Abstract
Background
Patients with anorexia nervosa forgo eating despite emaciation and severe health consequences. Such dysfunctional decision-making might be explained by an excessive level of self-control, alterations in homeostatic and hedonic regulation, or an interplay between these processes. We aimed to understand value-based decision-making in anorexia nervosa and its association with the gut hormone ghrelin. Besides its homeostatic function, ghrelin has been implicated in the hedonic regulation of appetite and reward via the modulation of phasic dopamine signalling.
Methods
In a cross-sectional design, we studied acutely underweight (n = 94) and recovered (n = 37) patients with anorexia nervosa of the restrictive subtype, as well as healthy control participants (n = 119). We assessed plasma concentrations of desacyl ghrelin and parameters of delay discounting, probability discounting for gains and losses, and loss aversion.
Results
Recovered patients displayed higher risk aversion for gains, but we observed no group differences for the remaining decision-making parameters. Desacyl ghrelin was higher in acutely underweight and recovered participants with anorexia nervosa relative to healthy controls. Moreover, we found a significant group × desacyl ghrelin interaction in delay discounting, indicating that in contrast to healthy controls, acutely underweight patients with anorexia nervosa who had high desacyl ghrelin concentrations preferably chose the delayed reward option.
Limitations
We probed decision-making using monetary rewards, but patients with anorexia nervosa may react differently to disorder-relevant stimuli. Furthermore, in contrast to acyl ghrelin, the functions of desacyl ghrelin are unclear. Therefore, the interpretation of the results is preliminary.
Conclusion
The propensity for risk aversion as found in recovered patients with anorexia nervosa could help them successfully complete therapy, or it could reflect sequelae of the disorder. Conversely, ghrelin findings might be related to a mechanism contributing to disease maintenance; that is, in acutely underweight anorexia nervosa, a hungry state may facilitate the ability to forgo an immediate reward to achieve a (dysfunctional) long-term goal.
Introduction
Anorexia nervosa is an eating disorder that frequently manifests during adolescence and is characterized by an intense fixation with body weight and self-starvation. Anorexia nervosa often takes a chronic course, associated with severe medical complications and high mortality rates.1 Clinically, a restricting subtype can be differentiated from a binge-eating/ purging subtype. Patients of the latter group eat large amounts of food in a short time period (“bingeing”) or attempt to counteract weight gain by vomiting or the use of certain medications (“purging”). All patients severely reduce food intake, despite their knowledge or experience of the serious health consequences.2 Such behaviour may be the result of impaired decision-making. However, the subcomponents and underlying mechanisms of impaired decision-making in anorexia nervosa remain elusive.
The unusual ability to resist hunger in anorexia nervosa might indicate relatively elevated and sustained self-control, or low levels of impulsivity.3 One behavioural index of impulsivity in the decision-making context is delay discounting, which characterizes how quickly a reward loses its value as a function of time and is linked to the ability to delay gratification. 4 Patients of the restrictive anorexia nervosa subtype have been reported to discount delayed rewards less than healthy controls,5–7 but others8,9 have reported negative findings. Such an internal disposition to choose delayed rewards may contribute to maladaptive eating choices or present a maintenance factor for anorexia nervosa.10 In contrast, overly steep discounting is observed in disorders associated with failures of impulse control (e.g., substance use, attention-deficit/hyperactivity disorder, binge-eating disorder or obesity).9
Decision-making in daily life can be characterized by options with temporally delayed rewards (e.g., saving and investing money in the stock market instead of spending what is immediately available), but most decisions involve a degree of uncertainty with respect to the likelihood of the expected outcome (e.g., what if the stocks do not rise in value?). It has been proposed that delay and risk are psychologically analogous, but not equivalent.11 Individual risk-taking propensities can be assessed via probability discounting, the degree to which the value of a reward (or loss) decreases as a function of the odds of receiving it when making choices between certain and probabilistic rewards.12 More recently, decision-making under risk has also been studied in the concept of loss aversion, initially derived from the framework of prospect theory in economics.13 Loss aversion can be described by the phrase “losses loom larger” than gains and reflects people’s tendency to prefer avoiding losses over acquiring equivalent gains. Although reports have shown altered probability discounting and unusual low or high rates of loss aversion for a range of psychiatric conditions,14–16 such measures of risky decision-making are understudied in anorexia nervosa research.
Owing to its high evolutionary relevance for survival, food intake and deprivation can profoundly alter decision-making processes. Accordingly, decision-making for delayed and probabilistic rewards has been shown to vary as a function of metabolic state.17,18 These associations are thought to be mediated via hormonal regulation of neural networks, in particular the gut–brain axis, implicated in reward and decision-making. 19,20 Ghrelin is a hormone produced predominantly in the stomach. In peripheral blood, 2 major subforms are present: acyl and desacyl ghrelin. Due to the biochemical instability and pharmacokinetics of acyl ghrelin, the majority of circulating ghrelin is desacylated. Ghrelin levels show a preprandial rise,21 and acyl ghrelin facilitates food intake via activation of the growth hormone secretagogue receptor 1a.22,23 The orexigenic effects arise from activation of hypothalamic circuitry. However, the effects of ghrelin are not limited to the control of basic food intake; they also include the modulation of other reward-related behaviour via the mesocorticolimbic dopamine system. In particular, ghrelin administration to the ventral tegmental area increases extracellular dopamine in the nucleus accumbens24 and, presumably via this mechanism, also increases food-motivated behaviour25 and food intake.26,27 In turn, dopamine mediates reward learning28 and has been implicated in the pathophysiology of anorexia nervosa.29 Indeed, previous studies in anorexia nervosa have found decreased cerebrospinal fluid levels of homovanillic acid, a major dopamine metabolite,30 and altered D2 receptor sensitivity during apomorphine stimulation. 31 Positron emission tomography data showed increased striatal D2/D3 receptor binding, which was also related to striatal responses during monetary choices and self-reported trait anxiety.32
Severe food restriction in anorexia nervosa is accompanied by significant changes in endocrine and metabolic signalling. 33 Plasma concentrations of ghrelin are consistently elevated23 and differ between clinical subtypes,34 while at the same time a reduced endocrine response, possibly reflecting an adaptive diminished sensitivity to ghrelin signalling, has been suggested.35 In line with this, patients with anorexia nervosa were reported to exhibit altered reward processing36 and seemed to lack normal brain responses to food, which may suggest a failure to integrate the homeostatic state into decision-making.37 Thus, altered central ghrelin function may help to explain altered decision-making in anorexia nervosa.
In particular, given the modulatory role of ghrelin for both internal state/food intake, as well as general reward-related behaviour and previously reported ghrelin dysfunction in anorexia nervosa, we hypothesized that the association between ghrelin plasma concentrations and value-based decision-making would be altered in anorexia nervosa. Given the abovementioned differences between the anorexia nervosa subtypes5,34 and the predominance of the restrictive subtype in predominantly adolescent samples (such as ours), the current study focused on restrictive anorexia nervosa. To systematically characterize subcomponents of value-based decision-making in anorexia nervosa, we used a validated test battery14,38 that reliably measures choice behaviour related to immediate versus delayed rewards, as well as under risk (probabilistic gains or losses and mixed gambles). To disentangle the acute effect of undernutrition from possible trait markers of risk for anorexia nervosa (or resilience against a chronic or fatal course), we also included patients who had recovered from anorexia nervosa as a patient control group.
Methods
Participants
The sample consisted of 263 female volunteers: 94 underweight patients with acute anorexia nervosa of the restrictive subtype (anorexia nervosa) according to DSM-V (12.1– 28.6 years old), 37 patients successfully recovered from anorexia nervosa (15.5–29.7 years old) and 119 healthy controls (12.1–29 years old). Given previously reported differences in decision-making5,10 and ghrelin signalling34 between patients of the different anorexia nervosa subtypes, we focused on a sample of patients with acute and recovered anorexia nervosa of the restrictive subtype and did not include participants with atypical anorexia nervosa or the binge/ purge subtype. We carefully compiled the healthy control sample according to age in an attempt to obtain an independent age-matched case–control sample for each patient group (acute, recovered). Then, we combined the 2 healthy control samples for a joint analysis of all 3 groups. This study was approved by the local institutional review board, and all participants (and if underage, their guardians) gave written informed consent.
All patients with acute anorexia nervosa were admitted to eating disorder programs of a university child and adolescent psychiatry and psychosomatic medicine department and were assessed within 96 hours after the beginning of a behaviourally oriented nutritional rehabilitation program. To be included in the acute group, participants needed to have a body mass index (BMI) less than 17.5 kg/m2 if they were older than 15.5 years, or a BMI in the 10th percentile (i.e., lower than at least 90% of the population of that age) if they were younger than 15.5 years. To be considered recovered, patients with anorexia nervosa had to maintain a BMI greater than 18.5 kg/m2 if they were older than 18 years, or a BMI above the 10th percentile if they were younger than 18 years for at least 6 months before the study; menstruate; and not engage in significant restrictive eating patterns. In fact, most of our recovered participants had been recovered for much longer than 6 months (mean ± standard deviation 60.2 ± 47.6 mo; range 9–168 mo). We assessed exclusion criteria, comorbid psychiatric diagnoses and possible confounding variables as in our previous studies,39,40 using the expert form of the Structured Interview for Anorexia and Bulimia Nervosa for DSM-IV (SIAB-EX),41 medical records and our own semi-structured research interview. Interviews were conducted by clinically experienced and trained research assistants under the supervision of the attending child and adolescent psychiatrist.
We recruited participants who had a normal weight and were eumenorrheic for inclusion in the healthy control group using advertisements among middle school, high school and university students. Participants in the healthy control group were excluded if they had any history of psychiatric illness; a life-time BMI below the 10th percentile (if younger than 18 years) or a BMI less than 18.5 kg/m2 (if older than 18 years); or were currently obese (BMI > 94th percentile if younger than 18 years or BMI > 28 kg/m2 if older than 18 years).
Exclusion criteria that applied to participants in all study groups were IQ less than 85; psychotropic medication within 2 weeks before the study (except 4 recovered and 4 acute patients who were taking selective serotonin reuptake inhibitors); current substance abuse; current inflammatory, neurologic or metabolic illness; chronic medical or neurologic illness that could affect appetite, eating behaviour or body weight (e.g., diabetes); clinically relevant anemia; pregnancy; breastfeeding; or lifetime history of any of the following diagnoses: organic brain syndrome, schizophrenia, substance dependence, psychosis not otherwise specified, bipolar disorder, bulimia nervosa or binge-eating disorder (or “regular” binge eating, defined as bingeing at least once weekly for 3 or more consecutive months).
Clinical measures
We assessed psychopathology specific to eating disorders using the short version of the Eating Disorders Inventory (EDI-2).42 We examined depressive symptoms using the Beck Depression Inventory (BDI-II).43 We used a proxy measure of socioeconomic status (SES), the ISCO 08.44 Finally, we used BMI standard deviation score (BMI-SDS) instead of BMI for statistical analysis (Appendix 1, available at jpn.ca/190031-a1).
Study protocol
After participants fasted overnight, blood samples were drawn into tubes containing EDTA (1.6 mg/mL) and aprotinin (270 KIU/mL) between 0730 h and 0800 h. Then, participants underwent a 1 h MRI scan. Afterward, they had breakfast and started the value-based decision-making battery at 0930 h. Patients with acute anorexia nervosa ate a standardized breakfast consisting of a sandwich and tea under supervision; patients recovered from anorexia nervosa and healthy controls ate a sandwich and water. Although the sandwiches were similar in caloric and nutrient content, they were not identical.
Ghrelin measurements
We assessed desacyl ghrelin (pg/mL) in 61 patients with acute anorexia nervosa, 32 patients recovered from anorexia nervosa and 102 healthy controls (Appendix 1, Figure S1) using the Ghrelin Human ELISA Kit (BioVendor GmbH) following standard procedures. We chose desacyl ghrelin to minimize measurement errors, because acyl ghrelin is highly unstable23 and quickly hydrolyzed to desacyl ghrelin.45 Therefore, whenever we discuss the findings of the present study, we are referring to desacyl ghrelin. Because we detected significant deviations from normality (Shapiro test: healthy controls < 0.001; recovered = 0.003; acute = 0.16), we applied a log-transformation before analysis of variance comparing ghrelin between groups and before Pearson correlations with clinical variables.
Value-based decision-making
We used an established task battery to estimate behavioural measures of value-based decision-making.14,38 The battery relies on classical forced-choice decisions with a trial-by-trial adaptive Bayesian approach. We assessed estimates of delay discounting (kDD) using a delay discounting (DD) task, in which participants repeatedly needed to choose between receiving a smaller amount of money right away or a larger amount after a delay (e.g., €3 now or €8 in 1 wk). Higher kDD values reflect steeper discounting of delayed outcomes and, hence, a preference for immediate rewards.
We assessed estimates of probability discounting (kPDG and kPDL) using tasks that tested risk aversion for probabilistic gains (PDG) and probabilistic losses (PDL). Here, participants needed to choose between a smaller but certain monetary win or loss and the probability of winning or losing a larger amount (e.g., winning €2 for sure or €5 with a 75% probability, or losing €3 for sure or €8 with a 50% probability). Higher kPDG values reflect a tendency to favour smaller but more certain rewards, which is considered a risk-averse behaviour. In contrast, higher kPDL values reflect a preference for probabilistic outcomes (larger monetary losses with a lower probability), and hence a more risk-seeking behaviour.
Finally, we obtained estimates of loss aversion (λ) from a mixed gambles task in which participants needed to accept or reject an offer with a 50/50 chance of gaining or losing another amount (e.g., refusing or accepting a gamble to either win €15 or lose €8). Higher λ values reflect higher loss aversion.
Further information on task settings, estimation of parameters and efficiency of inference are provided in Appendix 1 (methods and Figure S2); for details of the mathematical framework, see Pooseh and colleagues.38
Statistical analysis
We evaluated the differences between groups in terms of demographic and clinical variables using 1-way analysis of variance and post hoc t tests with Bonferroni correction. We employed histograms, box plots and Shapiro tests to verify that decision-making parameters were normally distributed (Appendix 1, Figure S3). We detected no significant violations from normality. To compare decision-making between groups, we implemented 4 general linear models with the parameters kDD, kPDG, kPDL and λ as independent variables and age as a covariate. To test our main hypothesis of ghrelin effects on decision-making in anorexia nervosa compared with healthy controls, we considered 4 additional general linear models with age and ghrelin concentration as covariates, while also including group × ghrelin interactions. For significant main or interaction effects, we computed Dunnett post hoc t tests. We tested associations between decision-making parameters and clinical variables using Spearman correlations.
Results
Demographic and clinical variables are summarized in Table 1. As expected, patients with acute anorexia nervosa had lower BMI and BMI-SDS than the patients recovered from anorexia nervosa and the healthy controls. The latter 2 groups did not differ in BMI, but the patients recovered from anorexia nervosa had a somewhat lower BMI-SDS than the healthy controls. Groups also differed with respect to eating disorder and depressive symptoms: patients with acute anorexia nervosa scored higher on the EDI-2 and BDI-II scales than patients recovered from anorexia nervosa, and patients recovered from anorexia nervosa scored higher than the healthy controls. Finally, patients with acute anorexia nervosa and patients recovered from anorexia nervosa had significantly higher log-transformed ghrelin concentrations than the healthy controls. We found no correlations between log-transformed ghrelin and BMI-SDS, EDI-2 or BDI-II scores in any of the groups (all p > 0.10).
Table 1.
Demographic and clinical variables of the sample and their differences between groups*
| Characteristic | Group; mean ± SD | Group comparison | |||
|---|---|---|---|---|---|
|
|
|
||||
| Control | Acute anorexia nervosa | Recovered anorexia nervosa | F(p) | Post hoc | |
| Age, yr | 18.6 ± 4.5 | 16.1 ± 3.1 | 22.2 ± 3.8 | < 0.001 | Control > acute† Control < recovered† Acute < recovered† |
| BMI, kg/m2 | 20.9 ± 2.2 | 14.6 ± 1.4 | 20.6 ± 1.6 | < 0.001 | Control > acute† Acute < recovered† |
| BMI-SDS | −0.14 ± 0.65 | −3.19 ± 1.16 | −0.54 ± 0.58 | < 0.001 | Control > acute† Control > recovered‡ Acute < recovered† |
| SES (ISCO) | 162 ± 366 | 172 ± 376 | 138 ± 345 | 0.89 | NA |
| BDI-II score | 4.5 ± 5.0 | 23.4 ± 10.4 | 8.8 ± 7.9 | < 0.001 | Control < acute† Control < recovered‡ Acute < recovered† |
| EDI-2 score | 139 ± 27 | 212 ± 43 | 176 ± 50 | < 0.001 | Control < acute† Control < recovered† Acute < recovered† |
| Ghrelin, pg/mL | 259 ± 121 | 417 ± 181 | 341 ± 162 | < 0.001 | Control < acute† Control < recovered‡ |
BDI-II = Beck Depression Inventory II; BMI = body mass index; BMI-SDS = body mass index standard deviation score; EDI-2 = Eating Disorder Inventory 2; ISCO = International Standard Classification of Occupations; NA = not applicable; SD = standard deviation; SES = socioeconomic status.
Descriptive statistics and results of 1-way ANOVA and post hoc t tests (Bonferroni corrected for multiple comparisons). Of the patients with acute anorexia nervosa, 14 (14.9%) had an associated psychiatric comorbidity (4 depressive disorder, 4 obsessive compulsive disorder, 2 anxiety disorder, 6 other disorder; Appendix 1). Of the patients recovered from anorexia nervosa, 10 (27%) had an associated psychiatric comorbidity at the time of treatment (6 depressive disorder, 1 obsessive compulsive disorder, 3 anxiety disorder, 4 other disorder; Appendix 1). Additional characteristics can be found in Appendix 1, Table S1.
p < 0.001.
p < 0.05.
Mean decision-making estimates for all tasks and groups are shown in Table 2. General linear model analysis showed a group effect for probabilistic gains: patients recovered from anorexia nervosa had significantly higher kPDG estimates, indicating that this patient group chose the safe option more often and thus were more risk-averse to probabilistic gains (Table 2 and Appendix 1, Figure S4). We found no other significant differences between groups. However, we also observed an expected age effect indicating lower delay discounting in older participants (Table 2; Appendix 1, Figure S4). Associations with clinical variables did not survive corrections for multiple testing ( Appendix 1, Figure S5), except for a positive correlation between λ and EDI-2 in patients recovered from anorexia nervosa.
Table 2.
Descriptive statistics and group differences in decision-making parameters*
| Task | Control | Acute anorexia nervosa | Recovered anorexia nervosa | Group, p value | Post hoc t | Age, p value |
|---|---|---|---|---|---|---|
| Delay discounting | −4.30 ± 1.22 | −3.92 ± 1.48 | −4.36 ± 1.39 | 0.39 | NA | 0.022 |
| Probability discounting for gains | −0.37 ± 0.69 | −0.26 ± 0.64 | 0.08 ± 0.91 | 0.007 | Recovered > control† | 0.78 |
| Probability discounting for losses | −0.60 ± 0.62 | −0.52 ± 0.63 | −0.45 ± 0.73 | 0.41 | NA | 0.22 |
| Mixed gamble | 0.42 ± 0.42 | 0.37 ± 0.46 | 0.51 ± 0.46 | 0.53 | NA | 0.46 |
NA = not applicable.
For each group and task, the mean ± standard deviation of the logarithmic decision-making parameter is reported. Further presented are the p values of F tests on general linear models containing group as the factor and mean subtracted age as a covariate.
p < 0.01.
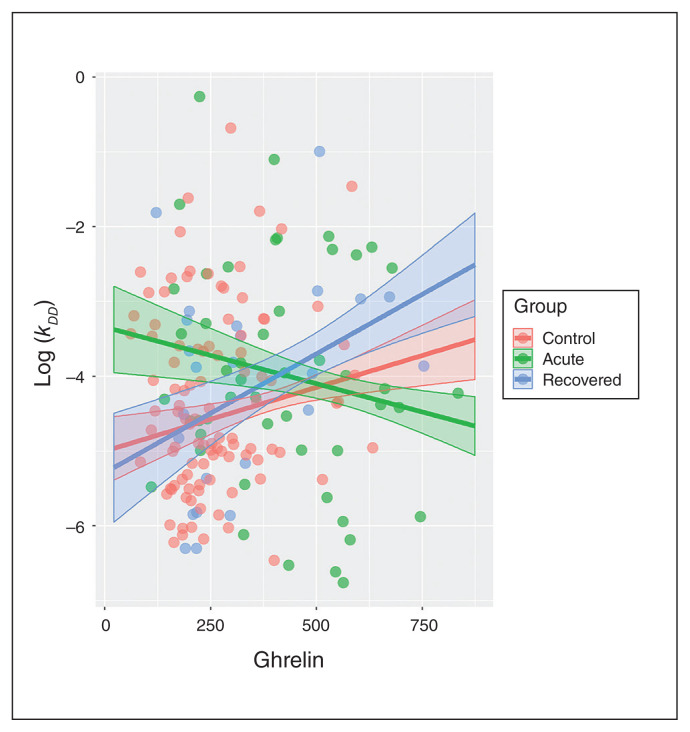
The results of the general linear model testing for main and interaction effects of ghrelin on decision-making are summarized in Table 3. For delay discounting, we found a significant group × ghrelin interaction. The linear coefficient relating ghrelin and kDD was decreased in patients with acute anorexia nervosa compared with healthy controls, resisting at the trend level when corrected for multiple testing. In other words, with increasing ghrelin concentrations, delay discounting seemed to decrease in patients with acute anorexia nervosa and increase in healthy controls (Figure 1). We found no further main effects or interactions of ghrelin on decision-making (Table 3). Inspection of quantile–quantile and residual plots revealed no significant deviations from normality (Appendix 1, Figure S6). To rule out the possibility that these results were driven by acute or recovered participants with psychiatric comorbidities, we repeated the same analyses after excluding these participants and found that the results were unchanged (Appendix 1, Tables S1 and S2). Furthermore, we tested the robustness of our findings for patients recovered from anorexia nervosa by excluding participants who had been recovered for less than 12 months. Again, the results were unchanged (Appendix 1, Table S3).
Table 3.
Ghrelin effect on decision-making parameters*
| Task | p value | |||
|---|---|---|---|---|
|
| ||||
| Group | Age | Ghrelin | Ghrelin × group | |
| Delay discounting | 0.60 | 0.024 | 0.17 | 0.036 |
| Probability discounting for gains | 0.005 | 0.82 | 0.20 | 0.74 |
| Probability discounting for losses | 0.26 | 0.29 | 0.49 | 0.15 |
| Mixed gamble | 0.16 | 0.43 | 0.32 | 0.07 |
Findings are presented as the p values of F tests on general linear models containing group as the factor, age and ghrelin as covariates, and the group × ghrelin interaction. The group × ghrelin interaction was significant for delay discounting (DD). The linear coefficient relating ghrelin and kDD was decreased in patients with acute anorexia nervosa compared with healthy controls, resisting at the trend level when correcting for multiple testing (t109 = −1.967; estimate [standard error] = −0.0029 (0.0015); p = 0.098; posthoc t test, Bonferroni corrected).
Fig. 1.
Associations between the estimated delay discounting parameter kDD and ghrelin in a general linear model, with group factor, age and ghrelin as covariates and group × ghrelin interaction. Error bands represent 95% confidence intervals. Ghrelin in pg/mL. DD = delay discounting.
Discussion
The present study investigated value-based decision-making in patients with acutely underweight anorexia nervosa and recovered from anorexia nervosa, as well as its modulation by the energy- and reward system–related gut hormone ghrelin. Two separate key findings were revealed. First, patients recovered from anorexia nervosa showed a higher risk aversion related to probabilistic monetary rewards. Second, although no general group differences in delay discounting were evident, ghrelin was increased in patients with acute anorexia nervosa and patients recovered from anorexia nervosa compared with healthy controls; most importantly, patients with acute anorexia nervosa who had high ghrelin levels showed less delay discounting than healthy controls with comparable ghrelin levels. Together, these results suggest a link between a dysfunctional metabolic/ endocrine modulation and unique alterations in self-regulatory decision-making in anorexia nervosa.
Patients recovered from anorexia nervosa were characterized by a more conservative choice behaviour for probabilistic rewards (i.e., they tend to choose the safe option), which theoretically could be driven by enhanced aversion to uncertainty (a construct related to risk that plays a crucial role in theoretical models of anorexia nervosa46) or aversion to negative outcomes (e.g., loss). However, loss aversion as measured with the mixed gambles task, and choice behaviour regarding probabilistic losses, were not altered in anorexia nervosa. Interestingly, patients with alcohol dependence seem to be characterized by a pattern of decision-making parameters that are somewhat the opposite of patients with anorexia nervosa. Specifically, in a previous study using the same probabilistic gains task, we found low risk aversion for probabilistic monetary gains but lower risk-seeking for probabilistic losses and a lower degree of loss aversion.14 Such deviant choice behaviour may thus reflect alterations underlying the pathology that are specific to the patient group. In contrast, a decision bias comparable to that observed in anorexia nervosa in the present study has been found in patients with generalized anxiety disorder.15 Similar to patients recovered from anorexia nervosa, patients with generalized anxiety disorder showed a more conservative choice pattern for probabilistic gains and also levels of loss aversion equivalent to controls. The authors of that study concluded that a reduced propensity to take risks underlies such alterations. Patients with acute anorexia nervosa may view the maintenance of weight loss as a desired but risky option. Patients who decide against this risky reward during treatment may be more likely to recover. Thus, a speculative interpretation of our findings would be that the patients recovered from anorexia nervosa represent a select group of former patients with anorexia nervosa, because those without increased risk aversion may have a lower probability of recovery. A disposition for risk-averse decision-making specifically in the domain of rewards may have served as protective factor. Alternatively, the observed higher discounting may reflect a result (or scar) of the illness (i.e., patients becoming more conservative given their clinical history). Furthermore, obsessive traits are frequently observed in anorexia nervosa,47 and based on theoretical models as well as empirical studies, such traits are expected to covary with risk aversion.48 However, the direction of the association could be either way; obsessive traits may underlie altered decision-making, or vice versa (i.e., altered decision-making may cause obsessive behaviour).
Consistent with previous reports,23 patients with acute anorexia nervosa in our study exhibited elevated ghrelin concentrations. Ghrelin is known to play an important role in the regulation of energy homeostasis, appetite and blood glucose levels, but recent work has shown that it also modulates reward processing.24 In rodents, systemic or central ghrelin administration triggered the release of dopamine in the nucleus accumbens, enhancing hedonic and incentive value.27 Comparably in humans, ghrelin injection is associated with heightened activation of reward-system structures (amygdala, orbitofrontal cortex, anterior insula, striatum and ventral tegmental area).49 Ghrelin also increases activity in brain areas involved in attention and memory and has been found to reinforce behaviour by mediating and facilitating the learning effects involved in the development of addictive behaviour, for example.50
Although we found no group differences in discounting of delayed rewards in a comparatively large sample of patients with restrictive acute anorexia nervosa, patients with higher ghrelin values showed reduced delay discounting compared to healthy controls. This association in anorexia nervosa stood in contrast to the suggested role of ghrelin in modulating a choice bias toward immediate rewards and the idea that endogenous ghrelin directly promotes impulsive decision-making. 51 Imaging studies in anorexia nervosa have illustrated altered neuronal activity related to food motivation,52 reward processing36 and executive control.40 In support of a breakdown in the interaction between ghrelin signalling and reward responsivity in anorexia nervosa, deviations in the relationship between circulating ghrelin concentrations and neural activity in limbic brain regions in response to food cues have been reported.53 It has been proposed that a relative resistance to ghrelin in anorexia nervosa may lead to reduced appetitive drive and restrictive eating.54 The current results may further suggest a reverse effect of ghrelin on choice behaviour in anorexia nervosa. Additional support for the speculative role of ghrelin in decision-making in anorexia nervosa comes from a recent study that reported an association between higher total ghrelin levels and impulsive choice as assessed with the Iowa gambling task.55
Of note, a recent pilot treatment study of underweight patients with anorexia nervosa has shown that the ghrelin agonist relamorelin — which may help to restore ghrelin sensitivity — increases hunger, leading to a trend in weight gain after only 4 weeks.56
Although other studies have reported intermediate53 or normalized ghrelin levels after weight recovery,57 patients recovered from anorexia nervosa in the present study still had somewhat elevated plasma ghrelin concentrations. In contrast to our findings in patients with acute anorexia nervosa, associations between delay discounting and ghrelin in patients recovered from anorexia nervosa were similar to healthy controls, compatible with the idea that ghrelin’s modulatory function on decision-making in anorexia nervosa normalizes with weight restoration. Taken together, our results suggest that an abnormal effect of endogenous ghrelin in patients with acute anorexia nervosa might help patients delay rewards. Whether or not such a mechanism would also extend to food rewards and support disease maintenance remains to be tested. A more complete understanding of ghrelin’s role in impaired decision-making in anorexia nervosa is needed and may help to unravel the therapeutic potential of pharmacological agents that bind to the ghrelin receptor.
Considered independent from ghrelin, the rate of delay discounting in our study did not differ significantly for patients with acute or recovered anorexia nervosa compared to healthy controls, in line with several previous reports.8,37,40 However a preference for larger-later over smaller-sooner rewards in anorexia nervosa has also been found.5–7 Possible explanations for these discrepancies include differences in employed tasks, parameter inference, hyperbolic versus exponential fitting, and differences in the sample composition and metabolic state of the patients. Decreased delay discounting has been found mostly in older patients with anorexia nervosa and likely chronic disease progression, as opposed to null findings in younger nonchronic (adolescent) anorexia nervosa.8,40 Indeed, there is compelling evidence that delay discounting is not a stable trait but can be affected within an individual by current physiologic needs, emotional state or external contextual influences.9
Limitations
Our results should be interpreted in the light of the following limitations. First, the cross-sectional design limits our ability to differentiate trait markers from scar effects of prolonged undernutrition. Longitudinal studies are warranted to further evaluate the predictive value of the different measures of decision-making on treatment outcome (in particular kPDG).
Second, we employed a relatively new adaptive assessment of decision-making. Nonetheless, results for delay discounting are comparable to those of previous studies in patients with anorexia nervosa from our laboratory obtained with a standard amount-adjusting procedure.8,40 Furthermore, the expected association with age and the correlation between discounting of delayed and probabilistic rewards58 were reproduced (Appendix 1, Figure S5). As in previous studies, participants weighed the risk for monetary losses 2-fold higher on average than for monetary gains.13
Third, we employed monetary rewards based on the assumption that all rewards can be translated to a universal scale of value. However, patients with eating disorders may ascribe very different (i.e., negative) values to disorder-specific stimuli such as food rewards. Therefore, although our results cannot be easily generalized to all reward categories in this population, using food stimuli may have its own challenges (e.g., food may not be perceived as rewarding by many anorexia nervosa patients).
Fourth, we measured the biochemically stable desacyl ghrelin. Although it acts as a growth hormone secretagogue receptor 1a agonist at supraphysiological levels,59,60 its physiologic functions are still a matter of debate.23 Consequently, our interpretations of the results are preliminary and future studies should aim to measure both forms.
Fifth, because patients with acute anorexia nervosa took part in the study within 96 h of admission to intensive treatment, nutritional state differed between groups, and it would be very difficult to appraise metabolic need. Furthermore, despite close supervision, we cannot guarantee that all patients were fully adherent during breakfast. Finally, we focused on ghrelin because of its demonstrated effect on the dopaminergic reward system. Other hormones (e.g., orexins and peptide YY) may also play important roles in this context,54 but the associations with brain networks are less clear.
Conclusion
We found that higher ghrelin levels did not increase choice impulsivity as measured using delay discounting in patients with acute anorexia nervosa, contrary to healthy controls. Therefore, an improved understanding of altered decision-making and its modulation by endocrine/homeostatic signals in anorexia nervosa might offer new avenues for the development of innovative treatment strategies (such as partial ghrelin agonists) for this chronic and devastating disorder. Furthermore, risk aversion for probabilistic wins was increased in patients recovered from anorexia nervosa, suggesting that psychological interventions aimed at increasing risk awareness may further help patients recognize that it is not worth taking (health) risks to achieve uncertain and dangerous goals (such as an increasingly thinner body).
Acknowledgments
The authors are grateful to all junior researchers and student workers for their assistance with data collection, and thank all participants for their time and cooperation. N. Bernhardt receives financial support from DFG grant FOR 1617 (grant numbers SM80/7-1). S. Ehrlich receives financial support from a collaborative research center grant (DFG, SFB 940/2) and from a DFG research grant: Hormonal modulation of neural networks in anorexia nervosa EH 367/5-1 (PI S. Ehrlich).
Footnotes
Competing interests: V. Roessner has received lecture fees from Eli Lilly, Janssen-Cilag, Medice and Novartis, and was a member of the advisory boards of Eli Lilly and Novartis. All other authors declared that they have no competing interests.
Contributors: S. Ehrlich designed the study with help from V. Roessner and M. Smolka. F. Bernardoni, J. King, D. Geisler, F. Ritschel, I. Boehm and M. Seidel acquired the data, which F. Bernardoni, N. Bernhardt, S. Pooseh and S. Ehrlich analyzed. F. Bernardoni, N. Bernhardt, J. King and S. Ehrlich wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Zipfel S, Giel KE, Bulik CM, et al. Anorexia nervosa: aetiology, assessment, and treatment. Lancet Psychiatry. 2015;2:1099–111. doi: 10.1016/S2215-0366(15)00356-9. [DOI] [PubMed] [Google Scholar]
- 2.Gregertsen EC, Mandy W, Serpell L. The egosyntonic nature of anorexia: an impediment to recovery in anorexia nervosa treatment. Front Psychol. 2017;8:2273. doi: 10.3389/fpsyg.2017.02273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wierenga CE, Ely A, Bischoff-Grethe A, et al. Are extremes of consumption in eating disorders related to an altered balance between reward and inhibition? Front Behav Neurosci. 2014;8:410. doi: 10.3389/fnbeh.2014.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ainslie G. Specious reward — behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–96. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- 5.Steinglass JE, Figner B, Berkowitz S, et al. Increased capacity to delay reward in anorexia nervosa. J Int Neuropsychol Soc. 2012;18:773–80. doi: 10.1017/S1355617712000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinglass JE, Lempert KM, Choo T-H, et al. Temporal discounting across three psychiatric disorders: anorexia nervosa, obsessive compulsive disorder, and social anxiety disorder. Depress Anxiety. 2017;34:463–70. doi: 10.1002/da.22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decker JH, Figner B, Steinglass JE. On weight and waiting: delay discounting in anorexia nervosa pre- and post-treatment. Biol Psychiatry. 2015;78:606–14. doi: 10.1016/j.biopsych.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritschel F, King JA, Geisler D, et al. Temporal delay discounting in acutely ill and weight-recovered patients with anorexia nervosa. Psychol Med. 2015;45:1229–39. doi: 10.1017/S0033291714002311. [DOI] [PubMed] [Google Scholar]
- 9.Lempert KM, Steinglass JE, Pinto A, et al. Can delay discounting deliver on the promise of RDoC? Psychol Med. 2019;49:190–9. doi: 10.1017/S0033291718001770. [DOI] [PubMed] [Google Scholar]
- 10.Steward T, Mestre-Bach G, Vintró-Alcaraz C, et al. Delay discounting of reward and impulsivity in eating disorders: from anorexia nervosa to binge eating disorder. Eur Eat Disord Rev. 2017;25:601–6. doi: 10.1002/erv.2543. [DOI] [PubMed] [Google Scholar]
- 11.Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull. 2004;130:769–92. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rachlin H, Raineri A, Cross D. Subjective-probability and delay. J Exp Anal Behav. 1991;55:233–44. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tversky A, Kahneman D. Advances in prospect theory: cumulative representation of uncertainty. J Risk Uncertain. 1992;5:297–323. [Google Scholar]
- 14.Bernhardt N, Nebe S, Pooseh S, et al. Impulsive decision making in young adult social drinkers and detoxified alcohol-dependent patients: a cross-sectional and longitudinal study. Alcohol Clin Exp Res. 2017;41:1794–807. doi: 10.1111/acer.13481. [DOI] [PubMed] [Google Scholar]
- 15.Charpentier CJ, Aylward J, Roiser JP, et al. Enhanced risk aversion, but not loss aversion, in unmedicated pathological anxiety. Biol Psychiatry. 2017;81:1014–22. doi: 10.1016/j.biopsych.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sip KE, Gonzalez R, Taylor SF, et al. Increased loss aversion in unmedicated patients with obsessive-compulsive disorder. Front Psychiatry. 2018;8:309. doi: 10.3389/fpsyt.2017.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Symmonds M, Emmanuel JJ, Drew ME, et al. Metabolic state alters economic decision making under risk in humans. PLoS One. 2010;5:e11090. doi: 10.1371/journal.pone.0011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang XT, Dvorak RD. Sweet future: fluctuating blood glucose levels affect future discounting. Psychol Sci. 2010;21:183–8. doi: 10.1177/0956797609358096. [DOI] [PubMed] [Google Scholar]
- 19.Berthoud H-R. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol. 2011;21:888–96. doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgs S, Spetter MS, Thomas JM, et al. Interactions between metabolic, reward and cognitive processes in appetite control: implications for novel weight management therapies. J Psychopharmacol. 2017;31:1460–74. doi: 10.1177/0269881117736917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummings DE, Purnell JQ, Frayo RS, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 22.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 23.Schalla MA, Stengel A. The role of ghrelin in anorexia nervosa. Int J Mol Sci. 2018;19:2117. doi: 10.3390/ijms19072117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jerlhag E, Egecioglu E, Dickson SL, et al. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12:6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- 25.Skibicka KP, Hansson C, Egecioglu E, et al. Role of ghrelin in food reward: impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addict Biol. 2012;17:95–107. doi: 10.1111/j.1369-1600.2010.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naleid AM, Grace MK, Cummings DE, et al. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–9. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 27.Egecioglu E, Jerlhag E, Salomé N, et al. Ghrelin increases intake of rewarding food in rodents: ghrelin and food reward. Addict Biol. 2010;15:304–11. doi: 10.1111/j.1369-1600.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13:900–13. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kontis D, Theochari E. Dopamine in anorexia nervosa: a systematic review. Behav Pharmacol. 2012;23:496–515. doi: 10.1097/FBP.0b013e328357e115. [DOI] [PubMed] [Google Scholar]
- 30.Kaye WH, Ebert MH, Raleigh M, et al. Abnormalities in CNS monoamine metabolism in anorexia nervosa. Arch Gen Psychiatry. 1984;41:350–5. doi: 10.1001/archpsyc.1984.01790150040007. [DOI] [PubMed] [Google Scholar]
- 31.Brambilla F, Bellodi L, Arancio C, et al. Central dopaminergic function in anorexia and bulimia nervosa: a psychoneuroendocrine approach. Psychoneuroendocrinology. 2001;26:393–409. doi: 10.1016/s0306-4530(00)00062-7. [DOI] [PubMed] [Google Scholar]
- 32.Bailer UF, Price JC, Meltzer CC, et al. Dopaminergic activity and altered reward modulation in anorexia nervosa-insight from multimodal imaging. Int J Eat Disord. 2017;50:593–6. doi: 10.1002/eat.22638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prince AC, Brooks SJ, Stahl D, et al. Systematic review and metaanalysis of the baseline concentrations and physiologic responses of gut hormones to food in eating disorders. Am J Clin Nutr. 2009;89:755–65. doi: 10.3945/ajcn.2008.27056. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka M, Naruo T, Yasuhara D, et al. Fasting plasma ghrelin levels in subtypes of anorexia nervosa. Psychoneuroendocrinology. 2003;28:829–35. doi: 10.1016/s0306-4530(02)00066-5. [DOI] [PubMed] [Google Scholar]
- 35.Broglio F, Gianotti L, Destefanis S, et al. The endocrine response to acute ghrelin administration is blunted in patients with anorexia nervosa, a ghrelin hypersecretory state. Clin Endocrinol (Oxf) 2004;60:592–9. doi: 10.1111/j.1365-2265.2004.02011.x. [DOI] [PubMed] [Google Scholar]
- 36.Wagner A, Aizenstein H, Venkatraman VK, et al. Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry. 2007;164:1842–9. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- 37.Wierenga CE, Bischoff-Grethe A, Melrose AJ, et al. Hunger does not motivate reward in women remitted from anorexia nervosa. Biol Psychiatry. 2015;77:642–52. doi: 10.1016/j.biopsych.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pooseh S, Bernhardt N, Guevara A, et al. Value-based decision-making battery: a Bayesian adaptive approach to assess impulsive and risky behavior. Behav Res Methods. 2018;50:236–49. doi: 10.3758/s13428-017-0866-x. [DOI] [PubMed] [Google Scholar]
- 39.Bernardoni F, King JA, Geisler D, et al. Nutritional status affects cortical folding: lessons learned from anorexia nervosa. Biol Psychiatry. 2018;84:692–701. doi: 10.1016/j.biopsych.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 40.King JA, Geisler D, Bernardoni F, et al. Altered neural efficiency of decision making during temporal reward discounting in anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 2016;55:972–9. doi: 10.1016/j.jaac.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Fichter M, Quadflieg N. The structured interview for anorexic and bulimic disorders for DSM-IV and ICD-10 (SIAB-EX): reliability and validity. Eur Psychiatry. 2001;16:38–48. doi: 10.1016/s0924-9338(00)00534-4. [DOI] [PubMed] [Google Scholar]
- 42.Paul T, Thiel A. Eating Disorder Inventory-2 (EDI-2): deutsche version. Boston (MA): Hogrefe; 2005. [Google Scholar]
- 43.Hautzinger M, Keller F, Beck AT, et al. Beck Depressions-Inventar: BDI II; manual. New York: Pearson Assessment; 2009. [Google Scholar]
- 44.ISCO: International Standard Classification of Occupations. Geneva, Switzerland: International Labour Organization; 2016. [accessed 2020 Jan. 27]. Available: www.ilo.org/public/english/bureau/stat/isco/isco08/ [Google Scholar]
- 45.Hosoda H, Kangawa K. Standard sample collections for blood ghrelin measurements. Methods Enzymol. 2012;514:113–26. doi: 10.1016/B978-0-12-381272-8.00008-8. [DOI] [PubMed] [Google Scholar]
- 46.Merwin RM, Timko CA, Moskovich AA, et al. Psychological inflexibility and symptom expression in anorexia nervosa. Eat Disord. 2011;19:62–82. doi: 10.1080/10640266.2011.533606. [DOI] [PubMed] [Google Scholar]
- 47.Halmi KA, Sunday SR, Klump KL, et al. Obsessions and compulsions in anorexia nervosa subtypes. Int J Eat Disord. 2003;33:308–19. doi: 10.1002/eat.10138. [DOI] [PubMed] [Google Scholar]
- 48.Frost RO, Steketee G, Cohn L, et al. Personality traits in subclinical and non-obsessive-compulsive volunteers and their parents. Behav Res Ther. 1994;32:47–56. doi: 10.1016/0005-7967(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 49.Malik S, McGlone F, Bedrossian D, et al. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–9. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Wittekind DA, Kluge M. Ghrelin in psychiatric disorders — a review. Psychoneuroendocrinology. 2015;52:176–94. doi: 10.1016/j.psyneuen.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 51.Anderberg RH, Hansson C, Fenander M, et al. The stomachderived hormone ghrelin increases impulsive behavior. Neuropsychopharmacology. 2016;41:1199–209. doi: 10.1038/npp.2015.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fladung A-K, Grön G, Grammer K, et al. A neural signature of anorexia nervosa in the ventral striatal reward system. Am J Psychiatry. 2010;167:206–12. doi: 10.1176/appi.ajp.2009.09010071. [DOI] [PubMed] [Google Scholar]
- 53.Holsen LM, Lawson EA, Christensen K, et al. Abnormal relationships between the neural response to high- and low-calorie foods and endogenous acylated ghrelin in women with active and weight-recovered anorexia nervosa. Psychiatry Res. 2014;223:94–103. doi: 10.1016/j.pscychresns.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berner LA, Brown TA, Lavender JM, et al. Neuroendocrinology of reward in anorexia nervosa and bulimia nervosa: beyond leptin and ghrelin. Mol Cell Endocrinol. 2019;497:110320. doi: 10.1016/j.mce.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paslakis G, Agüera Z, Granero R, et al. Associations between neuropsychological performance and appetite-regulating hormones in anorexia nervosa and healthy controls: ghrelin’s putative role as a mediator of decision-making. Mol Cell Endocrinol. 2019;497:110441. doi: 10.1016/j.mce.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 56.Fazeli PK, Lawson EA, Faje AT, et al. Treatment with a ghrelin agonist in outpatient women with anorexia nervosa: a randomized clinical trial. J Clin Psychiatry. 2018;79:17m11585. doi: 10.4088/JCP.17m11585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Otto B, Cuntz U, Fruehauf E, et al. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol. 2001;145:669–73. [PubMed] [Google Scholar]
- 58.Green L, Myerson J. How many impulsivities? A discounting perspective. J Exp Anal Behav. 2013;99:3–13. doi: 10.1002/jeab.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gauna C, van de Zande B, van Kerkwijk A, et al. Unacylated ghrelin is not a functional antagonist but a full agonist of the type 1a growth hormone secretagogue receptor (GHS-R) Mol Cell Endocrinol. 2007;274:30–4. doi: 10.1016/j.mce.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 60.Heppner KM, Piechowski CL, Muller A, et al. Both acyl and desacyl ghrelin regulate adiposity and glucose metabolism via central nervous system ghrelin receptors. Diabetes. 2014;63:122–31. doi: 10.2337/db13-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]