Table 1.
Inhibitory activities of compounds 3–5 against human AR and human PTP1B, expressed as IC50 a.
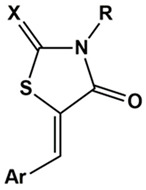
| X | R | Ar | AR IC50 (µM) |
PTP1B IC50 (µM) |
|
|---|---|---|---|---|---|
| 3a | O | (CH2)2COOH | 3-OC6H5-C6H4 | 11.9 ± 0.9 | 79% at 50 µM |
| 3b | O | (CH2)2COOH | 4-OC6H5-C6H4 | 43.8 ± 7.1 | 56% at 50 µM |
| 3c | O | (CH2)2COOH | 3-OCH2C6H5-C6H4 | 14.3 ± 1.0 | 76% at 50 µM |
| 3d | O | (CH2)2COOH | 4-OCH2C6H5-C6H4 | 35.7 ± 3.0 | 77% at 50 µM |
| 3e | O | (CH2)2COOH | 3-OCH2CH2C6H5-C6H4 | 27.9 ± 3.1 | 64% at 50 µM |
| 3f | O | (CH2)2COOH | 4-OCH2CH2C6H5-C6H4 | 50.2 ± 4.6 | 46% at 50 µM |
| 4a | S | (CH2)2COOH | 3-OC6H5-C6H4 | 2.2 ± 0.1 | 34.1 ± 0.5 |
| 4b | S | (CH2)2COOH | 4-OC6H5-C6H4 | 7.6 ± 0.6 | 29.5 ± 0.4 |
| 4c | S | (CH2)2COOH | 3-OCH2C6H5-C6H4 | 3.8 ± 0.1 | 42.8 ± 0.7 |
| 4d | S | (CH2)2COOH | 4-OCH2C6H5-C6H4 | 8.4 ± 0.7 | 34.9 ± 0.7 |
| 4e | S | (CH2)2COOH | 3-OCH2CH2C6H5-C6H4 | 2.3 ± 0.1 | 55.5 ± 0.8 |
| 4f | S | (CH2)2COOH | 4-OCH2CH2C6H5-C6H4 | 5.3 ± 0.4 | 12.7 ± 0.3 |
| 5a | O | CH2CH=CHCOOH | 3-OC6H5-C6H4 | 3.9 ± 0.2 | 42.1 ± 0.3 |
| 5b | O | CH2CH=CHCOOH | 4-OC6H5-C6H4 | 84% at 10 µM | 39.7 ± 0.1 |
| 5c | O | CH2CH=CHCOOH | 4-C6H5-C6H4 | 88% at 5 µM | 34.8 ± 0.5 |
| 5d | O | CH2CH=CHCOOH | 1-naphthyl | 3.7 ± 0.2 | 40.3 ± 0.5 |
| 5e | O | CH2CH=CHCOOH | 2-naphthyl | 86% at 10 µM | 37.1 ± 0.4 |
| Epalrestat | 0.102 ± 0.005 | ||||
| Vanadate | 0.4 ± 0.01 | ||||
a IC50 (µM) or % enzyme residual activity at the indicated concentration. Values are expressed as the mean ± S.E.M (see methods for details).
