Abstract
Aims
Positron emission tomography (PET) myocardial perfusion imaging (MPI) can non-invasively measure myocardial blood flow reserve (MBFR). We aimed to examine whether MBFR identifies patients with a survival benefit after revascularization, helping to guide post-test management.
Methods and results
We examined all-cause mortality in 12 594 consecutive patients undergoing Rb82 rest/stress PET MPI from January 2010 to December 2016, after excluding those with cardiomyopathy, prior coronary artery bypass surgery (CABG), and missing MBFR. Myocardial blood flow reserve was calculated as the ratio of stress to rest absolute myocardial blood flow. A Cox model adjusted for patient and test characteristics, early revascularization (percutaneous coronary intervention or CABG ≤90 days of MPI), and the interaction between MBFR and early revascularization was developed to identify predictors of all-cause mortality. After a median follow-up of 3.2 years, 897 patients (7.1%) underwent early revascularization and 1699 patients (13.5%) died. Ischaemia was present in 4051 (32.3%) patients, with 1413 (11.2%) having ≥10% ischaemia. Mean MBFR was 2.0 ± 1.3, with MBFR <1.8 in 4836 (38.5%). After multivariable adjustment, every 0.1 unit decrease in MBFR was associated with 9% greater hazard of all-cause death (hazard ratio 1.09, 95% confidence interval 1.08–1.10; P < 0.001). There was a significant interaction between MBFR and early revascularization (P < 0.001); such that patients with MBFR ≤1.8 had a survival benefit with early revascularization, regardless of type of revascularization or level of ischaemia.
Conclusion
Myocardial blood flow reserve on PET MPI is associated with all-cause mortality and can identify patients who receive a survival benefit with early revascularization compared to medical therapy. This may be used to guide revascularization, and prospective validation is needed.
Keywords: Myocardial blood flow reserve, Survival, Myocardial perfusion imaging, Positron emission tomography
See page 769 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz465)
Introduction
Myocardial blood flow reserve (MBFR), expressed as the ratio of maximal hyperaemic flow to myocardial blood flow at rest, assesses coronary vasomotor dysfunction. It integrates the haemodynamic effects of epicardial coronary stenoses, diffuse atherosclerotic disease, and microvascular dysfunction on myocardial perfusion.1 Positron emission tomography (PET) myocardial perfusion imaging (MPI) provides a non-invasive measurement of MBFR in addition to quantifying perfusion abnormalities and left ventricular function.1,2 Impaired MBFR on PET MPI is currently used to provide valuable information on the haemodynamic significance of coronary artery disease3,4 and also helps reclassify patients at an increased risk of major adverse cardiac events.5,6
While the diagnostic and prognostic benefits of MPI are extremely important, the ability to guide post-test management is equally valuable.7,8 Invasive coronary flow physiology and nuclear-derived ischaemic burden have identified patients with coronary disease who most benefit from revascularization.9,10 MBFR provides a non-invasive measure of change in coronary flow and may be ideally positioned to guide post-test medical or revascularization treatment.1 A prior study demonstrated that low MBFR was associated with improved outcomes with surgical revascularization in patients referred to angiography after PET MPI; however, it was limited by a small sample size and a low event rate.11 Whether MBFR helps identify patients who would benefit from early revascularization compared with medical therapy is not known. We examined a consecutive cohort of patients with suspected or known coronary artery disease undergoing PET MPI to identify whether MBFR was associated with mortality, and whether this association was modified by early revascularization.
Methods
Study population
A total of 19 221 unique consecutive patients who underwent rest/stress Rubidium (Rb)82 PET MPI within the Saint Luke’s Health System between January 2010 and December 2016 were included in our study (Supplementary material online, Figure S1). Saint Luke’s Health System includes fully functioning nuclear cardiology laboratories at four major metropolitan hospitals in the Kansas City area, with either a dedicated PET or PET/computed tomography (CT) system at each hospital. Patients with prior coronary artery bypass surgery (CABG) (n = 1707) and cardiomyopathy [ejection fraction (EF) < 40%] (n = 2854) were excluded due to concerns that the flow values might not be accurate in these patients.12 Patients with missing MBFR were also excluded (n = 2111), with 12 594 patients remaining in the final study cohort. For patients with multiple MPI tests, the earliest PET MPI in our system within the time period was included and subsequent studies were not. The study protocol was approved by the Institutional Review Board of the Saint Luke’s Hospital System, and requirement for informed consent was waived.
Study variables
Demographics, clinical risk factors, past medical history, recent and current symptoms, and active and held medications were collected by trained laboratory personnel at the time of the MPI test from medical chart review and confirmed by patient questioning using standardized intake forms, and entered into the database.
Rb82 positron emission tomography and positron emission tomography/computed tomography myocardial perfusion imaging protocols
All patients were studied using a PET (Siemens ECAT ACCEL) or PET/CT scanner (Siemens Biograph 16 or 64) after fasting for at least 6 h. Patients were asked to withhold caffeine containing beverages for 24 h prior to the test and beta-blockers, calcium channel blockers, and nitrates on the morning of the test. One site utilized dedicated cardiac PET for image acquisition with line source attenuation correction, while the remaining three sites utilized PET/CT cameras, where a low-dose transmission CT scan was acquired prior to obtaining rest images for attenuation correction. For the dedicated PET system, a 2.5 min, 2D dynamic study (8 × 12 s frames, 2 × 27 s frames) was acquired to measure the quantitative blood flow followed immediately by a 5 min, 3D ECG-gated perfusion study. For the PET/CT systems, a 3D, 7 min ECG-gated list mode study was acquired. Post-acquisition, list mode studies were rebinned into one dynamic study (8 × 12 s frames, 2 × 27 s frames), and an eight binned ECG-gated perfusion study (90–330 s). Average radiation dose per PET MPI was 3.5 ± 1.4 mSv.
On both PET and PET/CT systems, rest emission image acquisition was started at the same time as the beginning of the intravenous administration of Rb82 (20–50 mCi). All patients then underwent pharmacological stress testing using regadenoson (n = 8399), dipyridamole (n = 4107), or adenosine (n = 4). At peak stress, another dose of Rb82 (20–60 mCi) was injected, and stress images were acquired in a similar manner. Patient heart rate, blood pressure, and 12-lead ECG were acquired at baseline and every minute during and after pharmacological stress. After acquisition of images, all studies were electronically transmitted to the central nuclear cardiology laboratory where trained nuclear technologists processed and reconstructed the images for interpretation. Perfusion images were reconstructed from the list mode acquisitions starting 90–120 s after the beginning of Rb82 infusions at rest and peak stress using commercial software (Imagen Pro, Kansas City, MO, USA). In patients without known coronary artery disease (CAD) who underwent MPI tests at the three sites with a PET/CT camera, a separate CT scan was acquired for purposes of calcium scoring (scored using Siemens Syngo Calcium Scoring software, Erlangen, Germany), if no prior calcium score was available.
Myocardial perfusion and left ventricular ejection fraction analysis
Commercial software (Cedars Sinai QPET, Los Angeles, CA, USA) was used to quantify the perfusion images and measure left ventricular ejection fraction (LVEF). All images were interpreted by experienced observers using a 17-segment model and standard 5-point scoring system.12 Global Summed Rest Score (SRS), Summed Stress Score (SSS), and Summed Difference Score (SDS) were calculated. The percentage of myocardium with fixed defect/scar and the percentage of ischaemic myocardium were calculated from SRS and SDS by dividing these scores by a maximum score of 68. Rest and stress LVEF were calculated from gated myocardial perfusion images acquired with 8-frame gating (Cedars Sinai QGS software).
Myocardial blood flow reserve
Absolute myocardial blood flow (MBF, ml/min/g) was calculated at rest and peak stress using commercially available and previously validated software (Imagen Q, Kansas City, MO, USA).13,14 The software uses a net retention model and has previously been validated against other software based on 1- and 2-tissue compartment models.14–16 For every patient, global MBFR was calculated as the ratio of stress to rest absolute MBF for the entire left ventricle. MBFR values were not reported to the referring physician for the majority of the study period. MBFR values were reported to the referring physician at the discretion of the reading nuclear cardiologist beginning in 2016.
Outcomes
The date of last follow-up was 31 December 2017, so all patients had at least 1 year of follow-up. Data were censored at date of death or last follow-up visit. The primary study endpoint was all-cause mortality, determined from the patient medical record and the National Death Index. Cardiac mortality was a secondary endpoint. Early revascularization was defined as revascularization with percutaneous coronary intervention (PCI) or CABG within 90 days of the MPI test. Percutaneous coronary intervention/CABG were ascertained by trained personnel from the catheterization laboratory database and validated with 100% accuracy by medical chart review.
Statistical analysis
Baseline demographics, clinical risk factors, symptoms, and test results were compared across low (<1.8), intermediate (1.8–2), and normal (≥2) MBFR values using the Student’s t-test for continuous variables and χ2 test or Fisher’s exact test for categorical variables.
Cumulative survival for all-cause mortality were plotted using Kaplan–Meier curves according to MBFR categories. Kaplan–Meier curves were also generated according to MBFR categories within each stratum of ischaemia. A Cox proportional hazards model was used to assess the association of MBFR with all-cause mortality, after adjustment for 33 covariates, chosen a priori based upon previously published literature and clinical judgement. Assumptions for collinearity and proportional hazards were met. To account for selection bias for referral to early revascularization, factors affecting decision for revascularization were adjusted in the Cox model as covariates. We chose this method as our primary analysis as previous methodological studies have shown similar results with covariate adjustment and propensity matching, and we had adequate power to use covariate adjustment.17 Covariates included demographics (age, gender, and body mass index), clinical risk factors {known coronary artery disease [prior PCI or myocardial infarction (MI)], hypertension, diabetes mellitus, hyperlipidaemia, current smoker, family history of atherosclerotic cardiovascular disease, peripheral vascular disease, cerebrovascular accident, inpatient vs. outpatient status at the time of the test, and abnormal ECG at baseline}, symptoms (chest pain, dyspnoea, and syncope), medications (aspirin, beta-blockers, calcium channel blockers, and statins), stress data (resting rate-pressure product and ischaemic ECG response), coronary artery calcium score, perfusion data (% ischaemic and infarcted myocardium), gated data (rest LVEF and stress LVEF), global MBFR, and early revascularization. A sensitivity analysis was included global stress MBF was substituted in place of global MBFR in the Cox model. The interaction of global MBFR and early revascularization was tested in the final model. Global MBFR was used because of prior literature showing stronger associations between adverse outcomes with global MBFR, as compared with stress MBF.18 Subsequently, a sensitivity analysis was done by generating a propensity score for referral to early revascularization as an alternative means of analysis.
To evaluate whether the interaction effect of MBFR with early revascularization differs based on the amount of ischaemic myocardium on the PET MPI test, we evaluated a three-way interaction of % ischaemia * MBFR * early revascularization and further evaluated the effect of the interaction of MBFR * early revascularization within levels of 0–5%, >5–10%, and ≥10% ischaemic myocardium12 in the fully adjusted Cox model. We also tested for an interaction of MBFR with type of early revascularization (PCI vs. CABG) to assess if type of revascularization would influence the relationship between MBFR and survival. The most frequently missing variable was calcium score as it was only done in patients without prior CAD undergoing PET/CT MPI (missing rate = 40%), followed by LVEF at rest (missing rate = 10%). All other variables had either no missing data or rates <5%. Missing data were imputed using sequential regression models using the IVEWare software (Ann Arbor, MI, USA).
Two-sided P-values <0.05 were considered significant. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
A total of 12 549 patients were included in the study, with a median follow-up of 3.2 (1.8–5.0) years. The mean age of the cohort was 68.0 ± 12.1 years, body mass index was 29.7 ± 6.5 kg/m2 and more than half were women. Three-quarters of all patients had hypertension, and a quarter had diabetes. Chest pain was the predominant presenting symptom in ∼60% of all patients and half had dyspnoea. Ischaemia was present in 4051 (32.3%) patients with 1413 (11.3%) patients having ≥10% ischaemia on PET MPI.
Mean MBFR was 2.0 ± 1.3; 4836 (38.5%) patients had low (<1.8), 1888 (15.0%) had intermediate (1.8–2), and 5825 (46.4%) had normal (≥2) MBFR values. Baseline characteristics of patients with low, intermediate, and normal MBFR appears in Table 1. Patients with low MBFR were more likely to be older, female, have history of prior MI or PCI, hypertension, diabetes, peripheral vascular disease, cerebrovascular accidents, a greater burden of infarcted and ischaemic myocardium, and a coronary artery calcium score above 400.
Table 1.
Baseline characteristics of the study population
| Characteristics | Total | Myocardial blood flow reserve |
P-value | ||
|---|---|---|---|---|---|
| N = 12 549 | Low (<1.8) (n = 4836) | Intermediate (1.8–2) (n = 1888) | Normal (≥2) (n = 5825) | ||
| Age (years) | 68.0 ± 12.1 | 71.7 ± 11.4 | 68.8 ± 11.4 | 64.7 ± 11.9 | <0.001 |
| Male gender | 5927 (47.2) | 2155 (44.6) | 846 (44.8) | 2926 (50.2) | <0.001 |
| Body mass index (kg/m2) | 29.68 ± 6.47 | 29.26 ± 5.91 | 29.28 ± 5.91 | 30.16 ± 7.03 | <0.001 |
| Coronary artery disease | <0.001 | ||||
| No known CAD | 9711 (77.4) | 3636 (75.2) | 1450 (76.8) | 4625 (79.4) | |
| Prior MI | 216 (1.7) | 104 (2.2) | 25 (1.3) | 87 (1.5) | |
| Prior PCI | 2622 (20.9) | 1096 (22.7) | 413 (21.9) | 1113 (19.1) | |
| Hypertension | 9813 (78.2) | 4012 (83.0) | 1535 (81.3) | 4266 (73.2) | <0.001 |
| Diabetes | 3472 (27.7) | 1631 (33.7) | 494 (26.2) | 1347 (23.1) | <0.001 |
| Hyperlipidaemia | 9411 (75.0) | 3731 (77.2) | 1441 (76.3) | 4239 (72.8) | <0.001 |
| Current smoker | 1720 (13.7) | 627 (13.0) | 248 (13.1) | 845 (14.5) | 0.05 |
| Family history of CVD | 4743 (37.8) | 1715 (35.5) | 735 (38.9) | 2293 (39.4) | <0.001 |
| Peripheral vascular disease | 1452 (11.6) | 758 (15.7) | 205 (10.9) | 489 (8.4) | <0.001 |
| Cerebrovascular accident | 1071 (8.5) | 543 (11.2) | 148 (7.8) | 380 (6.5) | <0.001 |
| Patient status | <0.001 | ||||
| Inpatient | 4859 (38.7) | 1833 (37.9) | 650 (34.4) | 2376 (40.8) | |
| Outpatient | 7536 (60.1) | 2982 (61.7) | 1221 (64.7) | 3333 (57.2) | |
| Baseline abnormal ECG | 10 436 (83.2) | 4115 (85.1) | 1594 (84.4) | 4727 (81.2) | <0.001 |
| Symptoms | |||||
| Chest pain | <0.001 | ||||
| Atypical | 7398 (59.0) | 2578 (53.3) | 1086 (57.5) | 3734 (64.1) | |
| Non-anginal | 54 (0.4) | 19 (0.4) | 9 (0.5) | 26 (0.4) | |
| Typical | 62 (0.5) | 25 (0.5) | 14 (0.7) | 23 (0.4) | |
| Dyspnoea | 6151 (49.0) | 2529 (52.3) | 929 (49.2) | 2693 (46.2) | <0.001 |
| Syncope | 814 (6.5) | 329 (6.8) | 112 (5.9) | 373 (6.4) | 0.40 |
| Medications at baseline | |||||
| Aspirin | 8134 (64.8) | 3082 (63.7) | 1214 (64.3) | 3838 (65.9) | 0.06 |
| Beta-blocker | 5173 (41.2) | 2237 (46.3) | 783 (41.5) | 2153 (37.0) | <0.001 |
| Statin | 4304 (34.3) | 1732 (35.8) | 685 (36.3) | 1887 (32.4) | <0.001 |
| Calcium channel blocker | 2923 (23.3) | 1357 (28.1) | 452 (23.9) | 1114 (19.1) | <0.001 |
| Stress data | |||||
| Baseline heart rate (b.p.m.) | 70.26 ± 13.53 | 73.82 ± 14.19 | 70.33 ± 13.23 | 67.27 ± 12.30 | <0.001 |
| Peak heart rate (b.p.m.) | 89.93 ± 16.45 | 88.99 ± 16.63 | 89.69 ± 15.69 | 90.78 ± 16.50 | <0.001 |
| Rest systolic BP (mmHg) | 131.85 ± 20.99 | 136.21 ± 22.01 | 132.41 ± 20.68 | 128.06 ± 19.46 | <0.001 |
| Peak systolic BP (mmHg) | 122.19 ± 21.11 | 124.53 ± 22.51 | 122.88 ± 20.73 | 120.02 ± 19.76 | <0.001 |
| ECG response | <0.001 | ||||
| Ischaemic | 488 (3.9) | 263 (5.4) | 62 (3.3) | 163 (2.8) | |
| Non-diagnostic | 2239 (17.8) | 1094 (22.6) | 351 (18.6) | 794 (13.6) | |
| Non-ischaemic | 9644 (76.9) | 3415 (70.6) | 1451 (76.9) | 4778 (82.0) | |
| Equivocal | 178 (1.4) | 64 (1.3) | 24 (1.3) | 90 (1.5) | |
| Imaging data | |||||
| Rest LVEF (%) | 61.84 ± 13.61 | 60.84 ± 13.84 | 62.37 ± 12.76 | 62.51 ± 13.63 | <0.001 |
| % Scarred myocardium >0% | 2453 (19.5) | 1225 (25.3) | 363 (19.2) | 865 (14.8) | <0.001 |
| % Ischaemic myocardium | <0.001 | ||||
| None (0%) | 8498 (67.7) | 2708 (56.0) | 1305 (69.1) | 4485 (77.0) | |
| Mild–moderate (1–10%) | 2638 (21.0) | 1192 (24.6) | 424 (22.5) | 1022 (17.5) | |
| Severe (≥10+%) | 1413 (11.3) | 936 (19.4) | 159 (8.4) | 318 (5.5) | |
| Transient ischaemic dilatation | 1479 (12.1) | 792 (16.9) | 192 (10.5) | 495 (8.8) | <0.001 |
| LVEF reserve | 4.0 (1.0–8.0) | 3.0 (−1.0 to 6.0) | 4.0 (1.0–8.0) | 6.0 (2.0–9.0) | 0.73 |
| Coronary calcium score | <0.001 | ||||
| 0 | 1409 (19.5) | 331 (11.8) | 203 (19.4) | 875 (26.0) | |
| 1–99 | 2128 (29.5) | 640 (22.8) | 290 (27.8) | 1198 (35.6) | |
| 100–399 | 1470 (20.4) | 617 (22.0) | 233 (22.3) | 620 (18.4) | |
| ≥400 | 2211 (30.6) | 1219 (43.4) | 319 (30.5) | 673 (20.0) | |
| Rest MBF (ml/min/g) | 0.77 (0.61–0.97) | 0.88 (0.70–1.12) | 0.79 (0.64–0.99) | 0.69 (0.56–0.85) | <0.001 |
| Stress MBF (ml/min/g) | 1.51 (1.19–1.91) | 1.28 (1.00–1.62) | 1.50 (1.21–1.86) | 1.72 (1.39–2.11) | 0.01 |
Continuous variables presented as mean (SD) except median (IQR); categorical variables presented as frequency (%). Continuous variables compared using ANOVA or Wilcoxon rank sum test (non-normally distributed variables). Categorical variables compared using the χ2 or Fisher’s exact test.
CVD, cardiovascular disease; IQR: interquartile range; MI, myocardial infarction; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
There were 897 patients who underwent PCI or CABG (7.1%; 748 PCI, 149 CABG) within 90 days of PET MPI. Of those, 599 (66.8%), 97 (10.8%), and 201 (22.4%) had MBFR values of <1.8, 1.8–2, and >2, respectively. Baseline patient and test characteristics of patients who underwent early revascularization vs. medical therapy are shown in Supplementary material online, Table S1.
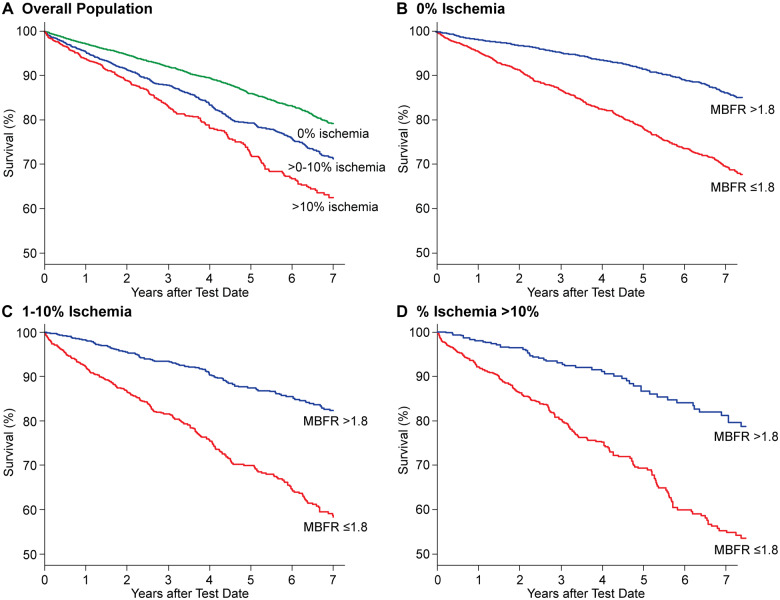
During study follow-up, there were 1699 (13.5%) deaths; 1061 (21.9%), 234 (12.4%), and 404 (6.9%) deaths in patients with low, intermediate, and normal MBFR groups, respectively (P < 0.001). Annualized mortality rates for patients with low, intermediate, and normal MBFR groups were 7.3, 3.8, and 2.1 deaths per 100-person years, respectively. Kaplan–Meier curves showed significantly lower overall survival with decreasing MBFR (Figure 1). Kaplan–Meier curves also show lower survival with increasing proportion of % ischaemic myocardium; however, within each strata of ischaemia (0%, 1–10%, and >10%), patients with lower MBFR had significantly lower survival than those with normal MBFR (Figure 2).
Figure 1.
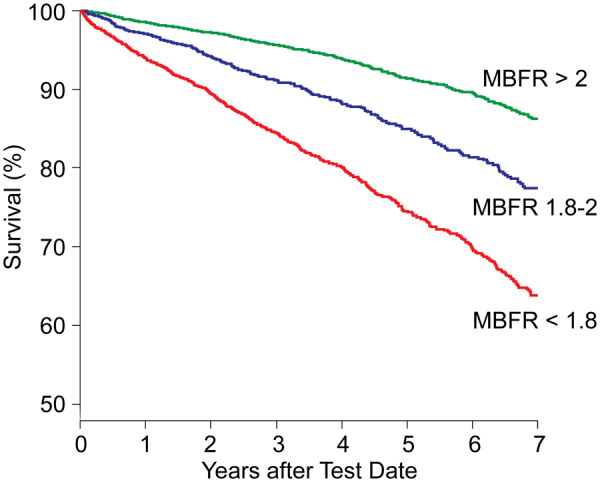
Kaplan–Meier unadjusted survival estimates as a function of myocardial blood flow reserve at baseline.
Figure 2.
Kaplan–Meier unadjusted survival estimates as a function of percent ischaemic myocardium at baseline (A), and substratified by myocardial blood flow reserve within 0% (B), 1–10% (C) and >10% (D) ischaemia.
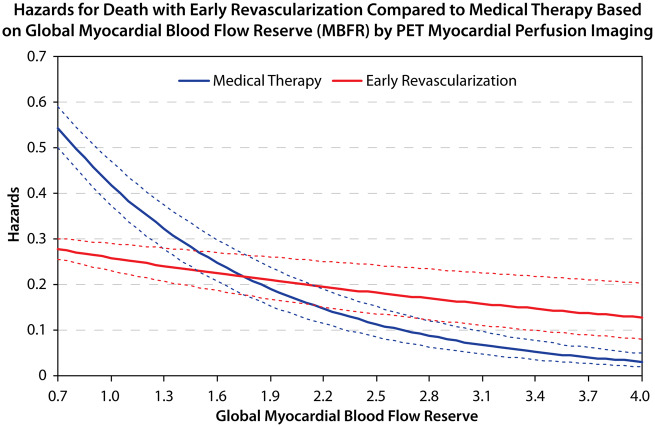
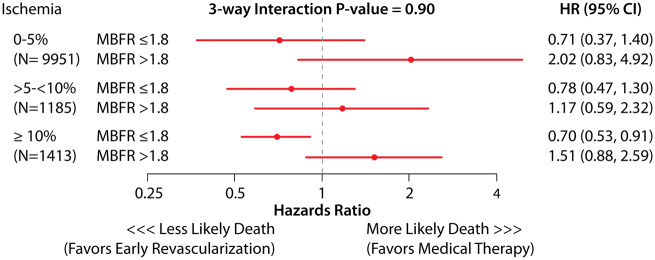
Table 2 shows hazard ratio (HR) estimates (main effects) for the risk-adjusted Cox proportional hazards model. After adjusting for patient and test characteristics, every 0.1 unit decrease in MBFR was associated with 9% greater hazard of all-cause death [HR 1.09, 95% confidence interval (CI) 1.08–1.10; P < 0.001, Table 2]. Of note, % ischaemia was not prognostic of survival in the adjusted model including MBFR. In the fully adjusted Cox model, there was presence of a significant interaction between MBFR and early revascularization (P = 0.001); such that patients with MBFR ≤1.8 had a survival benefit with early revascularization (HR 0.76, 95% CI 0.62–0.94), and those with MBFR >1.8 had similar or worse outcomes with early revascularization (HR 1.39, 95% CI 1.01–1.94). Figure 3 and Supplementary material online, Figure S2 demonstrates the hazards for death with early revascularization compared to medical therapy based on MBFR. Patients who underwent CABG had a higher likelihood of severe ischaemia, transient ischaemic dilatation, lower LVEF reserve, and lower MBFR compared with those who underwent PCI (Supplementary material online, Table S1B). However, the survival benefit with early revascularization with low MBFR was present regardless of the type of revascularization strategy that was used (three-way interaction with type of revascularization P = 0.99, Supplementary material online, Table S2). In the main model, the three-way interaction between % ischaemia, MBFR, and early revascularization was non-significant (P = 0.90), suggesting that % ischaemia on the PET MPI does not affect the MBFR * early revascularization association with survival. Figure 4 shows effect estimates for MBFR * revascularization across levels of ischaemia. Patients with low MBFR appear to have a survival benefit with revascularization compared to medical therapy across all strata (Figure 4), however, the CIs cross 1 in the 2 lower strata of ischaemia (<5% and 5–10%), due to lower numbers of revascularization and less precision within those strata.
Table 2.
Cox proportional hazards model estimates for all-cause and cardiac mortality
| Variable | Hazard ratio (95% CI) all-cause death | P-value | Hazard ratio (95% CI) cardiac death | P-value |
|---|---|---|---|---|
| Age (per 10 years) | 1.57 (1.49–1.66) | <0.0001 | 1.57 (1.46–1.7) | <0.0001 |
| Male gender | 0.98 (0.88–1.09) | 0.72 | 0.96 (0.83–1.11) | 0.58 |
| Body mass index (per 5 kg/m2 increase) | 0.86 (0.82–0.91) | <0.0001 | 0.83 (0.78–0.88) | <0.0001 |
| Hypertension | 1.21 (1.05–1.4) | 0.01 | 1.28 (1.07–1.54) | 0.008 |
| Diabetes mellitus | 1.44 (1.29–1.61) | <0.0001 | 1.63 (1.4–1.89) | <0.0001 |
| Coronary artery disease (prior MI or PCI) | 1.13 (1–1.27) | 0.04 | 0.91 (0.76–1.1) | 0.32 |
| Hyperlipidaemia | 0.79 (0.7–0.9) | 0.0003 | 0.74 (0.64–0.86) | 0.0001 |
| Current smoker | 1.57 (1.36–1.81) | <0.0001 | 1.58 (1.3–1.91) | <0.0001 |
| Family history of CVD | 0.84 (0.76–0.93) | 0.001 | 0.8 (0.69–0.93) | 0.004 |
| Peripheral vascular disease | 1.17 (1.03–1.33) | 0.01 | 1.23 (1.01–1.48) | 0.04 |
| Cerebrovascular accident | 1.24 (1.08–1.43) | 0.003 | 1.28 (1.05–1.57) | 0.01 |
| Chest pain symptoms | ||||
| Anginal chest pain vs. none | 0.91 (0.82–1.01) | 0.09 | 0.9 (0.78–1.04) | 0.16 |
| Non-anginal chest pain vs. none | 0.75 (0.4–1.41) | 0.38 | 0.62 (0.25–1.5) | 0.29 |
| Dyspnoea | 1.25 (1.14–1.38) | <0.0001 | 1.32 (1.15–1.51) | <0.0001 |
| Syncope | 0.96 (0.81–1.14) | 0.64 | 0.98 (0.78–1.23) | 0.87 |
| Aspirin | 0.9 (0.81–1) | 0.05 | 0.89 (0.78–1.02) | 0.08 |
| Beta-blocker | 0.97 (0.88–1.08) | 0.59 | 1.07 (0.93–1.22) | 0.36 |
| Other antiplatelet | 1.16 (0.85–1.59) | 0.34 | 1.6 (1.03–2.49) | 0.04 |
| Statin | 0.9 (0.81–1) | 0.06 | 0.87 (0.75–1.02) | 0.08 |
| Calcium channel blocker | 1.04 (0.93–1.16) | 0.47 | 1.1 (0.95–1.27) | 0.21 |
| Inpatient vs. outpatient | 1.65 (0.68–4.01) | 0.27 | 1.64 (1.42–1.89) | <0.0001 |
| Abnormal baseline ECG | 1.05 (0.92–1.21) | 0.47 | 1.04 (0.87–1.25) | 0.64 |
| Ischaemic ECG response | 0.81 (0.64–1.02) | 0.08 | 0.95 (0.68–1.32) | 0.76 |
| Resting rate-pressure product (per 10 000) | 1.01 (1–1.02) | 0.13 | 1.01 (1–1.03) | 0.17 |
| Early revascularization | 0.85 (0.7–1.03) | 0.09 | 0.26 (0.14–0.51) | <0.0001 |
| Coronary artery calcium score | ||||
| Calcium score: 1–99 vs. 0 | 1.15 (0.96–1.38) | 0.13 | 1.33 (1–1.76) | 0.05 |
| Calcium score: 100–399 vs. 0 | 1.32 (1.12–1.55) | 0.001 | 1.69 (1.28–2.25) | 0.0003 |
| Calcium score: ≥400 vs. 0 | 1.67 (1.45–1.93) | <0.0001 | 2.08 (1.58–2.75) | <0.0001 |
| Rest LVEF (per 10% increase) | 0.84 (0.8–0.88) | <0.0001 | 0.9 (0.85–0.96) | 0.001 |
| Stress LVEF (per 10% increase) | 0.97 (0.94–1.01) | 0.12 | 0.94 (0.9–0.98) | 0.002 |
| Percent ischaemic myocardium (per 5% increase) | 0.99 (0.96–1.03) | 0.59 | 1 (0.95–1.05) | 0.95 |
| Percent scarred myocardium (per 1% increase) | 1.01 (1–1.01) | 0.01 | 1.01 (1–1.02) | 0.08 |
| Myocardial blood flow reserve (per 0.1 decrease) | 1.09 (1.08–1.10) | <0.0001 | 1.03 (1.01–1.05) | <0.0001 |
All variables have been included in the Cox proportional hazards analysis.
CVD, cardiovascular disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Figure 3.
Hazards for death with early revascularization compared to medical therapy based on global myocardial blood flow reserve by positron emission tomography myocardial perfusion imaging.
Figure 4.
Analysis of interaction of myocardial blood flow reserve with early revascularization vs. medical therapy on long-term death, within levels of ischaemia. Estimates derived from the fully adjusted Cox proportional hazards analysis (three-way interaction of ischaemia * myocardial blood flow reserve * early revascularization P = 0.90).
In a sensitivity analysis, replacing global MBFR with global stress MBF (Supplementary material online, Table S3) showed similar results. For every 0.1 ml/min/g decrease in global stress MBF, there was 2% greater risk of all-cause death over the study period. Stress MBF also had a significant interaction with early revascularization on all-cause mortality (P = 0.001) such that patients with low stress MBF (<1.6 ml/g/min) had significantly lower hazards of death with early revascularization compared to medical therapy. The above results were found to be similar in a second sensitivity analysis which used a propensity score instead of a multivariable Cox model as the primary statistical analysis (Supplementary material online, Supplemental Methods).
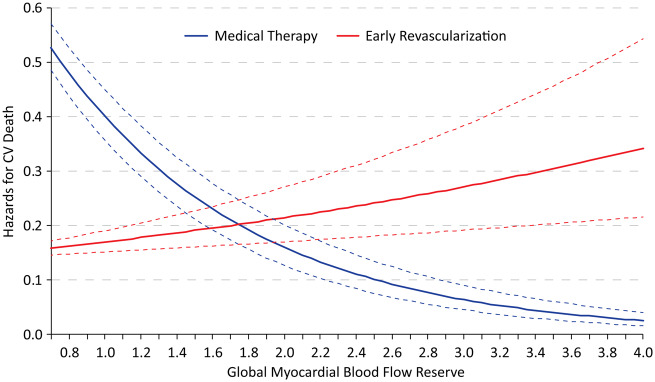
There were 489 cardiac deaths on follow-up. After adjusting for patient and test characteristics, every 0.1 unit decrease in MBFR was associated with 3% greater hazard of cardiac death (HR 1.03, 95% 1.01–1.05; P < 0.0001, Table 2). There was also a similar significant interaction of MBFR * early revascularization in the adjusted Cox model for cardiac death (P < 0.001). Figure 5 demonstrates the hazards for cardiac death with early revascularization compared to medical therapy based on MBFR, with similar threshold of MBFR (1.8) under which improved cardiac-survival was observed with early revascularization.
Figure 5.
Hazards for cardiac death with early revascularization compared to medical therapy based on global myocardial blood flow reserve by positron emission tomography myocardial perfusion imaging.
Discussion
Among 12 594 patients who underwent PET MPI testing for suspected or known coronary artery disease, MBFR was abnormal in more than half of patients, with 38.5% of all patients having values <1.8. MBFR was independently associated with a greater risk of cardiac and all-cause mortality with an increased hazard of 9% for every decrease in MBFR by 0.1 unit after robust adjustment. MBFR also modified the effect of early revascularization on long-term survival, such that patients with MBFR 1.8 or lower benefitted from revascularization with PCI or CABG within 90 days of testing compared to medical management. The interaction of MBFR with early revascularization was observed within levels of ischaemia, and regardless of the type of revascularization strategy that was used.
Myocardial blood flow reserve on PET MPI is a useful adjunct to diagnose obstructive coronary artery disease.3,4,19 Multiple studies have also evaluated the prognostic value of MBFR in identifying a high-risk cohort.5,6,20,21 Among 256 patients undergoing N13Ammonia PET MPI, Herzog et al.21 demonstrated the incremental prognostic value of MBFR over perfusion defects in predicting major adverse cardiac events. This was also seen in 275 and 704 patients, respectively, undergoing Rb82 PET MPI by Fukushima et al.20 and Ziadi et al.5 These studies, however, censored5 or excluded20,21 patients undergoing early revascularization. Furthermore, some endpoints included in the combined major adverse cardiac events outcome such as MI and heart failure-associated hospitalizations are subjective and suffer greatly from misclassification bias.20,21 Murthy et al.6 also demonstrated the incremental prognostic value of MBFR over perfusion abnormalities and LVEF reserve to predict cardiac death in 2783 patients with known or suspected coronary disease undergoing Rb82 PET MPI. This study did not exclude patients who underwent early revascularization, and showed similar results with all-cause mortality. Among 329 patients who underwent coronary angiography after Rb82 PET MPI, Taqueti et al.11 demonstrated that patients with low global coronary flow reserve were more likely to receive benefit in terms of reduced rates of heart failure hospitalizations and cardiac deaths with early revascularization with CABG, but not PCI, as compared with medical management. However, these results may have been limited by the inclusion of only patients who underwent angiography after MPI testing, a small sample size, and a low event rate. More recently, Gould et al.22 demonstrated that severely reduced regional artery-specific coronary flow capacity was associated with greater risk of death, MI, and stroke among 3774 PET MPI studies and that this risk was lowered with revascularization within 90 days of the test. The pre-determined thresholds for coronary flow reserve (CFR) and stress MBF used to define reduced coronary flow capacity in this study were very low compared to thresholds reported elsewhere in the literature.5,6 There was also possibly a significant influence of the flow results in the decision for revascularization which could bias the results, as all referring physicians received flow results with management recommendations based on flow measurements. In contrast, referring clinicians in our study did not have access to the MBFR results for the large majority of our study period, as our laboratory started reporting MBFR values near end of 2016. This should reduce the MBFR-related selection bias in revascularization decisions.
Our data extends the findings of these prior studies by analysing a large cohort of patients with adequate statistical power to examine interactions between MBFR and early revascularization. Similar to prior studies,5,6,21 we found MBFR was independently prognostic in predicting death, in addition to clinical risk factors and perfusion and EF results. We further found that the association of early revascularization on mortality differed among patients with low and normal MBFR, such that patients with MBFR 1.8 or lower had a survival benefit with early revascularization, whereas those with higher MBFR did not. Results were similar for cardiac death, further supporting the robustness of the association with different outcomes. Additionally, our models accounted for other high-risk markers available from PET MPI studies, including coronary calcium score, extent of myocardial ischaemia and infarcted myocardium, and changes in LVEF with stress. There was no evidence of a differential effect based on level of myocardial ischaemia on PET MPI.
In contrast to the prior study by Taqueti et al.,11 we did not find a differential effect of MBFR on outcome based on the type of revascularization with CABG vs. PCI. This may be due to the fact that low global MBFR values reflect haemodynamically significant disease affecting large enough regions of myocardium, which are prognostically significant (e.g. significant left main, left anterior descending artery, 2 or 3 vessel disease vs. isolated small right coronary artery territory). Coronary disease affecting arteries subtending a small amount of myocardium will result in reduced regional flow in that area, but may not be prognostically significant or change outcomes with revascularization. Regardless of how these territories are revascularized with PCI or CABG, reduction in region of subtended myocardium may explain the benefit in survival observed with revascularization in patients with low MBFR. Also, the decision to revascularize with CABG vs. PCI is complex and related to the overall burden of obstructive coronary disease, patient risk factors, frailty, and patient preference. Low global MBFR values may be due to multivessel coronary disease that should be revascularized with CABG or multivessel PCI in patients with higher comorbidity burden. In contrast, low global MBFR may be secondary to a single obstructive lesion with other comorbidities that lead to low global flows such as diabetes, obesity, and old age. We believe that the lack of differential effect of CABG vs. PCI on outcomes is related to this heterogeneity. Also, the MBFR threshold observed in our study was much greater compared to that used by Gould et al.22 in their recent study. Apart from differences in flow software and study design alluded to earlier, threshold for benefit was not pre-determined in our study, but suggested based on the observed association of MBFR, revascularization and long-term outcomes.
Our study provides further evidence to support physiology-based revascularization strategies. The COURAGE23 and BARI-2D24 trials failed to show a benefit of anatomy-based revascularization in reducing major adverse cardiac events compared with medical management in patients with stable ischaemic heart disease. There are conflicting data regarding the benefit of an ischaemia-guided revascularization strategy,9,25,26 with a randomized controlled trial investigating that strategy currently underway.27 In contrast, a physiologic evaluation with invasive fractional flow reserve (FFR) or MBFR provide the earliest and most direct measurement of myocardial flow-limiting ischaemic disease and, as such, has some inherent advantages over semi-quantitative techniques measuring perfusion abnormalities. This is especially true in cases of diffuse multivessel disease.1,28 The FAME-2 trial10 showed a benefit of an FFR-based threshold, although this was mainly driven by reduction in rates of urgent revascularization and not non-fatal MI or death. Invasive FFR reflects epicardial coronary artery disease, and may be normal in patients with microvascular dysfunction. In contrast, abnormal MBFR may reflect abnormalities in the epicardial vessels, microvasculature, or both.19,29–31 In the absence of a diseased microcirculation, functionally severe stenoses as measured by FFR may not manifest as perfusion abnormalities on myocardial perfusion scintigraphy.32 Conversely, in the presence of severely diseased microcirculation in addition to epicardial disease, patients may not be able to augment their myocardial blood flow with hyperaemia enough to produce a relative perfusion defect on MPI. Thus, our findings suggest that a revascularization decision based on MBFR, which captures the combined haemodynamic effects of epicardial stenoses and microvascular dysfunction, is significantly valuable.
Our study should be interpreted in context of the following limitations. All patients in our study received Rb82 radiotracer agent. As such, the MBFR cut-offs for benefit might be different for PET studies using other tracers such 13N-Ammonia, 15O-water, or 18F-flurpiridaz. However, Rb82 is the most widely used radiotracer for PET MPI imaging, especially in USA. Also, studies have shown a good correlation between flows derived from 13NH3 and Rb82 PET MPI.33 Our flow quantitation used a single software package, ImagenQ software, which uses a net retention model. While normal MBFR values are similar among tissue compartment model software packages, there may be variations on a patient-by-patient basis.14 We were unable to measure the dose and type of medical therapy during the follow-up period which could have influenced the outcome. We also did not assess the contribution of regional flow measurements. While revascularization theoretically affects cardiac events more directly than all-cause mortality, we chose the outcome of all-cause mortality as the primary outcome to prevent misclassification bias, but found similar results with cardiac death. Also, even though we extensively adjusted for >30 covariates to account for selection bias in referral to revascularization as well as possible confounders in the relationship between MBFR and death, given the observational nature of the study, it is still subject to residual confounding.
Conclusion
In conclusion, our study is the largest study to date evaluating the association of MBFR with all-cause and cardiovascular mortality, overall and as a function of subsequent treatment, in patients with known and suspected coronary artery disease. MBFR independently predicts mortality in these patients and may help identify patients with a survival benefit from early revascularization with PCI or CABG beyond perfusion defects on MPI. Prospective confirmation of an MBFR-based revascularization strategy is needed to confirm these results.
Conflict of interest: K.K.P. and F.A.B. are supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number T32HL110837. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.A.S. receives research grant support from Abbott Vascular, Novartis and is the PI of an analytic centre for the American College of Cardiology. He serves as a consultant to United Healthcare, Bayer, Janssen, AstraZeneca, and Novartis. He has an equity interest in Health Outcomes Sciences. T.M.B. receives research grant support from Astellas and GE Healthcare. He serves as a consultant to Curium and GE Healthcare. He has an equity interest in Cardiovascular Imaging Technologies. He has intellectual property rights for Imagen PET and SPECT software. J.A.C. has an equity interest in Cardiovascular Imaging Technologies and has intellectual property rights for Imagen PET and SPECT software. All other authors declared no conflict of interest.
Supplementary Material
References
- 1. Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, Camici PG, Cerqueira MD, Chow BJW, Di Carli MF, Dorbala S, Gewirtz H, Gropler RJ, Kaufmann PA, Knaapen P, Knuuti J, Merhige ME, Rentrop KP, Ruddy TD, Schelbert HR, Schindler TH, Schwaiger M, Sdringola S, Vitarello J, Williams KA, Gordon D, Dilsizian V, Narula J.. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol 2013;62:1639–1653. [DOI] [PubMed] [Google Scholar]
- 2. Murthy VL, Bateman TM, Beanlands RS, Berman DS, Borges-Neto S, Chareonthaitawee P, Cerqueira MD, deKemp RA, DePuey EG, Dilsizian V, Dorbala S, Ficaro EP, Garcia EV, Gewirtz H, Heller GV, Lewin HC, Malhotra S, Mann A, Ruddy TD, Schindler TH, Schwartz RG, Slomka PJ, Soman P, Di Carli MF;. SNMMI Cardiovascular Council Board of Directors; ASNC Board of Directors. Clinical quantification of myocardial blood flow using PET: joint position paper of the SNMMI cardiovascular council and the ASNC. J Nucl Cardiol 2018;25:269–297. [DOI] [PubMed] [Google Scholar]
- 3. Hajjiri MM, Leavitt MB, Zheng H, Spooner AE, Fischman AJ, Gewirtz H.. Comparison of positron emission tomography measurement of adenosine-stimulated absolute myocardial blood flow versus relative myocardial tracer content for physiological assessment of coronary artery stenosis severity and location. JACC Cardiovasc Imaging 2009;2:751–758. [DOI] [PubMed] [Google Scholar]
- 4. Morton G, Chiribiri A, Ishida M, Hussain ST, Schuster A, Indermuehle A, Perera D, Knuuti J, Baker S, Hedström E, Schleyer P, O'Doherty M, Barrington S, Nagel E.. Quantification of absolute myocardial perfusion in patients with coronary artery disease: comparison between cardiovascular magnetic resonance and positron emission tomography. J Am Coll Cardiol 2012;60:1546–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ziadi MC, deKemp RA, Williams KA, Guo A, Chow BJW, Renaud JM, Ruddy TD, Sarveswaran N, Tee RE, Beanlands RSB.. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58:740–748. [DOI] [PubMed] [Google Scholar]
- 6. Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF.. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124:2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shaw LJ, Blankstein R, Jacobs JE, Leipsic JA, Kwong RY, Taqueti VR, Beanlands RSB, Mieres JH, Flamm SD, Gerber TC, Spertus J, Di Carli MF; American Heart Association Cardiovascular Imaging and Intervention Subcommittee of the Council on Clinical Cardiology; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Disease in the Young; and Council on Quality of Care and Outcomes Research. Defining quality in cardiovascular imaging: a scientific statement from the American Heart Association. Circ Cardiovasc Imaging 2017;10. pii: e000017. doi: 10.1161/HCI.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schoenhagen P, Hachamovitch R.. Evaluating the clinical impact of cardiovascular imaging: is a risk-based stratification paradigm relevant? J Am Coll Cardiol 2013;61:185–186. [DOI] [PubMed] [Google Scholar]
- 9. Hachamovitch R, Rozanski A, Shaw LJ, Stone GW, Thomson LEJ, Friedman JD, Hayes SW, Cohen I, Germano G, Berman DS.. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J 2011;32:1012–1024. [DOI] [PubMed] [Google Scholar]
- 10. De Bruyne B, Pijls NHJ, Kalesan B, Barbato E, Tonino PAL, Piroth Z, Jagic N, Möbius-Winkler S, Mobius-Winckler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Jüni P, Fearon WF.. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991–1001. [DOI] [PubMed] [Google Scholar]
- 11. Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF.. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 2015;131:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, Gropler RJ, Knuuti J, Schelbert HR, Travin MI.. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol 2016;23:1187–1226. [DOI] [PubMed] [Google Scholar]
- 13. Yoshida K, Mullani N, Gould KL.. Coronary flow and flow reserve by PET simplified for clinical applications using rubidium-82 or nitrogen-13-ammonia. J Nucl Med 1996;37:1701–1712. [PubMed] [Google Scholar]
- 14. Nesterov SV, Deshayes E, Sciagrà R, Settimo L, Declerck JM, Pan X-B, Yoshinaga K, Katoh C, Slomka PJ, Germano G, Han C, Aalto V, Alessio AM, Ficaro EP, Lee BC, Nekolla SG, Gwet KL, deKemp RA, Klein R, Dickson J, Case JA, Bateman T, Prior JO, Knuuti JM.. Quantification of myocardial blood flow in absolute terms using (82)Rb PET imaging: the RUBY-10 study. JACC Cardiovasc Imaging 2014;7:1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lortie M, Beanlands RS, Yoshinaga K, Klein R, Dasilva JN, DeKemp RA.. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging 2007;34:1765–1774. [DOI] [PubMed] [Google Scholar]
- 16. Herrero P, Markham J, Shelton ME, Bergmann SR.. Implementation and evaluation of a two-compartment model for quantification of myocardial perfusion with rubidium-82 and positron emission tomography. Circ Res 1992;70:496–507. [DOI] [PubMed] [Google Scholar]
- 17. Elze MC, Gregson J, Baber U, Williamson E, Sartori S, Mehran R, Nichols M, Stone GW, Pocock SJ.. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol 2017;69:345–357. [DOI] [PubMed] [Google Scholar]
- 18. Gupta A, Taqueti VR, van de Hoef TP, Bajaj NS, Bravo PE, Murthy VL, Osborne MT, Seidelmann SB, Vita T, Bibbo CF, Harrington M, Hainer J, Rimoldi O, Dorbala S, Bhatt DL, Blankstein R, Camici PG, Di Carli MF.. Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation 2017;136:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee JM, Kim CH, Koo BK, Hwang D, Park J, Zhang J, Tong Y, Jeon KH, Bang JI, Suh M, Paeng JC, Cheon GJ, Na SH, Ahn JM, Park SJ, Kim HS. Integrated myocardial perfusion imaging diagnostics improve detection of functionally significant coronary artery stenosis by 13N-ammonia positron emission tomography. Circ Cardiovasc Imaging 2016;9. pii: e004768. doi: 10.1161/CIRCIMAGING.116.004768. [DOI] [PubMed] [Google Scholar]
- 20. Fukushima K, Javadi MS, Higuchi T, Lautamaki R, Merrill J, Nekolla SG, Bengel FM.. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med 2011;52:726–732. [DOI] [PubMed] [Google Scholar]
- 21. Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, Burkhard N, Wyss CA, Kaufmann PA.. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol 2009;54:150–156. [DOI] [PubMed] [Google Scholar]
- 22. Gould KL, Johnson NP, Roby AE, Nguyen T, Kirkeeide R, Haynie M, Lai D, Zhu H, Patel MB, Smalling R, Arain S, Balan P, Nguyen T, Estrera A, Sdringola S, Madjid M, Nascimbene A, Loyalka P, Kar B, Gregoric I, Safi H, McPherson D.. Regional, artery-specific thresholds of quantitative myocardial perfusion by PET associated with reduced myocardial infarction and death after revascularization in stable coronary artery disease. J Nucl Med 2019;60:410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GBJ, Weintraub WS.. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 24. Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TLZ, Molitch ME, Nesto RW, Sako EY, Sobel BE.. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS.. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003;107:2900–2907. [DOI] [PubMed] [Google Scholar]
- 26. Stergiopoulos K, Boden WE, Hartigan P, Möbius-Winkler S, Hambrecht R, Hueb W, Hardison RM, Abbott JD, Brown DL.. Percutaneous coronary intervention outcomes in patients with stable obstructive coronary artery disease and myocardial ischemia: a collaborative meta-analysis of contemporary randomized clinical trials. JAMA Intern Med 2014;174:232–240. [DOI] [PubMed] [Google Scholar]
- 27. Maron DJ, Hochman JS, O’Brien SM, Reynolds HR, Boden WE, Stone GW, Bangalore S, Spertus JA, Mark DB, Alexander KP, Shaw L, Berger JS, Ferguson TB, Williams DO, Harrington RA, Rosenberg Y.. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: rationale and design. Am Heart J 2018;201:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gould KL. Does coronary flow trump coronary anatomy? JACC Cardiovasc Imaging 2009;2:1009–1023. [DOI] [PubMed] [Google Scholar]
- 29. Johnson NP, Kirkeeide RL, Gould KL.. Is discordance of coronary flow reserve and fractional flow reserve due to methodology or clinically relevant coronary pathophysiology? JACC Cardiovasc Imaging 2012;5:193–202. [DOI] [PubMed] [Google Scholar]
- 30. van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SAJ, Voskuil M, Henriques JPS, Koch KT, de Winter RJ, Spaan JAE, Siebes M, Tijssen JGP, Meuwissen M, Piek JJ.. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv 2014;7:301–311. [DOI] [PubMed] [Google Scholar]
- 31. Ahn SG, Suh J, Hung OY, Lee HS, Bouchi YH, Zeng W, Gandhi R, Eshtehardi P, Gogas BD, Samady H.. Discordance between fractional flow reserve and coronary flow reserve: insights from intracoronary imaging and physiological assessment. JACC Cardiovasc Interv 2017;10:999–1007. [DOI] [PubMed] [Google Scholar]
- 32. van de Hoef TP, Nolte F, EchavarrÍa-Pinto M, van Lavieren MA, Damman P, Chamuleau SAJ, Voskuil M, Verberne HJ, Henriques JPS, van Eck-Smit BLF, Koch KT, de Winter RJ, Spaan JAE, Siebes M, Tijssen JGP, Meuwissen M, Piek JJ.. Impact of hyperaemic microvascular resistance on fractional flow reserve measurements in patients with stable coronary artery disease: insights from combined stenosis and microvascular resistance assessment. Heart 2014;100:951–959. [DOI] [PubMed] [Google Scholar]
- 33. El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, Fischman A, Coughlan M, Yasuda T, Di Carli MF.. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. J Nucl Med 2009;50:1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.