Abstract
Endothelial lipase (EL) is a strong modulator of the high-density lipoprotein (HDL) structure, composition, and function. Here, we examined the impact of EL on HDL paraoxonase 1 (PON1) content and arylesterase (AE) activity in vitro and in vivo. The incubation of HDL with EL-overexpressing HepG2 cells decreased HDL size, PON1 content, and AE activity. The EL modification of HDL did not diminish the capacity of HDL to associate with PON1 when EL-modified HDL was incubated with PON1-overexpressing cells. The overexpression of EL in mice significantly decreased HDL serum levels but unexpectedly increased HDL PON1 content and HDL AE activity. Enzymatically inactive EL had no effect on the PON1 content of HDL in mice. In healthy subjects, EL serum levels were not significantly correlated with HDL levels. However, HDL PON1 content was positively associated with EL serum levels. The EL-induced changes in the HDL-lipid composition were not linked to the HDL PON1 content. We conclude that primarily, the interaction of enzymatically active EL with HDL, rather than EL-induced alterations in HDL size and composition, causes PON1 displacement from HDL in vitro. In vivo, the EL-mediated reduction of HDL serum levels and the consequently increased PON1-to-HDL ratio in serum increase HDL PON1 content and AE activity in mice. In humans, additional mechanisms appear to underlie the association of EL serum levels and HDL PON1 content.
Keywords: endothelial lipase, high-density lipoprotein, paraoxonase 1, arylesterase activity, mass spectrometry, NMR spectroscopy
1. Introduction
The atheroprotective effects of serum high-density lipoprotein (HDL) are largely ascribed to its anti-oxidative, anti-inflammatory, cholesterol efflux, and endothelial function, which partially rely on HDL-associated paraoxonase 1 (PON1) [1,2,3,4,5]. PON1 is a serum enzyme synthetized by the liver. Binding of the secreted PON1 to HDL involves an interaction of HDL with the hepatocyte surface, which is a process facilitated by scavenger receptor class B type I (SR-BI) [6,7]. The protein–protein and protein–lipid interactions between PON1 and HDL have been found to be crucial for the stability and enzymatic activity of HDL-associated PON1 [8,9,10]. In addition to its HDL-associated form, PON1 exists also as a free enzyme, which can redistribute to cell membranes or can be taken up by cells [11,12].
Although the majority (95%) of secreted PON1 is associated with HDL, only a subset of circulating HDL particles (5–10%) contain PON1 [13,14,15,16]. It has been shown that the lipid and protein composition of PON1-containing HDL particles differ from HDL particles lacking PON1 [17].
In humans, a strong negative correlation between PON1 activity and serum concentrations of lipid oxidation markers was observed [18,19]. Similarly, PON1 deficiency in mice is associated with increased oxidative stress [20,21], and decreased oxidative stress is observed upon PON1 overexpression [22].
Endothelial lipase (EL) is a phospholipase expressed primarily by vascular endothelial cells, vascular smooth muscle cells, and macrophages [23,24]. Based on increased HDL serum levels in EL-deficient mice as well as in humans expressing EL variants with decreased enzymatic activity, EL emerged as a strong negative regulator of HDL plasma levels [25,26]. By hydrolyzing HDL, EL depletes primarily phospholipids generating lysophospholipids and free fatty acids, which partially remain associated with EL-modified HDL and partially distribute to albumin as well as underlying EL-expressing cells [27,28]. EL modification of HDL markedly alters the structural and functional properties of HDL [27,29,30,31]. EL modulates also plasma levels of apolipoprotein B-containing lipoproteins in mice and is associated with a proatherogenic lipid profile and subclinical atherosclerosis in humans [32,33,34,35]. While studies in mice generated inconclusive data regarding the role of EL in atherosclerosis [36,37], hepatic EL overexpression decreased cholesterol diet-induced hypercholesterolemia and atherosclerosis in transgenic rabbits [38].
HDL-associated PON1 content and activity rely on the interaction of PON1 with HDL phospholipids, cholesterol, and apolipoprotein A-I (apoA-I) [8,9]. Considering the pronounced impact of EL on structural and compositional features of HDL [27,30], it is conceivable that the interaction and association of PON1 with HDL and in turn the PON1 content and arylesterase (AE) activity of HDL are altered by EL. Indeed, in our recent study, in vitro EL modification of HDL decreased HDL PON1 content [30]. To study this phenomenon in more detail, in the present study, we conducted a more in-depth study in vitro and additionally investigated the relationship between EL and HDL PON1 content as well as AE activity in mice and humans in vivo.
2. Results
2.1. EL Decreases HDL-Associated PON1 Content and AE Activity In Vitro
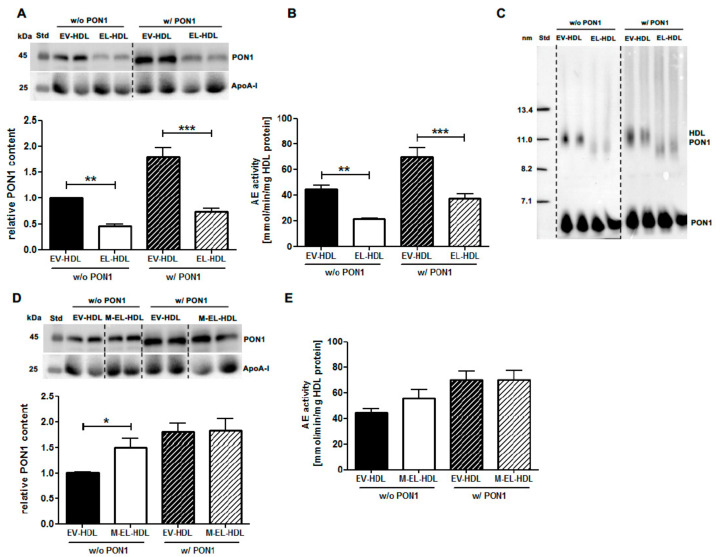
To examine the impact of EL on HDL-associated PON1, purified human HDL was incubated with EL-overexpressing or empty virus (EV)-infected control HepG2 cells in the absence or presence of PON1 co-overexpression. Following the isolation of modified HDL by ultracentrifugation, both the PON1 content (Figure 1A) and AE activity (Figure 1B) were significantly lower in EL-HDL compared to EV-HDL. This was irrespective of whether HDL modification was performed in the absence or presence of PON1 co-overexpression. Similar results were obtained with EL-HDL and EV-HDL generated in the presence of 4% bovine serum albumin (BSA) to prevent the accumulation of lipolytic products in HDL [30] (Supplementary Figure S1A,B). The decreased PON1 content of EL-HDL compared to EV-HDL was also observed when aliquots of the incubation media were analyzed by native gel electrophoresis followed by PON1 Western blotting (Figure 1C). Therefore, ultracentrifugation as a possible cause for the decreased PON1 content of EL-HDL can be excluded. In sharp contrast to the effects of enzymatically active EL, the incubation of HDL with HepG2 cells overexpressing enzymatically inactive EL (M-EL) resulted in significantly increased PON1 content (Figure 1D) but did not alter significantly the AE activity of M-EL-HDL (Figure 1E). In the presence of PON1 co-overexpression, EV-HDL and M-EL-HDL had comparable PON1 content (Figure 1D) and AE activity (Figure 1E). These results clearly suggest that the lipolytic activity of EL is crucial for decreasing HDL PON1 content and AE activity.
Figure 1.
Endothelial lipase (EL) decreases high-density lipoprotein (HDL) paraoxonase 1 (PON1) content and arylesterase (AE) activity. Empty virus (EV)-HDL, EL-HDL, and enzymatically inactive (M)-EL-HDL were generated by incubation of human HDL with EV, EL, or M-EL overexpressing cells in the absence (w/o) or presence (w/) of PON1 overexpression under cell culture conditions for 16 h. Modified HDL was purified by ultracentrifugation. (A) First, 10 µg HDL protein were separated by 12% SDS-PAGE followed by Western blotting analyses of PON1 and apolipoprotein A-I (apoA-I). (B) PON1 AE activity was measured in 2 µg HDL. (C) Aliquots (containing 15 µg protein) were electrophoresed on 4–20% non-denaturing polyacrylamide gels followed by Western blotting analyses of PON1. (D) Analyses were performed as in (A). (E) Analyses were performed as in (B). Densitometric values of PON1 in (A) and (D) were normalized to the corresponding apoA-I signals. Results obtained by densitometry and measurements of AE activity are presented as means + SEM of four independent modifications of human HDL each loaded onto gels (Western blotting) or measured (AE activity) in duplicate. The difference between EL-HDL and EV-HDL or M-EL-HDL and EV-HDL was analyzed by two-tailed unpaired t-test in samples w/ and w/o PON1 separately. * p < 0.05, ** p < 0.01, ***p < 0.001.
2.2. EL Decreases HDL Size and PON1 Content without Diminishing the Capacity of HDL to Associate with PON1
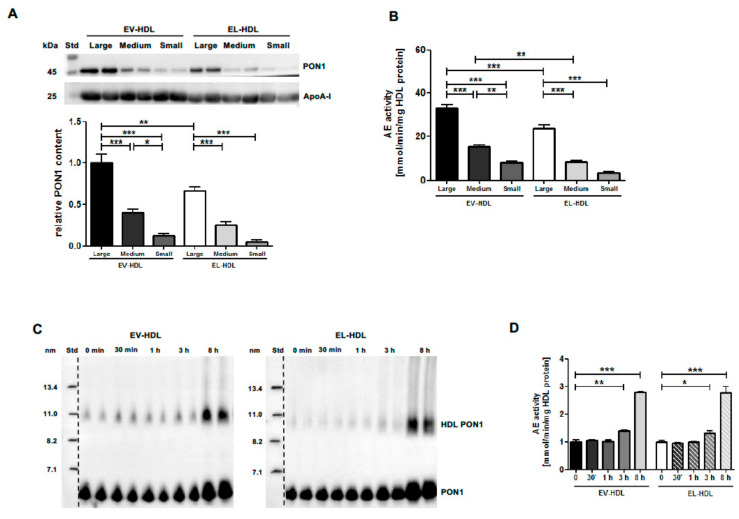
Since EL-induced HDL size reduction may explain PON1 displacement from HDL, we studied the relationship between HDL size and PON1 content. We analyzed the PON1 content and AE activity of EV-HDL and EL-HDL subpopulations with a defined size prepared by fast protein liquid chromatography (FPLC) (Supplementary Figure S2). As shown in Figure 2A,B, the decrease in particle size of both types of modified HDL was associated with a decrease in PON1 content and AE activity. These results strongly suggest the involvement of an EL-induced reduction of HDL size in diminishing HDL PON1 content and AE activity. However, the EL-induced HDL size and PON1 reduction did not diminish the capacity of EL-modified HDL to associate with PON1 when incubated with PON1-overexpressing cells (Figure 2C,D). Therefore, we concluded that primarily the interaction of enzymatically active EL with HDL, rather than EL-induced alterations in HDL size and composition, causes PON1 displacement from HDL.
Figure 2.
EL decreases HDL size and PON1 content but not the capacity of HDL to associate with PON1. (A,B) EV-HDL and EL-HDL were fractionated by fast protein liquid chromatography (FPLC) to obtain small, medium, and large EV-HDL and EL-HDL fractions. (A) 10 µg HDL protein were separated by 12% SDS-PAGE followed by Western blotting analyses of PON1 and apoA-I. Densitometric values of PON1 were normalized to the corresponding apoA-I signals. (B) PON1 AE activity was measured in 2 µg HDL protein. (C,D) Twenty-four hours after infection with PON1-Ad, HepG2 cells were washed and incubated with 100 µg/mL of EV-HDL protein or EL-HDL protein in Dulbecco’s modified Eagle medium (DMEM). Aliquots were collected after indicated time intervals followed by (C) 4–20% non-denaturing gradient-gel electrophoresis of 10 µg HDL protein and subsequent PON-1 Western blotting, and (D) measurements of AE activity in 2 µg HDL protein. Densitometry results and AE activity are presented as means + SEM of three (A,B) or two (C,D) independent modifications of human HDL each loaded onto gels (Western blotting) or measured (AE activity) in duplicate. The differences between FPLC-derived HDL fractions were analyzed by one-way ANOVA followed by Bonferroni post-hoc test, and the differences between 3 h or 8 h and 0 h time points were analyzed by two-tailed unpaired t-test. * p < 0.05, ** p < 0.01, *** p < 0.001.
2.3. EL Overexpression in Mice Increases HDL PON1 Content and AE Activity
To examine whether EL overexpression in vivo affects HDL PON1 content and AE activity, we analyzed PON1 and apoA-I levels as well as AE activity of serum of mice injected with EL-Ad or control EV-Ad. While apoA-I levels were below the detection limit, the PON1 levels were similar in EL serum and apoB-DS compared to the respective EV controls (Figure 3A). Interestingly, despite comparable PON1 levels, the AE activity of EL serum and apolipoprotein B-depleted serum (apoB-DS) was significantly lower compared to the respective EV controls (Figure 3B).
Figure 3.
EL overexpression in mice increases the PON1 content and AE activity of HDL. Mouse (m) serum was isolated from blood of EV- and EL-overexpressing mice 48 h after adenovirus (Ad) injection. (A) Serum (1.5 µL) and apolipoprotein B (apoB)-depleted serum (apoB-DS) (2 µL), were separated by 12% SDS-PAGE followed by Western blotting analyses of PON1 and apoA-I. (B) The AE activity was measured using 1.5 µL of 10-fold diluted serum or apoB-DS. (C) Total cholesterol concentrations in the FPLC fractions of mouse serum. (D) FPLC fractions 27–40 (10 µL) and (E) HDL isolated from serum by ultracentrifugation (10 µg) were analyzed as in (A). (F) The AE activity using 2 µg HDL protein isolated by ultracentrifugation was measured as described in (B). Densitometry and AE activity results are presented as mean + SEM of three independent in vivo modifications, with 12 EL-Ad and four EV-Ad-injected mice per modification. Samples from each modification were loaded onto gels in duplicate or used for AE activity measurements in triplicate. The difference between EL-HDL and EV-HDL was analyzed by two-tailed unpaired t-test. * p < 0.05, *** p < 0.001.
Unaltered PON1 serum levels despite the markedly decreased HDL serum levels in EL-overexpressing mice may suggest either an HDL overload with PON1 or an accumulation of PON1 that is not associated with HDL. To clarify this, we analyzed the PON1 content of HDL isolated from EV and EL mouse serum by FPLC. In line with markedly decreased HDL levels in EL compared to EV serum (Figure 3C), apoA-I levels in the EL-FPLC fractions 30–36 (corresponding to HDL) were drastically reduced, but PON1 levels were comparable when compared to the corresponding EV-FPLC fractions (Figure 3D). These findings suggested an EL-overexpression-driven overload of HDL with PON1. Indeed, mouse EL-HDL isolated by ultracentrifugation exhibited a 4.3-fold higher PON1 content and 1.6-fold higher AE activity when compared to control EV-HDL (Figure 3E,F). These in vivo findings led us to the conclusion that a marked EL-induced decrease in HDL serum levels increases the PON1-to-HDL ratio, leading in turn to a marked PON1 overload of HDL, which is accompanied by less profoundly increased PON1 AE activity.
2.4. HDL PON1 Content Is Positively Associated with EL Serum Levels in Humans
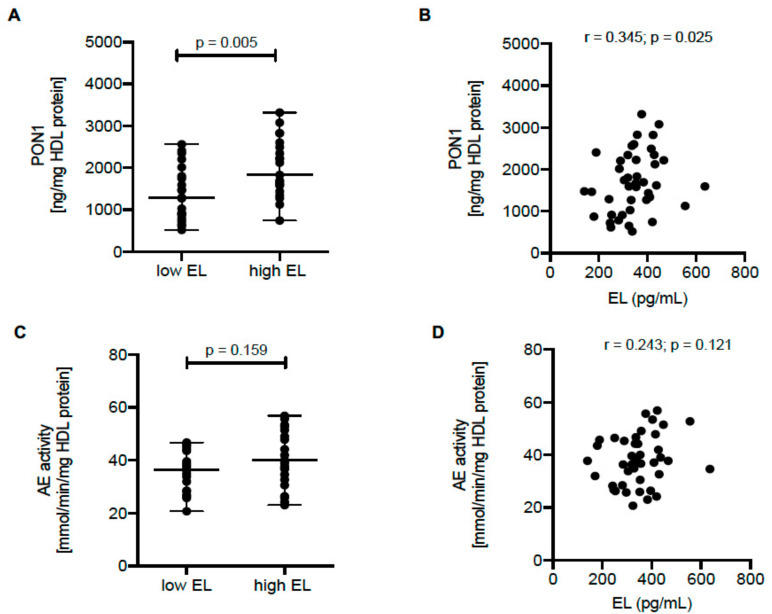
Next, we examined the relationship between EL serum levels, HDL PON1 content, and AE activity in humans. For this purpose, we recruited healthy volunteers (baseline characteristics are shown in Supplemental Materials Table S1)OK and measured EL serum levels as well as PON1 content and AE activity of isolated HDL. We found significantly higher PON1 content in HDL isolated from subjects with high (above median) compared to those with low (below median) EL serum levels (Figure 4A) and EL serum levels were significantly positively correlated with HDL PON1 content (Figure 4B). The HDL PON1 content was strongly correlated with HDL AE activity (r = 0.52, p < 0.001). However, HDL AE activity showed only a trend to be increased in subjects with above median EL expression (Figure 4C) and was not significantly correlated with EL serum levels (Figure 4D).
Figure 4.
Positive association between HDL PON1 content and EL serum levels in humans. (A) The differences in HDL PON1 content (measured by ELISA) between HDL isolated from subjects with high (above median; n = 21) or low (below median; n = 21) EL serum levels were analyzed by two-tailed unpaired t-test. (B) Correlation of EL serum levels with HDL PON1 content was determined by the Spearman correlation coefficient. (C) The differences in AE activity were analyzed as in (A). (D) Correlation of EL serum levels and AE activity was determined as in (B).
2.5. EL-Induced Alterations of HDL Lipidome and HDL Serum Levels Are Not Linked to HDL PON1 Content
We hypothesized that the positive association between EL serum levels and HDL PON1 content in humans reflects EL-induced alterations in the HDL lipid composition, which facilitates the loading of HDL with PON1. To test this hypothesis, we performed logistic regression analysis to identify HDL lipid species significantly associated with high PON1 content and then additionally adjusted the model for EL (low vs. high). We identified 16 lipid species that were significantly positively or negatively associated with high HDL PON1 content (Supplementary Figure S3). The levels of these species in samples containing high or low PON1 concentrations are shown in Supplementary Table S2. However, after adjusting for EL (high vs. low), the majority of the associations (with the exception of lysophosphatidylcholine (LPC) 22:4, phosphatidic acid (PA) 32:1, phosphatidylinositol (PI) 38:4, ceramide (Cer) d18:2/22:0, Cer d18:2/24:0, and Cer d18:2/26:0) remained significant (Supplementary Figure S3). In line with this, HDL particles that differ in lipid composition dependent on EL serum levels (low or high) had comparable content of PON1 (Supplementary Figure S4 and Supplementary Tables S3 and S4). These data clearly suggested that the association between the PON1 content and lipid composition of HDL is not significantly affected by EL. According to this, the observed positive relationship between EL serum levels and HDL PON1 content (Figure 4) cannot be explained by EL-induced alterations in the HDL lipid composition.
We observed that the EL-induced reduction of HDL levels was accompanied by increased HDL PON1 content in EL-overexpressing mice. Next, we assessed whether EL serum levels in humans are linked to serum concentrations of HDL parameters quantified by nuclear magnetic resonance (NMR) spectroscopy. Serum levels of the HDL parameters including total HDL as well as HDL subclasses quantified on the basis of their cholesterol, triacylglycerol, phospholipid, apoA-I or apoA-II content were comparable in the low and high EL sera (Supplementary Table S5) and were not significantly correlated with EL (Supplementary Table S6). Accordingly, the positive association between EL serum levels and HDL PON1 content cannot be directly attributed to the EL-induced HDL reduction and the concomitantly increased PON1-to-HDL ratio, as found in EL-overexpressing mice.
3. Discussion
In the present study, we provide evidence that EL modulates HDL PON1 content and AE activity and that marked differences are observed in vitro and in vivo.
In vivo, the concerted action of different enzymes and receptors as well as interactions of HDL with other lipoproteins modulate the HDL size and composition [1]. In our in vitro model, HDL remodeling is primarily due to EL and, to a lesser extent, the interaction of HDL with HepG2 cells as well as enzymes and lipids secreted by HepG2 cells [14]. However, EL-modified HDL generated by the incubation of human plasma with EL-overexpressing cells also exhibited decreased PON1 content and AE activity (Supplementary Figure S5). This finding indicates that the diminishing effect of EL on HDL PON1 content and AE activity in vitro was also operative under more physiological conditions and was not counteracted by the HDL remodeling machinery of human plasma.
It is well established that HDL phospholipids play an important role in the association of PON1 with HDL [9,10] and that EL by its profound phospholipase activity efficiently degrades HDL phospholipids [28]. Accordingly, the EL-mediated alterations in the HDL phospholipid abundance and composition [27,30] are likely the cause for the decreased PON1 content and AE activity in the in vitro EL-modified HDL.
Previous studies have shown that PON1-containing HDL particles contain decreased levels of unsaturated LPC species [17]. The fact that EL generates primarily unsaturated LPC species by cleaving HDL phospholipids [28,39] suggest that the accumulation of these EL-generated LPCs contributes to the reduction of PON1 content in EL-HDL. However, EL efficiently decreased HDL PON1 also in the presence of BSA (Supplementary Figure S1), which binds LPC and prevents their accumulation in EL-modified HDL [30]. Additionally, the observation that human HDL particles with high PON1 content contain higher levels of LPC 22:4 compared to those with low PON1 (Supplementary Table S2) supports the notion that EL-generated LPCs are not a likely cause for PON1 reduction in EL-modified HDL.
A positive relationship between HDL size and PON1 content, revealed by PON1 quantification in FPLC-generated HDL fractions of various size, implicated EL-mediated HDL size reduction in diminishing HDL PON1 content. The importance of the enzymatic activity of EL in displacing PON1 from HDL in vitro was also demonstrated by the increased, rather than decreased, PON1 content of HDL following the incubation of HDL with cells expressing enzymatically inactive EL. This most probably reflects EL-mediated bridging of HDL to the cells, which in turn facilitates HDL-mediated desorption of the secreted PON1 [6], as described for SR-BI [7].
Importantly, the EL-induced alterations in HDL size and composition, accompanied by a displacement of PON1, did not diminish the capacity of the EL-modified HDL to associate with PON1. Accordingly, it seems that the interaction of enzymatically active EL with HDL and/or an active EL-mediated lipolysis of HDL, rather than altered HDL size and composition, might be crucial for PON1 displacement from HDL.
In sharp contrast to the EL-mediated HDL PON1 reduction observed in vitro, EL overexpression in mice in vivo increased the PON1 content of HDL. It is conceivable that a massive EL-induced HDL reduction and a concomitantly increased serum PON1-to-HDL ratio drive the overload of HDL with PON1. The findings that endogenous PON1 expression was not increased by EL overexpression (Supplementary Figure S6), and that the overexpression of enzymatically inactive EL did not increase HDL PON1 content (Supplementary Figure S7), exclude the possibility that an increased endogenous PON1 synthesis or EL bridging function contributed to the HDL PON1 overload in EL-overexpressing mice. Moreover, comparable hepatic apoA-I mRNA levels in EL-overexpressing and control mice (Supplementary Figure S6) strongly argue against attenuated apoA-I synthesis as the cause of EL-induced HDL reduction. Previous studies have also shown that apoA-I synthesis is not affected by adenovirus-induced EL overexpression in mice and, importantly, that the reduced HDL serum concentrations are exclusively due to the EL-mediated acceleration of HDL catabolism [40,41]. Additionally, structural and compositional features of the in vivo EL-modified mouse HDL, including unaltered particle size, increased phospholipid and triacylglycerol as well as decreased lysophospholipid content (in contrast to reduced particle size, decreased phospholipid and triacylglycerol as well as increased lysophospholipid content observed in the in vitro EL-modified human EL-HDL) [27], may have facilitated the PON1 overload of the in vivo EL-modified mouse HDL.
Interestingly, however, the increased PON1 content of the EL-modified mouse HDL (4.3-fold higher compared to control mouse EV-HDL) was accompanied by only 1.6-fold increased AE activity. Considering that approximately only 5–10% of HDL particles in normal serum contain PON1 [13,14], the increased PON1 content of the in vivo EL-modified mouse HDL reflects either an increased number of PON1-containing HDL particles or an increased number of PON1 molecules per HDL particle. Accordingly, the observed discrepancy between the PON1 content and AE activity might be due to the EL-overexpression-driven association of PON1 with a subset of HDL particles, which under normal conditions would not contain PON1 and whose structure and composition do not provide a suitable micro-environment to support PON1 AE activity. Alternatively, the EL-overexpression-driven increase in PON1 load per HDL particle could preclude an appropriate interaction of PON1 with HDL lipids and proteins [8,9], resulting in an altered PON1 conformation and in turn decreased AE activity. Moreover, the decreased LPC levels in the in vivo EL-modified mouse HDL [27] may, considering the stimulating effect of LPC on PON1 AE activity [42], also, at least in part, be responsible for the decreased AE activity. However, in human HDL samples, AE activity was associated with several HDL lipid species (Supplementary Tables S7–S9) but not LPC (not shown).
Previous studies reported a negative correlation between EL and HDL serum levels in patients with cardiovascular disease but not in those without [35,43]. In line, the EL serum levels in the present study were not significantly correlated with either parameter indicative of HDL serum bioavailability measured by NMR spectroscopy (Supplementary Table S6). Nevertheless, significantly higher levels of LPC and LPE species (lipolytic products known to be generated by EL-mediated phospholipolysis [27,28,30]) in HDL isolated from subjects with high compared to low EL serum (Supplementary Table S10) strongly argue for the modulation of the HDL composition by EL in vivo. This is further supported by a significant positive association of the LPC and LPE species with high EL serum levels (Supplementary Table S11) However, results of the logistic regression analysis (Supplementary Figure S3) and the finding that HDL particles which differ in lipid composition, dependent on whether they originate from low or high EL serum, have comparable (high or low) content of PON1 (Supplementary Figure S4) suggest that in humans, the EL-related lipidomic signature of HDL is not a determinant of the HDL PON1 content.
What are the functional implications of EL-induced changes in HDL PON1 content? In line with a role of PON1 in the cholesterol efflux capacity of HDL [5,44], in the present study, we found a robust positive correlation between the cholesterol efflux capacity of HDL isolated from healthy subjects and the HDL PON1 content and AE activity, respectively (r = 0.4, p = 0.003 and r = 0.53, p < 0.001, respectively). Accordingly, the decreased cholesterol efflux capacity of the in vitro EL-modified HDL described in our previous report [27] was probably (at least in part) due to the decreased HDL PON1 content. In the same study [27], the cholesterol efflux capacity of the in vivo EL-modified mouse HDL was not altered, despite a PON1 overload. This indicates that physiological levels and/or the distribution of PON1 on the subset of HDL particles that provide an optimal micro-environment are crucial for the exertion of maximal biological PON1 activity.
In a previous study, we found an increased antioxidative capacity of in vitro EL-modified human HDL despite decreased PON1 content [30], disputing the antioxidative role of PON1. However, it is possible that the decreased HDL PON1 was compensated by the profound antioxidative activity of LPCs enriched in HDL [30]. In contrast to the in vitro EL-induced increase in the antioxidative capacity of HDL, the in vivo EL-modified mouse HDL overloaded with PON1 exhibited a strong prooxidative activity, which was comparable to control HDL (unpublished results). It is likely that the systemic inflammatory response induced by adenoviral transduction produced dysfunctional HDL, highlighting again the failure of PON1 overload to entail functional benefit. Further studies are warranted to understand the complex relationship between the antioxidative capacity of HDL and the HDL PON1 content, AE activity, and lipid composition in humans.
We demonstrated that EL profoundly modulates HDL PON1 content and AE activity in vitro and in vivo. We conclude that primarily the interaction of enzymatically active EL with HDL, rather than EL-induced alterations in HDL size and composition, causes PON1 displacement from HDL in vitro. In vivo, the EL-mediated reduction of HDL serum levels and consequently increased serum PON1-to-HDL ratio increase HDL PON1 content and AE activity in mice. In humans, additional mechanisms appear to underlie the association of EL serum levels and HDL PON1 content. Therefore, further larger studies are needed to understand the role of EL in modulation of HDL PON1 content in humans.
4. Materials and Methods
4.1. Cell Culture
HepG2 cells (ATCC® HB-8065TM, Manassas, VA, USA) were cultured as described [27].
4.2. Human Plasma
Normolipidemic plasma of 18 healthy donors was obtained as described [30].
4.3. Blood Collection from Healthy Volunteers
Fasting blood samples were collected by antecubital venipuncture into two 9 mL serum tubes (VACUETTE TUBE 9 mL Z Serum Clot Activator). Blood was incubated at 20 °C for 30 min followed by centrifugation at 1800 should be like this, please do not change× g at 20 °C for 10 min. Serum aliquots were frozen at −80 °C. The study was approved by the Ethics Committee of the Medical University of Graz (32-096 ex 19/20), and informed consent of all probands was obtained in accordance with the Declaration of Helsinki.
4.4. Isolation of HDL
HDL from human plasma or serum as well as mouse serum or cell culture media was prepared by a one-step density gradient ultracentrifugation as described [27].
4.5. Modification of HDL and Plasma
HDL and plasma were incubated with HepG2 cells overexpressing human EL or enzymatically inactive human EL or with control cells infected with empty adenovirus containing no recombinant cDNA as described [27,30]. Some modifications were performed in the presence of 4% (final concentration) of non-esterified fatty acid (NEFA)-free bovine serum albumin (BSA) (Sigma-Aldrich, Vienna, Austria) or PON1 co-overexpression achieved by co-infection of the cells with multiplicity of infection (MOI) 20 of PON1-adenovirus (Vector Biosystems Inc., Malvern, PA, USA). Modified HDLs were re-isolated and stored as described [27,30].
4.6. Preparation of In Vivo EL-Modified Serum and Control EV Serum
Modified serum was isolated from 12-week-old male C57BL/6J mice injected with EL-Ad or EV-Ad as described in our previous report [27]. The serum was used for the preparation of apoB-depleted serum (apoB-DS) [27] as well as for HDL isolation by ultracentrifugation or by fast protein liquid chromatography (FPLC). All experimental protocols related to animal experiments were approved by the Austrian Federal Ministry of Education, Science and Research (BMWF-66.010/0020-WF/V/3b/2016). All experiments were performed in accordance with relevant guidelines and regulations.
4.7. Fractionation of HDL by FPLC
HDL samples were loaded on a NGC QUEST FPLC System (Bio-Rad, Vienna, Austria) equipped with a Superdex 200 Increase 10/300 column (GE Healthcare Europe GmbH, Munich, Germany) using phosphate buffered saline (PBS) as running buffer. HDL fractionation was started after 18 min with a constant flow of 0.5 mL per fraction.
4.8. FPLC of Mouse Serum
A pool of 200 µL of mouse serum of EV- or EL-overexpressing mice was subjected to FPLC on a Pharmacia FPLC system (Pfizer Pharma, Karlsruhe, Germany) equipped with a Superose 6 column (Amersham Biosciences, Piscataway, NJ, USA). OK Lipoproteins were eluted with 10 mmol/L Tris-HCl, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), 0.9% NaCl, and 0.02% NaN3 (pH 7.4). The total cholesterol (TC) (Greiner Diagnostics AG, Bahlingen, Germany) concentrations in 0.5 mL fractions were determined spectrophotometrically.
4.9. SDS-PAGE and Western Blotting
HDL-associated proteins in isolated HDL (10 µg), FPLC fractions (10 µL), serum (1.5 µL), or apoB-DS (2 µL) were quantified using antibodies specific for human/mouse PON1 (abcam-ab24261, dilution 1:1000, Cambridge, UK), human apoA-I (Novus biological, NB100-65491, dilution 1:3000, Littleton, CO, USA), or mouse apoA-I (Santa Cruz Biotechnology, sc-30089, LOT D2913, dilution 1:500, Heidelberg, Germany) as described [27,30].
4.10. Non-Denaturing Gradient-Gel Electrophoresis and Western Blotting
Modified HDL (10 µg), FPLC fractions (10 µL), or incubation media (containing 15 µg protein) were electrophoresed on 4–20% non-denaturing polyacrylamide gels as described [27]. For the analyses of PON1 content, separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Carl Roth, Karlsruhe, Germany) followed by PON1 detection as described in Section 4.9.
4.11. AE Activity
Ca2+-dependent AE activities of serum, apoB-DS, and HDL were determined with a photometric assay using phenyl acetate as substrate as described previously [45]. Briefly, either 1.5 µL serum (1:10 diluted), apoB-DS (1:10 diluted), or 2 µg isolated HDL were added to 200 µL of buffer containing 100 mmol/L Tris, 2 mmol/L CaCl2 (pH 8.0), and 1 mmol/L phenylacetate. The rate of hydrolysis of phenylacetate was monitored by an increase of absorbance at 270 nm every 30 s. Activities were calculated from the slopes of the kinetic chart.
4.12. Cholesterol Efflux Capacity
The HDL cholesterol efflux capacity of HDL (50 µg isolated HDL protein) was assessed in J774 macrophages pretreated with 0.3 mmol/L 8-(4-chlorophenylthio)-cAMP no need to be defined (Sigma-Aldrich, Darmstadt, Germany) as described [27].
4.13. Association of EV-HDL and EL-HDL with PON1
Twenty-four hours after infection with PON1-Ad, HepG2 cells were washed and incubated with 100 µg/mL of EV-HDL or EL-HDL in DMEM w/o fetal calf serum (FCS). Aliquots of the incubation mixtures were collected after indicated time intervals followed by 4–20% non-denaturing gradient-gel electrophoresis, PON1 Western blotting, and measurements of AE activity.
4.14. PON1 ELISA
PON1 content of isolated human HDL (1 µg/well) was determined with a human PON1 ELISA kit (Biozol, Eiching, Germany) according to the manufacturer’s instruction.
4.15. EL ELISA
Serum EL concentrations were measured with Endothelial Lipase ELISA (Tecan; #JP27182, IBL International, Hamburg, Germany) as described previously [35].
4.16. RNA Isolation and Quantitative Real-Time PCR Analysis
RNA isolation from mouse liver, reverse transcription, and real-time PCR were performed as described [27]. Primers for mouse PON1 (fw: GGTGTTGGCACTTTACAAGAACC; rev: CGCAAAGGGCCACTTTTACTA), mouse apoA-I (fw. AGCTGAACCTGAATCTCCTG; rev CACTTCCTCTAGGTCCTTGT), and mouse cyclophilin A (fw:CCATCCAGCCATTCAGTCTT; rev: TTCCAGGATTCATGTGCCAG) were from Life Technologies (Vienna, Austria) and for human EL we used Primer Assay QT00078969 (Qiagen, Hilden, Germany).
4.17. Lipid and (Apo)Lipoprotein Profiling by Nuclear Magnetic Resonance (NMR) Spectroscopy
Blood serum lipoproteins were measured on a Bruker 600 MHz Avance Neo NMR spectrometer using the Bruker IVDr lipoprotein subclass analysis protocol. Serum samples were thawed, and 330 µL of each sample mixed with 330 µL of Bruker serum buffer (Bruker, Rheinstetten, Germany). The samples were mixed gently, and 600 µL of the mixed sample were transferred into a 5 mm SampleJet rack tube (Bruker). Proton spectra were obtained at a constant temperature of 310 K using a standard nuclear Overhauser effect spectroscopy (NOESY) pulse sequence (Bruker: noesygppr1d), a Carr–Purcedll–Meiboom–Gill (CPMG) pulse sequence with presaturation during the relaxation delay (Bruker: cpmgpr1d) to achieve water suppression, and a standard 2D J-resolved (JRES) pulse sequence (Bruker: jresgpprqf). Data analysis was carried out using the Bruker IVDr LIpoprotein Subclass Analysis (B.I.LISATM, Bruker, Rheinstetten, Germany) method.
4.18. HDL-Lipidome Analysis by Liquid Chromatography Coupled to Tandem Mass Spectrometry (LC-MS/MS)
HDL total lipids were extracted according to a modified Bligh and Dyer method: 50 µL of isolated HDL equivalent to 50 µg protein were supplemented with a mixture of internal standards, and lipids were extracted with 1.2 mL methanol/chloroform (2:1, v/v) in the presence of the antioxidant butylated hydroxytoluene and 310 µL HCL (0.01 N). Phase separation was triggered by the addition of 400 µL CHCl3 and 400 µL water. Lipids were quantified by LC-ESI/MS/MS using a Prominence UFLC (Shimadzu, Tokyo, Japan) and QTrap 4000 mass spectrometer (AB Sciex, Framingham, MA, USA). Quantification of phospholipids and sphingolipids was performed in positive-ion mode. A sample (4 µL) was injected to a Kinetex HILIC 2.6 µm 2.1 × 150 mm column (Phenomenex, CA, USA). Mobile phases consisted of water and acetonitrile containing ammonium acetate and acetic acid. The quantification of neutral lipids (triacylglycerols (TAG), diacylglycerols (DAG), cholesteryl ester (CE), and free cholesterol) was performed in positive-ion mode. A sample (4 µL) was injected into an Ascentis Express C18 column (150 × 2.1 mm, 2.7 µm) Sigma-Aldrich, Merck Group). Mobile phases consisted of (A) acetonitrile (ACN):H2O = (60:40 v/v) and (B) isopropanol (IPA)/ACN (90:10 v/v) supplemented with 10 mmol/L ammonium formate and 0.1% formic acid. Lipid species were detected using scheduled multiple reaction monitoring.
4.19. Statistical Analyses
Data of in vitro and mouse experiments are represented as the means ± standard error of the mean (S.E.M.). Differences between EL and EV samples were assessed by a two-tailed unpaired t-test between EL-HDL and EV-HDL or one-way ANOVA when comparing more than two groups (FPLC-derived EV-HDL and EL-HDL fractions) using Graph Pad Prism 5.0. Statistically significant differences between groups are indicated in the plots as follows: p < 0.05 (*), p < 0.01 (**), or p < 0.001 (***). The association between EL and PON1 as well as AE activity in human samples was investigated by correlation analyses using the Spearman correlation coefficient. We performed logistic regression analyses to identify HDL lipid species associated with high vs. low PON1 content, defined via the median, and additionally investigated the influence of EL on significant associations by adjusting these models for high vs. low EL (defined via the median). These analyses were performed using R version 3.6.1.
Acknowledgments
The authors wish to thank Claire Cannet for help and advice on lipoprotein analysis with B.I.LISATM, Christoph Russ, and Sabine Kern for the expert technical assistance and Isabella Hindler, Patricia Fellnhofer, and Arno Absenger for help with mice care.
Abbreviations
| HDL | high-density lipoprotein |
| Apo A-I | apolipoprotein A-I |
| PON1 | paraoxonase 1 |
| EL | endothelial lipase |
| FCS | fetal calf serum |
| BSA | bovine serum albumin |
| FPLC | fast protein liquid chromatography |
| MS | mass spectrometry |
| PC | phosphatidylcholine |
| PE | phosphatidylethanolamine |
| PI | phosphatidylinositol |
| TAG | triacylglycerols |
| LPC | lysophosphatidylcholine |
| LPE | lysophosphatidylethanolamine |
| FC | free cholesterol |
| CE | cholesteryl ester |
| Cer | ceramide |
| SM | sphingomyelin |
| NMR | nuclear magnetic resonance |
| Ad | adenovirus |
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/22/2/719/s1. Figure S1: EL decreases HDL PON1 content and AE activity in the presence of BSA; Figure S2: FPLC-derived EV-HDL and EL-HDL fractions; Figure S3: Association between HDL PON1 content and lipid composition is minimally affected by EL; Figure S4: HDL PON1 content is not determined by EL-related lipidomic signature of HDL; Figure S5: EL-modification of human plasma in vitro decreases HDL PON1 content and AE activity; Figure S6: Mouse PON1 mRNA levels are unaltered upon the overexpression of human EL in mice; Figure S7: HDL PON1 content is unaltered upon the overexpression of enzymatically inactive EL (M-EL) in mice; Table S1: Baseline characteristics of the blood donors; Table S2: Lipid species with levels significantly different in HDL with low compared to high PON1 (identified by logistic regression analysis); Table S3: Levels of lipid species significantly enriched in low PON1/low EL compared to low PON1/high EL HDL; Table S4: Levels of lipid species significantly enriched in high PON1/high EL compared to high PON1/low EL HDL; Table S5: Serum HDL parameters measured by NMR spectroscopy compared between high and low EL; Table S6: Correlation analysis of EL with serum HDL parameters measured by NMR spectroscopy; Table S7: Significant associations between HDL AE activity and HDL lipid species determined by univariable logistic regression analysis; Table S8: Lipid species with levels significantly different in HDL with high compared to low AE activity; Table S9: Significant correlations of AE activity with HDL lipid species; Table S10: Lipid species with levels significantly different in HDL isolated from high compared to low EL serum; Table S11: Significant associations between EL serum levels (high vs. low, determined by median) and HDL lipid species determined by univariable logistic regression analysis.
Author Contributions
Conceptualization, C.W., G.M. and S.F.; Data curation, A.B. and G.P.; Formal analysis, A.S., A.B. and G.P.; Funding acquisition, C.W., T.M., D.K., G.M. and S.F.; Investigation, I.S., J.T.S., M.L. (Margarete Lechleitner), A.H., M.L. (Marie Lhomme), M.H., M.K., F.R., A.S., and T.M.; Methodology, I.S., M.L. (Margarete Lechleitner), M.H., F.R., M.K., T.M. and A.K.; Project administration, M.L. (Margarete Lechleitner) and S.F.; Resources, M.L. (Marie Lhomme), C.W., T.M., D.K., A.K., G.M. and S.F.; Supervision, D.K., A.K., G.M. and S.F.; Validation, A.H.; Visualization, I.S., J.T.S., A.H., G.P., M.H., D.K., G.M. and S.F.; Writing—original draft, S.F.; Writing—review and editing, I.S., J.T.S., M.L. (Margarete Lechleitner), A.H., A.B., G.P., M.L. (Marie Lhomme), M.H., M.K., F.R., A.S., C.W., T.M., D.K., A.K., G.M. and S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Austrian Science Fund FWF (P27166-B23 to S.F., DP-iDP DOC 31 to C.W., D.K., G.M., and SFB F73 to D.K., and DK-MCD W1226 to T.M. and D.K., P28854 and I3792 to T.M.), Austrian Research Promotion Agency (FFG) (grants 864690 and 870454 to T.M.), and the PhD program Molecular Medicine of the Medical University of Graz. T.M. was additionally supported by the Integrative Metabolism Research Center Graz, Austrian Infrastructure Program 2016/2017 and the Styrian Government (Zukunftsfonds), and T.M. and D.K. by BioTechMed-Graz (Flagship project).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Medical University of Graz (protocol code 32-096 ex 19/20; date of approval: 02.12.2019). All experimental protocols related to animal experiments were approved by the Austrian Federal Ministry of Education, Science and Research (BMWF-66.010/0020-WF(V/3b/2016; date of approval 03.03.2016.).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fisher E.A., Feig J.E., Hewing B., Hazen S.L., Smith J.D. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 2012;32:2813–2820. doi: 10.1161/ATVBAHA.112.300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besler C., Luscher T.F., Landmesser U. Molecular mechanisms of vascular effects of High-density lipoprotein: Alterations in cardiovascular disease. Embo Mol. Med. 2012;4:251–268. doi: 10.1002/emmm.201200224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidson W.S., Silva R.A., Chantepie S., Lagor W.R., Chapman M.J., Kontush A. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: Relevance to antioxidative function. Arterioscler. Thromb. Vasc. Biol. 2009;29:870–876. doi: 10.1161/ATVBAHA.109.186031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aviram M., Rosenblat M., Bisgaier C.L., Newton R.S., Primo-Parmo S.L., La Du B.N. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J. Clin. Investig. 1998;101:1581–1590. doi: 10.1172/JCI1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenblat M., Vaya J., Shih D., Aviram M. Paraoxonase 1 (PON1) enhances HDL-mediated macrophage cholesterol efflux via the ABCA1 transporter in association with increased HDL binding to the cells: A possible role for lysophosphatidylcholine. Atherosclerosis. 2005;179:69–77. doi: 10.1016/j.atherosclerosis.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Deakin S., Leviev I., Gomaraschi M., Calabresi L., Franceschini G., James R.W. Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high affinity, saturable, desorption mechanism. J. Biol. Chem. 2002;277:4301–4308. doi: 10.1074/jbc.M107440200. [DOI] [PubMed] [Google Scholar]
- 7.James R.W., Brulhart-Meynet M.C., Singh A.K., Riederer B., Seidler U., Out R., Van Berkel T.J., Deakin S. The scavenger receptor class B, type I is a primary determinant of paraoxonase-1 association with high-density lipoproteins. Arterioscler. Thromb. Vasc. Biol. 2010;30:2121–2127. doi: 10.1161/ATVBAHA.110.209122. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y., Wu Z., Riwanto M., Gao S., Levison B.S., Gu X., Fu X., Wagner M.A., Besler C., Gerstenecker G., et al. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J. Clin. Investig. 2013;123:3815–3828. doi: 10.1172/JCI67478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu X., Huang Y., Levison B.S., Gerstenecker G., DiDonato A.J., Hazen L.B., Lee J., Gogonea V., DiDonato J.A., Hazen S.L. Identification of Critical Paraoxonase 1 Residues Involved in High Density Lipoprotein Interaction. J. Biol. Chem. 2016;291:1890–1904. doi: 10.1074/jbc.M115.678334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorenson R.C., Bisgaier C.L., Aviram M., Hsu C., Billecke S., La Du B.N. Human serum Paraoxonase/Arylesterase’s retained hydrophobic N-terminal leader sequence associates with HDLs by binding phospholipids: Apolipoprotein A-I stabilizes activity. Arterioscler. Thromb. Vasc. Biol. 1999;19:2214–2225. doi: 10.1161/01.ATV.19.9.2214. [DOI] [PubMed] [Google Scholar]
- 11.Deakin S.P., Bioletto S., Bochaton-Piallat M.L., James R.W. HDL-associated paraoxonase-1 can redistribute to cell membranes and influence sensitivity to oxidative stress. Free Radic. Biol. Med. 2011;50:102–109. doi: 10.1016/j.freeradbiomed.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Efrat M., Aviram M. Macrophage paraoxonase 1 (PON1) binding sites. Biochem. Biophys. Res. Commun. 2008;376:105–110. doi: 10.1016/j.bbrc.2008.08.106. [DOI] [PubMed] [Google Scholar]
- 13.Blatter M.C., James R.W., Messmer S., Barja F., Pometta D. Identification of a distinct human high-density lipoprotein subspecies defined by a lipoprotein-associated protein, K-45. Identity of K-45 with paraoxonase. Eur. J. Biochem. 1993;211:871–879. doi: 10.1111/j.1432-1033.1993.tb17620.x. [DOI] [PubMed] [Google Scholar]
- 14.Gugliucci A., Menini T. Paraoxonase 1 and HDL maturation. Clin Chim Acta. 2015;439:5–13. doi: 10.1016/j.cca.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Kontush A. HDL-mediated mechanisms of protection in cardiovascular disease. Cardiovasc. Res. 2014;103:341–349. doi: 10.1093/cvr/cvu147. [DOI] [PubMed] [Google Scholar]
- 16.Kingwell B.A., Chapman M.J., Kontush A., Miller N.E. HDL-targeted therapies: Progress, failures and future. Nat. Rev. Drug Discov. 2014;13:445–464. doi: 10.1038/nrd4279. [DOI] [PubMed] [Google Scholar]
- 17.Moren X., Lhomme M., Bulla A., Sanchez J.C., Kontush A., James R.W. Proteomic and lipidomic analyses of paraoxonase defined high density lipoprotein particles: Association of paraoxonase with the anti-coagulant, protein S. Proteom. Clin. Appl. 2016;10:230–238. doi: 10.1002/prca.201500062. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharyya T., Nicholls S.J., Topol E.J., Zhang R., Yang X., Schmitt D., Fu X., Shao M., Brennan D.M., Ellis S.G., et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang W.H., Hartiala J., Fan Y., Wu Y., Stewart A.F., Erdmann J., Kathiresan S., Consortium C.A., Roberts R., McPherson R., et al. Clinical and genetic association of serum paraoxonase and arylesterase activities with cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 2012;32:2803–2812. doi: 10.1161/ATVBAHA.112.253930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shih D.M., Gu L., Xia Y.R., Navab M., Li W.F., Hama S., Castellani L.W., Furlong C.E., Costa L.G., Fogelman A.M., et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 21.Rozenberg O., Rosenblat M., Coleman R., Shih D.M., Aviram M. Paraoxonase (PON1) deficiency is associated with increased macrophage oxidative stress: Studies in PON1-knockout mice. Free Radic. Biol. Med. 2003;34:774–784. doi: 10.1016/S0891-5849(02)01429-6. [DOI] [PubMed] [Google Scholar]
- 22.Mackness B., Quarck R., Verreth W., Mackness M., Holvoet P. Human paraoxonase-1 overexpression inhibits atherosclerosis in a mouse model of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2006;26:1545–1550. doi: 10.1161/01.ATV.0000222924.62641.aa. [DOI] [PubMed] [Google Scholar]
- 23.Jaye M., Lynch K.J., Krawiec J., Marchadier D., Maugeais C., Doan K., South V., Amin D., Perrone M., Rader D.J. A novel endothelial-derived lipase that modulates HDL metabolism. Nat. Genet. 1999;21:424–428. doi: 10.1038/7766. [DOI] [PubMed] [Google Scholar]
- 24.Hirata K., Dichek H.L., Cioffi J.A., Choi S.Y., Leeper N.J., Quintana L., Kronmal G.S., Cooper A.D., Quertermous T. Cloning of a unique lipase from endothelial cells extends the lipase gene family. J. Biol. Chem. 1999;274:14170–14175. doi: 10.1074/jbc.274.20.14170. [DOI] [PubMed] [Google Scholar]
- 25.Ishida T., Choi S., Kundu R.K., Hirata K., Rubin E.M., Cooper A.D., Quertermous T. Endothelial lipase is a major determinant of HDL level. J. Clin. Investig. 2003;111:347–355. doi: 10.1172/JCI16306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singaraja R.R., Sivapalaratnam S., Hovingh K., Dube M.P., Castro-Perez J., Collins H.L., Adelman S.J., Riwanto M., Manz J., Hubbard B., et al. The impact of partial and complete loss-of-function mutations in endothelial lipase on high-density lipoprotein levels and functionality in humans. Circ. Cardiovasc. Genet. 2013;6:54–62. doi: 10.1161/CIRCGENETICS.111.962613. [DOI] [PubMed] [Google Scholar]
- 27.Schilcher I., Kern S., Hrzenjak A., Eichmann T.O., Stojakovic T., Scharnagl H., Duta-Mare M., Kratky D., Marsche G., Frank S. Impact of Endothelial Lipase on Cholesterol Efflux Capacity of Serum and High-density Lipoprotein. Sci. Rep. 2017;7:12485. doi: 10.1038/s41598-017-12882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gauster M., Rechberger G., Sovic A., Horl G., Steyrer E., Sattler W., Frank S. Endothelial lipase releases saturated and unsaturated fatty acids of high density lipoprotein phosphatidylcholine. J. Lipid Res. 2005;46:1517–1525. doi: 10.1194/jlr.M500054-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Gauster M., Oskolkova O.V., Innerlohinger J., Glatter O., Knipping G., Frank S. Endothelial lipase-modified high-density lipoprotein exhibits diminished ability to mediate SR-BI (scavenger receptor B type I)-dependent free-cholesterol efflux. Biochem. J. 2004;382:75–82. doi: 10.1042/BJ20031882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schilcher I., Ledinski G., Radulovic S., Hallstrom S., Eichmann T., Madl T., Zhang F., Leitinger G., Kolb-Lenz D., Darnhofer B., et al. Endothelial lipase increases antioxidative capacity of high-density lipoprotein. Biochim. Biophys. Acta Mol Cell Biol. Lipids. 2019;1864:1363–1374. doi: 10.1016/j.bbalip.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radulovic S., Gottschalk B., Horl G., Zardoya-Laguardia P., Schilcher I., Hallstrom S., Vujic N., Schmidt K., Trieb M., Graier W.F., et al. Endothelial lipase increases eNOS activating capacity of high-density lipoprotein. Biochim. Biophys. Acta Mol Cell Biol. Lipids. 2020;1865:158612. doi: 10.1016/j.bbalip.2020.158612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adam R.C., Mintah I.J., Alexa-Braun C.A., Shihanian L.M., Lee J.S., Banerjee P., Hamon S.C., Kim H.I., Cohen J.C., Hobbs H.H., et al. Angiopoietin-like protein 3 governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. J. Lipid Res. 2020;61:1271–1286. doi: 10.1194/jlr.RA120000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badellino K.O., Wolfe M.L., Reilly M.P., Rader D.J. Endothelial lipase concentrations are increased in metabolic syndrome and associated with coronary atherosclerosis. PloS Med. 2006;3:e22. doi: 10.1371/journal.pmed.0030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paradis M.E., Badellino K.O., Rader D.J., Deshaies Y., Couture P., Archer W.R., Bergeron N., Lamarche B. Endothelial lipase is associated with inflammation in humans. J. Lipid Res. 2006;47:2808–2813. doi: 10.1194/jlr.P600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Potocnjak I., Trbusic M., Teresak S.D., Radulovic B., Pregartner G., Berghold A., Tiran B., Marsche G., Degoricija V., Frank S. Metabolic Syndrome Modulates Association between Endothelial Lipase and Lipid/Lipoprotein Plasma Levels in Acute Heart Failure Patients. Sci. Rep. 2017;7:1165. doi: 10.1038/s41598-017-01367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko K.W., Paul A., Ma K., Li L., Chan L. Endothelial lipase modulates HDL but has no effect on atherosclerosis development in apoE-/- and LDLR-/- mice. J. Lipid Res. 2005;46:2586–2594. doi: 10.1194/jlr.M500366-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Ishida T., Choi S.Y., Kundu R.K., Spin J., Yamashita T., Hirata K., Kojima Y., Yokoyama M., Cooper A.D., Quertermous T. Endothelial lipase modulates susceptibility to atherosclerosis in apolipoprotein-E-deficient mice. J. Biol. Chem. 2004;279:45085–45092. doi: 10.1074/jbc.M406360200. [DOI] [PubMed] [Google Scholar]
- 38.Wang C., Nishijima K., Kitajima S., Niimi M., Yan H., Chen Y., Ning B., Matsuhisa F., Liu E., Zhang J., et al. Increased Hepatic Expression of Endothelial Lipase Inhibits Cholesterol Diet-Induced Hypercholesterolemia and Atherosclerosis in Transgenic Rabbits. Arterioscler. Thromb. Vasc. Biol. 2017;37:1282–1289. doi: 10.1161/ATVBAHA.117.309139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riederer M., Kofeler H., Lechleitner M., Tritscher M., Frank S. Impact of endothelial lipase on cellular lipid composition. Biochim. Et Biophys. Acta. 2012;1821:1003–1011. doi: 10.1016/j.bbalip.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maugeais C., Tietge U.J., Broedl U.C., Marchadier D., Cain W., McCoy M.G., Lund-Katz S., Glick J.M., Rader D.J. Dose-dependent acceleration of high-density lipoprotein catabolism by endothelial lipase. Circulation. 2003;108:2121–2126. doi: 10.1161/01.CIR.0000092889.24713.DC. [DOI] [PubMed] [Google Scholar]
- 41.Nijstad N., Wiersma H., Gautier T., van der Giet M., Maugeais C., Tietge U.J. Scavenger receptor BI-mediated selective uptake is required for the remodeling of high density lipoprotein by endothelial lipase. J. Biol. Chem. 2009;284:6093–6100. doi: 10.1074/jbc.M807683200. [DOI] [PubMed] [Google Scholar]
- 42.Park C.H., Nguyen S.D., Kim M.R., Jeong T.S., Sok D.E. Differential effect of lysophospholipids on activities of human plasma paraoxonase1, either soluble or lipid-bound. Lipids. 2006;41:371–380. doi: 10.1007/s11745-006-5108-4. [DOI] [PubMed] [Google Scholar]
- 43.Ishida T., Miyashita K., Shimizu M., Kinoshita N., Mori K., Sun L., Yasuda T., Imamura S., Nakajima K., Stanhope K.L., et al. ELISA system for human endothelial lipase. Clin. Chem. 2012;58:1656–1664. doi: 10.1373/clinchem.2012.187914. [DOI] [PubMed] [Google Scholar]
- 44.Berrougui H., Loued S., Khalil A. Purified human paraoxonase-1 interacts with plasma membrane lipid rafts and mediates cholesterol efflux from macrophages. Free Radic. Biol. Med. 2012;52:1372–1381. doi: 10.1016/j.freeradbiomed.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 45.Trieb M., Horvath A., Birner-Gruenberger R., Spindelboeck W., Stadlbauer V., Taschler U., Curcic S., Stauber R.E., Holzer M., Pasterk L., et al. Liver disease alters high-density lipoprotein composition, metabolism and function. Biochim. Et Biophys. Acta. 2016;1861:630–638. doi: 10.1016/j.bbalip.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article or Supplementary Material.