Abstract
Background
Tetris has been proposed as a preventive intervention to reduce intrusive memories of a traumatic event. However, no neuroimaging study has assessed Tetris in patients with existing posttraumatic stress disorder (PTSD) or explored how playing Tetris may affect brain structure.
Methods
We recruited patients with combat-related PTSD before psychotherapy and randomly assigned them to an experimental Tetris and therapy group (n = 20) or to a therapy-only control group (n = 20). In the control group, participants completed therapy as usual: eye movement desensitization and reprocessing (EMDR) psychotherapy. In the Tetris group, in addition to EMDR, participants also played 60 minutes of Tetris every day from onset to completion of therapy, approximately 6 weeks later. Participants completed structural MRI and psychological questionnaires before and after therapy, and we collected psychological questionnaire data at follow-up, approximately 6 months later. We hypothesized that the Tetris group would show increases in hippocampal volume and reductions in symptoms, both directly after completion of therapy and at follow-up.
Results
Following therapy, hippocampal volume increased in the Tetris group, but not the control group. As well, hippocampal increases were correlated with reductions in symptoms of PTSD, depression and anxiety between completion of therapy and follow-up in the Tetris group, but not the control group.
Limitations
Playing Tetris may act as a cognitive interference task and as a brain-training intervention, but it was not possible to distinguish between these 2 potential mechanisms.
Conclusion
Tetris may be useful as an adjunct therapeutic intervention for PTSD. Tetris-related increases in hippocampal volume may ensure that therapeutic gains are maintained after completion of therapy.
Introduction
Recent work has provided evidence for the utility of the visuospatial video game Tetris as an early therapeutic intervention for posttraumatic stress disorder (PTSD).1–3 Holmes and colleagues have shown that playing Tetris directly after trauma exposure can reduce subsequent intrusive memories of the traumatic event, and they have demonstrated the efficacy of this “cognitive vaccine” in both experimental1 and real-world settings.2–4
Following exposure to an event, the memory trace of that event must be consolidated into long-term memory for it to be accessible for later recall.5 Shortly after the event, the memory trace remains in a labile state as it is consolidated, and it is susceptible or vulnerable to interference.6 Performing an unrelated task while the memory for an event is in a labile state can reduce subsequent retrieval.7 In addition, it has been proposed that following reactivation, a memory again enters a labile state and must be reconsolidated into long-term memory.8 During this reconsolidation process, the memory trace is also vulnerable to interference. Holmes and colleagues1 have proposed that by completing a demanding visuospatial task during memory consolidation or reconsolidation for a traumatic event, the memory trace is weakened because of competition for the cognitive resources required for consolidation.
To date, work using Tetris as an intervention has focused mainly on attempting to disrupt consolidation of the traumatic memory within the first 6 hours after the trauma exposure, 1–3 or reconsolidation of the traumatic memory the next day.8 However, playing a video game in the direct aftermath of a traumatic event is neither practical nor possible in every case. It is estimated that approximately 8 million adults have PTSD in the United States alone.9 As such, interventions for those who are already experiencing posttraumatic symptoms are sorely needed.
One study to date has assessed a Tetris intervention in people with existing PTSD.4 In this study, specific intrusions were targeted based on the concepts of “concurrent task interference and memory reconsolidation.” After a reminder for a specific intrusive memory, patients played 25 minutes of Tetris. The authors found that after completion of the study, the frequency of targeted intrusions was lower than that of nontargeted intrusions. In the current study, we investigate the utility of Tetris as an adjunct therapeutic intervention for people with current PTSD.
Current therapeutic interventions for PTSD have a number of limitations related to response rates and long-term efficacy. A significant minority of people with PTSD will not show significant improvement in symptoms directly following therapy, with rates of nonresponders estimated to be as high as 35% to 50% in some studies.10–12 In addition, the long-term prognosis for PTSD is poor: a majority of people continue to experience symptoms for months or years after initial diagnosis, and a substantial number never fully recovers.13 As such, there is a significant need for additional therapeutic interventions that may act as an adjunct to traditional psychotherapy for nonresponders and to ensure the long-term maintenance of therapy-related gains for responders.
The most widely used and most effective interventions for PTSD are psychotherapies such as trauma-focused cognitive behaviour therapy (CBT) and eye movement desensitization and reprocessing (EMDR) therapy. These therapies target memories of the traumatic event, along with the person’s cognitive and emotional interpretation of the event. Therapy with EMDR is particularly interesting, because it differs from other psychotherapies by incorporating a visuosensory attentional component.14 There are some inconsistencies in the literature regarding EMDR, particularly the therapeutic contribution of the visuosensory component:15 although reviews of studies comparing trauma-focused CBT and EMDR have failed to demonstrate increased efficacy for one over the other,10,15 separate reviews have provided evidence that the addition of eye movements results in significant improvements to treatment outcomes.16,17 As such, although EMDR is a common therapeutic intervention for the treatment of PTSD, the precise mechanisms underlying its efficacy remain somewhat unclear.
In the current study, we explored the use of Tetris as an adjunct to EMDR. Each EMDR session consisted of selecting a traumatic memory to work on. Given the visuosensory attentional component of EMDR, we considered that Tetris might complement EMDR better than other psychotherapeutic interventions, such as CBT.
At the neuroanatomical level, adult PTSD populations are characterized by smaller volumes in the hippocampus and in prefrontal regions, including the ventromedial prefrontal cortex and the anterior cingulate cortex.18–20
The hippocampus is hypothesized to play a key role in PTSD symptomatology: smaller hippocampal volumes have been associated with increased risk and poorer prognosis in PTSD, and with poorer prognosis.21–25 In addition, increases in grey matter volume in the hippocampus have been observed in response to psychological therapy, including EMDR26,27 and pharmacological interventions, and increases in hippocampal volume have been linked to improvements in memory.28
Training studies have demonstrated that increases in hippocampal volume can be produced with a wide variety of interventions, 29,30 including video-gaming interventions.31 As such, we hypothesized that the video-gaming intervention Tetris would increase hippocampal volume, which would in turn reduce PTSD symptomatology.
Smaller prefrontal regions have also been commonly observed in people with PTSD.18–20 Prefrontal regions have also been shown to increase in response to video-gaming interventions. 31 We therefore hypothesized that prefrontal regions in PTSD populations would also increase in response to therapeutic interventions, although the majority of relevant work in neuroplasticity and PTSD has demonstrated effects in the hippocampus rather than the prefrontal regions.
In the current study, we investigated the structural and behavioural effects of a Tetris intervention in people with PTSD who were undergoing psychotherapy, using a prospective design. We recruited people with combat-related PTSD and assessed them before and directly after EMDR therapy, as well as at follow-up, approximately 6 months later. We proposed that playing Tetris after therapy while the reactivated traumatic memory was in a labile state would weaken reconsolidation and aid recovery.8 It should be noted that playing Tetris has been shown to reduce the vivid, intrusive elements of a traumatic memory, but not of a declarative memory.1 As such, we expected that Tetris would not affect memory of the therapy sessions, or interfere with the clinical efficacy of EMDR. In addition, spatial memory training and video gaming have been linked to increases in hippocampal volume,31 and increases in hippocampal volume have been associated with improvements in memory and reductions in symptoms in PTSD.32 As such, we expected that Tetris would aid in recovery from PTSD by weakening the memory of the traumatic event, and by increasing hippocampal volume. We hypothesized that the Tetris group would show increases in hippocampal volume and reductions in symptoms — directly after completion of therapy and at follow-up.
Methods
Participants
We recruited 40 participants with combat-related PTSD from the German Federal Armed Forces before they started therapy. All participants were inpatients at the German Military Hospital, Berlin, Germany. All participants were male and had been deployed overseas to areas of conflict. For inclusion criteria, participants were screened by clinical psychologists and psychiatrists for the presence of mission-related trauma within the preceding 2 years and for a current diagnosis of PTSD according to ICD-10 criteria. For exclusion criteria, participants were screened for current or previous comorbid psychosis or substance abuse, use of psychotropic medication, a history of concussion or traumatic brain injury, and contraindications for MRI. Patients were assessed for inclusion and exclusion criteria and then randomly assigned to the experimental Tetris group (n = 20) or the control group (n = 20). The local ethics committee of Charité University Clinic (Berlin, Germany) approved the study, and we obtained written informed consent from each participant before they entered the study, in line with the declaration of Helsinki.
Participants in both groups completed EMDR therapy.14,33 Participants in the control group completed EMDR therapy only, and participants in the experimental Tetris group also played the video game Tetris for 60 minutes per day from the start of therapy to completion, approximately 6 weeks. After completion of therapy, all participants returned for neuroimaging and questionnaire assessment. Approximately 6 months after completion of therapy, participants also completed the questionnaire assessment only. Four participants — 2 in the Tetris group and 2 in the control group — did not complete the 6-month follow-up assessment.
Psychotherapy intervention
All participants completed EMDR therapy — one of the most common therapeutic interventions for PTSD34 and known to effectively reduce symptoms in a majority of individuals directly after completion of therapy.14,15,33 Psychotherapists and psychotherapy-specialized senior psychiatrists performed the EMDR in single sessions. Treatment was conducted according to standard EMDR protocol. A 1-week stabilization and preparation phase was followed by a 4-week exposure phase, with an average of 2 sessions per week for 60 to 90 minutes each. Participants completed a mean of 7.2 ± 1.8 sessions over approximately 6 weeks (duration 39.9 ± 4.8 d). When the number of sessions was not available from the clinical records (n = 3), we used participant self-report data. Each EMDR session consisted of selecting a memory to work on. The selected memory was then reactivated, during which time the individual attended briefly to certain elements of the memory while simultaneously engaging in a periodic set of eye movements by focusing on a moving visual stimulus controlled by the therapist.
Tetris intervention
Participants in the Tetris group were given a Nintendo DS XL console and the computer game Tetris. Participants were asked to play for 60 minutes per day. Participants reported playing a mean of 61 ± 14.6 minutes per day (data not available for 2 participants) and missing a mean of 1 ± 1.2 day (data not available for 2 participants). On days that participants completed an EMDR therapy session, they were required to play Tetris within 6 hours after they completed therapy.
Questionnaires
To assess participants’ duration of military deployment and their experiences during deployment, we asked them to complete a German version of the Combat Experiences Scale — a 33-item questionnaire that assesses the type and frequency of combat-related events during military deployment35 — and a study-specific questionnaire that included items about the number and duration of military deployments.
To assess psychological symptoms, participants also completed German versions of the following self-report questionnaires before each neuroimaging session: the Posttraumatic Diagnostic Scale (PDS), the Beck Depression Inventory II (BDI-II) and the State–Trait Anxiety Inventory (STAI).
The PDS36,37 is designed to aid in the diagnosis of PTSD and assess symptom severity. As part of the PDS, respondents rate 17 items representing the main symptoms of PTSD they have experienced in the preceding 30 days using a 4-point scale, ranging from 0 (“not at all or only 1 time”) to 3 (“5 or more times a week or almost always”).
The BDI-II38,39 is a 21-item inventory that assesses the characteristic behaviours and symptoms of depression participants experienced in the preceding 2 weeks using a 4-point scale, ranging from 0 (“I do not feel sad”) to 3 (“I am so sad or unhappy I can’t stand it”).
The STAI40,41 consists of 2 forms that assess state anxiety, experienced currently (Form X-1), and trait anxiety, experienced in general (Form X-2). In the current study, we used the trait anxiety subscale of the STAI (Form X-2) because we were interested in assessing changes in enduring states of anxiety and stress rather than temporary ones. In Form X-2, individuals rate 20 statements describing emotions of stress and worry using a 4-point scale, ranging from 1 (“almost never”) to 4 (“almost always”).
MRI scanning procedure
We acquired structural images using a 3 T Magnetom Tim Trio MRI scanner system (Siemens Medical Systems) and a 12-channel radiofrequency head coil. We obtained the images using a 3-dimensional T1-weighted magnetization-prepared gradient-echo (MPRAGE) sequence based on the Alzheimer’s Disease Neuroimaging Initiative protocol (www.adni-info.org; repetition time 2500 ms, echo time 4.77 ms, inversion time 1100 ms, acquisition matrix 256 × 256 × 176, flip angle 7°, voxel size 1 × 1 × 1 mm3).
MRI data analysis
We processed structural data using the computational anatomy toolbox (CAT12; http://dbm.neuro.uni-jena.de/cat/) and statistical parametric mapping (SPM12; http://www.fil.ion.ucl.ac.uk/spmb) with default parameters running on MATLAB 9.1 (Mathworks). We used voxel-based morphometry to estimate the local amount, or volume of grey matter. Voxel-based morphometry is a neuroimaging analytic technique that allows the investigation of focal differences in brain anatomy based on statistical parametric mapping of structural images. It involves bias correction, tissue classification and affine registration. We normalized images to the Montreal Neurological Institute (MNI) space using the ICBM152 template42 and segmented them into grey matter, white matter and cerebrospinal fluid using default parameters. We applied modulation to preserve the volume of a particular tissue within a voxel by multiplying voxel values in the segmented images by the Jacobian determinants derived from the spatial normalization step. We smoothed images with a full width at half maximum (FWHM) kernel of 8 mm.
We computed a whole-brain voxel-wise factorial analysis. We included age and total intracranial volume as covariates of no interest and applied an absolute grey matter probability threshold of 0.2 (CAT12 manual, http://dbm.neuro.uni-jena.de/cat12/CAT12-Manual.pdf). We thresholded the resulting maps at p < 0.001 at the voxel level and cluster-extent thresholded them at 100 voxels per cluster to control for type I error.
We also conducted region-of-interest (ROI) analysis in the hippocampus, a region commonly implicated in adult PTSD.19,25 We defined a bilateral anatomic hippocampal mask for the hippocampus using the anatomic automatic labelling (AAL)43 template. We defined a PTSD-specific hippocampal mask using the hippocampal cluster identified in a previous meta-analysis of whole-brain neuroimaging studies.20 We used the Region-of-Interest Extraction (REX) toolbox to extract grey matter volumes from the identified clusters and then entered the values for extracted volumes into repeatedmeasures analysis of variance.
We also assessed the relationship between increases in hippocampal volume and subsequent reductions in symptoms. We calculated change in the hippocampus by using the cluster identified in the whole-brain analysis and subtracting pretherapy from post-therapy hippocampal grey matter volumes; positive values indicated increases in hippocampal volume following completion of therapy. We also calculated change in symptoms by subtracting follow-up PDS, BDI-II and STAI scores from post-therapy scores; negative values indicated a decreases in symptoms from completion of therapy to follow-up. We then correlated these change scores separately for each group.
Results
Participants
The Tetris and control groups did not differ by age, sex, duration of military deployment, combat exposure, number of EMDR sessions or interval between assessments (Table 1).
Table 1.
Demographic characteristics of study participants
| Characteristic | Tetris, mean ± SD | Therapy only, mean ± SD | t test |
|---|---|---|---|
| Age, yr | 34.2 ± 7.3 | 32.5 ± 5.3 | t38 = 0.79; p = 0.43 |
| Military deployment, d | 354.9 ± 305.6 | 345.9 ± 214.2 | t38 = 0.11; p = 0.92 |
| CES score | 32.2 ± 12.9 | 34.4 ± 16.7 | t38 = 0.60; p = 0.55 |
| EMDR sessions, n | 7.0 ± 1.6 | 7.3 ± 1.9 | t38 = 0. 62; p = 0.54 |
| Pre/post interval, d | 39.9 ± 4.8 | 36.8 ± 6.6 | t38 = 1.74; p = 0.09 |
| Post/follow-up interval, d* | 204.1 ± 57.4 | 256.4 ± 138.5 | t34 = 1.48; p = 0.15 |
CES = Combat Experiences Scale; EMDR = eye movement desensitization and reprocessing; SD = standard deviation.
Follow-up data were missing for 4 participants (2 Tetris, 2 therapy-only controls).
Neuroimaging analyses
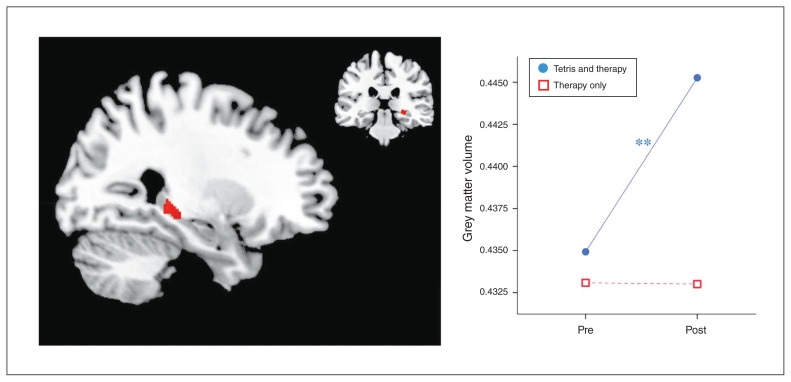
Whole-brain analysis revealed a significant increase in grey matter volume in a cluster in the right hippocampus after therapy in the Tetris group (k = 150, x = 27, y = −33, z = −3, p < 0.001 at the voxel level; and k > 100, non-isotropic smoothness corrected at the cluster level; Table 2 and Fig. 1), compared with the control group.
Table 2.
Grey matter volume from whole-brain and ROI hippocampal neuroimaging analyses
| Variable | Group | Pre, mm3* | Post, mm3* | Time | Time × group | Group |
|---|---|---|---|---|---|---|
| Voxel-based morphometry hippocampus cluster | Tetris | 0.43 ± 0.07 | 0.44 ± 0.07 | F1,38 = 9.04 | F1,38 = 9.30 | F1,38 = 0.167 |
| Therapy only | 0.43 ± 0.03 | 0.43 ± 0.03 |
p = 0.005§ ηp2 = 0.19 |
p = 0.004§ ηp2 = 0.19 |
p = 0.68 ηp2 = 0.01 |
|
| Meta-analysis hippocampus† | Tetris | 0.53 ± 0.05 | 0.54 ± 0.04 | F1,38 = 1.382 | F1,38 = 4.419 | F1,38 = 0.008 |
| Therapy only | 0.53 ± 0.03 | 0.53 ± 0.03 |
p = 0.25 ηp2 = 0.03 |
p = 0.042§ ηp2 = 0.10 |
p = 0.92 ηp2 = 0.01 |
|
| Bilateral anatomic hippocampus‡ | Therapy only | 0.45 ± 0.04 | 0.45 ± 0.04 | F1,38 = 2.13 | F1,38 = 7.07 | F1,38 = 0.006 |
| Tetris | 0.45 ± 0.02 | 0.45 ± 0.02 |
p = 0.15 ηp2 = 0.05 |
p = 0.011§ ηp2 = 0.16 |
p = 0.94 ηp2 = 0.01 |
PTSD = posttraumatic stress disorder; ROI = region of interest.
Values are given as mean ± standard deviation.
Left anterior hippocampal cluster identified in a meta-analysis of PTSD neuroimaging studies.20
Bilateral anatomic hippocampus defined using the anatomic automatic labelling template.43
Significant at p < 0.05.
Fig. 1.
Neuroimaging results of whole-brain analysis. Brain image displays the cluster from a whole-brain analysis across all participants, comparing increases in the Tetris group to the therapy-only control group after treatment (k = 150; x = 27, y = −33, z = −3; p < 0.001 at the voxel level, and k > 100, non-isotropic smoothness corrected at the cluster level). Post = directly after therapy; pre = before therapy.
As well, ROI analysis of the hippocampus revealed a significant group × time interaction. Compared to the control group, we found larger volumes in the Tetris group after therapy, in the hippocampal cluster identified in a meta-analysis of PTSD neuroimaging studies20 where PTSD patients were compared to trauma-exposed controls (F1,38 = 4.42, p = 0.042, ηp2 = 0.10), and the bilateral anatomic hippocampus defined using the AAL template (F1,38 = 7.07, p = 0.011, ηp2 = 0.16; Table 2 and Appendix 1, Fig. S1, available at jpn.ca/190027-a1).43
We also conducted ROI analyses on clusters in the ventromedial prefrontal cortex and anterior cingulate cortex identified in the meta-analysis of PTSD neuroimaging studies mentioned above20 and the bilateral anatomic amygdala defined using the AAL template.43 However, we observed no significant results (Appendix 1, Table S1).
Psychological questionnaire analyses
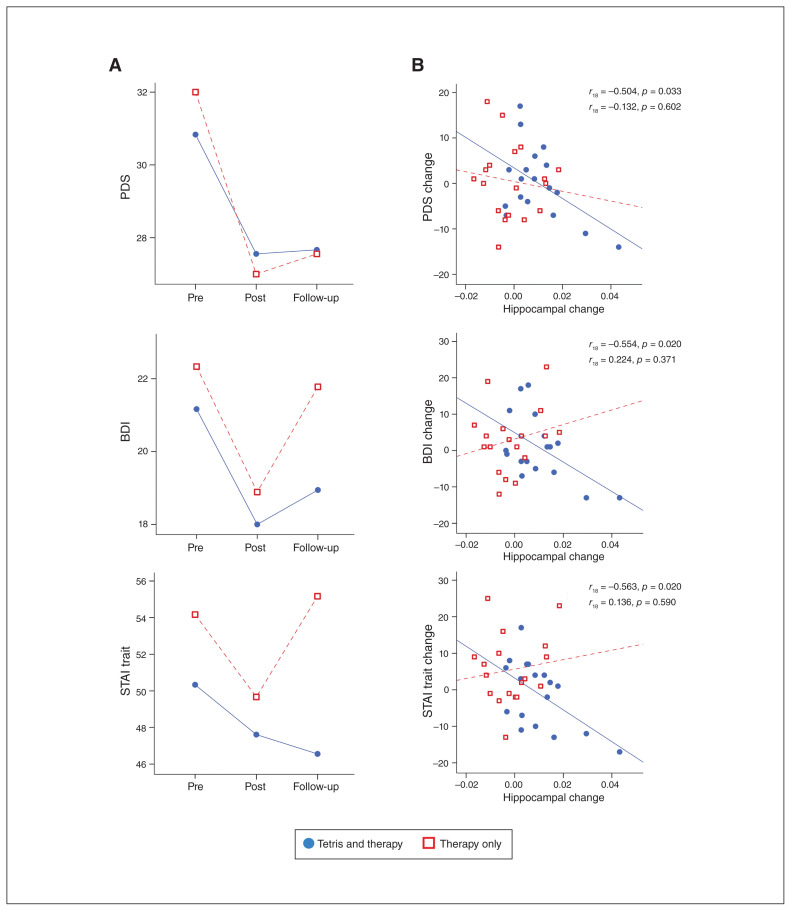
Repeated-measures analysis of variance revealed a main effect of time, with significant reductions in PTSD symptoms (PDS: F2,68 = 7.19, p = 0.001, ηp2 = 0.17) and trait anxiety (STAI Form X-2: F2,68 = 3.64, p = 0.031, ηp2 = 0.09), but no significant reductions in depression symptoms (BDI: F2,68 = 2.67, p = 0.076, ηp2 = 0.07). The group × time interaction was not significant for the PDS (F2,68 = 0.27, p = 0.76, ηp2 = 0.01) or BDI (F2,68 = 0.27, p = 0.765, ηp2 = 0.01), but we did find a significant interaction for the STAI (F2,68 = 3.15, p = 0.049, ηp2 = 0.08). We then conducted post hoc paired-sample t tests to compare symptom levels pre-therapy to those at 6-month follow-up. Both groups continued to show a significant improvement in PTSD symptoms from pre-therapy levels at 6-month follow-up (PDS Tetris t17 = 1.93, p = 0.036; PDS control t17 = 3.39, p = 0.002; significance 1-tailed). Only the Tetris group continued to show a significant reduction in anxiety symptoms (STAI Tetris t17 = 1.93, p = 0.035; STAI control t17 = 0.57, p = 0.29; significance 2-tailed), but no significant reduction in depression symptoms (BDI Tetris t17 = 1.00, p = 0.165; BDI control t17 = 0.24, p = 0.41; significance 1-tailed) at 6-month follow-up (Fig. 2A).
Fig. 2.
Psychological questionnaire scores and hippocampal grey matter. (A) Psychological questionnaire scores are displayed separately for the Tetris and therapy-only control groups, at 3 time points: before therapy (pre), directly after therapy (post) and approximately 6 months later (follow-up). (B) Increases in hippocampal volume after therapy were correlated with decreases in symptoms at follow-up in the Tetris group, but not in the therapy-only control group. Follow-up data were missing for 4 participants (2 Tetris, 2 therapy-only controls). Analyses based on 36 participants. BDI = Beck Depression Inventory; PDS = Post-traumatic Diagnostic Scale; STAI = State Trait Anxiety Inventory.
To explore associations between neural and clinical change, we correlated changes in hippocampal volume with changes in psychological symptoms. Using the cluster identified in the whole-brain analysis, we found that increased hippocampal volume after therapy was correlated with further reduction of symptoms between completion of therapy and follow-up in the Tetris group, but not in the control group (Fig. 2B).
Discussion
We found evidence that completing a visuospatial videogaming intervention during psychological therapy for PTSD led to increases in hippocampal volume and maintenance of a wider range of therapy-related gains, and increases in hippocampal volume correlated with further reductions in symptoms at 6-month follow-up. At the brain structural level, we found larger hippocampal volumes in the Tetris group in both the whole-brain and the ROI analyses. At the psychological level, directly after therapy both groups showed reductions in PTSD, depression and anxiety symptoms. At 6-month follow-up, both groups continued to show reduced PTSD symptoms, but only the Tetris group continued to show reduced anxiety symptoms. In addition, hippocampal grey matter increases during therapy were correlated with further reductions in PTSD, depression and anxiety symptoms from discharge to follow-up in the Tetris group but not the control group.
We observed a volume increase in the hippocampus but not in prefrontal regions such as the ventromedial prefrontal cortex and anterior cingulate cortex, in both the whole-brain and ROI analyses. The hippocampus is involved in memory, learning and fear extinction,44 and the ventromedial prefrontal cortex and anterior cingulate cortex are involved in affective and cognitive processing and the regulation of fear expression. 45 Smaller hippocampal volumes have been associated with increased risk for PTSD25 and with poorer prognosis; 21–24 smaller volumes in the prefrontal cortex may represent a more general effect of stress exposure.46,47 At a structural level, the hippocampus is one of the most plastic regions in the human brain; it is rich in glucocorticoid receptors, making it highly susceptible to the effects of stress. In addition, reduction in glucocorticoid receptor function may lead to hyperactivity in the hypothalamus–pituitary–adrenal axis, leading to increased levels of glucocorticoids and further reductions in hippocampal volume.48 Stress has been shown to produce dendritic atrophy and reductions in neurogenesis in the hippocampus,49 and stress-induced alterations in the hippocampus have been linked to reductions in memory and cognition50 and increases in anxiety-related behaviour. 51 Conversely, training studies have demonstrated that increases in hippocampal volume can be produced with a wide variety of interventions,29,30 including video-gaming interventions.31 The hippocampus is also involved in spatial orientation and navigation, and spatial learning has been linked to increases in hippocampal volume.52 As such, sustained focus on a demanding visuospatial task such as Tetris may produce increases in hippocampal volume, driven in part by increases in neurogenesis. In animal models, hippocampal neurogenesis has been shown to mediate forgetting and support new learning.53 Newly formed neurons compete with existing neurons for connections, and these newly formed connections may come to replace old ones, weakening existing memories and strengthening new ones.53 Therefore, we propose that video-gaming-related increases in hippocampal neurogenesis may lead to subsequent reductions in PTSD symptoms by weakening memories of the traumatic event and strengthening memories formed during therapy.
Tetris provides a promising therapeutic intervention for a number of practical reasons. The video-gaming consoles required are inexpensive and mobile, and they can be reused multiple times. Tetris does not require a clinician to administer; it can even be self-administered. Tetris does not produce adverse effects and is enjoyable to play, so adherence is likely to be high. Tetris is also adaptive; as a person continues to play, the difficulty level (the speed at which the blocks fall) automatically increases until the game ends (the blocks fill the screen), at which point the game restarts at the lowest difficulty. As such, by adapting the level of difficulty to the skill of the player, it is possible to minimize frustration that the game is too difficult and boredom that the game is too easy, and the player should remain engaged in the game.
The current study builds on previous work using Tetris as the visuospatial task of choice.1–4 However, other demanding visuospatial working-memory tasks, including other video games, could also be used successfully for PTSD. Attention and engagement with a task is known to be a key factor in producing training-related gains at both the behavioural and neural levels.54 Providing a range of games from which a person could choose could help maintain motivation and compliance, particularly for those outside of a clinical setting.
In the current study, we assessed combat-exposed young adult males. It is possible that trauma type, age, sex and military status could mediate the effect of a video-gaming intervention on the brain. Sex and age have been shown to play a role in resilience to stress49 and hippocampal neuroplasticity; 55 as well, military and civilian PTSD populations have been shown to differ in risk factors56 and response to psychotherapy.11 Future work may seek to explore the utility of video game interventions for female and civilian PTSD populations.
Limitations
At the time of study initiation, no study had attempted to use Tetris as an intervention to target memory reconsolidation or for people with current PTSD. We considered Tetris to be a promising adjunct to EMDR therapy, both as a cognitive interference task targeting reconsolidation of traumatic memories after reactivation during therapy, and as a braintraining intervention targeting the hippocampus. We consider the current results to be an initial proof of concept of the utility of Tetris as an adjunct therapeutic intervention. However, with the current design it was not possible to distinguish the cognitive-interference elements of the Tetris intervention from the brain-training elements. In addition, given the novel nature of this work, we used a standard-of-care control group. A standard-of-care control group is a widely accepted approach for assessing the effectiveness of a novel therapeutic intervention in conditions with existing therapeutic interventions. Future work may seek to employ a game active control group to test whether the observed effects were specific for Tetris and to distinguish potential brain-training effects from cognitive-interference effects. In addition, patients with PTSD may show signs of cognitive impairment,57 and increases in hippocampal volume have been linked to increases in cognitive performance after therapy for PTSD.28 Future work may seek to explore if Tetris-related changes in hippocampal volume are associated with improvements in cognitive performance in patients with PTSD.
It should be noted that more conservative approaches could be adopted for the neuroimaging analyses. However, the current sample size was small, because of the non-trivial challenge of recruiting this patient population. As such, we sought to balance concerns of type I and type II errors in our analysis by applying the less conservative approach of a voxel-level threshold of p < 0.005 combined with cluster-size correction, a method that is accepted and published.47,58
Conversely, we also note that we did not observe effects in the prefrontal cortex, despite the fact that these regions are reduced in PTSD populations18–20 and have been shown to respond to video-gaming interventions.31 The timeline of changes in the hippocampus and prefrontal cortex may differ, with the prefrontal cortex showing an initial period of expansion followed by a period of renormalization.59 Future studies may seek to use multiple neuroimaging assessments over the course of therapy to assess the rate and pattern of neural changes. Alternatively, owing to the small sample size the current study may simply have been underpowered to detect changes in the prefrontal cortex.
At the behavioural level, we observed an interesting trend toward reductions in depression symptoms at 6-month follow-up in the Tetris group, although this finding was not statistically significant. As previously noted, we consider the current results to be an initial proof of concept. However, future work with larger sample sizes is needed further to explore the utility of Tetris as an adjunct therapeutic intervention and the underlying neural mechanisms.
Conclusion
Current interventions for PTSD have a number of limitations with respect to response rates and long-term efficacy, and there is a significant need for additional therapeutic interventions that can act as an adjunct to traditional psychotherapy. We provide evidence that Tetris may be useful as an intervention for people with current PTSD, alongside EMDR psychotherapy. After completion of psychotherapy, symptoms were reduced across all participants, and both groups continued to show reductions in PTSD symptoms at 6-month follow-up. However, only the Tetris group continued to show reductions in anxiety symptoms and a trend toward reductions in depression symptoms at 6-month follow-up. Playing Tetris was correlated with increases in hippocampal volume, and hippocampal increases were correlated with continued reduction of PTSD, depression and anxiety symptoms between completion of therapy and 6-month follow-up. As such, Tetris playing may ensure that a wider range of symptom improvements are maintained after therapy, through increases in hippocampal volume.
Acknowledgments
The authors thank Sarah Polk for her helpful contributions.
Footnotes
Competing interests: J. Gallinat has received research funding from AstraZeneca and speaker fees from Lundbeck, Janssen-Cilag, Lilly and Otsuka, outside the submitted work. K. Herr, G. Willmund and P. Zimmermann are employed by the German Armed Forces; their employment had no influence on the study design. No other competing interests were declared.
Funding: O. Butler received a PhD student stipend from the International Max Planck Research School on the Life Course (LIFE). J. Gallinat has received research funding from the German Federal Ministry of Education and Research and the German Science Foundation. S. Kühn has been funded by a Heisenberg grant from the German Science Foundation (DFG KU 3322/1-1), the European Union (ERC-2016-StG-Self-Control-677804) and the Jacobs Foundation (JRF 2016–2018). The study was funded by the Military Medical Academy of German Armed Forces, German Ministry of Defense.
Data sharing: Key data and materials have been made publicly available via the Open Science Framework and can be accessed at osf.io/xz32u, or are otherwise available from the authors on request (with the exception of questionnaire measures subject to third-party copyright or potentially identifying patient information).
Contributors: All authors designed the study. O. Butler, K. Herr, G. Willmund and S. Kühn acquired the data, which O. Butler, K. Herr, G. Willmund, J. Galllinat and S. Kühn analyzed. O. Butler, K. Herr and S. Kühn wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Holmes EA, James EL, Kilford EJ, et al. Key steps in developing a cognitive vaccine against traumatic flashbacks: visuospatial Tetris versus Verbal pub quiz. PLoS One. 2010;5:e13706. doi: 10.1371/journal.pone.0013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iyadurai L, Blackwell SE, Meiser-Stedman R, et al. Preventing intrusive memories after trauma via a brief intervention involving Tetris computer game play in the emergency department: a proof-ofconcept randomized controlled trial. Mol Psychiatry. 2018;23:674–82. doi: 10.1038/mp.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horsch A, Vial Y, Favrod C, et al. Reducing intrusive traumatic memories after emergency caesarean section: a proof-of-principle randomized controlled study. Behav Res Ther. 2017;94:36–47. doi: 10.1016/j.brat.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler H, Holmes EA, Blackwell SE, et al. Reducing intrusive memories of trauma using a visuospatial interference intervention with inpatients with posttraumatic stress disorder (PTSD) J Consult Clin Psychol. 2018;86:1076–90. doi: 10.1037/ccp0000340. [DOI] [PubMed] [Google Scholar]
- 5.McGaugh JL. Memory—a century of consolidation. Science. 2000;287:248–51. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 6.Wixted JT. The psychology and neuroscience of forgetting. Annu Rev Psychol. 2004;55:235–69. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]
- 7.Walker MP, Brakefield T, Hobson JA, et al. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–20. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- 8.James EL, Bonsall MB, Hoppitt L, et al. Computer game play reduces intrusive memories of experimental trauma via reconsolidation-update mechanisms. Psychol Sci. 2015;26:1201–15. doi: 10.1177/0956797615583071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilpatrick DG, Resnick HS, Milanak ME, et al. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26:537–47. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bisson JI, Roberts NP, Andrew M, et al. Psychological therapies for chronic post-traumatic stress disorder (PTSD) in adults. Cochrane Database Syst Rev. 2013;12:CD003388. doi: 10.1002/14651858.CD003388.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley R, Greene J, Russ E, et al. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry. 2005;162:214–27. doi: 10.1176/appi.ajp.162.2.214. [DOI] [PubMed] [Google Scholar]
- 12.Schottenbauer MA, Glass CR, Arnkoff DB, et al. Nonresponse and dropout rates in outcome studies on PTSD: review and methodological considerations. Psychiatry. 2008;71:134–68. doi: 10.1521/psyc.2008.71.2.134. [DOI] [PubMed] [Google Scholar]
- 13.Kessler RC. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro F. Eye movement desensitization and reprocessing (EMDR): evaluation of controlled PTSD research. J Behav Ther Exp Psychiatry. 1996;27:209–18. doi: 10.1016/s0005-7916(96)00029-8. [DOI] [PubMed] [Google Scholar]
- 15.Seidler GH, Wagner FE. Comparing the efficacy of EMDR and trauma-focused cognitive-behavioral therapy in the treatment of PTSD: a meta-analytic study. Psychol Med. 2006;36:1515–22. doi: 10.1017/S0033291706007963. [DOI] [PubMed] [Google Scholar]
- 16.Jeffries FW, Davis P. What is the role of eye movements in eye movement desensitization and reprocessing (EMDR) for post-traumatic stress disorder (PTSD)? A review. Behav Cogn Psychother. 2013;41:290–300. doi: 10.1017/S1352465812000793. [DOI] [PubMed] [Google Scholar]
- 17.Lee CW, Cuijpers P. A meta-analysis of the contribution of eye movements in processing emotional memories. J Behav Ther Exp Psychiatry. 2013;44:231–9. doi: 10.1016/j.jbtep.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Karl A, Schaefer M, Malta LS, et al. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–31. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Kitayama N, Vaccarino V, Kutner M, et al. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Kühn S, Gallinat J. Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol Psychiatry. 2013;73:70–4. doi: 10.1016/j.biopsych.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Apfel BA, Ross J, Hlavin J, et al. Hippocampal volume differences in Gulf War veterans with current versus lifetime posttraumatic stress disorder symptoms. Biol Psychiatry. 2011;69:541–8. doi: 10.1016/j.biopsych.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chao LL, Yaffe K, Samuelson K, et al. Hippocampal volume is inversely related to PTSD duration. Psychiatry Res. 2014;222:119–23. doi: 10.1016/j.pscychresns.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Rubin M, Shvil E, Papini S, et al. Greater hippocampal volume is associated with PTSD treatment response. Psychiatry Res Neuroimaging. 2016;252:36–9. doi: 10.1016/j.pscychresns.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Rooij SJH, Kennis M, Sjouwerman R, et al. Smaller hippocampal volume as a vulnerability factor for the persistence of post-traumatic stress disorder. Psychol Med. 2015;45:2737–46. doi: 10.1017/S0033291715000707. [DOI] [PubMed] [Google Scholar]
- 25.Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–7. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bossini L, Tavanti M, Calossi S, et al. EMDR treatment for post-traumatic stress disorder, with focus on hippocampal volumes: a pilot study. J Neuropsychiatry Clin Neurosci. 2011;23:E1–2. doi: 10.1176/jnp.23.2.jnpe1. [DOI] [PubMed] [Google Scholar]
- 27.Butler O, Willmund G, Gleich T, et al. Hippocampal gray matter increases following multimodal psychological treatment for combat-related post-traumatic stress disorder. Brain Behav. 2018;8:e00956. doi: 10.1002/brb3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vermetten E, Vythilingam M, Southwick SM, et al. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Draganski B, Gaser C, Kempermann G, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26:6314–7. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kühn S, Gleich T, Lorenz R, et al. Playing Super Mario induces structural brain plasticity: gray matter changes resulting from training with a commercial video game. Mol Psychiatry. 2014;19:265–71. doi: 10.1038/mp.2013.120. [DOI] [PubMed] [Google Scholar]
- 32.Levy-Gigi E, Szabó C, Kelemen O, et al. Association among clinical response, hippocampal volume, and FKBP5 gene expression in individuals with posttraumatic stress disorder receiving cognitive behavioral therapy. Biol Psychiatry. 2013;74:793–800. doi: 10.1016/j.biopsych.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro F. Efficacy of the eye movement desensitization procedure in the treatment of traumatic memories. J Trauma Stress. 1989;2:199–223. [Google Scholar]
- 34.Foa EB, Keane TM, Friedman MJ, et al., editors. Effective treatments for PTSD: practice guidelines from the International Society for Traumatic Stress Studies. 2nd ed. New York: Guilford Press; 2009. [Google Scholar]
- 35.Guyker WM, Donnelly K, Donnelly JP, et al. Dimensionality, reliability, and validity of the Combat Experiences Scale. Mil Med. 2013;178:377–84. doi: 10.7205/MILMED-D-12-00223. [DOI] [PubMed] [Google Scholar]
- 36.Foa EB, Cashman L, Jaycox L, et al. The validation of a self-report measure of posttraumatic stress disorder: the Posttraumatic Diagnostic Scale. Vol. 9. Psychol Assess. 1997;9:445–51. [Google Scholar]
- 37.Ehlers A, Steil R, Winter H, et al. Deutsche Übersetzung der Posttraumatic Stress Diagnostic Scale (PDS) Oxford, United Kingdom: Warneford Hospital, Department of Psychiatry; 1996. [Google Scholar]
- 38.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio (TX): Psychological Corp; 1996. [Google Scholar]
- 39.Hautzinger M, Bailer MHW, Keller F. Beck-Depressions-Inventar (BDI). Bearbeitung der deutschen Ausgabe. Testhandbuch. Bern, Switzerland: Huber; 1994. [Google Scholar]
- 40.Spielberger C. Manual for the State-Trait Anxiety Inventory (STAI) Mountain View (CA): Consult Psychology Press; 1983. [Google Scholar]
- 41.Laux L, Glanzmann P, Schaffner P, et al. Das State-Trait-Angstinventar: STAI. Weinheim, Germany: Beltz; 1981. [Google Scholar]
- 42.Mazziotta J, Toga A, Evans A, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos Trans R Soc Lond B Biol Sci. 2001;356:1293–322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 44.Pohlack ST, Nees F, Liebscher C, et al. Hippocampal but not amygdalar volume affects contextual fear conditioning in humans. Hum Brain Mapp. 2012;33:478–88. doi: 10.1002/hbm.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Butler O, Adolf J, Gleich T, et al. Military deployment correlates with smaller prefrontal gray matter volume and psychological symptoms in a subclinical population. Transl Psychiatry. 2017;7:e1031. doi: 10.1038/tp.2016.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Butler O, Yang X-F, Laube C, et al. Community violence exposure correlates with smaller gray matter volume and lower IQ in urban adolescents. Hum Brain Mapp. 2018;39:2088–99. doi: 10.1002/hbm.23988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anacker C, Zunszain PA, Cattaneo A, et al. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatry. 2011;16:738–50. doi: 10.1038/mp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McEwen BS, Morrison J. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JJ, Diamond DM, Haven N, et al. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–62. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 51.Lagace DC, Donovan MH, DeCarolis NA, et al. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci U S A. 2010;107:4436–41. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kühn S, Gallinat J. Segregating cognitive functions within hippocampal formation: a quantitative meta-analysis on spatial navigation and episodic memory. Hum Brain Mapp. 2014;35:1129–42. doi: 10.1002/hbm.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akers KG, Martinez-Canabal A, Restivo L, et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- 54.Park DC, Bischof GN. The aging mind: neuroplasticity in response to cognitive training. Dialogues Clin Neurosci. 2013;15:109–19. doi: 10.31887/DCNS.2013.15.1/dpark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lisofsky N, Mårtensson J, Eckert A, et al. Hippocampal volume and functional connectivity changes during the female menstrual cycle. Neuroimage. 2015;118:154–62. doi: 10.1016/j.neuroimage.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 56.Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748–66. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- 57.Qureshi SU, Long ME, Bradshaw MR, et al. Does PTSD impair cognition beyond the effect of trauma? J Neuropsychiatry Clin Neurosci. 2011;23:16–28. doi: 10.1176/jnp.23.1.jnp16. [DOI] [PubMed] [Google Scholar]
- 58.Butler O, Herr K, Willmund G, et al. Neural correlates of response bias: larger hippocampal volume correlates with symptom aggravation in combat-related posttraumatic stress disorder. Psychiatry Res Neuroimaging. 2018;279:1–7. doi: 10.1016/j.pscychresns.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 59.Wenger E, Brozzoli C, Lindenberger U, et al. Expansion and renormalization of human brain structure during skill acquisition. Trends Cogn Sci. 2017;21:930–9. doi: 10.1016/j.tics.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]