Abstract
Gilles de la Tourette syndrome (GTS) is a complex neurodevelopmental disorder characterized by motor and vocal tics. Most of the GTS individuals have comorbid diagnoses, of which obsessive-compulsive disorder (OCD) and attention deficit-hyperactivity disorder (ADHD) are the most common. Several neurotransmitter systems have been implicated in disease pathogenesis, and amongst these, the dopaminergic and the serotonergic pathways are the most widely studied. In this study, we aimed to investigate whether the serotonin transporter (SERT) gene (SLC6A4) was differentially expressed among GTS individuals compared to healthy controls, and whether DNA variants (the SERT-linked polymorphic region 5-HTTLPR, together with the associated rs25531 and rs25532 variants, and the rare Ile425Val variant) or promoter methylation of SLC6A4 were associated with gene expression levels or with the presence of OCD as comorbidity. We observed that SLC6A4 expression is upregulated in GTS individuals compared to controls. Although no specific genotype, allele or haplotype was overrepresented in GTS individuals compared to controls, we observed that the LAC/LAC genotype of the 5-HTTLPR/rs25531/rs25532 three-locus haplotype was associated with higher SLC6A4 mRNA expression levels in GTS individuals, but not in the control group.
Keywords: SERT, SLC6A4, 5-HTT, Gilles de la Tourette syndrome, GTS, OCD, obsessive compulsive disorder, methylation, expression, serotonin
1. Introduction
Gilles de la Tourette syndrome (GTS) is a childhood-onset neurodevelopmental disorder characterized by at least one vocal and multiple motor tics, which begin before age 18 years and persist at least 1 year. Average age of onset is between 3 and 9 years with a male to female ratio of around 3:1 [1]. Comorbid conditions including obsessive-compulsive disorder (OCD), attention deficit-hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) are present in more than 70% of the GTS individuals [1,2].
GTS is a complex disorder, with a largely unknown aetiology: several environmental factors are thought to interact with multiple genes in yet undiscovered ways. GTS has a high heritability estimate (>0.5) [3,4], but identification of susceptibility genes has been challenging likely due to the complex and heterogeneous genetic architecture, wherein common and rare variants in various genes and biological pathways are involved [5,6,7,8].
Neuroimaging and neurophysiology studies suggest that GTS is associated with altered synaptic neurotransmission systems involving dopamine, serotonin, inhibitory neurotransmitter γ-aminobutyric acid (GABA) and excitatory neurotransmitter glutamate in the cortico-striato-thalamo-cortical circuits [9,10,11]. Furthermore, candidate gene studies suggest involvement of dopaminergic [12,13,14,15], serotonergic [16,17], glutamatergic [18] and histaminergic [19] pathways in GTS pathogenesis. Dopamine neurotransmission is extensively studied in GTS pathology and an alteration of the tonic-phasic dopamine release is considered as a hallmark leading to the designation of the “dopaminergic hypothesis” [20,21]. The connection between serotonin neurotransmission and GTS has, however, been characterized to a lesser extent. As early as 1990, Comings reported decreased serotonin/platelet ratio in a large cohort of GTS individuals and family members [22], and a range of drugs with high affinity for serotonin receptors, mainly atypical antipsychotics, have been used to relieve tics [23]. However, even though the dysfunction of the serotonergic system is thought to be a primary cause in OCD [24], the extent of its involvement in GTS is yet unknown. Serotonin (5-hydroxytryptamine, 5-HT) receptors have been found to both facilitate and inhibit dopamine activity [25,26,27,28]. The serotonin transporter (5-HTT, SERT), which regulates serotonergic neurotransmission by retrieving serotonin from the synaptic cleft back to the presynaptic neuron, is capable of dopamine uptake, meaning that it also functions as a dopamine transporter [29]. Furthermore, SERT knockout rats have reduced expression of proteins essential for the glutamatergic synapses [30]. SERT binding potential (BP) has been investigated in GTS [31,32,33,34] and reduced SERT BP was observed in both GTS-only and GTS+OCD individuals [32,33] and negatively correlated with tic severity [31]. However, in a recent study, increased SERT BP was observed in GTS+OCD, but not in GTS-only or OCD-only individuals [34], and the differences in methodologies were suggested as an explanation [34]. The serotonin system may thus be involved in GTS pathology directly and/or indirectly through regulation of other neurotransmitter systems, especially the dopaminergic system.
SERT is encoded by SLC6A4, which has been implicated in GTS aetiology by several studies: higher blood SLC6A4 mRNA expression levels were found to correlate with tic severity in GTS [35], elevated SLC6A4 expression was found in the striatum of rat models of GTS [36] and the rare SLC6A4 gain-of-function (GOF) variant Ile425Val known to modulate SERT activity was reported to have a higher prevalence in GTS individuals compared to controls [17,37]. The SERT-linked polymorphic region (5-HTTLPR) in the promoter region immediately upstream to SLC6A4 has been implicated in both OCD [38,39,40] and GTS aetiology [17]. The 5-HTTLPR polymorphic region is a 43 bp repeat with two common alleles, a long (L) and a short (S) allele. The L allele has been associated with higher SLC6A4 mRNA expression in blood leading to increased SERT mediated serotonin clearance, and ultimately resulting in reduced serotonergic neurotransmission [41]. SLC6A4 expression is further modulated by two 5-HTTLPR-adjacent single nucleotide polymorphisms (SNPs) rs25531 (A>G) and rs25532 (C>T). Initially, the 5-HTTLPR/rs25531 LG allele was found to mimic the 5-HTTLPR S allele regarding expression levels, and only the LA allele was found to have higher SLC6A4 expression [39]. Later, when Wendland et al. investigated the variants of the SLC6A4 promoter region including rs25532, and their effect on mRNA levels, they identified the three-locus haplotype LAC (5-HTTLPR/rs25531/rs25532) as the highest expressing haplotype [38]. At the same time, they reported the LAC haplotype to be overrepresented in OCD individuals compared to controls, and the same haplotype was later found to be more prevalent in GTS individuals compared to controls [17].
Methylation of the SLC6A4 promoter region has been previously investigated in the peripheral blood of individuals with major depressive disorder [42], children with childhood physical aggression [43] and ADHD [44] and in the saliva of paediatric OCD [45]. In general, increased methylation was observed in affected individuals compared to controls, and hypermethylation of two CpG-sites was also correlated with increased SLC6A4 mRNA expression levels in affected individuals [42]. Furthermore, methylation of SLC6A4 in peripheral blood was correlated with in vivo human brain serotonin synthesis [43]. So far, there are not any studies investigating the methylation of the SLC6A4 promoter region in GTS individuals, but elevated blood methylation levels of the dopamine D2 receptor gene (DRD2) was correlated with tic severity, while DNA methylation of the dopamine transporter (DAT) gene (SLC6A3) was lower in more severely affected individuals [46].
As associations between SLC6A4 methylation, expression and gene variants have not been studied in GTS previously, we investigated whether SLC6A4 was differentially expressed in GTS individuals with or without OCD compared to healthy controls and assessed whether gene variants or promoter methylation of SLC6A4 were associated with gene expression levels.
2. Materials and Methods
2.1. GTS and Control Cohort
In this study, we included only male individuals to exclude sex-specific methylation differences, as SLC6A4 promoter region methylation has been shown to be higher in females [47] and GTS is more common in males [1]. The affected individuals are referred to as GTS individuals regardless of the presence of comorbid OCD unless otherwise noted (i.e., GTS-only or GTS+OCD). The GTS cohort comprised 72 male individuals (aged 16.1 ± 4.0) of whom 50 had only GTS (GTS-only), while 22 had GTS and OCD (GTS+OCD). RNA was available from 57 GTS individuals (43 GTS-only and 14 GTS+OCD). GTS individuals were recruited through the Herlev Tourette Clinic (Denmark) and the GTS diagnosis was established by an experienced neuropediatrician using DSM-IV-TR criteria (DSM-IV-TR, 2000) and validated clinical instruments were used to assess the presence of comorbidities as described previously [48]. The study was approved by the Danish Institutional Review Board (2011 H-2-2010-144). Control material comprised DNA from 87 anonymized male individuals (aged 17.7 ± 7.1), and RNA was available from 36 of them. The GTS and control cohort are summarized in Supplementary Table S1.
2.2. Genotyping
DNA was extracted from peripheral blood following standard procedures. Genotyping of 5-HTTLPR, rs25531, rs25532 (Supplementary Figure S1), and Ile425Val variants in SLC6A4 were carried out with PCR followed by Sanger sequencing. PCR was carried out using the HotStarTaq® DNA Polymerase kit (Qiagen, Hilden, Germany) with a no-template-control (NTC) included in each run. PCR products were purified using the MultiScreen® PCRµ96 Plate (Millipore, Burlington, MA, USA) according to the manufacturer’s instructions and run on a 2% agarose gel (Sigma Aldrich, St. Louis, MO, USA). The long (L allele) and the short (S allele) fragments were excised from the gel and Sanger sequenced using the BigDye™ Terminator v3.1 Cycle Sequencing kit and analysed on an ABI 3730 DNA analyser (Applied Biosystems, Foster City, CA, USA). Only individuals homozygous for either the S or the L allele were sequenced for rs25531 and rs25532 determination. PCR and Sanger sequencing primers and conditions are listed in Supplementary Tables S2 and S3.
2.3. Expression Analysis
RNA was extracted from peripheral blood following standard procedures. mRNA expression levels of SLC6A4 were quantified using reverse-transcription quantitative PCR (RT-qPCR). For this, 1 µg of total RNA was used for cDNA synthesis using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems) according to manufacturer’s instructions, with minor modifications (Supplementary Table S4). qPCR was carried out using TaqMan probes against either SLC6A4 (#Hs00169010_m1; Applied Biosystems) or GUSB (#Hs00939627_m1; Applied Biosystems). All samples were amplified in triplicates on a 7500 Fast Real-Time PCR system (Applied Biosystems). The relative standard curve method was used for calculation and SLC6A4 mRNA expression levels were normalized to GUSB mRNA levels. qPCR conditions are shown in Supplementary Table S5.
2.4. Methylation Analysis
Bisulphite pyrosequencing was used to quantify the degree of DNA methylation at eight CpG-sites within the 799 bp CpG-island at the promoter region of SLC6A4 (chromosome position chr17: 28562388–28563186, GRCh37/hg19) (Supplementary Figure S2). DNA (200 ng) was bisulphite converted using an EZ DNA Methylation-Gold™ Kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s instructions. PCR was performed using 1 µL of bisulphite-converted DNA with a PyroMark PCR kit (Qiagen) according to the manufacturer’s instructions, with minor modifications. Methylation levels were quantified using PyroMark Q48 Autoprep and PyroMark software. Primer sequences and PCR conditions are shown in Supplementary Table S6.
2.5. Statistical Analyses
For statistical analysis of the distribution of 5-HTTLPR (/rs25531/rs25532) genotypes, the Chi-square test was applied. Nonparametric tests (Mann–Whitney U test or Kruskal–Wallis test) were applied to test for difference in continuous variables between two or more categorical variables. The Dunn test with adjustment for multiple comparisons was used as a post hoc pairwise test after significant results in the Kruskal–Wallis test, to determine which categorical variables differed from each other. Linear regression was used to assess whether one continuous variable had an impact on another continuous variable.
To avoid type I errors, Bonferroni correction was applied to the p-values where multiple testing was conducted. As eight CpG-sites together with the mean value of all sites were considered in each methylation test, a p-value of 0.05/9 = 0.0056 was taken as threshold for statistical significance. Statistical results of methylation analyses were only reported for the mean of all CpG-sites.
All statistical analyses were performed in either SPSS (IBM, Armonk, NY, USA) or R (http://www.r-project.org). Figures were generated using R and RStudio [49], using the packages ggplot2 and ggpubr [50,51].
3. Results
3.1. Genotyping
To investigate whether there was an association between SLC6A4 promoter variants and GTS, we genotyped all the GTS individuals and the controls, and there was no statistically significant difference neither in the genotype distribution (n = 159, p = 0.243) nor in the allele frequencies of the 5-HTTLPR polymorphism (n = 318, p = 0.177) (Table 1).
Table 1.
Genotyping summary.
| Variable | GTS Individuals | Controls | chi2 | p-Value | OR | |
|---|---|---|---|---|---|---|
| Genotype: no. (%) | ||||||
| Total number of individuals | 72 | 87 | ||||
| L/L | 28 (38.9) | 23 (26.4) | ||||
| S/L | 31 (43.1) | 46 (52.9) | ||||
| S/S | 13 (18.1) | 18 (20.7) | 2.829 | 0.243 | ||
| Allele: no. (%) | ||||||
| L | 87 (60.4) | 92 (52.9) | ||||
| S | 57 (39.6) | 82 (47.1) | 1.822 | 0.177 | 1.360 | |
| Haplotype: no. (%) | ||||||
| Total number of individuals | 41 | 40 | ||||
| LAC | 47 (57) | 42 (52.5) | ||||
| LAT, LG, S | 35 (43) | 38 (47.5) | 0.380 | 0.538 | 1.2150 | |
L, long allele; S, short allele; A and G, A- or G-allele in rs25531; T and C, T- or C-allele in rs25532; OR, odds ratio; chi2, chi-square.
To determine the distribution of 5-HTTLPR/rs25531/rs25532 haplotypes (LAC compared to all other combinations), we genotyped the two 5-HTTLPR-adjacent SNPs rs25531 (A>G) and rs25532 (C>T) in 41 GTS individuals and 40 controls homozygous for either the S or the L allele. We did not observe any significant difference in the haplotype distribution (n = 162, p = 0.538) in GTS individuals compared to controls (Table 1). As the rs25532 T allele was not observed in the LG background, the LGC haplotype will be referred to as LG, which is in line with the nomenclature from previous publications [17,38]. Finally, we investigated all the GTS individuals and 51 controls for the Ile425Val variant, which was not present in anyone.
3.2. Expression Analysis
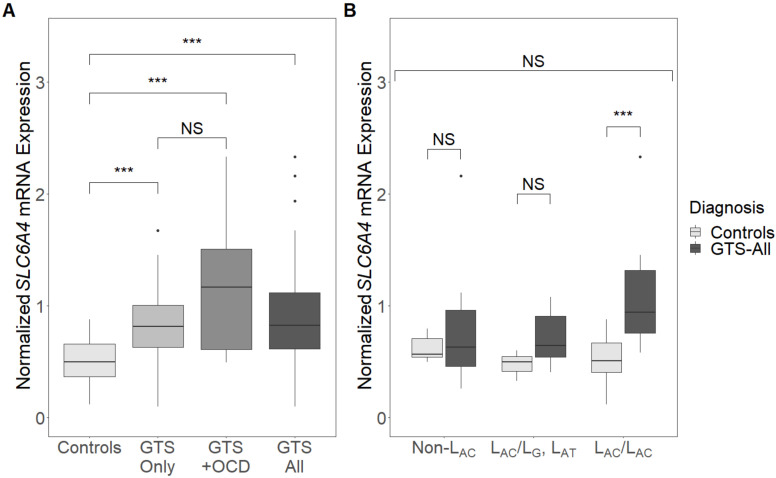
We analysed SLC6A4 mRNA expression levels using RT-qPCR in 57 GTS individuals (43 GTS-only and 14 GTS+OCD) and 36 controls from whom RNA was available and observed a significant difference (n = 93, p < 0.001) (Figure 1A). A three-way analysis between GTS-only, GTS+OCD and control individuals followed by a pairwise comparison showed significantly higher expression levels in both GTS-only and GTS+OCD individuals compared to controls ((n = 79, p.adj < 0.001) and (n = 50, p.adj < 0.001), respectively), while there was no significant difference between GTS-only and GTS+OCD individuals (n = 57, p.adj = 0.368) (Figure 1A).
Figure 1.
Expression levels of SLC6A4 normalized to GUSB expression levels in (A) Gilles de la Tourette syndrome (GTS)-only, GTS+obsessive-compulsive disorder (OCD) or GTS—all individuals and controls and (B) GTS—all individuals and controls with different three-locus genotypes (LAC/LAC, LAC/LG or LAT, or Non-LAC). Box plots indicate median, quartiles and outliers. ***, p < 0.001; NS, not significant.
To assess whether SLC6A4 variants had a modifying effect on gene expression, we examined SLC6A4 expression levels in GTS individuals and controls with regards to their 5-HTTLPR genotype and three-locus genotype (LAC/LAC, LAC/LG or LAT, or non-LAC) and did not detect any statistically significant difference (GTS: n = 57, p = 0.555; controls: n = 36, p = 0.162) (Supplementary Figure S3). SLC6A4 expression levels were, however, significantly higher in GTS individuals than in controls when only individuals with the LAC/LAC three-locus genotype were considered (n = 27, p < 0.001) (Figure 1B). A difference in expression levels between GTS individuals and controls was not observed for the other genotypes (LAC/LG or LAT: (n = 9, p = 0.167); Non-LAC: (n = 16, p = 1)).
3.3. Methylation Analyses
To investigate whether the observed differences in SLC6A4 expression could be associated with epigenetic regulation, we assessed the DNA methylation levels of eight CpG-sites at the promoter region of SLC6A4 in all the GTS individuals (50 GTS-only and 22 GTS+OCD) and the controls. One individual (GTS+OCD) was omitted from the analysis, as the DNA sample repeatedly failed the pyrosequencing quality control. The eight CpG sites assessed in this study were selected from a region of the SLC6A4 CpG island previously investigated in individuals with major depressive disorder [42] and ADHD [44].
There was no significant difference in the mean DNA methylation levels between GTS-only, GTS+OCD and controls (n = 158, p = 0.725, Supplementary Figure S4), and we did not observe any association between the mean methylation levels and the SLC6A4 expression (n = 92, p = 0.992) or the 5-HTTLPR genotype (n = 158, p = 0.253). Furthermore, methylation levels were not associated with GTS when considering individuals with the LAC/LAC three-locus genotype (n = 38, p = 0.884) nor any of the other three-locus genotypes (LAC/LG or LAT: (n = 11, p = 0.850); Non-LAC: (n = 31, p = 0.842)).
4. Discussion
To investigate the involvement of the serotonin transporter SERT in GTS pathology, we performed expression analysis, genotyping and methylation analysis of SLC6A4 in GTS individuals with and without OCD compared to healthy controls. We observed significantly higher SLC6A4 mRNA levels in GTS individuals compared to controls (p < 0.001), with a tendency of higher expression levels of SLC6A4 mRNA in GTS+OCD individuals compared to GTS-only, although this difference was not statistically significant. Elevated expression levels of SLC6A4 were previously observed in GTS rat models [36], and taken together these results suggest that increased serotonin clearance due to overexpression of SLC6A4 may contribute to GTS aetiology. The tendency of higher SLC6A4 expression levels in GTS+OCD individuals than GTS-only may explain why selective serotonin reuptake inhibitors (SSRIs) are more effective in the treatment of OCD symptoms than the treatment of tics [52]. SSRIs increase the level of serotonin in the synaptic cleft by inhibiting reuptake of serotonin to the presynaptic neuron. If GTS+OCD individuals indeed do have a higher SLC6A4 expression than those with only GTS, the use of SSRIs would also have a larger counteracting effect on the hyposerotonergic state resulting from the increased SLC6A4 expression. These results are also in line with the recent study by Müller-Vahl andcolleagues, who have shown increased SERT BP in TS+OCD individuals, but not in TS-only individuals, and a significant overall reduction in SERT binding following SSRI treatment [34].
When only considering the individuals with the same genotype, higher expression levels were only observed in GTS individuals with the LAC/LAC genotype compared to the controls. In a previous study, overall higher SLC6A4 expression levels were detected in human and rat cell lines with the LAC/LAC genotype when using reporter constructs [38], while in our study, the increased expression levels were only observed in GTS individuals, but not in the control individuals. This indicates that an overexpression of SLC6A4 in the presence of the LAC/LAC genotype is more protruding in GTS individuals with or without OCD, whereas the different three-locus genotypes do not seem to affect SLC6A4 expression in healthy controls.
In line with some of the other studies [16,53], we did not find any association between GTS diagnosis (with or without OCD) and SLC6A4 5-HTTLPR genotype nor allele distribution. Previously, the 5-HTTLPR/rs25331/rs25332 LAC haplotype was associated with OCD [38], and it was shown to be more frequent in GTS individuals compared to controls, in particular in GTS individuals without OCD [17]. In this study, we did not find an association between GTS diagnosis and any of the 5-HTTLPR/rs25331/rs25332 haplotype variants. This is likely to be due to the small size of the present cohort, especially with regards to the number of GTS+OCD individuals (n = 14) included in the haplotype analysis. The third locus, rs25532, was included only in a few studies with OCD and/or GTS individuals [17,38,54], but in several other studies, only the distribution of the 5-HTTLPR or 5-HTTLPR/rs25531 variants were investigated [39,53,55,56]. This challenges comparison across studies, which will either be limited by the number of studies, or is potentially erroneous due to different genotyping methodologies. Further studies investigating the distribution and the effect of the three-locus haplotype in larger cohorts of GTS individuals with or without OCD are warranted to provide a clearer picture.
Promoter methylation of SLC6A4 has not been investigated previously in individuals with GTS. In the present study, we did not observe any differences in the mean DNA methylation levels between GTS individuals with or without OCD and controls. We cannot, however, exclude that inclusion of further sites within the promoter CpG-island may affect the mean methylation levels, as we have investigated only eight selected CpG-sites, which were also employed in other studies [42,44]. In this study, differential methylation was investigated in blood, the only material available from the individuals. It is possible that brain regions, such as caudate nucleus tissue of the basal ganglia, the prefrontal cortex, the thalamus and the putamen known to be involved in GTS pathology, may be differentially methylated [57,58,59,60,61]. Another plausible explanation of the negative findings can be that serotonin expression in GTS individuals is not regulated by DNA methylation.
The SLC6A4 mRNA upregulation reported in the current study is furthermore in line with the dopamine hypothesis of GTS. Upregulation of SERT would result in increased serotonin clearance, and such a hyposerotonergic state is suggested to cause upregulation post-synaptic 5-HT2A receptors, which may facilitate dopamine release [17,33]. At the same time, SERT is also capable of dopamine uptake [29]. An upregulation of SLC6A4 expression could thus ultimately lead to abnormal levels of dopamine, a key component in GTS pathology. It is though warranted to investigate larger cohorts, as the size of the present cohort is relatively small.
5. Conclusions
In this study, we show that SLC6A4 expression is upregulated in GTS individuals, both with and without OCD, compared to controls. Although we did not observe any overrepresentation of any specific genotype in GTS individuals compared to controls, increased expression of SLC6A4 in GTS individuals may be modulated by the LAC/LAC genotype, as controls with this genotype had normal expression levels. DNA methylation levels at the promoter region of SLC6A4 were not associated with the presence of GTS (with or without OCD), mRNA expression levels or individual genotypes, suggesting that SLC6A4 expression is not regulated by DNA methylation of the investigated CpG-sites in the promotor region of the gene.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/12/1/86/s1: Table S1—Patients and controls, Table S2—Genotyping SLC6A4 (Ile425Val)—Primers and PCR conditions, Table S3—Genotyping SLC6A4 (5-HTTLPR)—Primers and PCR condition, Table S4—cDNA synthesis, Table S5—Quantitative PCR of SLC6A4 and GUSB—Probes and qPCR conditions, Table S6—Methylation analysis—Primers and PCR conditions, Figure S1—Genomic location of 5-HTTLPR, rs25531 and rs25532, Figure S2—Genomic location of CpG-sites, Figure S3—Expression levels and 5-HTTLPR genotypes, Figure S4—Mean SLC6A4 methylation levels.
Author Contributions
Conceptualization Z.T.; methodology Z.T.; formal analysis M.H. and A.M.L.; investigation, M.H. and A.M.L.; patient data N.M.D.; data curation M.H. and A.M.L.; writing—original draft preparation M.H. and A.M.L.; writing—review and editing Z.T.; visualization M.H. and A.M.L.; supervision Z.T., P.G., C.D., L.B.M. and V.A.B.; project administration Z.T.; funding acquisition Z.T. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Lundbeck Fonden, grant number R100-2011-9332 and Jascha Fonden, grant number 3674.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Danish Institutional Review Board (2011 H-2-2010-144. Date of approval: 3/3-2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hirschtritt M.E., Lee P.C., Pauls D.L., Dion Y., Grados M.A., Illmann C., King R.A., Sandor P., McMahon W.M., Lyon G.J., et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in tourette syndrome. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2014.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burd L., Li Q., Kerbeshian J., Klug M.G., Freeman R.D. Tourette syndrome and comorbid pervasive developmental disorders. J. Child Neurol. 2009 doi: 10.1177/0883073808322666. [DOI] [PubMed] [Google Scholar]
- 3.Mataix-Cols D., Isomura K., Pérez-Vigil A., Chang Z., Rück C., Johan Larsson K., Leckman J.F., Serlachius E., Larsson H., Lichtenstein P. Familial risks of tourette syndrome and chronic tic disorders a population-based cohort study. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0627. [DOI] [PubMed] [Google Scholar]
- 4.Davis L.K., Yu D., Keenan C.L., Gamazon E.R., Konkashbaev A.I., Derks E.M., Neale B.M., Yang J., Lee S.H., Evans P., et al. Partitioning the Heritability of Tourette Syndrome and Obsessive Compulsive Disorder Reveals Differences in Genetic Architecture. PLoS Genet. 2013 doi: 10.1371/journal.pgen.1003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertelsen B., Stefánsson H., Riff Jensen L., Melchior L., Mol Debes N., Groth C., Skov L., Werge T., Karagiannidis I., Tarnok Z., et al. Association of AADAC Deletion and Gilles de la Tourette Syndrome in a Large European Cohort. Biol. Psychiatry. 2016 doi: 10.1016/j.biopsych.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Yu D., Sul J.H., Tsetsos F., Nawaz M.S., Huang A.Y., Zelaya I., Illmann C., Osiecki L., Darrow S.M., Hirschtritt M.E., et al. Interrogating the genetic determinants of Tourette’s syndrome and other tiC disorders through genome-wide association studies. Am. J. Psychiatry. 2019 doi: 10.1176/appi.ajp.2018.18070857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang A.Y., Yu D., Davis L.K., Sul J.H., Tsetsos F., Ramensky V., Zelaya I., Ramos E.M., Osiecki L., Chen J.A., et al. Rare Copy Number Variants in NRXN1 and CNTN6 Increase Risk for Tourette Syndrome. Neuron. 2017 doi: 10.1016/j.neuron.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scharf J.M., Yu D., Mathews C.A., Neale B.M., Stewart S.E., Fagerness J.A., Evans P., Gamazon E., Edlund C.K., Service S.K., et al. Genome-wide association study of Tourette’s syndrome. Mol. Psychiatry. 2013 doi: 10.1038/mp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Draper A., Stephenson M.C., Jackson G.M., Pépés S., Morgan P.S., Morris P.G., Jackson S.R. Increased GABA contributes to enhanced control over motor excitability in tourette syndrome. Curr. Biol. 2014 doi: 10.1016/j.cub.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordstrom E.J., Bittner K.C., McGrath M.J., Parks C.R., Burton F.H. Hyperglutamatergic cortico-striato-thalamo-cortical circuit breaker drugs alleviate tics in a transgenic circuit model of Tourette’s syndrome. Brain Res. 2015 doi: 10.1016/j.brainres.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 11.Kanaan A.S., Gerasch S., García-García I., Lampe L., Pampel A., Anwander A., Near J., Möller H.E., Müller-Vahl K. Pathological glutamatergic neurotransmission in Gilles de la Tourette syndrome. Brain. 2017 doi: 10.1093/brain/aww285. [DOI] [PubMed] [Google Scholar]
- 12.Herzberg I., Valencia-Duarte A.V., Kay V.A., White D.J., Müller H., Rivas I.C., Mesa S.C., Cuartas M., García J., Bedoya G., et al. Association of DRD2 variants and Gilles de la Tourette syndrome in a family-based sample from a South American population isolate. Psychiatr. Genet. 2010 doi: 10.1097/YPG.0b013e32833a215a. [DOI] [PubMed] [Google Scholar]
- 13.Lee C.C., Chou I.C., Tsai C.H., Wang T.R., Li T.C., Tsai F.J. Dopamine receptor D2 gene polymorphisms are associated in Taiwanese children with Tourette syndrome. Pediatr. Neurol. 2005 doi: 10.1016/j.pediatrneurol.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Liu S., Cui J., Zhang X., Wu W., Niu H., Ma X., Xu H., Yi M. Variable number tandem repeats in dopamine receptor D4 in Tourette’s syndrome. Mov. Disord. 2014 doi: 10.1002/mds.26027. [DOI] [PubMed] [Google Scholar]
- 15.Yoon D.Y., Rippel C.A., Kobets A.J., Morris C.M., Lee J.E., Williams P.N., Bridges D.D., Vandenbergh D.J., Shugart Y.Y., Singer H.S. Dopaminergic polymorphisms in Tourette syndrome: Association with the DAT gene (SLC6A3) Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2007 doi: 10.1002/ajmg.b.30466. [DOI] [PubMed] [Google Scholar]
- 16.Dehning S., Müller N., Matz J., Bender A., Kerle I., Benninghoff J., Musil R., Spellmann I., Bondy B., Möller H.J., et al. A genetic variant of HTR2C may play a role in the manifestation of Tourette syndrome. Psychiatr. Genet. 2010 doi: 10.1097/YPG.0b013e32833511ce. [DOI] [PubMed] [Google Scholar]
- 17.Moya P.R., Wendland J.R., Rubenstein L.M., Timpano K.R., Heiman G.A., Tischfield J.A., King R.A., Andrews A.M., Ramamoorthy S., Mcmahon F.J., et al. Common and rare alleles of the serotonin transporter gene, SLC6A4, associated with Tourette’s disorder. Mov. Disord. 2013 doi: 10.1002/mds.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crane J., Fagerness J., Osiecki L., Gunnell B., Stewart S.E., Pauls D.L., Scharf J.M., Cath D., Heutink P., Grados M., et al. Family-based genetic association study of DLGAP3 in Tourette Syndrome. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2011 doi: 10.1002/ajmg.b.31134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karagiannidis I., Dehning S., Sandor P., Tarnok Z., Rizzo R., Wolanczyk T., Madruga-Garrido M., Hebebrand J., Nöthen M.M., Lehmkuhl G., et al. Support of the histaminergic hypothesis in tourette syndrome: Association of the histamine decarboxylase gene in a large sample of families. J. Med. Genet. 2013 doi: 10.1136/jmedgenet-2013-101637. [DOI] [PubMed] [Google Scholar]
- 20.Buse J., Schoenefeld K., Münchau A., Roessner V. Neuromodulation in Tourette syndrome: Dopamine and beyond. Neurosci. Biobehav. Rev. 2013;37:1069–1084. doi: 10.1016/j.neubiorev.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Maia T.V., Conceição V.A. Dopaminergic Disturbances in Tourette Syndrome: An Integrative Account. Biol. Psychiatry. 2018;84:332–344. doi: 10.1016/j.biopsych.2018.02.1172. [DOI] [PubMed] [Google Scholar]
- 22.Comings D.E. Blood serotonin and tryptophan in Tourette syndrome. Am. J. Med. Genet. 1990 doi: 10.1002/ajmg.1320360410. [DOI] [PubMed] [Google Scholar]
- 23.Budman C.L. The role of atypical antipsychotics for treatment of Tourette’s syndrome: An overview. Drugs. 2014;74:1177–1193. doi: 10.1007/s40265-014-0254-0. [DOI] [PubMed] [Google Scholar]
- 24.Sinopoli V.M., Burton C.L., Kronenberg S., Arnold P.D. A review of the role of serotonin system genes in obsessive-compulsive disorder. Neurosci. Biobehav. Rev. 2017;80:372–381. doi: 10.1016/j.neubiorev.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Bortolozzi A., Díaz-Mataix L., Scorza M.C., Celada P., Artigas F. The activation of 5-HT2A receptors in prefrontal cortex enhances dopaminergic activity. J. Neurochem. 2005 doi: 10.1111/j.1471-4159.2005.03485.x. [DOI] [PubMed] [Google Scholar]
- 26.De Deurwaerdère P., Navailles S., Berg K.A., Clarke W.P., Spampinato U. Constitutive Activity of the Serotonin2C Receptor Inhibits In Vivo Dopamine Release in the Rat Striatum and Nucleus Accumbens. J. Neurosci. 2004 doi: 10.1523/JNEUROSCI.0112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esposito E., Di Matteo V., Di Giovanni G. Serotonin-dopamine interaction: An overview. Prog. Brain Res. 2008;172:3–6. doi: 10.1016/S0079-6123(08)00901-1. [DOI] [PubMed] [Google Scholar]
- 28.Sorensen S.M., Kehne J.H., Fadayel G.M., Humphreys T.M., Ketteler H.J., Sullivan C.K., Taylor V.L., Schmidt C.J. Characterization of the 5-HT2 receptor antagonist MDL 100907 as a putative atypical antipsychotic: Behavioral, electrophysiological and neurochemical studies. J. Pharmacol. Exp. Ther. 1993;266:684–691. [PubMed] [Google Scholar]
- 29.Larsen M.B., Sonders M.S., Mortensen O.V., Larson G.A., Zahniser N.R., Amara S.G. Dopamine transport by the serotonin transporter: A Mechanistically distinct mode of substrate translocation. J. Neurosci. 2011 doi: 10.1523/JNEUROSCI.0576-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brivio P., Homberg J.R., Riva M.A., Calabrese F. Alterations of Glutamatergic Markers in the Prefrontal Cortex of Serotonin Transporter Knockout Rats: A Developmental Timeline. Cell. Mol. Neurobiol. 2019 doi: 10.1007/s10571-019-00673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinz A., Knable M.B., Wolf S.S., Jones D.W., Gorey J.G., Hyde T.M., Weinberger D.R. Tourette’s syndrome: [I-123]β-CIT SPECT correlates of vocal tic severity. Neurology. 1998 doi: 10.1212/WNL.51.4.1069. [DOI] [PubMed] [Google Scholar]
- 32.Müller-Vahl K.R., Meyer G.J., Knapp W.H., Emrich H.M., Gielow P., Brücke T., Berding G. Serotonin transporter binding in Tourette Syndrome. Neurosci. Lett. 2005 doi: 10.1016/j.neulet.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 33.Wong D.F., Brašić J.R., Singer H.S., Schretlen D.J., Kuwabara H., Zhou Y., Nandi A., Maris M.A., Alexander M., Ye W., et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: Clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology. 2008 doi: 10.1038/sj.npp.1301528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Müller-Vahl K.R., Szejko N., Wilke F., Jakubovski E., Geworski L., Bengel F., Berding G. Serotonin transporter binding is increased in Tourette syndrome with Obsessive Compulsive Disorder. Sci. Rep. 2019 doi: 10.1038/s41598-018-37710-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunther J., Tian Y., Stamova B., Lit L., Corbett B., Ander B., Zhan X., Jickling G., Bos-Veneman N., Liu D., et al. Catecholamine-related gene expression in blood correlates with tic severity in tourette syndrome. Psychiatry Res. 2012 doi: 10.1016/j.psychres.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 36.Li J., Li Z., Li A., Wang S., Qi F., Zhao L., Lv H. Abnormal expression of dopamine and serotonin transporters associated with the pathophysiologic mechanism of Tourette syndrome. Neurol. India. 2010 doi: 10.4103/0028-3886.68663. [DOI] [PubMed] [Google Scholar]
- 37.Kilic F., Murphy D.L., Rudnick G. A human serotonin transporter mutation causes constitutive activation of transport activity. Mol. Pharmacol. 2003 doi: 10.1124/mol.64.2.440. [DOI] [PubMed] [Google Scholar]
- 38.Wendland J.R., Moya P.R., Kruse M.R., Ren-Patterson R.F., Jensen C.L., Timpano K.R., Murphy D.L. A novel, putative gain-of-function haplotype at SLC6A4 associates with obsessive-compulsive disorder. Hum. Mol. Genet. 2008;17:717–723. doi: 10.1093/hmg/ddm343. [DOI] [PubMed] [Google Scholar]
- 39.Hu X.Z., Lipsky R.H., Zhu G., Akhtar L.A., Taubman J., Greenberg B.D., Xu K., Arnold P.D., Richter M.A., Kennedy J.L., et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am. J. Hum. Genet. 2006 doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bengel D., Greenberg B.D., Corá-Locatelli G., Altemus M., Heils A., Li Q., Murphy D.L. Association of the serotonin transporter promoter regulatory region polymorphism and obsessive-compulsive disorder. Mol. Psychiatry. 1999 doi: 10.1038/sj.mp.4000550. [DOI] [PubMed] [Google Scholar]
- 41.Lesch K.P., Bengel D., Heils A., Sabol S.Z., Greenberg B.D., Petri S., Benjamin J., Müller C.R., Hamer D.H., Murphy D.L. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science (80-.) 1996 doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 42.Iga J.I., Watanabe S.Y., Numata S., Umehara H., Nishi A., Kinoshita M., Inoshita M., Shimodera S., Fujita H., Ohmori T. Association study of polymorphism in the serotonin transporter gene promoter, methylation profiles, and expression in patients with major depressive disorder. Hum. Psychopharmacol. 2016 doi: 10.1002/hup.2527. [DOI] [PubMed] [Google Scholar]
- 43.Wang D., Szyf M., Benkelfat C., Provençal N., Turecki G., Caramaschi D., Côté S.M., Vitaro F., Tremblay R.E., Booij L. Peripheral SLC6A4 DNA methylation is associated with in vivo measures of human brain serotonin synthesis and childhood physical aggression. PLoS ONE. 2012 doi: 10.1371/journal.pone.0039501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park S., Lee J.M., Kim J.W., Cho D.Y., Yun H.J., Han D.H., Cheong J.H., Kim B.N. Associations between serotonin transporter gene (SLC6A4) methylation and clinical characteristics and cortical thickness in children with ADHD. Psychol. Med. 2015 doi: 10.1017/S003329171500094X. [DOI] [PubMed] [Google Scholar]
- 45.Grünblatt E., Marinova Z., Roth A., Gardini E., Ball J., Geissler J., Wojdacz T.K., Romanos M., Walitza S. Combining genetic and epigenetic parameters of the serotonin transporter gene in obsessive-compulsive disorder. J. Psychiatr. Res. 2018 doi: 10.1016/j.jpsychires.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 46.Müller-Vahl K.R., Loeber G., Kotsiari A., Müller-Engling L., Frieling H. Gilles de la Tourette syndrome is associated with hypermethylation of the dopamine D2 receptor gene. J. Psychiatr. Res. 2017 doi: 10.1016/j.jpsychires.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Palma-Gudiel H., Peralta V., Deuschle M., Navarro V., Fañanás L. Epigenetics-by-sex interaction for somatization conferred by methylation at the promoter region of SLC6A4 gene. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019 doi: 10.1016/j.pnpbp.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Mol Debes N.M.M., Hjalgrim H., Skov L. Validation of the presence of comorbidities in a danish clinical cohort of children with Tourette syndrome. J. Child Neurol. 2008 doi: 10.1177/0883073808316370. [DOI] [PubMed] [Google Scholar]
- 49.Rstudio T. RStudio: Integrated Development for R. Rstudio Team, PBC; Boston, MA, USA: 2020. [(accessed on 8 January 2021)]. Available online: http//www.rstudio.com. [Google Scholar]
- 50.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer; New York, NY, USA: 2016. [Google Scholar]
- 51.Kassambara A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 0.4.0. [(accessed on 8 January 2021)];2020 Available online: https://CRAN.R-project.org/package=ggpubr.
- 52.Steeves T.D.L., Fox S.H. Neurobiological basis of serotonin-dopamine antagonists in the treatment of Gilles de la Tourette syndrome. Prog. Brain Res. 2008;172:495–513. doi: 10.1016/S0079-6123(08)00924-2. [DOI] [PubMed] [Google Scholar]
- 53.Cavallini M.C., Di Bella D., Catalano M., Bellodi L. An association study between 5-HTTLPR polymorphism, COMT polymorphism, and Tourette’s syndrome. Psychiatry Res. 2000 doi: 10.1016/S0165-1781(00)00220-1. [DOI] [PubMed] [Google Scholar]
- 54.Rashidi F.S., Ahmadipour E., Shiravand S., Ahmadiani A., Asadi S., Shams J. Association of the functional serotonin transporter haplotype with familial form of obsessive compulsive disorder in Iranian patients. Int. J. Psychiatry Clin. Pract. 2018 doi: 10.1080/13651501.2017.1353634. [DOI] [PubMed] [Google Scholar]
- 55.Voyiaziakis E., Evgrafov O., Li D., Yoon H.J., Tabares P., Samuels J., Wang Y., Riddle M.A., Grados M.A., Bienvenu O.J., et al. Association of SLC6A4 variants with obsessive-compulsive disorder in a large multicenter US family study. Mol. Psychiatry. 2011 doi: 10.1038/mp.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wendland J.R., Kruse M.R., Cromer K.C., Murphy D.L. A large case-control study of common functional SLC6A4 and BDNF variants in obsessive-compulsive disorder. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301394. [DOI] [PubMed] [Google Scholar]
- 57.Makki M.I., Behen M., Bhatt A., Wilson B., Chugani H.T. Microstructural abnormalities of striatum and thalamus in children with tourette syndrome. Mov. Disord. 2008 doi: 10.1002/mds.22264. [DOI] [PubMed] [Google Scholar]
- 58.Draganski B., Martino D., Cavanna A.E., Hutton C., Orth M., Robertson M.M., Critchley H.D., Frackowiak R.S. Multispectral brain morphometry in Tourette syndrome persisting into adulthood. Brain. 2010 doi: 10.1093/brain/awq300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Müller-Vahl K.R., Kaufmann J., Grosskreutz J., Dengler R., Emrich H.M., Peschel T. Prefrontal and anterior cingulate cortex abnormalities in Tourette Syndrome: Evidence from voxel-based morphometry and magnetization transfer imaging. BMC Neurosci. 2009 doi: 10.1186/1471-2202-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Müller-Vahl K.R., Grosskreutz J., Prell T., Kaufmann J., Bodammer N., Peschel T. Tics are caused by alterations in prefrontal areas, thalamus and putamen, while changes in the cingulate gyrus reflect secondary compensatory mechanisms. BMC Neurosci. 2014 doi: 10.1186/1471-2202-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Segura B., Strafella A.P. International Review of Neurobiology. Academic Press; Cambridge, MA, USA: 2013. Functional imaging of dopaminergic neurotransmission in tourette syndrome. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.