Abstract
Plant growth and productivity are orchestrated by a network of signaling cascades involved in balancing responses to perceived environmental changes with resource availability. Vascular plants are divided into the shoot, an aboveground organ where sugar is synthesized, and the underground located root. Continuous growth requires the generation of energy in the form of carbohydrates in the leaves upon photosynthesis and uptake of nutrients and water through root hairs. Root hair outgrowth depends on the overall condition of the plant and its energy level must be high enough to maintain root growth. TARGET OF RAPAMYCIN (TOR)-mediated signaling cascades serve as a hub to evaluate which resources are needed to respond to external stimuli and which are available to maintain proper plant adaptation. Root hair growth further requires appropriate distribution of the phytohormone auxin, which primes root hair cell fate and triggers root hair elongation. Auxin is transported in an active, directed manner by a plasma membrane located carrier. The auxin efflux carrier PIN-FORMED 2 is necessary to transport auxin to root hair cells, followed by subcellular rearrangements involved in root hair outgrowth. This review presents an overview of events upstream and downstream of PIN2 action, which are involved in root hair growth control.
Keywords: TOR signaling, auxin, PIN-FORMED 2, root hair growth, polar cell elongation, ROP2, ROS, root growth, plant adaptation
1. Introduction
Plant growth, and, more critically, its adaptation to ever-changing environmental conditions, is maintained by the ability of plants to perceive exogenous signals, to evaluate the availability of resources, and to initiate signaling cascades orchestrating changes in growth rate and direction [1,2,3]. The most crucial aspect of plant fitness is to keep the potential of balancing the amount of cell division in meristematic regions and elongation processes and differentiation to mature cells, which depends on environmental conditions and available resources [2,4]. Efficient growth requires the generation of energy in form of sugars in the leaves of the plant upon photosynthesis, which is followed by the biosynthesis of the phytohormone and growth regulator auxin [2,5,6]. Furthermore, the plant needs to regulate nutrient and water uptake through the root from the soil [7,8]. To enhance uptake efficiency, the root has the ability to grow tubular structures from specialized epidermis cells, the root hairs [7,8]. Root hair cell priming and outgrowth are highly dependent on proper auxin distribution and abundance, whereby both are dependent on environmental conditions and the overall condition of the plant [2,8,9,10,11]. Distribution of the phytohormone auxin through the plant creates auxin gradients, which orchestrate the activity of meristems, adaptational growth via cell expansion, and differentiation of specialized cells [12,13,14,15]. PIN-FORMED (PIN) proteins, auxin efflux carriers, ensure on-point and polar distribution of the phytohormone, whereby auxin signaling underpins the fine-tuning of various cellular processes involved in plant growth adaptation under changing environmental conditions [3,16,17,18]. The role of the root-specific PIN family member PIN2 during root hair growth adaptation in response to environmental conditions will be discussed in particular in the scope of this review. The establishment of auxin gradients is crucial for plant growth from the first asymmetric cell division of the zygote [19,20] and tissue organization [21] through fine-tuning cell proliferation and cell elongation [10,22,23,24,25], which in the end ensures that the shoot will grow above the soil, whereas the root anchors the plant in the soil [1]. Shoot growth is primarily oriented towards the light (positive phototropic, negative gravitropic) to ensure the most efficient exposure to the sun to harvest energy, which is converted to sugar and powers plant growth [1,2,26]. In contrast, the root grows along the gravity vector (positive gravitropic, negative phototropic) to anchor it in the soil, where it absorbs nutrients and water [2,3]. Auxin biosynthesis correlates with the sugar levels produced depending on light quality and quantity perceived by the leaves and transported to the roots [1,27,28]. The available auxin orchestrates the establishment of root system architecture including, among others, the length of the primary root, the number of lateral roots, and the outgrowth of root hairs [3,10,28,29,30]. Auxin is actively transported towards the root meristem, where it modulates root growth depending on the requirements in a balance between environmental stimuli and available resources, such as carbohydrates, minerals, and water [2,31]. The information about the energy status and available resources of the plant are sensed by the TARGET OF RAPAMYCIN (TOR) complex, which then activates cellular responses upstream of root hair outgrowth [2] (Figure 1).
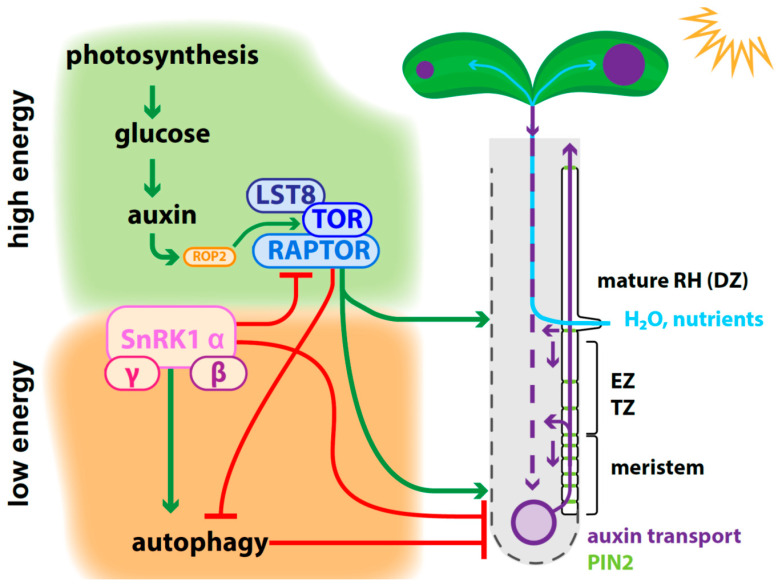
Figure 1.
Energy status and resource uptake are orchestrated by an interplay of TARGET OF RAPAMYCIN (TOR) and SNF1-RELATED KINASE 1 (SnRK1) complexes, which antagonistically modulate root and root hair growth. High energy status results from sugar synthesis derived from photosynthesis in the leaves, which is followed by elevated auxin biosynthesis. Auxin signaling enhances RHO-RELATED PROTEIN FROM PLANTS 2 (ROP2) activity, and ROP2 binds TOR and promotes its phosphorylation. This results in the activation of the TOR complex, which further consists of the substrate-presenting subunit REGULATORY-ASSOCIATED PROTEIN OF TOR (RAPTOR) and the complex stabilizer LETHAL WITH SEC THIRTEEN 8 (LST8), described in Section 2.1. TOR signaling positively correlates with root meristem activity and therefore with root length. TOR stabilizes the auxin efflux carrier PIN2 by phosphorylation at the plasma membrane, and thereby modulates shootward auxin transport. Auxin levels in the meristem enhance the cell proliferation rate and are involved in root hair cell priming (described in Section 3.1). Furthermore, efficient auxin delivery and signaling trigger root hair elongation, which is responsible for root surface enlargement, allowing enhanced uptake of water and nutrients to ensure plant growth and productivity. Under high energy levels, TOR signaling inhibits autophagy, which would trigger the engulfment and recycling of existing plant material to gain resources, and this is accompanied by the end of root growth. When the energy levels in the root drop, reduced sugar availability is sensed over the SNF1-RELATED KINASE 1 (SnRK1) complex, which balances between TOR complex deactivation and autophagy activation. SnRK1 complex activity results in RAPTOR deactivation by phosphorylation, and the downregulation of auxin efflux transporters involved in auxin transport from the shoot to the root, followed by reduced root meristem activity (described in Section 3.2). EZ = elongation zone; TZ = transition zone; DZ = differentiation zone.
2. Plant Growth Is Dynamic and Orchestrated by Interwoven Signaling Cascades Orchestrated by TOR
Successful plant growth and productivity are highly dependent on the establishment and maintenance of polarity on the tissue, cell, and subcellular levels [13,32,33]. Plants evolved a fine-tuned network of intracellular processes to adapt to environmental changes, leading to fast adaptation responses and manifesting in the structural reorganization of plant architecture to ensure proper nutrient distribution and growth [16,25,34]. Manifold signaling cascades are simultaneously active along the plant body, which implement exogenous signals and information of available resources and the energy status of the shoot and the root [2,25]. TARGET OF RAPAMYCIN (TOR) is a core hub among signaling cascades to balance cellular responses upon hormonal and nutrient signaling, including root meristem activity and root hair outgrowth [2,35,36,37,38].
2.1. TOR Complex Acts as a Hub for Signaling Pathways Sensing the Nutrient Status of the Plant
TOR is a Ser/Thr protein kinase, structurally and functionally conserved among all eukaryotes, and acts as a master regulator of transition between cell proliferation and elongation [39,40]. Furthermore, it inhibits autophagy in nutrient-rich conditions [38] (Figure 1). TOR associates with two other proteins in plants, REGULATORY-ASSOCIATED PROTEIN OF TOR (RAPTOR), which presents substrate proteins to TOR, and LETHAL WITH SEC THIRTEEN 8 (LST8), responsible for stabilizing the complex [2,41,42] (Figure 1). TOR signaling networks are crucial to adjusting metabolism rates during plant growth [2,39,43,44,45]. As a crucial part of the glucose signaling network, the TOR complex modulates the activity of transcription factors, such as E2Fα, to enhance root meristem activity [46]. TOR signaling positively regulates the energy-demanding protein translation machinery, but is inhibited when the energy status of the plant drops [11,47]. In elongated starvation, the plant needs to shut down de novo protein synthesis, and furthermore, the TOR inhibitory effect on autophagy is reversed in Arabidopsis thaliana [48], as well in the green alga Chlamydomonas reinhardtii [49,50,51]. When the available resources do not cover the plant’s energy demand anymore, cell material undergoes recycling [41,52,53]. The drop in energy levels of the plant is sensed by the SNF1-RELATED KINASE 1 (SnRK1) complex, which serves as a plant’s metabolic sensor to keep track of carbohydrate level changes [39,47,54]. The heterotrimeric SnRK1 complex is composed of one catalytic and two regulatory subunits [41] (Figure 1). Three isoforms of the catalytic subunit are known to be encoded in the Arabidopsis thaliana genome, but only SnRK1α1/AKIN10/KIN10 is responsible for most of the SnRK1 activity [41,55]. Under nutrient-rich conditions, SnRK1 activity is kept low, but the reduction of available resources leads to the transcriptional upregulation of SnRK1 levels [41,55]. SnRK1 complex activity further results in the activation of autophagy to regain resources [41,54]. Recently, it has been shown that the overexpression of SnRK1α1 leads to the downregulation of the TOR/S6K/RPS6 phosphorylation cascade, while SnRK1α1 suppression causes the opposite effect [47] The TOR/S6K/RPS6 phosphorylation cascade is supposed to be involved in high energy-demanding polysome abundance and protein translation activity as well as stress responses [56,57]. It has been shown that SnRK1α1 physically interacts with RAPTOR and is able to phosphorylate the TOR complex, thereby potentially downregulating its activity [47] (Figure 1). When energy levels rise again, as is the case of elevated photosynthetic activity, rising sugar levels are followed by an enhanced auxin biosynthesis, whereas elevated auxin levels are connected with the activation of TOR by phosphorylation [2,11,46,56] (Figure 1). Besides sugar levels, the availability of amino acids underpins TOR activity, like tryptophan, which is an important precursor for the biosynthesis of auxins [57,58,59,60,61]. TOR signaling and auxin abundance and distribution are tightly interconnected, as auxin levels decrease upon treatment with TOR inhibitors in the root [62]. Among other amino acids, the abundance of cysteine is critical for plant productivity and is also highly dependent on sufficient sulfur uptake [63]. Sulfur depletion is transduced over TOR signaling, resulting in the downregulation of glucose metabolism and the upregulation of autophagy, followed by reduced meristem activity [63]. Sulfate uptake is tightly coupled with carbohydrate and nitrogen metabolism [63], and TOR signaling is thereby a centerpiece of the integration and coordination of light, hormone, and metabolism sensing and signaling upstream of root and shoot architecture adaptation [11].
2.2. TOR Activity Depends on ROP2 Action
TOR activity is also regulated by phosphorylation, which, as described above, correlates with auxin abundance in the presence of glucose [46,64]. Enhanced TOR activity is coupled to increased plant growth rate, fitness, and size [11,65]. In the root, shoot-derived glucose and auxin signaling together activate TOR signaling pathways, leading to the upregulation of cell proliferation in the meristem, which further positively correlates with root hair growth [2,66]. Estradiol-inducible tor-es mutant and raptor1b roots are impaired in glucose-activated root hair elongation [44,46,67]. Furthermore, TOR inhibitor treatments also suggest a link to auxin biosynthesis and signaling in root hair development [11,62]. Auxin activates the TOR complex over RHO-RELATED PROTEIN FROM PLANTS 2 (ROP2) action [45,68] (Figure 1). To date, the interaction between auxin, ROP2, and TOR upstream of TOR signaling on the whole plant level has been well studied [35], but how ROP2 activity and polar auxin transport are precisely regulated during root hair growth initiation depending on TOR signaling must be still determined. In upcoming paragraphs of the review, we will dissect how far the interplay of auxin signaling, TOR activity, and ROP2 mediate root hair outgrowth in more detail [8,69]. Auxin distribution to the root tip and the redistribution towards the root hair zone are, on the other hand, dependent on TOR activity, showing how exogenous and hormonal signals influence each other [2,11].
3. Fine-Tuned Root Hair Outgrowth Ensures Plant Growth and Productivity
Roots are very plastic and can adjust their tissue organization and cell appearance during abiotic stress responses [3,70]. The root is divided into individual zones depending on the cell’s maturation status [2]. The meristematic zone continually delivers new cells, and its activity arrests when the environmental conditions are not beneficial for the plant [2]. After leaving the meristem, cells pass through the transition and elongation zone towards the differentiation zone, undergoing maturation and adaptation steps according to the growth conditions of the whole plant [2]. Epidermis cells of the root can be primed while outgrowing the meristem by hormonal signaling events to become root hair cells [9]. Root hair length and density are crucial to anchor the plant in the soil and to enlarge the surface for nutrient and water uptake [2,71]. Both auxin- and TOR-regulated signaling events are dominant regulators of root development and adaptation, mediating tight communications between shoot and root [2,11,72] (Figure 1).
3.1. Root Hair Cell Priming, Root Hair Positioning, and Elongation Are Regulated by Auxin
Auxin gradients in the root tip prime root hair cells, the trichoblasts, which grow polar expanding, tubular extensions, the root hairs to enlarge the surface of the root [8,73,74,75,76]. The root epidermis is divided into trichoblasts, root hair cells, and atrichoblasts, non-root hair cells [8]. Cell fate establishment requires fine-tuned cellular events, including the activity of specific receptor-like protein kinases and transcriptional regulators, substantially depending on auxin availability and signaling [75,77,78,79], which are described in Section 4. PIN2 facilitates auxin distribution from the very root tip shootwards, over the lateral root cap and epidermis cells, and back to the root tip over the cortex, resembling a reverse fountain [21,80,81]. In the two cell files of the epidermis, the trichoblasts and atrichoblasts, PIN2 undergoes differential intracellular trafficking, showing higher internalization rates, followed by lower protein abundance at the plasma membrane (PM) in trichoblasts [82]. Auxin transport rates differ among the cell files, and it is suspected that the atrichoblasts act as auxin reservoirs for the trichoblasts [83]. Fine-tuned spatial and temporal auxin distribution triggers transcriptional events responsible for priming root hair cells or orchestrating polar root hair outgrowth in mature cells [9,10] (Figure 1). Mutants of key players of auxin signaling and transport show severe root hair morphology, spacing, and length phenotypes [10,18,84,85]. When shootward auxin transport is impaired, root hairs are shorter, and this is visible in the knockout mutants of the auxin efflux carrier PIN2 as well as for the auxin influx carrier AUXIN1 (AUX1) [10,86]. With further impairment of auxin distribution, like in the triple mutant aux1 ethylene-insensitive2 (ein2) gnomeb (gneb), root hairs appear randomly along the root hair cell, and correct positioning can be restored by auxin application rootward of the trichoblast [10,87]. Auxin signaling depending on TIR1/AFB-Aux/IAA action underpins root hair density (initiation) and length (elongation) [86]. Finally, auxin regulates cell wall properties by initiating its loosening, a crucial event during cell elongation, by activating signaling cascades and transport of structural proteins to the PM, which modify cell wall composition [88,89].
3.2. Polar Auxin Transport Is Crucial for On-Point Auxin Distribution
Auxin levels and on-point distribution are modulated by polar auxin transport, biosynthesis, conjugation, perception, and signaling [14,25,90]. Auxin signaling events underpin the reorganization of root hair cells on a subcellular level, including cytoskeleton bundling, vacuolar morphology, cell wall properties, endomembrane trafficking, and membrane composition [25,72,89,91,92,93,94,95]. Polar auxin transport is characterized by active, PM protein-dependent distribution from auxin source to sink, including importers, like the AUXIN1/LIKE-AUX1 (AUX1/LAX) protein family [96,97,98,99] or the nitrate and auxin transporter NITRATE TRANSPORTER 1.1 (NRT1.1) [100,101,102]. AUX1 in the root epidermis is predominantly expressed in non-root hair cells, but its overexpression still enhances root hair outgrowth, while the knockout mutant shows shorter root hairs [9,79,103,104]. Several proteins of the ATP-BINDING CASSETTE SUBFAMILYB-TYPE (ABCB) proteins were found to be involved in auxin efflux and influx [105,106,107,108,109]. ABCB4 and ABCB21 may switch from influx to efflux functions, depending on internal auxin concentrations [110,111,112] and ABCB19 was shown to stabilize PIN1 at the PM [113]. The main occurring auxin in plants is INDOLE-3-ACETIC ACID (IAA), and as a weak acid it requires active export from one cell towards another to maintain long-distance, cell-to-cell transport because it is deprotonated and trapped in the cytoplasm [91,114,115]. Auxin efflux carriers from the PIN family, which are located at the PM, are substantially involved in polarizing auxin flow through the plant body [31,116,117,118,119,120]. Two PIN types can be distinguished by the presence (PIN1, PIN2, PIN3, PIN4, and PIN7) or absence (PIN5 and PIN8) of a long hydrophilic loop in the cytoplasm, whereas PIN6 presents a partially reduced hydrophilic loop [22,81]. PIN1 is involved in the distribution of auxin through the plant and plays a crucial role during embryogenesis [20] and post-embryonic development and growth adaptation [119,121,122,123]. Loss-of-function mutations in PIN1 exhibit a pointed inflorescence stem that fails to produce flowers, emphasizing not only the role of auxin, but also of auxin transport in this process [121,122]. In the root, the redistribution of auxin towards the elongation zone takes place in the root tip and involves the activity of PIN3, PIN4, and PIN7 that localize to the PM of root cap columella cells [81,124,125] Auxin flow continues through PIN2 over the lateral root cap cells and epidermis shootward towards the differentiation zone, and back over the cortex to the root meristem [21,80,81]. Auxin enhances the cell proliferation rate in the root meristem [119,123], and when the energy status of the whole plant drops, auxin distribution from the shoot to the root is reused to stop root growth by downregulating PIN expression over the SnRK1 signaling pathway [126]. A recent study showed that glucose-activated TOR phosphorylates and stabilizes PIN2 at the PM, and thereby enhances shootward auxin transport to the root hair zone [127] (Figure 1 and Figure 2).
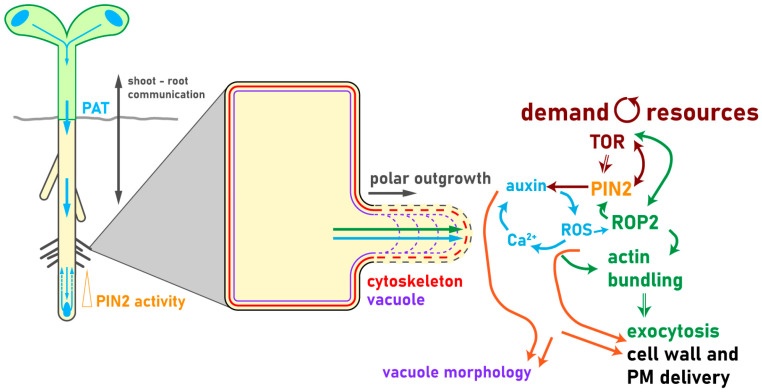
Figure 2.
PIN2 orchestrates shootward polar auxin transport as s mediator between exogenous stimuli and root hair outgrowth.
3.3. Auxin Crosstalk with Other Hormones Is Mandatory to Regulate Root Hair Growth
Root hairs allow a rapid expansion of the root surface to elevate the amount of nutrient and water uptake to adjust plant growth and maximize overall plant fitness. The uptake of inorganic phosphate (Pi) is up to 60 times more efficient when root hairs emerge [128]. Therefore, low Pi availability triggers root hair growth [129] and auxin was linked to the orchestration of root hair growth on Pi-deficient media [79]. Furthermore, the integration of environmental stimuli on root hair growth is highly fine-tuned by hormonal crosstalk with other phytohormones, such as ethylene, strigolactones (SLs), and brassinosteroids (BRs) [86,129,130,131,132,133]. BR signaling was shown to stabilize PIN2 at the PM [132,133,134,135]. Cell elongation is modulated by influencing cell wall biosynthesis and cytoskeleton-related functions [136,137]. Auxin and BR activate overlapping sets of genes involved in synergistic interactions in root elongation [138,139]. Crosstalk between auxin and BR signaling changes on a subcellular level the arrangements of actin cytoskeleton bundles [137]. Proper actin bundling is crucial for the elongation ability of root hairs, as actin2 (act2) mutants possess shorter root hairs [137], as well as the pin2 mutant [140]. BR signaling is indispensable to define trichoblast cell fate by regulating the WEREWOLF/GLABRA3/ ENHANCER OF GLABRA3-TRANSPARENT TESTA GLABRA1 transcriptional complex [141]. SL signaling was also linked to cytoskeleton rearrangement [142] and to increased root hair length and density [143]. Cytokinins and light perception of the root were furthermore linked to an important role in root hair growth. cis-Zeatin is needed for root hair elongation and Pi allocation in the root during Pi starvation, which is masked under light, causing stress and interfering with root growth [144]. Most of the published studies regarding root and root hair growth are performed on roots continuously exposed to light, therefore, more detailed studies of root hair emergence and elongation under more natural growth conditions are needed [144,145].
4. Cellular Events Downstream of Auxin and TOR Signaling Orchestrate Root Hair Growth
Root hairs serve as a model system for polarity in plant cells [146]. They expand upon the fine-tuned delivery of PM and cell wall components to the root hair tip, driven by cytoskeleton rearrangements and exocytosis [146,147,148]. Auxin gradients and signaling (also dependent on PIN2 action), ROP2 positioning, cytoskeleton rearrangements, the signaling molecules REACTIVE OXYGEN SPECIES (ROS) and Ca2+, cell wall remodeling and signaling at PM and cell wall, and, finally, vacuole expansion are well-studied cellular components required for efficient root hair growth and therefore elongation (Figure 2).
Polar auxin transport (PAT) delivers auxin from the shoot to the root depending on the environmental conditions and available resources of the plant (described in Section 3.2). In the root tip, the auxin efflux carrier PIN-FORMED 2 (PIN2) mediates shootward auxin distribution, which is crucial to deliver auxin to the root hair cell. Auxin signaling events underpin subcellular rearrangement that mediates proper root hair tip outgrowth (described in Section 4). Efficient, polar root hair tip elongation primarily depends on the proper establishment of secondary messenger gradients (REACTIVE OXYGEN SPECIES (ROS) and Ca2+, described in Section 4.1), subcellular relocalization of ROP2, and rearrangement of the cytoskeleton (described in Section 4.2). Auxin signaling mediates the gene expression of ROS-producing enzymes, ROP2, and further key elements, keeping a positive feedback loop at the elongating root hair tip plasma membrane (PM). Cell wall modifications, including loosening by ROS and pH changes and the delivery of new cell wall material by exocytosis, are crucial to allow a steady enlargement of the root tip without bursting. The vacuole stabilizes the expanding root tip from the inside, which is mediated by signaling cascades communicating at the plasma membrane between cell wall and cytoplasm (described in Section 4.3).
4.1. Signaling Molecules Initiate a Positive Feedback Loop during Root Hair Growth
ROS regulate root and root hair development downstream of auxin signaling [149,150]. Changed levels of ROS and Ca2+ are the primary regulators of root hair emergence and elongation [151,152,153,154], followed immediately by cytoskeleton bundling and rearrangement at the root hair tip [18]. ROS signaling is a fundamental regulatory element of plant growth, including the fine-tuning of tropistic reactions via auxin flow regulation [8,144,155,156]. ROS produced by NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE-OXIDASE(NOX) is needed to stimulate root hair elongation [150,157,158]. Furthermore, ROS accumulation is crucial for root hair tip outgrowth by modulating cell wall properties [152], regulating the rearrangement and abundance of the secondary messenger, cytoskeleton arrangement, and PM remodeling [150,159,160], and pH changes in the apoplast upon auxin efflux [8]. The calcium gradient at the root hair tip controls the direction of growth, and Ca2+ is a universal second messenger, crucial to modulate developmental plasticity in plants [8]. In the emerging root hair tip, it facilitates the fusion of exocytotic vesicles with the apical plasma membrane and subsequent delivery of cell wall cargo to the expanding cell wall [161].
4.2. Molecular Key Players of Root Hair Initiation and Elongation
ROPs are GTP-binding proteins acting as molecular switches, and some of them are crucial for root elongation events by regulating cell shape and auxin responses [159,162,163]. ROPs interact with membrane lipids upon posttranslational modifications and thereby transduce external and intracellular stimuli [164]. ROP2 and TOR can interact physically, resulting in TOR phosphorylation, activating TOR signaling pathways [68]. ROP2 is targeted to the PM region of the trichoblast where the root hair will emerge, to polarize subcellular organization to allow root hair outgrowth [165] and is followed by enhanced ROS production at the expanding root hair tip [166,167]. The activation of Ca2+ influx followed by increased cytoplasmic Ca2+ levels further enhance ROS production, creating a positive feedback loop [168]. Overexpression of ROP2 results in PIN2 accumulation in the root tip, and glucose activation of BR through the actin configuration stabilizes PIN2 at the PM [137,169]. ROP2 is a positive regulator of root hair growth and occurs slightly before the emerging bulge is visible [170] to initiate the positive feedback loop of ROS and Ca2+ fluxes responsible for triggering root hair elongation [8,165,166,167]. This triggers changes in the PM composition [171], resulting in the differential bundling of microtubules and actin at the root hair tip [172,173]. ROP2 activates ROS generation through NADPH oxidase action [150,166,174]. Plants overexpressing ROP2 have longer hairs than wild-type plants [69] and multiple root hair outgrowths per trichoblast [175] suggest that its function is needed during all stages of root hair growth. ROP2 is linked to the fine-tuning of filamentous actin (F-actin) bundling [172,176]. The tubular shape and polar growth of root hairs are maintained by correctly assembled microtubules [177] as well as actin bundles [172]. Actin is further essential for bulge site selection and tip growth [18,178,179,180]. F-actin supports cytoplasmic streaming in the cortical cytoplasm, and the distribution of actin bundles results in root hair tip growth inhibition [8,147,181].
4.3. Membrane Composition and Endomembrane Trafficking Is Fine-Tuned during Root Hair Growth
Correct membrane composition and fine-tuned endomembrane trafficking are key components of root hair positioning and elongation and are characterized by specific phosphoinositide patterning [182]. Membrane composition is maintained by an interplay of several kinases, like PHOSPATIDYLINOSITOL 4-OH KINASE BETA1 and 2 (PI4Kβ1, PI4Kβ2), which play an essential role in the regulation and delivery of secretory cargo to the tips of growing root hairs [183,184,185]. Furthermore, 1-PHOSPHATIDYLINOSITOL-4-PHOSPHATE 5-KINASE3 (PIP5K3) is required for appropriate tip growth control in root hairs [186,187] by mediating the recruitment of proteins with PI-4P or PI-4,5P2 binding domains, which modulate tip elongation [188,189]. PIP5K3 defines the accumulation site for ROP2, and overexpression of PIP5K3 results in multiple root hair outgrowths from a single trichoblast, whereas the mutants possess shorter root hairs compared to the wild type [69,186]. Vesicles transported to the expanding root hair tip by exocytosis secure polar tip growth by delivering freshly synthesized PM and cell wall components [8,190,191,192]. TOR signaling events modulate cell wall composition by orchestrating galactan/rhamnogalacturonan-I and arabinogalactan protein components over a signaling cascade including REPRESSOR OF LRX1 (ROL5), a mitochondrial protein and major source of ROS, and the extracellular protein LRR-EXTENSIN1 (LRX1) [193]. Lrx1 mutants develop aberrant root hairs, and TOR signaling can reverse the phenotype by changing the cell wall composition in the same way as it is detected in rol5 mutants [193]. The maintenance of cell wall integrity (CWI) during controlled root hair tip expansion is crucial to prevent the expanding root hair tip from bursting [89,194]. The PM located kinase FERONIA (FER) modulates the positioning of ROP2, followed by stabilizing actin filaments at the PM [167,195]. FER signaling incorporates cell growth, sensing, and the maintenance of CWI and is linked to ROS activity regulated by ROP2, as fer mutants exhibit impaired auxin-induced root hair growth, which can be rescued by overexpressing the FER binding partner ROP2 [167]. Recently, it was also shown how crucial FER is to maintain root hair elongation under elevated temperatures, as the mutant stops root hair tip growth [196]. In the root tip, fer-4 shows aberrant PIN2 polarity, probably because of diminished F-actin cytoskeleton assembly, which interferes with proper auxin gradient establishment [197]. The expanding root hair tip is almost fully filled with the vacuole, surrounded by a thin layer of highly polarized cytoplasm [8]. Vacuolar morphology has to date been extensively studied during the elongation processes of cells in the primary root tip, where it is crucial to maintain the expansion of the elongating cell without investing excessive resources to build up cytoplasmic content and stability [94,196,197,198,199,200]. It highly depends on intracellular auxin concentration [23,82,201] and signaling pathways partially regulated by FER [202,203]. To date, detailed studies on vacuolar integrity in the expanding root hair tip are lacking.
5. Summary and Outlook
Root hair outgrowth is orchestrated upon the appropriate spatial distribution of auxin, which is modulated depending on the overall status of the plant. Energy in the form of sugar and other available nutrients is sensed through the whole plant body and the information is transmitted over the molecular hub TOR complex, which is involved in auxin flow modulation between shoot and roots. In the root tip, auxin is redistributed by the auxin efflux carrier shootwards to the root hairs, where auxin-mediated cellular processes orchestrate polar hair outgrowth through specialized cells. Although manifold studies were published describing every aspect leading to proper root hair outgrowth, some questions are still open. Recent studies showed that direct light illumination interferes with root hair elongation, which is more evident during additive stress responses, therefore, more intense studies on roots grown shaded from light are needed. Furthermore, although much is known about the vacuolar fusion of elongating cells in the elongation zone, further studies are necessary to unravel the mechanisms controlling root hair branching, and the expansion of the vacuole during root hair tip elongation.
Acknowledgments
The authors would like to thank Kasper van Gelderen for constructive comments, and Verena Ibl for critical reading.
Author Contributions
Conceptualization, K.R. and W.W.; resources, K.R.; writing—original draft preparation, K.R. and W.W.; writing—review and editing, K.R.; visualization, K.R.; funding acquisition, K.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Youth and Sports of the Czech Republic from the European Regional Development Fund “Centre for Experimental Plant Biology”: Project no. CZ.02.1.01/0.0/0.0/16_019/0000738 and the Czech Science Foundation (19-13375Y).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van Gelderen K., Kang C., Pierik R. Light Signaling, Root Development, and Plasticity. Plant Physiol. 2018 doi: 10.1104/pp.17.01079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrada A., Montané M.H., Robaglia C., Menand B. Spatial Regulation of Root Growth: Placing the Plant TOR Pathway in a Developmental Perspective. Int. J. Mol. Sci. 2015;16:19671–19697. doi: 10.3390/ijms160819671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Retzer K., Korbei B., Luschnig C. Auxin and Its Role in Plant Development. Springer; Berlin, Germany: 2014. Auxin and Tropisms. [Google Scholar]
- 4.Calleja-Cabrera J., Boter M., Oñate-Sánchez L., Pernas M. Root Growth Adaptation to Climate Change in Crops. Front. Plant Sci. 2020 doi: 10.3389/fpls.2020.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kircher S., Schopfer P. Photosynthetic Sucrose Acts as Cotyledon-Derived Long-Distance Signal to Control Root Growth during Early Seedling Development in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2012 doi: 10.1073/pnas.1203746109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett T., Leyser O. Auxin and Its Role in Plant Development. Springer; Berlin, Germany: 2014. The Auxin Question: A Philosophical Overview. [Google Scholar]
- 7.Dolan L., Duckett C.M., Grierson C., Linstead P., Schneider K., Lawson E., Dean C., Poethig S., Roberts K. Clonal Relationships and Cell Patterning in the Root Epidermis of Arabidopsis. Development. 1994;120:2465–2474. [Google Scholar]
- 8.Grierson C., Nielsen E., Ketelaarc T., Schiefelbein J. Root Hairs. Arab. Book. 2014;12:e0172. doi: 10.1199/tab.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones A.R., Kramer E.M., Knox K., Swarup R., Bennett M.J., Lazarus C.M., Leyser H.M.O., Grierson C.S. Auxin Transport through Non-Hair Cells Sustains Root-Hair Development. Nat. Cell Biol. 2009 doi: 10.1038/ncb1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leyser O. Auxin Signaling. Plant Physiol. 2018 doi: 10.1104/pp.17.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y., Shi L., Li L., Fu L., Liu Y., Xiong Y., Sheen J. Integration of Nutrient, Energy, Light, and Hormone Signalling via TOR in Plants. J. Exp. Bot. 2019 doi: 10.1093/jxb/erz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauer M., Kleine-Vehn J. PIN-FORMED and PIN-LIKES Auxin Transport Facilitators. Development. 2019 doi: 10.1242/dev.168088. [DOI] [PubMed] [Google Scholar]
- 13.Muroyama A., Bergmann D. Plant Cell Polarity: Creating Diversity from inside the Box. Annu. Rev. Cell Dev. Biol. 2019 doi: 10.1146/annurev-cellbio-100818-125211. [DOI] [PubMed] [Google Scholar]
- 14.Adamowski M., Friml J. PIN-Dependent Auxin Transport: Action, Regulation, and Evolution. Plant Cell. 2015 doi: 10.1105/tpc.114.134874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi J., Greb T. Cell Polarity in Plants: The Yin and Yang of Cellular Functions. Curr. Opin. Plant Biol. 2017 doi: 10.1016/j.pbi.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ötvös K., Marconi M., Vega A., O’Brien J., Johnson A., Abualia R., Antonielli L., Montesinos J.C., Zhang Y., Tan S., et al. Modulation of Root Growth by Nutrient-Defined Fine-Tuning of Polar Auxin Transport. bioRxiv. 2020 doi: 10.1101/2020.06.19.160994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Band L.R., Wells D.M., Larrieu A., Sun J., Middleton A.M., French A.P., Brunoud G., Sato E.M., Wilson M.H., Peŕet B., et al. Root Gravitropism Is Regulated by a Transient Lateral Auxin Gradient Controlled by a Tipping-Point Mechanism. Proc. Natl. Acad. Sci. USA. 2012 doi: 10.1073/pnas.1201498109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park E., Nebenführ A. Cytoskeleton and Root Hair Growth. Plant Cytoskelet. 2011 doi: 10.1007/978-1-4419-0987-9_12. [DOI] [Google Scholar]
- 19.Friml J., Vieten A., Sauer M., Weijers D., Schwarz H., Hamann T., Offringa R., Jürgens G. Efflux-Dependent Auxin Gradients Establish the Apical-Basal Axis of Arabidopsis. Nature. 2003 doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 20.Smit M.E., Weijers D. The Role of Auxin Signaling in Early Embryo Pattern Formation. Curr. Opin. Plant Biol. 2015 doi: 10.1016/j.pbi.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. Local, Efflux-Dependent Auxin Gradients as a Common Module for Plant Organ Formation. Cell. 2003 doi: 10.1016/S0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 22.Armengot L., Marquès-Bueno M.M., Jaillais Y. Regulation of Polar Auxin Transport by Protein and Lipid Kinases. J. Exp. Bot. 2016 doi: 10.1093/jxb/erw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fendrych M., Akhmanova M., Merrin J., Glanc M., Hagihara S., Takahashi K., Uchida N., Torii K.U., Friml J. Rapid and Reversible Root Growth Inhibition by TIR1 Auxin Signalling. Nat. Plants. 2018 doi: 10.1038/s41477-018-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallei M., Luschnig C., Friml J. Auxin Signalling in Growth: Schrödinger’s Cat out of the Bag. Curr. Opin. Plant Biol. 2020 doi: 10.1016/j.pbi.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Semeradova H., Montesinos J.C., Benkova E. All Roads Lead to Auxin: Post-Translational Regulation of Auxin Transport by Multiple Hormonal Pathways. Plant Commun. 2020 doi: 10.1016/j.xplc.2020.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Gelderen K., Kang C., Paalman R., Keuskamp D., Hayes S., Pierik R. Far-Red Light Detection in the Shoot Regulates Lateral Root Development through the HY5 Transcription Factor. Plant Cell. 2018 doi: 10.1105/tpc.17.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sairanen I., Novák O., Pěnčík A., Ikeda Y., Jones B., Sandberg G., Ljung K. Soluble Carbohydrates Regulate Auxin Biosynthesis via PIF Proteins in Arabidopsis. Plant Cell. 2013 doi: 10.1105/tpc.112.104794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierik R., Testerink C. The Art of Being Flexible: How to Escape from Shade, Salt, And Drought1. Plant Physiol. 2014 doi: 10.1104/pp.114.239160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Smet I. Lateral Root Initiation: One Step at a Time. New Phytol. 2012 doi: 10.1111/j.1469-8137.2011.03996.x. [DOI] [PubMed] [Google Scholar]
- 30.Orman-Ligeza B., Parizot B., Gantet P.P., Beeckman T., Bennett M.J., Draye X. Post-Embryonic Root Organogenesis in Cereals: Branching out from Model Plants. Trends Plant Sci. 2013 doi: 10.1016/j.tplants.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Habets M.E.J., Offringa R. PIN-Driven Polar Auxin Transport in Plant Developmental Plasticity: A Key Target for Environmental and Endogenous Signals. New Phytol. 2014:362–377. doi: 10.1111/nph.12831. [DOI] [PubMed] [Google Scholar]
- 32.Rakusová H., Fendrych M., Friml J. Intracellular Trafficking and PIN-Mediated Cell Polarity during Tropic Responses in Plants. Curr. Opin. Plant Biol. 2015:116–123. doi: 10.1016/j.pbi.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Wallner E.-S. The Value of Asymmetry: How Polarity Proteins Determine Plant Growth and Morphology. J. Exp. Bot. 2020;71:5733–5739. doi: 10.1093/jxb/eraa329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta A., Rico-Medina A., Caño-Delgado A.I. The Physiology of Plant Responses to Drought. Science. 2020 doi: 10.1126/science.aaz7614. [DOI] [PubMed] [Google Scholar]
- 35.Bögre L., Henriques R., Magyar Z. TOR tour to auxin. EMBO J. 2013 doi: 10.1038/emboj.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokawa K., Baluška F. The TOR Complex: An Emergency Switch for Root Behavior. Plant Cell Physiol. 2016 doi: 10.1093/pcp/pcv191. [DOI] [PubMed] [Google Scholar]
- 37.Ryabova L.A., Robaglia C., Meyer C. Target of Rapamycin Kinase: Central Regulatory Hub for Plant Growth and Metabolism. J. Exp. Bot. 2019 doi: 10.1093/jxb/erz108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y., Wang X.Q. The Hot Issue: TOR Signalling Network in Plants. Funct. Plant Biol. 2020 doi: 10.1071/FP20071. [DOI] [PubMed] [Google Scholar]
- 39.Roustan V., Jain A., Teige M., Ebersberger I., Weckwerth W. An Evolutionary Perspective of AMPK-TOR Signaling in the Three Domains of Life. J. Exp. Bot. 2016 doi: 10.1093/jxb/erw211. [DOI] [PubMed] [Google Scholar]
- 40.Dobrenel T., Caldana C., Hanson J., Robaglia C., Vincentz M., Veit B., Meyer C. TOR Signaling and Nutrient Sensing. Annu. Rev. Plant Biol. 2016;67:261–285. doi: 10.1146/annurev-arplant-043014-114648. [DOI] [PubMed] [Google Scholar]
- 41.Soto-Burgos J., Bassham D.C. SnRK1 Activates Autophagy via the TOR Signaling Pathway in Arabidopsis Thaliana. PLoS ONE. 2017 doi: 10.1371/journal.pone.0182591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Leene J., Han C., Gadeyne A., Eeckhout D., Matthijs C., Cannoot B., De Winne N., Persiau G., Van De Slijke E., Van de Cotte B., et al. Capturing the phosphorylation and protein interaction landscape of the plant TOR kinase. Nat. Plants. 2019;5:316–327. doi: 10.1038/s41477-019-0378-z. [DOI] [PubMed] [Google Scholar]
- 43.Mair A., Pedrotti L., Wurzinger B., Anrather D., Simeunovic A., Weiste C., Valerio C., Dietrich K., Kirchler T., Nägele T., et al. SnRK1-Triggered Switch of BZIP63 Dimerization Mediates the Low-Energy Response in Plants. eLife. 2015 doi: 10.7554/eLife.05828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiong Y., Sheen J. Novel Links in the Plant TOR Kinase Signaling Network. Curr. Opin. Plant Biol. 2015 doi: 10.1016/j.pbi.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi L., Wu Y., Sheen J. TOR Signaling in Plants: Conservation and Innovation. Development. 2018 doi: 10.1242/dev.160887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiong Y., McCormack M., Li L., Hall Q., Xiang C., Sheen J. Glucose-TOR Signalling Reprograms the Transcriptome and Activates Meristems. Nature. 2013 doi: 10.1038/nature12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nukarinen E., Ngele T., Pedrotti L., Wurzinger B., Mair A., Landgraf R., Börnke F., Hanson J., Teige M., Baena-Gonzalez E., et al. Quantitative Phosphoproteomics Reveals the Role of the AMPK Plant Ortholog SnRK1 as a Metabolic Master Regulator under Energy Deprivation. Sci. Rep. 2016 doi: 10.1038/srep31697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y., Bassham D.C. TOR Is a Negative Regulator of Autophagy in Arabidopsis Thaliana. PLoS ONE. 2010 doi: 10.1371/journal.pone.0011883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pérez-Pérez M.E., Florencio F.J., Crespo J.L. Inhibition of Target of Rapamycin Signaling and Stress Activate Autophagy in Chlamydomonas Reinhardtii. Plant Physiol. 2010 doi: 10.1104/pp.109.152520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roustan V., Bakhtiari S., Roustan P.J., Weckwerth W. Quantitative in Vivo Phosphoproteomics Reveals Reversible Signaling Processes during Nitrogen Starvation and Recovery in the Biofuel Model Organism Chlamydomonas Reinhardtii. Biotechnol. Biofuels. 2017 doi: 10.1186/s13068-017-0949-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roustan V., Weckwerth W. Quantitative Phosphoproteomic and System-Level Analysis of TOR Inhibition Unravel Distinct Organellar Acclimation in Chlamydomonas Reinhardtii. Front. Plant Sci. 2018 doi: 10.3389/fpls.2018.01590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batoko H., Dagdas Y., Baluska F., Sirko A. Understanding and Exploiting Autophagy Signaling in Plants. Essays Biochem. 2017 doi: 10.1042/EBC20170034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masclaux-Daubresse C. Autophagy Controls Carbon, Nitrogen, and Redox Homeostasis in Plants. Autophagy. 2016 doi: 10.4161/auto.36261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Margalha L., Confraria A., Baena-González E. SnRK1 and TOR: Modulating Growth–Defense Trade-Offs in Plant Stress Responses. J. Exp. Bot. 2019 doi: 10.1093/jxb/erz066. [DOI] [PubMed] [Google Scholar]
- 55.Margalha L., Valerio C., Baena-González E. Plant SnRK1 Kinases: Structure, Regulation, and Function. Act. Protein Kinase. 2016 doi: 10.1007/978-3-319-43589-3_17. [DOI] [PubMed] [Google Scholar]
- 56.Dobrenel T., Mancera-Martínez E., Forzani C., Azzopardi M., Davanture M., Moreau M., Schepetilnikov M., Chicher J., Langella O., Zivy M., et al. The Arabidopsis TOR Kinase Specifically Regulates the Expression of Nuclear Genes Coding for Plastidic Ribosomal Proteins and the Phosphorylation of the Cytosolic Ribosomal Protein S6. Front. Plant Sci. 2016 doi: 10.3389/fpls.2016.01611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahfouz M.M., Kim S., Delauney A.J., Verma D.P.S. Arabidopsis TARGET of RAPAMYCIN Interacts with RAPTOR, Which Regulates the Activity of S6 Kinase in Response to Osmotic Stress Signals. Plant Cell. 2006 doi: 10.1105/tpc.105.035931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schepetilnikov M., Dimitrova M., Mancera-Martínez E., Geldreich A., Keller M., Ryabova L.A. TOR and S6K1 Promote Translation Reinitiation of UORF-Containing MRNAs via Phosphorylation of EIF3h. EMBO J. 2013 doi: 10.1038/emboj.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ljung K. Auxin Metabolism and Homeostasis during Plant Development. Development. 2013 doi: 10.1242/dev.086363. [DOI] [PubMed] [Google Scholar]
- 60.Simon S., Petrášek J. Why Plants Need More than One Type of Auxin. Plant Sci. 2011 doi: 10.1016/j.plantsci.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Morffy N., Strader L.C. Old Town Roads: Routes of Auxin Biosynthesis across Kingdoms. Curr. Opin. Plant Biol. 2020 doi: 10.1016/j.pbi.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deng K., Yu L., Zheng X., Zhang K., Wang W., Dong P., Zhang J., Ren M. Target of Rapamycin Is a Key Player for Auxin Signaling Transduction in Arabidopsis. Front. Plant Sci. 2016 doi: 10.3389/fpls.2016.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong Y., Silbermann M., Speiser A., Forieri I., Linster E., Poschet G., Allboje Samami A., Wanatabe M., Sticht C., Teleman A.A., et al. Sulfur Availability Regulates Plant Growth via Glucose-TOR Signaling. Nat. Commun. 2017 doi: 10.1038/s41467-017-01224-w. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Schepetilnikov M., Ryabova L.A. Auxin Signaling in Regulation of Plant Translation Reinitiation. Front. Plant Sci. 2017 doi: 10.3389/fpls.2017.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deprost D., Yao L., Sormani R., Moreau M., Leterreux G., Bedu M., Robaglia C., Meyer C. The Arabidopsis TOR Kinase Links Plant Growth, Yield, Stress Resistance and MRNA Translation. EMBO Rep. 2007 doi: 10.1038/sj.embor.7401043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mishra B.S., Singh M., Aggrawal P., Laxmi A. Glucose and Auxin Signaling Interaction in Controlling Arabidopsis Thaliana Seedlings Root Growth and Development. PLoS ONE. 2009 doi: 10.1371/journal.pone.0004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salem M.A., Li Y., Wiszniewski A., Giavalisco P. Regulatory-Associated Protein of TOR (RAPTOR) Alters the Hormonal and Metabolic Composition of Arabidopsis Seeds, Controlling Seed Morphology, Viability and Germination Potential. Plant J. 2017 doi: 10.1111/tpj.13667. [DOI] [PubMed] [Google Scholar]
- 68.Schepetilnikov M., Makarian J., Srour O., Geldreich A., Yang Z., Chicher J., Hammann P., Ryabova L.A. GTP Ase ROP 2 Binds and Promotes Activation of Target of Rapamycin, TOR, in Response to Auxin. EMBO J. 2017 doi: 10.15252/embj.201694816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones M.A., Shen J.J., Fu Y., Li H., Yang Z., Grierson C.S. The Arabidopsis Rop2 GTPase Is a Positive Regulator of Both Root Hair Initiation and Tip Growth. Plant Cell. 2002 doi: 10.1105/tpc.010359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Korver R.A., Koevoets I.T., Testerink C. Out of Shape During Stress: A Key Role for Auxin. Trends Plant Sci. 2018 doi: 10.1016/j.tplants.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freschet G., Pagès L., Iversen C., Comas L., Rewald B., Roumet C., Klimešová J., Zadworny M., Poorter H., Postma J. A Starting Guide to Root Ecology: Strengthening Ecological Concepts and Standardizing Root Classification, Sampling, Processing and Trait Measurements. Hal; Lyon, France: 2020. Hal-02918834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schepetilnikov M., Ryabova L.A. Recent Discoveries on the Role of Tor (Target of Rapamacin) Signaling in Translation in Plants. Plant Physiol. 2018 doi: 10.1104/pp.17.01243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Datta S., Kim C.M., Pernas M., Pires N.D., Proust H., Tam T., Vijayakumar P., Dolan L. Root Hairs: Development, Growth and Evolution at the Plant-Soil Interface. Plant Soil. 2011 doi: 10.1007/s11104-011-0845-4. [DOI] [Google Scholar]
- 74.Dolan L. How and Where to Build a Root Hair. Curr. Opin. Plant Biol. 2001 doi: 10.1016/S1369-5266(00)00214-4. [DOI] [PubMed] [Google Scholar]
- 75.Salazar-Henao J.E., Vélez-Bermúdez I.C., Schmidt W. The Regulation and Plasticity of Root Hair Patterning and Morphogenesis. Development. 2016 doi: 10.1242/dev.132845. [DOI] [PubMed] [Google Scholar]
- 76.Boutté Y., Grebe M. Cellular Processes Relying on Sterol Function in Plants. Curr. Opin. Plant Biol. 2009 doi: 10.1016/j.pbi.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 77.Lee R.D.-W., Cho H.-T. Auxin, the Organizer of the Hormonal/Environmental Signals for Root Hair Growth. Front. Plant Sci. 2013;4:448. doi: 10.3389/fpls.2013.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei Z., Li J. Receptor-like Protein Kinases: Key Regulators Controlling Root Hair Development in Arabidopsis thaliana. J. Integr. Plant Biol. 2018 doi: 10.1111/jipb.12663. [DOI] [PubMed] [Google Scholar]
- 79.Ganguly A., Lee S.H., Cho M., Lee O.R., Yoo H., Cho H.T. Differential Auxin-Transporting Activities of PIN-FORMED Proteins in Arabidopsis Root Hair Cells. Plant Physiol. 2010 doi: 10.1104/pp.110.156505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jozef L., Katarzyna R., Christian L., Eva Z. Encyclopedia of Life Sciences. John Wiley Sons; Hoboken, NJ, USA: 2017. Polar Auxin Transport. [Google Scholar]
- 81.Petrášek J., Friml J. Auxin Transport Routes in Plant Development. Development. 2009 doi: 10.1242/dev.030353. [DOI] [PubMed] [Google Scholar]
- 82.Löfke C., Scheuring D., Dünser K., Schöller M., Luschnig C., Kleine-Vehn J. Tricho- and Atrichoblast Cell Files Show Distinct PIN2 Auxin Efflux Carrier Exploitations and Are Jointly Required for Defined Auxin-Dependent Root Organ Growth. J. Exp. Bot. 2015 doi: 10.1093/jxb/erv282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dolan L., Davies J. Cell Expansion in Roots. Curr. Opin. Plant Biol. 2004 doi: 10.1016/j.pbi.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 84.Fischer U., Ikeda Y., Grebe M. Planar Polarity of Root Hair Positioning in Arabidopsis. Biochem. Soc. Trans. 2007 doi: 10.1042/BST0350149. [DOI] [PubMed] [Google Scholar]
- 85.Pitts R.J., Cernac A., Estelle M. Auxin and Ethylene Promote Root Hair Elongation in Arabidopsis. Plant J. 1998 doi: 10.1046/j.1365-313x.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- 86.Velasquez S.M., Barbez E., Kleine-Vehn J., Estevez J.M. Auxin and Cellular Elongation. Plant Physiol. 2016 doi: 10.1104/pp.15.01863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fischer U., Ikeda Y., Ljung K., Serralbo O., Singh M., Heidstra R., Palme K., Scheres B., Grebe M. Vectorial Information for Arabidopsis Planar Polarity Is Mediated by Combined AUX1, EIN2, and GNOM Activity. Curr. Biol. 2006 doi: 10.1016/j.cub.2006.08.091. [DOI] [PubMed] [Google Scholar]
- 88.Majda M., Robert S. The Role of Auxin in Cell Wall Expansion. Int. J. Mol. Sci. 2018;19:951. doi: 10.3390/ijms19040951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Franck C.M., Westermann J., Boisson-Dernier A. Plant Malectin-Like Receptor Kinases: From Cell Wall Integrity to Immunity and Beyond. Annu. Rev. Plant Biol. 2018 doi: 10.1146/annurev-arplant-042817-040557. [DOI] [PubMed] [Google Scholar]
- 90.Grones P., Friml J. Auxin Transporters and Binding Proteins at a Glance. J. Cell Sci. 2015 doi: 10.1242/jcs.159418. [DOI] [PubMed] [Google Scholar]
- 91.Geisler M., Wang B., Zhu J. Auxin Transport during Root Gravitropism: Transporters and Techniques. Plant Biol. 2014 doi: 10.1111/plb.12030. [DOI] [PubMed] [Google Scholar]
- 92.Retzer K., Butt H., Korbei B., Luschnig C. The Far Side of Auxin Signaling: Fundamental Cellular Activities and Their Contribution to a Defined Growth Response in Plants. Protoplasma. 2014 doi: 10.1007/s00709-013-0572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luschnig C., Vert G. The Dynamics of Plant Plasma Membrane Proteins: PINs and Beyond. Development. 2014 doi: 10.1242/dev.103424. [DOI] [PubMed] [Google Scholar]
- 94.Kaiser S., Scheuring D. To Lead or to Follow: Contribution of the Plant Vacuole to Cell Growth. Front. Plant Sci. 2020 doi: 10.3389/fpls.2020.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boutté Y., Jaillais Y. Metabolic Cellular Communications: Feedback Mechanisms between Membrane Lipid Homeostasis and Plant Development. Dev. Cell. 2020 doi: 10.1016/j.devcel.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 96.Péret B., Swarup K., Ferguson A., Seth M., Yang Y., Dhondt S., James N., Casimiro I., Perry P., Syed A., et al. AUX/LAX Genes Encode a Family of Auxin Influx Transporters That Perform Distinct Functions during Arabidopsis Development. Plant Cell. 2012;24:2874–2885. doi: 10.1105/tpc.112.097766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Swarup R., Bhosale R. Developmental Roles of AUX1/LAX Auxin Influx Carriers in Plants. Front. Plant Sci. 2019 doi: 10.3389/fpls.2019.01306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singh G., Retzer K., Vosolsobě S., Napier R. Advances in Understanding the Mechanism of Action of the Auxin Permease Aux1. Int. J. Mol. Sci. 2018;19:3391. doi: 10.3390/ijms19113391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kleine-Vehn J., Dhonukshe P., Swarup R., Bennett M., Friml J. Subcellular Trafficking of the Arabidopsis Auxin Influx Carrier AUX1 Uses a Novel Pathway Distinct from PIN1. Plant Cell. 2006 doi: 10.1105/tpc.106.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krouk G., Lacombe B., Bielach A., Perrine-Walker F., Malinska K., Mounier E., Hoyerova K., Tillard P., Leon S., Ljung K., et al. Nitrate-Regulated Auxin Transport by NRT1.1 Defines a Mechanism for Nutrient Sensing in Plants. Dev. Cell. 2010 doi: 10.1016/j.devcel.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 101.Parker J.L., Newstead S. Molecular Basis of Nitrate Uptake by the Plant Nitrate Transporter NRT1.1. Nature. 2014 doi: 10.1038/nature13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun J., Bankston J.R., Payandeh J., Hinds T.R., Zagotta W.N., Zheng N. Crystal Structure of the Plant Dual-Affinity Nitrate Transporter NRT1.1. Nature. 2014 doi: 10.1038/nature13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Swarup R., Kramer E.M., Perry P., Knox K., Leyser H.M.O., Haseloff J., Beemster G.T.S., Bhalerao R., Bennett M.J. Root Gravitropism Requires Lateral Root Cap and Epidermal Cells for Transport and Response to a Mobile Auxin Signal. Nat. Cell Biol. 2005 doi: 10.1038/ncb1316. [DOI] [PubMed] [Google Scholar]
- 104.Lee S.H., Cho H.-T. Auxin and Root Hair Morphogenesis. Root Hairs. 2009 doi: 10.1007/978-3-540-79405-9_16. [DOI] [Google Scholar]
- 105.Rea P.A. Plant ATP-Binding Cassette Transporters. Annu. Rev. Plant Biol. 2007 doi: 10.1146/annurev.arplant.57.032905.105406. [DOI] [PubMed] [Google Scholar]
- 106.Remy E., Duque P. Beyond Cellular Detoxification: A Plethora of Physiological Roles for MDR Transporter Homologs in Plants. Front. Physiol. 2014 doi: 10.3389/fphys.2014.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fukui K., Hayashi K.I. Manipulation and Sensing of Auxin Metabolism, Transport and Signaling. Plant Cell Physiol. 2018 doi: 10.1093/pcp/pcy076. [DOI] [PubMed] [Google Scholar]
- 108.Geisler M., Aryal B., Di Donato M., Hao P. A Critical View on ABC Transporters and Their Interacting Partners in Auxin Transport. Plant Cell Physiol. 2017 doi: 10.1093/pcp/pcx104. [DOI] [PubMed] [Google Scholar]
- 109.Cho M., Cho H.T. The Function of ABCB Transporters in Auxin Transport. Plant Signal. Behav. 2013 doi: 10.4161/psb.22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang H., Murphy A.S. Functional Expression and Characterization of Arabidopsis ABCB, AUX 1 and PIN Auxin Transporters in Schizosaccharomyces pombe. Plant J. 2009 doi: 10.1111/j.1365-313X.2009.03856.x. [DOI] [PubMed] [Google Scholar]
- 111.Kamimoto Y., Terasaka K., Hamamoto M., Takanashi K., Fukuda S., Shitan N., Sugiyama A., Suzuki H., Shibata D., Wang B., et al. Arabidopsis ABCB21 Is a Facultative Auxin Importer/Exporter Regulated by Cytoplasmic Auxin Concentration. Plant Cell Physiol. 2012;53:2090–2100. doi: 10.1093/pcp/pcs149. [DOI] [PubMed] [Google Scholar]
- 112.Kubeš M., Yang H., Richter G.L., Cheng Y., Młodzińska E., Wang X., Blakeslee J.J., Carraro N., Petrášek J., Zažímalová E., et al. The Arabidopsis Concentration-Dependent Influx/Efflux Transporter ABCB4 Regulates Cellular Auxin Levels in the Root Epidermis. Plant J. 2012 doi: 10.1111/j.1365-313X.2011.04818.x. [DOI] [PubMed] [Google Scholar]
- 113.Titapiwatanakun B., Blakeslee J.J., Bandyopadhyay A., Yang H., Mravec J., Sauer M., Cheng Y., Adamec J., Nagashima A., Geisler M., et al. ABCB19/PGP19 Stabilises PIN1 in Membrane Microdomains in Arabidopsis. Plant J. 2009 doi: 10.1111/j.1365-313X.2008.03668.x. [DOI] [PubMed] [Google Scholar]
- 114.Zazímalová E., Murphy A.S., Yang H., Hoyerová K., Hosek P. Auxin Transporters—Why so Many? Cold Spring Harb. Perspect. Biol. 2010 doi: 10.1101/cshperspect.a001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Parry G., Delbarre A., Marchant A., Swarup R., Napier R., Perrot-Rechenmann C., Bennett M.J. Novel Auxin Transport Inhibitors Phenocopy the Auxin Influx Carrier Mutation Aux1. Plant J. 2001 doi: 10.1046/j.1365-313X.2001.00970.x. [DOI] [PubMed] [Google Scholar]
- 116.Petrášek J., Mravec J., Bouchard R., Blakeslee J.J., Abas M., Seifertová D., Wiśniewska J., Tadele Z., Kubeš M., Čovanová M., et al. PIN Proteins Perform a Rate-Limiting Function in Cellular Auxin Efflux. Science. 2006 doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- 117.Zourelidou M., Absmanner B., Weller B., Barbosa I.C.R., Willige B.C., Fastner A., Streit V., Port S.A., Colcombet J., van Bentem S., et al. Auxin Efflux by PIN-FORMED Proteins Is Activated by Two Different Protein Kinases, D6 PROTEIN KINASE and PINOID. eLife. 2014 doi: 10.7554/eLife.02860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wisniewska J., Xu J., Seifartová D., Brewer P.B., Růžička K., Blilou L., Rouquié D., Benková E., Scheres B., Friml J. Polar PIN Localization Directs Auxin Flow in Plants. Science. 2006 doi: 10.1126/science.1121356. [DOI] [PubMed] [Google Scholar]
- 119.Vieten A., Vanneste S., Wiśniewska J., Benková E., Benjamins R., Beeckman T., Luschnig C., Friml J. Functional Redundancy of PIN Proteins Is Accompanied by Auxin-Dependent Cross-Regulation of PIN Expression. Development. 2005 doi: 10.1242/dev.02027. [DOI] [PubMed] [Google Scholar]
- 120.Dettmer J., Friml J. Cell Polarity in Plants: When Two Do the Same, It Is Not the Same.... Curr. Opin. Cell Biol. 2011 doi: 10.1016/j.ceb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 121.Okada K., Ueda J., Komaki M.K., Bell C.J., Shimura Y. Requirement of the Auxin Polar Transport System in Early Stages of Arabidopsis Floral Bud Formation. Plant Cell. 1991 doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gälweiler L., Guan C., Müller A., Wisman E., Mendgen K., Yephremov A., Palme K. Regulation of Polar Auxin Transport by AtPIN1 in Arabidopsis Vascular Tissue. Science. 1998 doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- 123.Billou I., Xu J., Wildwater M., Willemsen V., Paponov I., Frimi J., Heldstra R., Aida M., Palme K., Scheres B. The PIN Auxin Efflux Facilitator Network Controls Growth and Patterning in Arabidopsis Roots. Nature. 2005 doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 124.Friml J., Wiŝniewska J., Benková E., Mendgen K., Palme K. Lateral Relocation of Auxin Efflux Regulator PIN3 Mediates Tropism in Arabidopsis. Nature. 2002 doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- 125.Kleine-Vehn J., Ding Z., Jones A.R., Tasaka M., Morita M.T., Friml J. Gravity-Induced PIN Transcytosis for Polarization of Auxin Fluxes in Gravity-Sensing Root Cells. Proc. Natl. Acad. Sci. USA. 2010 doi: 10.1073/pnas.1013145107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weiste C., Pedrotti L., Selvanayagam J., Muralidhara P., Fröschel C., Novák O., Ljung K., Hanson J., Dröge-Laser W. The Arabidopsis BZIP11 Transcription Factor Links Low-Energy Signalling to Auxin-Mediated Control of Primary Root Growth. PLoS Genet. 2017 doi: 10.1371/journal.pgen.1006607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yuan X., Xu P., Yu Y., Xiong Y. Glucose-TOR signaling regulates PIN2 stability to orchestrate auxin gradient and cell expansion in Arabidopsis root. Proc. Natl. Acad. Sci. USA. 2020 doi: 10.1073/pnas.2015400117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bieleski R.L. Phosphate Pools, Phosphate Transport, and Phosphate Availability. Annu. Rev. Plant Physiol. 1973 doi: 10.1146/annurev.pp.24.060173.001301. [DOI] [Google Scholar]
- 129.Ma Z., Bielenberg D.G., Brown K.M., Lynch J.P. Regulation of Root Hair Density by Phosphorus Availability in Arabidopsis thaliana. Plant Cell Environ. 2001 doi: 10.1046/j.1365-3040.2001.00695.x. [DOI] [Google Scholar]
- 130.Zhang Y.J., Lynch J.P., Brown K.M. Ethylene and Phosphorus Availability Have Interacting yet Distinct Effects on Root Hair Development. J. Exp. Bot. 2003 doi: 10.1093/jxb/erg250. [DOI] [PubMed] [Google Scholar]
- 131.Zhang S., Huang L., Yan A., Liu Y., Liu B., Yu C., Zhang A., Schiefelbein J., Gan Y. Multiple Phytohormones Promote Root Hair Elongation by Regulating a Similar Set of Genes in the Root Epidermis in Arabidopsis. J. Exp. Bot. 2016 doi: 10.1093/jxb/erw400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kapulnik Y., Resnick N., Mayzlish-Gati E., Kaplan Y., Wininger S., Hershenhorn J., Koltai H. Strigolactones Interact with Ethylene and Auxin in Regulating Root-Hair Elongation in Arabidopsis. J. Exp. Bot. 2011 doi: 10.1093/jxb/erq464. [DOI] [PubMed] [Google Scholar]
- 133.Pandey A., Devi L.L., Singh A.P. Review: Emerging Roles of Brassinosteroid in Nutrient Foraging. Plant Sci. 2020 doi: 10.1016/j.plantsci.2020.110474. [DOI] [PubMed] [Google Scholar]
- 134.Li L., Xu J., Xu Z.H., Xue H.W. Brassinosteroids Stimulate Plant Tropisms through Modulation of Polar Auxin Transport in Brassica and Arabidopsis. Plant Cell. 2005 doi: 10.1105/tpc.105.034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Retzer K., Akhmanova M., Konstantinova N., Malínská K., Leitner J., Petrášek J., Luschnig C. Brassinosteroid Signaling Delimits Root Gravitropism via Sorting of the Arabidopsis PIN2 Auxin Transporter. Nat. Commun. 2019 doi: 10.1038/s41467-019-13543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fridman Y., Savaldi-Goldstein S. Brassinosteroids in Growth Control: How, When and Where. Plant Sci. 2013 doi: 10.1016/j.plantsci.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 137.Lanza M., Garcia-Ponce B., Castrillo G., Catarecha P., Sauer M., Rodriguez-Serrano M., Páez-García A., Sánchez-Bermejo E., Tc M., Leo del Puerto Y., et al. Role of Actin Cytoskeleton in Brassinosteroid Signaling and in Its Integration with the Auxin Response in Plants. Dev. Cell. 2012 doi: 10.1016/j.devcel.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 138.Müssig C., Shin G.H., Altmann T. Brassinosteroids Promote Root Growth in Arabidopsis. Plant Physiol. 2003 doi: 10.1104/pp.103.028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang Z.Y., Zhu J.Y., Sae-Seaw J. Brassinosteroid Signaling. Development. 2013 doi: 10.1242/dev.060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rigas S., Ditengou F.A., Ljung K., Daras G., Tietz O., Palme K., Hatzopoulos P. Root Gravitropism and Root Hair Development Constitute Coupled Developmental Responses Regulated by Auxin Homeostasis in the Arabidopsis Root Apex. New Phytol. 2013 doi: 10.1111/nph.12092. [DOI] [PubMed] [Google Scholar]
- 141.Cheng Y., Zhu W., Chen Y., Ito S., Asami T., Wang X. Brassinosteroids Control Root Epidermal Cell Fate via Direct Regulation of a MYB-BHLH-WD40 Complex by GSK3-like Kinases. eLife. 2014 doi: 10.7554/eLife.02525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kumar M., Pandya-Kumar N., Dam A., Haor H., Mayzlish-Gati E., Belausov E., Wininger S., Abu-Abied M., McErlean C.S.P., Bromhead L.J., et al. Arabidopsis Response to Low-Phosphate Conditions Includes Active Changes in Actin Filaments and PIN2 Polarization and Is Dependent on Strigolactone Signalling. J. Exp. Bot. 2015 doi: 10.1093/jxb/eru513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kapulnik Y., Delaux P.M., Resnick N., Mayzlish-Gati E., Wininger S., Bhattacharya C., Séjalon-Delmas N., Combier J.P., Bécard G., Belausov E., et al. Strigolactones Affect Lateral Root Formation and Root-Hair Elongation in Arabidopsis. Planta. 2011 doi: 10.1007/s00425-010-1310-y. [DOI] [PubMed] [Google Scholar]
- 144.Silva-Navas J., Conesa C.M., Saez A., Navarro-Neila S., Garcia-Mina J.M., Zamarreño A.M., Baigorri R., Swarup R., del Pozo J.C. Role of Cis-Zeatin in Root Responses to Phosphate Starvation. New Phytol. 2019 doi: 10.1111/nph.16020. [DOI] [PubMed] [Google Scholar]
- 145.Silva-Navas J., Moreno-Risueno M.A., Manzano C., Pallero-Baena M., Navarro-Neila S., Téllez-Robledo B., Garcia-Mina J.M., Baigorri R., Gallego F.J., Del Pozo J.C. D-Root: A System for Cultivating Plants with the Roots in Darkness or under Different Light Conditions. Plant J. 2015 doi: 10.1111/tpj.12998. [DOI] [PubMed] [Google Scholar]
- 146.Rounds C.M., Bezanilla M. Growth Mechanisms in Tip-Growing Plant Cells. Annu. Rev. Plant Biol. 2013 doi: 10.1146/annurev-arplant-050312-120150. [DOI] [PubMed] [Google Scholar]
- 147.Takatsuka H., Ito M. Cytoskeletal Control of Planar Polarity in Root Hair Development. Front. Plant Sci. 2020 doi: 10.3389/fpls.2020.580935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Žárský V., Cvrčková F., Potocký M., Hála M. Exocytosis and Cell Polarity in Plants–Exocyst and Recycling Domains. New Phytol. 2009 doi: 10.1111/j.1469-8137.2009.02880.x. [DOI] [PubMed] [Google Scholar]
- 149.Dvorak P., Krasylenko Y., Zeiner A., Samaj J., Takac T. Signaling toward ROS-Scavenging Enzymes in Plants. Front. Plant Sci. 2020 doi: 10.3389/fpls.2020.618835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhou X., Xiang Y., Li C., Yu G. Modulatory Role of Reactive Oxygen Species in Root Development in Model Plant of Arabidopsis thaliana. Front. Plant Sci. 2020 doi: 10.3389/fpls.2020.485932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bibikova T.N., Jacob T., Dahse I., Gilroy S. Localized Changes in Apoplastic and Cytoplasmic PH Are Associated with Root Hair Development in Arabidopsis thaliana. Development. 1998;125:2925–2934. doi: 10.1242/dev.125.15.2925. [DOI] [PubMed] [Google Scholar]
- 152.Monshausen G.B., Bibikova T.N., Messerli M.A., Shi C., Gilroy S. Oscillations in Extracellular PH and Reactive Oxygen Species Modulate Tip Growth of Arabidopsis Root Hairs. Proc. Natl. Acad. Sci. USA. 2007 doi: 10.1073/pnas.0708586104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Felle H.H., Hepler P.K. The Cytosolic Ca2+ Concentration Gradient of Sinapis Alba Root Hairs as Revealed by Ca2+-Selective Microelectrode Tests and Fura-Dextran Ratio Imaging. Plant Physiol. 1997 doi: 10.1104/pp.114.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cole R.A., Fowler J.E. Polarized Growth: Maintaining Focus on the Tip. Curr. Opin. Plant Biol. 2006 doi: 10.1016/j.pbi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 155.Yokawa K., Koshiba T., Baluška F. Light-Dependent Control of Redox Balance and Auxin Biosynthesis in Plants. Plant Signal. Behav. 2014 doi: 10.4161/psb.29522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Silva-Navas J., Moreno-Risueno M.A., Manzano C., Téllez-Robledo B., Navarro-Neila S., Carrasco V., Pollmann S., Gallego F.J., Del Pozo J.C. Flavonols Mediate Root Phototropism and Growth through Regulation of Proliferation-to-Differentiation Transition. Plant Cell. 2016 doi: 10.1105/tpc.15.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chapman J.M., Muhlemann J.K., Gayomba S.R., Muday G.K. RBOH-Dependent ROS Synthesis and ROS Scavenging by Plant Specialized Metabolites to Modulate Plant Development and Stress Responses. Chem. Res. Toxicol. 2019 doi: 10.1021/acs.chemrestox.9b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Foreman J., Demidchik V., Bothwell J.H.F., Mylona P., Miedema H., Angel Torres M., Linstead P., Costa S., Brownlee C., Jones J.D.G., et al. Reactive Oxygen Species Produced by NADPH Oxidase Regulate Plant Cell Growth. Nature. 2003 doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 159.Vernoud V., Horton A.C., Yang Z., Nielsen E. Analysis of the Small GTPase Gene Superfamily of Arabidopsis. Plant Physiol. 2003 doi: 10.1104/pp.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yoo C.M., Blancaflor E.B. Overlapping and Divergent Signaling Pathways for ARK1 and AGD1 in the Control of Root Hair Polarity in Arabidopsis thaliana. Front. Plant Sci. 2013 doi: 10.3389/fpls.2013.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pei W., Du F., Zhang Y., He T., Ren H. Control of the Actin Cytoskeleton in Root Hair Development. Plant Sci. 2012 doi: 10.1016/j.plantsci.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 162.Vanneste S., Friml J. Auxin: A Trigger for Change in Plant Development. Cell. 2009 doi: 10.1016/j.cell.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 163.Xu T., Wen M., Nagawa S., Fu Y., Chen J.G., Wu M.J., Perrot-Rechenmann C., Friml J., Jones A.M., Yang Z. Cell Surface- and Rho GTPase-Based Auxin Signaling Controls Cellular Interdigitation in Arabidopsis. Cell. 2010 doi: 10.1016/j.cell.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bloch D., Yalovsky S. Cell Polarity Signaling. Curr. Opin. Plant Biol. 2013 doi: 10.1016/j.pbi.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 165.Denninger P., Reichelt A., Schmidt V.A.F., Mehlhorn D.G., Asseck L.Y., Stanley C.E., Keinath N.F., Evers J.F., Grefen C., Grossmann G. Distinct RopGEFs Successively Drive Polarization and Outgrowth of Root Hairs. Curr. Biol. 2019 doi: 10.1016/j.cub.2019.04.059. [DOI] [PubMed] [Google Scholar]
- 166.Jones M.A., Raymond M.J., Yang Z., Smirnoff N. NADPH Oxidase-Dependent Reactive Oxygen Species Formation Required for Root Hair Growth Depends on ROP GTPase. J. Exp. Bot. 2007 doi: 10.1093/jxb/erl279. [DOI] [PubMed] [Google Scholar]
- 167.Duan Q., Kita D., Li C., Cheung A.Y., Wu H.M. FERONIA Receptor-like Kinase Regulates RHO GTPase Signaling of Root Hair Development. Proc. Natl. Acad. Sci. USA. 2010 doi: 10.1073/pnas.1005366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Takeda S., Gapper C., Kaya H., Bell E., Kuchitsu K., Dolan L. Local Positive Feedback Regulation Determines Cell Shape in Root Hair Cells. Science. 2008 doi: 10.1126/science.1152505. [DOI] [PubMed] [Google Scholar]
- 169.Singh A.P., Fridman Y., Friedlander-Shani L., Tarkowska D., Strnad M., Savaldi-Goldstein S. Activity of the Brassinosteroid Transcription Factors Brassinazole Resistant1 and Brassinosteroid Insensitive1-Ethyl Methanesulfonate-Suppressor1/Brassinazole Resistant2 Blocks Developmental Reprogramming in Response to Low Phosphate Availability. Plant Physiol. 2014;166:678–688. doi: 10.1104/pp.114.245019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fu Y., Li H., Yang Z. The ROP2 GTPase Controls the Formation of Cortical Fine F-Actin and the Early Phase of Directional Cell Expansion during Arabidopsis Organogenesis. Plant Cell. 2002 doi: 10.1105/tpc.001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yalovsky S., Bloch D., Sorek N., Kost B. Regulation of Membrane Trafficking, Cytoskeleton Dynamics, and Cell Polarity by ROP/RAC GTPases. Plant Physiol. 2008 doi: 10.1104/pp.108.122150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Baluška F., Salaj J., Mathur J., Braun M., Jasper F., Šamaj J., Chua N.H., Barlow P.W., Volkmann D. Root Hair Formation: F-Actin-Dependent Tip Growth Is Initiated by Local Assembly of Profilin-Supported F-Actin Meshworks Accumulated within Expansin-Enriched Bulges. Dev. Biol. 2000 doi: 10.1006/dbio.2000.9908. [DOI] [PubMed] [Google Scholar]
- 173.Molendijk A.J., Bischoff F., Rajendrakumar C.S.V., Friml J., Braun M., Gilroy S., Palme K. Arabidopsis Thaliana Rop GTPases Are Localized to Tips of Root Hairs and Control Polar Growth. EMBO J. 2001 doi: 10.1093/emboj/20.11.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gu F., Nielsen E. Targeting and Regulation of Cell Wall Synthesis during Tip Growth in Plants. J. Integr. Plant Biol. 2013 doi: 10.1111/jipb.12077. [DOI] [PubMed] [Google Scholar]
- 175.Yang G., Gao P., Zhang H., Huang S., Zheng Z.L. A Mutation in MRH2 Kinesin Enhances the Root Hair Tip Growth Defect Caused by Constitutively Activated ROP2 Small GTPase in Arabidopsis. PLoS ONE. 2007 doi: 10.1371/journal.pone.0001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Miller R.K., Matheos D., Rose M.D. The Cortical Localization of the Microtubule Orientation Protein, Kar9p, Is Dependent upon Actin and Proteins Required for Polarization. J. Cell Biol. 1999 doi: 10.1083/jcb.144.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bruaene N., Joss G., Van Oostveldt P. Reorganization and In Vivo Dynamics of Microtubules during Arabidopsis Root Hair Development. Plant Physiol. 2004 doi: 10.1104/pp.103.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ringli C., Baumberger N., Diet A., Frey B., Keller B. ACTIN2 Is Essential for Bulge Site Selection and Tip Growth during Root Hair Development of Arabidopsis. Plant Physiol. 2002 doi: 10.1104/pp.005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wasteneys G.O., Yang Z. The Cytoskeleton Becomes Multidisciplinary. Plant Physiol. 2004 doi: 10.1104/pp.104.900130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kiefer C.S., Claes A.R., Nzayisenga J.C., Pietra S., Stanislas T., Hüser A., Ikeda Y., Grebe M. Arabidopsis AIP1-2 Restricted by WER-Mediated Patterning Modulates Planar Polarity. Development. 2015 doi: 10.1242/dev.122697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ketelaar T. The Actin Cytoskeleton in Root Hairs: All Is Fine at the Tip. Curr. Opin. Plant Biol. 2013 doi: 10.1016/j.pbi.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 182.Noack L.C., Jaillais Y. Precision Targeting by Phosphoinositides: How PIs Direct Endomembrane Trafficking in Plants. Curr. Opin. Plant Biol. 2017 doi: 10.1016/j.pbi.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 183.Preuss M.L., Serna J., Falbel T.G., Bednarek S.Y., Nielsen E. The Arabidopsis Rab GTPase RabA4b Localizes to the Tips of Growing Root Hair Cells. Plant Cell. 2004 doi: 10.1105/tpc.021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Preuss M.L., Schmitz A.J., Thole J.M., Bonner H.K.S., Otegui M.S., Nielsen E. A Role for the RabA4b Effector Protein PI-4Kβ1 in Polarized Expansion of Root Hair Cells in Arabidopsis thaliana. J. Cell Biol. 2006 doi: 10.1083/jcb.200508116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Thole J.M., Vermeer J.E.M., Zhang Y., Gadella T.W.J., Nielsen E. Root Hair Defective4 Encodes a Phosphatidylinositol-4-Phosphate Phosphatase Required for Proper Root Hair Development in Arabidopsis thaliana. Plant Cell. 2008 doi: 10.1105/tpc.107.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kusano H., Testerink C., Vermeer J.E.M., Tsuge T., Shimada H., Oka A., Munnik T., Aoyama T. The Arabidopsis Phosphatidylinositol Phosphate 5-Kinase PIP5K3 Is a Key Regulator of Root Hair Tip Growth. Plant Cell. 2008 doi: 10.1105/tpc.107.056119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Stenzel I., Ischebeck T., König S., Hołubowska A., Sporysz M., Hause B., Heilmanna I. The Type B Phosphatidylinositol-4-Phosphate 5-Kinase 3 Is Essential for Root Hair Formation in Arabidopsis thaliana. Plant Cell. 2008 doi: 10.1105/tpc.107.052852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yoo C.M., Quan L., Cannon A.E., Wen J., Blancaflor E.B. AGD1, a Class 1 ARF-GAP, Acts in Common Signaling Pathways with Phosphoinositide Metabolism and the Actin Cytoskeleton in Controlling Arabidopsis Root Hair Polarity. Plant J. 2012 doi: 10.1111/j.1365-313X.2011.04856.x. [DOI] [PubMed] [Google Scholar]
- 189.Bubb M.R., Baines I.C., Korn E.D. Localization of Actobindin, Profilin I, Profilin II, and Phosphatidylinositol-4,5-Bisphosphate (PIP2) in Acanthamoeba castellanii. Cell Motil. 1998 doi: 10.1002/(SICI)1097-0169(1998)39:2134::AID-CM43.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 190.Synek L., Schlager N., Eliáš M., Quentin M., Hauser M.T., Žárský V. AtEXO70A1, a Member of a Family of Putative Exocyst Subunits Specifically Expanded in Land Plants, Is Important for Polar Growth and Plant Development. Plant J. 2006 doi: 10.1111/j.1365-313X.2006.02854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Drdová E.J., Synek L., Pečenková T., Hála M., Kulich I., Fowler J.E., Murphy A.S., Žárský V. The Exocyst Complex Contributes to PIN Auxin Efflux Carrier Recycling and Polar Auxin Transport in Arabidopsis. Plant J. 2013 doi: 10.1111/tpj.12074. [DOI] [PubMed] [Google Scholar]
- 192.Ovečka M., Lang I., Baluška F., Ismail A., Illeš P., Lichtscheidl I.K. Endocytosis and Vesicle Trafficking during Tip Growth of Root Hairs. Protoplasma. 2005 doi: 10.1007/s00709-005-0103-9. [DOI] [PubMed] [Google Scholar]
- 193.Leiber R.M., John F., Verhertbruggen Y., Diet A., Knox J.P., Ringli C. The TOR Pathway Modulates the Structure of Cell Walls in Arabidopsis. Plant Cell. 2010 doi: 10.1105/tpc.109.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Westermann J., Streubel S., Franck C.M., Lentz R., Dolan L., Boisson-Dernier A. An Evolutionarily Conserved Receptor-like Kinases Signaling Module Controls Cell Wall Integrity During Tip Growth. Curr. Biol. 2019 doi: 10.1016/j.cub.2019.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhu S., Martínez Pacheco J., Estevez J.M., Yu F. Autocrine Regulation of Root Hair Size by the RALF-FERONIA-RSL4 Signaling Pathway. New Phytol. 2020 doi: 10.1111/nph.16497. [DOI] [PubMed] [Google Scholar]
- 196.Kim D., Yang J., Gu F., Park S., Combs J., Adams A., Mayes H.B., Jeon S.J., Bahk J.D., Nielsen E. A Temperature-Sensitive FERONIA Mutant Allele That Alters Root Hair Growth. Plant Physiol. 2020 doi: 10.1093/plphys/kiaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dong Q.K., Zhang Z.W., Liu Y.T., Tao L.Z., Liu H.L. FERONIA Regulates Auxin-Mediated Lateral Root Development and Primary Root Gravitropism. FEBS Lett. 2019 doi: 10.1002/1873-3468.13292. [DOI] [PubMed] [Google Scholar]
- 198.Krüger F., Schumacher K. Pumping up the Volume—Vacuole Biogenesis in Arabidopsis thaliana. Semin. Cell Dev. Biol. 2018 doi: 10.1016/j.semcdb.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 199.Kaiser S., Eisa A., Kleine-Vehn J., Scheuring D. NET4 Modulates the Compactness of Vacuoles in Arabidopsis thaliana. Int. J. Mol. Sci. 2019;20:4752. doi: 10.3390/ijms20194752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dünser K., Gupta S., Herger A., Feraru M.I., Ringli C., Kleine-Vehn J. Extracellular Matrix Sensing by FERONIA and Leucine-Rich Repeat Extensins Controls Vacuolar Expansion during Cellular Elongation in Arabidopsis thaliana. EMBO J. 2019 doi: 10.15252/embj.2018100353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Barbez E., Dünser K., Gaidora A., Lendl T., Busch W. Auxin Steers Root Cell Expansion via Apoplastic PH Regulation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2017 doi: 10.1073/pnas.1613499114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Höfte H. The Yin and Yang of Cell Wall Integrity Control: Brassinosteroid and FERONIA Signaling. Plant Cell Physiol. 2015 doi: 10.1093/pcp/pcu182. [DOI] [PubMed] [Google Scholar]
- 203.Yu M., Li R., Cui Y., Chen W., Li B., Zhang X., Bu Y., Cao Y., Xing J., Jewaria P.K., et al. The RALF1-FERONIA Interaction Modulates Endocytosis to Mediate Control of Root Growth in Arabidopsis. Develompent. 2020 doi: 10.1242/dev.189902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.