Abstract
Lipid rafts are critical cell membrane lipid platforms enriched in sphingolipid and cholesterol content involved in diverse cellular processes. They have been proposed to influence membrane properties and to accommodate receptors within themselves by facilitating their interaction with ligands. Over the past decade, technical advances have improved our understanding of lipid rafts as bioactive structures. In this review, we will cover the more recent findings about cholesterol, sphingolipids and lipid rafts located in cellular and nuclear membranes in cancer. Collectively, the data provide insights on the role of lipid rafts as biomolecular targets in cancer with good perspectives for the development of innovative therapeutic strategies.
Keywords: cholesterol, sphingolipid, cancer, lipid raft, therapeutic target
1. Introduction
Over the last two decades, scientific research has conceptually changed the structure and function of the cell membranes described about 40 years ago. It was the faraway 1972 when Singer and Nicolson described the fluid mosaic theory, hypothesizing that the cell membrane is composed of a homogeneous lipid bilayer formed by glycerophospholipids and cholesterol, and inside proteins playing the main communication and response roles of the cell membrane. Over time, a mosaic of domains with unique biochemical compositions has been demonstrated, and among them, lipid rafts. Originally, lipid rafts were identified as ordered structures due to the aggregation of sphingolipids that were responsible for their molecular composition and properties, different from those of the surrounding membrane. Later, the presence of cholesterol within a liquid-ordered phase that interacted with sphingolipids by stabilizing lipid rafts was demonstrated.
2. Lipid Rafts
2.1. Lipid Rafts in Cell Membrane
In the cell membrane, lipid rafts or cholesterol-sphingolipids enriched domains act as platforms for cellular and/or exogenous proteins with different functions, including the shuttling of molecules on the cell surface, organization of cell signal transduction pathways, entry for pathogens and toxins, and the formation of pathological forms of proteins [1]. For example, sphingomyelinase influences cell proliferation and migration by regulating the ceramide/sphingomyelin balance in lipid rafts and thereby changing receptor-mediated signal transduction [2].
In the brain, lipid rafts are found in neurons, astrocytes, and microglia and play a role in the organization of multiprotein complexes involved in signal transduction. Cognitive decline in aging and neurodegenerative diseases is associated with the destructuration of lipid rafts [3]. Interestingly, gangliosides are enriched in lipid rafts and their levels change in healthy aging, Huntington’s disease, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, stroke, multiple sclerosis and epilepsy [4]. Among gangliosides, monosialoganglioside 1 (GM1) is the most common in lipid rafts of the brain. GM1 in lipid rafts mediate the pathophysiology of several cellular processes, including development, differentiation, and protection of neuronal tissue. GM1 has been proposed to play a negative role in Alzheimer’s disease by changing spatial organization of extracellular amyloid β [5].
In the inflammation process, lipid rafts contribute to permitting of the entry of bacterial pathogens into non-phagocytic cells, i.e., Listeria monocytogenes. In this way, the microorganism is capable of interacting and crossing physiological barriers, as intestinal, blood-brain, and placental barriers with severe tissue damage [6]. Moreover, some recognition receptors for viruses, including coronavirus, cluster into lipid rafts [7]. Thus, lipid platforms are considered as potential targets to block virus entry into endothelial cells [8].
2.2. Lipid Rafts in Nuclear Membrane
Over the past 15 years, significant research has focused on the presence cholesterol-sphingolipids enriched domains in the inner nuclear membrane. These lipid domains are now clearly appreciated to function as essential platforms for nuclear function. The inner nuclear membrane is a highly functional structure [9]. It is internally covered by the nuclear lamina associated with transcriptionally active chromatin, which includes the site for the nascent pre-mRNA transcripts. The “textbook” blueprint of nuclear structure and function describes the periphery of the nucleus and provides a platform for sequestering transcription factors away from chromatin, thereby modulating gene expression [10]. However, the reality is much more complex. The link of active chromatin (chromatin whose DNA is being duplicated or transcribed) to the nuclear lamina is due to specific lipid domains enriched in sphingomyelin and cholesterol [11]. In this way, sphingomyelin participates in maintaining the internal nuclear organization and function. Using sphingomyelinase, coupled with colloidal gold grains and immune anti-bromodeoxyuridine (BrdU), colocalization of sphingomyelin and nascent RNA has been demonstrated [12]. The intranuclear injection of sphingomyelinase destructures the active chromatin with alteration of the RNA transcription [12]. However, nuclear lipid domains contain transcription factors that regulate mRNA synthesis, i.e., signal transducer and activator of transcription 3 [11]. Interestingly, it has been demonstrated that both vitamin D3 and its receptor, which is a transcription factor, are localized in nuclear lipid domains [13,14]. Moreover, dexamethasone, a drug that acts directly in the nucleus by influencing RNA transcription, localizes in nuclear lipid domains [15].
3. Sphingolipids in Cancer
Sphingolipids have been identified as key regulators of a wide variety of cellular processes, including proliferation, differentiation, adhesion, autophagy, inflammation, migration and angiogenesis, which are crucial in cancer [16].
3.1. Sphingomyelin
Increased levels of sphingomyelin have been found in tumoral tissues such as hepatocellular carcinomas [17], compared to normal ones, but the specific changes depend on the type of cancers. However, sphingomyelin levels appear to be inversely correlated with the aggressiveness of some colorectal and breast cancer cell lines. Lower levels of sphingomyelin were found in prostate cancer, in nonsmallcell lung cancerand in esophageal tumor compared with their respective normal tissue due to the dysregulation of its biosynthesis [18]. Interestingly, sphingomyelin biosynthesis might be a downstream target of oncogenes and might also alter oncogenic activity. For example, upregulation of sphingomyelin synthase 1 transcription, translation and activity by the oncogene Bcr-abl has been reported in chronic myelogenous leukemia cell lines [19,20]. Pharmacological and siRNA-mediated inhibition of sphingomyelin synthase 1 in these cells reduced cell growth and induced the accumulation of ceramide, together with a reduction in diacylglycerol levels [19]. However, the maintenance of the correct localization and/or level of sphingomyelin at the plasma membrane is important to preserve the localization of oncogenic KRAS at the inner leaflet of the membrane and its protumorigenic effects [21]. Interestingly, the breakdown of sphingomyelin, due to the action of both acid and neutral sphingomyelinases, with the consequent production of different species of ceramides, is involved in the apoptosis of cancer cells. Notably, ingastric cancer cells gentamicin stimulates acid sphingomyelinase [22] and vitamin D3 neutral sphingomyelinase [23]. Moreover, vitamin D3 reduces glioblastoma cell aggressivenessvia neutral sphingomyelinase [24].
3.2. Ceramide
Ceramide is considered to be a mediator of cell death and senescence. Radiation treatment and chemotherapicdrugs, such as doxorubicin, etoposide, vincristine, and celecoxi band taxol, are known to induce ceramide accumulation, while decreased levels of ceramide are associated with drug resistance [25]. However, accumulating evidence indicates that distinct ceramide species, which are generated in different subcellular compartments and/or by different metabolic pathways, might exert different functions. Indeed, the effects of ceramides with specific chain lengths on cell fate appear to be cell-type specific. For example, a shift in ceramide composition from C24 to C16 increases the susceptibility to apoptosis in HeLa cells [26], but C16:0-ceramide generated by ceramide synthase 1 suppresses tumor growth, while C18:0-ceramide generated by ceramide synthases 5/6 protects tumors from apoptosis in human head and neck squamous cell carcinomas [27]. Celecoxib mediates its anti-proliferative effects, in part, by selectively activating ceramide synthase 6 in human colon carcinoma cells, leading to an increase in C16:0-ceramide [28]. Ceramide synthase 6 and C16-ceramide levels are higher in acute lymphoblastic leukemia cells compared to peripheral blood mononuclear cells and T lymphocytes derived from healthy human volunteers and it has been demonstrated that ceramide synthase 6 interferes with Fas-associated protein with death domain, death-inducing signaling complex (FADD DISC). FADD DISC assembly inhibiting the extrinsic pathway of apoptosis upon drug treatment, and thus inducing chemo-resistance in T-acute lymphoblastic leukemia cell lines [29]. The levels of C16:0-ceramide, C24:1-ceramide and C24:0-ceramide were found to be higher in malignant breast tumors than in benign and normal tissue and to correlate with disease severity [28]. By contrast, Moro et al. (2018) have found that the increased ceramide levels are inversely associated with aggressive phenotypes in breast cancers [30]. The authors also reported that a high ratio sphingosine-1-phosphate (S1P)/ceramide correlates with high proliferation and that patients with high expression of ceramide-generating enzymes exhibit worse prognosis, suggesting that this effect was probably due to the proliferative effect of S1P originating from ceramide. Acid ceramidase, the enzyme that catalyzes the formation of sphingosine, is over-expressed in 70% of head and neck squamous cell tumors compared with normal tissues, suggesting that this enzyme may play an important role in facilitating head and neck squamous cell cancer growth. Indeed, the over-expression of acid ceramidase in SCC-1 cells increases resistance to Fas-induced cell death, whereas down-regulation or chemical inhibition of acid ceramidase sesensitizes the SCC-1 cancer cell line to Fas-induced apoptosis [31]. Immunohistochemical analysis of primary prostate cancer samples showed that higher levels of acid ceramidase were associated with more advanced stages of this neoplasia [32]. Moreover, radiation-induced acid ceramidasee confers prostate cancer resistance and tumor relapse [33]. It has been reported that acid ceramidase promotes the nuclear export of phosphatase and tensin homolog (PTEN) through S1P- mediated Akt signaling, leading to tumor cell proliferation, and resistance to therapy [34]. Elevated expression of acid ceramidase in breast and epithelial ovarian cancers has been associated with positive outcomes [35,36].
3.3. Ceramide-1-Phosphate
Ceramide kinase (CerK) phosphorylates ceramide to form ceramide-1-phosphate (C1P), a molecule involved in proliferation, migration and inflammation [37] via the production of several cytokines and chemokines [38]. C1P-enhanced pancreatic tumor cell migration and invasion are dependent upon PI3K, Akt1, mTOR1, ERK1-2, and RhoA/ROCK signaling pathways [39]. CerK activity is required in the proliferation of neuroblastoma cells [40], of breast cancer cells [41], and of Kaposi sarcoma stem cells [42]. In metastatic breast cancer cells, CerK is upregulated and contributes to the migration and invasion through PI3K/Akt/Rho kinase activation [43]. By contrast, downregulation of CerK increases migration in A549 lung cancer cells by stimulating Rac1 activation and lamellipodium formation [44].
3.4. Sphingosine-1-Phosphate
Sphingosine 1-phosphate (S1P) is generated by sphingosine kinases 1 and 2 (SphK1 and SphK2) and is a bioactive lipid mediator, which acts both as a second and a first messenger. It is able to bind to five G-protein coupled receptors S1P1R-5 and to regulate different aspects of cancer progression, including cell proliferation, apoptosis, migration, inflammation and angiogenesis [16,45]. Many studies have suggested that the SphK1/S1P axis could play a role in the promotion of several types of tumors, based on the overexpression of SphK1 in tumor cell lines and in patient samples, and sometimes, on the measured increased S1P levels in tumors and/or in patient blood. These studies have recently been reviewed for breast [46], ovarian, [47], and gastrointestinal cancers [48], hepatocellular carcinoma [49], melanoma [50] and glioblastoma [51]. High S1P in breast tumor tissue is significantly associated with lymphnode metastasis [52]. In addition, S1P increased angiogenesis. Thus, in breast cancer, the SphK inhibitor decreases angiogenesis around the primary tumor and in lymph nodes [53]. From these observations, SphK/S1P molecules have been considered cancer biomarkers or therapeutic targets. However, the utility of targeting S1P in tumors is hindered by the role of S1P1 on immune cell trafficking [54]. Recently, it has been proposed that targeting S1PR4, which has a minor effect on immune cell trafficking, could be a successful alternative. Indeed, S1PR4 ablation reduces mammary and colorectal tumor growth and improves chemotherapy [55].
3.5. Gangliosides
Some O-acetylated gangliosides, which are transiently expressed during development, reemerge during tumorigenesis and can be used as tumor markers [56]. GM3 is expressed in melanoma, but not in normal melanocytes [57]. Expression of disialoganglioside 2 (GD2) and GD3 leads to cell growth and invasion [58]. GD2 might have a role in the fixation of melanoma cells at metastasized sites [59]. Moreover, it is highly expressed in neuroblastoma [60] and an anti-GD2 antibody Dinutuximab (Unituxin™) has been approved by Food Drug Administration and European Medicines Agency for the treatment of high-risk neuroblastoma patients [61]. GM3 is associated with aggressiveness and negative outcomes in many types of tumors, such as neuroblastoma, astrocytoma, thyroid carcinoma, sarcoma, cutaneous melanoma, and non-small cell lung cancer [62]. In addition, GM3 inhibits tumor cell proliferation through angiogenesis inhibition or decreases in cell motility [63].
4. Cholesterol in Cancer
Today, the dysregulation of cholesterol metabolism, pathologically associated with cancer, is the object of extensive discussion. Epidemiological studies reported an association between cancer and serum cholesterol levels but these results have not been confirmed by other authors [64]. Differences in the results might partly depend on the investigated tumour. A positive association between elevated serum cholesterollevels and increased cancer risk has been found in melanoma, prostate cancer, non-Hodgkin’s lymphoma, endometrial and breast cancer [65]. In other types of cancers, no association with hypercholesterolemia [66] or hypocholesterolemia [67] was found. Another crucial aspect about cholesterol in cancer relates to the diet. Even if several studies suggest a positive correlation between dietary cholesterol uptake and cancer risk, these studies are considered to be questionable, given the poor reliability of dietary investigations [68,69].
Interestingly, cancer development and progression is closely related to cholesterol metabolism at the cellular level. Less important is the blood cholesterol level [70,71]. Furthermore, studies on intracellular cholesterol traffic inhibitors showed the importance of subcellular cholesterol distribution [72]. Once synthesized in the endoplasmic reticulum, cholesterol is distributed to cell organelles, and to the plasma membrane, by sterol transfer protein [73]. Intracellular cholesterol levels and its compartmentalization are modulated by a complex and are integrated by a metabolic network involving de novo biosynthesis, the LDL-cholesterol entry by LDL receptors, cholesterol distribution by sterol transferring proteins, and cholesterol efflux by ABCA1 and ABCG1 proteins [74]. More than one gene and/or protein of this complex network can be deregulated in cancer. Indeed, it has been observed that many enzymes involved in the de novo cholesterol biosynthesis are deregulated in cancer cells, especially 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR), the rate-limiting enzyme of the entire biosynthesis process [75,76]. Statins, a class of HMGCR inhibitors, have anti-cancer activity in a wide range of both liquid and solid tumours [77], not including invasive breast cancer in postmenopausal women [78]. Oncogenic and tumor suppressor factors activate or inhibit, respectively, sterol regulatory element-binding proteins, consequently regulating cholesterol synthesis [79]. Notably, the tumor suppressor protein p53 inhibits the sterol regulatory element-binding proteins-mevalonate pathway [80]. The analysis of more than 800 human cancer cell lines showed that inhibition of the mevalonate pathway by p53 activates mechanisms of tumor suppression in many types of cancer [81]. A role of the interaction between p53 and Hippo tumor-suppressor pathway in the coordination of cell proliferation has been reported [82]. In addition, several molecules derived from the cholesterol metabolism have also been implicated in the development of several types of cancer [83], including oxisterols [84], estrogens [85] and bile acids [86].
Moreover, a lot of experimental evidence underlines a close correlation between lipid rafts present in tumor-associated macrophages, i.e., macrophages that change phenotypes in the tumor microenvironment, and tumor progression and invasion [87]. This is relevant, considering that tumor-associated macrophages play a role in immunological suppression [88].
5. Lipid Rafts in Cancer
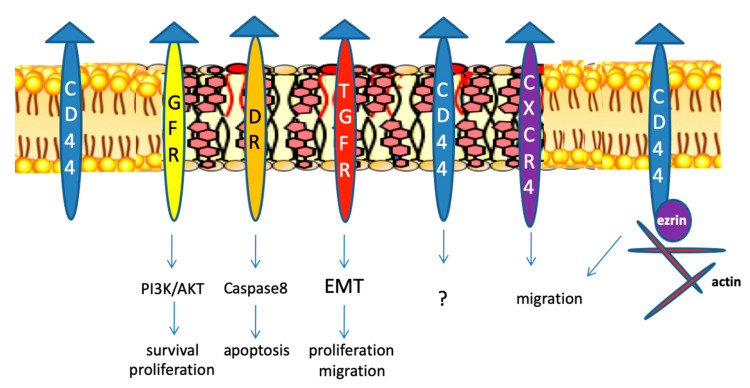
A large body of work has netted several significant advances in the study of the role of lipid rafts in cancer. These include molecular mechanisms that have led to the elucidation of key functions of sphingolipids and cholesterol in the cell membrane and nuclear membrane platforms. Unwittingly, these advances have highlighted previously unappreciated complexities of interactions between lipid and protein components of lipid rafts. Thus, an intensive 10 years of study has led to the discovery that well known proteins involved in migration, cell adhesion, invasion, angiogenesis and metastasis are localized in lipid rafts (Figure 1) [89].
Figure 1.
Roles of lipid rafts in cancer NO. Lipid rafts are involved in proliferation, apoptosis, migration, cell adhesion, and metastasis. Many proteins involved in cancer are localized in rafts. For example, growth factors receptors (GFR) through activation of PI3K/AKT or MAPK pathways lead to survival and proliferation; dead receptors (DR) such as TRAIL and Fas activate caspase 8 and the apoptotic cascade; the chemokine receptor CXCR4 activation leads to migration; the transforming growth factor-1 β receptor (TGFR) is involved in epithelial to mesenchimal transition (EMT). CD44 is localized in rafts, but its role is unclear (2). When it moves out of them, it is able to interact with the metalloproteinase 10 [90] and with ezrin, leading to actin remodelling and cell migration. Triangles: ligands of the receptors. See explanations and references in the text.
A proteomic analysis of lipid rafts and cytoskeleton demonstrated a stronger protein–protein interaction network in breast cancer, melanoma, and renal carcinoma cells than in the respective normal cells [91]. Simulation analysis showed a higher stabilization of lipid rafts in cancer cells than in normal cells [91].
Significant research has focused on cholesterol and sphingolipids metabolism enzymes in lipid rafts and has demonstrated that they act as stimulus responders/coordinators, involved in the cell reaction to several pathophysiological conditions. Recently, cholesterol metabolism is clearly appreciated to function as a regulator of lipid rafts in cell membranes. Farnesyl-diphosphate farnesyltransferase 1, a key enzyme of cholesterol synthesis, increases lipid raft content in the membranes of cancer cells [92]. Goossens et al. (2019) demonstrated that, in ovarian cancer cells, cholesterol depletion from lipid rafts, caused by increased cholesterol efflux due to specific transporters, is responsible for the phenotypic reprogramming of macrophages into tumor-associated macrophages, making them more responsive to pro-tumor signals such as interleukin-4 and more resistant to the action of anti-tumor cytokines such as interferon-gamma [93].
Moreover, molecular localization of enzymes of sphingolipid metabolism in lipid rafts have led to elucidation of their key function in cancer cells and/or in drug sensitivity. SphKs and S1PRss are localized both inside and outside lipid microdomains [94]. SphK1 phosphorylation and translocation to plasma membrane microdomains is important for its oncogenic effects while localization outside the microdomains leads to survival with inhibition of proliferation [95]. GMs present in lipid rafts of cancer cells are involved in signaling, cell-to-cell recognition, and invasion [57,60]. Notably, GD3 accumulation in rafts has been associated with apoptosis induction [96]. GD3 is normally localized at the plasma membrane but, it translocates to the mitochondrial membranes following CD95/Fas-induced apoptosis [97]. It has been hypothesized that GD3, by interacting with mitochondrial raft-like microdomains, may trigger events associated with apoptosis, including changes in mitochondrial membrane potential, mitochondrial fission, and release of apoptogenic factors. Then, some GD3-containing “small” mitochondria, possibly derived from their fission process, could reach the nuclear envelope and contribute to the execution of apoptosis [98].
Thus, significant progress has been achieved in the past few years in understanding specific cholesterol-sphingolipid pathways mediated by specific enzymes. This progress is being translated in the development and optimization of novel therapeutic strategies based on targeting lipid rafts of cancer cell membranes [99].
5.1. Lipid Rafts in Metastasis
Many processes involved in cancer metastasis are modulated by lipid rafts. They include cell adhesion, migration and epithelial-to-mesenchimal transition (EMT), which is characterized by the loss of contact between cells and acquisition of fibroblastic phenotype. There are recent reviews on the role of lipid rafts in metastasis and EMT [89,90]. For example, disrupting lipid rafts with cyclodestrin or nystatin reverses EMT induced by TGF-β1 in breast cancer and gastric cancer cells, respectively [100,101]. Many studies demonstrate that proteins localized in lipid rafts such as podoplanin, caveolin-1, flotillins and CD44 are essential for EMT. For example, raft localization of podoplanin is necessary for the recruitment and activation of proteins of the Ezrin family, induction of EMT and thereby invadopodia function [102]. Upregulation of caveolin-1 is associated with EMT, increased migration of bladder cancer cell lines and metastatic bladder cancer [103]. Overexpression of caveolin-1 enhances EMT by increasing the transcription factor Slug, through activation of the PI3K/AKT pathway [103], while knockdown of caveolin-1 reduces Slug expression and EMT. Flotillins are upregulated in a variety of cancers [104], including gastric cancer. Flotillin-2 is is required for TGFβ-induced EMT in gastric cancer cells [105] since downregulation of flotillin-2 reduced the expression of the EMT markers vimentin and N-cadherin. CD44 is a biomarker of cancer stem cells, it is involved in the regulation of proliferation, invasion, metastasis and resistance and it is usually a marker of adverse prognosis [106]. The proteolytic cleavage of CD44 is required for the migration of tumor cells. CD44 localized in lipid rafts while its processing enzyme, the metalloproteinase 10, is mainly found outside rafts. Localization of CD44 outside lipid rafts in human glioblastoma allows metalloproteinase-mediated CD44 shedding and tumor cell migration [107] while raft disruption by cyclodestrins or simvastatin increase CD44 shedding and migration. Localization of CD44 in lipid rafts by palmitoylation limits the interaction of CD44 with its migratory binding partner ezrin, and reduces breast cancer cell migration [108].
Other proteins involved in invasion and metastasis, including potassium channels, urokinase-type plasminogen activator receptor and the type 1 transmembrane glycoprotein mucin 1, are found in lipid rafts [90]. The association of the potassium channel, SK3 with the calcium channel Orai1 in lipid rafts leads to the upregulation of calcium influx, increase of breast cancer cell migration and bone metastasis [109]. The treatment with the alkyl-lipid ohmline results in the translocation of the SK3–Orai1 complex out of lipid rafts, calcium entry reduction and cell migration impairment [109].
Interestingly, Tisza et al. [110] have reported that lipid raft destabilization is necessary for EMT. In breast cancer cells, this reduction in stability was necessary for the maintenance of the stem cell phenotype and EMT-induced remodeling. When raft stability was increased by adding docosahexaenoic acid, the metastatic capacity of the cells was reduced. Therefore, not only disrupting lipid rafts prevents EMT signaling, but EMT also requires modification of the physical properties of the lipid microdomains.
5.2. Lipid Rafts as Therapeutic Targets in Cancer
There are multiple lines of evidence that lipid rafts are promising targets in cancer therapy (Table 1).
Table 1.
Main papers showing lipid rafts of cellular and nuclear membranes as targets of anticancer molecules.
| MOLECULES TARGETING LIPID RAFTS IN CANCER | |
|---|---|
| Cell Membrane Lipid Rats | |
| Hydroxypropyl-β-cyclodextrin | [100] |
| Nystatin | [101] |
| TGF-β1 | [100,101] |
| Podoplanin | [102] |
| Caveolin-1 | [103] |
| Flotillin | [104,105] |
| CD44 | [106,107,108] |
| SK3 | [109] |
| Orai1 | [109] |
| TRAIL | [111,117] |
| Edelfosine | [112] |
| Endocannabinoids | [113] |
| Liver X receptor | [118] |
| γ-tocotrienol | [119] |
| Bufalin | [120] |
| Resveratrol | [121] |
| Methyl beta cyclodextrin | [122] |
| Epidermal growth factor receptor | [123] |
| β-elemene | [124] |
| Fumonisin B1 | [125] |
| Antagonist 20S-protopanaxadiol | [126] |
| Lovastatin | [127] |
| CXCL12 | [128] |
| Temozolomide | [129] |
| Nuclear membrane lipid rafts | |
| Gentamicin | [130] |
| Daunorubicin | [131] |
One interesting feature to consider in anti-cancer therapy is the recent use of anti-cancer compounds with clinical efficacy as tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) that selectively induces the apoptosis pathway in tumor cells leading to tumor cell death. However, some compounds have entered clinical trials in oncology [111]. However, they have had some problems in their application due to drug resistance, off-target toxicities, short half-life and limited uptake of TRAIL genes. Some interesting results have involved lipid rafts. Edelfosine, aalkylphospholipid analog, recruiting the death receptor into lipid rafts, is able to induce cancer cells apoptosis [112]. Moreover the anticancer effect of endocannabinoids is mediated by the perturbation of lipid rafts [113]. Thus, the recruitment of death receptors into lipid rafts is essential for drug response through apoptosis activation in cancer cells. However, the involvement of lipid raft is not limited to death receptors, but includes other signaling molecules [114]. Upregulation of lipid and protein metabolism enzymes of lipid rafts is involved in drug transport [115]. Gajate and Mollinedo (2015) reported that the presence of Fas/CD95 in lipid rafts is a putative target for hematologic cancer therapy [116]. These data suggest that the quantity and structure of cellular and nuclear lipid rafts are important for the therapeutic response of cancer cells. This emerging role of lipid rafts has significant implications in different liquid and solid tumors.
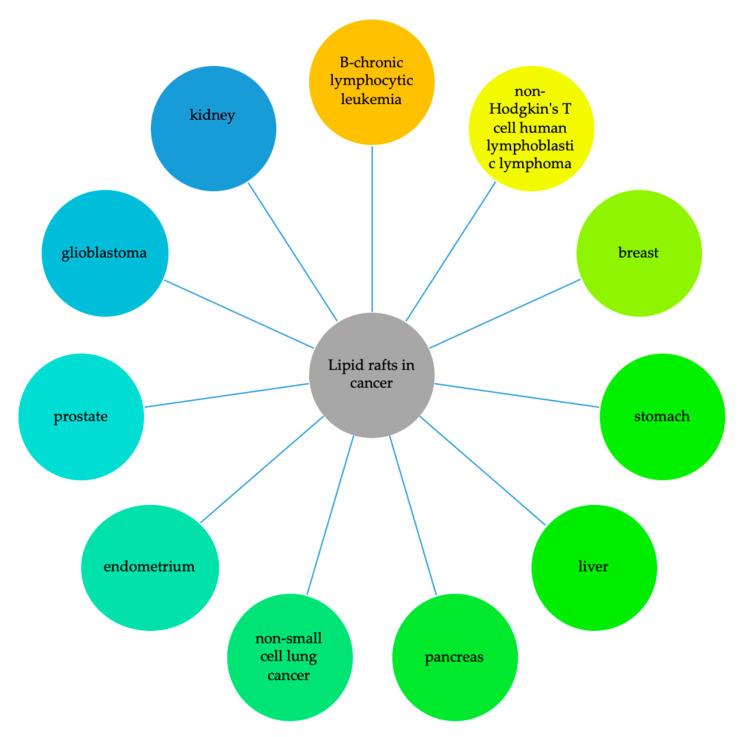
Lipid rafts are involved in liquid and solid cancers (Figure 2).
Figure 2.
Schematic representation of the main cancers in which lipid rafts are involved. Lipid rafts are involved in liquid [117,130,132] and solid tumors [118,119,120,121,122,123,124,125,126,127,128,129,131,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148] including tumors of breast [118,119,120,121,122,133,134,135,136,137,138,139], stomach [123,124], liver [125,126,131,140,141], pancreas [127,142,143], lung [144,145], endometrium [146], prostate [128,147], nervous system [129] and kidney [148].
5.2.1. Liquid Tumors
Lipid rafts were shown to control the drug response of patients affected by B-chronic lymphocytic leukemia. It has been demonstrated that the susceptibility of patients with B-chronic lymphocytic leukemia is linked to the embedding of the promoting death receptor in lipid rafts and its exclusion is responsible for TRAIL resistance [117]. In non-Hodgkin’s T cell human lymphoblastic lymphoma cells, serum cholesterol reaches inner nuclear membrane by enriching it with lipid rafts content and regulating gene expression with the overexpression of antioxidant proteins as superoxide dismutase 1 and 2, copper chaperone for superoxide dismutase, peroxiredoxins 1, glutathione reductase, glutathione synthase, catalase and polynucleotide kinase/phosphatase [132]. In the same cells, gentamicin inhibits nuclear nSMase and stimulates SM-synthase by increasing SM content and thereby making nuclear lipid rafts more rigid structures useful in regulating gene expression [130].
5.2.2. Solid Tumors
Several recent studies have disclosed specific functional and patho-biological roles of lipid rafts in solid tumors, including breast, stomach, liver, and pancreas cancer [118,119,120,121,122,123,124,125,126,127,131,133,134,135,136,137,138,139,140,141,142,143].
5.2.3. Breast Cancer
The multiple advances in breast cancer have led to a better understanding of the molecular mechanisms that occur in lipid rafts involved in migration, invasion, angiogenesis, and metastasis. A proteomic study on lipid rafts purified from breast cancer cells showed an abundance of groups of proteins specific for the progression toward malignancy [133]. Promotion of breast cancer cell migration by TNF-α-MAPK/ERK signaling pathway requires translocation of membrane-type metalloproteinase 1 and matrix metalloproteinase 2 proteins into lipid rafts [134]. Moreover, localization of the epidermal growth factor receptor in lipid rafts induces activation of Akt epidermal growth factor receptor kinase-independent [135]. However, downregulation of caveolin 1 in lipid rafts of lysosome is responsible for enhanced autophagy in human breast cancer cells and tissues [136].
As a consequence of the interest in lipid rafts of breast cancer cells, the study of pharmacological targets has also expanded. The liver X receptor that is implicated in the cholesterol metabolism modulates lipid rafts integrity with consequent inhibition of breast cancer cell growth [118]. It has been demonstrated that anticancer activity of γ-tocotrienol is not only due to its accumulation in the lipid rafts, resulting in disrupting of HER2 dimerization and activation in lipid rafts [119], but also to the reduction of heregulin content in exosome with subsequent decrease in autocrine/paracrine mitogenic stimulation [137]. Interestingly, bufalin stimulates the entry of death receptor 4 (DR4) and DR5 into lipid rafts by promoting breast cancer cell apoptosis. In this way, the cells that were TRAIL resistant became TRAIL sensitive [120]. Li et al. (2017) showed that miR-3908 is involved in the migration process via lipid rafts that act as platform for interaction between adiponectin receptor 1 and Flotillin-1 [138]. Thus, the authors suggest that targeting miR-3908 and the lipid raft, might be the correct way to prevent and/or treat breast cancer. Later is has been shown that resveratrol changes the distribution of flotillin-1 and flotillin-2 in lipid rafts with consequent anticancer effects [121]. The use of methyl beta cyclodextrin, which extracts cholesterol from lipid rafts destroying them, has permitted the identification of the location in lipid rafts of urokinase-type plasminogen activator receptor and matrix metalloprotease-9 essential for breast cancer cell migration, invasion and angiogenesis [139]. Nano assembly of methyl beta cyclodextrin with hyaluronic acid-ceramide, which targets the CD44 receptor and destroys lipid rafts, has been developed for breast cancer therapy [122].
5.2.4. Gastric Cancer
Gastric cancer cells are insensitive to TRAIL, but inhibition of epidermal growth factor receptor activation and redistribution in lipid rafts can increase the sensitivity and therefore stimulate apoptosis [123]. The anti-epidermal growth factor receptor monoclonal antibody cetuximab facilitates the action of TRAIL in gastric cancer cells by promoting death receptor 4 clustering and FADD protein translocation into lipid rafts [123]. A drug extracted from traditional Chinese medicinal herb, β-elemene, by acting on lipid rafts, reduces the drug resistance to TRAIL by inhibiting proliferation and stimulating apoptosis in gastric cancer cells [124].
5.2.5. Liver Cancer
In hepatocarcinoma cells, localization of CD44 in lipid rafts is promoted by cholesterol with consequent inhibition of invasion and metastasis [140]. Sphingomyelin of lipid rafts localized in inner nuclear membrane of hepatoma cells is richer in palmitic acid than that of hepatocytes. It results in different dynamic properties of nuclear lipid rafts in the two cell types and increased import-export of protein signaling molecules in cancer cells respect to normal cells [141]. In the same nuclear rafts, daunorubicin changes the neutral sphingomyelinase activity, resulting in the saturated long chain fatty acid sphingomyelin decrease. Thus, nuclear lipid rafts change their function as platforms for active chromatin and for transcription process [131]. Fumonisin B1, a mycotoxin that inhibits Cer-synthases, promotes liver cancer by reducing sphingomyelin and by increasing free cholesterol with the consequent modulation of lipid rafts [125]. Inhibition of Toll-like receptor 7, a molecule belonging to a family of receptors playing an important role in innate immune responses, with specific antagonist 20S-protopanaxadiol changes caveolin-1 and flotillin-1 molecules in lipid rafts of hepatic cancer cells delaying, consequently, cell proliferation and migration [126].
5.2.6. Pancreatic Cancer
CD133 pancreatic cancer marker co-localizes with signaling proteins for cancer aggression in lipid rafts [142]. Lovastatin by inhibiting mevalonic-acid-pathway in cholesterol synthesis reduces chemoresistance in CD133Hi cells and in CD133lo cells [127]. Recently, it has been reported that localization of complement component 1, q subcomponent binding protein (C1QBP) in lipid rafts regulates the liver metastatic process induced by IGF-1/IGF-1R [143]. The authors suggest that targeting C1QBP in lipid rafts could be a strategy to limit hepatic metastasis [143].
5.2.7. Others
The attention of lipid rafts in lung cancer has been focused in non-small cell lung cancer cell lines. The cells resistant to gefitinib have higher cholesterol levels than the gefitinib-sensitive cell lines [144]. Squalene synthase, a key enzyme for the cholesterol synthesis, enriches the content of tumor necrosis factor receptor 1 in lipid rafts, thereby cancer metastasis. Activation of c-Met in rafts represents a key metabolic pathway responsible for resistance to radiation [145]. Additionally, in endometrial cancer cells, CD24-mediated amplification of the Met signaling is responsible for drug resistance [146].
The higher cholesterol content in prostate cancer cells than in results in increased lipid raft formation and regulation of tumor progression [147]. In lipid rafts of prostate cancer cells CXCL12 interacts with its receptor CXCR4, a G protein coupled receptor. CXCL12/CXCR4 interaction transactivates EGRF2/HER2 stimulating invasion [128].
Pirmoradi et al. (2019) have suggested that targeting cholesterol metabolism in glioblastoma cells might be promising since CHO regulates temozolomide-induced cell death due to the accumulation and activation of death receptor 5 in lipid rafts [129].
In renal cell cancers, GalNAc-disialyl Lc4 forms a molecular complex with integrin β1 and caveolin-1 in lipid rafts and increases malignant properties that can be reverted by antibodies [148].
6. Conclusions
In this review, we have attempted to highlight novel insights into the role of lipid rafts in cancer cells. The involvement of lipid rafts in signaling processes modulating proliferation, death and metastasis suggests that they might be promising targets in cancer therapy. Changes of rafts by manipulation of sphingolipid and cholesterol content or by antibodies against gangliosides alter the signaling of the growth factor and death receptors localized inside. Chemotherapy drugs may act on death receptor clustering and/or disrupting receptor signaling. In the last years, the existence of lipid rafts in different cell compartments such as plasma membrane, mitochondrion and nucleus and their involvement in apoptosis and in the modulation of gene expression has become clear in normal and cancer cells. However, further investigation is necessary to understand the possible communication between rafts of different subcellular organelles and how this can be used to improve cancer therapy.
Abbreviations
| C1P | Ceramide 1 phosphate |
| CerK | Ceramide kinase |
| GD | Disialoganglioside |
| FADD DISC | Fas-associated protein with death domain, death-inducing signaling complex |
| GM | Monosialoganglioside |
| S1P | Sphingosine 1 phosphate |
| S1PR | Sphingosine-1-phosphate receptor |
| SphK | Sphingosine kinase |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
Author Contributions
E.A. and M.G.-G. equally contributed to this work. Conceptualization, E.A. and M.G.-G.; Writing, M.C., E.A. and M.G.-G.; Review & editing, E.A. and M.G.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fantini J., Garmy N., Mahfoud R., Yahi N. Lipid rafts: Structure, function and role in HIV, Alzheimer’s and prion diseases. Expert Rev. Mol. Med. 2002;4:1–22. doi: 10.1017/S1462399402005392. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi M., Okazaki T. Ceramide/Sphingomyelin Rheostat Regulated by Sphingomyelin Synthases and Chronic Diseases in Murine Models. J. Lipid Atheroscler. 2020;9:380–405. doi: 10.12997/jla.2020.9.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grassi S., Giussani P., Mauri L., Prioni S., Sonnino S., Prinetti A. Lipid rafts and neurodegeneration: Structural and functional roles in physiologic aging and neurodegenerative diseases. J. Lipid Res. 2020;61:636–654. doi: 10.1194/jlr.TR119000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sipione S., Monyror J., Galleguillos D., Steinberg N., Kadam V. Gangliosides in the Brain: Physiology, Pathophysiology and Therapeutic Applications. Front. Neurosci. 2020;14:572965. doi: 10.3389/fnins.2020.572965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudajev V., Novotný J. The Role of Lipid Environment in Ganglioside GM1-Induced Amyloid β Aggregation. Membranes. 2020;10:226. doi: 10.3390/membranes10090226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai Y.-H., Chen W.-L. Host Lipid Rafts as the Gates for Listeria monocytogenes Infection: A Mini-Review. Front. Immunol. 2020;11:1666. doi: 10.3389/fimmu.2020.01666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fecchi K., Anticoli S., Peruzzu D., Iessi E., Gagliardi M.C., Matarrese P., Ruggieri A. Coronavirus Interplay With Lipid Rafts and Autophagy Unveils Promising Therapeutic Targets. Front. Microbiol. 2020;11:821. doi: 10.3389/fmicb.2020.01821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filippini A., D’Alessio A. Caveolae and Lipid Rafts in Endothelium: Valuable Organelles for Multiple Functions. Biomolecules. 2020;10:1218. doi: 10.3390/biom10091218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomassoni M.L., Albi E., Magni M.V. Changes of nuclear membrane fluidity during rat liver regeneration. Biochem. Mol. Biol. Int. 1999;47:1049–1059. doi: 10.1080/15216549900202173. [DOI] [PubMed] [Google Scholar]
- 10.Heessen S., Fornerod M. The inner nuclear envelope as a transcription factor resting place. EMBO Rep. 2007;8:914–919. doi: 10.1038/sj.embor.7401075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cascianelli G., Villani M., Tosti M., Marini F., Bartoccini E., Magni M.V., Albi E. Lipid Microdomains in Cell Nucleus. Mol. Biol. Cell. 2008;19:5289–5295. doi: 10.1091/mbc.e08-05-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scassellati C., Albi E., Cmarko D., Tiberi C., Cmarkova J., Bouchet-Marquis C., Verschure P.J., Driel R., Magni M.V., Fakan S. Intranuclear sphingomyelin is associated with transcriptionally active chromatin and plays a role in nuclear integrity. Biol. Cell. 2010;102:361–375. doi: 10.1042/BC20090139. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Gil M., Albi E. Nuclear Lipids in the Nervous System: What they do in Health and Disease. Neurochem. Res. 2016;42:321–336. doi: 10.1007/s11064-016-2085-8. [DOI] [PubMed] [Google Scholar]
- 14.Conte C., Arcuri C., Cataldi S., Mecca C., Codini M., Ceccarini M.R., Patria F., Beccari T., Albi E. Niemann-Pick Type A Disease: Behavior of Neutral Sphingomyelinase and Vitamin D Receptor. Int. J. Mol. Sci. 2019;20:2365. doi: 10.3390/ijms20092365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albi E., Alessenko A., Grösch S. Sphingolipids in Inflammation. Mediat. Inflamm. 2018;2018:7464702. doi: 10.1155/2018/7464702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannun Y.A., Obeid L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018;19:175–191. doi: 10.1038/nrm.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z., Guan M., Lin Y., Cui X., Zhang Y., Zhao Z., Zhu J. Aberrant Lipid Metabolism in Hepatocellular Carcinoma Revealed by Liver Lipidomics. Int. J. Mol. Sci. 2017;18:2550. doi: 10.3390/ijms18122550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Angelo G., Moorthi S., Luberto C. Role and function of sphingomyelin biosynthesis in the development of cancer. Adv. Cancer Res. 2018;140:61–96. doi: 10.1016/bs.acr.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Burns T.A., Subathra M., Signorelli P., Choi Y., Yang X., Wang Y., Villani M., Bhalla K., Zhou D., Luberto C. Sphingomyelin synthase 1 activity is regulated by theBCR-ABLoncogene. J. Lipid Res. 2013;54:794–805. doi: 10.1194/jlr.M033985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moorthi S., Burns T.A., Yu G.Q., Luberto C. Bcr-Abl regulation of sphingomyelin synthase 1 reveals a novel oncogenic-driven mechanism of protein up-regulation. FASEB J. 2018;32:4270–4283. doi: 10.1096/fj.201701016R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Der Hoeven D., Cho K.-J., Zhou Y., Ma X., Chen W., Naji A., Montufar-Solis D., Zuo Y., Kovar S.E., Levental K.R., et al. Sphingomyelin Metabolism Is a Regulator of K-Ras Function. Mol. Cell. Biol. 2017;38:e00373-17. doi: 10.1128/MCB.00373-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albi E., Cataldi S., Ceccarini M.R., Conte C., Ferri I., Fettucciari K., Patria F., Beccari T., Codini M. Gentamicin Targets Acid Sphingomyelinase in Cancer: The Case of the Human Gastric Cancer NCI-N87 Cells. Int. J. Mol. Sci. 2019;20:4375. doi: 10.3390/ijms20184375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albi E., Cataldi S., Ferri I., Sidoni A., Traina G., Fettucciari K., Ambesi-Impiombato F.S., Lazzarini A., Curcio F., Ceccarini M.R., et al. VDR independent induction of acid-sphingomyelinase by 1,23(OH)2 D3 in gastric cancer cells: Impact on apoptosis and cell morphology. Biochimie. 2018;146:35–42. doi: 10.1016/j.biochi.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Cataldi S., Arcuri C., Lazzarini A., Nakashidze I., Ragonese F., Fioretti B., Ferri I., Conte C., Codini M., Beccari T., et al. Effect of 1α,25(OH)2 Vitamin D3 in Mutant P53 Glioblastoma Cells: Involvement of Neutral Sphingomyelinase1. Cancers. 2020;12:3163. doi: 10.3390/cancers12113163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beckham T.H., Cheng J.C., Marrison S.T., Norris J.S., Liu X. Interdiction of Sphingolipid Metabolism to Improve Standard Cancer Therapies. Adv. Cancer Res. 2013;117:1–36. doi: 10.1016/B978-0-12-394274-6.00001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sassa T., Suto S., Okayasu Y., Kihara A. A shift in sphingolipid composition from C24 to C16 increases susceptibility to apoptosis in HeLa cells. Biochim. Biophys. Acta. 2012;1821:1031–1037. doi: 10.1016/j.bbalip.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Senkal C.E., Ponnusamy S., Bielawski J., Hannun Y.A., Ogretmen B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2010;24:296–308. doi: 10.1096/fj.09-135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiffmann S., Ziebell S., Sandner J., Birod K., Deckmann K., Hartmann D., Rode S., Schmidt H., Angioni C., Geisslinger G., et al. Activation of ceramide synthase 6 by celecoxib leads to a selective induction of C16:0-ceramide. Biochem. Pharmacol. 2010;80:1632–1640. doi: 10.1016/j.bcp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Verlekar D., Wei S.-J., Cho H.E., Yang S., Kang M.H. Ceramide synthase-6 confers resistance to chemotherapy by binding to CD95/Fas in T-cell acute lymphoblastic leukemia. Cell Death Dis. 2018;9:925. doi: 10.1038/s41419-018-0964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moro K., Kawaguchi T., Tsuchida J., Gabriel E., Qi Q., Yan L., Wakai T., Takabe K., Nagahashi M. Ceramide species are elevated in human breast cancer and are associated with less aggressiveness. Oncotarget. 2018;9:19874–19890. doi: 10.18632/oncotarget.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elojeimy S., Liu X., McKillop J.C., El-Zawahry A.M., Holman D.H., Cheng J.Y., Meacham W.D., Mahdy A.E., Saad A.F., Turner L.S., et al. Role of Acid Ceramidase in Resistance to FasL: Therapeutic Approaches Based on Acid Ceramidase Inhibitors and FasL Gene Therapy. Mol. Ther. 2007;15:1259–1263. doi: 10.1038/sj.mt.6300167. [DOI] [PubMed] [Google Scholar]
- 32.Camacho L., Meca-Cortés Ó., Abad J.L., García S., Rubio N., Díaz A., Celià-Terrassa T., Cingolani F., Bermudo R., Fernández P.L., et al. Acid ceramidase as a therapeutic target in metastatic prostate cancer. J. Lipid Res. 2013;54:1207–1220. doi: 10.1194/jlr.M032375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng J.C., Bai A., Beckham T.H., Marrison S.T., Yount C.L., Young K., Lu P., Bartlett A.M., Wu B.X., Keane B.J., et al. Radiation-induced acid ceramidase confers prostate cancer resistance and tumor relapse. J. Clin. Investig. 2013;123:4344–4358. doi: 10.1172/JCI64791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beckham T.H., Cheng J.C., Lu P., Marrison S.T., Norris J.S., Liu X. Acid ceramidase promotes nuclear export of PTEN through sphingosine 1-phosphate mediated Akt signaling. PLoS ONE. 2013;8:e76593. doi: 10.1371/journal.pone.0076593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sänger N., Ruckhäberle E., Györffy B., Engels K., Heinrich T., Fehm T., Graf A., Holtrich U., Becker S., Karn T. Acid ceramidase is associated with an improved prognosis in both DCIS and invasive breast cancer. Mol. Oncol. 2014;9:58–67. doi: 10.1016/j.molonc.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanker L., Karn T., Holtrich U., Gätje R., Rody A., Heinrich T., Ruckhäberle E., Engels K. Acid Ceramidase (AC)—A Key Enzyme of Sphingolipid Metabolism—Correlates With Better Prognosis in Epithelial Ovarian Cancer. Int. J. Gynecol. Pathol. 2013;32:249–257. doi: 10.1097/PGP.0b013e3182673982. [DOI] [PubMed] [Google Scholar]
- 37.Presa N., Gomez-Larrauri A., Dominguez-Herrera A., Trueba M., Gomez-Muñoz A. Novel signaling aspects of ceramide 1-phosphate. Biochim. Biophys. Acta. 2020;1865:158630. doi: 10.1016/j.bbalip.2020.158630. [DOI] [PubMed] [Google Scholar]
- 38.Newcomb B., Rhein C., Mileva I., Ahmad R., Clarke C.J., Snider J., Obeid L.M., Hannun Y.A. Identification of an acid sphingomyelinase ceramide kinase pathway in the regulation of the chemokine CCL5. J. Lipid Res. 2018;59:1219–1229. doi: 10.1194/jlr.M084202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivera I.-G., Ordoñez M., Presa N., Gangoiti P., Gomez-Larrauri A., Trueba M., Fox T., Kester M., Gómez-Muñoz A. Ceramide 1-phosphate regulates cell migration and invasion of human pancreatic cancer cells. Biochem. Pharmacol. 2016;102:107–119. doi: 10.1016/j.bcp.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Bini F., Frati A., Garcia-Gil M., Battistini C., Granado M., Martinesi M., Mainardi M., Vannini E., Luzzati F., Caleo M., et al. New signalling pathway involved in the anti-proliferative action of vitamin D3 and its analogues in human neuroblastoma cells. A role for ceramide kinase. Neuropharmacology. 2012;63:524–537. doi: 10.1016/j.neuropharm.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 41.Pastukhov O., Schwalm S., Zangemeister-Wittke U., Fabbro D., Bornancin F., Japtok L., Kleuser B., Pfeilschifter J., Huwiler A. The ceramide kinase inhibitor NVP-231 inhibits breast and lung cancer cell proliferation by inducing M phase arrest and subsequent cell death. Br. J. Pharmacol. 2014;171:5829–5844. doi: 10.1111/bph.12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadi L.A., Calcaterra F., Brambilla L., Carenza C., Marfia G., Della Bella S., Riboni L. Enhanced phosphorylation of sphingosine and ceramide sustains the exuberant proliferation of endothelial progenitors in Kaposi sarcoma. J. Leukoc. Biol. 2018;103:525–533. doi: 10.1002/JLB.2MA0817-312R. [DOI] [PubMed] [Google Scholar]
- 43.Schwalm S., Erhardt M., Römer I., Pfeilschifter J., Zangemeister-Wittke U., Huwiler A. Ceramide Kinase Is Upregulated in Metastatic Breast Cancer Cells and Contributes to Migration and Invasion by Activation of PI 3-Kinase and Akt. Int. J. Mol. Sci. 2020;21:1396. doi: 10.3390/ijms21041396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomizawa S., Tamori M., Tanaka A., Utsumi N., Sato H., Hatakeyama H., Hisaka A., Kohama T., Yamagata K., Honda T., et al. Inhibitory effects of ceramide kinase on Rac1 activation, lamellipodium formation, cell migration, and metastasis of A549 lung cancer cells. Biochim. Biophys. Acta. 2020;1865:158675. doi: 10.1016/j.bbalip.2020.158675. [DOI] [PubMed] [Google Scholar]
- 45.Pyne N.J., Pyne S. Recent advances in the role of sphingosine 1-phosphate in cancer. FEBS Lett. 2020;594:3583–3601. doi: 10.1002/1873-3468.13933. [DOI] [PubMed] [Google Scholar]
- 46.Moro K., Nagahashi M., Gabriel E., Takabe K., Wakai T. Clinical application of ceramide in cancer treatment. Breast Cancer. 2019;26:407–415. doi: 10.1007/s12282-019-00953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knapp P., Chomicz K., Świderska M., Chabowski A., Jach R. Unique Roles of Sphingolipids in Selected Malignant and Nonmalignant Lesions of Female Reproductive System. BioMed Res. Int. 2019;2019:4376583. doi: 10.1155/2019/4376583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sukocheva O.A., Furuya H., Ng M.L., Friedemann M., Menschikowski M., Tarasov V.V., Chubarev V.N., Klochkov S.G., Neganova M.E., Mangoni A.A., et al. Sphingosine kinase and sphingosine-1-phosphate receptor signaling pathway in inflammatory gastrointestinal disease and cancers: A novel therapeutic target. Pharmacol. Ther. 2020;207:107464. doi: 10.1016/j.pharmthera.2019.107464. [DOI] [PubMed] [Google Scholar]
- 49.Maceyka M., Rohrbach T., Milstien S., Spiegel S. Role of Sphingosine Kinase 1 and Sphingosine-1-Phosphate Axis in Hepatocellular Carcinoma. Handb. Exp. Pharmacol. 2020;259:3–17. doi: 10.1007/164_2019_217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carrié L., Virazels M., Dufau C., Montfort A., Levade T., Ségui B., Andrieu-Abadie N. New Insights into the Role of Sphingolipid Metabolism in Melanoma. Cells. 2020;9:1967. doi: 10.3390/cells9091967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hawkins C.C., Ali T., Ramanadham S., Hjelmeland A.B. Sphingolipid Metabolism in Glioblastoma and Metastatic Brain Tumors: A Review of Sphingomyelinases and Sphingosine-1-Phosphate. Biomolecules. 2020;10:1357. doi: 10.3390/biom10101357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsuchida J., Nagahashi M., Nakajima M., Moro K., Tatsuda K., Ramanathan R., Takabe K., Wakai T. Breast cancer sphingosine-1-phosphate is associated with phospho-sphingosine kinase 1 and lymphatic metastasis. J. Surg. Res. 2016;205:85–94. doi: 10.1016/j.jss.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagahashi M., Ramachandran S., Kim E.Y., Allegood J.C., Rashid O.M., Yamada A., Zhao R., Milstien S., Zhou H., Spiegel S., et al. Sphingosine-1-Phosphate Produced by Sphingosine Kinase 1 Promotes Breast Cancer Progression by Stimulating Angiogenesis and Lymphangiogenesis. Cancer Res. 2012;72:726–735. doi: 10.1158/0008-5472.CAN-11-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rivera J., Proia R.L., Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olesch C., Sirait-Fischer E., Berkefeld M., Fink A.F., Susen R.M., Ritter B., Michels B.E., Steinhilber D., Greten F.R., Savai R., et al. S1PR4 ablation reduces tumor growth and improves chemotherapy via CD8+ T cell expansion. J. Clin. Investig. 2020:5461–5476. doi: 10.1172/JCI136928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cavdarli S., Delannoy P., Groux-Degroote S. O-acetylated Gangliosides as Targets for Cancer Immunotherapy. Cells. 2020;9:741. doi: 10.3390/cells9030741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cavdarli S., Groux-Degroote S., Delannoy P. Gangliosides: The Double-Edge Sword of Neuro-Ectodermal Derived Tumors. Biomolecules. 2019;9:311. doi: 10.3390/biom9080311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iwasawa T., Zhang P., Ohkawa Y., Momota H., Wakabayashi T., Ohmi Y., Bhuiyan R.H., Furukawa K., Furukawa K. Enhancement of malignant properties of human glioma cells by ganglioside GD3/GD2. Int. J. Oncol. 2018;52:1255–1266. doi: 10.3892/ijo.2018.4266. [DOI] [PubMed] [Google Scholar]
- 59.Ohmi Y., Kambe M., Ohkawa Y., Hamamura K., Tajima O., Takeuchi R., Furukawa K., Furukawa K. Differential roles of gangliosides in malignant properties of melanomas. PLoS ONE. 2018;13:e0206881. doi: 10.1371/journal.pone.0206881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schengrund C.-L. Gangliosides and Neuroblastomas. Int. J. Mol. Sci. 2020;21:5313. doi: 10.3390/ijms21155313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sait S., Modak S. Anti-GD2 immunotherapy for neuroblastoma. Exp. Rev. Anticancer Ther. 2017;17:889–904. doi: 10.1080/14737140.2017.1364995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Labrada M., Dorvignit D., Hevia G., Rodríguez N., Hernández A.M., Vázquez A.M., Fernández L.E. GM3(Neu5Gc) ganglioside: An evolution fixed neoantigen for cancer immunotherapy. Semin. Oncol. 2018;45:41–51. doi: 10.1053/j.seminoncol.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Zheng C., Terreni M., Sollogoub M., Zhang Y. Ganglioside GM3 and Its Role in Cancer. Curr. Med. Chem. 2019;26:2933–2947. doi: 10.2174/0929867325666180129100619. [DOI] [PubMed] [Google Scholar]
- 64.Borgquist S., Butt T., Almgren P., Shiffman D., Stocks T., Orho-Melander M., Manjer J., Melander O. Apolipoproteins, lipids and risk of cancer. Int. J. Cancer. 2016;138:2648–2656. doi: 10.1002/ijc.30013. [DOI] [PubMed] [Google Scholar]
- 65.Kuzu O.F., Noory M.A., Robertson G.P. The Role of Cholesterol in Cancer. Cancer Res. 2016;76:2063–2070. doi: 10.1158/0008-5472.CAN-15-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qin W.-H., Yang Z.-S., Li M., Chen Y., Zhao X.-F., Qin Y.-Y., Song J.-Q., Wang B.-B., Yuan B., Cui X.-L., et al. High Serum Levels of Cholesterol Increase Antitumor Functions of Nature Killer Cells and Reduce Growth of Liver Tumors in Mice. Gastroenterology. 2020;158:1713–1727. doi: 10.1053/j.gastro.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 67.Gao R., Liang J.-H., Wang L., Zhu H.-Y., Wu W., Cao L., Fan L., Li J., Yang T., Xu W. Low serum cholesterol levels predict inferior prognosis and improve NCCN-IPI scoring in diffuse large B cell lymphoma. Int. J. Cancer. 2018;143:1884–1895. doi: 10.1002/ijc.31590. [DOI] [PubMed] [Google Scholar]
- 68.Lin X., Liu L., Fu Y., Gao J., He Y., Wu Y., Lian X. Dietary Cholesterol Intake and Risk of Lung Cancer: A Meta-Analysis. Nutrients. 2018;10:185. doi: 10.3390/nu10020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu Y., Jeong S., Wu M., Jin Z., Zhou J.-Y., Han R.-Q., Yang J., Zhang X.-F., Wang X.-S., Liu A.-M., et al. Dietary Intake of Fatty Acids, Total Cholesterol, and Stomach Cancer in a Chinese Population. Nutrients. 2019;11:1730. doi: 10.3390/nu11081730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freed-Pastor W.A., Mizuno H., Zhao X., Langerød A., Moon S.-H., Rodriguez-Barrueco R., Barsotti A.M., Chicas A., Li W., Polotskaia A., et al. Mutant p53 Disrupts Mammary Tissue Architecture via the Mevalonate Pathway. Cell. 2012;148:244–258. doi: 10.1016/j.cell.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sorrentino G., Ruggeri N., Specchia V., Cordenonsi M., Mano M., Dupont S., Manfrin A., Ingallina E., Sommaggio R., Piazza S., et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat. Cell Biol. 2014;16:357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- 72.Lyu J., Yang E.J., Shim J.S. Cholesterol Trafficking: An Emerging Therapeutic Target for Angiogenesis and Cancer. Cells. 2019;8:389. doi: 10.3390/cells8050389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 74.Riscal R., Skuli N., Simon M.C. Even Cancer Cells Watch Their Cholesterol! Mol. Cell. 2019;76:220–231. doi: 10.1016/j.molcel.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mullen P.J., Yu R., Longo J., Archer M.C., Penn L.Z. The interplay between cell signalling and the mevalonate pathway in cancer. Nat. Rev. Cancer. 2016;16:718–731. doi: 10.1038/nrc.2016.76. [DOI] [PubMed] [Google Scholar]
- 76.Bathaie S.Z., Ashrafi M., Azizian M., Tamanoi F. Mevalonate Pathway and Human Cancers. Curr. Mol. Pharmacol. 2017;10:77–85. doi: 10.2174/1874467209666160112123205. [DOI] [PubMed] [Google Scholar]
- 77.Clendening J.W., Penn L.Z. Targeting tumor cell metabolism with statins. Oncogene. 2012;31:4967–4978. doi: 10.1038/onc.2012.6. [DOI] [PubMed] [Google Scholar]
- 78.McDougall J.A., Malone K.E., Daling J.R., Cushing-Haugen K.L., Porter P.L., Li C.I. Long-Term Statin Use and Risk of Ductal and Lobular Breast Cancer among Women 55 to 74 Years of Age. Cancer Epidemiol. Biomark. Prev. 2013;22:1529–1537. doi: 10.1158/1055-9965.EPI-13-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aylon Y., Oren M. The Hippo pathway, p53 and cholesterol. Cell Cycle. 2016;15:2248–2255. doi: 10.1080/15384101.2016.1207840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bieging K.T., Mello S.S., Attardi L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer. 2014;14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moon S.-H., Huang C.-H., Houlihan S.L., Regunath K., Freed-Pastor W.A., Morris J.P., Tschaharganeh D.F., Kastenhuber E.R., Barsotti A.M., Culp-Hill R., et al. p53 Represses the Mevalonate Pathway to Mediate Tumor Suppression. Cell. 2019;176:564–580.e19. doi: 10.1016/j.cell.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Furth N., Aylon Y., Oren M. p53 shades of Hippo. Cell Death Differ. 2018;25:81–92. doi: 10.1038/cdd.2017.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang B., Song B.-L., Xu C. Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab. 2020;2:132–141. doi: 10.1038/s42255-020-0174-0. [DOI] [PubMed] [Google Scholar]
- 84.Chimento A., Casaburi I., Avena P., Trotta F., De Luca A., Rago V., Pezzi V., Sirianni R. Cholesterol and Its Metabolites in Tumor Growth: Therapeutic Potential of Statins in Cancer Treatment. Front. Endocrinol. 2019;9:807. doi: 10.3389/fendo.2018.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Filardo E.J. A role for G-protein coupled estrogen receptor (GPER) in estrogen-induced carcinogenesis: Dysregulated glandular homeostasis, survival and metastasis. J. Steroid Biochem. Mol. Biol. 2018;176:38–48. doi: 10.1016/j.jsbmb.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 86.Silvente-Poirot S., Dalenc F., Poirot M. The Effects of Cholesterol-Derived Oncometabolites on Nuclear Receptor Function in Cancer. Cancer Res. 2018;78:4803–4808. doi: 10.1158/0008-5472.CAN-18-1487. [DOI] [PubMed] [Google Scholar]
- 87.Noy R., Pollard J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 89.Greenlee J.D., Subramanian T., Liu K., King M.R. Rafting Down the Metastatic Cascade: The Role of Lipid Rafts in Cancer Metastasis, Cell Death, and Clinical Outcomes. Cancer Res. 2020 doi: 10.1158/0008-5472.CAN-20-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mollinedo F., Gajate C. Lipid rafts as signaling hubs in cancer cell survival/death and invasion: Implications in tumor progression and therapy. J. Lipid Res. 2020;61:611–635. doi: 10.1194/jlr.TR119000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shah A.D., Inder K.L., Shah A.K., Cristino A.S., McKie A.B., Gabra H., Davis M.J., Hill M.M. Integrative Analysis of Subcellular Quantitative Proteomics Studies Reveals Functional Cytoskeleton Membrane–Lipid Raft Interactions in Cancer. J. Proteome Res. 2016;15:3451–3462. doi: 10.1021/acs.jproteome.5b01035. [DOI] [PubMed] [Google Scholar]
- 92.Tuzmen S., Hostetter G., Watanabe A., Ekmekçi C., Carrigan E.P., Shechter I., Kallioniemi O., Miller L.J., Mousses S. Characterization of farnesyl diphosphate farnesyl transferase 1 (FDFT1) expression in cancer. Pers. Med. 2019;16:51–65. doi: 10.2217/pme-2016-0058. [DOI] [PubMed] [Google Scholar]
- 93.Goossens P., Rodriguez-Vita J., Etzerodt A., Masse M., Rastoin O., Gouirand V., Ulas T., Papantonopoulou O., Van Eck M., Auphan-Anezin N., et al. Membrane Cholesterol Efflux Drives Tumor-Associated Macrophage Reprogramming and Tumor Progression. Cell Metab. 2019;29:1376–1389.e4. doi: 10.1016/j.cmet.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 94.Hengst J.A., Guilford J.M., Fox T.E., Wang X., Conroy E.J., Yun J.K. Sphingosine kinase 1 localized to the plasma membrane lipid raft microdomain overcomes serum deprivation induced growth inhibition. Arch. Biochem. Biophys. 2009;492:62–73. doi: 10.1016/j.abb.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pitson S.M. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem. Sci. 2011;36:97–107. doi: 10.1016/j.tibs.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 96.Kim J.K., Kim S.-H., Cho H.-Y., Shin H.-S., Sung H.-R., Jung J.-R., Quan M.-L., Jiang D.-H., Bae H.-R. GD3 Accumulation in Cell Surface Lipid Rafts Prior to Mitochondrial Targeting Contributes to Amyloid-β-induced Apoptosis. J. Korean Med Sci. 2010;25:1492–1498. doi: 10.3346/jkms.2010.25.10.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sorice M., Matarrese P., Tinari A., Giammarioli A.M., Garofalo T., Manganelli V., Ciarlo L.G., Gambardella L., Maccari G., Botta M., et al. Raft component GD3 associates with tubulin following CD95/Fas ligation. FASEB J. 2009;23:3298–3308. doi: 10.1096/fj.08-128140. [DOI] [PubMed] [Google Scholar]
- 98.Garofalo T., Tinari A., Matarrese P., Giammarioli A.M., Manganelli V., Ciarlo L., Misasi R., Sorice M., Malorni W. Do mitochondria act as “cargo boats” in the journey of GD3 to the nucleus during apoptosis? FEBS Lett. 2007;581:3899–3903. doi: 10.1016/j.febslet.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 99.Preta G. New Insights Into Targeting Membrane Lipids for Cancer Therapy. Front. Cell Dev. Biol. 2020;8:571237. doi: 10.3389/fcell.2020.571237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu Y., Zhao Y., He X., He Z., Wang T., Wan L., Chen L., Yan N. Hydroxypropyl-β-cyclodextrin attenuates the epithelial-to-mesenchymal transition via endoplasmic reticulum stress in MDA-MB-231 breast cancer cells. Mol. Med. Rep. 2019;21:249–257. doi: 10.3892/mmr.2019.10802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cheng Y., Song Y., Qu J., Che X., Song N., Fan Y., Wen T., Xu L., Gong J., Wang X., et al. The Chemokine Receptor CXCR4 and c-MET Cooperatively Promote Epithelial-Mesenchymal Transition in Gastric Cancer Cells. Transl. Oncol. 2018;11:487–497. doi: 10.1016/j.tranon.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fernández-Muñoz B., Yurrita M.M., Martín-Villar E., Carrasco-Ramírez P., Megías D., Renart J., Quintanilla M. The transmembrane domain of podoplanin is required for its association with lipid rafts and the induction of epithelial-mesenchymal transition. Int. J. Biochem. Cell Biol. 2011;43:886–896. doi: 10.1016/j.biocel.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 103.Liang W., Hao Z., Han J.-L., Zhu D.-J., Jin Z.-F., Xie W. CAV-1 contributes to bladder cancer progression by inducing epithelial-to-mesenchymal transition. Urol. Oncol. Semin. Orig. Investig. 2014;32:855–863. doi: 10.1016/j.urolonc.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 104.Gauthier-Rouvière C., Bodin S., Comunale F., Planchon D. Flotillin membrane domains in cancer. Cancer Metastasis Rev. 2020;39:361–374. doi: 10.1007/s10555-020-09873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu T., Peng J., Li X., Leng A., Liu T. miR-449a targets Flot2 and inhibits gastric cancer invasion by inhibiting TGF-β-mediated EMT. Diagn. Pathol. 2015;10:202. doi: 10.1186/s13000-015-0435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu H., Niu M., Yuan X., Wu K., Liu A. CD44 as a tumor biomarker and therapeutic target. Exp. Hematol. Oncol. 2020;9:36. doi: 10.1186/s40164-020-00192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murai T., Maruyama Y., Mio K., Nishiyama H., Suga M., Sato C. Low Cholesterol Triggers Membrane Microdomain-dependent CD44 Shedding and Suppresses Tumor Cell Migration. J. Biol. Chem. 2011;286:1999–2007. doi: 10.1074/jbc.M110.184010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Babina I.S., McSherry A.E., Donatello S., Hill A.D.K., Hopkins A.M. A novel mechanism of regulating breast cancer cell migration via palmitoylation-dependent alterations in the lipid raft affiliation of CD44. Breast Cancer Res. 2014;16:R19. doi: 10.1186/bcr3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chantôme A., Potier-Cartereau M., Clarysse L., Fromont G., Marionneau-Lambot S., Guéguinou M., Pagès J.-C., Collin C., Oullier T., Girault A., et al. Pivotal Role of the Lipid Raft SK3–Orai1 Complex in Human Cancer Cell Migration and Bone Metastases. Cancer Res. 2013;73:4852–4861. doi: 10.1158/0008-5472.CAN-12-4572. [DOI] [PubMed] [Google Scholar]
- 110.Tisza M.J., Zhao W., Fuentes J.S.R., Prijic S., Chen X., Levental I., Chang J.T. Motility and stem cell properties induced by the epithelial-mesenchymal transition require destabilization of lipid rafts. Oncotarget. 2016;7:51553–51568. doi: 10.18632/oncotarget.9928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dai X., Zhang J., Arfuso F., Chinnathambi A., Zayed E.M., Alharbi S.A., Kumar A.P., Ahn K.S., Sethi G. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp. Biol. Med. 2015;240:760–773. doi: 10.1177/1535370215579167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gajate C., Mollinedo F. Lipid Rafts, Endoplasmic Reticulum and Mitochondria in the Antitumor Action of the Alkylphospholipid Analog Edelfosine. Anti-Cancer Agents Med. Chem. 2014;14:509–527. doi: 10.2174/1871520614666140309222259. [DOI] [PubMed] [Google Scholar]
- 113.Grimaldi C., Capasso A. Role of lipid rafts/caveolae in the anticancer effect of endocannabinoids. Mini Rev. Med. Chem. 2012;12:1119–1126. doi: 10.2174/138955712802762158. [DOI] [PubMed] [Google Scholar]
- 114.Mollinedo F., Gajate C. Lipid rafts and clusters of apoptotic signaling molecule-enriched rafts in cancer therapy. Futur. Oncol. 2010;6:811–821. doi: 10.2217/fon.10.34. [DOI] [PubMed] [Google Scholar]
- 115.Lavie Y., Liscovitch M. Changes in lipid and protein constituents of rafts and caveolae in multidrug resistant cancer cells and their functional consequences. Glycoconj. J. 2001;17:253–259. doi: 10.1023/A:1026553626537. [DOI] [PubMed] [Google Scholar]
- 116.Gajate C., Mollinedo F. Lipid raft-mediated Fas/CD95 apoptotic signaling in leukemic cells and normal leukocytes and therapeutic implications. J. Leukoc. Biol. 2015;98:739–759. doi: 10.1189/jlb.2MR0215-055R. [DOI] [PubMed] [Google Scholar]
- 117.Marconi M., Ascione B., Ciarlo L., Vona R., Garofalo T., Sorice M., Gianni A.M., Locatelli S.L., Carlo-Stella C., Malorni W., et al. Constitutive localization of DR4 in lipid rafts is mandatory for TRAIL-induced apoptosis in B-cell hematologic malignancies. Cell Death Dis. 2013;4:e863. doi: 10.1038/cddis.2013.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carbonnelle D., Luu T.H., Chaillou C., Huvelin J.-M., Bard J.-M., Nazih H. LXR Activation Down-regulates Lipid Raft Markers FLOT2 and DHHC5 in MCF-7 Breast Cancer Cells. Anticancer. Res. 2017;37:4067–4073. doi: 10.21873/anticanres.11792. [DOI] [PubMed] [Google Scholar]
- 119.Alawin O.A., Ahmed R.A., Ibrahim B.A., Briski K.P., Sylvester P.W. Antiproliferative effects of γ-tocotrienol are associated with lipid raft disruption in HER2-positive human breast cancer cells. J. Nutr. Biochem. 2016;27:266–277. doi: 10.1016/j.jnutbio.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 120.Yan S., Qu X., Xu L., Che X., Ma Y., Zhang L., Teng Y., Zou H., Liu Y. Bufalin enhances TRAIL-induced apoptosis by redistributing death receptors in lipid rafts in breast cancer cells. Anti-Cancer Drugs. 2014;25:683–689. doi: 10.1097/CAD.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 121.Gomes L., Sorgine M., Passos C.L.A., Ferreira C., De Andrade I.R., Silva J.L., Atella G.C., Mermelstein C., Fialho E. Increase in fatty acids and flotillins upon resveratrol treatment of human breast cancer cells. Sci. Rep. 2019;9:13960. doi: 10.1038/s41598-019-50416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee S.Y., Ko S.-H., Shim J.-S., Kim D.-D., Cho H.-J. Tumor Targeting and Lipid Rafts Disrupting Hyaluronic Acid-Cyclodextrin-Based Nanoassembled Structure for Cancer Therapy. ACS Appl. Mater. Interfaces. 2018;10:36628–36640. doi: 10.1021/acsami.8b08243. [DOI] [PubMed] [Google Scholar]
- 123.Xu L., Hu X., Qu X., Hou K., Zheng H., Liu Y. Cetuximab enhances TRAIL-induced gastric cancer cell apoptosis by promoting DISC formation in lipid rafts. Biochem. Biophys. Res. Commun. 2013;439:285–290. doi: 10.1016/j.bbrc.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 124.Xu L., Guo T., Qu X., Hu X., Zhang Y., Che X., Song H., Gong J., Ma R., Li C., et al. β-elemene increases the sensitivity of gastric cancer cells to TRAIL by promoting the formation of DISC in lipid rafts. Cell Biol. Int. 2018;42:1377–1385. doi: 10.1002/cbin.11023. [DOI] [PubMed] [Google Scholar]
- 125.Burger H.-M., Abel S., Gelderblom W. Modulation of key lipid raft constituents in primary rat hepatocytes by fumonisin B1—Implications for cancer promotion in the liver. Food Chem. Toxicol. 2018;115:34–41. doi: 10.1016/j.fct.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 126.Liu Y., Guo X., Wu L., Yang M., Li Z., Gao Y., Liu S., Zhou G., Zhao J. Lipid rafts promote liver cancer cell proliferation and migration by up-regulation of TLR7 expression. Oncotarget. 2016;7:63856–63869. doi: 10.18632/oncotarget.11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gupta V.K., Sharma N.S., Kesh K., Dauer P., Nomura A., Giri B., Dudeja V., Banerjee S., Bhattacharya S., Saluja A., et al. Metastasis and chemoresistance in CD133 expressing pancreatic cancer cells are dependent on their lipid raft integrity. Cancer Lett. 2018;439:101–112. doi: 10.1016/j.canlet.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 128.Chinni S.R., Yamamoto H., Dong Z., Sabbota A., Bonfil R.D., Cher M.L. CXCL12/CXCR4 Transactivates HER2 in Lipid Rafts of Prostate Cancer Cells and Promotes Growth of Metastatic Deposits in Bone. Mol. Cancer Res. 2008;6:446–457. doi: 10.1158/1541-7786.MCR-07-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pirmoradi L., Seyfizadeh N., Ghavami S., Zeki A.A., Shojaei S. Targeting cholesterol metabolism in glioblastoma: A new therapeutic approach in cancer therapy. J. Investig. Med. 2019;67:715–719. doi: 10.1136/jim-2018-000962. [DOI] [PubMed] [Google Scholar]
- 130.Codini M., Cataldi S., Ambesi-Impiombato F.S., Lazzarini A., Floridi A., Lazzarini R., Curcio F., Beccari T., Albi E. Gentamicin Arrests Cancer Cell Growth: The Intriguing Involvement of Nuclear Sphingomyelin Metabolism. Int. J. Mol. Sci. 2015;16:2307–2319. doi: 10.3390/ijms16022307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Codini M., Conte C., Cataldi S., Arcuri C., Lazzarini A., Ceccarini M.R., Patria F., Floridi A., Mecca C., Ambesi-Impiombato F.S., et al. Nuclear Lipid Microdomains Regulate Daunorubicin Resistance in Hepatoma Cells. Int. J. Mol. Sci. 2018;19:3424. doi: 10.3390/ijms19113424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Odini M., Cataldi S., Lazzarini A., Tasegian A., Ceccarini M.R., Floridi A., Lazzarini R., Ambesi-Impiombato F.S., Curcio F., Beccari T., et al. Why high cholesterol levels help hematological malignancies: Role of nuclear lipid microdomains. Lipids Health Dis. 2016;15:4. doi: 10.1186/s12944-015-0175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Caruso J.A., Stemmer P.M. Proteomic profiling of lipid rafts in a human breast cancer model of tumorigenic progression. Clin. Exp. Metastasis. 2011;28:529–540. doi: 10.1007/s10585-011-9389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wolczyk D., Zaremba-Czogalla M., Hryniewicz-Jankowska A., Tabola R., Grabowski K., Sikorski A.F., Augoff K. TNF-α promotes breast cancer cell migration and enhances the concentration of membrane-associated proteases in lipid rafts. Cell. Oncol. 2016;39:353–363. doi: 10.1007/s13402-016-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Irwin M.E., Bohin N., Boerner J.L. Src family kinases mediate epidermal growth factor receptor signaling from lipid rafts in breast cancer cells. Cancer Biol. Ther. 2011;12:718–726. doi: 10.4161/cbt.12.8.16907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shi Y., Tan S.-H., Ng S., Zhou J., Yang N.-D., Koo G.-B., McMahon K.-A., Parton R.G., Hill M.M., Del Pozo M.A., et al. Critical role of CAV1/caveolin-1 in cell stress responses in human breast cancer cells via modulation of lysosomal function and autophagy. Autophagy. 2015;11:769–784. doi: 10.1080/15548627.2015.1034411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Alawin O.A., Ahmed R.A., Dronamraju V., Briski K.P., Sylvester P.W. γ-Tocotrienol-induced disruption of lipid rafts in human breast cancer cells is associated with a reduction in exosome heregulin content. J. Nutr. Biochem. 2017;48:83–93. doi: 10.1016/j.jnutbio.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 138.Li Y., Shan F., Chen J. Lipid raft-mediated miR-3908 inhibition of migration of breast cancer cell line MCF-7 by regulating the interactions between AdipoR1 and Flotillin-1. World J. Surg. Oncol. 2017;15:69. doi: 10.1186/s12957-017-1120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Raghu H., Sodadasu P.K., Malla R., Gondi C.S., Estes N., Rao J.S. Localization of uPAR and MMP-9 in lipid rafts is critical for migration, invasion and angiogenesis in human breast cancer cells. BMC Cancer. 2010;10:647. doi: 10.1186/1471-2407-10-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang Z., Qin W., Chen Y., Yuan B., Song X., Wang B., Shen F., Fu J., Wang H. Cholesterol inhibits hepatocellular carcinoma invasion and metastasis by promoting CD44 localization in lipid rafts. Cancer Lett. 2018;429:66–77. doi: 10.1016/j.canlet.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 141.Lazzarini A., Macchiarulo A., Floridi A., Coletti A., Cataldi S., Codini M., Lazzarini R., Bartoccini E., Cascianelli G., Ambesi-Impiombato F.S., et al. Very Long Chain Fatty Acid Sphingomyelin in Nuclear Lipid Microdomains of Hepatocytes and Hepatoma Cells: Can the Exchange from C24:0 To C16:0 Affect Signal Proteins and Vitamin D Receptor? Mol. Biol. Cell. 2015;26:2418–2425. doi: 10.1091/mbc.e15-04-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gupta V.K., Banerjee S. Isolation of Lipid Raft Proteins from CD133+ Cancer Stem Cells. Methods Mol. Biol. 2017;1609:25–31. doi: 10.1007/978-1-4939-6996-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shi H., Fang W., Liu M., Fu D. Complement component 1, q subcomponent binding protein (C1QBP) in lipid rafts mediates hepatic metastasis of pancreatic cancer by regulating IGF-1/IGF-1R signaling. Int. J. Cancer. 2017;141:1389–1401. doi: 10.1002/ijc.30831. [DOI] [PubMed] [Google Scholar]
- 144.Chen Q., Pan Z., Zhao M., Wang Q., Qiao C., Miao L., Ding X. High cholesterol in lipid rafts reduces the sensitivity to EGFR-TKI therapy in non-small cell lung cancer. J. Cell. Physiol. 2018;233:6722–6732. doi: 10.1002/jcp.26351. [DOI] [PubMed] [Google Scholar]
- 145.Zeng J., Zhang H., Tan Y., Sun C., Liang Y., Yu J., Zou H. Aggregation of lipid rafts activates c-met and c-Src in non-small cell lung cancer cells. BMC Cancer. 2018;18:611. doi: 10.1186/s12885-018-4501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ono Y.J., Tanabe A., Tanaka T., Tanaka Y., Hayashi M., Terai Y., Ohmichi M. Met Signaling Cascade Is Amplified by the Recruitment of Phosphorylated Met to Lipid Rafts via CD24 and Leads to Drug Resistance in Endometrial Cancer Cell Lines. Mol. Cancer Ther. 2015;14:2353–2363. doi: 10.1158/1535-7163.MCT-15-0187. [DOI] [PubMed] [Google Scholar]
- 147.Hryniewicz-Jankowska A., Augoff K., Sikorski A.F. The role of cholesterol and cholesterol-driven membrane raft domains in prostate cancer. Exp. Biol. Med. 2019;244:1053–1061. doi: 10.1177/1535370219870771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tsuchida A., Senda M., Ito A., Saito S., Kiso M., Ando T., Harduin-Lepers A., Matsuda A., Furukawa K., Furukawa K. Roles of GalNAc-disialyl Lactotetraosyl Antigens in Renal Cancer Cells. Sci. Rep. 2018;8:7017. doi: 10.1038/s41598-018-25521-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.