Abstract
As the age of child-bearing increases and correlates with infertility, cryopreservation of female gametes is becoming common-place in ART. However, the developmental competence of vitrified oocytes has remained low. The underlying mechanisms responsible for reduced oocyte quality post-vitrification are largely unknown. Mouse cumulus–oocyte complexes were vitrified using a cryoloop technique and a mixture of dimethylsulphoxide, ethylene glycol and trehalose as cryoprotectants. Fresh and vitrified/thawed oocytes were compared for chromosome alignment, spindle morphology, kinetochore-microtubule attachments, spindle assembly checkpoint (SAC) and aneuploidy. Although the majority of vitrified oocytes extruded the first polar body (PB), they had a significant increase of chromosome misalignment, abnormal spindle formation and aneuploidy at metaphase II. In contrast to controls, vitrified oocytes extruded the first PB in the presence of nocodazole and etoposide, which should induce metaphase I arrest in a SAC-dependent manner. The fluorescence intensity of mitotic arrest deficient 2 (MAD2), an essential SAC protein, at kinetochores was reduced in vitrified oocytes, indicating that the SAC is weakened after vitrification/thawing. Furthermore, we found that vitrification-associated stress disrupted lysosomal function and stimulated cathepsin B activity, with a subsequent activation of caspase 3. MAD2 localization and SAC function in vitrified oocytes were restored upon treatment with a cathepsin B or a caspase 3 inhibitor. This study was conducted using mouse oocytes, therefore confirming these results in human oocytes is a prerequisite before applying these findings in IVF clinics. Here, we uncovered underlying molecular pathways that contribute to an understanding of how vitrification compromises oocyte quality. Regulating these pathways will be a step toward improving oocyte quality post vitrification and potentially increasing the efficiency of the vitrification program.
Keywords: vitrification, mouse oocyte, meiosis, spindle assembly checkpoint, cathepsin B
Introduction
As the age of child-bearing has increased, cryopreservation of female gametes has become more common place in the assisted reproduction setting to preserve or extend fertility. Moreover, oocyte cryopreservation offers a solution for the premature loss of ovarian function caused by ovarian cancer, chemotherapy-induced ovarian insufficiency or autoimmune diseases. There are two methods of oocyte cryopreservation: slow freezing and vitrification. Slow freezing is the procedure of exposing the oocyte gradually to various phases of freezing using low concentrations of cryoprotectants. On the other hand, vitrification uses high concentrations of cryoprotectants and submerging the oocyte rapidly in liquid nitrogen (−196°C), omitting the need for sophisticated and expensive equipment required for slow freezing (Vajta and Nagy, 2006; Son and Tan, 2009). Because vitrification induces less damage and results in higher fertilization and pregnancy rates when compared with slow freezing, it has become the technique of choice for oocyte cryopreservation (Yoon et al., 2003; Kuwayama, 2007; Fadini et al., 2009; Smith et al., 2010). However, the rates of fertilization and embryonic development for vitrified oocytes remains lower compared to fresh oocytes (Son et al., 2019). The underlying effect of vitrification on oocyte quality is not fully understood.
Mammalian oocytes enter meiosis during early fetal life prior to arrest at the dictyate stage of meiotic prophase. After puberty and under hormonal stimulation, prophase I (germinal vesicle; GV)-arrested oocytes resume meiosis I (MI, oocyte maturation) and undergo a series of orchestrated processes that end upon the extrusion of the first polar body (1st PB) and arrest of the fertilization-competent egg at metaphase (Met) II. Current clinic protocols are most successful when Met II eggs are vitrified. Although cryopreservation of Met II eggs is the most widely used method for preserving female gametes in human IVF clinics, growing evidence suggests that Met II eggs are vulnerable to cryopreservation (Kuwayama et al., 2005; Edgar and Gook, 2012; Chang et al., 2013). An alternative approach is the cryopreservation of, immature, prophase I-arrested oocytes, which has the advantage of avoiding the need for hormonal stimulation in cases of poor ovarian response or urgent cases of ovariectomy in the oncofertility setting. However, cryopreservation of oocytes at the GV stage can cause deleterious effects. The rates of IVM and embryonic cleavage were significantly lower in vitrified GV oocytes when compared with fresh non-vitrified gametes (Wang et al., 2012; Song et al., 2016). This protocol, therefore, demands that we understand the impacts of vitrification on oocyte meiotic maturation and improve these procedures.
To complete MI, the nuclear envelope of GV oocytes first undergoes break down (GVBD). Following GVBD, chromosomes condense and align at the metaphase plate and a bipolar spindle is assembled. During Met I, each homologous chromosome is oriented toward the opposing spindle poles through kinetochore microtubule (K-MT) attachments. This bi-orientation of homologous chromosomes ensures faithful chromosome segregation. The surveillance mechanism that protects against cell-cycle progression when a kinetochore is not attached to MTs is called the spindle assembly checkpoint (SAC). The SAC creates a diffusible signal to delay anaphase I (Ana I) onset until the oocyte establishes proper K-MT attachments (Brunet et al., 2003; Homer et al., 2005; Lane et al., 2012). A non-functional or weakened SAC, or defects in establishing correct K-MT, are known causes of developing aneuploid Met II eggs (Leland et al., 2009; Lane et al., 2012). Cryopreservation of mouse oocytes significantly increased the rate of aneuploidy (Kola et al., 1988; Van der Elst et al., 1993). Therefore, it is important to understand the underlying molecular mechanisms of cryopreservation-induced aneuploidy in mouse oocytes in order to improve the quality of vitrified oocytes.
We show here that although the majority of vitrified oocytes at GV stage extruded the 1st PB following IVM, they had a significant increase of chromosome misalignment, abnormal spindle formation, abnormal K-MT attachments at Met I and aneuploidy at Met II. We found that vitrification disrupts lysosomal function and stimulates both cathepsin B (CTSB) and cytosolic caspase 3 activities. Importantly, treating vitrified oocytes with E-64, a CTSB inhibitor or Z-VAD-FMK, a caspase 3 inhibitor, during IVM restored SAC function and decreased the rate of aneuploidy in IVM eggs.
Materials and methods
Ethics
All animals were kept, and experiments were conducted, in accordance with the Animal Care and Use Committee at the University of Hokkaido and the University of Missouri (Animal Care Quality Assurance Ref. Number, 9695).
Oocyte collection and culture
GV-arrested oocytes were obtained from CD-1 female mice (6- to 8-week-old) previously primed (44 h) with pregnant mare’s serum gonadotrophin (Lee BioSolutions #493-10-10) according to (Schultz et al., 1983). Cumulus–oocyte complexes (COCs) were denuded partially by mechanical pipetting leaving a few layers of cumulus cells surrounding the oocyte. The collection medium for oocytes and the base medium for vitrification and thawing was bicarbonate-free minimal essential medium (MEM) containing 3 mg/ml polyvinylpyrolidone (PVP) and 25 mM Hepes (pH 7.3). In vitro meiotic maturation was carried out in Chatot, Ziomek and Bavister (CZB) medium (Chatot et al., 1989) in a humidified incubator with 5% CO2 in air at 37°C. Met I oocytes were collected 7 h after oocyte culture, whereas Met II eggs were collected 16 h after oocyte culture.
Nocodazole (MilliporeSigma, MO, USA, #M1404), Etoposide (MilliporeSigma # E1383), E-64 (MilliporeSigma # E3132) and Z-VAD-FMK (Promega, Madison, WI # G7231) were added to medium to a final concentration of 1 µM, 25 μg/ml, 1 μM and 1 µM, respectively. In all experiments, both Etoposide and Nocodazole were added to the culture medium at GVBD (∼2 h after IVM initiation) until the end of the experiment.
Oocyte vitrification and thawing
Vitrification of mouse oocytes was carried out using a cryoloop device and a mixture of dimethylsulphoxide (DMSO) (MilliporeSigma #276855), ethylene glycol (EG; MilliporeSigma #102466) and trehalose (MilliporeSigma #T0167) as previously described (Lane et al., 1999; Moawad et al., 2013). Briefly, COCs were incubated in the equilibration solution (MEM + 7.5% EG + 7.5% DMSO + 0.25 M trehalose) for 3 min. The COCs were then transferred to the vitrification solution (MEM + 15% EG + 15% DMSO) for 40–45 s prior to loading into the cryoloop (5–10 COCs/cryoloop) and submerging in liquid nitrogen. COCs were kept in liquid nitrogen at least for 1 h before thawing. Vitrified COCs were thawed in gradual concentrations of trehalose (1 M, 0.5 M and 0 M) in MEM for 3 min each, prior to washing and culturing in CZB medium.
Immunocytochemistry and confocal microscopy
Met I or Met II oocytes were fixed in freshly prepared 2% paraformaldehyde solution (PFA, MilliporeSigma #P6148) dissolved in PBS for 20 min at room temperature. Fixed oocytes were placed in permeabilization solution (0.1% Triton X-100 in PBS) for 20 min prior to incubation in blocking solution (PBS + 0.3% bovine serum albumin +0.01% Tween-20) for another 20 min. After blocking, the oocytes were incubated with primary antibodies for 1 h at room temperature. After washing three times (8 min each) in blocking solution, the oocytes were then incubated with secondary antibodies for 1 h. Oocytes were then washed again in blocking solutions three times, 8 min each, prior to mounting onto a 5 μl drop of Vectashieled with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI; Vector Laboratories, Burlingame, CA, USA). Omitting the primary antibody served as a negative control. Fluorescence signals were detected using a Leica SP5 confocal microscope under a 63× objective. The laser power was selected to the level that induces a signal intensity just below saturation for the group that displayed the highest intensity, and all oocytes were scanned on the same day and with the same laser power to allow accurate intensity quantification. The intensity of fluorescence was quantified using NIH image J software (National Institute of Health, Bethesda, MD, USA) and the same processing parameters were applied during experimental analysis.
Cold-stable microtubule assay
Met I oocytes were incubated for 7 min on ice in MEM-PVP, and then fixed for 20 min at room temperature in 2% PFA. Fixed oocytes were immunostained as mentioned above with CREST autoimmune serum to detect kinetochores and α-tubulin-Alexa Fluor 488 conjugate to detect α-tubulin. Immunostained oocytes were mounted onto a glass slide using Vectashield with DAPI (Vector Laboratories) to label DNA. Images were collected at 0.5-µm Z-intervals to capture the entire region of the Met I spindle. Serial confocal sections were analyzed to assess K-MT attachments using NIH image J software.
In situ chromosome counting
Met II eggs were incubated for 2 h in CZB medium containing 100 µM Eg5-kinesin inhibitor (monastrol, MilliporeSigma #M8515) to induce monopolar spindle formation and allow chromosome dispersion (Duncan et al., 2009; Balboula and Schindler, 2014). Oocytes were fixed in freshly prepared 2% PFA and immunostained as mentioned above with CREST autoimmune serum to detect kinetochores. Immunostained oocytes were mounted onto a glass slide using Vectashield with DAPI (Vector Laboratories) to stain DNA. Images were collected at 0.7-µm Z-intervals to capture the entire region of the chromosomes. Serial confocal sections were analyzed to count the total number of kinetochores using NIH image J software.
Antibodies
The following primary antibodies were used in immunofluorescence: α-tubulin-Alexa Fluor 488 conjugate (Life Technologies #322 588; 1:100), CREST autoimmune serum (Antibodies Incorporated #15-234; 1:25), mitotic arrest deficient 2 (MAD2) (Biolegends #PRB-452C; 1:500).
Detection of intracellular cathepsin B and caspase activities
A magic red CTSB assay kit (Immunochemistry Technologies, Bloomington, MN, USA #938) and Caspase 3 (DEVD)2 (Immunochemistry Technologies) were used to detect CTSB activity and caspase 3 activity, respectively, following the manufacturer’s instructions with some modifications. Briefly, Met I oocytes were incubated with the reaction mix (1 μl of DMSO-diluted stock solution in 250 μl CZB culture medium) for 25 min in a humidified atmosphere of 5% CO2 at 37°C. Hoechst 33342 (25 μg/ml) was added to the same staining solution for an additional 10 min to label the DNA. The omission of CTSB or caspase 3 substrates served as a negative control. Stained oocytes were washed in 0.05% PBS-PVP prior to mounting onto a glass slide and observed under a fluorescence microscope (Leica, Germany). Images within the same experiment were captured and analyzed under the same parameters using NIH image J software.
Assessment of lysosomal function
To assess lysosomal function, Met I oocytes were incubated with LysoSensor Green DND (Molecular Probes, OR, USA #L7535) at a final concentration of 2 μM in CZB medium for 30 min at 37 °C in a humidified atmosphere of 5% CO2. Stained oocytes were then rinsed in 0.05% PBS-PVP prior to mounting onto a glass slide and were observed under the fluorescence microscope (Leica, Germany). The omission of lysosensor served as a negative control. Images within the same experiment were captured and analyzed under the same parameters using NIH Image J software.
Terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling
Met II eggs were assessed for apoptosis using a terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) assay kit (Roche Applied Science, Indianapolis, IN, USA). Met II oocytes were fixed in 4% PFA (pH 7.4) for 40 min. Fixed oocytes were washed three times (5 min each) in 0.05% PBS-PVP followed by permeabilization in PBS-PVP containing 0.5% TritonX-100 for 20 min. The oocytes were washed three times in PVP-PBS for 5 min each. The oocytes were then incubated with fluorescein-dUTP for 60 min at 37 °C to label fragmented DNA ends. After rinsing three times in PVP-PBS for 5 min each, oocytes were mounted onto glass slides using Vectashield with DAPI (Vector Laboratories). Positive control oocytes were pretreated with DNase I recombinant enzyme, whereas omitting fluorescein-dUTP served as a negative control. The fluorescence of fragmented DNA ends was detected using a Leica SP5 confocal microscope.
Statistical analysis
One-way ANOVA, Student’s t-test and χ2 contingency test were used to evaluate the differences between groups using GraphPad Prism (GraphPad Software, San Diego, USA). The ANOVA test was followed by the Tukey post hoc test to allow the comparison among groups. The differences with a value of P < 0.05 were considered significant. The data were expressed as mean ± SEM.
Results
Vitrification of prophase I oocytes impairs meiotic progression, spindle formation and chromosome alignment at Met II
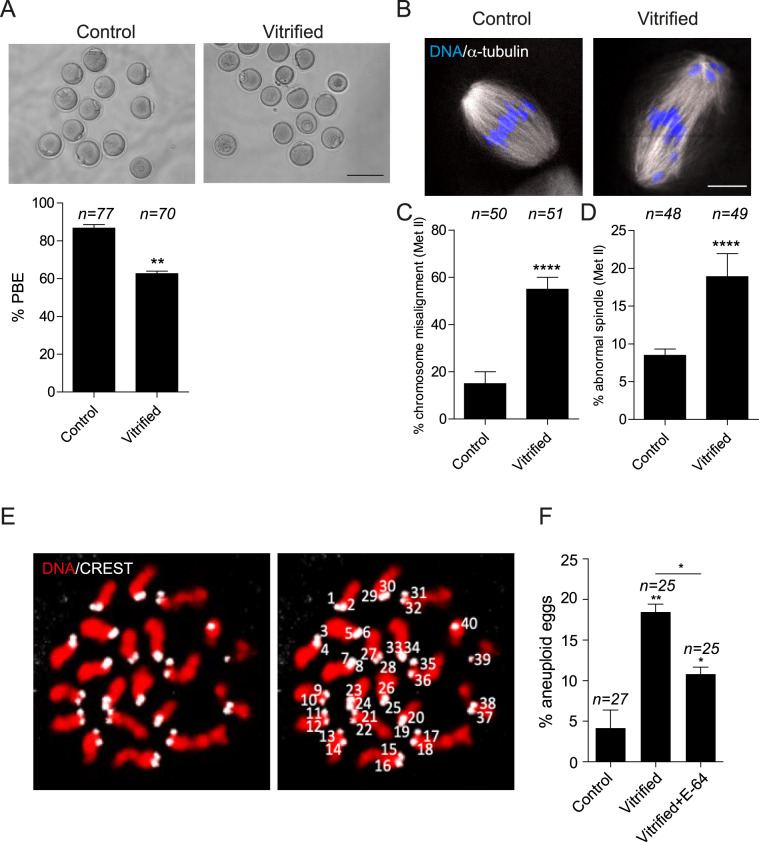
To assess the effect of oocyte vitrification on MI, prophase I (immature) oocytes were vitrified and thawed followed by in vitro meiotic maturation; controls were isolated and matured without freeze-thaw. Vitrification of oocytes at prophase I did not affect the survival rate (92.12% ± 1.21% vs 95.83% ± 1.4% in controls), the timing of GVBD (Supplementary Fig. S1A) or the timing of PB extrusion (PBE, Supplementary Fig. S1B and C), but significantly (P < 0.05) decreased the percentage of oocytes that extruded the 1st PB (∼60%) when compared with control oocytes (∼87%; Fig. 1A). Vitrified oocytes that extruded the 1st PB showed a significant increase of chromosome misalignment and abnormal spindles at Met II when compared with controls (Fig. 1B–D). When we assessed chromosome alignment, as described previously (Lane et al., 2012), we frequently observed chromosomes not at the Met II plate and instead at spindle poles. To assess spindle morphology, only eggs with barrel-shaped spindles containing two clearly defined poles were considered normal. We more frequently observed Met II eggs that lacked polar definition, had short pole-to-pole distances or contained more than two poles. Chromosome misalignment and abnormal spindle configurations at Met II are two phenotypes that are commonly associated with aneuploidy. To investigate whether vitrification of prophase I oocytes increases the risk of developing aneuploid gametes, chromosome numbers from vitrified eggs were assessed using an in situ chromosome counting assay. Similar to previous reports (Kola et al., 1988; Van der Elst et al., 1993), oocyte vitrification increased the percentage of aneuploid eggs (Fig. 1E and F), indicating that vitrification perturbs faithful chromosome segregation during MI.
Figure 1.
Effect of mouse oocyte vitrification at germinal vesicle stage. Full-grown germinal vesicle (GV) oocytes were vitrified for at least 24 h followed by thawing and IVM for 16 h and assessed for first polar body extrusion (PBE) (A; upper panel is representative images). The scale bars represent 100 μm. (B) Fresh and vitrified/thawed oocytes were fixed and stained with an anti-α-tubulin antibody (white) to detect spindles and with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) to detect DNA (blue). The scale bars represent 10 μm. The confocal images were quantified for the percentage of misaligned chromosomes (C) and abnormal spindle morphology (D). Each experiment was performed three times. The data are expressed as mean ± SEM; χ2 contingency test was used to analyze the data. Values with asterisks vary significantly, **P < 0.01, ****P < 0.0001. (E, F) Vitrified/thawed GV oocytes underwent IVM for 16 h in the presence or absence of E-64 (cathepsin B inhibitor) prior to assessing the incidence of aneuploidy in the developed eggs. Eggs were treated with monastrol for 2 h prior to fixation and staining of kinetochores with CREST anti-serum (white) and DNA with DAPI (red). (E) Shown is a representative image of euploid egg with (right panel) or without (left panel) numbered kinetochores. Eggs with greater or less than 40 kinetochores were considered aneuploid. (F) Quantification of the percentage of aneuploid eggs. Each experiment was performed two times. The data are expressed as mean ± SEM; one-way ANOVA were used to analyze the data. Values with asterisks vary significantly, *P < 0.05, **P < 0.01. Unless otherwise specified, asterisks above each group denote the statistical analysis of P-value against the control group. The total number of analyzed oocytes in each group (from all replicates) is specified above each bar within each graph.
Oocyte vitrification perturbs the establishment of stable K-MT attachments
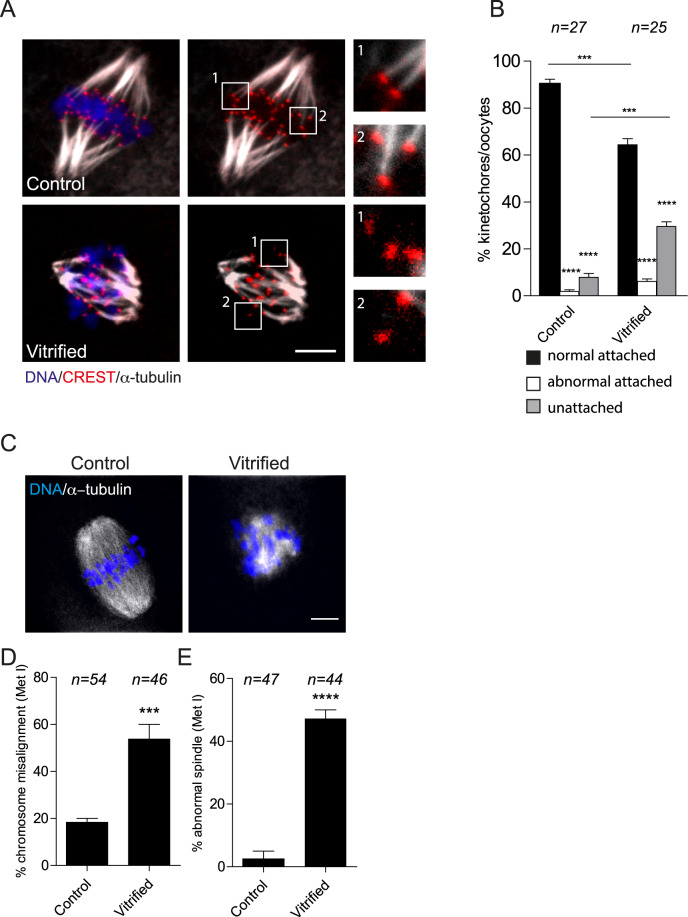
To understand the underlying mechanism of vitrification-induced aneuploidy, we focused on Met I, the stage in which most of errors causing aneuploidy arises. During chromosome segregation, each bivalent chromosome must bi-orient, with respect to the spindle poles. Establishing correct and stable K-MT attachments is critical for bivalent biorientation and faithful chromosome segregation (Compton, 2011; Thompson and Compton, 2011; Lane et al., 2012). To assess the status of K-MT attachments, fresh (control) and vitrified Met I oocytes were exposed to cold culture medium to depolymerize labile microtubules while maintaining stable K-MT fibers, followed by fixation and immunostaining to detect kinetochores and microtubules. We did not observe a significant difference in the percentage abnormal K-MT attachments (neither merotelic nor syntelic) between fresh and vitrified oocytes. However, compared to fresh oocytes, vitrified oocytes had a significantly greater percentage of unattached kinetochores (Fig. 2A and B), suggesting that vitrification-associated aneuploidy, at least partially, occurs through perturbing the ability of oocyte to establish stable K-MT attachments at Met I. Accordingly, we analyzed chromosome alignment and spindle configuration at Met I. Similar to eggs at Met II, Met I oocytes exhibited a significant increase of chromosome misalignment and abnormal spindles when compared with controls (Fig. 2C–E).
Figure 2.
Effect of mouse oocyte vitrification on metaphase I (Met I). Fresh and vitrified/thawed GV oocytes underwent IVM for 7 h until Met I. (A) Representative confocal images from the cold-stable microtubule assay from the indicated treatment groups. Kinetochores were detected with CREST anti-sera (red), microtubules with an anti-α-tubulin antibody (white) and DNA with DAPI (blue). The scale represents 10 μm. (B) Quantification of images in A. The data are expressed as mean ± SEM; one-way ANOVA was used to analyze the data. Values with asterisks vary significantly, ***P < 0.001, ****P < 0.0001. Unless otherwise specified, asterisks above each group denote the statistical analysis of P-value against the ‘normal attached’ group. (C) Met I oocytes were fixed and stained with an anti-α-tubulin antibody (white) to detect spindles and DAPI to detect DNA (blue) using confocal microscopy. The confocal images were quantified for the percentage of misaligned chromosomes (D) and abnormal spindle morphology (E). The scale represents 10 μm. The data are expressed as mean ± SEM; χ2 contingency test was used to analyze the data. Values with asterisks vary significantly, ***P < 0.001, ****P < 0.0001. The total number of analyzed oocytes in each group (from two independent replicates) is specified above each condition within each graph. Unless otherwise specified, asterisks above each group denote the statistical analysis of P-value against the control group
The SAC is weakened in vitrified oocytes
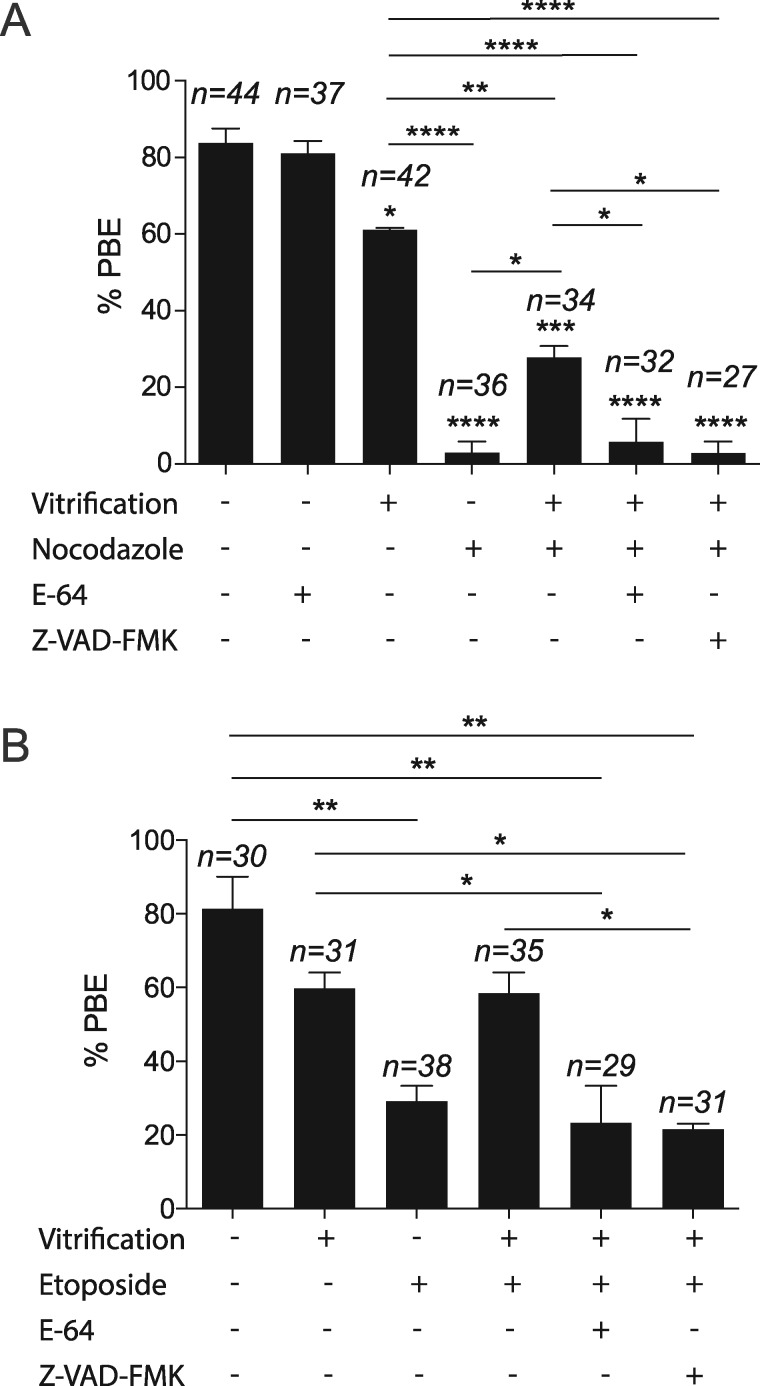
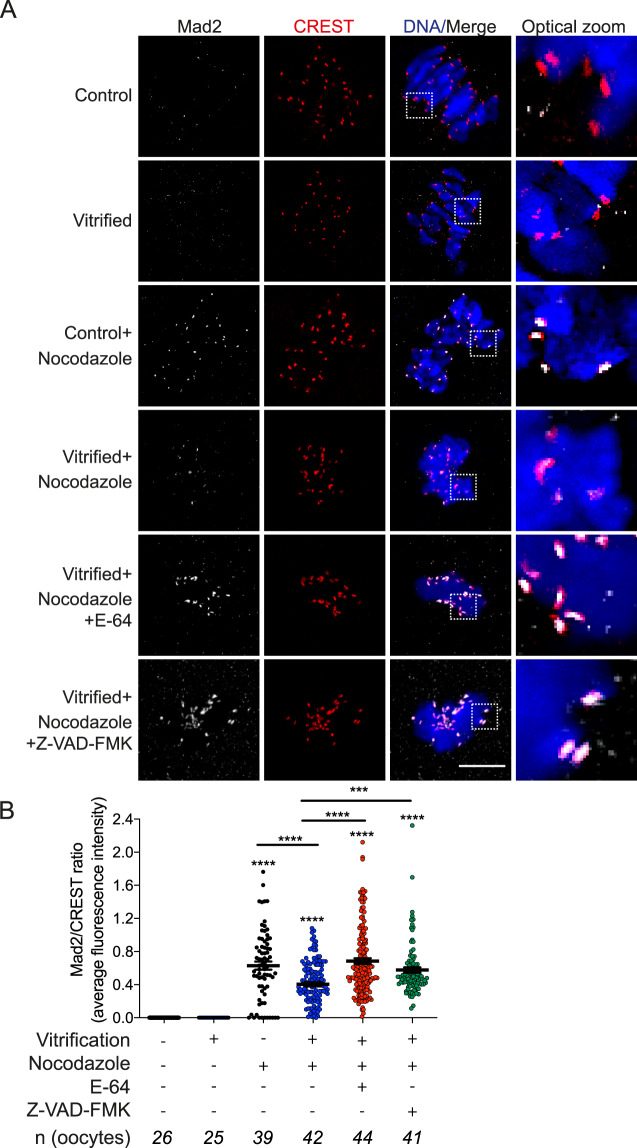
Establishing correct K-MT attachments is an indispensable step for accurate chromosome segregation. Therefore, the oocyte relies on the SAC to prevent Ana I onset until MTs establish correct interactions with kinetochores (Lane et al., 2012). The ability of vitrified oocytes to extrude the 1st PB with a high incidence of unattached kinetochores and the resulting Met II aneuploidy suggests that the SAC surveillance mechanism is perturbed. To investigate our hypothesis, we challenged the SAC using nocodazole and etoposide DNA damage assays. Treating mouse oocytes with nocodazole or etoposide maintains SAC activity by depolymerizing microtubules or by inducing DNA damage, respectively (Homer et al., 2005; Marangos et al., 2015). Expectedly, compared to untreated fresh oocytes, treatment of fresh oocytes with nocodazole or etoposide significantly decreased the percentage of PBE after 16 h of IVM (Fig. 3A and B). Importantly, in the presence of nocodazole or etoposide many vitrified oocytes extruded PBs (27.88% vs 2.94% in controls; 59.46% vs 28.74% in controls, respectively Fig. 3A and B). These results suggest that the SAC is weakened in vitrified oocytes. To further confirm these findings, we examined the localization of MAD2, an essential component of SAC signaling in Met I oocytes (Homer et al., 2005). MAD2 localizes to unattached kinetochores during pro-Met I and then is released upon establishment of end-on K-MT attachments. Although vitrified oocytes had a significant increase of unattached kinetochores (Fig. 2A and B), we did not detect an increase of MAD2 localization at kinetochores in vitrified oocytes (Fig. 4A and B). We also created a condition where we forced SAC activity by depolymerizing microtubules using nocodazole. As anticipated, MAD2 was enriched at the kinetochores in nocodazole-treated fresh oocytes. MAD2 localization was increased in vitrified oocytes exposed to nocodozole but, importantly, to a lesser extent than the control oocytes (Fig. 4A and B). Taken together, these results indicate that the SAC is weakened in vitrified oocytes.
Figure 3.
Effect of mouse oocyte vitrification on PBE. Fresh and vitrified/thawed GV oocytes were treated with nocodazole (A) or etoposide (B) to maintain the SAC active, and divided into the indicated treatment groups (dimethylsulphoxde (DMSO), E-64 or Z-VAD-FMK), in vitro matured for 16 h and scored to quantify the percentage extrusion of the first polar body (PBE). The total number of analyzed oocytes in each group (from two independent replicates) is specified above each condition within each graph. The data are expressed as mean ± SEM; one-way ANOVA was used to analyze the data. Values with asterisks vary significantly, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Unless otherwise specified, asterisks above each group denote the statistical analysis of P-value against the control group.
Figure 4.
Effect of mouse oocyte vitrification on mitotic arrest deficient 2 localization. (A) Fresh and vitrified/thawed GV oocytes underwent IVM with or without microtubule depolymerizing agent nocodazole (added at GV break down) until Met I (7 h). Vitrified oocytes were also treated during maturation with or without E-64 or Z-VAD-FMK. Met I oocytes were fixed and stained with an anti-MAD2 (mitotic arrest deficient 2) antibody (white), CREST anti-sera (red) to label kinetochores and DAPI to label DNA (blue). Images were obtained using confocal microscopy. (B) Quantification of images in A. The experiment was performed four times. The total number of analyzed oocytes in each group (from all replicates) is specified above each condition within each graph. The scale bar represents 10 μm. The data are expressed as mean ± SEM; one-way ANOVA was used to analyze the data. Values with asterisks vary significantly, ***P < 0.001, ****P < 0.0001. Unless otherwise specified, asterisks above each group denote the statistical analysis of P-value against the control and vitrified groups.
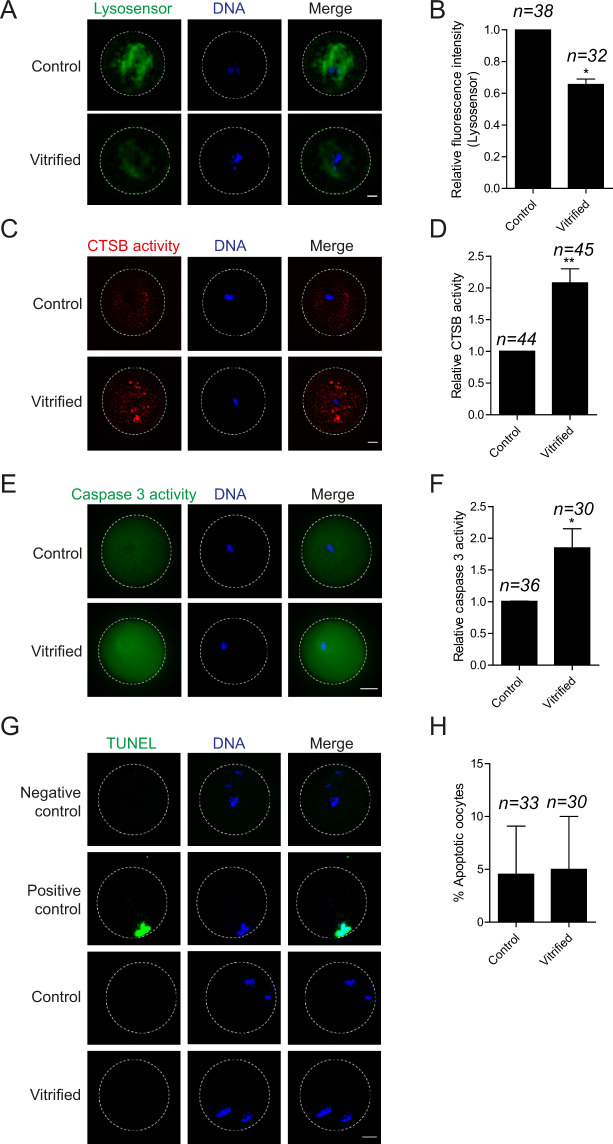
Oocyte vitrification perturbs lysosomal function and stimulates the cathepsin B/caspase 3 pathway
In mitotic cells, activation of the caspase 3 pathway results in impaired SAC function (Perera and Freire, 2005). We previously demonstrated that exposing bovine oocytes to moderate stress induces CTSB leakage from lysosomes to stimulate cytosolic caspase 3 and initiate the apoptotic pathway (Balboula et al., 2013). Taken together, we hypothesized that vitrification-associated stress might stimulate the lysosomal CTSB/caspase 3 pathway which, in turn, perturbs SAC function. We assessed lysosomal integrity in fresh and vitrified Met I oocytes using a LysoSensor kit. In contrast to fresh oocytes, vitrified oocytes exhibited a significant decrease of lysosomal activity (Fig. 5A and B), suggesting that lysosomal integrity is perturbed in vitrified oocytes. We also assessed CTSB and caspase 3 activities in Met I vitrified oocytes using the Magic Red CTSB detection kit and the Caspase 3 (DEVD)2 detection kit, respectively. Oocyte vitrification significantly increased CTSB (Fig. 5C and D) and caspase 3 (Fig. 5E and F) activities at Met I when compared with control oocytes. In mitotic cells, the activation of caspase 3, an executioner caspase, induces apoptosis (McIlwain et al., 2013). We assessed the apoptotic status in Met II eggs using the TUNEL apoptotic assay. Interestingly, vitrification did not affect the percentage of TUNEL positive eggs (Fig. 5G and H) suggesting that, in mouse oocytes, the level of vitrification-induced increase of cathepsin B/caspase 3 is not enough to reach the threshold of completing the apoptosis.
Figure 5.
Effect of mouse oocyte vitrification on lysosomal activity, cathepsin B, caspase 3 and apoptotic status. Fresh and vitrified/thawed GV oocytes underwent IVM for 7 h until Met I or for 16 h until Met II. Met I oocytes were stained with LysoSensor, Magic red cathepsin B detection kit and Caspase 3 (DEVD)2 detection kit to detect lysosomal activity (A), cathepsin B activity (C) and caspase 3 activity (E). Met II oocytes were stained with TUNEL to assess the apoptotic status (G). Positive control oocytes were pretreated with DNase I recombinant enzyme, whereas omitting fluorescein-dUTP served as a negative control. (B, D, F, H) Quantifications for A, C, E and G, respectively. Each experiment was performed two times. The total number of analyzed oocytes in each group (from all replicates) is specified above each condition within each graph. The scale bar represents 10 μm. The data are expressed as mean ± SEM; Student’s t-test and one-way ANOVA were used to analyze the data. Values with asterisks vary significantly, *P < 0.05, **P < 0.01. Unless otherwise specified, asterisks above each group denote the statistical analysis of P-value against the control and vitrified groups. CTSB, cathepsin B.
CTSB/caspase 3 pathway is involved in the weakened SAC function in vitrified oocytes
Because activation of the caspase 3 pathway results in impaired SAC function in mitotic cells (Perera and Freire, 2005) and because we found a significant increase of CTSB/caspase 3 in vitrified oocytes, we asked whether CTSB/caspase 3 inhibition restores the SAC function in vitrified oocytes. Vitrification of oocytes overrides the ability of nocodazole or etoposide to induce Met I arrest (Fig. 3A and B). Importantly, inhibition of CTSB with E-64 or caspase 3 with Z-VAD-FMK in vitrified oocytes treated with nocodazole or etoposide induced a Met I arrest. These oocytes were more similar to nocodazole or etoposide-treated fresh oocytes, suggesting that CTSB or caspase 3 inhibition restored the SAC function in vitrified oocytes (Fig. 3A and B). We also confirmed that CTSB or caspase 3 inhibition does not affect the percentage of PBE in fresh or vitrified oocytes (Supplementary Fig. S2). Importantly, inhibition of CTSB activity or caspase 3 activity fully restored MAD2 localization at the kinetochores of nocodazole-treated vitrified oocytes (Fig. 4A and B), suggesting that CTSB and caspase 3 activation causes the weakened SAC function upon vitrification. We next asked whether CTSB inhibition during IVM can rescue the high rate of aneuploidy in vitrified oocytes. In bovine oocytes, supplementation of the IVM medium with the CTSB inhibitor, E-64, did not affect the maturation rate but improved the developmental competence and the quality of produced blastocysts after IVF (Bettegowda et al., 2008; Balboula et al., 2010a). Similarly, addition of E-64 to the maturation media did not affect the maturation rate significantly (Fig. 3A). However, it decreased the rate of aneuploidy in vitrified oocytes significantly (Fig. 1F).
Discussion
Oocyte cryopreservation is a well-established technique in IVF clinics for fertility preservation purposes. Although much progress has been achieved during recent years, the overall efficiency of IVF following cryopreservation at Met II is lower than that of non-vitrified oocytes. Vitrification of prophase I-arrested oocytes is an alternative approach. Because little is known about how vitrification compromises the quality of prophase I-arrested oocytes, this approach remains technically challenging. Therefore, it is critical to understand the underlying molecular mechanisms of cryopreservation-induced oocyte alterations in order to improve the efficiency of this program. Here, we find that vitrification of GV mouse oocytes causes a series of phenotypes during MI, including chromosome misalignment, abnormal spindle formation, inability to establish stable K-MT attachments and weakened SAC function, which are the likely causes of increased aneuploidy in vitrified eggs. We also found that vitrification disrupts lysosomal function and stimulates CTSB and caspase 3 activities. Importantly, inhibition of CTSB or caspase 3 activity rescued the SAC function in vitrified oocytes, indicating that CTSB/caspase 3 activation is the cause, at least partially, of weakened SAC in vitrified oocytes. These results contribute to our understanding of how vitrification adversely affects oocyte quality and shed light on different directions to further improve oocyte cryopreservation protocols.
At prophase I, the chromatin is decondensed and there is no bipolar spindle. In contrast, at Met II, chromosomes are condensed and aligned at the metaphase plate with a bipolar spindle. Because spindle MTs at Met II are sensitive to depolymerization when exposed to low temperatures (Gomes et al., 2012; Tamura et al., 2013), it is plausible that manipulating the vitrification program of GV stage oocytes would be a way to evade this adverse effect on Met II eggs. However, we found that the vitrification of GV oocytes also induces abnormal spindle formation at Met I. Interestingly, although mammalian oocytes during MI are transcriptionally silent, many mRNA transcripts are differently increased or decreased following vitrification (Monzo et al., 2012; Wang et al., 2017), likely through perturbing transcription repression or RNA stability. Moreover, the vitrification of human oocytes severely perturbed the protein ubiquitination pathway, which might affect the expression of certain proteins (Monzo et al., 2012). Taken together, it is possible that vitrification of GV oocytes affects the expression level of cytoskeletal components, which might perturb spindle formation at a later time point. Alternatively, vitrification could perturb the integrity of MT organizing centers (MTOCs) that nucleate MTs and help establish spindle structure. Indeed, vitrification of Met II eggs caused key MTOC components, namely γ-tubulin, pericentrin and NEDD1 (Tamura et al., 2013), to transiently disperse. Whether vitrification of GV oocytes perturbs early perinuclear MTOCs, MTOC fragmentation or clustering is unknown and further investigations are required to understand the underlying mechanism of this critical phenotype.
The inability of the cell to establish correct K-MT attachments before entering Ana I is considered an important cause of aneuploidy in mitosis and meiosis (Compton, 2011; Thompson and Compton, 2011; Lane et al., 2012). To our knowledge, there is no data regarding the effect of vitrification on oocyte ability to establish correct K-MT attachments. We found that vitrification increased the percentage of unattached kinetochores during Met I but did not affect the percentage of abnormal attachments. These results suggest that MTs and the chromosome interface might be perturbed in vitrified oocytes, or that the activities of MT destabilizing proteins, such as Aurora kinases, are up-regulated. Consistent with the former observation, the gene expression of spindle and centromere-associated proteins was differentially expressed in cryopreserved oocytes when compared with fresh oocytes (Monzo et al., 2012). Under normal conditions, cells respond to unattached kinetochores by activating the SAC to delay Ana I onset and to ensure establishment of correct, stable K-MT attachments. Strikingly, vitrified oocytes did not have the ability to maintain SAC activity because MAD2 at kinetochores of vitrified oocytes either with or without nocodazole was decreased. These results confirm the previous observation that the mRNA expression of SAC genes was decreased following vitrification (Wu et al., 2019). However, our finding that MAD2 recruitment to kinetochores was largely restored by inhibiting the CTSB/caspase 3 pathway suggests that the weakened SAC function in vitrified oocytes is not only caused by the alteration of SAC-related gene expression but also the inability to recruit MAD2 or other SAC-related proteins to kinetochores.
The localization of MAD2 to kinetochores depends on a series of complicated steps regulated by many kinases and scaffold proteins. One of these proteins is BUB1, which is required to recruit SAC proteins such as MAD1, MAD2, BUB3 and centromere-associated protein E to kinetochores (Sharp-Baker and Chen, 2001). Accordingly, Bub1 depletion in mouse oocytes perturbed the SAC mechanism resulting in precocious anaphase-promoting complex/cyclosome activation and early PBE (McGuinness et al., 2009). Interestingly, activation of caspase 3 in mitotic cells resulted in BUB1 cleavage, rendering BUB1 non-functional (Perera and Freire, 2005). Importantly, inhibition of caspase 3 using Z-VAD-FMK abolished BUB1 cleavage (Perera and Freire, 2005). Taken together, these observations explain our finding that inhibition of CTSB/caspase 3 restored the SAC function and MAD2 localization in vitrified oocytes, likely through preventing BUB1 cleavage, and further support our conclusion that the CTSB/caspase 3 pathway is the likely cause of impaired SAC function in vitrified oocytes.
CTSB is a lysosomal cysteine protease that is ubiquitously expressed in different types of cells including oocytes and cumulus cells (Balboula et al., 2010a). Exposure of the cell to mild or moderate stress perturbs lysosomal membrane integrity causing severe leakage of CTSs, including CTSB, into the cytoplasm (Brunk et al., 1997). Although a minor controlled leakage of lysosomal CTSB (in cancer cell lines and murine intestinal cells but not pancreatic cells) seems to be required for proper chromosome segregation in certain cell types (Hamalisto et al., 2020), severe leakage of CTSB into the cytoplasm increases mitochondrial membrane permeability leading to the release of cytochrome C and proapoptotic components to the cytoplasm which, in turn, activate initiator and executionary caspases with subsequent initiation of the apoptotic cascade (Bossy-Wetzel et al., 1998; Steemans et al., 1998; Vancompernolle et al., 1998). Given that CTSB/caspase 3 activities were increased in bovine COCs exposed to moderate heat stress (Balboula et al., 2013), it is plausible that vitrification-associated stress causes an elevation of CTSB/caspase 3 activities. We previously showed that CTSB activity is inversely correlated with oocyte and embryo quality (Balboula et al., 2010a, b). Here, we implicated SAC inactivation as an underlying mechanism of how the CTSB/apoptotic pathway compromises oocyte quality. Our results also shed light on why CTSB inhibition during IVM did not improve the maturation rate but significantly improved the blastocyst rate and embryo quality after IVF (Bettegowda et al., 2008; Balboula et al., 2010a), which is likely through supporting a robust SAC function during MI to protect against aneuploidy (Fig. 6).
Figure 6.
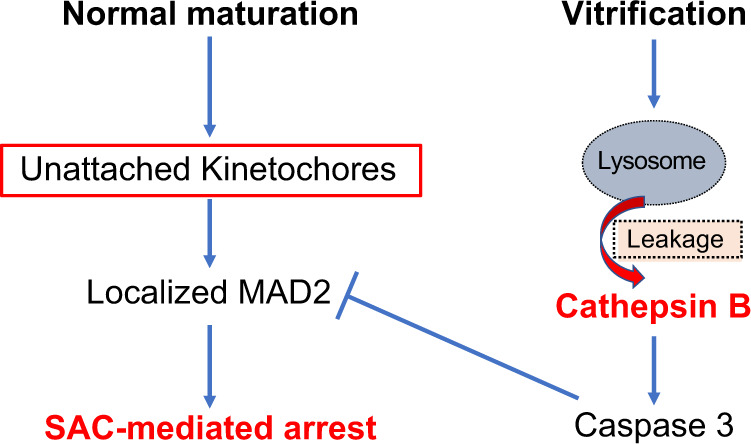
Schematic diagram of the effect of vitrification-induced cathepsin B activation on oocyte meiosis. SAC, spindle assembly checkpoint.
Supplementary data
Supplementary data are available at Molecular Human Reproduction online.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Susanta Behura (University of Missouri) for his help with data analyses. The authors thank the members of the laboratory of Animal Breeding and Reproduction, Hokkaido University and the Balboula lab for valuable help and discussions.
Authors’ roles
A.Z.B., K.S. and M.T. designed experiments. A.Z.B. carried out and analyzed the experiments. A.Z.B., K.S., T.K., M.K. and M.T. wrote the manuscript and provided resources.
Funding
This study was supported by grant-aid for scientific research from JSPS (KAKENHI, 24580439 and 24780265) to M.T. and A.Z.B. (P15400), NIH funding (R01 GM112801) to K.S., and laboratory start-up funding from the University of Missouri to A.Z.B.
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Balboula AZ, Schindler K.. Selective disruption of aurora C kinase reveals distinct functions from aurora B kinase during meiosis in mouse oocytes. PLoS Genet 2014;10:e1004194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboula AZ, Yamanaka K, Sakatani M, Hegab AO, Zaabel SM, Takahashi M.. Cathepsin B activity is related to the quality of bovine cumulus oocyte complexes and its inhibition can improve their developmental competence. Mol Reprod Dev 2010. a;77:439–448. [DOI] [PubMed] [Google Scholar]
- Balboula A Z., Yamanaka K., Sakatani M., Hegab A O., Zaabel S M., Takahashi M. Intracellular cathepsin B activity is inversely correlated with the quality and developmental competence of bovine preimplantation embryos.. Mol Reprod Dev 2010b;77:1031–1039. [DOI] [PubMed] [Google Scholar]
- Balboula AZ, Yamanaka K, Sakatani M, Kawahara M, Hegab AO, Zaabel SM, Takahashi M.. Cathepsin B activity has a crucial role in the developmental competence of bovine cumulus-oocyte complexes exposed to heat shock during in vitro maturation. Reproduction 2013;146:407–417. [DOI] [PubMed] [Google Scholar]
- Bettegowda A, Patel OV, Lee KB, Park KE, Salem M, Yao J, Ireland JJ, Smith GW.. Identification of novel bovine cumulus cell molecular markers predictive of oocyte competence: functional and diagnostic implications. Biol Reprod 2008;79:301–309. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Newmeyer DD, Green DR.. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J 1998;17:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet S, Pahlavan G, Taylor S, Maro B.. Functionality of the spindle checkpoint during the first meiotic division of mammalian oocytes. Reproduction 2003;126:443–450. [DOI] [PubMed] [Google Scholar]
- Brunk UT, Dalen H, Roberg K, Hellquist HB.. Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic Biol Med 1997;23:616–626. [DOI] [PubMed] [Google Scholar]
- Chang CC, Elliott TA, Wright G, Shapiro DB, Toledo AA, Nagy ZP.. Prospective controlled study to evaluate laboratory and clinical outcomes of oocyte vitrification obtained in in vitro fertilization patients aged 30 to 39 years. Fertil Steril 2013;99:1891–1897. [DOI] [PubMed] [Google Scholar]
- Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I.. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil 1989;86:679–688. [DOI] [PubMed] [Google Scholar]
- Compton DA. Mechanisms of aneuploidy. Curr Opin Cell Biol 2011;23:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan FE, Chiang T, Schultz RM, Lampson MA.. Evidence that a defective spindle assembly checkpoint is not the primary cause of maternal age-associated aneuploidy in mouse eggs. Biol Reprod 2009;81:768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar DH, Gook DA.. A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos. Hum Reprod Update 2012;18:536–554. [DOI] [PubMed] [Google Scholar]
- Fadini R, Brambillasca F, Renzini MM, Merola M, Comi R, De Ponti E, Dal Canto MB.. Human oocyte cryopreservation: comparison between slow and ultrarapid methods. Reprod Biomed Online 2009;19:171–180. [DOI] [PubMed] [Google Scholar]
- Gomes C, Merlini M, Konheim J, Serafini P, Motta EL, Baracat EC, Smith GD.. Oocyte meiotic-stage-specific differences in spindle depolymerization in response to temperature changes monitored with polarized field microscopy and immunocytochemistry. Fertil Steril 2012;97:714–719. [DOI] [PubMed] [Google Scholar]
- Hamalisto S, Stahl JL, Favaro E, Yang Q, Liu B, Christoffersen L, Loos B, Guasch Boldu C, Joyce JA, Reinheckel T. et al. Spatially and temporally defined lysosomal leakage facilitates mitotic chromosome segregation. Nat Commun 2020;11:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer HA, McDougall A, Levasseur M, Murdoch AP, Herbert M.. Mad2 is required for inhibiting securin and cyclin B degradation following spindle depolymerisation in meiosis I mouse oocytes. Reproduction 2005;130:829–843. [DOI] [PubMed] [Google Scholar]
- Kola I, Kirby C, Shaw J, Davey A, Trounson A.. Vitrification of mouse oocytes results in aneuploid zygotes and malformed fetuses. Teratology 1988;38:467–474. [DOI] [PubMed] [Google Scholar]
- Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology 2007;67:73–80. [DOI] [PubMed] [Google Scholar]
- Kuwayama M, Vajta G, Kato O, Leibo SP.. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online 2005;11:300–308. [DOI] [PubMed] [Google Scholar]
- Lane M, Bavister BD, Lyons EA, Forest KT.. Containerless vitrification of mammalian oocytes and embryos. Nat Biotechnol 1999;17:1234–1236. [DOI] [PubMed] [Google Scholar]
- Lane SI, Yun Y, Jones KT.. Timing of anaphase-promoting complex activation in mouse oocytes is predicted by microtubule-kinetochore attachment but not by bivalent alignment or tension. Development 2012;139:1947–1955. [DOI] [PubMed] [Google Scholar]
- Leland S, Nagarajan P, Polyzos A, Thomas S, Samaan G, Donnell R, Marchetti F, Venkatachalam S.. Heterozygosity for a Bub1 mutation causes female-specific germ cell aneuploidy in mice. Proc Natl Acad Sci USA 2009;106:12776–12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangos P, Stevense M, Niaka K, Lagoudaki M, Nabti I, Jessberger R, Carroll J.. DNA damage-induced metaphase I arrest is mediated by the spindle assembly checkpoint and maternal age. Nat Commun 2015;6:8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness BE, Anger M, Kouznetsova A, Gil-Bernabe AM, Helmhart W, Kudo NR, Wuensche A, Taylor S, Hoog C, Novak B. et al. Regulation of APC/C activity in oocytes by a Bub1-dependent spindle assembly checkpoint. Curr Biol 2009;19:369–380. [DOI] [PubMed] [Google Scholar]
- McIlwain DR, Berger T, Mak TW.. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 2013;5:a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moawad AR, Tan SL, Xu B, Chen HY, Taketo T.. L-carnitine supplementation during vitrification of mouse oocytes at the germinal vesicle stage improves preimplantation development following maturation and fertilization in vitro. Biol Reprod 2013;88:104. [DOI] [PubMed] [Google Scholar]
- Monzo C, Haouzi D, Roman K, Assou S, Dechaud H, Hamamah S.. Slow freezing and vitrification differentially modify the gene expression profile of human metaphase II oocytes. Hum Reprod 2012;27:2160–2168. [DOI] [PubMed] [Google Scholar]
- Perera D, Freire R.. Human spindle checkpoint kinase Bub1 is cleaved during apoptosis. Cell Death Differ 2005;12:827–830. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Montgomery RR, Belanoff JR.. Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol 1983;97:264–273. [DOI] [PubMed] [Google Scholar]
- Sharp-Baker H, Chen RH.. Spindle checkpoint protein Bub1 is required for kinetochore localization of Mad1, Mad2, Bub3, and CENP-E, independently of its kinase activity. J Cell Biol 2001;153:1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Serafini PC, Fioravanti J, Yadid I, Coslovsky M, Hassun P, Alegretti JR, Motta EL.. Prospective randomized comparison of human oocyte cryopreservation with slow-rate freezing or vitrification. Fertil Steril 2010;94:2088–2095. [DOI] [PubMed] [Google Scholar]
- Son WY, Henderson S, Cohen Y, Dahan M, Buckett W.. Immature oocyte for fertility preservation. Front Endocrinol (Lausanne) 2019;10:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son WY, Tan SL.. Comparison between slow freezing and vitrification for human embryos. Expert Rev Med Devices 2009;6:1–7. [DOI] [PubMed] [Google Scholar]
- Song WY,, Peng ZF, Chen XM, Jin HX,, Yao GD, Shi SL, Yang HY, Zhang XY, Sun YP.. Effects of vitrification on outcomes of in vivo-mature, in vitro-mature and immature human oocytes. Cell Physiol Biochem 2016;38:2053–2062. [DOI] [PubMed] [Google Scholar]
- Steemans M, Goossens V, Van de Craen M, Van Herreweghe F, Vancompernolle K, De Vos K, Vandenabeele P, Grooten J.. A caspase-activated factor (CAF) induces mitochondrial membrane depolarization and cytochrome c release by a nonproteolytic mechanism. J Exp Med 1998;188:2193–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura AN, Huang TT, Marikawa Y.. Impact of vitrification on the meiotic spindle and components of the microtubule-organizing center in mouse mature oocytes. Biol Reprod 2013;89:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SL, Compton DA.. Chromosome missegregation in human cells arises through specific types of kinetochore-microtubule attachment errors. Proc Natl Acad Sci USA 2011;108:17974–17978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajta G, Nagy ZP.. Are programmable freezers still needed in the embryo laboratory? Review on vitrification. Reprod Biomed Online 2006;12:779–796. [DOI] [PubMed] [Google Scholar]
- Van der Elst J, Nerinckx S, Van Steirteghem AC.. Association of ultrarapid freezing of mouse oocytes with increased polyploidy at the pronucleate stage, reduced cell numbers in blastocysts and impaired fetal development. J Reprod Fertil 1993;99:25–32. [DOI] [PubMed] [Google Scholar]
- Vancompernolle K, Van Herreweghe F, Pynaert G, Van de Craen M, De Vos K, Totty N, Sterling A, Fiers W, Vandenabeele P, Grooten J.. Atractyloside-induced release of cathepsin B, a protease with caspase-processing activity. FEBS Lett 1998;438:150–158. [DOI] [PubMed] [Google Scholar]
- Wang H, Racowsky C, Combelles CM.. Is it best to cryopreserve human cumulus-free immature oocytes before or after in vitro maturation? Cryobiology 2012;65:79–87. [DOI] [PubMed] [Google Scholar]
- Wang N, Li CY, Zhu HB, Hao HS, Wang HY, Yan CL, Zhao SJ, Du WH, Wang D, Liu Y. et al. Effect of vitrification on the mRNA transcriptome of bovine oocytes. Reprod Domest Anim 2017;52:531–541. [DOI] [PubMed] [Google Scholar]
- Wu Z, Pan B, Qazi IH, Yang H, Guo S, Yang J, Zhang Y, Zeng C, Zhang M, Han H. et al. Melatonin improves in vitro development of vitrified-warmed mouse germinal vesicle oocytes potentially via modulation of spindle assembly checkpoint-related genes. Cells 2019;8:1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon TK, Kim TJ, Park SE, Hong SW, Ko JJ, Chung HM, Cha KY.. Live births after vitrification of oocytes in a stimulated in vitro fertilization-embryo transfer program. Fertil Steril 2003;79:1323–1326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.