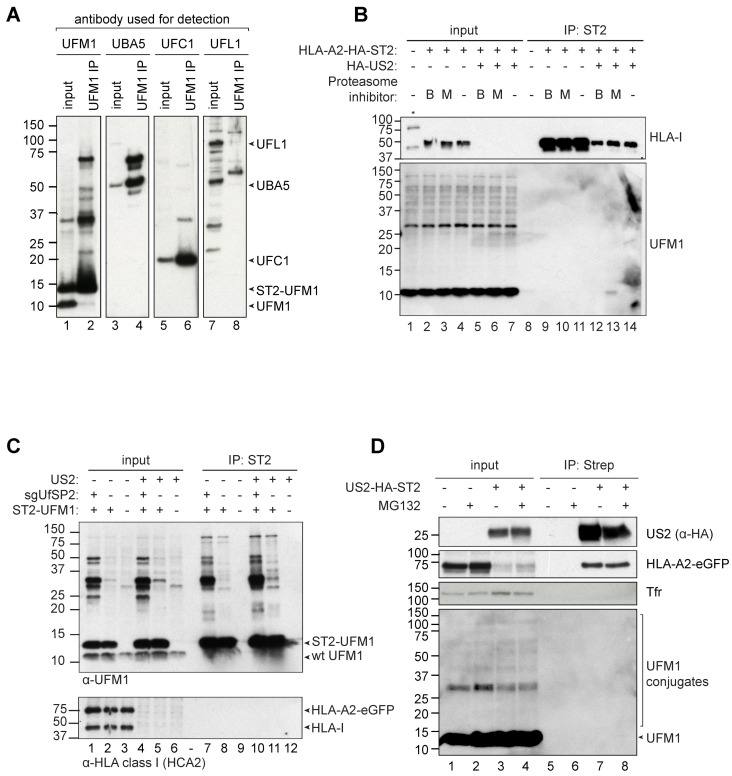
Figure 4.
HLA class I, US2, and p97 are not UFMylated in US2-expressing cells. (A) ST2-UFM1 was immunoprecipitated from U937 cells co-expressing HLA-A2-eGFP and US2 in 1% LMNG lysis buffer. Samples were subsequently blotted for UFM1 to detect the interaction between UFM1 and its E1 (UBA5), E2 (UFC1) and E3 (UFL1) enzymes. Whereas UBA5 and UFL1 interact with UFM1, this could not be established for UFL1. (B) U937 cells expressing HLA-A2-HA-ST2 in the presence or absence of US2 were incubated with the proteasome inhibitors Bortezomib (B) or MG132 (M) to accumulate ubiquitinated HLA class I that would otherwise be degraded by the proteasome. HLA-A2-HA-StrepII was immunoprecipitated from these cells in 1% Digitonin lysis buffer, immunoblotted, and stained for UFMylation. (C) ST2-UFM1 was immunoprecipitated from U937 cells also expressing HLA-A2-eGFP, in the presence or absence of US2. For immunoprecipitation, 1% Digitonin lysis buffer was used. Indicated cell lines also contained a sgRNA targeting UfSP2. Samples were stained with a UFM1-specific antibody (top panel) or with HCA2, an HLA class I-specific antibody (bottom panel). (D) ST2-HA-US2 was immunoprecipitated from U937 cells also expressing eGFP-HLA-A2 in 1% Digitonin lysis buffer. Samples were immunoblotted and stained for US2, the HLA-A2-eGFP chimera (HCA2 antibody), and UFM1. Tfr was used as a loading control.