Abstract
Background
While acute kidney injury (AKI) is commonly reported following hematopoietic stem cell transplant (HCT), the incidence and impact of AKI on mortality among patients undergoing HCT are not well described. We conducted this systematic review to assess the incidence and impact of AKI on mortality risk among patients undergoing HCT.
Methods
Ovid MEDLINE, EMBASE and the Cochrane Databases were searched from database inceptions through August 2019 to identify studies assessing the incidence of AKI and mortality risk among adult patients who developed AKI following HCT. Random-effects and generic inverse variance method of DerSimonian–Laird were used to combine the effect estimates obtained from individual studies.
Results
We included 36 cohort studies with a total of 5144 patients undergoing HCT. Overall, the pooled estimated incidence of AKI and severe AKI (AKI Stage III) were 55.1% (95% confidence interval (CI) 46.6–63.3%) and 8.3% (95% CI 6.0–11.4%), respectively. The pooled estimated incidence of AKI using contemporary AKI definitions (RIFLE, AKIN and KDIGO criteria) was 49.8% (95% CI 41.6–58.1%). There was no significant correlation between study year and the incidence of AKI (P = 0.12) or severe AKI (P = 0.97). The pooled odds ratios of 3-month mortality and 3-year mortality among patients undergoing HCT with AKI were 3.05 (95% CI 2.07–4.49) and 2.23 (95% CI 1.06–4.73), respectively.
Conclusion
The incidence of AKI among patients who undergo HCT remains high, and it has not changed over the years despite advances in medicine. AKI after HCT is associated with increased short- and long-term mortality.
Introduction
Hematopoietic stem cell transplant (HCT) is being used for multiple malignant and non-malignant conditions.1–4 In the current era, indications have been extended to metabolic, immune-related, autoimmune and other inflammatory disorders.5 More than 50 000 patients undergo HCT every year, and its rate is increasing by 20–30% annually.6 Despite the widespread use of preventive measures, acute kidney injury (AKI) remains a substantial problem after HCT. AKI is associated with significant cost burden, morbidity and mortality.7,8 Survivors of AKI could sustain recurrent episodes of AKI, leading to multiple hospitalizations.9 In long-term survivors after HCT, chronic kidney disease is prevalent in up to 20% of the patients.10–12 They are at further risk for the development of hypertension, albuminuria and nephrotic range proteinuria.13,14 Severe AKI requiring renal replacement therapy (RRT) is associated with significant mortality of about 80%.15–18
Multiple steps are involved in successful hematopoietic stem cell transplantation.19 The process begins with the procurement of stem cells from the donor, while the recipient undergoes intensive chemotherapy (myeloablative)3,20 vs. less intensive chemo (non-myeloablative)21 depending on age and other comorbidities. The second stage includes the infusion of graft stem cells to the recipient (engraftment). Finally, the recipient receives immunosuppression to suppress rejection or graft vs. host disease. AKI can occur during any of the above-mentioned steps.22 AKI following HCT is traditionally defined as ‘Doubling of serum creatinine in the first hundred days’. However, in order to standardize AKI risk stratification, RIFLE, KDIGO and AKIN definitions were developed.18,23–27 The reported incidence of AKI after HCT varies widely from 12% to 66%.18,20,28–30 This wide variation is likely related to not using a standardized AKI definition, various conditioning regimens, allogeneic vs. autologous donor and retrospective nature of studies.25,26 It is reported that the incidence of AKI after autologous stem cell transplant is 12–50%, non-myeloablative allogeneic 29–54% and myeloablative allogeneic at 19–66%.
Given the variability in the reported incidence of AKI post-HCT, we performed a systematic review and meta-analysis of the existing cohort studies up to August 2019 to assess the pooled incidence of AKI and its associated mortality.
Materials and methods
Search strategy
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement31 was followed in conducting this systematic review. Ovid MEDLINE, EMBASE and the Cochrane Databases were systematically searched from database inceptions through August 2019 to identify studies fulfilled the following inclusion criteria: (i) clinical trials or observational studies published as original articles or conference abstracts; (ii) studies that assessed the incidence of AKI or AKI-associated mortality among patients undergoing HCT; (iii) adult patient population (age ≥ 18 years old). The primary outcome was AKI post-HCT. Mortality risk was also assessed among the studies that reported AKI-outcome. Two investigators (S.K. and K.K.) performed independent literature search using the search terms of ((‘bone morrow’ OR ‘stem cell’) AND (‘transplant’ OR ‘transplantation’)) AND (‘acute kidney injury’ OR ‘acute renal failure’ OR ‘renal replacement therapy’). Supplementary Data S1 provide information on the detailed search strategy. The data for this meta-analysis are publicly available through the Open Science Framework (URL: <seurld>https://osf.io/qfgj9/</seurld>). Language restriction was not applied. Potentially related studies were manually reviewed using the references. Gray literature was additionally searched for further relevant information.
Study selection
Observational studies and clinical trials providing 95% confidence intervals (CI) data on the incidence of AKI and mortality risk of AKI in adult patients undergoing HCT were included in the meta-analysis. Two investigators (S.K. and K.K.) independently reviewed retrieved articles for eligibility. A third reviewer (W.C.) solved inconsistencies by collective agreement. AKIN,32 RIFLE33 and KDIGO34 definitions of AKI were used for subgroup analysis.
Data collection
The collected data from individual studies included title, name of authors, year of the study, publication year, the country where the study was conducted, patient characteristics, AKI definition, the incidence of AKI and severe AKI requiring RRT and finally reported death rate among patients with AKI following HCT.
Statistical analysis
Meta-analysis was performed using Comprehensive Meta-Analysis software version 3.3.070 (Biostat Inc., NJ, USA). Adjusted point estimates of included studies were incorporated by the generic inverse variance method of DerSimonian–Laird, which assigned the weight of individual study based on its variance.35 Due to the probability of between-study variance, we applied a random-effects model to pool outcomes of interest, including the incidence of AKI and mortality risk. Cochran’s Q test (P < 0.05 for a statistical significance) and I2 statistic (≤25% represents insignificant heterogeneity, 26–50% represents low heterogeneity, 51–75% represents moderate heterogeneity and ≥75% represents high heterogeneity) were used to assess statistical heterogeneity.36 Publication bias was assessed by funnel plot and the Egger test.37
Results
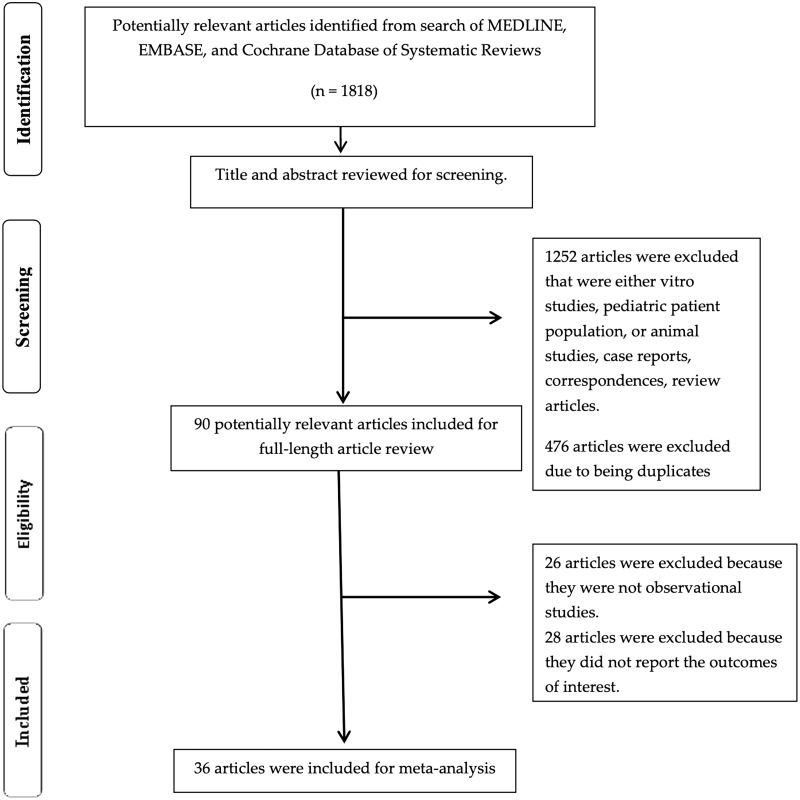
The search yielded a total of 1818 articles for initial screening. Four hundred seventy-eight duplicates were removed, and 1262 articles were excluded for the following reasons: in vitro studies, pediatric patient population, animal studies, case reports, correspondences or review articles. Full-length reviews of 90 studies were performed. Twenty-six studies were not observational studies and 28 studies were excluded due to not providing the outcome of interest; thus, 36 cohort studies15–18,20,25,26,38–65 with a total of 5144 patients undergoing HCT were enrolled. Figure 1 outlines the flowchart of paper selection for inclusion. Table 1 provides details of the included studies.
Figure 1.
Outlines the flowchart of paper selection for inclusion.
Table 1.
The main characteristic of studies included in this meta-analysis of AKI incidence and mortality among patients with hematopoietic stem cell transplantation
| Study | Year | Country | Patients | Indication for HCT | Number | AKI definition | AKI incidence | Mortality |
|---|---|---|---|---|---|---|---|---|
| Merouani et al.38 | 1995 | Colorado, USA, 1991–1994 | Autologous hematopoietic cell transplant | Breast cancer | 232 |
|
|
|
| Gruss et al.39 | 1995 | Madrid, Spain | Allogeneic and autologous BMT | AL, CML, AA, other | 275 | Doubling of serum creatinine or creatinine > 2 mg/dl or AKI requiring HD |
|
|
| Parikh et al.40 | 2002 | Colorado, USA | Allogeneic hematopoietic cell transplant | Hematological malignancy | 88 |
|
|
|
| Schrier et al.53 | 2005 | New Haven, CT, USA | Autologous HCT | Breast cancer | 232 |
|
|
|
| Lopes et al.54 | 2006 | Portugal | Autologous and allogeneic HCT | Hematological malignancy | 140 | RIFLE |
|
N/A |
| Parikh et al.41 | 2004 | Colorado, USA, 1998–2001 | Non-myeloablative HCT | CML ALL | 253 |
|
|
N/A |
| Caliskan et al.20 | 2006 | Turkey, 2001–2003 | Myeloablative allogeneic and autologous | Hematological malignancy | 47 |
|
|
|
| Liu et al.42 | 2007 | China, 2002–2005 | Non-myeloablative peripheral blood stem cell transplant | CML | 26 |
|
|
|
| Kersting et al.43 | 2008 | Netherlands | Non-myeloablative HCT | CML, AA | 150 |
|
|
NA |
| Kersting et al.44 | 2007 | Netherlands, 1993–2004 | Allogeneic myeloablative | AML, ALL, CML, OTHERS | 363 |
|
|
|
| Lopes et al.17 | 2008 | Portugal, 1999–2005 | Reduced-intensity conditioning, HCT | AML, CML | 82 | KDIGO |
|
|
| Yakushijin et al.55 | 2009 | Tokyo, Japan | Reduced-intensity stem cell transplant | AML, ALL, CML, MDS | 286 |
|
|
N/A |
| Tokgoz et al.56 | 2009 | Turkey, 2007–2008 | Allogeneic myeloablative | AML, ALL | 39 |
|
Overall AKI = 20/39 = (51.3) |
|
| Ando et al.25 | 2010 | Japan, 2004–2007 | Autologous and allogeneic HCT | Hematological malignancy | 249 | AKIN |
|
|
| Lui et al.18 | 2010 | China, 2002–2007 | Non-myeloablative HCT | CML, ALL, CLL | 62 | AKIN |
|
|
| Yu et al.66 | 2010 | China, 2003–2008 | Allogeneic HCT | Hematological malignancy | 96 |
|
|
NA |
| Irazabal et al.57 | 2011 | Rochester, USA, 1997–2009 | Autologous stem cell transplant | Light chain amyloidosis | 29 | AKIN |
|
100-day AKI mortality = 2/28 = (7.1%) |
| Helal et al.45 | 2011 | France | Hematopoietic stem cell transplant | AML, ALL, CML, MM | 101 | Requiring RRT | AKI requiring RRT: 12/101 = (11.8%) | N/A |
| Morito et al.47 | 2011 | Japan | Allogeneic HCT | Hematological malignancy | 40 | RIFLE |
|
|
| Bao et al.46 | 2011 | China, 2003–2008 | Allogeneic hematopoietic stem cell transplant | CML, ALL, MDS, MM | 143 | RIFLE |
|
|
| Kagoya et al.58 | 2011 | Tokyo | Autologous and allogeneic | Hematological malignancy | 207 | RIFLE |
|
3-year AKI mortality, 24/158 (16.6%) |
| Durate et al.59 | 2012 | Brazil, 2008–2011 | Autologous BMT |
|
70 | AKIN |
|
N/A |
| Mori et al.48 | 2012 | Japan, 2004–2009 | Allogeneic hematopoietic stem cell transplant | ALL, CML, ATL, MDS, MM | 289 | AKIN |
|
|
| Canet et al.60 | 2014 | Paris, France, 2007–2011 | Allo—HCT | ALL, AML and lymphoma | 75 | KDIGO |
|
N/A |
| Chapchap et al.61 | 2016 | Brazil, 2007–2014 | Allogeneic HCT | Hematological malignancy | 111 | Requiring RRT | RRT = 20/111 = (18.3%) | N/A |
| Esposito et al.62 | 2016 | Pavia, Italy, 2013–2015 | Allogeneic HCT | Hematological malignancy | 57 |
|
|
N/A |
| Liu et al.26 | 2017 | China, May 2013–June 2014 |
|
|
353 |
|
|
N/A |
| Myhre et al.63 | 2017 | Norway, 2004–2016 | Non-myeloablative allogeneic | Lymphoma | 108 | RIFLE | Overall AKI = 75/108 = (69.4%) | N/A |
| Pinana et al.52 | 2017 | Spain, 2008–2015 | Allo- HCT |
|
186 | KDIGO |
|
Grade 2 KDIGO = HR 2.8; P = 0.05, Grade 3 KDIGO (HR 6.6; P < 0.001). |
| Sehgal et al.49 | 2017 | India, 2008–2014 | Hematopoietic stem cell transplant | MM, leukemia, lymphoma, aplastic anemia | 65 | RIFLE |
|
|
| Deger et al.50 | 2017 | Turkey, 2009–2011 | Allogeneic HCT | Hematological malignancies | 50 | AKIN |
|
NA |
| Cekdemi et al.64 | 2018 | Turkey, 2010–2017 | Autologous and allogeneic | Hematological malignancy | 155 | AKIN | Overall AKI = 78/155 = (50.3%) | N/A |
| Khalil et al.51 | 2019 | Jordan, 2002–2016 | Hematopoietic stem cell transplant | CML, MM, ALL, AML, HL, NHL | 60 | RIFLE |
|
|
| Pereira et al.65 | 2018 | Brazil, 2010–2014 | Hematopoietic stem cell transplant | Multiple myeloma | 132 | Rise in serum creatinine >0.3 mg/dl | Overall AKI = 21/132 = (16%) | N/A |
| Andronesi et al.15 | 2019 | Romania, 2016–2017 | Autologous stem cell transplant | Multiple myeloma | 185 | KDIGO |
|
|
| Mima et al.16 | 2019 | Japan, 2006–2016 | Hematopoietic stem cell transplant | AML, ALL, CML, MM, AA | 108 | KDIGO |
|
N/A |
AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; AKIN, acute kidney injury network; KDIGO, Kidney Disease Improving Global Outcomes; RIFLE, Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease; BMT, bone marrow transplant; HCT, hematopoietic stem cell transplantation; RRT, renal replacement therapy; GFR, glomerular filtration rate; HD, hemodialysis; CML, chronic myelogenous leukemia; CLL, chronic lymphocytic leukemia; AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia; MDS, myelodysplastic syndrome; MM, multiple myeloma; ATL, adult T-cell leukemia/lymphoma; HL, Hodgkin’s lymphoma; NHL, non-Hodgkin’s lymphoma; TX, transplant; NA, not applicable; USA, United States of America.
Incidence of AKI among patients undergoing HCT
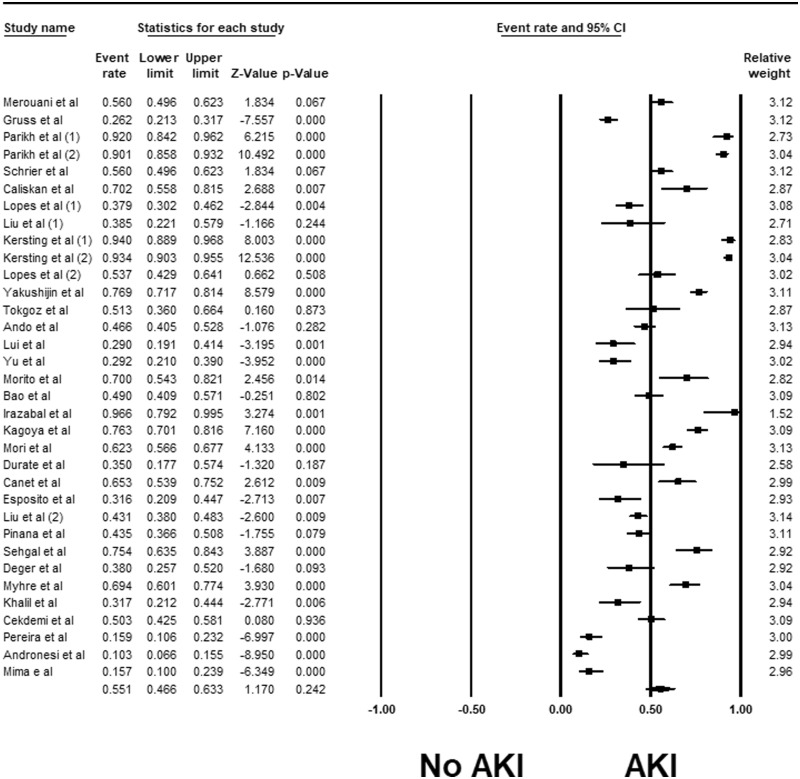
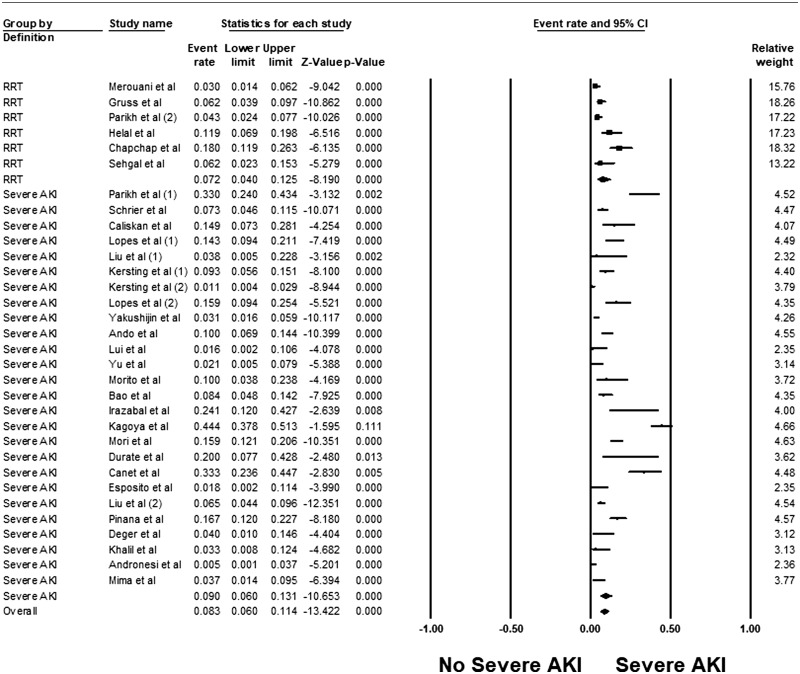
Overall, the pooled estimated incidence of AKI and severe AKI among patients undergoing HCT were 55.1% (95% CI 46.6–63.3%, I2 = 96%, Figure 2) and 8.3% (95% CI 6.0–11.4%, I2 = 92%, Figure 3), respectively. The pooled estimated incidence of AKI using standard AKI definitions was 49.8% (95% CI 41.6–58.1%, I2 = 93%, Supplementary Figure S1). The pooled estimated incidence of RRT among patients undergoing HCT was 7.2% (95% CI: 4.0–12.5%, I2 = 83%, Figure 3).
Figure 2.
Forest plots of the included studies evaluating incidence rates of AKI among patients undergoing HCT.
Figure 3.
Forest plots of the included studies evaluating incidence rates of severe AKI among patients undergoing HCT.
Subgroup analyses were performed according to AKI definitions. The pooled estimated incidence rates of AKI by RIFLE, AKIN and KDIGO criteria were 59.2% (95% CI 44.5–72.5%, I2 = 93%, Supplementary Figure S1), 48.2% (95% CI 37.4–59.1%, I2 = 86%, Supplementary Figure S1) and 34.1% (95% CI 16.7–57.2%, I2 = 96%, Supplementary Figure S1), respectively.
Meta-regression of all studies using standard AKI definitions showed that the year of the study did not significantly affect the incidence of AKI (P = 0.12, Supplementary Figure S2A) and severe AKI (P = 0.97, Supplementary Figure S2B).
Mortality risk of AKI in patients after HCT
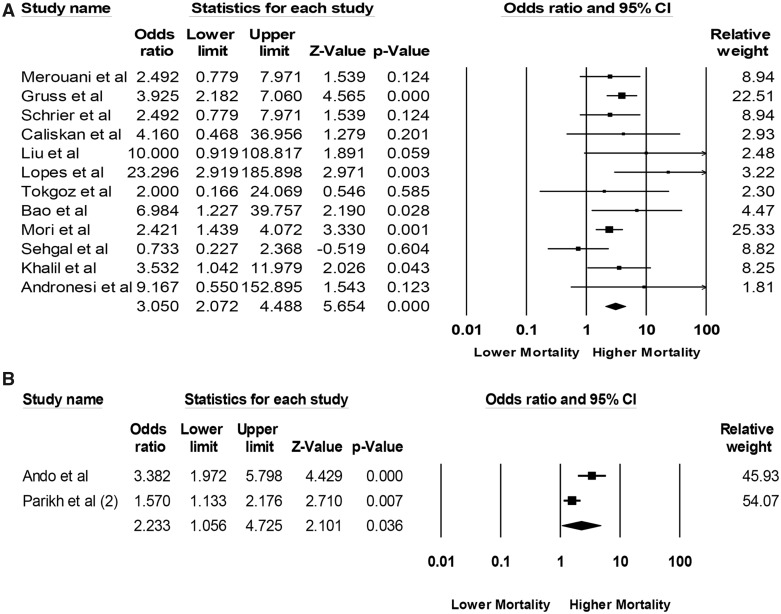
Data on mortality risk from included studies are shown in Table 1. The pooled odds ratios (ORs) of 3-month mortality and 3-year mortality among patients undergoing HCT with AKI were 3.05 (95% CI 2.07–4.49, I2 = 19%, Figure 4A) and 2.23 (95% CI 1.06–4.73, I2 = 82%, Figure 4B), respectively.
Figure 4.
Forest plots of the included studies evaluating (A) mortality risk of AKI within 3 months and (B) mortality risk of AKI within 3 years after HCT.
Evaluation for publication bias
The funnel plot (Supplementary Figure S3) and Egger’s regression asymmetry tests were performed to assess publication bias in analysis evaluating the 3-month mortality of AKI in patients undergoing HCT. We found no significant publication bias in the meta-analysis evaluating the mortality risk of patients after HCT with AKI (P = 0.30).
Discussion
In this systematic review and meta-analysis, we found that the incidence of overall AKI and severe AKI requiring RRT after HCT is very high. Overall, the pooled estimated incidence of AKI and severe AKI among patients undergoing HCT are 55.1% and 8.3%, respectively. The pooled estimated incidence of AKI using standard AKI definition (KDIGO, RIFLE and AKIN) is 49.8%. Our findings showed significant increased short- and long-term mortality among patients with AKI after HCT. Meta-regression analyses showed that the year of the study did not significantly affect the incidence of AKI after HCT among included studies (published between years 1995 and 2019).
The etiology and mechanism of acute renal failure after HCT remain complex and multifactorial. Multiple risk factors are linked to the incidence of AKI after HCT. Major risk factors include diabetes,67 hypertension,44 preexisting chronic kidney disease,68 nephrotoxic medications including amphotericin B,10 acyclovir for viral prophylaxis,69 aminoglycosides,70 calcineurin inhibitors for prophylaxis of graft vs. host effect,71 intravenous immune globulin,28 underlying sepsis,18 admission to intensive care unit,44 use of mechanical ventilation,41 preexisting lung toxicity,43 incomplete human leukocyte antigen (HLA) matched transplant,18 female sex, weight gain > 10% and cytomegalovirus infections (Table 2).28
Table 2.
Risk factors linked to incidence of AKI after HCT.
| Risk factors linked to incidence of AKI after HCT |
|---|
In general, the etiology of AKI varies based on the different phases of HCT.10,72–74 Tumor lysis syndrome and marrow intoxication syndrome manifest between 0–5 days of the pre-conditioning phase. Tumor lysis syndrome is rare in patients following HCT, as most are in the remission phase.75–77 The incidence of tumor lysis is <1 in 400 patients.5 Marrow intoxication syndrome is specifically seen in patients after HCT. Dimethyl sulfoxide (DMSO) is used as a freezing solvent to store stem cells and could contribute to RBC and granulocyte lysis.76 With modified stem cell storing options and limiting the amount of DMSO, the incidence of marrow intoxication syndrome has reduced.78
In the early phase between 1 and 4 weeks, the etiology of AKI is attributed to chemo-induced volume loss, pre-renal AKI,79, ischemic acute tubular necrosis (ATN), septic ATN,80,81 engraftment syndrome,82 hepatic veno-occlusive disease,83–87 use of nephrotoxic medications including69 aminoglycosides,70,88 amphotericin10 and acyclovir.5,89 Acute graft vs. host disease (GVHD) can be seen first 100 days post-HCT. Acute GVHD post-HCT is associated with significant renal dysfunction and rejection episodes post-transplant.6 Viral infections, including adenovirus and BK virus leading to AKI post-HCT, are worth mentioning. Calcineurin inhibitors play a significant role in causing renal vasoconstriction, tubular toxicity contributing to AKI post-HCT.28,71 Transplant thrombotic microangiopathy, chronic calcineurin inhibitor nephrotoxicity and chronic GVHD are being noticed after 6–12 months post-transplant and could lead to chronic kidney disease.1,19,78,79,90–94
Our meta-analysis included some limitations. This systematic review was based on cohort studies. Thus, it is not identifying any causal relationship between AKI and death rate, but it reports associations. The missing data from the included studies related to the novel AKI biomarkers may be another limitation. Due to the presence of statistical heterogeneities among the studies, subgroup analyses were performed using standardized definitions of AKI (RIFLE, AKIN and KDIGO) to mitigate the risk of bias.
As demonstrated in our meta-analysis, AKI post-HCT is associated with increased risk of mortality especially if RRT is needed. Despite medical advances, the overall incidence has not decreased since 1995. Our effort is to increase awareness about the continued high incidence of AKI in hopes that identifying at risk patients and implementing naive preventive measures through continued research might mitigate some AKI-associated poor outcomes.
Supplementary material
Supplementary material is available at QJMED online.
Conflict of interest: None declared.
Supplementary Material
References
- 1. Kogon A, Hingorani S.. Acute kidney injury in hematopoietic cell transplantation. Semin Nephrol 2010; 30:615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burt RK, Traynor A, Statkute L, Barr WG, Rosa R, Schroeder J, et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA 2006; 295:527–35. [DOI] [PubMed] [Google Scholar]
- 3. Burt RK, Verda L, Statkute L, Quigley K, Yaung K, Brush M, et al. Stem cell transplantation for autoimmune diseases. Clin Adv Hematol Oncol 2004; 2:313–9. [PubMed] [Google Scholar]
- 4. Oyama Y, Craig RM, Traynor AE, Quigley K, Statkute L, Halverson A, et al. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn’s disease. Gastroenterology 2005; 128:552–63. [DOI] [PubMed] [Google Scholar]
- 5. Parikh CR, Coca SG.. Acute renal failure in hematopoietic cell transplantation. Kidney Int 2006; 69:430–5. [DOI] [PubMed] [Google Scholar]
- 6. Hingorani S. Renal complications of hematopoietic-cell transplantation. N Engl J Med 2016; 374:2256–67. [DOI] [PubMed] [Google Scholar]
- 7. Cheungpasitporn W Kashani K. Electronic data systems and acute kidney injury. Contrib Nephrol 2016; 187:73. [DOI] [PubMed] [Google Scholar]
- 8. Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 2018; 14:607–25. [DOI] [PubMed] [Google Scholar]
- 9. Sawhney S, Marks A, Fluck N, Levin A, McLernon D, Prescott G, et al. Post-discharge kidney function is associated with subsequent ten-year renal progression risk among survivors of acute kidney injury. Kidney Int 2017; 92:440–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hingorani SR, Guthrie K, Batchelder A, Schoch G, Aboulhosn N, Manchion J, et al. Acute renal failure after myeloablative hematopoietic cell transplant: incidence and risk factors. Kidney Int 2005; 67:272–7. [DOI] [PubMed] [Google Scholar]
- 11. Hingorani S, Guthrie KA, Schoch G, Weiss NS, McDonald GB.. Chronic kidney disease in long-term survivors of hematopoietic cell transplant. Bone Marrow Transplant 2007; 39:223–9. [DOI] [PubMed] [Google Scholar]
- 12. Ando M, Ohashi K, Akiyama H, Sakamaki H, Morito T, Tsuchiya K, et al. Chronic kidney disease in long-term survivors of myeloablative allogeneic haematopoietic cell transplantation: prevalence and risk factors. Nephrol Dial Transplant 2010; 25:278–82. [DOI] [PubMed] [Google Scholar]
- 13. Momoki K, Yamaguchi T, Ohashi K, Ando M, Nitta K.. Emergence of dipstick proteinuria predicts overt nephropathy in patients following stem cell transplantation. Nephron 2017; 135:31–8. [DOI] [PubMed] [Google Scholar]
- 14. Morito T, Ando M, Kobayashi T, Kakihana K, Ohashi K, Akiyama H, et al. New-onset microalbuminuria following allogeneic myeloablative SCT is a sign of near-term decrease in renal function. Bone Marrow Transplant 2013; 48:972–6. [DOI] [PubMed] [Google Scholar]
- 15. Andronesi AG, Tanase AD, Sorohan BM, Craciun OG, Stefan L, Varady Z, et al. Incidence and risk factors for acute kidney injury following autologous stem cell transplantation for multiple myeloma. Cancer Med 2019; 8:3278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mima A, Tansho K, Nagahara D, Tsubaki K.. Incidence of acute kidney disease after receiving hematopoietic stem cell transplantation: a single-center retrospective study. PeerJ 2019; 7:e6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lopes JA, Goncalves S, Jorge S, Raimundo M, Resende L, Lourenco F, et al. Contemporary analysis of the influence of acute kidney injury after reduced intensity conditioning haematopoietic cell transplantation on long-term survival. Bone Marrow Transplant 2008; 42:619–26. [DOI] [PubMed] [Google Scholar]
- 18. Liu H, Li YF, Liu BC, Ding JH, Chen BA, Xu WL, et al. A multicenter, retrospective study of acute kidney injury in adult patients with nonmyeloablative hematopoietic SCT. Bone Marrow Transplant 2010; 45:153–8. [DOI] [PubMed] [Google Scholar]
- 19. Singh N, McNeely J, Parikh S, Bhinder A, Rovin BH, Shidham G.. Kidney complications of hematopoietic stem cell transplantation. Am J Kidney Dis 2013; 61:809–21. [DOI] [PubMed] [Google Scholar]
- 20. Caliskan Y, Besisik SK, Sargin D, Ecder T.. Early renal injury after myeloablative allogeneic and autologous hematopoietic cell transplantation. Bone Marrow Transplant 2006; 38:141–7. [DOI] [PubMed] [Google Scholar]
- 21. Blaise D, Bay JO, Faucher C, Michallet M, Boiron JM, Choufi B, et al. Reduced-intensity preparative regimen and allogeneic stem cell transplantation for advanced solid tumors. Blood 2004; 103:435–41. [DOI] [PubMed] [Google Scholar]
- 22. Carella AM, Champlin R, Slavin S, McSweeney P, Storb R.. Mini-allografts: ongoing trials in humans. Bone Marrow Transplant 2000; 25:345–50. [DOI] [PubMed] [Google Scholar]
- 23. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 2006; 10:R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ando M, Mori J, Ohashi K, Akiyama H, Morito T, Tsuchiya K, et al. A comparative assessment of the RIFLE, AKIN and conventional criteria for acute kidney injury after hematopoietic SCT. Bone Marrow Transplant 2010; 45:1427–34. [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Xu L, Zhang X, Wang Y, Liu K, Chen H, et al. Acute kidney injury following haplo stem cell transplantation: incidence, risk factors and outcome. Bone Marrow Transplant 2018; 53:483–6. [DOI] [PubMed] [Google Scholar]
- 27. Kellum JA, Lameire N; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013; 17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wanchoo R, Stotter BR, Bayer RL, Jhaveri KD.. Acute kidney injury in hematopoietic stem cell transplantation. Curr Opin Crit Care 2019; 25:531–8. [DOI] [PubMed] [Google Scholar]
- 29. Ataei S, Hadjibabaie M, Moslehi A, Taghizadeh-Ghehi M, Ashouri A, Amini E, et al. A double-blind, randomized, controlled trial on N-acetylcysteine for the prevention of acute kidney injury in patients undergoing allogeneic hematopoietic stem cell transplantation. Hematol Oncol 2015; 33:67–74. [DOI] [PubMed] [Google Scholar]
- 30. Kang SH, Park HS, Sun IO, Choi SR, Chung BH, Choi BS, et al. Changes in renal function in long-term survivors of allogeneic hematopoietic stem-cell transplantation: single-center experience. Clin Nephrol 2012; 77:225–30. [DOI] [PubMed] [Google Scholar]
- 31. Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bagshaw SM, George C, Bellomo R; ANZICS Database Management Committee. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant 2008; 23:1569–74. [DOI] [PubMed] [Google Scholar]
- 33. Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C.. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 2006; 34:1913–7. [DOI] [PubMed] [Google Scholar]
- 34. Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 2013; 61:649–72. [DOI] [PubMed] [Google Scholar]
- 35. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–88. [DOI] [PubMed] [Google Scholar]
- 36. Higgins JP, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR.. Publication bias in clinical research. Lancet 1991; 337:867–72. [DOI] [PubMed] [Google Scholar]
- 38. Merouani A, Shpall EJ, Jones RB, Archer PG, Schrier RW.. Renal function in high dose chemotherapy and autologous hematopoietic cell support treatment for breast cancer. Kidney Int 1996; 50:1026–31. [DOI] [PubMed] [Google Scholar]
- 39. Gruss E, Bernis C, Tomas JF, Garcia-Canton C, Figuera A, Motellon JL, et al. Acute renal failure in patients following bone marrow transplantation: prevalence, risk factors and outcome. Am J Nephrol 1995; 15:473–9. [DOI] [PubMed] [Google Scholar]
- 40. Parikh CR, McSweeney PA, Korular D, Ecder T, Merouani A, Taylor J, et al. Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int 2002; 62:566–73. [DOI] [PubMed] [Google Scholar]
- 41. Parikh CR, Sandmaier BM, Storb RF, Blume KG, Sahebi F, Maloney DG, et al. Acute renal failure after nonmyeloablative hematopoietic cell transplantation. J Am Soc Nephrol 2004; 15:1868–76. [DOI] [PubMed] [Google Scholar]
- 42. Liu H, Ding JH, Liu BC, Zhao G, Chen BA.. Early renal injury after nonmyeloablative allogeneic peripheral blood stem cell transplantation in patients with chronic myelocytic leukemia. Am J Nephrol 2007; 27:336–41. [DOI] [PubMed] [Google Scholar]
- 43. Kersting S, Dorp SV, Theobald M, Verdonck LF.. Acute renal failure after nonmyeloablative stem cell transplantation in adults. Biol Blood Marrow Transplant 2008; 14:125–31. [DOI] [PubMed] [Google Scholar]
- 44. Kersting S, Koomans HA, Hene RJ, Verdonck LF.. Acute renal failure after allogeneic myeloablative stem cell transplantation: retrospective analysis of incidence, risk factors and survival. Bone Marrow Transplant 2007; 39:359–65. [DOI] [PubMed] [Google Scholar]
- 45. Helal I, Byzun A, Rerolle JP, Morelon E, Kreis H, Bruneel-Mamzer MF.. Acute renal failure following allogeneic hematopoietic cell transplantation: incidence, outcome and risk factors. Saudi J Kidney Dis Transpl 2011; 22:437–43. [PubMed] [Google Scholar]
- 46. Bao YS, Xie RJ, Wang M, Feng SZ, Han MZ.. An evaluation of the RIFLE criteria for acute kidney injury after myeloablative allogeneic haematopoietic stem cell transplantation. Swiss Med Wkly 2011; 141:w13225. [DOI] [PubMed] [Google Scholar]
- 47. Morito T, Ando M, Tsuchiya K, Nitta K.. [Early identification of acute kidney injury after hematopoietic stem cell transplantation by the measurement of urinary biomarkers]. Nihon Jinzo Gakkai Shi 2011; 53:1150–8. [PubMed] [Google Scholar]
- 48. Mori J, Ohashi K, Yamaguchi T, Ando M, Hirashima Y, Kobayashi T, et al. Risk assessment for acute kidney injury after allogeneic hematopoietic stem cell transplantation based on Acute Kidney Injury Network criteria. Intern Med 2012; 51:2105–10. [DOI] [PubMed] [Google Scholar]
- 49. Sehgal B, George P, John MJ, Samuel C.. Acute kidney injury and mortality in hematopoietic stem cell transplantation: a single-center experience. Indian J Nephrol 2017; 27:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Deger SM, Erten Y, Suyani E, Aki SZ, Ulusal Okyay G, Pasaoglu OT, et al. Early diagnostic markers for detection of acute kidney injury in allogeneic hematopoietic stem cell transplant recipients. Exp Clin Transplant 2017; doi: 10.6002/ect.2016.0161. [DOI] [PubMed] [Google Scholar]
- 51. Khalil AA, Khalil LT, Awidi A.. Incidence, risk factors and prognosis of acute kidney injury following hematopoietic stem cell transplant: a pilot study. Int J Stem Cells 2019; 12:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pinana JL, Perez-Pitarch A, Garcia-Cadenas I, Barba P, Hernandez-Boluda JC, Esquirol A, et al. A time-to-event model for acute kidney injury after reduced-intensity conditioning stem cell transplantation using a tacrolimus- and sirolimus-based graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant 2017; 23:1177–85. [DOI] [PubMed] [Google Scholar]
- 53. Schrier RW, Parikh CR.. Comparison of renal injury in myeloablative autologous, myeloablative allogeneic and non-myeloablative allogeneic haematopoietic cell transplantation. Nephrol Dial Transplant 2005; 20:678–83. [DOI] [PubMed] [Google Scholar]
- 54. Lopes JA, Jorge S, Silva S, de Almeida E, Abreu F, Martins C, et al. An assessment of the RIFLE criteria for acute renal failure following myeloablative autologous and allogeneic haematopoietic cell transplantation. Bone Marrow Transplant 2006; 38:395. [DOI] [PubMed] [Google Scholar]
- 55. Yakushijin K, Fukuda T, Asakura Y, Kurosawa S, Hiramoto N, Nakamura D, et al. Renal complications after Busulfan-based reduced-intensity stem cell transplantation in 286 patients with hematological disorders. Blood 2009; 114:3353–3353. [Google Scholar]
- 56. Tokgoz B, Kocyigit I, Polat G, Eser B, Unal A, Kaynar L, et al. Acute renal failure after myeloablative allogeneic hematopoietic stem cell transplantation: incidence, risk factors, and relationship with the quantity of transplanted cells. Ren Fail 2010; 32:547–54. [DOI] [PubMed] [Google Scholar]
- 57. Irazabal MV, Eirin A, Gertz MA, Dispenzieri A, Kumar S, Buadi FK, et al. Acute kidney injury during leukocyte engraftment after autologous stem cell transplantation in patients with light-chain amyloidosis. Am J Hematol 2012; 87:51–4. [DOI] [PubMed] [Google Scholar]
- 58. Kagoya Y, Kataoka K, Nannya Y, Kurokawa M.. Pretransplant predictors and posttransplant sequels of acute kidney injury after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2011; 17:394–400. [DOI] [PubMed] [Google Scholar]
- 59. Duarte P, Duarte FB, Barros EM, Castro FQ, Silva Junior G, Daher E. Clinical: Acute kidney injury after autologous bone marrow transplantation in patients using lower dosage di-methyl sulfoxide. Nephrol Dial Transplant 2012; 27- SAP 162:ii348–77. [Google Scholar]
- 60. Canet E, Lengline E, Zafrani L, Peraldi MN, Socie G, Azoulay E.. Acute kidney injury in critically ill allo-HSCT recipients. Bone Marrow Transplant 2014; 49:1121–2. [DOI] [PubMed] [Google Scholar]
- 61. Chapchap EC, Kerbauy LN, Esteves I, Belucci TR, Rodrigues M, Kerbauy FR, et al. Clinical outcomes in allogeneic haematopoietic stem cell transplantation: a comparison between young and elderly patients. Observational study. Eur J Cancer Care (Engl) 2019; 28:e13122. [DOI] [PubMed] [Google Scholar]
- 62. Esposito V, Garlando MA, Catucci D, Colucci M, Torreggiani M, Colombo AA, et al. Risk Factors for AKI in HSCT recipients, a single center experience. Nephrol Dial Transplant 2016; 31:i421–i. [Google Scholar]
- 63. Myhre A, Kolstad A, Holte H, Gedde-Dahl T, Fløisand Y. Acute kidney injury after nonmyeloablative allogenic stem cell transplantation for lymphoma. Bone Marrow Transplant 2017; 52. [Google Scholar]
- 64. Cekdemir D, Atasoyu E, Gulbas Z.. Acute kidney injury developing in the early period in the patients undergoing autolog and allogenic hematopoietic stem cell transplantation. Nephrol Dial Transplant 2018; 33:i25. [Google Scholar]
- 65. Pereira B, Silva M, Hellmeister M, Duarte T, Oliveira V, Murari P, et al. Acute renal injury in patients after bone marrow transplantation due to multiple myeloma. Nephrol Dial Transplant 2018; 33- SP 217:i416–i. [Google Scholar]
- 66. Yu ZP, Ding JH, Chen BA, Liu BC, Liu H, Li YF, et al. Risk factors for acute kidney injury in patients undergoing allogeneic hematopoietic stem cell transplantation. Chin J Cancer 2010; 29:946–51. [DOI] [PubMed] [Google Scholar]
- 67. Pinana JL, Valcarcel D, Martino R, Barba P, Moreno E, Sureda A, et al. Study of kidney function impairment after reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation. A single-center experience. Biol Blood Marrow Transplant 2009; 15:21–9. [DOI] [PubMed] [Google Scholar]
- 68. Fadia A, Casserly LF, Sanchorawala V, Seldin DC, Wright DG, Skinner M, et al. Incidence and outcome of acute renal failure complicating autologous stem cell transplantation for AL amyloidosis. Kidney Int 2003; 63:1868–73. [DOI] [PubMed] [Google Scholar]
- 69. Izzedine H, Launay-Vacher V, Deray G.. Antiviral drug-induced nephrotoxicity. Am J Kidney Dis 2005; 45:804–17. [DOI] [PubMed] [Google Scholar]
- 70. Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ.. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int 2011; 79:33–45. [DOI] [PubMed] [Google Scholar]
- 71. Wolff D, Wilhelm S, Hahn J, Gentilini C, Hilgendorf I, Steiner B, et al. Replacement of calcineurin inhibitors with daclizumab in patients with transplantation-associated microangiopathy or renal insufficiency associated with graft-versus-host disease. Bone Marrow Transplant 2006; 38:445–51. [DOI] [PubMed] [Google Scholar]
- 72. Lopes JA, Jorge S, Silva S, de Almeida E, Abreu F, Martins C, et al. Acute renal failure following myeloablative autologous and allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2006; 38:707–707. [DOI] [PubMed] [Google Scholar]
- 73. Zhou T, Cen XN, Qiu ZX, Ou JP, Wang WS, Xu WL, et al. Clinical analysis of acute renal failure after allogeneic hematopoietic stem cell transplantation. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2009; 17:723–8. [PubMed] [Google Scholar]
- 74. Ikegawa S, Inomata T, Ikeda N, Sugiura H, Kuroi T, Asano T, et al. Successful outcome of allogeneic hematopoietic stem cell transplantation in patients with mild renal dysfunction calculated by creatinin clearance. Blood 2016; 128:4656–4656. [Google Scholar]
- 75. Mughal TI, Ejaz AA, Foringer JR, Coiffier B.. An integrated clinical approach for the identification, prevention, and treatment of tumor lysis syndrome. Cancer Treat Rev 2010; 36:164–76. [DOI] [PubMed] [Google Scholar]
- 76. Zager RA. Acute renal failure in the setting of bone marrow transplantation. Kidney Int 1994; 46:1443–58. [DOI] [PubMed] [Google Scholar]
- 77. Linck D, Basara N, Tran V, Vucinic V, Hermann S, Hoelzer D, et al. Peracute onset of severe tumor lysis syndrome immediately after 4 Gy fractionated TBI as part of reduced intensity preparative regimen in a patient with T-ALL with high tumor burden. Bone Marrow Transplant 2003; 31:935–7. [DOI] [PubMed] [Google Scholar]
- 78. Lopes JA, Jorge S, Neves M.. Acute kidney injury in HCT: an update. Bone Marrow Transplant 2016; 51:755–62. [DOI] [PubMed] [Google Scholar]
- 79. Kemmner S, Verbeek M, Heemann U.. Renal dysfunction following bone marrow transplantation. J Nephrol 2017; 30:201–9. [DOI] [PubMed] [Google Scholar]
- 80. Schrier RW, Wang W.. Acute renal failure and sepsis. N Engl J Med 2004; 351:159–69. [DOI] [PubMed] [Google Scholar]
- 81. Wan L, Bagshaw SM, Langenberg C, Saotome T, May C, Bellomo R.. Pathophysiology of septic acute kidney injury: what do we really know? Crit Care Med 2008; 36:S198–203. [DOI] [PubMed] [Google Scholar]
- 82. Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant 2001; 27:893–8. [DOI] [PubMed] [Google Scholar]
- 83. Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant 2010; 16:157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118:255–67. [DOI] [PubMed] [Google Scholar]
- 85. Carreras E, Bertz H, Arcese W, Vernant JP, Tomas JF, Hagglund H, et al. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukemia Working Party. Blood 1998; 92:3599–604. [PubMed] [Google Scholar]
- 86. DeLeve LD, Shulman HM, McDonald GB.. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis 2002; 22:027–42. [DOI] [PubMed] [Google Scholar]
- 87. Fink JC, Cooper MA, Burkhart KM, McDonald GB, Zager RA.. Marked enzymuria after bone marrow transplantation: a correlate of veno-occlusive disease-induced “hepatorenal syndrome”. J Am Soc Nephrol 1995; 6:1655–60. [DOI] [PubMed] [Google Scholar]
- 88. Olsen KM, Rudis MI, Rebuck JA, Hara J, Gelmont D, Mehdian R, et al. Effect of once-daily dosing vs. multiple daily dosing of tobramycin on enzyme markers of nephrotoxicity. Crit Care Med 2004; 32:1678–82. [DOI] [PubMed] [Google Scholar]
- 89. Zager RA, O'Quigley J, Zager BK, Alpers CE, Shulman HM, Gamelin LM, et al. Acute renal failure following bone marrow transplantation: a retrospective study of 272 patients. Am J Kidney Dis 1989; 13:210–6. [DOI] [PubMed] [Google Scholar]
- 90. Labrador J, Lopez-Corral L, Lopez-Godino O, Vazquez L, Cabrero-Calvo M, Perez-Lopez R, et al. Risk factors for thrombotic microangiopathy in allogeneic hematopoietic stem cell recipients receiving GVHD prophylaxis with tacrolimus plus MTX or sirolimus. Bone Marrow Transplant 2014; 49:684–90. [DOI] [PubMed] [Google Scholar]
- 91. Wanchoo R, Bayer RL, Bassil C, Jhaveri KD.. Emerging concepts in hematopoietic stem cell transplantation-associated renal thrombotic microangiopathy and prospects for new treatments. Am J Kidney Dis 2018; 72:857–65. [DOI] [PubMed] [Google Scholar]
- 92. Cho BS, Yahng SA, Lee SE, Eom KS, Kim YJ, Kim HJ, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation 2010; 90:918–26. [DOI] [PubMed] [Google Scholar]
- 93. Changsirikulchai S, Myerson D, Guthrie KA, McDonald GB, Alpers CE, Hingorani SR.. Renal thrombotic microangiopathy after hematopoietic cell transplant: role of GVHD in pathogenesis. Clin J Am Soc Nephrol 2009; 4:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Keir L, Coward RJ.. Advances in our understanding of the pathogenesis of glomerular thrombotic microangiopathy. Pediatr Nephrol 2011; 26:523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.