Abstract
Introduction
With the advancement of technology, peripheral nerve stimulation (PNS) has been increasingly used to treat various chronic pain conditions. Its origin is based on the gate control theory postulated by Wall and Melzack in 1965. However, the exact mechanism behind PNS’ analgesic effect is largely unknown. In this article, we performed a comprehensive literature review to overview the PNS mechanism of action.
Design
A comprehensive literature review on the mechanism of PNS in chronic pain.
Methods
Comprehensive review of the available literature on the mechanism of PNS in chronic pain. Data were derived from database searches of PubMed, Scopus, and the Cochrane Library and manual searches of bibliographies and known primary or review articles.
Results
Animal, human, and imaging studies have demonstrated the peripheral and central analgesic mechanisms of PNS by modulating the inflammatory pathways, the autonomic nervous system, the endogenous pain inhibition pathways, and involvement of the cortical and subcortical areas.
Conclusions
Peripheral nerve stimulation exhibits its neuromodulatory effect both peripherally and centrally. Further understanding of the mechanism of PNS can help guide stimulation approaches and parameters to optimize the use of PNS.
Keywords: Peripheral Nerve Stimulation, Chronic Pain, Mechanism
Introduction
Electrical stimulation of peripheral nerves is widely used in various medical settings, ranging from testing of neuromuscular function and somatic nerve stimulation for treatment of paralysis to vagal nerve stimulation for treatment of intractable epilepsy and refractory depression [1–4]. Due to recent technological advancements, there has been an increasing interest in the utility of peripheral nerve stimulation (PNS) for pain control. While the neuromodulatory effect of PNS was first explored in 1965, its principles share similarities with acupuncture and transcutaneous electrical nerve stimulation (TENS), which have long been used for pain control before the invention of PNS. Its origin is based on the gate control theory postulated by Melzack and Wall in 1965, in which innocuous sensory input carried by large Aβ fibers disrupts transmission of nociceptive input from small pain fibers [5]. In 1967, Wall and Sweet then demonstrated that nonpainful electrical stimulation of a peripheral nerve does indeed suppress pain perception by delivering electrical stimulation to the infraorbital nerves via percutaneous needle electrodes [6]. Now, PNS is used in many chronic pain conditions including peripheral nerve injury, complex regional pain syndrome, phantom limb pain, and even fibromyalgia [7–10]. Miniaturized devices that are less invasive than previous generations have also brought this treatment modality into mainstream use. Despite the increasingly common use of PNS, the exact mechanism behind its analgesic effect is largely unknown. In this article, we performed a comprehensive literature review to overview the PNS mechanism of action.
Methods
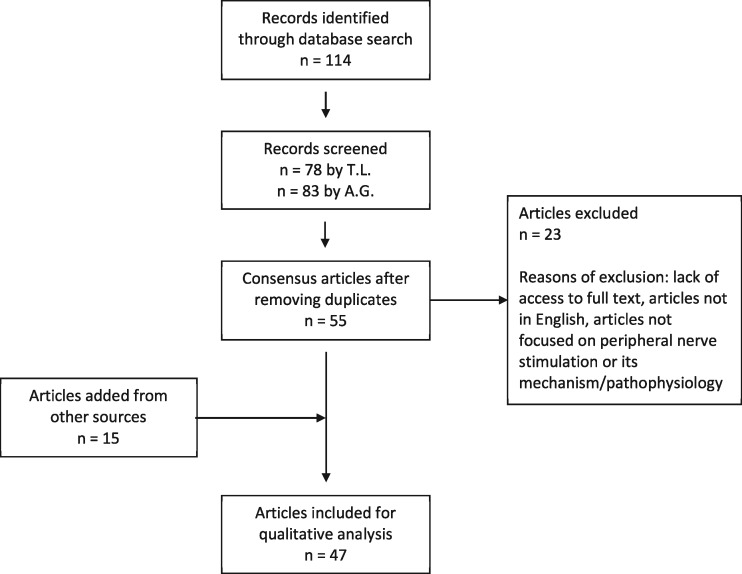
This review utilized the PubMed, Scopus, and Cochrane Library databases using the search terms “peripheral nerve stimulation,” “mechanism,” AND “pain.” One hundred fourteen references including original studies, case reports, and review articles were found. The references were then independently screened by two authors (TL and AG). Articles with literature related to the mechanism of PNS specifically were included. Only PNS modalities with extraspinal targets were included. Therefore, dorsal root ganglion and visceral peripheral targets for stimulation were excluded from our search criteria. After deliberation of those articles not in initial agreement, 55 consensus articles were identified for detailed review. Additional articles were derived from manual searches of bibliographies and known primary or review articles. Figure 1 shows a PRISMA flow chart of the review process.
Figure 1.
PRISMA flow diagram of systematic literature search using PubMed, Scopus, and the Cochrane Library.
Results
Foundation of Peripheral Nerve Stimulation
The following articles were obtained and referenced below, with a summary of findings in Table 1.
Table 1.
Pathways involved in the mechanism of peripheral nerve stimulation
| Studies | Subjects | Targeted Nerves | Pathways Involved | |
|---|---|---|---|---|
| EA | ||||
| Goldman, 2010 [13] | Animal | N/A | Adenosine A1 receptor | |
| Hu, 2017 [14] | Animal | N/A | p38MAPK | |
| Dong, 2005 [15] | Animal | N/A | Somatostatin | |
| TENS | ||||
| Sluka, 2005 [18] | Animal | N/A | Delta-opioid receptors, glutamate, aspartate | |
| Maeda, 2007 [19] | Animal | N/A | GABA | |
| Radhakrishnan, 2003 [20] | Animal | N/A | 5-HT2, 5-HT3 receptors | |
| Han, 1991 [21] | Human | N/A | Met-enkephalin-Arg-Phe, dynorphin-A | |
| Gurgen, 2014 [22] | Animal | N/A | TNFa, IL-1b, IL-6 | |
| Silva, 2014 [23] | Human | N/A | Parietal cortex | |
| PNS | ||||
| Torebjork, 1974 [25] | Human | Radial nerve, saphenous nerve | Excitation failure in peripheral nerve fibers | |
| Wall, 1974 [26] | Animal | Sciatic nerve neuroma | Silent period after brief antidromic tetanus applied | |
| Swett, 1983 [27] | Animal, human | Radial nerve | Mechanism depends on normal conduction of large diameter fibers | |
| Jeong, 1995 [28] | Animal | Common peroneal nerve, tibial nerve | GABA | |
| Meyer-Friebem, 2019 [29] | Human | Femoral, ulnar, median, radial nerves | Antihyperalgesic effect, endogenous pain inhibition | |
| Sun, 2018 [30] | Animal | Sciatic nerve | Arc, GluA1 | |
| Yang, 2013 [31] | Animal | Tibial nerve | Suppression of wind-up | |
| Chung, 1984 [33] | Animal | Common peroneal nerve, tibial nerve | Spinothalamic tract cells | |
| Ristic, 2008 [34] | Human | Radial nerve | Combination of peripheral and central antinociceptive mechanism | |
| Kupers, 2011 [35] | Human | Various | Somatosensory cortex, anterior cingulate cortex, insular cortex | |
| Bandeira, 2019 [36] | Human | Accessory spinal nerve | Sensorimotor cortex, dorsolateral prefrontal cortex | |
| ONS | ||||
| Lyubashina, 2017 [37] | Animal | Greater occipital nerve | Inhibition of nociceptive processing at the spinal trigeminal nucleus | |
| Storer, 2004 [38] | Animal | Superior sagittal sinus | Inhibition of trigeminocervical nucleus | |
| Walling, 2017 [39] | Animal | Greater occipital nerve | Decreased activity in ventral posteromedial nucleus of thalamus | |
| Matharu, 2004 [40] | Human | Greater occipital nerve | Dorsal rostral pons, anterior cingulate cortex, cuneus, left pulvinar | |
| Kovacs, 2009 [43] | Human | Greater occipital nerve | Decreased activity in bilateral primary visual, auditory, and somatosensory cortices, amygdala; increased activity in bilateral thalamus, frontal, parietal areas and cerebellum | |
| VNS | ||||
| Henry, 1999 [46] | Human | Vagus nerve | Bilateral thalami | |
| Henry, 2004 [47] | Human | Vagus nerve | Bilateral thalami, hypothalami, inferior cerebellar hemispheres, right postcentral gyrus |
EA = electroacupuncture; ONS = occipital nerve stimulation; PNS = peripheral nerve stimulation; TENS = transcutaneous electrical nerve stimulation; VNS = vagal nerve stimulation.
Electroacupuncture
Acupuncture has been practiced in China for >2,000 years to treat a variety of illnesses based on the meridian theory. Acupoints have been shown to overlie major neuronal bundles, which correlate with cutaneous branches of major nerves [11]. These nerves converge and interact with visceral nociceptive inputs at the spinal cord level. For example, spinal nerves that carry cutaneous branches to the thorax and abdomen stem from the same spinal segments that receive nociceptive afferent input from splanchnic organs [11]. This anatomical correlation provides the basis on which acupuncture applied to a specific region could treat a variety of conditions remote to the site of treatment.
On a molecular level, acupuncture needles, either manipulated manually or stimulated using a low current and frequency in the case of electroacupuncture (EA), have been documented to modulate the activity of peripheral and central neural pathways. Peripherally, EA activates the sympathetic nervous system (SNS), which increases adhesion of immune cells to the blood vessels, stimulates opioid release from adrenergic receptors and fibroblasts, upregulates cannabinoid CB2 receptors, and inhibits COX2 [12]. Increased adenosine-mediated activation of antinociceptive ascending pathways has been also demonstrated [13].
Centrally, EA interacts with neural transmission of analgesia via modulation of the SNS and the inflammatory pathway, effectively inhibiting central sensitization. Norepinephrine activation by EA stimulates ɑ-2a receptors and the serotonergic pathway, which downregulates phosphorylation of GluN1 and thus the expression of NMDA receptors [12]. Levels of pro-inflammatory cytokines including interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF) ɑ are decreased in the spinal cord in setting of EA [12]. IL-1β enhances GluN1 phosphorylation, whereas TNFɑ promotes NMDA activity in neurons in the lamina II of the spinal cord. Furthermore, EA has been demonstrated to decrease p38MAPK phosphorylation in the spinal dorsal horn, periaqueductal gray, and rostral ventromedial medulla [14]. p38MAPK is involved in intracellular signaling pathways that promote transcription of TNFɑ, IL-1, and COX-2. Lastly, EA has also been shown to increase expression of somatostatin and its precursor in the spinal cord [15].
Transcutaneous Electrical Nerve Stimulation
Transcutaneous electrical nerve stimulation (TENS) involves direct application of electrical current of various frequencies to the surface of the skin. The analgesic effect of TENS is mediated via different mechanisms depending on the type of stimulus applied. Conventional TENS at high frequency (10–200 Hz) and low intensity (5–10 mA) produces a strong and comfortable paresthesia sensation and stimulates the large myelinated Aβ fibers, modulating pain at a spinal cord level via the gate control theory [16,17]. It has also been shown to cause δ-opioidergic blockade of glutamate and aspartate and to increase serum and cerebrospinal fluid (CSF) enkephalins and β-endorphins [12,18]. Acupuncture-like TENS at low frequency (4-20Hz) and high intensity (15–60 mA) produces strong and uncomfortable muscle contractions and stimulates the Aδ fibers, modulating pain by inducing endogenous opioid expression and depression of nociceptive pathways via the μ-opioid receptors, GABA, serotonin, and muscarinic receptors [12,19,20]. An increased level of dynorphin A in the CSF has been demonstrated in humans after high-frequency TENS, with its effect reversed by kappa receptor antagonist in animal studies [21]. Burst TENS, where higher-frequency stimulation is delivered at a rate of two to three bursts per second, stimulates both the Aβ and Aδ fibers, producing analgesia via both the gate control mechanism and the endogenous opioid pathway [17].
TENS has been shown to promote wound healing by its effects on temperature, blood flow, and reduction in proinflammatory cytokines including IL-1, IL-6, and TNFɑ [22]. The analgesic effect of TENS persists even when applied to the contralateral side of injury, demonstrating its central mechanism [12]. Electrophysiological studies have also demonstrated decreased slow and fast alpha waveforms in the parietal cortex during TENS, an area responsible for orientation and attention toward painful sensory stimuli [23].
Peripheral Nerve Stimulation
Peripheral nerve stimulation involves electrical stimulation of a specific nerve trunk via implanted subcutaneous electrodes targeting a named nerve. Its mechanism is based on the postulated gate control theory proposed by Melzack and Wall in 1965 [5]. Stimulation of large-diameter low-threshold non-nociceptive Aβ fibers results in excitation of inhibitory dorsal horn interneurons that are involved in the processing and transmission of nociceptive information from the Aδ and C nerve fibers, thus inhibiting pain signal transmission from the spinal cord to higher centers in the central nervous system (CNS) [24]. Despite increasing use, the exact mechanism of PNS is unknown. However, studies done in the last several decades have proposed several potential pathways.
Peripheral Mechanism
Studies have demonstrated that PNS disrupts transmission of nociceptive afferent fibers at a peripheral level. A human study by Torebjork and Hallin demonstrated that repeated electrical stimulation of intact radial nerves and saphenous nerves resulted in excitation failure of A and C fibers [25]. In rat models, experimentally induced neuromas containing an abundance of hyperirritable small myelinated fibers also showed prolonged silent periods after a brief antidromic tetanus was applied to the ganglion of the neuroma containing the sciatic nerve [26]. However, in a human and animal study, Swett found that the analgesic effect of PNS occurred with stimulus intensities above the threshold of perception but below the threshold for pain, arguing against the theory that PNS exerts its effect by disrupting nociceptive afferent nerve conduction [27]. On a molecular level, PNS has been shown to modulate the biochemistry of the local microenvironment by downregulating neurotransmitters, endorphins, and local inflammatory mediators [24]. Electrophysiological studies have also demonstrated reduced ectopic discharges with PNS [24].
Central Mechanism
In the spinal cord, animal research has demonstrated that the analgesic effects of PNS may involve the serotonergic (5HT2, 5HT3), GABAergic, and glycinergic pathways [20,28]. PNS has also been shown to improve endogenous pain inhibition by interfering with the interaction of large nociceptive fibers and central pathways at the spinal dorsal level via increased inhibition of dorsal wide dynamic range neurons [29]. In a bone cancer rat model, PNS has been shown to induce Arc protein expression in the spinal cord dorsal horn, which inhibits AMPAR, a receptor that facilitates neuropathic, inflammatory, and bone cancer pain [30]. Furthermore, PNS decreases central sensitization and hyperalgesia by reducing excessive peripheral nociceptive activity in the spinal cord, inhibiting wide dynamic range neurons in the dorsal horn, and decreasing Aβ fiber–induced activity in the medial lemniscal pathway in the brain [29,31,32]. Repetitive PNS in high-intensity and low-frequency (30 Hz every 10 seconds) pulses inhibits the spinothalamic tract cells as well [33].
PNS most likely exhibits its analgesic effect in a combination of peripheral and central mechanisms. An experiment done by Ristic et al. on 23 volunteers demonstrated late cortical laser-evoked potential amplitude regardless of PNS location. However, N2 latency and laser ratings were increased exclusively with ipsilateral PNS [34]. The divergent and common effects of ipsilateral vs contralateral PNS suggest a combination of peripheral and central antinociceptive mechanisms [34].
Imaging Studies
In positron emission tomography (PET) studies, peripheral nerve stimulation has been demonstrated to increase cerebral blood flow in the contralateral primary somatosensory cortex and other pain-modulating areas including the anterior cingulate and insular cortices, antero-ventral insula, and thalamus [35]. These findings further indicate that PNS modulates supraspinal areas beyond the dorsal columns. Other brain imaging studies have used functional near infrared spectroscopy (fNIRS) and functional magnetic resonance imaging (fMRI) to measure the effect of PNS on nerves such as the accessory spinal nerve [36]. These studies have shown that electrical stimulation to peripheral nerves activates critical cortical areas related to sensory–discriminative and affective–motivational pain dimensions, similar to noninvasive brain stimulation for pain [36].
Craniofacial PNS
Occipital Nerve Stimulation
Occipital nerve stimulation (ONS) is extensively studied and has provided useful insight into the analgesic mechanism of PNS. The greater occipital nerve (GON) enters the C2 spinal cord at the level of the nucleus caudalis of the trigeminal nerve, forming the trigeminocervical complex. The nucleus caudalis then projects to the thalamus, which relays sensory information to the cortex [10]. ONS has been successfully used in many headache disorders including migraine and trigeminal neuralgia. In a rat model, Lyubashina et al. demonstrated that GON stimulation inhibits nociceptive processing at the spinal trigeminal nucleus [37]. It was also found that ONS stimulates central pain-processing regions outside of the nerve distribution [38]. In PET studies, Matharu et al. showed that migraine patients had changes in cerebral blood flow in the dorsal rostral pons, anterior cingulate cortex, and cuneus in response to suboccipital stimulation [39]. Other areas that may also be involved in pain modulation for headaches include the thalamus, hypothalamus, orbitofrontal cortex, amygdala, and cerebral reward areas such as the ventral striatum and nucleus accumbens [40–42].
ONS has also been recently used to treat fibromyalgia due to its central analgesic properties [10]. It has been postulated that C2 stimulation can modulate autonomic nervous system (ANS) involvement in fibromyalgia as most of the brain structures that regulate the ANS are connected monosynaptically to neurons at the C2 level [10]. Also, PET and electroencephalogram (EEG) studies have shown changes in the anterior cingulate with ONS. Therefore, it is possible that ONS can interfere with the dopaminergic modulation of the area, which has been shown to be implicated in the pathophysiology of fibromyalgia [10].
Vagal Nerve Stimulation
The vagus nerve is an important modulator in the autonomic nervous system and the innate immune response and inflammatory pathways [12]. It carries the afferent signals from peripheral inflammation to the brainstem/CNS and thus provides the circuitry to link the peripheral nervous system with the CNS [43]. Vagus nerve stimulation (VNS) attenuates the inflammatory response through activation of the cholinergic anti-inflammatory pathway (CAP) [12]. CAP follows the path of the vagus afferent nerve, which synapses at the nucleus tractus solitarius and projects to the dorsal motor nucleus and parabrachial nucleus, with further projections to the amygdala, hypothalamus, and limbic system. The efferent limb of CAP then projects from the dorsal motor nucleus to the celiac-superior mesenteric plexus ganglion, splenic nerve, and the spleen, stimulating the hypothalamus–pituitary–adrenal axis and the SNS [12,43].
Cervical VNS has been shown to reduce pain response by modulating peripheral and central nociceptive functions. Peripherally, it inhibits the inflammatory cascades, resulting in reduced TNFɑ, IL-1β, IL-18, HMGB1 protein, and other cytokines [12,43]. Centrally, imaging studies have demonstrated that chronic VNS decreases thalamic activity [44–46]. VNS has shown promising results in treating migraine, in which it decreases pain-induced activation of neurons in the trigeminal nucleus caudalis, reducing pain symptoms and trigeminal allodynia [47]. It has also shown benefit in other chronic inflammatory diseases including rheumatoid arthritis and Crohn’s disease [12].
Limitations
This is a literature review on the mechanism of peripheral nerve stimulation. Clinical studies were not included in this article. Most of the studies presented were animal studies. Further research on human models is required to investigate this correlation. Different waveforms of peripheral nerve stimulation and their differential mechanisms of action were not discussed.
Conclusions
Peripheral nerve stimulation is an established neuromodulatory approach for treatment of chronic pain. Its origin is based on the gate control theory by Wall and Melzack from 1965. However, further studies have demonstrated PNS’ peripheral and central analgesic mechanisms by modulating the inflammatory pathways, the autonomic nervous system, the endogenous pain inhibition pathways, and involvement of the cortical and subcortical areas. Further understanding of the mechanism of PNS can help guide stimulation approaches and parameters to optimize the use of PNS.
Funding sources: This work was supported, in part, by the National Cancer Institute Cancer Center Support Grant P30CA008748.
Disclosure and conflicts of interest: Consultant, Medtronic; Advisory Board, AIS HealthCare; Consultant, Flowonix Medical Inc.; Consultant, Nalu Medical Inc.; Consultant, SPR Therapeutics.
Supplement sponsorship: This article appears as part of the supplement entitled “Peripheral Nerve Stimulation: Update for the 21st Century” sponsored by Bioness and by SPR Therapeutics, Inc.
References
- 1. Slavin KV. History of peripheral nerve stimulation In: Slavin KV, ed. Peripheral Nerve Stimulation. Basel: Krager; 2011:1–15. [DOI] [PubMed] [Google Scholar]
- 2. Rozand V, Grosprêtre S, Stapley PJ, et al. Assessment of neuromuscular function using percutaneous electrical nerve stimulation. J Vis Exp 2015;(103):52974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carrico C, Chelette KC 2nd, Westgate PM, Salmon-Powell E, Nichols L, Sawaki L.. Randomized trial of peripheral nerve stimulation to enhance modified constraint-induced therapy after stroke. Am J Phys Med Rehabil 2016;95(6):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Milby AH, Halpern CH, Baltuch GH.. Vagus nerve stimulation for epilepsy and depression. Neurotherapeutics 2008;5(1):75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Melzack R, Wall PD.. Pain mechanism: A new theory. Science 1965;150(3699):971–9. [DOI] [PubMed] [Google Scholar]
- 6. Wall PD, Sweet WH.. Temporary abolition of pain in man. Science 1967;155(3758):108–9. [DOI] [PubMed] [Google Scholar]
- 7. Campbell JN, Taub A.. Local analgesia from percutaneous electrical stimulation: A peripheral mechanism. Arch Neurol 1973;28(5):347–50. [DOI] [PubMed] [Google Scholar]
- 8. Fritz A, Ferreira-Dos-Santos G, Hurdle M, Clendenen S.. Ultrasound-guided percutaneous peripheral nerve stimulation for the treatment of complex regional pain syndrome type 1 following a crush injury to the fifth digit: A rare case report. Cureus 2019;11(12):e6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilmore C, Ilfeld B, Rosenow J, et al. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic postamputation pain: A multicenter, randomized, placebo-controlled trial. Reg Anesth Pain Med 2019;44(6):637–45. [DOI] [PubMed] [Google Scholar]
- 10. Plazier M, Vanneste S, Dekelver I, et al. Peripheral nerve stimulation for fibromyalgia. In: Slavin KV, ed. Peripheral Nerve Stimulation. Progress in Neurological Surgery. Basel: Krager 2011;24:133–46. [DOI] [PubMed] [Google Scholar]
- 11. Zhou W, Benharash P.. Effects and mechanisms of acupuncture based on the principle of meridians. J Acupunct Meridian Stud 2014;7(4):190–3. [DOI] [PubMed] [Google Scholar]
- 12. Chakravarthy KV, Xing F, Bruno K, et al. A review of spinal and peripheral neuromodulation and neuroinflammation: Lessons learned thus far and future prospects of biotype development. Neuromodulation 2019;22(3):235–43. [DOI] [PubMed] [Google Scholar]
- 13. Goldman N, Chen M, Fujita T, et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci 2010;13(7):883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu ML, Zhou FY, Liu JJ, et al. Electroacupuncture inhibits the activation of p38MAPK in the central descending facilitatory pathway in rats with inflammatory pain. Evid Based Complement Alternat Med 2017;2017:7531060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dong Z, Xie H, Ma F, Li W, Wang Y, Wu G.. Effects of electroacupuncture on expression of somatostatin and preprosomatostatin mRNA in dorsal root ganglions and spinal dorsal horn in neuropathic pain rats. Neurosci Lett 2005;385(3):189–94. [DOI] [PubMed] [Google Scholar]
- 16. Coutaux A. Non-pharmacological treatments for pain relief: TENS and acupuncture. Joint Bone Spine 2017;84(6):657–61. [DOI] [PubMed] [Google Scholar]
- 17. Claydon LS, Chesterton LS, Barlas P, Sim J.. Dose-specific effects of transcutaneous electrical nerve stimulation (TENS) on experimental pain: A systematic review. Clin J Pain 2011;27(7):635–47. [DOI] [PubMed] [Google Scholar]
- 18. Sluka K, Vance C, Lisi T.. High-frequency, but not low-frequency, transcutaneous electrical nerve stimulation reduces aspartate and glutamate release in the spinal cord dorsal horn. J Neurochem 2005;95(6):1794–801. [DOI] [PubMed] [Google Scholar]
- 19. Maeda Y, Lisi T, Vance C, Sluka K.. Release of GABA and activation of GABAA in the spinal cord mediates the effects of TENS in rats. Brain Res 2007;1136:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Radhakrishnan R, King E, Dickman J, et al. Spinal 5-HT2 and 5-HT3 receptors mediate low, but not high, frequency TENS-induced antihyperalgesia in rats. Pain 2003;105(1):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han JS, Chen XH, Sun SL, et al. Effect of low- and high-frequency TENS on Met-enkephalin-Arg-Phe and dynorphin A immunoreactivity in human lumbar CSF. Pain 1991;47(3):295–8. [DOI] [PubMed] [Google Scholar]
- 22. Gurgen SG, Sayin O, Cetin F, Tuc Yucel A. Transcutaneous electrical nerve stimulation (TENS) accelerates cutaneous wound healing and inhibits pro-inflammatory cytokines. Inflammation 2014;37:775–84. [DOI] [PubMed] [Google Scholar]
- 23. Silva JG, Santana CG, Inocencio KR, Orsini M, Machado S, Bergmann A.. Electrocortical analysis of patients with intercostobrachial pain treated with TENS after breast cancer surgery. J Phys Ther Sci 2014;26(3):349–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deer TR, Jain S, Hunter C, et al. Neurostimulation for intractable chronic pain. Brain Sci 2019;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torebjork HE, Hallin RG.. Responses in human A and C fibres to repeated electrical intradermal stimulation. J Neurol Neurosurg Psychiatry 1974;37(6):653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wall PD, Gutnick M.. Properties of afferent nerve impulses originating from a neuroma. Nature 1974;248(5451):740–3. [DOI] [PubMed] [Google Scholar]
- 27. Swett JE, Law JD.. Analgesia with peripheral nerve stimulation: Absence of a peripheral mechanism. Pain 1983;15(1–4):55–70. [Google Scholar]
- 28. Jeong Y, Baik E, Nam T, Paik K.. Effects of iontophoretically applied naloxone, picrotoxin and strychnine on dorsal horn neuron activities treated with high frequency conditioning stimulation in cats. Yonsei Med J 1995;36(4):336–47. [DOI] [PubMed] [Google Scholar]
- 29. Meyer-Friebem CH, Wiegand T, Eitner L, et al. Effects of spinal cord and peripheral nerve stimulation reflected in sensory profiles and endogenous pain modulation. Clin J Pain 2019;35(2):111–20. [DOI] [PubMed] [Google Scholar]
- 30. Sun KF, Feng WW, Liu YP, et al. Electrical peripheral nerve stimulation relieves bone cancer pain by inducing Arc protein expression in the spinal cord dorsal horn. J Pain Res 2018;11:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang F, Zhang T, Tiwari V, et al. Effects of combined electrical stimulation of the dorsal column and dorsal roots on wide-dynamic-range neuronal activity in nerve-injured rats. Neuromodulation 2015;18(7):592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang F, Xu Q, Cheong Y, et al. Comparison of intensity-dependent inhibition of spinal wide-dynamic range neurons by dorsal column and peripheral nerve stimulation in a rat model of neuropathic pain. Eur J Pain 2014;18(7):978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chung J, Fang Z, Hori Y, Lee K, Willis W.. Prolonged inhibition of primate spinothalamic tract cells by peripheral nerve stimulation. Pain 1984;19(3):259–75. [DOI] [PubMed] [Google Scholar]
- 34. Ristic D, Spangenberg P, Ellrich J.. Analgesic and antinociceptive effects of peripheral nerve neurostimulation in an advanced human experimental model. Eur J Pain 2008;12(4):480–90. [DOI] [PubMed] [Google Scholar]
- 35. Kupers R, Laerel K, Calenberghl F, et al. Multimodal therapeutic assessment of peripheral nerve stimulation in neuropathic pain: Five case reports with a 20-year follow-up. Eur J Pain 2011;15(2):161.e1–9. [DOI] [PubMed] [Google Scholar]
- 36. Bandeira J, Antunes L, Soldatelli M, Sato J, Fregni F, Caumo W.. Functional spectroscopy mapping of pain processing cortical areas during non-painful peripheral electrical stimulation of the accessory spinal nerve. Front Hum Neurosci 2019;13:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lyubashina OA, Panteleev SS, Sokolov A.. Inhibitory effect of high-frequency greater occipital nerve electrical stimulation on trigeminovascular nociceptive processing in rats. J Neural Transm 2017;124(2):171–83. [DOI] [PubMed] [Google Scholar]
- 38. Walling I, Smith H, Gee L, et al. Occipital nerve stimulation attenuates neuronal firing response to mechanical stimuli in the ventral posteromedial thalamus of a rodent model of chronic migraine. Neurosurgery 2017;81(4):696–701. [DOI] [PubMed] [Google Scholar]
- 39. Matharu M. Central neuromodulation in chronic migraine patients with suboccipital stimulators: A PET study. Brain 2004;127(1):220–30. [DOI] [PubMed] [Google Scholar]
- 40. Magis D, Schoenen J.. Neurostimulation in chronic cluster headache. Curr Pain Headache Rep 2008;12(2):145–53. [DOI] [PubMed] [Google Scholar]
- 41. Magis D, Gerardy P, Remacle J, Schoenen J.. Sustained effectiveness of occipital nerve stimulation in drug-resistant chronic cluster headache. Headache 2011;51(8):1191–201. [DOI] [PubMed] [Google Scholar]
- 42. Kovacs S, Peeters R, De Ridder D, Plazier M, Menovsky T, Sunaert S.. Central effects of occipital nerve electrical stimulation studied by functional magnetic resonance imaging. Neuromodulation 2011;14(1):46–57. [DOI] [PubMed] [Google Scholar]
- 43. Johnson RL, Wilson CG.. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res 2018; 11:203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bari A, Pouratian N.. Brain imaging correlates of peripheral nerve stimulation. Surg Neurol Int 2012;3(5):S260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Henry T, Votaw J, Pennell P, et al. Acute blood flow changes and efficacy of vagus nerve stimulation in partial epilepsy. Neurology 1999;52(6):1166–73. [DOI] [PubMed] [Google Scholar]
- 46. Henry T, Bakay R, Pennell P, Epstein C, Votaw J.. Brain blood-flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: II. Prolonged effects at high and low levels of stimulation. Epilepsia 2004;45(9):1064–1070. [DOI] [PubMed] [Google Scholar]
- 47. Lendvai IS, Maier A, Scheele D, et al. Spotlight on cervical vagus nerve stimulation for the treatment of primary headache disorders: A review. J Pain Res 2018;11:1613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]