. 2001;83:667–676. doi: 10.1016/S0300-9084(01)01289-5. [DOI] [PubMed] [Google Scholar]
- 5.Boscher C., Zheng Y.Z., Lakshminarayan R., Johannes L., Dennis J.W., Foster L.J., Nabi I.R. Galectin-3 Protein Regulates Mobility of N-cadherin and GM1 Ganglioside at cell-cell junctions of mammary carcinoma cells. J. Biol. Chem. 2012;287:32940–32952. doi: 10.1074/jbc.M112.353334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johannes L., Jacob R., Leffler H. Galectins at a glance. J. Cell Sci. 2018;131:jcs208884. doi: 10.1242/jcs.208884. [DOI] [PubMed] [Google Scholar]
- 7.Hirabayashi J., Kasai K.-I. The family of metazoan metal-independent β-galactoside-binding lectins: Structure, function and molecular evolution. Glycobiology. 1993;3:297–304. doi: 10.1093/glycob/3.4.297. [DOI] [PubMed] [Google Scholar]
- 8.Wada J., Kanwar Y.S. Identification and characterization of galectin-9, a novel β-galactoside-binding mammalian lectin. J. Biol. Chem. 1997;272:6078–6086. doi: 10.1074/jbc.272.9.6078. [DOI] [PubMed] [Google Scholar]
- 9.Zhu C., Anderson A.C., Schubart A., Xiong H., Imitola J., Khoury S.J., Zheng X.X., Strom T.B., Kuchroo V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 10.Matsuoka N., Kozuru H., Koga T., Abiru S., Yamasaki K., Komori A., Fujita Y., Tenmoku J., Asano T., Sato S., et al. Galectin-9 in autoimmune hepatitis. Medicine. 2019;98:e16924. doi: 10.1097/MD.0000000000016924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panda S.K., Facchinetti V., Voynova E., Hanabuchi S., Karnell J.L., Hanna R.N., Kolbeck R., Sanjuan M.A., Ettinger R., Liu Y.-J. Galectin-9 inhibits TLR7-mediated autoimmunity in murine lupus models. J. Clin. Investig. 2018;128:1873–1887. doi: 10.1172/JCI97333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamauchi A., Kontani K., Kihara M., Nishi N., Yokomise H., Hirashima M. Galectin-9, a novel prognostic factor with antimetastatic potential in breast cancer. Breast J. 2006;12:S196–S200. doi: 10.1111/j.1075-122X.2006.00334.x. [DOI] [PubMed] [Google Scholar]
- 13.Lai J.-H., Luo S.F., Wang M.-Y., Ho L.-J. translational implication of galectin-9 in the pathogenesis and treatment of viral infection. Int. J. Mol. Sci. 2017;18:2108. doi: 10.3390/ijms18102108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdel-Mohsen M., Chavez L., Tandon R., Chew G.M., Deng X., Danesh A., Keating S., Lanteri M., Samuels M.L., Hoh R., et al. Human galectin-9 is a potent mediator of hiv transcription and reactivation. PLoS Pathog. 2016;12:e1005677. doi: 10.1371/journal.ppat.1005677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z.-Y., Dong J.-H., Chen Y.-W., Wang X.-Q., Li C.-H., Wang J., Wang G.-Q., Li H.-L., Wang X.-D. Galectin-9 Acts as a prognostic factor with antimetastatic potential in hepatocellular carcinoma. Asian Pac. J. Cancer Prev. 2012;13:2503–2509. doi: 10.7314/APJCP.2012.13.6.2503. [DOI] [PubMed] [Google Scholar]
- 16.Irie A., Yamauchi A., Kontani K., Kihara M., Liu D., Shirato Y., Seki M., Nishi N., Nakamura T., Yokomise H., et al. Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin. Cancer Res. 2005;11:2962–2968. doi: 10.1158/1078-0432.CCR-04-0861. [DOI] [PubMed] [Google Scholar]
- 17.O’Farrell C., Vaghefi N., Cantonnet M., Buteau B., Boudinot P., Benmansour A. Survey of transcript expression in rainbow trout leukocytes reveals a major contribution of interferon-responsive genes in the early response to a rhabdovirus infection. J. Virol. 2002;76:8040–8049. doi: 10.1128/JVI.76.16.8040-8049.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong H.J., Kim W.-J., Kim H.S., Lee Y.J., Kim C.H., Nam B.-H., Kim Y.-O., Kim D.-G., Lee S.-J., Lim S.-G., et al. Molecular characterization of a tandem-repeat galectin-9 (RuGlec9) from Korean rose bitterling (Rhodeus uyekii) Fish Shellfish Immunol. 2012;32:939–944. doi: 10.1016/j.fsi.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y., Ke F., Ma J., Zhou S. A tandem-repeat galectin-9 involved in immune response of yellow catfish, Pelteobagrus fulvidraco, against Aeromonas hydrophila. Fish Shellfish Immunol. 2016;51:153–160. doi: 10.1016/j.fsi.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Zhang D.L., Lv C.H., Yu D.H., Wang Z. Characterization and functional analysis of a tandem-repeat galectin-9 in large yellow croaker Larimichthys crocea. Fish Shellfish Immunol. 2016;52:167–178. doi: 10.1016/j.fsi.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 21.Mushtaq Z., Krishnan R., Prasad K.P., Bedekar M.K., Pavan-Kumar A. Molecular cloning, characterization and expression profiling of galectin-9 gene from Labeo rohita (Hamilton, 1822) Fish Shellfish Immunol. 2018;76:287–292. doi: 10.1016/j.fsi.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 22.Niu J., Huang Y., Li Y., Wang Z., Tang J., Wang B., Lu Y., Cai J., Jian J. Characterization of a tandem-repeat galectin-9 from Nile tilapia (Oreochromis niloticus) involved in the immune response against bacterial infection. Fish Shellfish Immunol. 2019;92:216–223. doi: 10.1016/j.fsi.2019.05.061. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y.J., Jung S.J., Choi T.J., Kim H.R., Rajendran K.V., Oh M.J. PCR amplification and sequence analysis of irido-like virus infecting fish in Korea. J. Fish Dis. 2002;25:121–124. doi: 10.1046/j.1365-2761.2002.00345.x. [DOI] [Google Scholar]
- 24.Shin G.-H., Shin Y., Jung M., Hong J.-M., Lee S., Subramaniyam S., Noh E.-S., Shin E.-H., Park E.-H., Park J.Y., et al. First draft genome for red sea bream of family Sparidae. Front. Genet. 2018;9:643. doi: 10.3389/fgene.2018.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabinovich G.A. Galectins: An evolutionarily conserved family of animal lectins with multifunctional properties; a trip from the gene to clinical therapy. Cell Death Differ. 1999;6:711–721. doi: 10.1038/sj.cdd.4400535. [DOI] [PubMed] [Google Scholar]
- 26.Nagae M., Nishi N., Murata T., Usui T., Nakamura T., Wakatsuki S., Kato R. Crystal structure of the galectin-9 n-terminal carbohydrate recognition domain from mus musculus reveals the basic mechanism of carbohydrate recognition. J. Biol. Chem. 2006;281:35884–35893. doi: 10.1074/jbc.M606648200. [DOI] [PubMed] [Google Scholar]
- 27.Bi S., Earl L.A., Jacobs L., Baum L.G. Structural features of galectin-9 and galectin-1 that determine distinct t cell death pathways. J. Biol. Chem. 2008;283:12248–12258. doi: 10.1074/jbc.M800523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishi N., Itoh A., Fujiyama A., Yoshida N., Araya S.-I., Hirashima M., Shoji H., Nakamura T. Development of highly stable galectins: Truncation of the linker peptide confers protease-resistance on tandem-repeat type galectins. FEBS Lett. 2005;579:2058–2064. doi: 10.1016/j.febslet.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 29.Labrie M., Vladoiu M., Leclerc B.G., Grosset A.-A., Gaboury L., Stagg J., St-Pierre Y. A mutation in the carbohydrate recognition domain drives a phenotypic switch in the role of galectin-7 in prostate cancer. PLoS ONE. 2015;10:e0131307. doi: 10.1371/journal.pone.0131307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang E.Y., Jeong M.S., Park S.K., Ha S.C., Yu H.S., Jang S.B. Structural basis for carbohydrate recognition and anti-inflammatory modulation by gastrointestinal nematode parasite Toxascaris leonina galectin. J. Biol. Chem. 2016;291:25326–25338. doi: 10.1074/jbc.M116.743773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wada J., Ota K., Kumar A.I., Wallner E., Kanwar Y.S. Developmental regulation, expression, and apoptotic potential of galectin-9, a beta-galactoside binding lectin. J. Clin. Investig. 1997;99:2452–2461. doi: 10.1172/JCI119429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perillo N.L., Marcus M.E., Baum L.G. Galectins: Versatile modulators of cell adhesion, cell proliferation, and cell death. J. Mol. Med. 1998;76:402–412. doi: 10.1007/s001090050232. [DOI] [PubMed] [Google Scholar]
- 33.Spitzenberger F., Graessler J., Schroeder H.-E. Molecular and functional characterization of galectin 9 mRNA isoforms in porcine and human cells and tissues. Biochimie. 2001;83:851–862. doi: 10.1016/S0300-9084(01)01335-9. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto R., Matsumoto H., Seki M., Hata M., Asano Y., Kanegasaki S., Stevens R.L., Hirashima M. Human ecalectin, a variant of human galectin-9, is a novel eosinophil chemoattractant produced by T lymphocytes. J. Biol. Chem. 1998;273:16976–16984. doi: 10.1074/jbc.273.27.16976. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Fueyo A., Tian J., Picarella D., Domenig C., Zheng X.X., Sabatos C.A., Manlongat N., Bender O., Kamradt T., Kuchroo V.K., et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 36.Cano L.Q., Tagit O., Dolen Y., Van Duffelen A., Dieltjes S., Buschow S.I., Niki T., Hirashima M., Joosten B., van den Dries K., et al. Intracellular galectin-9 controls dendritic cell function by maintaining plasma membrane rigidity. iScience. 2019;22:240–255. doi: 10.1016/j.isci.2019.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inagawa H., Kuroda A., Nishizawa T., Honda T., Ototake M., Yokomizo Y., Nakanishi T., Soma G.-I. Cloning and characterisation of tandem-repeat type galectin in rainbow trout (Oncorhynchus mykiss) Fish Shellfish Immunol. 2001;11:217–231. doi: 10.1006/fsim.2000.0307. [DOI] [PubMed] [Google Scholar]
- 38.Wang F., He W., Zhou H., Yuan J., Wu K., Xu L., Chen Z.K. The Tim-3 ligand galectin-9 negatively regulates CD8+ alloreactive T cell and prolongs survival of skin graft. Cell. Immunol. 2007;250:68–74. doi: 10.1016/j.cellimm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Vega-Carrascal I., Bergin D.A., McElvaney O.J., McCarthy C., Banville N., Pohl K., Hirashima M., Kuchroo V.K., Reeves E.P., McElvaney N.G. Galectin-9 Signaling through TIM-3 Is involved in neutrophil-mediated gram-negative bacterial killing: An effect abrogated within the cystic fibrosis lung. J. Immunol. 2014;192:2418–2431. doi: 10.4049/jimmunol.1300711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steichen A.L., Simonson T.J., Salmon S.L., Metzger D.W., Mishra B.B., Sharma J. Alarmin Function of Galectin-9 in murine respiratory tularemia. PLoS ONE. 2015;10:e0123573. doi: 10.1371/journal.pone.0123573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inouye K., Yamano K., Maeno Y., Nakajima K., Matsuoka M., Wada Y., Sorimachi M. Iridovirus infection of cultured red sea bream, Pagrus major. Fish Pathol. 1992;27:19–27. doi: 10.3147/jsfp.27.19. [DOI] [Google Scholar]
- 42.Chou H.-Y., Hsu C.-C., Peng T.-Y. Isolation and characterization of a pathogenic iridovirus from cultured grouper (Epinephelus sp.) in Taiwan. Fish Pathol. 1998;33:201–206. doi: 10.3147/jsfp.33.201. [DOI] [Google Scholar]
- 43.Sudthongkong C., Miyata M., Miyazaki T. Viral DNA sequences of genes encoding the ATPase and the major capsid protein of tropical iridovirus isolates which are pathogenic to fishes in Japan, South China Sea and Southeast Asian countries. Arch. Virol. 2002;147:2089–2109. doi: 10.1007/s00705-002-0883-6. [DOI] [PubMed] [Google Scholar]
- 44.Girisha S.K., Puneeth T.G., Nithin M.S., Naveen-Kumar B.T., Ajay S.K., Vinay T.N., Suresh T., Venugopal M.N., Ramesh K. S Red sea bream iridovirus disease (RSIVD) outbreak in Asian seabass (Lates calcarifer) cultured in open estuarine cages along the west coast of India: First report. Aquaculture. 2020;520:734712. doi: 10.1016/j.aquaculture.2019.734712. [DOI] [Google Scholar]
- 45.Alcendor D.J., Knobel S.M., Desai P., Zhu W.Q., Vigil H.E., Hayward G.S. KSHV downregulation of galectin-3 in Kaposi’s sarcoma. Glycobiology. 2009;20:521–532. doi: 10.1093/glycob/cwp204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toledo K.A., Fermino M.L., Andrade C.D.C., Riul T.B., Alves R.T., Muller V.D.M., Russo R.R., Stowell S.R., Cummings R.D., Aquino V.H., et al. Galectin-1 exerts inhibitory effects during denv-1 infection. PLoS ONE. 2014;9:e112474. doi: 10.1371/journal.pone.0112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uluca U., Sen V., Ece A., Tan I., Karabel D., Aktar F., Karabel M., Balık H., Güneş A. Serum galectin-3 levels in children with chronic hepatitis B infection and inactive hepatitis B carriers. Med. Sci. Monit. 2015;21:1376–1380. doi: 10.12659/msm.894035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimmoto H., Kawai K., Ikawa T., Oshima S.-I. Protection of red sea bream Pagrus major against red sea bream iridovirus infection by vaccination with a recombinant viral protein. Microbiol. Immunol. 2010;54:135–142. doi: 10.1111/j.1348-0421.2010.00204.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.