The authors would like to make corrections to their published paper [1].
There were mistakes in some usages of the chemical name “isoplumbagin” in the original version in Sections 2.1, 3.1, and 3.2. These “isoplumbagin” words should be changed to “plumbagin”.
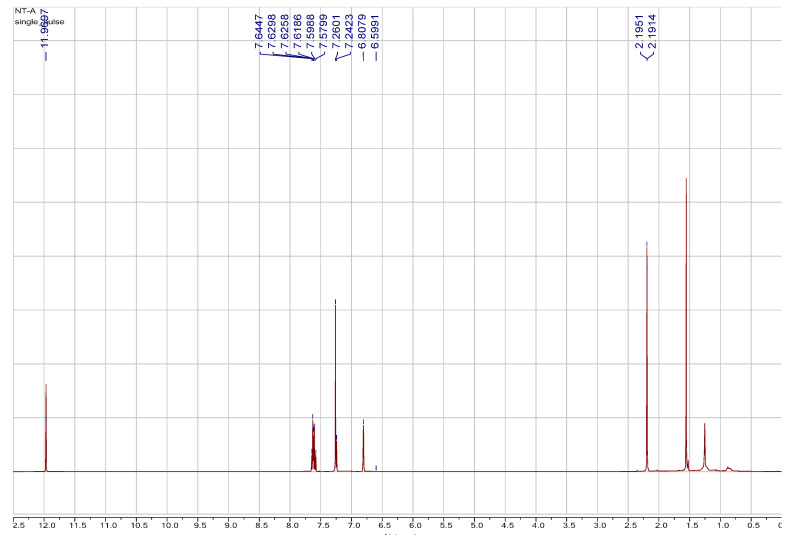
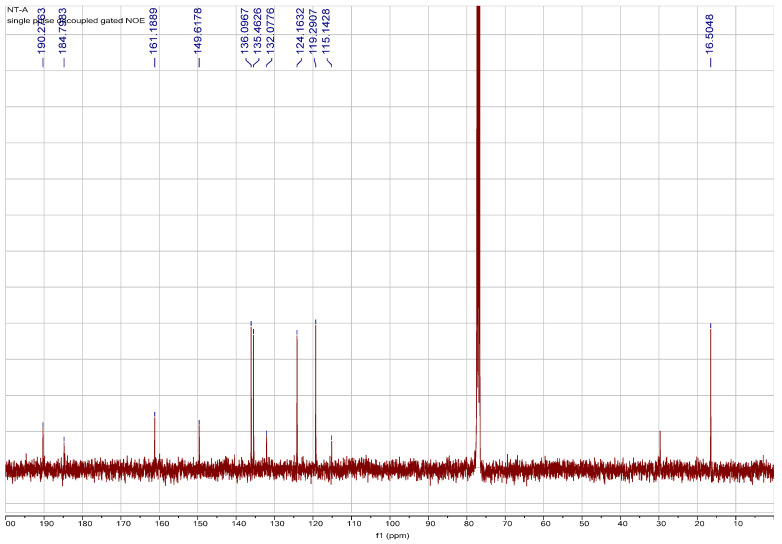
Literature reported that the difference between plumbagin and isoplumbagin is the coupling constant of methyl group (1.5 Hz for plumbagin and 1.2 Hz for isoplumbagin) [2]. We found that the 1H and 13C spectra data (Figure 1 and Figure 2) of the main compound of EANT is plumbagin because our compound shows a J value of 1.5. Therefore, we confirmed the major compound of EANT to be “plumbagin” based on spectra data rather than isoplumbagin.
Figure 1.
Spectrum data of 1H of the major compound isolated from EANT.
Figure 2.
Spectrum data of 13C of the major compound isolated from EANT.
We also further performed the detailed physical characters such as melting temperature. The melting points of these two compounds are different (74–75 °C for plumbagin and 158–159 °C for isoplumbagin) [2]. After checking the melting point (77–78 °C) of the major compound isolated from EANT, we realized it should be plumbagin rather than isoplumbagin.
Additionally, the paragraph for the Supplementary Materials also needs to be corrected due to missing words. The authors have corrected the error as shown below. The change does not affect the scientific results. The authors would like to apologize for any inconvenience that may have been caused to readers of the journal. The manuscript will be updated, and the original will remain online on the article webpage.
Please find the correct sentences below (only isoplumbagin in the original paper has been corrected to plumbagin):
2.1. The Identified Components from Fingerprint Profiles of EANT (Page 2: Line 2 of the First Paragraph)
According to HPLC fingerprinting assay (Supplementary Figure S1), the major bioactive components of EANT are plumbagin, cis-isoshinanolone, quercetin 3-O-(6″-n-butyl β-d-glucuronide), and fatty acids.
3.1. EANT Preferentially Inhibits Proliferation of Breast Cancer Cells (page 8: line 6 of the Second Paragraph of 3.1)
In the current study, we found that the major bioactive components of EANT identified by HPLC fingerprinting method were plumbagin [21], cis-isoshinanolone [22], and quercetin 3-O-(6″-n-butyl β-d-glucuronide) [23] (Supplementary Figure S1).
3.2. EANT Induces Oxidative Stress on Breast Cancer Cells (Page 8: line 1 of the Third Paragraph of 3.2)
Plumbagin is a common naphthoquinone in Nepenthes. Moreover, plumbagin but not isoplumbagin is identified in EANT.
Supplementary Materials (page 11)
The following are available online at http://www.mdpi.com/1422-0067/20/13/3238/s1, Table S1: The HPLC method for fingerprint profile of N. thorellii x (ventricosa x maxima), Figure S1: Components of EANT. (A) Fingerprint profile of EANT. It is monitored at 365 nm. (B) Retention time of plumbagin (NT-A). Volume is 50 μL. It is monitored at 400 nm. (C) Retention time of cis-isoshinanolone (NT-B). Volume is 10 μL. It is monitored at 254 nm.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ou-Yang F., Tsai I.H., Tang J.Y., Yen C.Y., Cheng Y.B., Farooqi A.A., Chen S.R., Yu S.Y., Kao J.K., Chang H.W. Antiproliferation for breast cancer cells by ethyl acetate extract of Nepenthes thorellii x (ventricosa x maxima) Int. J. Mol. Sci. 2019;20:3238. doi: 10.3390/ijms20133238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J.L., Yang Z.J., Wang J.J., Tang W.X., Zhao M., Zhang S.J. Synthesis of juglone and its derivatives. Appl. Mech. Mater. 2012;138–139:1139–1141. doi: 10.4028/www.scientific.net/AMM.138-139.1139. [DOI] [Google Scholar]