Abstract
Arthropod-borne viruses (Arboviruses) continue to generate significant health and economic burdens for people living in endemic regions. Of these viruses, some of the most important (e.g., dengue, Zika, chikungunya, and yellow fever virus), are transmitted mainly by Aedes mosquitoes. Over the years, viral infection control has targeted vector population reduction and inhibition of arboviral replication and transmission. This control includes the vector control methods which are classified into chemical, environmental, and biological methods. Some of these control methods may be largely experimental (both field and laboratory investigations) or widely practised. Perceptively, one of the biological methods of vector control, in particular, Wolbachia-based control, shows a promising control strategy for eradicating Aedes-borne arboviruses. This can either be through the artificial introduction of Wolbachia, a naturally present bacterium that impedes viral growth in mosquitoes into heterologous Aedes aegypti mosquito vectors (vectors that are not natural hosts of Wolbachia) thereby limiting arboviral transmission or via Aedes albopictus mosquitoes, which naturally harbour Wolbachia infection. These strategies are potentially undermined by the tendency of mosquitoes to lose Wolbachia infection in unfavourable weather conditions (e.g., high temperature) and the inhibitory competitive dynamics among co-circulating Wolbachia strains. The main objective of this review was to critically appraise published articles on vector control strategies and specifically highlight the use of Wolbachia-based control to suppress vector population growth or disrupt viral transmission. We retrieved studies on the control strategies for arboviral transmissions via arthropod vectors and discussed the use of Wolbachia control strategies for eradicating arboviral diseases to identify literature gaps that will be instrumental in developing models to estimate the impact of these control strategies and, in essence, the use of different Wolbachia strains and features.
Keywords: Aedes-borne, arboviruses, Wolbachia, vectors, controls
1. Introduction
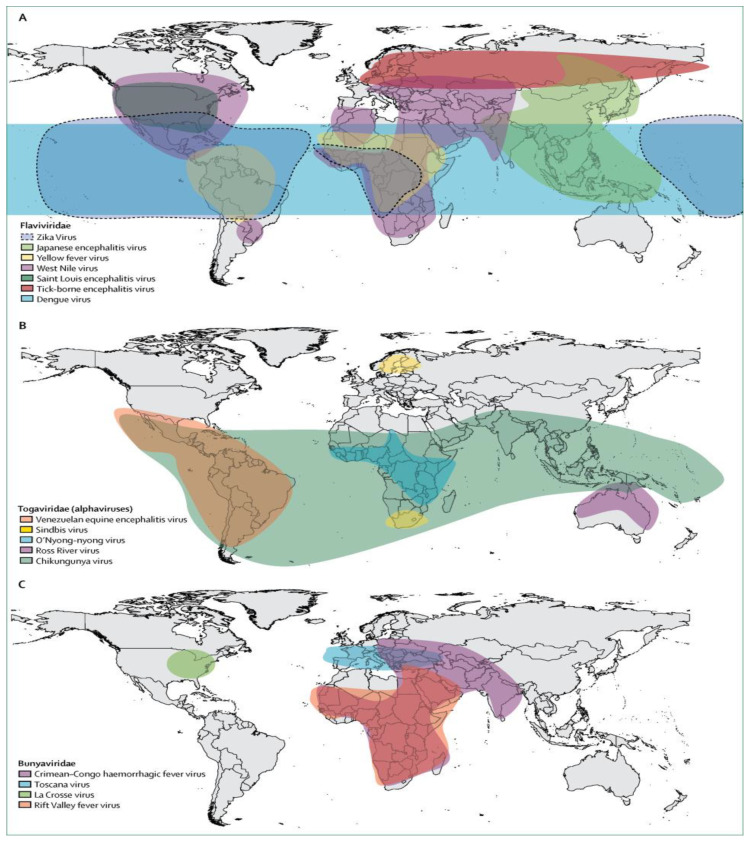
Arboviruses (arthropod-borne viruses) are transmitted via blood feeding arthropods such as Aedes mosquitoes, flies, and ticks [1]. These viruses are characterised by either a double-stranded DNA or a RNA genome [2]. One and only example of the DNA genome of medical significance is the African swine fever virus (ASFV) which is mainly transmitted by ticks and belongs to the Asfarviridae family of viruses [3]. Except for the double-stranded DNA arboviruses, all other arboviruses have RNA genomes and are members of either of the Flaviviridae, Togaviridae, Bunyaviridae, Rhabdoviridae, and Reoviridae families [4]. Specifically, Aedes-borne viruses are a subset of arboviruses that are mostly transmitted by female Aedes aegypti mosquitoes and sometimes by female Aedes albopictus mosquitoes [5,6]. Examples of Aedes-borne arboviruses having RNA genomes are dengue virus (DENV), Zika virus (ZIKV), chikungunya virus (CHIKV), yellow fever virus (YFV), and Ross River virus (RRV) [7] (Figure 1). Other RNA arboviruses which are not Aedes-borne include West Nile virus (WNV) and Sindbis virus (Culex-borne) [8,9], Tick-borne encephalitis virus and Crimean-Congo haemorrhagic fever virus (tick-borne) [10,11], and Toscana virus (fly-borne) [12].
Figure 1.
Global distribution of major arboviruses [7]. The Aedes-borne arboviruses described in this review belong to the Flaviviridae (ZIKV—light blue; YFV—yellow; DENV—blue) and Togaviridiae (CHIKV—green) families. Reproduced with permission from Elsevier; License no. 4892881108152.
Aedes-borne viruses are fast spreading diseases that pose significant health problems globally [13,14,15] (Figure 1). These viruses can be life-threatening as dengue virus alone currently infects approximately 390 million people annually with 96 million of these showing clinical symptoms [16,17,18,19]. The global spread of these viruses is being fuelled by human migration, urbanization, and animal transportation [14,20,21]. Presently, there are no specific treatments for Aedes-borne infections [4,22]. However, supportive care for symptoms such as headache, seizure, and fever management and maintaining vital organs is available [2,20]. Additionally, some vaccines with high efficacy have been developed to prevent arboviral infections. They include 17D YFV [23], Japanese encephalitis [24] and tetravalent DENV vaccines [25]. However, research on the development of other arboviral infection vaccines such as ZIKV [26], WNV [27], RRV [28], and CHIKV [29] is still in progress but not yet approved. Full details of disease symptoms and available treatment alternatives are presented in Section 2.
To control the spread of Aedes-borne infections, several approaches such as those targeting human hosts, human-vector interactions and vectors specifically can be used [2]. Primarily, vector control strategies are used since they induce direct or biological reduction/elimination of the vectors without causing significant harm to human hosts [30]. The vector control strategies are classified into chemical, environmental, and biological control methods [2,30]. Presently, some of these methods are either widely practised or largely experimental (laboratory or field investigations).
Interestingly, one of the biological methods of vector control is the Wolbachia-based control method, which works by replacing existing wild-type mosquito vector populations with a Wolbachia-infected variant for which viral proliferation in its midgut is prohibited, rendering them less capable of transmitting the virus [31,32,33]. Several field studies have demonstrated the feasibility and effectiveness of Wolbachia introduction into native mosquito populations [34,35,36]. The main objective of this review is to critically appraise published literature on the available vector control methods and specifically highlight the use of the Wolbachia-based control method as a natural control measure for eradicating arboviral diseases. This includes both theoretical investigations of the potential efficacy of Wolbachia-based strategies and field trials that provide concrete demonstration. We also aim to identify literature gaps that will be instrumental in developing models to estimate the impact of this strategy. Therefore, we provide important background information on the types, scale, severity, and treatment of Aedes-borne arboviral infections, focusing on vector control methods and specifically highlighting those amenable to Wolbachia-type control.
2. Aedes-Borne Arboviruses
Aedes-transmitted arboviruses can be life-threatening when contracted by human hosts depending on the infection severity [37,38,39,40]. The primary vector responsible for the transmission of these arboviruses such as DENV, ZIKV, YFV, and CHIKV is the female Aedes aegypti (Yellowfever) mosquito, while female Aedes albopictus (Asian Tiger) mosquitoes also contribute to transmission [5,6]. DENV, in particular, is the most widespread Flavivirus, and also the most recognisable and deadly among the known Aedes-borne viruses [14] (Figure 1). Dengue viral infection can lead to health complications such as dengue haemorrhagic fever with shock syndrome and even cause circulatory failure and death [41]. The mean estimated intrinsic incubation period of dengue virus in humans is 5.9 days, while the estimated extrinsic (temperature-dependent) incubation period of the virus in the mosquito vectors is 15 and 6.5 days at 25 °C and 30 °C, respectively [42] (Table 1). In recent decades, the incidence of dengue viral infection has continued to increase. Modelling studies recently estimated that approximately 390 million dengue infections occur per year, with 96 million of these exhibiting clinical symptoms [16,17], and that the global population at risk of dengue is 3.9 billion [43].
Table 1.
Aedes-borne arboviral incubation periods and the asymptomatic fraction of infections
| Aedes-Borne Arboviruses | Virus Type | Transmitted By | Symptoms | Supportive Treatment | Coinfection with Other Arboviruses | Intrinsic Incubation Period (Days) | Extrinsic Incubation Period (Days) | Asymptomatic Proportion in Infected Humans (%) |
|---|---|---|---|---|---|---|---|---|
| Dengue |
Flavivirus [14] |
Ae. Aegypti Ae. albopictus [5,6] |
Sudden high grade fever, Headache, Nausea, Arthralgia, Eye and Muscle pain [65] | DENV vaccine and drug administration [66] | Yes (e.g., DENV and ZIKV) [67] | Median: 5.3 [68] Mean: 5.9 [42] |
Mean: 15 (at 25 °C) 6.5 (at 30 °C) [42] |
75 [69] |
| Zika |
Flavivirus [70] |
Ae. aegypti Ae. albopictus Human (via blood transfusion) [45,46,51] |
Fever, Conjunctivitis, Muscle pain, Headache, Joint pain, Rash and Microcephaly [71,72] | Fluid intake and drug administration (such as acetaminophen) [73] | Yes (e.g., ZIKV and CHIKV) [74] | Median: 6.8 [75] 6.2 [76] |
Median: 5.1 (at 30 °C) 9.6 (at 26 °C) 24.2 (at 21 °C) [77] |
80 [78] |
| Chikungunya |
Alphavirus [79] |
Ae. aegypti Ae. albopictus [80] |
High fever, Joint pain, Myalgia, Arthritis, Conjunctivitis, and Dermatologic manifestations [81,82] | Plenty of rest, Fluid intake and Acetaminophen [52,80] | Yes (e.g., CHIKV and DENV) [83] | Median: 3.0 [68] | Median: 2 [84] | Approx. 18 to 28 [85] |
| Yellow Fever |
Flavivirus [56] |
Ae. aegypti Ae. Albopictus [86] |
Headache, Nausea, Vomiting, Fever, Dizziness and Joint pain [87,88] | YFV vaccine and Ribavirin [89,90] | Yes (e.g., YFV and CHIKV) [91] | Median: 4.3 [92] 4.4 [68] |
Median: 10 (at 25 °C) [92] | 55 [93] |
Similar to DENV, ZIKV is transmitted through the infectious bite of Aedes mosquitoes. It was first isolated from a rhesus monkey in 1947 in an Ugandan forest: Zika [44]. Also, the vectors responsible for ZIKV transmission are Ae. aegypti and Ae. albopictus [45,46]. Although Zika viral infection is mainly transmitted via mosquito bites, instances of human-to-human and perinatal transmission have been observed [47,48,49,50]. There is evidence that ZIKV infection is associated with microcephaly, a congenital condition causing abnormal smallness of the head due to improper development of a baby’s brain during pregnancy or after childbirth [51]. Other symptoms of ZIKV are shown in Table 1.
Unlike DENV, a Flavivirus, chikungunya (meaning “to become contorted” in the Kimakonde language) virus: CHIKV is an Alphavirus that causes incapacitating joint pain and is transmitted by Aedes mosquitoes [52]. CHIKV transmission has also been reported through blood exposure [53]; infection of the human cornea [54]; and maternal transmissions—the latter of which can lead to miscarriage [55].
Furthermore, YFV is a member of the Flaviviridae family and is usually transmitted by Aedes mosquitoes [56]. The YFV infection can be severe, causing a high proportion of deaths in endemic populations [56]. YFV is a single-stranded RNA virus with a single serotype whose antigens are conserved [57]. The single serotypic nature of YFV allows the developed vaccine to protect the infected host against all the virus strains [58]. Human hosts are highly susceptible to contracting yellow fever infections as well as some non-human primates and rodents [59,60,61]. Recently, some studies have suggested that coinfection of arboviruses (Table 1) can not only occur, but can also generate cross-protective immunity where initial exposure to the first viral infection activates the immune response and confers acquired immunity against the next viral infection, and can also reduce the risk of subsequent infections for some arboviruses, in particular, dengue [62]. However, not all arboviral antibody responses are cross-protective as the interaction between some arboviruses and antiviral antibodies may result in a phenomenon known as antibody-dependent enhancement (ADE) of infection, which allows viruses to enter into the host cell [63]. This effect modulates the immune response of the host, facilitates viral production and may increase the severity of the viral disease [64].
3. Control Strategies for Aedes-Borne Viral Infections
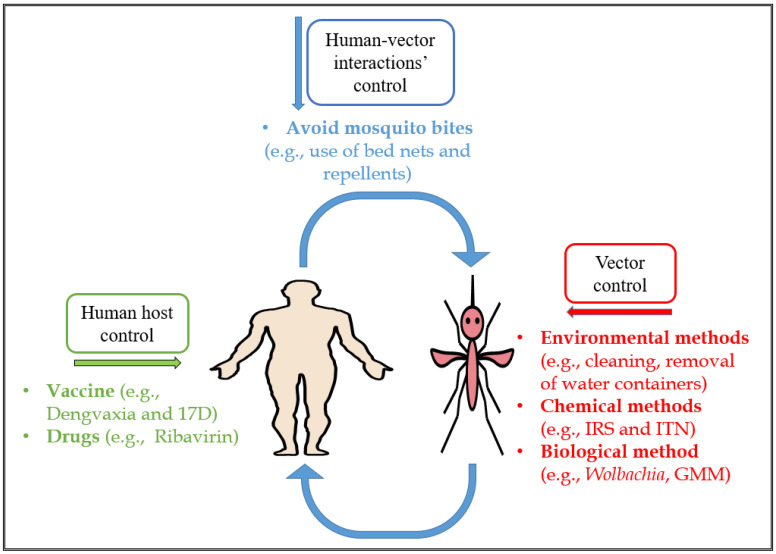
Aedes-borne viral infection control has proven effective in reducing disease burden [94]. These strategies include taking preventive measures such as ensuring environmental cleanliness and adequate drainage, avoiding contact with vectors, vaccinating susceptible individuals and using genetic control of mosquitoes and paratransgenesis [41,95]. These measures can be grouped into three types of control measures depending on the stage of the transmission cycle that they target: (i) the human host; (ii) human-vector interactions; and (iii) vector control categories [2] (Figure 2).
Figure 2.
Diagram showing the summary of methods and examples (in brackets) that could be used to control Aedes-borne arboviral transmission: The green text refers to the human-host strategies, which could be used to prevent arboviral transmission via vaccines and drugs. The blue text refers to the human-vector interactions’ strategies that could be used for arboviral transmission control via humans avoiding bites from infectious mosquitoes. The red text refers to the vector control strategies that include chemical, environmental or biological methods to control arboviral transmission (IRS—indoor residual spraying; ITN—insecticide-treated bed nets; GMM—genetically modified mosquitoes).
Firstly, human host control strategies typically focus on reducing the susceptibility of humans to contracting Aedes-borne viral infections. This can be achieved through the use of vaccines and chemoprophylaxis (drug use) [23,24,25,96]. These control measures are used to inhibit, suppress or clear the virus, preventing replication in the human host [97]. Some vaccines with high efficacy have been developed, including the 17D yellow fever vaccine [23] and the Japanese encephalitis vaccine [24]. Notably, the tetravalent dengue vaccine has high protective efficacy rates of 56.5% and 60.8% against virologically-confirmed dengue but lower for DENV-2 [25]. A modelling study explored the third-year results of phase III trials of Dengvaxia and suggested that the vaccine generated protection against dengue within partially-immune persons but also increased hospitalizations among vaccine-sensitized individuals infected with dengue [66]. However, this vaccine is still controversial as it has been linked to significant side effects in the Philippines for instance [98]. Research on the development of vaccines for other Aedes-borne viral infections such as ZIKV [26], Ross-River virus (RRV) [28] and CHIKV [29] is still in progress.
Secondly, human-vector preventive measures prevent contact between susceptible human hosts and infected mosquitoes (and vice-versa), particularly mosquito bites. Examples include insecticide-treated bed nets, repellents [99] and sensitization of people in areas with high transmission rates to take preventive measures such as ensuring a clean environment and a good drainage system to avoid water stagnancy during rainfall [100]. Other preventive measures include the use of metofluthrin in the home as this has been shown to produce a rapid decrease in the observed biting frequency and increased kills among mosquitoes trapped inside the house [101]. Some studies in Australia and Vietnam have shown a significant reduction in mosquito population densities in homes treated with metofluthrin compared with those that were untreated [102].
Lastly, the vector control approach focuses on reducing the abundance, and inhibiting the transmission capacities, of virus-carrying mosquitoes [2]. Vector control can be challenging in endemic areas due to inadequate and haphazard implementation. Nevertheless, vector control techniques remain the main control strategies for suppressing dengue transmission, but often require a great deal of both financial and labour investment to achieve sustainability and sometimes do pose an environmental contamination risk, such as the use of chemical larvicides [30,103].
4. Vector Control Methods for Aedes-Borne Viral Infections
Vector control approaches are classified into three categories: environmental, chemical, and biological control methods [2]. Environmental methods include: cleaning of the environment, particularly, the mosquito vector breeding sites; covering or emptying water containers; and implementing strategic waste management schemes [30]. Chemical and biological control methods involve the use of insecticides such as Temephos and pyrethroids/organophosphates used in outdoor fogging [104]. Biological methods include the use of biological agents such as copepods, larvivorous fish, genetically modified mosquitoes and intracellular endosymbionts, e.g., Wolbachia, for control purposes [104,105,106]. Some of the environmental and chemical methods of vector control are widely practised while some biological methods are presently largely experimental.
4.1. Established Vector Control Methods
Environmental control methods for host vectors include common practices such as emptying, covering, or destroying water-filled containers, providing piped water, clearing and cleaning of the vectors’ breeding sites, and setting up strategies to ensure waste management implementation [30].
The chemical method generally involves use of a chemical mixture, solution or material to directly expel or, in most cases, kill arthropods such as mosquito vectors [107]. This method may be grouped into the use of: (a) durable treated materials such as door curtains and insecticide-treated bed nets (ITN); (b) insecticides for residual spraying, which include peri-domestic space treatments and indoor residual spraying (IRS) [108,109]; and (c) larval breeding control that includes the application of chemical larvicides such as Temephos to destroy breeding habitats [95,104]. Insecticides such as organophosphates and pyrethroids are most commonly used for the chemical control of Aedes mosqutoes [104]. However, there are limitations to chemical control methods. These limitations include environmental pollution, contamination, and toxicity [104], which may cause irritation to humans and endanger aquatic animal species.
Presently, chemical control methods are the most widely practised form of vector control, in particular the use of pyrethroids for outdoor fogging [110]. The direct killing of vectors using insecticides has been used for a long time and some studies have reported increased resistance to insecticides in mosquitoes, especially Aedes aegypti [108,109]. One of these studies investigated the insecticide resistance in Aedes aegypti mosquitoes in Ceara, Brazil and reported that these mosquitoes are subjected to selective pressure by the larvicide used, Temephos, as they reduce its effectiveness in the field. This resistance may be difficult to reverse as it may take more than seven years [108].
4.2. Experimental Vector Control Methods
Experimental vector control methods include the introduction of biological agents such as larvivorous fish [111], copepods (a group of small crustaceans) [112] or Bacillus thuringiensis [113], typically for larvae control. A study investigating the community effectiveness and efficacy of the use of larvivorous fish for dengue vector control reported that although the use of larvivorous fish could be effective in reducing the immature vector stages in small settings such as containers, these results could be minimal as it would require large coverage of multiple production of larvivorous fish containers to achieve any impact in an area of dengue endemicity [111]. Another similar study systematically reviewed the community effectiveness of copepods for dengue vector control in Vietnam [114]. The authors concluded that although there was an effective control of dengue transmission, the impact is difficult to determine as other control measures such as increased educational campaigns were combined with copepods [114]. A controlled study investigated the effectiveness of using Bacillus thuringiensis israelensis (BTI) spray to control the population of Aedes mosquitoes [113]. They showed that, although BTI treatment kills larvae, and thus suppresses adult mosquitoes indirectly, this effect is not sustainable over time [113]. Therefore, it can only be used together with other control measures as a supplement.
Other biological vector controls that are largely experimental at present may include both laboratory and field investigations. These investigations include the introduction of sterile insect techniques (SITs), genetically modified mosquitoes (GMM) and control agents that are incapable of transmitting viral pathogens [115], such as the Wolbachia-based strategy for disease control [31,116].
SITs are a method of insect control involving the rearing of large numbers of sterilized male mosquitoes that are released to mate with wild-type female mosquitoes resulting in the reduction of the reproductive advantage of the females [117]. This may lead to vector population suppression if sufficient releases of sterile male mosquitoes are rolled out. Sterilization can be achieved using radiation in dedicated facilities [118]. There are some drawbacks to SITs, which include difficulties in isolating male mosquitoes for sterilization and transportation problems, and overdose of radiation as this may also affect the physical strength of the sterilized mosquitoes [119,120]. The release of insects with dominant lethality (RIDL) which involves introducing a lethal trait into the female mosquitoes has emerged as a technique to overcome SITs difficulties [119,121]. A resulting example of the RIDL technique is the production of female flightless Aedes mosquitoes [122]. These flightless mosquitoes are created via RIDL using an indirect flight muscle gene Act4 of the Aedes aegypti mosquito [122]. When this gene is switched on in developing female mosquitoes, it incapacitates the flight muscles leading to the death of the muscle cells and rendering the mosquitoes flightless [123]. This physical disability makes it difficult to fly, to find blood from human host, find a mate, and the mosquito easily becomes a prey for insectivores [123].
Studies have shown that genetic engineering can be used to modify the genetic features of mosquitoes to: resist viral infection; vaccinate humans against infection; and produce infertility in males [124]. However, studies describing ethical issues surrounding field trials of viral-resistant GMM deduced that for this technique to be rolled out, the disease of interest must pose a significant threat to public health in an area of isolation, as the greatest concern was for the protection of other community members who may be impacted but not enrolled in the study [125]. Additionally, the use of drives has interestingly increased the zeal for genetic control of mosquito vectors [126,127]. A study tested the first gene drives developed in Aedes aegypti mosquitoes. The authors confirmed that these drives, which are split so as to allow for drive safety performed excellently at very high frequency and also predicts that the split drives can be suitable for field trials to control local disease spread once the effectors are linked [126].
Another vector control technique that requires the introduction of a biological agent such as bacteria to control arthropod vectors is Wolbachia-based control [128,129]. Realistically, Wolbachia-based control is self-sustaining and the bacterium Wolbachia can be transmitted via transinfection to other insect species and is endosymbiotic in nature [130]. Although this strategy may require transinfection to successfully infect host vectors such as Aedes mosquitoes, it is not considered to be genetically modified because the Wolbachia bacterium is a natural endosymbiont that exists in most insect species [2].
5. Wolbachia Control Strategy
Wolbachia is an intracellular bacterium belonging to the Anaplasmataceae family [2]. This endosymbiotic bacterium naturally infects a wide range of invertebrate organisms such as arthropods and nematodes [130]. Wolbachia bacteria are found within the cytoplasm of the cells of their hosts, and they naturally exist in more than 50% of all insect species [131,132]. The Wolbachia endosymbiont is maternally (vertically) transmitted—the female Wolbachia-carrying arthropod passes the bacteria through the eggs to their offspring [133]. However, paternal (horizontal) transmission, which is very rare, has been observed in Drosophila simulans [134]. Paternal transmission can also occur under rare ecological circumstances such as the transmission of Wolbachia from infected to uninfected larvae of wasps sharing the same source of food [135]. While Wolbachia infection is not naturally present in all arbovirus-transmitting vectors such as Aedes aegypti, it can be introduced through stable transinfections of Wolbachia strains via microinjection [136]. The Wolbachia bacteria can be extracted from native hosts such as Aedes albopictus [137] and Drosophila melanogaster [133,138] and then injected into heterologous arthropods as with Aedes aegypti.
Most Wolbachia strains have derived their names from the host in which they were first discovered (Table 2). The first Wolbachia strain to be discovered was wPip (Wolbachia pipientis) found in Culex pipiens mosquitoes [139]. Other strains include: wMel found in Drosophila melanogaster (Fruit fly), wAlbA and wAlbB found in Aedes albopictus (Asian Tiger mosquito), and wAu found in Drosophila simulans [140]. The features of these Wolbachia strains may vary in their mosquito hosts due to high fitness cost and environmental factors such as high temperature (Table 2) [141,142,143].
Table 2.
Description of different Wolbachia strains, the arthropod (origin) in which they were found and the presence of the means of control of arboviral transmissions
| Wolbachia Strain | Origin | Means of Control of Arboviral Transmission (CI, MT, WIR, VB, F) |
References |
|---|---|---|---|
| wAu | Drosophila simulans/Fruit fly | (No, Yes, High, High, Partial) | [141,144] |
| wMel | Drosophila melanogaster/Fruit fly | (Yes, Yes, Low, Partial, Partial) | [31,133,145] |
| wAlbA | Aedes albopictus/Asian Tiger mosquito | (Yes, Yes, Medium, Partial, High) | [140,141] |
| wAlbB | Aedes albopictus/Asian Tiger mosquito | (Yes, Yes, Medium, High, Partial) | [129,141] |
| wMelPop | Drosophila melanogaster/Fruit fly | (Yes, Yes, Low, High, High) | [141,146,147] |
| wPip | Culex pipiens/Mosquito | (Yes, Yes, -, Low, Low) | [129,148,149] |
| wRi | Drosophila simulans (Riverside)/Fly | (Yes, Yes, -, Partial, Low) | [129,140] |
| wInn | Drosophila innubila/Vinegar fly | (Yes [only males], Yes, -, Partial, Low) | [129,150] |
CI—cytoplasmic incompatibility (Yes or No); MT—maternal transmission (Yes or No); WIR—Wolbachia infection retention (None, Low, Partial, High); VB—viral blockage (None, Low, Partial, High); and F—Fitness (None, Low, Partial, High). None—0, Low—<20%, medium—20%–90%, high—>90%.
The potential benefits of Wolbachia-based control techniques may be twofold: Wolbachia infection can disrupt arboviral replication and transmission; and the bacteria can also suppress vector populations [2,129,151].
5.1. Wolbachia-Based Disruption of Arboviral Transmission
The transinfection of Aedes aegypti with the endosymbiotic bacterium, Wolbachia could disrupt or inhibit arboviral transmission through four mechanisms [2]. The first is the competition for intracellular resource. Once present, Wolbachia bacteria can induce autophagy (cleaning or eating up damaged cells) in the arthropod’s cells [152]. To be able to survive, Wolbachia typically hijacks and regulates the autophagy system both within and outside the cell [153]. Similarly, arboviruses such as DENV and CHIKV rely on the autophagy system to replicate [154]. However, Wolbachia has the ability to manipulate the autophagy system set-up and interfere with some arboviral replications. This in turn, reduces the nutritional resources, such as cholesterol and iron, essential for viral growth [155]. Like Wolbachia, which is dependent on the arthropod cell cholesterol to multiply, Aedes-borne viruses such as DENV and CHIKV have been observed to manipulate the arthropod vector’s cell iron reserves [155]. In each event, both Wolbachia bacteria and arboviruses continually compete for limited host intracellular nutrients, resources, and space [156].
The second arboviral inhibitory mechanism is immune-priming. Immune-priming—also known as immune system preactivation—occurs when Wolbachia infection is transmitted into heterologous arthropods (i.e., non-native hosts of Wolbachia such as Aedes aegypti) via transinfection [157]. This mechanism preactivates the arthropod host immune system, which allows it to defend itself against arboviral pathogens [157]. According to a recent study, immune-priming can be induced by signalling pathways such as Immune deficiency (IMD), Toll and Janus kinase-signal transducer and activator of transcription (JAK-STAT) [2]. One study investigated the response of innate immune-priming in Aedes aegypti mosquitoes in the presence of Wolbachia-dengue interference [158]. It was shown that Wolbachia induced some immune genes involved in melanisation, Toll pathways genes and antimicrobial proteins such as peptides. The JAK-STAT pathway, which regulates the antiviral immunity and growth processes in arthropods has been shown to prevent DENV infection in Aedes aegypti mosquitoes [159]. An experimental study recently showed that immune-priming during the aquatic (larval) stage of Aedes aegypti mosquitoes with dormant DENV induced protection against the virus in the adult Aedes mosquitoes [160].
The third disruptive mechanism induces phenoloxidase (PO—an enzyme that increases the rate of phenol oxidation) cascade [161,162]. The importance of the PO cascade is that it produces melanin that accumulates around invading pathogens and at wound sites as this is known to have antipathogenic characteristics [162]. This cascade plays a critical role in the mosquito’s innate immune response to arboviruses. Studies have shown that Wolbachia bacteria increase melanization via the phenoloxidase activities in both homologous and heterologous arthropod vectors [161,162]. Therefore, a Wolbachia-induced phenoloxidase cascade may likely serve as protection against several arboviral infections [132].
The fourth mechanism is the miRNA-dependent immune pathway [163]. This pathway is an important component that modulates the arthropod hosts’ genes to control arboviral infection in many mosquito vectors [164]. Several miRNA-dependent immune responses and various metabolic processes needed for arboviral growth and replication are regulated in the presence of arboviral infections [165,166].
5.2. Wolbachia-Based Vector Population Suppression
The transinfection of Wolbachia into arthropod vectors such as Aedes mosquitoes may decrease their fitness, which in turn, leads to a reduction in the mosquito population [151]. One study previously reported that the introduction of a particular Wolbachia strain (wMelPop) into a mosquito could halve its life-span [167]. Another study conducted a survival experiment for three different Wolbachia-infected mosquito populations (wMel, wAlbB, wMelPop) and wild-type mosquitoes stratified by sex (male and female). They showed that for the females, all Wolbachia-infected mosquitoes had significantly higher mortality rates compared with their wild-type counterparts. Similar results were observed for males, except for wMel-infected mosquitoes whose lifespans did not differ significantly from the wild-type [151].
In practice, infecting Aedes mosquitoes with Wolbachia may also alter their reproductive lifecycle—potentially conferring Wolbachia-infected variants a competitive advantage over wild-type populations. One such mechanism is cytoplasmic incompatibility (CI) [129,168,169]. CI is a mechanism that induces incompatibility between the eggs and sperm of arthropods, in particular mosquitoes, enabling them to produce unviable offspring (no offspring) [170,171]. There are two types of CI: unidirectional and bidirectional CI. The former occurs when a Wolbachia infected male is crossed (mates) with an uninfected female mosquito (usually Wolbachia uninfected) and the resulting embryos are unable to mature into viable offspring [172,173]. However, the latter (bidirectional CI) describes the above inhibition mechanism but happening between crosses with infected mosquitoes with different strains of Wolbachia [174,175,176]. For example, the mating combination of a male and female mosquitoes infected with different Wolbachia strains are incompatible, thereby producing no viable offspring.
In general, the CI effect is not always dominant in all Wolbachia-infected arthropods as some Wolbachia strains do not exhibit this effect in some insect vectors. The CI effect may be fully present or absent depending on the Wolbachia strain and the arthropod host. For instance, in Aedes mosquitoes, studies have shown that the Wolbachia strains (wAlbA, wAlbB, wMel) exhibit complete CI while wAu does not [141,144]. Several studies, in the case of mosquitoes, have shown that CI fuels the persistence of Wolbachia-infected mosquito populations and also confers a reproductive advantage on Wolbachia-infected female mosquitoes over the uninfected ones [128,129,177,178,179]. This persistence phenomenon in the presence of CI occurs because all mating patterns except crosses between uninfected male and female mosquito lines, produce Wolbachia-infected offspring [129,141].
Other features of Wolbachia infection that may suppress the vector population, include imperfect maternal transmission (IMT) [133,169,180], loss of Wolbachia infection (LWI) due to unbearable conditions (such as high temperature) [179], and superinfection of two strains of Wolbachia (which can occur in Ae. albopictus hosts) [181]. IMT rates may vary for different Wolbachia strains depending on some abiotic conditions such as altitude (higher IMT at high altitude compared to lower altitude) [182] and environmental factors (very low IMT under laboratory conditions but high IMT in the field) [183]. However, a particularly novel strain of Wolbachia: wAu, does not possess the CI feature [141]. Despite the non-induction of CI, wAu has been shown to produce high viral blockage and maintain Wolbachia infection at higher temperatures while other strains do not [141].
The effects created by the transinfection of Wolbachia in arthropods, which, in particular, resulted in viral blockage [141,144] and population reduction in arthropod vectors [31], make it a promising control strategy for the reduction and elimination of Aedes-borne infections [2].
6. Wolbachia-Based Field and Experimental Studies
Recent field studies have reported that Wolbachia can be used to suppress vector-borne disease transmission [36,129,132,133,138,141,184,185,186]. These studies showed that suppression can be achieved by introducing a Wolbachia strain into wild mosquito populations in the hopes of replacing the vector transmitting agent Ae. aegypti with one that is incapable of transmission [36,133,138,187]. The use of Wolbachia strains to control Aedes-borne viral infections such as dengue is categorized into three strategies: (a) introduction of Wolbachia-infected male mosquitos together with uninfected female mosquitoes causing CI [188]; (b) invasion of a strain of Wolbachia generating fitness reduction in an area of varying seasonality [167,189], e.g., by halving the life-span of mosquitoes after the introduction of a Wolbachia strain; and (c) invasion of a strain of Wolbachia that inhibits transmission by reducing the ability of the virus-carrying vectors to transmit infections [133,138,186,187]. These control strategies, which are not mutually exclusive, have reportedly been effective in Australia, Indonesia, Brazil, and Vietnam, leading policy makers, including The WHO, to encourage the use of these strategies in controlling the spread of Aedes-borne viral infections [129,190,191].
Previously, a study investigated the introduction of wAlbB Wolbachia strain into transgenic Aedes aegypti mosquitoes [192]. The study showed that the wAlbB infection in mosquitoes activates both IMD and Toll pathways and infection is maintained through maternal transmission (MT) [192]. Another study also showed that Wolbachia boosts immune responses and increase mosquitoes’ resistance to viruses, which allows the immune system to actively fight against the viruses in the arthropod host [193]. In a study series of blood-feeding mosquito trials in response to the human host, it was shown that as mosquitoes infected with wMelPop-Wolbachia strain age, they feed less compared to their uninfected counterparts as a result of the observed bent proboscis. This defect may cause tissue damage in mosquitoes as they age leading to a decreased bite rate [194]. One study [133] described the successful transinfection of Aedes aegypti mosquitoes with a wMel-Wolbachia strain. It showed that this strain induces CI and high MT and also provides viral blockage of dengue serotype 2 infection transmission in Aedes aegypti mosquitoes.
Unlike other Wolbachia strains, the novel wAu strain displays some different characteristics in Aedes mosquitoes [141,144]. Some of the features include high retainment of wAu-Wolbachia infection at high temperatures and IMT [141]. In particular, this Wolbachia strain has been shown to be highly efficient in blocking arboviral transmission in Aedes aegypti [141] and Aedes albopictus [144] mosquitoes. However, the wAu strain does not induce CI [141,195] but may allow superinfection as bidirectional CI is ineffective in the presence of paternal transmission which itself is rare [134,141,144].
A study compared different Wolbachia strain features, such as high viral blockage and infection retention under high temperature, in transinfected Aedes aegypti mosquitoes [141]. The authors concluded that the wAu-Wolbachia strain was highly efficient in blocking DENV and ZIKV transmission and also provided more resilience to varying high temperature than the wMel strain [141]. A similar study conducted in Aedes albopictus also showed that the special triple strain line (the generation of wAlbA-wAlbB-wAu line) created via wAu transinfection was completely resistant to arboviral infections like dengue and ZIKV [144]. Therefore, the wAu strain is a potentially promising control candidate as it maintains high frequency at high temperature and allows Wolbachia co-infection [141,144]. To support this reasoning, modelling the transmission dynamics between different Wolbachia strains possessing different features could contribute to the global reduction and elimination of Aedes-borne arboviral diseases.
7. Previous Studies on Mathematical Models of Wolbachia
In recent years, human and animal invasions of new ecosystems, environmental degradation, global warming, and downward economic trends such as financial recession have given rise to various types of arboviral diseases. These trends not only exacerbate infectious disease transmission, but also reduce access to efficient therapy due to poorer treatment retention or poorer living circumstances during recession periods [196]. In response to disease emergence, many researchers, epidemiologists in particular, have formulated and analysed mathematical models to understand the dynamics of disease transmission and to identify useful solutions [197,198,199,200].
In general, the introduction of mathematical models to understand the infection dynamics of diseases has long been helpful in the area of disease control [201]. One of the applicable concepts of mathematical models is computing the basic reproduction number (R0). R0 is the expected number of secondary cases produced, in a completely susceptible population, by a typical infective individual throughout his/her entire infectious lifetime. R0 can be used as a threshold to determine disease persistence (R0 > 1) or extinction (R0 < 1) [202].
In the context of arboviral control, mathematical models have been formulated to study the population dynamics of Wolbachia-infected mosquitoes invading naïve mosquito populations [180,203,204,205,206,207,208,209,210]. Some of these models introduced Wolbacbia strain(s) into a mosquito population and classified them into age-structured Wolbachia-infected and uninfected mosquito compartments [180,205,206,209]. These models were constructed to accommodate the population progression from offspring maturing to adult mosquitoes and reproducing; and examine the effects of IMT and CI which may determine the status and production of offspring respectively following the adult mosquitoes’ mating. A study by Ndii et al. [205] formulated a mathematical model for the Wolbachia interaction between the immature stages (aquatic stage), and the adult male and female mosquito populations to determine the persistence of mosquitoes infected with Wolbachia when competing with the uninfected ones. To do this, the authors derived the steady state solutions of the model and showed that maternal transmission, death, maturation, and reproductive rates determine the dominance of Wolbachia-infected mosquitoes. In a similar study, Xue et al. [209] considered the Wolbachia-induced fitness change and the CI effect. They showed that if the basic reproduction number is less than one, at which the disease typically dies out, an endemic Wolbachia infection can still occur if a sufficient number of the mosquitoes are introduced into the population. This is caused by backward bifurcation, where stable disease free and endemic equilibria co-exist [211].
Modelling investigations that estimate the impact of Wolbachia introductions in arboviral-endemic countries are surging by the day, as these studies tend to give insights into the appropriate time-dependent strategy in deploying Wolbachia as a means of controlling Aedes-borne infections [34,35,36]. In their study, O’Relly et al. [36] combined multiple modelling methods to first estimate the burden of dengue disease across separate jurisdictions in Indonesia, and then forecast the change in dengue prevalence following a wide-scale Wolbachia release program. They predicted a dramatic reduction in dengue transmission after a nationwide release of the wMel Wolbachia strain. In particular, they estimated that there were approximately 7.8 million cases of symptomatic dengue in Indonesia in 2015 and attributed most of the gap in previous estimates of disease burden to underreporting (that is, asymptomatic and non-severe clinical cases that were challenging to diagnose in walk-in patients in hospitals or instances where patients did not go for treatment). The nationwide rollout of Wolbachia over the long term was estimated to avert 86.2% of these dengue cases [36].
A combined modelling-field study investigating the release of mosquitoes infected with the wAlbB strain was carried out in six different areas in Kuala Lumpur, the capital city of Malaysia [35]. The study showed that wAlbB-Wolbachia establishment was a success, maintained at high frequency in some sites and dominating at other sites following subsequent releases to overcome initial fluctuations.
Recently, one study modelled how the insecticide resistance of mosquitoes infected with Wolbachia could contribute to the local establishment of Wolbachia in a secluded area of Rio de Janeiro, Brazil, and validated the model results with experimental data [34]. After the release of two Aedes aegypti mosquito cohorts with different Wolbachia strains, wMelRio and wMelBr, the model clearly showed that wMelRio, which is resistant to pyrethroid pesticides, was able to establish while wMelBr, which is pyrethroid-susceptible, did not [34]. This implies that Wolbachia-infected mosquitoes resistant to pesticides may drive and establish Wolbachia infections in wild-type mosquito populations more readily than their pesticide-susceptible counterparts.
Another Wolbachia invasion model incorporated IMT and the loss of Wolbachia infection and showed that CI alone does not guarantee the establishment of Wolbachia-infected mosquitoes as IMT and Wolbachia loss could be more deleterious than CI is advantageous [180]. In effect, CI is not enough for Wolbachia-infected mosquitoes to dominate as both their intrinsic fitness and the possibility of mixed offspring play a critical role. Hence, we are interested in understanding how different features of Wolbachia infection, such as non-induction of CI, the high maintenance of the Wolbachia infection at high temperature, and the superinfection with different Wolbachia strains (wAu and wMel) in mosquitoes could drive a reduction in arboviral transmission.
8. Literature Gap, Future Research, and Conclusion
Aedes-borne arboviral infections continue to be a public health problem globally [2,7,212,213,214,215]. Various control mechanisms for these viral infections are targeted at either suppressing the population of the virus-carrying vectors or inhibiting the viral replication in the vector hosts thereby hampering transmission [2]. Herein, we have typically described these control methods as: human host; human-vector interactions; and vector-focused (Figure 2). Of these control measures, the vector control methods, including environmental; chemical; and biological approaches, are the most widely used. Furthermore, this review highlights the importance of biological methods, specifically Wolbachia-based methods, in controlling Aedes-borne viral transmission.
The intracellular bacterium Wolbachia has been shown to reduce Aedes-borne viral infections such as DENV, ZIKV, CHIKV, and YFV in their endemic regions [129,133,138,141,145,186,194]. Although promising, the Wolbachia control strategy is not guaranteed to succeed as it faces the challenge of degrading potency at unfavourable weather conditions, among other limiting factors [179,216]. However, a novel Wolbachia strain, wAu, does not induce CI [141] yet is maintained even at high temperatures. This strain has been shown to produce high viral blockage, and induces stable superinfection when combined with other Wolbachia strains such as wAlbB in the vector host [141].
To better understand the dynamics of Aedes-borne viral infection both in human and vector hosts, there is a need to investigate the strategies of introducing Wolbachia-infected mosquitoes to control arboviral infection transmission. This can be done by formulating and analyzing mathematical models of different Wolbachia strains to capture the various important infection-driven features and validate these models using experimental data.
The research gaps identified in this review are: no modelling work on the combined three-vector control methods and no introduction of two Wolbachia strains with different characteristics such as the novel strain wAu-Wolbachia infected mosquitoes and its combination with other Wolbachia strains to quantify arboviral infection burden and control, have yet been performed. Therefore, in this review, we focus on the vector control methods together with different strains of Wolbachia-based control. Apart from greatly controlling virus proliferation in the midgut of Wolbachia-infected mosquitoes, the CI-absent Wolbachia strain, when subjected to high temperature is being retained in mosquitoes. This could be a successful strategy towards eliminating Aedes-borne infections. Hence, the need for in-depth insight and understanding of the different Wolbachia mosquito infection and superinfection dynamics and its impact when introduced into a mixed mosquito and human populations in arboviral endemic regions is sought in this regard.
Therefore, future work will include developing and comparing models for vector control methods incorporating the chemical, biological, and environmental control methods and comparing interventions. This would give great insights as it may require combining strategies such as outdoor fogging or use of chemical larvicides, educational campaigns to ensure clean drainages and covering of waterlogged containers, and sterile insect release or Wolbachia-infected mosquito rollout. In addition, the development of Wolbachia transmission models that describe the competitive dynamics between Wolbachia-infected and uninfected mosquitoes with different characteristics. It will also investigate the impact of releasing CI-absent Wolbachia-infected mosquitoes and its combination with other CI-present Wolbachia-infected mosquitoes in a human population infected with dengue and explore how single or combined strategies will impact on disease dynamics, in particular, the effectiveness of Wolbachia introduction in dengue endemic areas. These investigations will reveal the interactions between the different characteristics of Wolbachia-infected mosquitoes and dengue virus serotypes in the human host. These revelations will further contribute to the global effort to reduce or eliminate arboviral transmission.
Acknowledgments
The authors of this paper gratefully acknowledge the James Cook University Postgraduate Research Scholarship (JCUPRS), Queensland, Australia, awarded to STO (PhD scholarship), College of Medicine and Dentistry, College of Public Health, Medical and Veterinary Sciences and Australian Institute of Tropical Health and Medicine (AITHM), James Cook University, Australia.
Author Contributions
Conceptualization, S.T.O. and E.S.M.; Methodology, S.T.O., E.S.M., M.T.M., A.I.A., and D.P.R.; Software, S.T.O., E.S.M., M.T.M., and A.I.A.; Validation, S.T.O., E.S.M., M.T.M., A.I.A., and D.P.R.; Investigation, S.T.O.; Resources, S.T.O., E.S.M., M.T.M., A.I.A., D.P.R., and O.A.A.; Data curation, writing—original draft preparation, S.T.O.; Writing—reviewing and editing, S.T.O., E.S.M., M.T.M., A.I.A., D.P.R., and O.A.A.; Supervision, E.S.M., M.T.M., A.I.A., and D.P.R.; Project administration, E.S.M.; Funding acquisition, E.S.M. All authors have read and agreed to the published version of the manuscript.
Funding
M.T.M. is funded by a postdoctoral research fellowship from the National Health and Medical Research Council’s Centre of Research Excellence in Tuberculosis Control on Both Sides of Our Border (Project ID: 1153493).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hanley K.A. Origin and Evolution of Viruses. Elsevier; Amsterdam, The Netherlands: 1998. pp. 351–391. [Google Scholar]
- 2.Kamtchum-Tatuene J., Makepeace B.L., Benjamin L., Baylis M., Solomon T. The potential role of Wolbachia in controlling the transmission of emerging human arboviral infections. Curr. Opin. Infect. Dis. 2017;30:108–116. doi: 10.1097/QCO.0000000000000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costard S., Mur L., Lubroth J., Sanchez-Vizcaino J., Pfeiffer D. Epidemiology of African swine fever virus. Virus Res. 2013;173:191–197. doi: 10.1016/j.virusres.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Wasay M., Khatri I.A., Abd-Allah F. Arbovirus infections of the nervous system: Current trends and future threats. Neurology. 2015;84:421–423. doi: 10.1212/WNL.0000000000001177. [DOI] [PubMed] [Google Scholar]
- 5.Guzman M.G., Harris E. Dengue. Lancet. 2015;385:453–465. doi: 10.1016/S0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- 6.Simmons C.P., Farrar J.J., Chau N.V.V., Wills B. Dengue. N. Engl. J. Med. 2012;366:1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 7.Charlier C., Beaudoin M.-C., Couderc T., Lortholary O., Lecuit M. Arboviruses and pregnancy: Maternal, fetal, and neonatal effects. Lancet Child. Adolesc. Heal. 2017;1:134–146. doi: 10.1016/S2352-4642(17)30021-4. [DOI] [PubMed] [Google Scholar]
- 8.Bergman A., Dahl E., Lundkvist Å., Hesson J.C. Sindbis Virus Infection in Non-Blood-Fed Hibernating Culex pipiens Mosquitoes in Sweden. Viruses. 2020;12:1441. doi: 10.3390/v12121441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snyder R.E., Feiszli T., Foss L., Messenger S., Fang Y., Barker C.M., Reisen W.K., Vugia D.J., Padgett K.A., Kramer V.L. West Nile virus in California, 2003–2018: A persistent threat. PLoS Negl. Trop. Dis. 2020;14:e0008841. doi: 10.1371/journal.pntd.0008841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiuya T., Masiga D.K., Falzon L.C., Bastos A.D., Fèvre E.M., Villinger J. Tick-Borne pathogens, including Crimean-Congo haemorrhagic fever virus, at livestock markets and slaughterhouses in western Kenya. Transbound. Emerg. Dis. 2020 doi: 10.1111/tbed.13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deviatkin A.A., Kholodilov I.S., Vakulenko Y.A., Karganova G.G., Lukashev A.N. Tick-Borne Encephalitis Virus: An Emerging Ancient Zoonosis? Viruses. 2020;12:247. doi: 10.3390/v12020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayhan N., Charrel R.N. An update on Toscana virus distribution, genetics, medical and diagnostic aspects. Clin. Microbiol. Infect. 2020;26:1017–1023. doi: 10.1016/j.cmi.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Ciota A.T., Kramer L.D. Insights into Arbovirus Evolution and Adaptation from Experimental Studies. Viruses. 2010;2:2594–2617. doi: 10.3390/v2122594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould E., Pettersson J., Higgs S., Charrel R., Lamballerie X.d. Emerging arboviruses: Why today? One Health. 2017;4:1–13. doi: 10.1016/j.onehlt.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mavian C., Dulcey M., Munoz O., Salemi M., Vittor A.Y., Capua I. Islands as Hotspots for Emerging Mosquito-Borne Viruses: A One-Health Perspective. Viruses. 2018;11:11. doi: 10.3390/v11010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M., Brownstein J.S., Hoen A.G., Sankoh O., et al. The global distribution and burden of dengue. Nat. Cell Biol. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Y.P., Luo Y., Chen Y., Lamers M.M., Zhou Q., Yang X.H., Sanyal S., Mok C.K.P., Liu Z.M. Clinical and epidemiological features of the 2014 large-Scale dengue outbreak in Guangzhou city, China. BMC Infect. Dis. 2016;16:1–8. doi: 10.1186/s12879-016-1379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messina J.P., Brady O.J., Golding N., Kraemer M.U.G., Wint G.R.W., Ray S.E., Pigott D.M., Shearer F.M., Johnson K., Earl L., et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019;4:1508–1515. doi: 10.1038/s41564-019-0476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Z., Bambrick H., Frentiu F.D., Devine G., Yakob L., Williams G., Hu W. Projecting the future of dengue under climate change scenarios: Progress, uncertainties and research needs. PLoS Negl. Trop. Dis. 2020;14:e0008118. doi: 10.1371/journal.pntd.0008118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckham J.D., Tyler K.L. Arbovirus Infections. Continu. (Minneap. Minn) 2015;21:1599–1611. doi: 10.1212/CON.0000000000000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weaver S.C., Reisen W.K. Present and future arboviral threats. Antivir. Res. 2010;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rust R.S. Human Arboviral Encephalitis. Semin. Pediatr. Neurol. 2012;19:130–151. doi: 10.1016/j.spen.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Wieten R.W., Goorhuis A., Jonker E.F.F., De Bree G.J., Visser A.W.d., Van Genderen P.J.J., Remmerswaal E.B.M., Berge I.J.M.T., Visser L.G., Grobusch M.P., et al. 17D yellow fever vaccine elicits comparable long-Term immune responses in healthy individuals and immune-Compromised patients. J. Infect. 2016;72:713–722. doi: 10.1016/j.jinf.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Chen H.-L., Chang J.-K., Tang R.-B. Current recommendations for the Japanese encephalitis vaccine. J. Chin. Med. Assoc. 2015;78:271–275. doi: 10.1016/j.jcma.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Scott L.J. Tetravalent Dengue Vaccine: A Review in the Prevention of Dengue Disease. Drugs. 2016;76:1301–1312. doi: 10.1007/s40265-016-0626-8. [DOI] [PubMed] [Google Scholar]
- 26.Marston H.D., Lurie N., Borio L.L., Fauci A.S. Considerations for Developing a Zika Virus Vaccine. N. Engl. J. Med. 2016;375:1209–1212. doi: 10.1056/NEJMp1607762. [DOI] [PubMed] [Google Scholar]
- 27.Brandler S., Tangy F. Vaccines in Development against West Nile Virus. Viruses. 2013;5:2384–2409. doi: 10.3390/v5102384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wressnigg N., Van Der Velden M.V.W., Portsmouth D., Draxler W., O’Rourke M., Richmond P., Hall S., McBride W.J.H., Redfern A., Aaskov J., et al. An Inactivated Ross River Virus Vaccine Is Well Tolerated and Immunogenic in an Adult Population in a Randomized Phase 3 Trial. Clin. Vaccine Immunol. 2015;22:267–273. doi: 10.1128/CVI.00546-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia A., Diego L., Judith B. New Approaches to Chikungunya Virus Vaccine Development. Recent Pat. Inflamm. Allergy Drug Discov. 2015;9:31–37. doi: 10.2174/1872213X09666150223114226. [DOI] [PubMed] [Google Scholar]
- 30.Buhler C., Winkler V., Runge-Ranzinger S., Boyce R., Horstick O. Environmental methods for dengue vector control–A systematic review and meta-Analysis. PLoS Negl. Trop. Dis. 2019;13:e0007420. doi: 10.1371/journal.pntd.0007420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blagrove M.S.C., Arias-Goeta C., Failloux A.-B., Sinkins S.P. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc. Natl. Acad. Sci. USA. 2011;109:255–260. doi: 10.1073/pnas.1112021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crain P.R., Mains J.W., Suh E., Huang Y., Crowley P.H., Dobson S.L. Wolbachia infections that reduce immature insect survival: Predicted impacts on population replacement. BMC Evol. Biol. 2011;11:290. doi: 10.1186/1471-2148-11-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joubert D.A., Walker T., Carrington L.B., De Bruyne J.T., Kien D.H.T., Hoang N.L.T., Chau N.V.V., Iturbe-Ormaetxe I., Simmons C.P., O’Neill S.L. Establishment of a Wolbachia Superinfection in Aedes aegypti Mosquitoes as a Potential Approach for Future Resistance Management. PLoS Pathog. 2016;12:e1005434. doi: 10.1371/journal.ppat.1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia G.A., Hoffmann A.A., Maciel-De-Freitas R., Villela D.A.M. Aedes aegypti insecticide resistance underlies the success (and failure) of Wolbachia population replacement. Sci. Rep. 2020;10:1–9. doi: 10.1038/s41598-019-56766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nazni W.A., Hoffmann A.A., NoorAfizah A., Cheong Y.L., Mancini M.V., Golding N., Kamarul G.M.R., Arif M.A.K., Thohir H., NurSyamimi H., et al. Establishment of Wolbachia Strain wAlbB in Malaysian Populations of Aedes aegypti for Dengue Control. Curr. Biol. 2019;29:4241–4248. doi: 10.1016/j.cub.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Reilly K.M., Hendrickx E., Kharisma D.D., Wilastonegoro N.N., Carrington L.B., Elyazar I.R.F., Kucharski A.J., Lowe R., Flasche S., Pigott D.M., et al. Estimating the burden of dengue and the impact of release of wMel Wolbachia-Infected mosquitoes in Indonesia: A modelling study. BMC Med. 2019;17:1–14. doi: 10.1186/s12916-019-1396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lowe R., Lee S., Lana R.M., Codeço C.T., Castro M.C., Pascual M. Emerging arboviruses in the urbanized Amazon rainforest. BMJ. 2020;371:m4385. doi: 10.1136/bmj.m4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madewell Z.J. Arboviruses and Their Vectors. South Med. J. 2020;113:520–523. doi: 10.14423/SMJ.0000000000001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Causa R., Ochoa-Diaz-Lopez H., Dor A., Rodríguez-León F., Solís-Hernández R., Pacheco-Soriano A.L. Emerging arboviruses (dengue, chikungunya, and Zika) in Southeastern Mexico: Influence of socio-Environmental determinants on knowledge and practices. Cad. Saúde Pública. 2020;36:e00110519. doi: 10.1590/0102-311x00110519. [DOI] [PubMed] [Google Scholar]
- 40.Silva P.A.N.d., Ito C.R.M., Barbosa M.S., Santos M.d.O., Carneiro L.C. Arboviruses (chikungunya, dengue, and Zika) associated with ophthalmic changes: A focus on aqueous fluid and vitreous humor. Eur. J. Clin. Microbiol. Infect. Dis. 2019;39:827–833. doi: 10.1007/s10096-019-03792-9. [DOI] [PubMed] [Google Scholar]
- 41.Kularatne S. Dengue fever. BMJ. 2015;351:h4661. doi: 10.1136/bmj.h4661. [DOI] [PubMed] [Google Scholar]
- 42.Chan M., Johansson M.A. The Incubation Periods of Dengue Viruses. PLoS ONE. 2012;7:e50972. doi: 10.1371/journal.pone.0050972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brady O.J., Gething P.W., Bhatt S., Messina J.P., Brownstein J.S., Hoen A.G., Moyes C.L., Farlow A.W., Scott T.W., Hay S.I. Refining the Global Spatial Limits of Dengue Virus Transmission by Evidence-Based Consensus. PLoS Negl. Trop. Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dick G.W.A., Kitchen S.F., Haddow A.J. Zika Virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 45.Bogoch I.I., Brady O.J., Kraemer M.U.G., German M.J., Creatore M.I., Kulkarni M.A., Brownstein J.S., Mekaru S.R., Hay S.I., Groot E., et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387:335–336. doi: 10.1016/S0140-6736(16)00080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guerbois M., Fernandez-Salas I., Azar S.R., Danis-Lozano R., Alpuche-Aranda C.M., Leal G., Garcia-Malo I.R., Diaz-Gonzalez E.E., Casas-Martinez M., Rossi S.L., et al. Outbreak of Zika Virus Infection, Chiapas State, Mexico, 2015, and First Confirmed Transmission byAedes aegyptiMosquitoes in the Americas. J. Infect. Dis. 2016;214:1349–1356. doi: 10.1093/infdis/jiw302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamel R., Liégeois F., Wichit S., Pompon J., Diop F., Talignani L., Thomas F., Després P., Yssel H., Missé D. Zika virus: Epidemiology, clinical features and host-Virus interactions. Microbes Infect. 2016;18:441–449. doi: 10.1016/j.micinf.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Musso D., Nhan T., Robin E., Roche C., Bierlaire D., Zisou K., Yan A.S., Cao-Lormeau V.M., Broult J. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Eurosurveillance. 2014;19:20761. doi: 10.2807/1560-7917.ES2014.19.14.20761. [DOI] [PubMed] [Google Scholar]
- 49.Brooks J.T., Friedman A., Kachur R.E., LaFlam M., Peters P.J., Jamieson D.J. Update: Interim Guidance for Prevention of Sexual Transmission of Zika Virus-United States. Morb. Mortal. Wkly. Rep. 2016;65:745–747. doi: 10.15585/mmwr.mm6529e2. [DOI] [PubMed] [Google Scholar]
- 50.Motta I.J., Spencer B.R., Silva S.G.C.d., Arruda M.B., Dobbin J.A., Gonzaga Y.B., Arcuri I.P., Tavares R.C., Atta E.H., Fernandes R.F., et al. Evidence for Transmission of Zika Virus by Platelet Transfusion. N. Engl. J. Med. 2016;375:1101–1103. doi: 10.1056/NEJMc1607262. [DOI] [PubMed] [Google Scholar]
- 51.Brady O.J., Osgood-Zimmerman A., Kassebaum N.J., Ray S.E., De Araújo V.E.M., Da Nóbrega A.A., Frutuoso L.C.V., Lecca R.C.R., Stevens A., De Oliveira B.Z., et al. The association between Zika virus infection and microcephaly in Brazil 2015–2017: An observational analysis of over 4 million births. PLoS Med. 2019;16:e1002755. doi: 10.1371/journal.pmed.1002755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.WHO . Fact Sheets: Chikungunya. World Health Organization; 2017. [(accessed on 15 June 2020)]. Available online: https://www.who.int/news-room/fact-sheets/detail/chikungunya. [Google Scholar]
- 53.Parola P., De Lamballerie X., Jourdan J., Rovery C., Vaillant V., Minodier P., Brouqui P., Flahault A., Raoult D., Charrel R.N. Novel Chikungunya Virus Variant in Travelers Returning from Indian Ocean Islands. Emerg. Infect. Dis. 2006;12:1493–1499. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Couderc T., Gangneux N., Caro V., Ducloux B., Tolou H., Chrétien F., Luong T.L., Lecuit M., Grandadam M. Chikungunya Virus Infection of Corneal Grafts. J. Infect. Dis. 2012;206:851–859. doi: 10.1093/infdis/jis296. [DOI] [PubMed] [Google Scholar]
- 55.Lenglet Y., Barau G., Robillard P.Y., Randrianaivo H., Michault A., Bouveret A., Gerardin P., Boumahni B., Touret Y., Kauffmann E., et al. Chikungunya infection in pregnancy: Evidence for intrauterine infection in pregnant women and vertical transmission in the parturient. Survey of the Reunion Island outbreak. J. Gynecol. Obstet. Biol. Rep. (Paris) 2006;35:578–583. doi: 10.1016/S0368-2315(06)76447-X. [DOI] [PubMed] [Google Scholar]
- 56.Mutebi J.-P., Wang H., Li L., Bryant J.E., Barrett A.D.T. Phylogenetic and Evolutionary Relationships among Yellow Fever Virus Isolates in Africa. J. Virol. 2001;75:6999–7008. doi: 10.1128/JVI.75.15.6999-7008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathai D., Vasanthan A.G. State of the globe: Yellow fever is still around and active! J. Glob. Infect. Dis. 2009;1:4–6. doi: 10.4103/0974-777X.52975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rogers D., Wilson A.J., Hay S.I., Graham A. The Global Distribution of Yellow Fever and Dengue. Adv. Parasitol. 2006;62:181–220. doi: 10.1016/s0065-308x(05)62006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bae H.-G., Drosten C., Emmerich P., Colebunders R., Hantson P., Pest S., Parent M., Schmitz H., Warnat M.-A., Niedrig M. Analysis of two imported cases of yellow fever infection from Ivory Coast and The Gambia to Germany and Belgium. J. Clin. Virol. 2005;33:274–280. doi: 10.1016/j.jcv.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Xiao S.-Y., Zhang H., Guzman H., Tesh R.B. Experimental Yellow Fever Virus Infection in the Golden Hamster (Mesocricetus auratus). II. Pathology. J. Infect. Dis. 2001;183:1437–1444. doi: 10.1086/320200. [DOI] [PubMed] [Google Scholar]
- 61.Tesh R., Guzman H., Rosa A.P.A.T.d., Vasconcelos P.F.C., Dias L.B., Bunnell J.E., Zhang H., Xiao S.-Y. Experimental Yellow Fever Virus Infection in the Golden Hamster (Mesocricetus auratus). I. Virologic, Biochemical, and Immunologic Studies. J. Infect. Dis. 2001;183:1431–1436. doi: 10.1086/320199. [DOI] [PubMed] [Google Scholar]
- 62.Barba-Spaeth G., Dejnirattisai W., Rouvinski A., Vaney M.-C., Medits I., Sharma A., Simon-Lorière E., Sakuntabhai A., Cao-Lormeau V.-M., Haouz A., et al. Structural basis of potent Zika–Dengue virus antibody cross-Neutralization. Nat. Cell Biol. 2016;536:48–53. doi: 10.1038/nature18938. [DOI] [PubMed] [Google Scholar]
- 63.Kulkarni R. Dynamics of Immune Activation in Viral Diseases. Springer; Singapore: 2020. Antibody-Dependent Enhancement of Viral Infections; pp. 9–41. [DOI] [Google Scholar]
- 64.Smatti M.K., Al Thani A.A., Yassine H.M. Viral-Induced Enhanced Disease Illness. Front. Microbiol. 2018;9:2991. doi: 10.3389/fmicb.2018.02991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fukusumi M., Arashiro T., Arima Y., Matsui T., Shimada T., Kinoshita H., Arashiro A., Takasaki T., Sunagawa T., Oishi K. Dengue Sentinel Traveler Surveillance: Monthly and Yearly Notification Trends among Japanese Travelers, 2006–2014. PLoS Negl. Trop. Dis. 2016;10:e0004924. doi: 10.1371/journal.pntd.0004924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aguiar M., Stollenwerk N., Halstead S.B. The Impact of the Newly Licensed Dengue Vaccine in Endemic Countries. PLoS Negl. Trop. Dis. 2016;10:e0005179. doi: 10.1371/journal.pntd.0005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Priyamvada L., Hudson W., Ahmed R., Wrammert J. Humoral cross-Reactivity between Zika and dengue viruses: Implications for protection and pathology. Emerg. Microbes Infect. 2017;6:1–6. doi: 10.1038/emi.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rudolph K.E., Moloney R.M., Kmush B., Cummings D.A.T., Lessler J. Incubation Periods of Mosquito-Borne Viral Infections: A Systematic Review. Am. J. Trop. Med. Hyg. 2014;90:882–891. doi: 10.4269/ajtmh.13-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schaefer T.J., Panda P.K., Wolford R.W. Dengue Fever. StatPearls Publishing; Treasure Island, FL, USA: 2019. [PubMed] [Google Scholar]
- 70.Goh G.K.-M., Dunker A.K., Foster J.A., Uversky V.N. Zika and Flavivirus Shell Disorder: Virulence and Fetal Morbidity. Biomolecules. 2019;9:710. doi: 10.3390/biom9110710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brasil P., Calvet G.A., Siqueira A.M., Wakimoto M., Sequeira P.C.d., Nobre A., Quintana M.D.S.B., Mendonça M.C.L.d., Lupi O., Souza R.V.d., et al. Zika Virus Outbreak in Rio de Janeiro, Brazil: Clinical Characterization, Epidemiological and Virological Aspects. PLoS Negl. Trop. Dis. 2016;10:e0004636. doi: 10.1371/journal.pntd.0004636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen L.H. Zika Virus Infection in a Massachusetts Resident After Travel to Costa Rica: A Case Report. Ann. Intern. Med. 2016;164:574–576. doi: 10.7326/L16-0075. [DOI] [PubMed] [Google Scholar]
- 73.CDC Centers for Disease Control and Prevention: Zika Virus, Symptoms Testing & Treatment. [(accessed on 21 October 2019)];2019 Available online: https://www.cdc.gov/zika/symptoms/treatment.html.
- 74.Waggoner J.J., Gresh L., Vargas M.J., Ballesteros G., Tellez Y., Soda K.J., Sahoo M.K., Nuñez A., Balmaseda A., Harris E., et al. Viremia and Clinical Presentation in Nicaraguan Patients Infected with Zika Virus, Chikungunya Virus, and Dengue Virus. Clin. Infect. Dis. 2016;63:1584–1590. doi: 10.1093/cid/ciw589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fourié T., Grard G., Leparc-Goffart I., Briolant S., Fontaine A. Variability of Zika Virus Incubation Period in Humans. Open Forum Infect. Dis. 2018;5:261. doi: 10.1093/ofid/ofy261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krow-Lucal E.R., Biggerstaff B.J., Staples J.E. Estimated Incubation Period for Zika Virus Disease. Emerg. Infect. Dis. 2017;23:841–845. doi: 10.3201/eid2305.161715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winokur O.C., Main B.J., Nicholson J., Barker C.M. Impact of temperature on the extrinsic incubation period of Zika virus in Aedes aegypti. PLoS Negl. Trop. Dis. 2020;14:e0008047. doi: 10.1371/journal.pntd.0008047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duffy M.R., Chen T.-H., Hancock W.T., Powers A.M., Kool J.L., Lanciotti R.S., Pretrick M., Marfel M., Holzbauer S., DuBray C., et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 79.Ganesan V.K., Duan B., Reid S.P. Chikungunya Virus: Pathophysiology, Mechanism, and Modeling. Viruses. 2017;9:368. doi: 10.3390/v9120368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weaver S.C., Lecuit M. Chikungunya Virus and the Global Spread of a Mosquito-Borne Disease. N. Engl. J. Med. 2015;372:1231–1239. doi: 10.1056/NEJMra1406035. [DOI] [PubMed] [Google Scholar]
- 81.Javelle E., Tiong T.H., Leparc-Goffart I., Savini H., Simon F. Inflammation of the external ear in acute chikungunya infection: Experience from the outbreak in Johor Bahru, Malaysia, 2008. J. Clin. Virol. 2014;59:270–273. doi: 10.1016/j.jcv.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 82.Miner J.J., Yeang H.X.A., Fox J.M., Taffner S., Malkova O.N., Oh S.T., Kim A.H.J., Diamond M.S., Lenschow D.J., Yokoyama W.M. Brief Report: Chikungunya Viral Arthritis in the United States: A Mimic of Seronegative Rheumatoid Arthritis. Arthritis Rheumatol. 2015;67:1214–1220. doi: 10.1002/art.39027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nayar S.K., Noridah O., Paranthaman V., Ranjit K., Norizah I., Chem Y.K., Mustafa B., Chua K.B. Co-Infection of dengue virus and chikungunya virus in two patients with acute febrile illness. Med. J. Malays. 2007;62:335–336. [PubMed] [Google Scholar]
- 84.Dubrulle M., Mousson L., Moutailler S., Vazeille M., Failloux A.-B. Chikungunya Virus and Aedes Mosquitoes: Saliva Is Infectious as soon as Two Days after Oral Infection. PLoS ONE. 2009;4:e5895. doi: 10.1371/journal.pone.0005895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakkhara P., Chongsuvivatwong V., Thammapalo S. Risk factors for symptomatic and asymptomatic chikungunya infection. Trans. R. Soc. Trop. Med. Hyg. 2013;107:789–796. doi: 10.1093/trstmh/trt083. [DOI] [PubMed] [Google Scholar]
- 86.Kamgang B., Vazeille M., Yougang A.P., Tedjou A.N., Wilson-Bahun T.A., Mousson L., Wondji C.S., Failloux A.-B. Potential of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) to transmit yellow fever virus in urban areas in Central Africa. Emerg. Microbes Infect. 2019;8:1636–1641. doi: 10.1080/22221751.2019.1688097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barnett E.D. Yellow Fever: Epidemiology and Prevention. Clin. Infect. Dis. 2007;44:850–856. doi: 10.1086/511869. [DOI] [PubMed] [Google Scholar]
- 88.Klitting R., Gould E.A., Paupy C., Lamballerie X.d. What Does the Future Hold for Yellow Fever Virus? (I) Genes. 2018;9:291. doi: 10.3390/genes9060291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Melo A.B.d., Silva M.D.P.C.D., Bertani G.R., Gil L.H.V.G., Magalhães M.C.F., Cordeiro M.T., Marques E.T.A., Carvalho E.M.F.d., Braga-Neto U.M. Description of a Prospective 17DD Yellow Fever Vaccine Cohort in Recife, Brazil. Am. J. Trop. Med. Hyg. 2011;85:739–747. doi: 10.4269/ajtmh.2011.10-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sbrana E., Xiao S.Y., Guzman H., Ye M., Rosa A.P.T.d., Tesh R.B. Efficacy of post-Exposure treatment of yel-Low fever with ribavirin in a hamster model of the disease. Am. J. Trop Med. Hyg. 2004;71:306–312. doi: 10.4269/ajtmh.2004.71.306. [DOI] [PubMed] [Google Scholar]
- 91.Gould L.H., Osman M.S., Farnon E.C., Griffith K.S., Godsey M.S., Karch S., Mulenda B., Kholy A.E., Grandesso F., Radiguès X.d., et al. An outbreak of yellow fever with concurrent chikungunya virus transmission in South Kordofan, Sudan, 2005. Trans. R. Soc. Trop. Med. Hyg. 2008;102:1247–1254. doi: 10.1016/j.trstmh.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 92.Johansson M.A., Biggerstaff B.J., Arana-Vizcarrondo N., Staples J.E. Incubation Periods of Yellow Fever Virus. Am. J. Trop. Med. Hyg. 2010;83:183–188. doi: 10.4269/ajtmh.2010.09-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johansson M.A., Vasconcelos P.F.C., Staples J.E. The whole iceberg: Estimating the incidence of yellow fever virus infection from the number of severe cases. Trans. R. Soc. Trop. Med. Hyg. 2014;108:482–487. doi: 10.1093/trstmh/tru092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roiz D., Wilson A.L., Scott T.W., Fonseca D.M., Jourdain F., Müller P., Velayudhan R., Corbel V. Integrated Aedes management for the control of Aedes-borne diseases. PLoS Negl. Trop. Dis. 2018;12:e0006845. doi: 10.1371/journal.pntd.0006845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rather I.A., Parray H.A., Lone J.B., Paek W.K., Lim J., Bajpai V.K., Park Y.-H. Prevention and Control Strategies to Counter Dengue Virus Infection. Front. Cell. Infect. Microbiol. 2017;7:336. doi: 10.3389/fcimb.2017.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Whitehorn J., Yacoub S., Anders K.L., Macareo L.R., Cassetti M.C., Van V.C.N., Shi P.-Y., Wills B., Simmons C.P. Dengue Therapeutics, Chemoprophylaxis, and Allied Tools: State of the Art and Future Directions. PLoS Negl. Trop. Dis. 2014;8:e3025. doi: 10.1371/journal.pntd.0003025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hladish T.J., Pearson C.A.B., Ben Toh K., Rojas D.P., Manrique-Saide P., Vazquez-Prokopec G.M., Halloran M.E., Longini I.M. Designing effective control of dengue with combined interventions. Proc. Natl. Acad. Sci. USA. 2020;117:3319–3325. doi: 10.1073/pnas.1903496117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilder-Smith A., Flasche S., Smith P.G. Vaccine-attributable severe dengue in the Philippines. Lancet. 2019;394:2151–2152. doi: 10.1016/S0140-6736(19)32525-5. [DOI] [PubMed] [Google Scholar]
- 99.Heydari N., Larsen D.A., Neira M., Ayala E.B., Fernandez P., Adrian J., Rochford R., Stewart-Ibarra A.M. Household Dengue Prevention Interventions, Expenditures, and Barriers to Aedes aegypti Control in Machala, Ecuador. Int. J. Environ. Res. Public Heal. 2017;14:196. doi: 10.3390/ijerph14020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen L.H., Kozarsky P.E., Freedman D.O. Medical Considerations before International Travel. N. Engl. J. Med. 2016;375:e32. doi: 10.1056/nejmc1610671. [DOI] [PubMed] [Google Scholar]
- 101.Rapley L.P., Russell R.C., Montgomery B.L., Ritchie S.A. The Effects of Sustained Release Metofluthrin on the Biting, Movement, and Mortality of Aedes aegypti in a Domestic Setting. Am. J. Trop. Med. Hyg. 2009;81:94–99. doi: 10.4269/ajtmh.2009.81.94. [DOI] [PubMed] [Google Scholar]
- 102.Achee N.L., Gould F., Perkins T.A., Reiner R.C., Jr., Morrison A.C., Ritchie S.A., Gubler D.J., Teyssou R., Scott T.W. A Critical Assessment of Vector Control for Dengue Prevention. PLoS Negl. Trop. Dis. 2015;9:e0003655. doi: 10.1371/journal.pntd.0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hunter P. Challenges and options for disease vector control: The outbreak of Zika virus in South America and increasing insecticide resistance among mosquitoes have rekindled efforts for controlling disease vectors. EMBO Rep. 2016;17:1370–1373. doi: 10.15252/embr.201643233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Horstick O., Boyce R., Runge-Ranzinger S. Dengue vector control: Assessing what works? Southeast Asian J. Trop Med. Public Health. 2017;48:181–195. [Google Scholar]
- 105.Flores H., Neill S.L.O. Controlling vector-Borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol. 2018;16:508–518. doi: 10.1038/s41579-018-0025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yen P.-S., Failloux A.-B. A Review: Wolbachia-Based Population Replacement for Mosquito Control Shares Common Points with Genetically Modified Control Approaches. Pathogens. 2020;9:404. doi: 10.3390/pathogens9050404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Choi L., Majambere S., Wilson A.L. Larviciding to prevent malaria transmission. Cochrane Database Syst. Rev. 2019;8:CD012736. doi: 10.1002/14651858.CD012736.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lima E.P., Paiva M.H., Araujo A.P.d., Silva E.V.d., Silva U.M.d., Oliveira L.N.d., Santana A.E., Barbosa C.N., Neto C.C.d.P., Goulart M.O., et al. Insecticide resistance in Aedes aegypti populations from Ceará, Brazil. Parasites Vectors. 2011;4:5. doi: 10.1186/1756-3305-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dusfour I., Vontas J., David J.-P., Weetman D., Fonseca D.M., Corbel V., Raghavendra K., Coulibaly M.B., Martins A.J., Kasai S., et al. Management of insecticide resistance in the major Aedes vectors of arboviruses: Advances and challenges. PLoS Negl. Trop. Dis. 2019;13:e0007615. doi: 10.1371/journal.pntd.0007615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Karunamoorthi K. Vector control: A cornerstone in the malaria elimination campaign. Clin. Microbiol. Infect. 2011;17:1608–1616. doi: 10.1111/j.1469-0691.2011.03664.x. [DOI] [PubMed] [Google Scholar]
- 111.Han W.W., Lazaro A., McCall P.J., George L., Runge-Ranzinger S., Toledo J., Velayudhan R., Horstick O. Efficacy and community effectiveness of larvivorous fish for dengue vector control. Trop. Med. Int. Heal. 2015;20:1239–1256. doi: 10.1111/tmi.12538. [DOI] [PubMed] [Google Scholar]
- 112.Udayanga L., Ranathunge T., Iqbal M.C.M., Abeyewickreme W., Hapugoda M.D. Predatory efficacy of five locally available copepods on Aedes larvae under laboratory settings: An approach towards bio-Control of dengue in Sri Lanka. PLoS ONE. 2019;14:e0216140. doi: 10.1371/journal.pone.0216140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tissera H.A., Samaraweera P.C., Jayamanne B.D.W., Janaki M.D.S., Chulasiri M.P.P.U., Rodrigo C., Fernando S.D. Use of Bacillus thuringiensis israelensis in integrated vector control of Aedes sp. in Sri Lanka: A prospective controlled effectiveness study. Trop. Med. Int. Heal. 2017;23:229–235. doi: 10.1111/tmi.13015. [DOI] [PubMed] [Google Scholar]
- 114.Lazaro A., Han W.W., Manrique-Saide P., George L., Velayudhan R., Toledo J., Ranzinger S.R., Horstick O. Community effectiveness of copepods for dengue vector control: Systematic review. Trop. Med. Int. Heal. 2015;20:685–706. doi: 10.1111/tmi.12485. [DOI] [PubMed] [Google Scholar]
- 115.Alphey L., McKemey A., Nimmo D., Oviedo M.N., Lacroix R., Matzen K., Beech C. Genetic control ofAedesmosquitoes. Pathog. Glob. Heal. 2013;107:170–179. doi: 10.1179/2047773213Y.0000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Atyame C.M., Cattel J., Lebon C., Flores O., Dehecq J.-S., Weill M., Gouagna L.C., Tortosa P. Wolbachia-Based Population Control Strategy Targeting Culex quinquefasciatus Mosquitoes Proves Efficient under Semi-Field Conditions. PLoS ONE. 2015;10:e0119288. doi: 10.1371/journal.pone.0119288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Perez-Staples D., Diaz-Fleischer F., Montoya P. The Sterile Insect Technique: Success and Perspectives in the Neotropics. Néotrop. Èntomol. 2020;10:1–14. doi: 10.1007/s13744-020-00817-3. [DOI] [PubMed] [Google Scholar]
- 118.Kittayapong P., Ninphanomchai S., Limohpasmanee W., Chansang C., Chansang U., Mongkalangoon P. Combined sterile insect technique and incompatible insect technique: The first proof-Of-Concept to suppress Aedes aegypti vector populations in semi-Rural settings in Thailand. PLoS Negl. Trop. Dis. 2019;13:e0007771. doi: 10.1371/journal.pntd.0007771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mustafa M.S., Bansal A., Rastogi V. Flightless Aedes mosquitoes in dengue control. Med. J. Armed Forces India. 2011;67:192–193. doi: 10.1016/S0377-1237(11)60035-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chung H.-N., Rodriguez S.D., Gonzales K.K., Vulcan J., Cordova J.J., Mitra S., Adams C.G., Moses-Gonzales N., Tam N., Cluck J.W., et al. Toward Implementation of Mosquito Sterile Insect Technique: The Effect of Storage Conditions on Survival of MaleAedes aegyptiMosquitoes (Diptera: Culicidae) During Transport. J. Insect Sci. 2018;18 doi: 10.1093/jisesa/iey103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dickens B.L., Yang J., Cook A.R., Carrasco L.R. Time to Empower Release of Insects Carrying a Dominant Lethal and Wolbachia Against Zika. Open Forum Infect. Dis. 2016;3:ofw103. doi: 10.1093/ofid/ofw103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Navarro-Payá D., Flis I., Anderson M., Hawes P., Li M., Akbari O.S., Basu S., Alphey L. Targeting female flight for genetic control of mosquitoes. PLoS Negl. Trop. Dis. 2020;14:e0008876. doi: 10.1371/journal.pntd.0008876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fu G., Lees R.S., Nimmo D., Aw D., Jin L., Gray P., Berendonk T.U., White-Cooper H., Scaife S., Phuc H.K., et al. Female-Specific flightless phenotype for mosquito control. Proc. Natl. Acad. Sci. USA. 2010;107:4550–4554. doi: 10.1073/pnas.1000251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Resnik D.B. Ethical issues in field trials of genetically modified disease-Resistant mosquitoes. Dev. World Bioeth. 2012;14:37–46. doi: 10.1111/dewb.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Reeves R.G., Denton J.A., Santucci F., Bryk J., Reed F.A. Scientific Standards and the Regulation of Genetically Modified Insects. PLoS Negl. Trop. Dis. 2012;6:e1502. doi: 10.1371/journal.pntd.0001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li M., Yang T., Kandul N.P., Bui M., Gamez S., Raban R.R., Bennett J., Sánchez C H.M., Lanzaro G.C., Schmidt H., et al. Development of a confinable gene drive system in the human disease vector Aedes aegypti. Elife. 2020;9 doi: 10.7554/eLife.51701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Terradas G., McGraw E.A. Using genetic variation in Aedes aegypti to identify candidate anti-Dengue virus genes. BMC Infect. Dis. 2019;19:1–14. doi: 10.1186/s12879-019-4212-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hoffmann A.A., Montgomery B.L., Popovici J., Iturbeormaetxe I., Johnson P.H., Muzzi F., Greenfield M., Durkan M., Leong Y.S., Dong Y., et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 129.Hoffmann A.A., Ross P.A., Rašić G. Wolbachia strains for disease control: Ecological and evolutionary considerations. Evol. Appl. 2015;8:751–768. doi: 10.1111/eva.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Werren J.H. Biology of Wolbachia. Annu. Rev. Èntomol. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- 131.Hilgenboecker K., Hammerstein P., Schlattmann P., Telschow A., Werren J.H. How many species are infected with Wolbachia? –A statistical analysis of current data. FEMS Microbiol. Lett. 2008;281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rainey S.M., Shah P., Kohl A., Dietrich I. Understanding the Wolbachia-Mediated inhibition of arboviruses in mosquitoes: Progress and challenges. J. Gen. Virol. 2014;95:517–530. doi: 10.1099/vir.0.057422-0. [DOI] [PubMed] [Google Scholar]
- 133.Walker T.G., Johnson P.H., Moreira L.A., Iturbeormaetxe I., Frentiu F.D., McMeniman C.J., Leong Y.S., Dong Y., Axford J.K., Kriesner P., et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nat. Cell Biol. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- 134.Kriesner P., Hoffmann A.A., Lee S.F., Turelli M., Weeks A.R. Rapid Sequential Spread of Two Wolbachia Variants in Drosophila simulans. PLoS Pathog. 2013;9:e1003607. doi: 10.1371/journal.ppat.1003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huigens M.E., Luck R.F., Klaassen R.H.G., Maas M.F.P.M., Timmermans M.J.T.N., Stouthamer R. Infectious parthenogenesis. Nat. Cell Biol. 2000;405:178–179. doi: 10.1038/35012066. [DOI] [PubMed] [Google Scholar]
- 136.Zabalou S., Riegler M., Theodorakopoulou M., Stauffer C., Savakis C., Bourtzis K. Wolbachia-Induced cytoplasmic incompatibility as a means for insect pest population control. Proc. Natl. Acad. Sci. USA. 2004;101:15042–15045. doi: 10.1073/pnas.0403853101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sinkins S.P., Braig H.R., O’Neill S.L. Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proc. R. Soc. B: Boil. Sci. 1995;261:325–330. doi: 10.1098/rspb.1995.0154. [DOI] [PubMed] [Google Scholar]
- 138.Moreira L.A., Iturbe-Ormaetxe I., Jeffery J.A., Lu G., Pyke A.T., Hedges L.M., Rocha B.C., Hall-Mendelin S., Day A., Riegler M., et al. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 139.Hertig M., Wolbach S.B. Studies on Rickettsia-Like Micro-Organisms in Insects. J. Med. Res. 1924;44:329–374.7. [PMC free article] [PubMed] [Google Scholar]
- 140.Zhou W., Rousset F., O’Neill S.L. Phylogeny and PCR-Based classification of Wolbachia strains using wsp gene sequences. Proc. Biol. Sci. 1998;265:509–515. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ant T.H., Herd C.S., Geoghegan V., Hoffmann A.A., Sinkins S.P. The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog. 2018;14:e1006815. doi: 10.1371/journal.ppat.1006815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ulrich J.N., Beier J.C., Devine G.J., Hugo L.E. Heat Sensitivity of wMel Wolbachia during Aedes aegypti Development. PLoS Negl. Trop. Dis. 2016;10:e0004873. doi: 10.1371/journal.pntd.0004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nguyen T.H., Le Nguyen H., Nguyen T.Y., Vu S.N., Tran N.D., Le T.N., Vien Q.M., Bui T.C., Le H.T., Kutcher S., et al. Field evaluation of the establishment potential of wmelpop Wolbachia in Australia and Vietnam for dengue control. Parasites Vectors. 2015;8:563. doi: 10.1186/s13071-015-1174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mancini M.V., Herd C.S., Ant T.H., Murdochy S.M., Sinkins S.P. Wolbachia strain wAu efficiently blocks arbovirus transmission in Aedes albopictus. PLoS Negl. Trop. Dis. 2020;14:e0007926. doi: 10.1371/journal.pntd.0007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hurk A.F.v.d., Hall-Mendelin S., Pyke A.T., Frentiu F.D., McElroy K., Day A., Higgs S., O’Neill S.L. Impact of Wolbachia on Infection with Chikungunya and Yellow Fever Viruses in the Mosquito Vector Aedes aegypti. PLoS Negl. Trop. Dis. 2012;6:e1892. doi: 10.1371/journal.pntd.0001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ferguson N.M., Kien D.T.H., Clapham H., Aguas R., Trung V.T., Chau T.N.B., Popovici J., Ryan P.A., O’Neill S.L., McGraw E.A., et al. Modeling the impact on virus transmission ofWolbachia-Mediated blocking of dengue virus infection ofAedes aegypti. Sci. Transl. Med. 2015;7:279ra37. doi: 10.1126/scitranslmed.3010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yeap H.L., Mee P., Walker T.W., Weeks A.R., Oneill S.L., Johnson P.R.S., Ritchie S.A., Richardson K.M., Doig C.J., Endersby N.M., et al. Dynamics of the “Popcorn” Wolbachia Infection in Outbred Aedes aegypti Informs Prospects for Mosquito Vector Control. Genetics. 2011;187:583–595. doi: 10.1534/genetics.110.122390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rasgon J.L., Scott T.W. Wolbachia and cytoplasmic incompatibility in the California Culex pipiens mosquito species complex: Parameter estimates and infection dynamics in natural populations. Genetics. 2003;165:2029–2038. doi: 10.1093/genetics/165.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Almeida F.d., Moura A.S., Cardoso A.F., Winter C.E., Bijovsky A.T., Suesdek L. Effects of Wolbachia on fitness of Culex quinquefasciatus (Diptera; Culicidae) Infect. Genet. Evol. 2011;11:2138–2143. doi: 10.1016/j.meegid.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 150.Dyer K.A., Jaenike J. Evolutionarily stable infection by a male-killing endosymbiont in Drosophila innubila: Molecular evidence from the host and parasite genomes. Genetics. 2004;168:1443–1455. doi: 10.1534/genetics.104.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Axford J.K., Callahan A.G., Hoffmann A.A., Yeap H.L., Ross P.A. Fitness of wAlbB Wolbachia Infection in Aedes aegypti: Parameter Estimates in an Outcrossed Background and Potential for Population Invasion. Am. J. Trop. Med. Hyg. 2016;94:507–516. doi: 10.4269/ajtmh.15-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Parzych K.R., Klionsky D.J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antioxid. Redox Signal. 2014;20:460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Voronin D., Cook D.A.N., Steven A., Taylor M.J. Autophagy regulates Wolbachia populations across diverse symbiotic associations. Proc. Natl. Acad. Sci. USA. 2012;109:E1638–E1646. doi: 10.1073/pnas.1203519109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Krejbich-Trotot P., Gay B., Li-Pat-Yuen G., Hoarau J.-J., Jaffar-Bandjee M.-C., Briant L., Gasque P., Denizot M. Chikungunya triggers an autophagic process which promotes viral replication. Virol. J. 2011;8:432. doi: 10.1186/1743-422X-8-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gill A.C., Darby A.C., Makepeace B.L. Iron Necessity: The Secret of Wolbachia’s Success? PLoS Negl. Trop. Dis. 2014;8:e3224. doi: 10.1371/journal.pntd.0003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zug R., Hammerstein P. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol. Rev. Camb. Philos. Soc. 2015;90:89–111. doi: 10.1111/brv.12098. [DOI] [PubMed] [Google Scholar]
- 157.Sim S., Jupatanakul N., Dimopoulos G. Mosquito Immunity against Arboviruses. Viruses. 2014;6:4479–4504. doi: 10.3390/v6114479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rancès E., Ye Y.H., Woolfit M., McGraw E.A., O’Neill S.L. The Relative Importance of Innate Immune Priming in Wolbachia-Mediated Dengue Interference. PLoS Pathog. 2012;8:e1002548. doi: 10.1371/journal.ppat.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jupatanakul N., Sim S., Angleró-Rodríguez Y.I., Souza-Neto J., Das S., Poti K.E., Rossi S.L., Bergren N., Vasilakis N., Dimopoulos G. Engineered Aedes aegypti JAK/STAT Pathway-Mediated Immunity to Dengue Virus. PLoS Negl. Trop. Dis. 2017;11:e0005187. doi: 10.1371/journal.pntd.0005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vargas V., Cime-Castillo J., Lanz-Mendoza H. Immune priming with inactive dengue virus during the larval stage of Aedes aegypti protects against the infection in adult mosquitoes. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-63402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Thomas P., Kenny N., Eyles D.W., Moreira L.A., O’Neill S.L., Asgari S. Infection with the wMel and wMelPop strains of Wolbachia leads to higher levels of melanization in the hemolymph of Drosophila melanogaster, Drosophila simulans and Aedes aegypti. Dev. Comp. Immunol. 2011;35:360–365. doi: 10.1016/j.dci.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 162.Rodriguez-Andres J., Rani S., Varjak M., Chase-Topping M.E., Beck M.H., Ferguson M.C., Schnettler E., Fragkoudis R., Barry G., Merits A., et al. Phenoloxidase Activity Acts as a Mosquito Innate Immune Response against Infection with Semliki Forest Virus. PLoS Pathog. 2012;8:e1002977. doi: 10.1371/journal.ppat.1002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hussain M., Frentiu F.D., Moreira L.A., O’Neill S.L., Asgari S. Wolbachia uses host microRNAs to manipulate host gene expression and facilitate colonization of the dengue vector Aedes aegypti. Proc. Natl. Acad. Sci. USA. 2011;108:9250–9255. doi: 10.1073/pnas.1105469108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee W.S., Webster J.A., Madzokere E.T., Skinner E.B., Herrero L.J. Mosquito antiviral defense mechanisms: A delicate balance between innate immunity and persistent viral infection. Parasites Vectors. 2019;12:1–12. doi: 10.1186/s13071-019-3433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Saldaña M.A., Etebari K., Hart C.E., Widen S.G., Wood T.G., Thangamani S., Asgari S., Hughes G.L. Zika virus alters the microRNA expression profile and elicits an RNAi response in Aedes aegypti mosquitoes. PLoS Negl. Trop. Dis. 2017;11:e0005760. doi: 10.1371/journal.pntd.0005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Campbell C.L., Harrison T., Hess A.M., Ebel G.D. MicroRNA levels are modulated inAedes aegyptiafter exposure to Dengue-2. Insect Mol. Biol. 2013;23:132–139. doi: 10.1111/imb.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.McMeniman C.J., Lane R.V., Cass B.N., Fong A.W.C., Sidhu M., Wang Y.-F., O’Neill S.L. Stable Introduction of a Life-Shortening Wolbachia Infection into the Mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 168.Duron O., Bernard C., Unal S., Berthomieu A., Berticat C., Weill M. Tracking factors modulating cytoplasmic incompatibilities in the mosquito Culex pipiens. Mol. Ecol. 2006;15:3061–3071. doi: 10.1111/j.1365-294X.2006.02996.x. [DOI] [PubMed] [Google Scholar]
- 169.Turelli M., Hoffmann A.A. Cytoplasmic Incompatibility in Drosophila Simulans: Dynamics and Parameter Estimates from Natural Populations. Genetics. 1995;140:1319–1338. doi: 10.1093/genetics/140.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rousset F., Raymond M. Cytoplasmic incompatibility in insects: Why sterilize females? Trends Ecol. Evol. 1991;6:54–57. doi: 10.1016/0169-5347(91)90123-F. [DOI] [PubMed] [Google Scholar]
- 171.Hoffmann A.A., Clancy D.J., Merton E. Cytoplasmic Incompatibility in Australian Populations of Drosophi-La-Melanogaster. Genetics. 1994;136:993–999. doi: 10.1093/genetics/136.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Flor M., Hammerstein P., Telschow A. Wolbachia-Induced unidirectional cytoplasmic incompatibility and the stability of infection polymorphism in parapatric host populations. J. Evol. Biol. 2007;20:696–706. doi: 10.1111/j.1420-9101.2006.01252.x. [DOI] [PubMed] [Google Scholar]
- 173.Telschow A., Flor M., Kobayashi Y., Hammerstein P., Werren J.H. Wolbachia-Induced Unidirectional Cytoplasmic Incompatibility and Speciation: Mainland-Island Model. PLoS ONE. 2007;2:e701. doi: 10.1371/journal.pone.0000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Branca A., Vavre F., Silvain J.-F., Dupas S. Maintenance of adaptive differentiation by Wolbachia induced bidirectional cytoplasmic incompatibility: The importance of sib-Mating and genetic systems. BMC Evol. Biol. 2009;9:185. doi: 10.1186/1471-2148-9-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sicard M., Bouchon D., Ceyrac L., Raimond R., Thierry M., Clec’H W.L., Marcadé I., Caubet Y., Grève P. Bidirectional cytoplasmic incompatibility caused by Wolbachia in the terrestrial isopod Porcellio dilatatus. J. Invertebr. Pathol. 2014;121:28–36. doi: 10.1016/j.jip.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 176.Zhong Y., Li Z.-X. Bidirectional Cytoplasmic Incompatibility Induced by Cross-Order Transfection of Wolbachia: Implications for Control of the Host Population. Microb. Ecol. 2014;68:463–471. doi: 10.1007/s00248-014-0425-2. [DOI] [PubMed] [Google Scholar]
- 177.Dorigatti I., McCormack C., Nedjati-Gilani G., Ferguson N.M. Using Wolbachia for Dengue Control: Insights from Modelling. Trends Parasitol. 2018;34:102–113. doi: 10.1016/j.pt.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ogunlade S.T., Adekunle A.I., Meehan M.T., Rojas D.P., McBryde E.S. Modeling the potential of wAu-Wolbachia strain invasion in mosquitoes to control Aedes-Borne arboviral infections. Sci. Rep. 2020;10:1–16. doi: 10.1038/s41598-020-73819-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ross P.A., Ritchie S.A., Axford J.K., Hoffmann A.A. Loss of cytoplasmic incompatibility in Wolbachia-Infected Aedes aegypti under field conditions. PLoS Negl. Trop. Dis. 2019;13:e0007357. doi: 10.1371/journal.pntd.0007357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Adekunle A.I., Meehan M.T., McBryde E.S. Mathematical analysis of a Wolbachia invasive model with imperfect maternal transmission and loss of Wolbachia infection. Infect. Dis. Model. 2019;4:265–285. doi: 10.1016/j.idm.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dutton T.J., Sinkins S.P. Strain-Specific quantification of Wolbachia density in Aedes albopictus and effects of larval rearing conditions. Insect Mol. Biol. 2004;13:317–322. doi: 10.1111/j.0962-1075.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 182.Hague M.T.J., Mavengere H., Matute D.R., Cooper B.S. Environmental and Genetic Contributions to Imperfect wMel-Like Wolbachia Transmission and Frequency Variation. Genetics. 2020;215:1117–1132. doi: 10.1534/genetics.120.303330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Meany M.K., Conner W.R., Richter S.V., Bailey J.A., Turelli M., Cooper B.S. Loss of cytoplasmic incompatibility and minimal fecundity effects explain relatively low Wolbachia frequencies in Drosophila mauritiana. Evolution. 2019;73:1278–1295. doi: 10.1111/evo.13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hedges L.M., Brownlie J.C., O’Neill S.L., Johnson K.N. Wolbachia and Virus Protection in Insects. Science. 2008;322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- 185.Shaw A.E., Veronesi E., Maurin G., Ftaich N., Guiguen F., Rixon F., Ratinier M., Mertens P., Carpenter S., Palmarini M., et al. Drosophila melanogaster as a Model Organism for Bluetongue Virus Replication and Tropism. J. Virol. 2012;86:9015–9024. doi: 10.1128/JVI.00131-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Teixeira L.A., Ferreira Á., Ashburner M. The Bacterial Symbiont Wolbachia Induces Resistance to RNA Viral Infections in Drosophila melanogaster. PLoS Biol. 2008;6:e1000002. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kambris Z., Cook P.E., Phuc H.K., Sinkins S.P. Immune Activation by Life-Shortening Wolbachia and Reduced Filarial Competence in Mosquitoes. Science. 2009;326:134–136. doi: 10.1126/science.1177531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.O’Connor L., Plichart C., Sang A.C., Brelsfoard C.L., Bossin H.C., Dobson S.L. Open release of male mosquitoes in-Fected with a wolbachia biopesticide: Field performance and infection containment. PLoS Negl. Trop. Dis. 2012;6:e1797. doi: 10.1371/journal.pntd.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rasic G., Filipovic I., Weeks A.R., Hoffmann A.A. Genome-Wide SNPs lead to strong signals of geographic structure and relatedness patterns in the major arbovirus vector, Aedes aegypti. BMC Genom. 2014;15:275. doi: 10.1186/1471-2164-15-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Frentiu F.D., Zakir T., Walker T., Popovici J., Pyke A.T., Hurk A.V.D., McGraw E.A., O’Neill S.L. Limited Dengue Virus Replication in Field-Collected Aedes aegypti Mosquitoes Infected with Wolbachia. PLoS Negl. Trop. Dis. 2014;8:e2688. doi: 10.1371/journal.pntd.0002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.WHO . World Health Organization Mosquito Control: Can. It Stop Zika at Source? WHO; Geneva, Switzerland: 2016. [(accessed on 17 October 2019)]. Available online: http://www.who.int/emergencies/zika-virus/articles/mosquito-control/en/ [Google Scholar]
- 192.Pan X., Pike A., Joshi D., Bian G., McFadden M.J., Lu P., Liang X., Zhang F., Raikhel A.S., Xiaoling P. The bacterium Wolbachia exploits host innate immunity to establish a symbiotic relationship with the dengue vector mosquito Aedes aegypti. ISME J. 2018;12:277–288. doi: 10.1038/ismej.2017.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang D., Wang Y., He K., Yang Q., Gong M., Ji M., Chen L. Wolbachia limits pathogen infections through induction of host innate immune responses. PLoS ONE. 2020;15:e0226736. doi: 10.1371/journal.pone.0226736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Turley A.P., Moreira L.A., O’Neill S.L., McGraw E.A. Wolbachia Infection Reduces Blood-Feeding Success in the Dengue Fever Mosquito, Aedes aegypti. PLoS Negl. Trop. Dis. 2009;3:e516. doi: 10.1371/journal.pntd.0000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sutton E.R., Harris S.R., Parkhill J., Sinkins S.P. Comparative genome analysis of Wolbachia strain wAu. BMC Genom. 2014;15:1–15. doi: 10.1186/1471-2164-15-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Suhrcke M., Stuckler D., Suk J.E., Desai M., Senek M., McKee M., Tsolova S., Basu S., Abubakar I., Hunter P., et al. The Impact of Economic Crises on Communicable Disease Transmission and Control: A Systematic Review of the Evidence. PLoS ONE. 2011;6:e20724. doi: 10.1371/journal.pone.0020724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Anderson R.M., May R.M., Gupta S. Non-Iinear phenomena in host—Parasite interactions. Parasitology. 1989;99:S59–S79. doi: 10.1017/S0031182000083426. [DOI] [PubMed] [Google Scholar]
- 198.May R.M. Infectious Disease: Can We Avert a Lethal Flu Pandemic? Curr. Biol. 2005;15:R922–R924. doi: 10.1016/j.cub.2005.10.063. [DOI] [PubMed] [Google Scholar]
- 199.Hethcote H.W., Driessche P.V.D. Two SIS epidemiologic models with delays. J. Math. Biol. 2000;40:3–26. doi: 10.1007/s002850050003. [DOI] [PubMed] [Google Scholar]
- 200.Anderson R.M., May R.M. Population biology of infectious diseases: Part I. Nat. Cell Biol. 1979;280:361–367. doi: 10.1038/280361a0. [DOI] [PubMed] [Google Scholar]
- 201.Siettos C., Russo L. Mathematical modeling of infectious disease dynamics. Virulence. 2013;4:295–306. doi: 10.4161/viru.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Driessche P.V.D., Watmough J. Reproduction numbers and sub-Threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002;180:29–48. doi: 10.1016/S0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 203.Campo-Duarte D.E., Vasilieva O., Cardona-Salgado D., Svinin M. Optimal control approach for establishing wMelPop Wolbachia infection among wild Aedes aegypti populations. J. Math. Biol. 2018;76:1907–1950. doi: 10.1007/s00285-018-1213-2. [DOI] [PubMed] [Google Scholar]
- 204.Hughes H., Britton N.F. Modelling the Use of Wolbachia to Control Dengue Fever Transmission. Bull. Math. Biol. 2013;75:796–818. doi: 10.1007/s11538-013-9835-4. [DOI] [PubMed] [Google Scholar]
- 205.Ndii M.Z., Hickson R.I., Mercer G.N. Modelling the Introduction of Wolbachia into Aedes Aegypti Mosquitoes to Reduce Dengue Transmission. ANZIAM J. 2012;53:213–227. doi: 10.1017/s1446181112000132. [DOI] [Google Scholar]
- 206.Qu Z., Xue L., Hyman J.M. Modeling the Transmission of Wolbachia in Mosquitoes for Controlling Mosquito-Borne Diseases. SIAM J. Appl. Math. 2018;78:826–852. doi: 10.1137/17M1130800. [DOI] [Google Scholar]
- 207.Schraiber J.G., Kaczmarczyk A.N., Kwok R., Park M., Silverstein R., Rutaganira F.U., Aggarwal T., Schwemmer M.A., Hom C.L., Grosberg R.K., et al. Constraints on the use of lifespan-Shortening Wolbachia to control dengue fever. J. Theor. Biol. 2012;297:26–32. doi: 10.1016/j.jtbi.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 208.Telschow A., Yamamura N., Werren J.H. Bidirectional cytoplasmic incompatibility and the stable coexistence of two Wolbachia strains in parapatric host populations. J. Theor. Biol. 2005;235:265–274. doi: 10.1016/j.jtbi.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 209.Xue L., Manore C.A., Thongsripong P., Hyman J.M. Two-Sex mosquito model for the persistence ofWolbachia. J. Biol. Dyn. 2017;11:216–237. doi: 10.1080/17513758.2016.1229051. [DOI] [PubMed] [Google Scholar]
- 210.Zheng B., Tang M., Yu J., Qiu J. Wolbachia spreading dynamics in mosquitoes with imperfect maternal transmission. J. Math. Biol. 2018;76:235–263. doi: 10.1007/s00285-017-1142-5. [DOI] [PubMed] [Google Scholar]
- 211.Gumel A.B. Causes of backward bifurcations in some epidemiological models. J. Math. Anal. Appl. 2012;395:355–365. doi: 10.1016/j.jmaa.2012.04.077. [DOI] [Google Scholar]
- 212.Costa M.C.D.S., Maia L.M.S., De Souza V.C., Gonzaga A.M., De Azevedo V.C., Martins L.R., Pavoni J.H.C., Naveca F.G., Slhessarenko R.D. Arbovirus investigation in patients from Mato Grosso during Zika and Chikungunya virus introdution in Brazil, 2015–2016. Acta Trop. 2019;190:395–402. doi: 10.1016/j.actatropica.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 213.Eckerle I., Briciu V., Ergonul O., Lupse M., Papa A., Radulescu A., Tsiodras S., Tsitou C., Drosten C., Nussenblatt V., et al. Emerging souvenirs—Clinical presentation of the returning traveller with imported arbovirus infections in Europe. Clin. Microbiol. Infect. 2018;24:240–245. doi: 10.1016/j.cmi.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 214.Eder M., Cortes F., Filha N.T.D.S., De França G.V.A., DeGroote S., Braga M.C., Ridde V., Martelli C.M.T. Scoping review on vector-Borne diseases in urban areas: Transmission dynamics, vectorial capacity and co-Infection. Infect. Dis. Poverty. 2018;7:1–24. doi: 10.1186/s40249-018-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Iwashita H., Higa Y., Futami K., Lutiali P.A., Njenga S.M., Nabeshima T., Minakawa N. Mosquito arbovirus survey in selected areas of Kenya: Detection of insect-Specific virus. Trop. Med. Heal. 2018;46:1–15. doi: 10.1186/s41182-018-0095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ross P.A., Wiwatanaratanabutr I., Axford J.K., White V.L., Endersby-Harshman N.M., Hoffmann A.A. Wolbachia Infections in Aedes aegypti Differ Markedly in Their Response to Cyclical Heat Stress. PLoS Pathog. 2017;13:e1006006. doi: 10.1371/journal.ppat.1006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.