Abstract
BACKGROUND:
The safety and efficacy of convalescent plasma in severe coronavirus disease 2019 (COVID-19) remain uncertain. To support a guideline on COVID-19 management, we conducted a systematic review and meta-analysis of convalescent plasma in COVID-19 and other severe respiratory viral infections.
METHODS:
In March 2020, we searched international and Chinese biomedical literature databases, clinical trial registries and prepublication sources for randomized controlled trials (RCTs) and nonrandomized studies comparing patients receiving and not receiving convalescent plasma. We included patients with acute coronavirus, influenza and Ebola virus infections. We conducted a meta-analysis using random-effects models and assessed the quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.
RESULTS:
Of 1099 unique records, 6 studies were eligible, and none of these included patients with COVID-19. One nonrandomized study (n = 40) on convalescent plasma in severe acute respiratory syndrome coronavirus (SARS-CoV) provided uninformative results regarding mortality (relative risk [RR] 0.10, 95% confidence interval [CI] CI 0.01 to 1.70). Pooled estimates from 4 RCTs on influenza (n = 572) showed no convincing effects on deaths (4 RCTs, RR 0.94, 95% CI 0.49 to 1.81), complete recovery (2 RCTs, odds ratio 1.04, 95% CI 0.69 to 1.64) or length of stay (3 RCTs, mean difference −1.62, 95% CI −3.82 to 0.58, d). The quality of evidence was very low for all efficacy outcomes. Convalescent plasma caused few or no serious adverse events in influenza RCTs (RR 0.85, 95% CI 0.56 to 1.29, low-quality evidence).
INTERPRETATION:
Studies of non-COVID-19 severe respiratory viral infections provide indirect, very low-quality evidence that raises the possibility that convalescent plasma has minimal or no benefit in the treatment of COVID-19 and low-quality evidence that it does not cause serious adverse events.
Coronavirus disease 2019 (COVID-19) has been diagnosed in nearly 3 million individuals around the globe, of whom around 0.2 million have died.1 Many patients with COVID-19 develop severe acute respiratory illness requiring admission to intensive care units (ICU) and often mechanical ventilation.2 The case fatality rate in COVID-19 may be as high as 2.3% overall2 and from 10% to 40% among severely affected individuals. 3,4 There is an urgent need for effective therapies.
Emerging epidemiologic and clinical data show both similarities and differences between severe COVID-19 and severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS).5 Similarly, treatment strategies for severe influenza infections tested during the H1N1 pandemic and H5N1 and H7N9 outbreaks could inform the care of patients with severe COVID-19.6
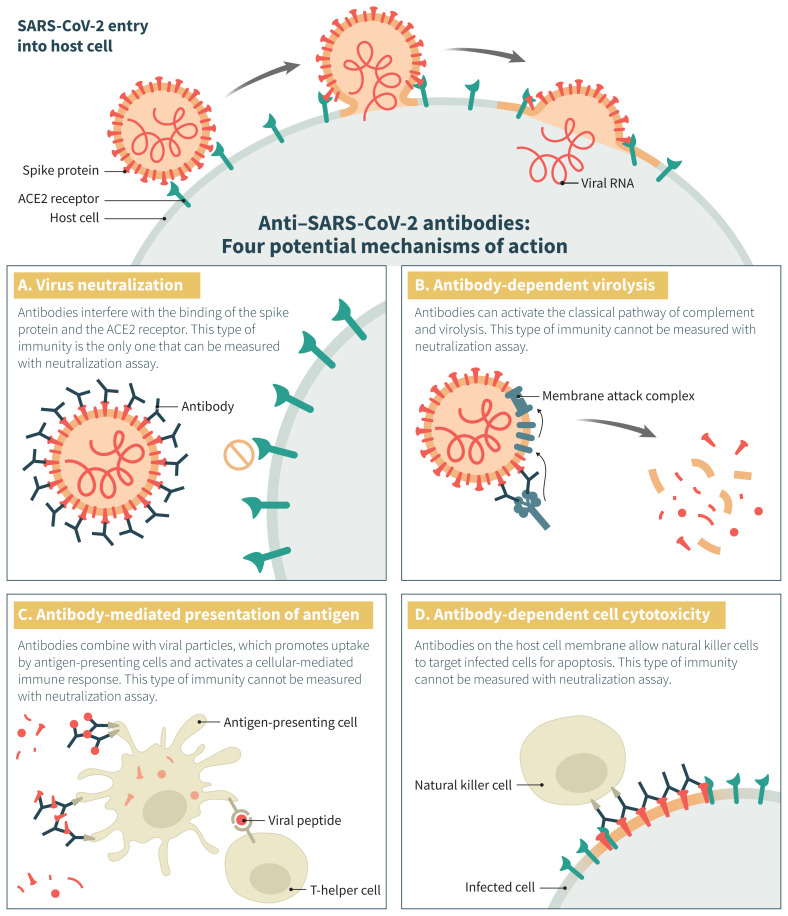
Of the treatment options proposed for COVID-19,7 convalescent plasma has evidence suggesting a mortality benefit for Ebola virus infection.8 This intervention has also been tested in other severe acute viral respiratory infections.6,9,10 “Convalescent plasma” refers to plasma obtained from individuals recently recovered from a viral illness, which is expected to contain the highest levels of polyclonal antibodies directed against the virus.11 Similarly, “hyperimmune plasma” is collected from donors exhibiting high titres of neutralizing antibodies, independent of time elapsed since viral illness. Authors have used the terms interchangeably, and because viral neutralization is only one of the postulated mechanisms by which antibodies exert their antiviral effect, the importance of the distinction between the 2 products remains unclear (Figure 1).
Figure 1:
Potential mechanisms of action of anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in coronavirus disease 2019 (COVID-19). This figure illustrates the normal entry of SARS-CoV-2 in a host cell, in which membrane fusion is mediated by the interaction between the SARS-CoV-2 spike glycoprotein (red) and the angiotensin-converting enzyme 2 (ACE2) receptor (green) on the host cell, either through the cytoplasmic or endosomal route. Antibodies directed against the receptor-binding domain (RBD) of the spike protein can interfere with its interaction with the ACE2 receptor and prevent viral entry in the host cell (panel A). Antibodies directed against epitopes outside the RBD can also exert antiviral functions through other mechanisms (panels B, C and D). The relative importance of these various functions in rescuing patients from an active SARS-CoV-2 infection is unknown. Importantly, neutralization assays generally used to qualify hyperimmune products measure only 1 of the 4 mechanisms depicted here and do not necessarily correlate with the others.
Clinicians have typically administered convalescent plasma to patients with viral infections whose condition deteriorated despite supportive care.6 Although the primary postulated mechanism of action of convalescent plasma is reduction in viremia (passive immunity),12 an increase in host immune response (active immunity) has also been proposed.13 We describe in Figure 1 the possible mechanisms by which convalescent plasma inhibits severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Systematic summaries of the available evidence regarding safety and effectiveness can inform the use of convalescent plasma in patients with COVID-19. We therefore conducted a systematic review to summarize the evidence for convalescent plasma to support a guideline on COVID-19 management.14 Because we anticipated a paucity of direct evidence addressing the use of convalescent plasma in COVID-19, we summarized the available evidence addressing convalescent plasma in the treatment of SARS, MERS and influenza, including H1N1, H7N9 and H5N1, as well as addressing possible adverse effects in patients with Ebola disease.
Methods
This systematic review and meta-analysis follows PRISMA (Preferred reporting items for systematic reviews and meta-analyses) reporting guidelines.15 A supplementary file presents the systematic review protocol (Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.200642/-/DC1).
Study selection
We included studies of patients infected with SARS-CoV-2, SARS-CoV, MERS-CoV or influenza virus with severe respiratory infection. For safety outcomes we also included data from Ebola virus infection. We used the COVID-19 definition of severe respiratory infection from the World Health Organization: fever or suspected respiratory infection, plus 1 of the following: respiratory rate > 30 breaths/min, severe respiratory distress, or peripheral oxygen saturation (SpO2) ≤ 93% on room air.16 Eligible studies compared intravenous convalescent plasma or hyperimmune immunoglobulin against standard care management without use of convalescent plasma.
Outcomes of interest included mortality at longest follow-up, extent of recovery, length of ICU stay, length of hospital stay, days of mechanical ventilation and viral load. We also sought evidence of serious adverse outcomes, including complications related to intravascular volume overload and transfusion-related acute lung injury, allergy or anaphylaxis, and other serious adverse events.
For each patient population, we included only randomized controlled trials (RCTs) if they were available and dealt satisfactorily with issues of both benefit and harm. If RCTs were not available, or if nonrandomized comparative studies yielded important complementary information, we included nonrandomized studies with adjusted analysis and, if these were not available, any nonrandomized studies. We excluded studies with no comparator arm.
Data sources and searches
With the assistance of a medical librarian (R.C.), we searched health care databases (MEDLINE, Embase, PubMed for nonindexed studies and the Cochrane Central Register of Controlled Trials [CENTRAL]) on Mar. 6 and updated on Apr. 19, medRxiv (for non–peer reviewed prepublication sources) on Mar. 11 and updated on Apr. 26, Chinese databases (China National Knowledge Infrastructure [CNKI], Wanfang, Chongqing VIP Information [CQVIP] and SinoMed) on Mar. 23 and updated on Apr. 21, and ChinaXiv on Apr. 26, 2020. The key search words were virus-related terms (coronavirus, influenza, Ebola, MERS, SARS) and intervention-related terms (convalescent plasma and hyperimmune plasma). There were no restrictions by language. Appendix 1 presents the complete search strategy and summary of search results.
Two reviewers independently assessed titles and abstracts and, subsequently, for potentially eligible articles, the full text. Reviewers manually searched reference lists of eligible articles and published systematic reviews for eligible studies.
Data extraction and quality assessment
Two reviewers independently abstracted data related to study and participant characteristics, collection method of plasma and methods to quantify antibodies in the convalescent or hyperimmune plasma, study outcomes and risk of bias assessment, using Excel spreadsheets. Reviewers resolved disagreement through discussion.
A modified Cochrane risk-of-bias tool for RCTs provided guidance for risk-of-bias assessment.17 The instrument identifies 6 possible sources of bias in RCTs: sequence generation, concealment of allocation, blinding, loss to follow-up, selective outcome reporting and other problems. A modified Newcastle–Ottawa tool for comparative cohort studies18 that identifies possible sources of bias in selection of participants, temporality of outcome relative to exposure, measurement of outcome, exposure and prognostic factors, balance in prognostic factors, balance in concomitant therapy across groups, and completeness of follow-up provided guidance for observational studies. For both instruments, responses for each item were “definitely yes” or “probably yes” (low risk of bias), and “probably no” or “definitely no” (high risk of bias). We judged the overall risk of bias for each outcome in each study as “low risk” if all domains were rated as low risk of bias and otherwise as high risk of bias. Details are available at www.evidencepartners.com/resources/methodological-resources.
Quality of evidence
We evaluated the quality of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach19 including considerations of risk-of-bias assessment, indirectness, inconsistency, imprecision and publication bias. We reported the level of evidence in Summary of Findings tables.20,21
Data analysis
DerSimonian and Laird random-effects models were used for calculation of relative risks (RRs) for binary outcomes and proportional odds ratios (ORs) for ordinal outcomes, and 95% confidence intervals (CIs). For continuous outcomes, we computed weighted mean differences (MDs) using DerSimonian and Laird random-effects models. For the purposes of pooling, if studies reported medians and interquartile ranges rather than means and standard deviations, we approximated means and standard deviation using the following formula: 75th percentile–25th percentile)/1.35.22 We pooled effect estimates for each population by virus type. Anticipating a small number of studies, we did not plan funnel plots or statistical tests to explore publication bias, and we did not postulate subgroup effects. For computing risk differences (RDs) and 95% CIs, we applied pooled RRs and ORs to baseline risk estimates from studies of COVID-193 and, when not available, to the median baseline risk of control arms from the eligible studies. We used Stata 15 software for all analyses.
Ethics approval
Ethics approval was not required for this systematic review.
Results
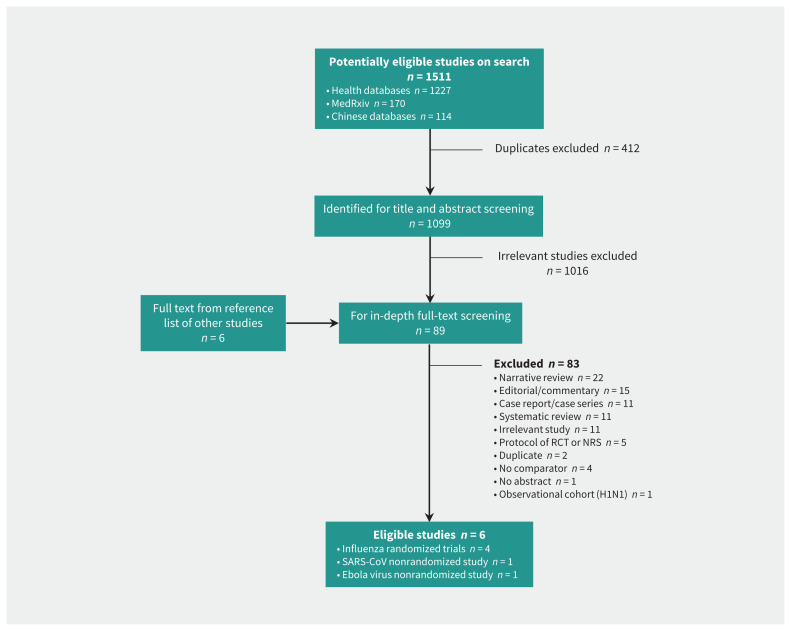
The search yielded 1099 articles after removal of duplicates. The PRISMA flow chart (Figure 2), presents the reasons for excluding studies at the time of full-text review. No eligible studies of COVID-19 were identified. Six studies23–28 (Table 1) proved eligible, including 4 RCTs involving patients infected with influenza virus.24–27 One nonrandomized study related to SARS-CoV23 also provided evidence of effectiveness. We obtained safety data from the influenza RCTs24–27 and nonrandomized studies of Ebola virus disease28 and SARS-CoV.23
Figure 2:
Study selection flow chart. Note: NRS = nonrandomized study, RCT = randomized controlled trial, SARS-CoV = severe acute respiratory syndrome coronavirus.
Table 1:
Characteristics of included studies
| Study; year; country | Study design | Disease | Intervention | Comparators | Sample size | Median age (IQR),* yr | ICU scoring system; median (IQR) score | Time since symptom onset, median (IQR), d |
|---|---|---|---|---|---|---|---|---|
| Soo et al., 2004,23 Hong Kong | Retrospective cohort | Hospital-admitted patients with SARS-CoV | 200–400 mL of CP (titre 1:160–1:2560) | Methylprednisolone (≥ 4 doses; each dose is a 500-mg pulse) | CP arm: n = 19 Control arm: n = 21 |
CP arm: mean 38.7 (SD 12.39) Control arm: mean 47.9 (SD 19.6) |
NR | NR |
| Hung et al., 2013,24 Hong Kong | RCT | Hospital-admitted patients with H1N1 influenza | Single infusion of 0.4 g/kg of H-IVIG solution over 4 hr; NAbT > 1:40 | 0.4 g/kg normal IVIG prepared by pharmacists; NAbT < 1:10 | CP arm: n = 17 Control arm: n = 18 |
CP arm: 43 (36.5 to 56) Control arm: 52 (40.5 to 58.5) |
APACHE II; CP arm: 12 (8 to 17.5) Control arm: 13 (9 to 19) |
CP arm: 2 (1 to 4) Control arm: 3 (2 to 5) |
| Beigel et al., 2017,25 US | RCT | Hospital-admitted patients with influenza A (H1N1, H3N2), influenza B | 2 units of CP infusion with at least 1-hr gap | Standard care that included a neuraminidase inhibitor | CP arm: n = 49 Control arm: n = 49 |
CP arm: 50 (38 to 66) Control arm: 57 (39 to 71) |
APACHE II; CP arm: 11 (8 to 21) Control arm: 16 (10 to 22) |
CP arm: 3 (2 to 5) Control arm: 4 (2 to 6) |
| Beigel et al., 2019,26 US | RCT | Hospital-admitted patients with influenza A (H1N1, H3N2) | 2 units high-titre anti-influenza plasma (hemagglutination inhibition titre ≥ 1:80) | 2 units of low titre plasma (hemagglutination inhibition titre, ≤ 1:10) | CP arm: n = 91 Control arm: n = 47 |
CP arm: 43 (36.5 to 56) Control arm: 52 (40.5 to 58.5) |
NEWS; CP arm: 5 (4 to 8) Control arm: 5 (3 to 7) PELOD score; CP arm: 0 (0 to 1) Control arm: 3 (1.5 to 12) |
CP arm: 3 (2 to 5) Control arm: 3 (2 to 4) |
| Davey et al., 2019,27 multi-national | RCT | Hospital-admitted patients with influenza A (H1N1, H3N2), influenza B | Single infusion 25 g/kg H-IVIG (up to 24.75 g in saline (500 mL) over 2 hr | Saline placed in 500-mL bag (coloured sleeve) | CP arm: n = 156 Control arm: n = 152 |
CP arm: 55 (41 to 68) Control arm: 57 (48 to 68) |
NEWS; CP arm: 4 (2 to 6) Control arm: 4 (2 to 6) |
CP arm: 3 (2 to 5) Control arm: 4 (2 to 5) |
| van Griensven et al., 2016,28 Guinea | Prospective non-randomized study | Laboratory-confirmed Ebola virus disease | 2 infusions 200–250 mL ABO-compatible CP (gap of 2 d) | Standard care without CP | CP arm: n = 99 Control arm: n = 418 |
CP arm: 29 (0 to 75) Control arm: 28 (0 to 87) |
NR | CP arm: 19% with > 6 d Control arm: 49% with > 6 d |
Note: APACHE II = Acute Physiology and Chronic Health Evaluation II, CP = convalescent plasma, H-IVIG = hyperimmune intravenous immunoglobulin, ICU = intensive care unit, IQR = interquartile range, IVIG = intravenous immunoglobulin, NAbT = neutralizing antibody titres, NEWS = National Early Warning Score, NR = not reported, PELOD = Pediatric Logistic Organ Dysfunction, RCT = randomized controlled trial, SARS-CoV = severe acute respiratory distress syndrome coronavirus, SD = standard deviation.
Unless stated otherwise.
Of the 4 RCTs, 3 blinded patients and physicians24,26,27 — 2 using low-titre intravenous immunoglobulin24,26 and 1 using saline infusion as a control27 — and 1 used an open-label design.25 Table 2 presents the risk of bias associated with mortality and length of stay. All RCTs except for 125 were low in risk of bias (Table 2).
Table 2:
Risk-of-bias assessment for mortality and length-of-stay outcomes using modified risk-of-bias criteria for randomized controlled trials and nonrandomized studies
| Influenza trials, mortality outcome | Sequence generation | Allocation concealment | Blinding (patients) | Blinding (health care providers) | Blinding (outcome assessors) | Blinding (data collectors) | Blinding (data analyst) | Loss to follow-up |
|---|---|---|---|---|---|---|---|---|
| Beigel et al., 201725 | Definitely yes | Definitely yes | Definitely yes | Definitely no | Probably no | Definitely yes | Probably no | Definitely no |
| Beigel et al., 201926 | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Probably yes |
| Davey et al., 201927 | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes |
| Hung et al., 201324 | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes |
| Non-RCT (SARS-CoV), mortality outcome | Selection of groups | Exposure assessment | Outcome following exposure | Baseline comparability | Measurement of prognostic variables | Outcome assessment | Completeness of follow-up | Concomitant treatments |
| Soo et al., 200423 | Definitely no | Probably yes | Definitely yes | Definitely no | Definitely no | Definitely no | Definitely no | Definitely no |
| Influenza trials, length of stay | Sequence generation | Allocation concealment | Blinding (patients) | Blinding (health care providers) | Blinding (outcome assessors) | Blinding (data collectors) | Blinding (data analyst) | Loss to follow-up |
| Beigel et al., 201926 | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Probably yes |
| Hung et al., 201324 | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes |
| Beigel et al., 201725 | Definitely yes | Definitely yes | Definitely yes | Definitely no | Probably no | Definitely yes | Probably no | Definitely no |
Note: RCT = randomized controlled trial, SARS-CoV = severe acute respiratory syndrome coronavirus.
The method of obtaining the hyperimmune sera varied across the RCTs. Only the trial reported by Hung and colleagues24 collected plasma from recently recovered donors (within 2 weeks) and quantified the antibody activity using neutralizing antibody titres. The other 3 trials25–27 obtained plasma from blood banks and included plasma from donors who recorded high doses of hemagglutination inhibition (HAI) titres and not necessarily from recently recovered individuals. All 3 RCTs reported very high levels of HAI titres (Table 1).
Mortality
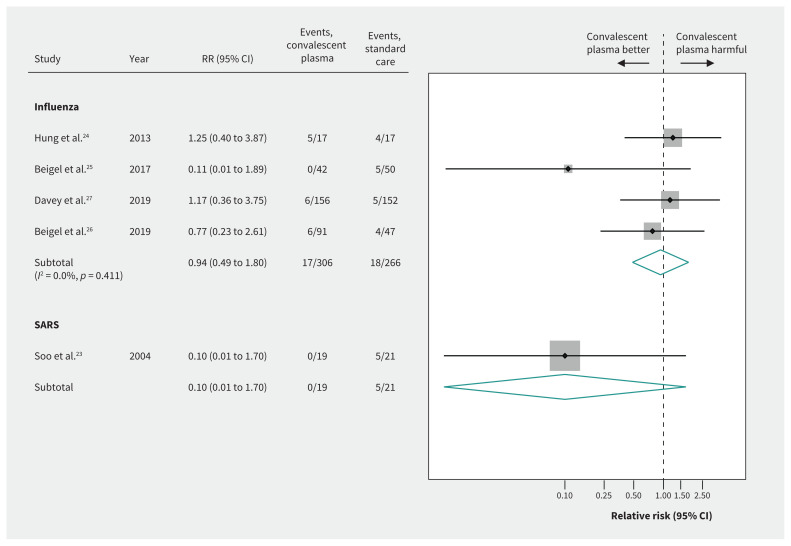
For SARS-CoV, a small nonrandomized comparative study suggested very uncertain effects of convalescent plasma on mortality, based on unadjusted estimates (RR 0.10, 95% CI 0.01 to 1.70, very low-quality evidence)23 (Figure 3 and Table 3).
Figure 3:
Efficacy of convalescent plasma on mortality in acute viral respiratory infections. Weights are from random-effects analysis. Note: CI = confidence interval, RR = relative risk, SARS = severe acute respiratory syndrome.
Table 3:
GRADE summary of findings on use of convalescent plasma in COVID-19
| Patient or population: Children or adults with severe COVID-19 infection Intervention: Convalescent or hyperimmune intravenous immunoglobulin Comparison: Usual care + placebo (saline or intravenous immunoglobulin) | |||||
|---|---|---|---|---|---|
| Outcome | Relative effects, source of evidence | Absolute effects | Certainty/quality of evidence | Plain-language summary | |
| Baseline risk for control group (per 1000) | Difference (95% CI) (per 1000) | ||||
| Mortality (7–28 d) | RR 0.94 (95% CI 0.49 to 1.80) Based on 572 patients with influenza in 4 RCTs |
104* | −6 (−53 to 84) | Very low ⊕⊖⊖⊖ (Very serious indirectness and serious imprecision)† | Convalescent plasma may have little to no effect on mortality, but the evidence is very uncertain. |
| Mortality (22 d) | RR 0.10 (95% CI 0.01 to 1.70) Based on 40 patients with SARS in 1 observational study |
104* | −94 (−103 to 73) | Very low ⊕⊖⊖⊖ (Serious indirectness, very serious risk of bias and serious imprecision)‡ | Convalescent plasma could have an important effect on decreasing or increasing mortality, but the evidence is very uncertain. |
| Recovery by 28 days as measured by a 6-point ordinal scale§ | Proportional OR for recovery§ OR 1.05 (95% CI 0.67 to 1.64) Based on 438 patients with influenza from 2 RCTs |
104* | 5 (−30 to 56) | Very low ⊕⊖⊖⊖ (Very serious indirectness and serious imprecision)† | Convalescent plasma may have little to no effect on recovery, but the evidence is very uncertain. |
| Length of hospital stay, d | Based on 259 patients with influenza in 3 RCTs | Median 13¶ | MD −1.62 (−3.82 to 0.58) | Very low ⊕⊖⊖⊖ (Very serious indirectness and serious imprecision)† | Convalescent plasma may confer a small reduction in hospital length of stay, but the evidence is very uncertain. |
| Length of ICU stay, d | Based on 149 patients with influenza in 2 RCTs | Median 7** | MD −0.32 (CI −3.20 to 2.56) | Very low ⊕⊖⊖⊖ (Very serious indirectness and serious imprecision)† | Convalescent plasma may have little to no effect in reducing duration of ICU stay, but the evidence is very uncertain. |
| Time on mechanical ventilation, d | Based on 83 patients with influenza in 2 RCTs | Median 9.25** | MD −3.67 (CI −7.70 to 0.36) | Very low ⊕⊖⊖⊖ (Very serious indirectness and serious imprecision)† | Convalescent plasma may reduce days of mechanical ventilation, but the evidence is very uncertain. |
| Serious adverse events | RR 0.85 (95% CI 0.56 to 1.29) Based on 576 patients with influenza in 3 RCTs |
80†† | −12 (−35 to 23) | Low ⊕⊕⊖⊖ (Serious indirectness and imprecision)‡‡ | Convalescent plasma may result in little or no difference in the number of serious adverse events. |
Note: CI = confidence interval; COVID-19 = coronavirus disease 2019; GRADE = Grading of Recommendations Assessment, Development, and Evaluation; ICU = intensive care unit; MD = mean difference; OR = odds ratio; RCT = randomized controlled trial; RR = relative risk; SARS = severe acute respiratory syndrome.
We chose the baseline risk from patients admitted to hospital with COVID-19 who did not receive convalescent plasma and steroids from the article by Guan et al.3 This paper reports 96/173 severely ill patients who did not receive steroids or hyperimmune plasma, of whom 10 patients died (Dr. W. Guan, State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong Province, China: personal communication, 2020). Hence, the baseline mortality risk is 10/96 = 10.4%. The median duration of hospital admission was 12.0 days (mean 12.8 d).
We rated down 2 levels for indirectness because clinical and epidemiologic characteristics of patients with influenza vary in COVID-19. We rated down 1 level for imprecision because the confidence interval included both important benefit and important harm.
Evidence from observational studies begins as low-quality evidence. We rated down 1 level for indirectness because evidence came from SARS rather than COVID-19. We rated 1 level down for imprecision because the confidence intervals included both important benefit and important harm.
Recovery defined by an ordinal outcome (6 mutually exclusive categories) at 28 days: death, in ICU, in hospital with oxygen support, in hospital without oxygen support, discharged but not fully recovered, discharged and fully recovered. An OR of > 1 indicates treatment is better than control, interpreted as odds of better recovery is 1.24 times higher among those treated with hyperimmune plasma than control arm. This OR is similar across categories. We also assumed the risk differences between treatment groups is the same across categories of the outcome.
We chose the median duration of hospital admission from hospital-admitted COVID-19 patients with severe disease from the article by Guan et al.3
This is the median number of days in ICU obtained from the control arm of RCTs including patients with severe influenza.
The baseline risk of serious adverse events obtained from the control arm of studies including influenza (3 studies).
We rated down 1 level for indirectness for this safety outcome, inferring that the adverse effects are likely to be similar across viral illnesses, and 1 level down for imprecision because the confidence intervals included both important benefit and important harm.
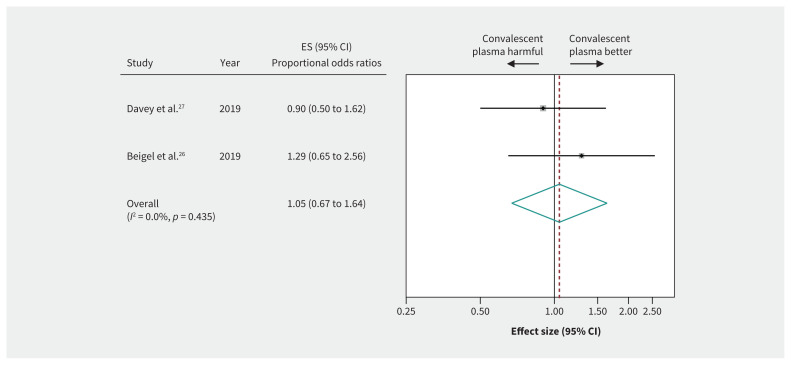
Evidence from 4 RCTs (n = 572) of severely ill patients with influenza showed inconclusive effects of convalescent plasma on mortality between 7 and 28 days (RR 0.94, 95% CI 0.49 to 1.80, very low-quality evidence) (Figure 3 and Table 3). Two RCTs in patients with influenza reported a 6-item ordinal outcome relating to extent of recovery (death, in ICU, in hospital with oxygen support, in hospital without oxygen support, discharged but not fully recovered, discharged and fully recovered) at 28 days.25–27 The pooled OR for recovery (438 patients from 2 RCTs26,27) was 1.05 (95% CI 0.67 to 1.64), representing very low-quality evidence (Figure 4 and Table 3).
Figure 4:
Efficacy of convalescent plasma in recovery from acute viral respiratory illness. Weights are from random-effects analysis. Note: CI = confidence interval, ES = effect size.
Length of hospital and ICU stay
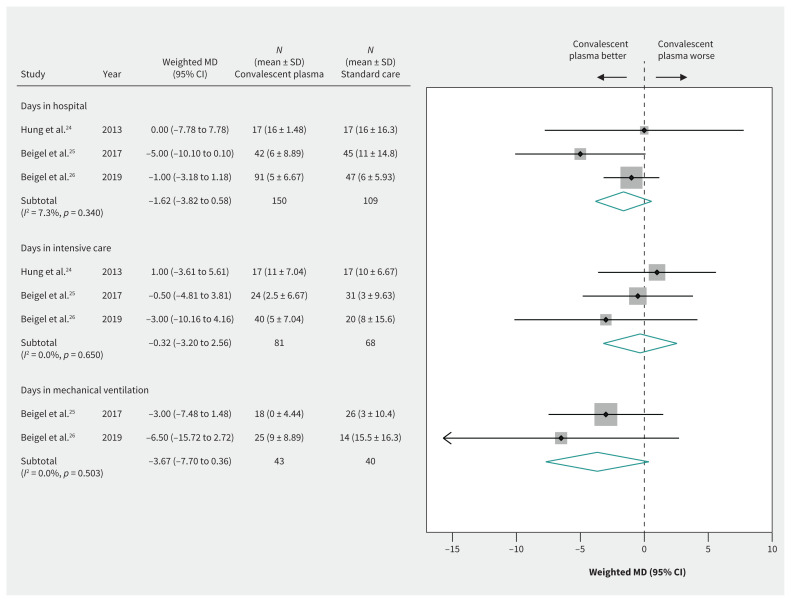
Evidence from 3 RCTs24–26 in patients with severe influenza suggested possible but uncertain effects of convalescent plasma on decreasing length of hospital stay (weighted MD −1.62, 95% CI −3.82 to 0.58, d), ICU stay (weighted MD −0.32, 95% CI −3.20 to 2.56, d) and duration of mechanical ventilation (weighted MD −3.67, 95% CI −7.70 to 0.36, d), providing very low-quality evidence in all cases (Figure 5 and Table 3).
Figure 5:
Efficacy of convalescent plasma in reducing length of hospital stay. Weights are from random-effects analysis. Note: CI = confidence interval, MD = mean difference, SD = standard deviation.
Reduction in viral load
Pooled estimates from 2 RCTs25,27 in patients with severe influenza (n = 334) showed inconclusive effects of convalescent plasma on the proportion of patients with nondetectable levels of virus in nasopharyngeal specimens on day 3 (RR 1.07, 95% CI 0.58 to 1.8, very low-quality evidence) and a possible but uncertain increase in patients with undetectable virus on day 725 (RR 1.32, 95% CI 0.97 to 1.81, very low-quality evidence).
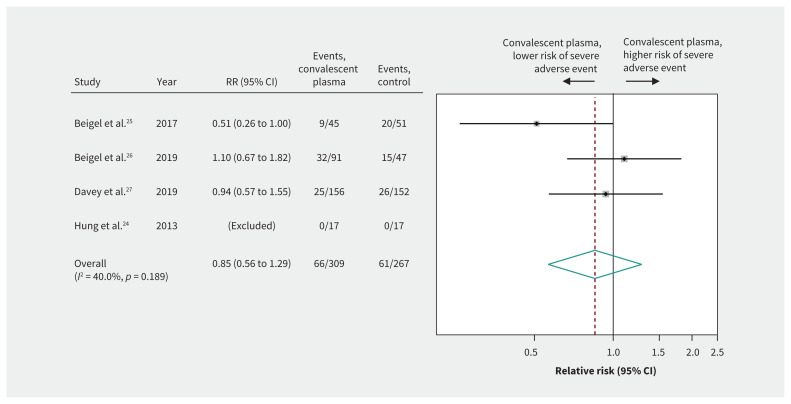
Harm
Pooled estimates from the 4 RCTs24–27 of patients with severe influenza (n = 576) suggested that convalescent plasma caused few or no serious adverse events (RR 0.85, 95% CI 0.56 to 1.29, low-quality evidence) (Figure 6 and Table 3). Two RCTs25,26 reported transfusion-specific serious adverse reactions in 8 of 140 (6%) patients in the convalescent plasma arm versus 6 of 96 (6%) patients in the control arm. Nonrandomized studies of patients with SARS-CoV23 or Ebola virus28 infections and 1 RCT in patients with influenza24 reported no serious adverse events related to intravascular volume overload and transfusion-related acute lung injury or serious allergic reactions due to plasma transfusion.
Figure 6:
Safety of convalescent plasma. Weights are from random-effects analysis. Note: CI = confidence interval, RR = relative risk.
Interpretation
Existing studies provide only very low-quality evidence to support inferences regarding benefits of convalescent plasma in patients with COVID-19. Based on low-quality evidence, there is no suggestion that convalescent plasma would cause any serious adverse events in patients with COVID-19.
On Mar. 25, 2020, the US Food and Drug Administration approved the use of convalescent plasma for COVID-19 under the emergency investigational new drug category and not for routine clinical use.29 Its inclusion in the expanded access program should not be perceived as an endorsement of efficacy, as the criteria for the expanded access program is not demonstrated efficacy but rather lack of other approved therapeutic options or access to a clinical trial. Whether the limited evidence of benefit — or, in this case, of little or no benefit — is sufficient to justify compassionate use of an unproven intervention is a matter of debate.30
Strengths of this systematic review include the comprehensive search across databases for emerging and past evidence and use of the GRADE approach to assess the quality of evidence with attention to indirectness of the study populations in relation to patients with COVID-19. Eligibility decisions, risk-of-bias assessment, and data abstraction were all conducted in duplicate. We limited risk of bias by excluding single-arm studies.
A systematic review published in 2006 summarized evidence from 8 comparative observational studies addressing convalescent plasma (n = 1703 patients) conducted during the Spanish flu pandemic of 1918.10 The range of absolute RDs in mortality between patients who received convalescent plasma and control groups was 8% to 26% (pooled RD 21%, 95% CI 15% to 27%).10 However, these observational studies with evident imbalance in prognostic factors provide only very low-quality evidence even for influenza, and thus the evidence is even lower quality when applied to COVID-19.
Similarly, a 2015 systematic review6 reported a very large mortality reduction (OR 0.25, 95% CI 0.14 to 0.45; I2 = 0%) in a post hoc meta-analysis of observational studies (n = 8) of convalescent plasma in severe SARS (1 retrospective cohort23 and 1 case series31) and influenza A. Once again, this evidence is very low quality even for SARS, and thus even lower quality for COVID-19.
A recent systematic review32 that appeared on a non–peer reviewed preprint server33 pooled 5 influenza RCTs, of which 4 RCTs24–27 are included in our review.32 The pooled mortality estimates were very similar to those of our review, OR 1.06 (95% CI 0.51 to 2.23). However, the authors included a pilot trial of the International Network for Strategic Initiatives in Global HIV Trials, INSIGHT FLU005,34 thus double-counting patients included in the main trial.27 Further, this review did not pool the ordinal outcome (extent of recovery) and did not use GRADE methodology for rating the quality of the evidence. Most relevant for our review is the GRADE focus on assessing the indirectness of the evidence.
There are 3 studies reporting on the use of convalescent plasma in patients with severe COVID-19. One case series35 reported on 5 patients critically ill with COVID-19, of whom 3 were discharged and 2 improved, coincident with receipt of convalescent plasma. Another uncontrolled phase 1 study reported clinical improvement with resolution of lung lesions in 10 patients with severe COVID-19 who received convalescent plasma.36 Finally a case series of 4 patients, including 1 pregnant woman, also reported clinical improvement of all patients after transfusion.37 These uncontrolled case series are insufficient to question the inference from this review that benefits from convalescent plasma in patients with COVID-19 remain very uncertain. Recently, experimental monoclonal antibodies have shown promise in reducing mortality in Ebola virus disease.38 Generalization of results from these forms of passive immunization using highly standardized monoclonal antibodies selected for its antiviral potency to COVID-19 is, at best, very questionable.
Limitations
One important limitation of this review stems from the level of indirectness given that the majority of evidence comes from trials in influenza. Although this is the best evidence available in this urgent situation, the different biological, clinical and epidemiologic characteristics of influenza versus COVID-19 severely limit inferences regarding effects of convalescent plasma against the new virus. The unique 2-phase immune response observed in patients with severe coronavirus infections has direct implications for the potential effect of immune-boosting strategies.39 In addition, the small number of studies and events led to very wide confidence intervals, further lowering the quality of the evidence. Finally, because observational studies may provide additional evidence of harms, our restriction to randomized influenza trials represents, in this regard, a limitation.
Another limitation is that the method of plasma collection offers a possible explanation for lack of efficacy observed in the influenza trials. Only 1 trial24 used plasma from individuals recovered from influenza in the prior 2 weeks. The other trials25–27 referred to their intervention as hyperimmune plasma and reported HAI titres that were quite high.25–27 The use of HAI for quantifying antibody activity of donor plasma has, however, proven controversial.40 Those who argue that HAI is of limited use might explain the negative results of 3 of the trials as a result of their failure to use truly convalescent plasma.40 This line of reasoning would suggest that trials studying the efficacy of convalescent plasma in COVID-19 should obtain plasma from recently recovered individuals and report the number of days since recovery of the donor along with information regarding serologic and functional assays on the plasma. Such a study design would markedly reduce doubts regarding the explanation of such studies failing to detect benefit.
Conclusion
The merit of convalescent plasma is its apparent low rate of serious adverse effects. Although this may be an advantage over other unproven therapies for COVID-19, it is insufficient to justify its use without associated evidence of efficacy. Collection of convalescent plasma consumes resources that, pending trustworthy evidence of positive effects, could be better allocated elsewhere. Widespread use of convalescent plasma should therefore await high-quality evidence from randomized trials, ideally testing the effect of plasma obtained from individuals recently recovered from COVID-19. Prioritization of clinical trials for testing efficacy of convalescent plasma over testing of other unproven therapeutic options for COVID-19 should be based not only on existing evidence but also on the sociopolitical context. The fact that clinicians have started using convalescent plasma in patients with COVID-19 in hospitals outside clinical trials makes it urgent to address its therapeutic value.
Acknowledgement
The authors thank Quazi Ibrahim for statistical advice. Yingqi Xiao is supported by the China Scholarship Council (no. 201906240082).
See related article at www.cmaj.ca/lookup/doi/10.1503/cmaj.200648
Footnotes
Competing interests: Mark Loeb has received personal fees and nonfinancial support from Sanofi, nonfinancial support from the World Health Organization, grant funding and personal fees from Seqirus, and personal fees from Pfizer, Medicago and the National Institutes of Health. Philippe Bégin is the co–principal investigator of a multicentre randomized controlled trial investigating the use of convalescent plasma in coronavirus disease 2019 (COVID-19). Philippe Bégin reports personal fees from Novartis, Pfizer, Sanofi, ALK and Aralez, as well as grants from DBV Technologies, Regeneron and Sanofi outside the submitted work. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Niveditha Devasenapathy was responsible for the systematic review, performed the meta-analyses, interpreted the results, wrote the first draft of the manuscript and had final responsibility for the decision to submit for publication. Gordon Guyatt approved the review methodology, interpreted the results, provided critical comments on the manuscript and approved the final draft. Fang Fang and Yingqi Xiao were involved in the search of Chinese databases. Borna Tadayon Najafabadi was involved in the screening of eligible studies and data extraction. Zhikang Ye was involved in the systematic review design and interpretation of results. Fang Fang, Yingqi Xiao and Borna Tadayon Najafabadi were involved in screening and data extraction. Mark Loeb and Philippe Bégin were involved in the interpretation of the study results and provided critical comments on the manuscript. Rachel Couban is a medical information specialist and designed the search strategy and conducted the search. All of the authors revised the manuscript for important intellectual content. Niveditha Devasenapathy, Gordon Guyatt and Zhikang Ye had full access to all the data in the study. All of the authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Data sharing: Data extracted from the included studies are presented in the results; however, full extraction data tables are available on reasonable request from the corresponding author.
References
- 1.Coronavirus disease (COVID-2019) situation reports. Geneva: World Health Organization; 2020. Available: www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed 2020 Apr. 27). [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020. Feb. 24 [Epub ahead of print]. 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, et al. China Medical Treatment Expert Group for COVID-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Wang Y, Chen Y, et al. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol 2020. Mar. 5 [Epub ahead of print]. 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. Convalescent Plasma Study Group. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis 2015; 211: 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol 2020;92:479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JS, Adhikari NKJ, Kwon HY, et al. Anti-Ebola therapy for patients with Ebola virus disease: a systematic review. BMC Infect Dis 2019;19:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med 2006;3:e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luke TC, Kilbane EM, Jackson JL, et al. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: A future H5N1 treatment? Ann Intern Med 2006;145:599–609. [DOI] [PubMed] [Google Scholar]
- 11.Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an empirical treatment during outbreaks. Geneva: World Health Organization; 2014. Available: www.who.int/csr/resources/publications/ebola/convalescent-treatment/en (accessed 2020 Mar. 25). [Google Scholar]
- 12.Cheng Y, Wong R, Soo YO, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis 2005;24:44–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoofs T, Klein F, Braunschweig M, et al. HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science 2016; 352:997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye Z, Rochwerg B, Wang Y, et al. Treatment of patients with nonsevere and severe coronavirus disease 2019: an evidence-based guideline. CMAJ 2020. May 4 [Epub ahead of print]. 10.1503/cmaj.200648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- 16.Clinical management of severe acute respiratory infection when COVID-19 is suspected [Interim guidance]. Geneva: World Health Organization; 2020. Available: www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (accessed 2020 Mar. 21). [Google Scholar]
- 17.Guyatt G, Busse JW. Risk of bias in randomized trials. GROWTH Evidence; 2016. Available: https://growthevidence.com/gordon-h-guyatt-md-msc-and-jason-w-busse-dc-phd (accessed 2020 Mar. 24).
- 18.Guyatt GH, Busse J. Methods commentary: risk of bias in cohort studies. Ottawa: Evidence Partners; Available: www.evidencepartners.com/resources/methodological-resources/risk-of-bias-in-cohort-studies (accessed 2020 Mar. 24). [Google Scholar]
- 19.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64: 383–94. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Santesso N, et al. GRADE guidelines: 12. Preparing summary of findings tables — binary outcomes. J Clin Epidemiol 2013;66:158–72. [DOI] [PubMed] [Google Scholar]
- 21.Guyatt GH, Thorlund K, Oxman AD, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles — continuous outcomes. J Clin Epidemiol 2013;66:173–83. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Green S, editors. 7.7.3.5. Medians and interquartile ranges. In: Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Oxford (UK): Cochrane Collaboration; 2011. Available: https://handbook-5-1.cochrane.org/chapter_7/7_7_3_5_mediansand_interquartile_ranges.htm (accessed 2020 Mar. 20). [Google Scholar]
- 23.Soo YO, Cheng Y, Wong R, et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect 2004;10:676–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hung IFN, To KKW, Lee CK, et al. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest 2013;144:464–73. [DOI] [PubMed] [Google Scholar]
- 25.Beigel JH, Tebas P, Elie-Turenne MC, et al. IRC002 Study Team. Immune plasma for the treatment of severe influenza: an open-label, multicentre, phase 2 randomised study. Lancet Respir Med 2017;5:500–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beigel JH, Aga E, Elie-Turenne MC, et al. IRC002 Study Team. Anti-influenza immune plasma for the treatment of patients with severe influenza A: a randomised, double-blind, phase 3 trial. Lancet Respir Med 2019;7:941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davey RT, Jr, Fernández-Cruz E, Markowitz N, et al. INSIGHT FLU-IVIG Study Group. Anti-influenza hyperimmune intravenous immunoglobulin for adults with influenza A or B infection (FLU-IVIG): a double-blind, randomised, placebo-controlled trial. Lancet Respir Med 2019;7:951–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Griensven J, Edwards T, de Lamballerie X, et al. Ebola-Tx Consortium. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med 2016; 374:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Recommendations for investigational COVID-19 convalescent plasma. Silver Sprint (MD): US Food and Drug Administration; 2020. Available: www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/investigational-covid-19-convalescent-plasma-emergency-inds (accessed 2020 Mar. 26). [Google Scholar]
- 30.Kalil AC. Treating COVID-19-off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA 2020. Mar. 24 [Epub ahead of print]. 10.1001/jama.2020.4742. [DOI] [PubMed] [Google Scholar]
- 31.Zhou XZ, et al. Epidemiologic features, clinical diagnosis and therapy of first cluster of patients with severe acute respiratory syndrome in Beijing area [article in Chinese]. Zhonghua Yi Xue Za Zhi 2003;83:1018–22. [PubMed] [Google Scholar]
- 32.Xu Z, Zhou J, Huang Y, et al. The efficacy of convalescent plasma for the treatment of severe influenza. medRxiv 2020. Feb. 25. 10.1101/2020.02.20.20025593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.COVID-19 SARS-CoV-2 preprints from medRxiv and bioRxiv. medRxiv; 2020. Available: https://connect.medrxiv.org/relate/content/181 (accessed 2020 Mar. 11).
- 34.INSIGHT FLU005 IVIG Pilot Study Group. INSIGHT FLU005: an anti-influenza virus hyperimmune intravenous immunoglobulin pilot study. J Infect Dis 2016; 213: 574–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 2020. Mar. 27 [Epub ahead of print]. 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A 2020;117:9490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang B, Liu S, Tan T, et al. Treatment with convalescent plasma for critically ill patients with SARSCoV-2 infection. Chest 2020. Mar. 31 [Epub ahead of print]. pii S0012-3692(20)30571–7. 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulangu S, Dodd LE, Davey RT, Jr, et al. PALM Consortium Study Team. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med 2019;381:2293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y, Wang Y, Shao C, et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ 2020;27:1451–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanjilal S, Mina MJ. Passive immunity for the treatment of influenza: quality not quantity. Lancet Respir Med 2019;7:922–3. [DOI] [PubMed] [Google Scholar]