Abstract
Background
Resting-state functional MRI (fMRI) studies commonly report alterations in 3 core networks in obsessive–compulsive disorder (OCD) — the frontoparietal network, the default mode network and the salience network — defined by functionally connected infra-slow oscillations in ongoing brain activity. However, most of these studies observed static functional connectivity in the brains of patients with OCD.
Methods
To investigate dynamic functional connectivity alterations and widen the evidence base toward the triple network model in OCD, we performed group-based independent component and sliding time window analyses in 49 patients with OCD and 41 healthy controls.
Results
The traditional independent component analysis showed alterations in the left frontoparietal network as well as between the left and right frontoparietal networks in patients with OCD compared with healthy controls. For dynamic functional connectivity, the sliding time window approach revealed peak dysconnectivity between the left and right frontoparietal networks and between the left frontoparietal network and the salience network.
Limitations: The number of independent components, noise in the resting-state fMRI images, the heterogeneity of the OCD sample, and comorbidities and medication status in the patients could have biased the results.
Conclusion
Disrupted modulation of these intrinsic brain networks may contribute to the pathophysiology of OCD.
Introduction
In recent decades, increased use of functional MRI (fMRI) has contributed to our understanding of the human brain and the pathophysiology of many psychiatric disorders. Studies typically measure brain activity using blood-oxygen-level-dependent (BOLD) signals while participants perform various tasks. More recent studies have focused on brain activity while participants lie in the scanner performing no tasks (i.e., during the resting state).1 These studies investigated intrinsic functional connectivity (iFC), reflecting coherent spontaneous BOLD fluctuations at low frequencies (0.01–0.1 Hz). Brain regions that are coupled with iFC form large-scale intrinsic brain networks,2 reflecting a basic functional organization in the brain.3
Obsessive–compulsive disorder (OCD) has been associated with altered resting-state connectivity in several brain networks. Consistent findings converge toward iFC alterations in the default mode network (DMN), the frontoparietal network (FPN) and the salience network (SN).4–6 The DMN consists of the medial frontal cortex, the posterior cingulate cortex and the precuneus, and is associated with self-referential thoughts, daydreaming and internal processes.7 The FPN, including frontal and parietal areas, has been linked to external processes and goal-driven actions.8,9 The SN encompasses the insular cortex and the anterior cingulate cortex, and is involved in switching between internal attention (modulated by the DMN) and external, goal-oriented behaviour (modulated by the FPN). Communication and interplay between these 3 networks have been discussed recently in the framework of the “triple network model.” Alterations described in this model have been found for many psychiatric disorders, including depression, schizophrenia, attention-deficit/hyperactivity disorder and autism.10
In OCD, such intrinsic brain network alterations have been found for both intraconnectivity (i.e., within a network) and interconnectivity (i.e., between networks). For instance, decreased intraconnectivity has been found in the SN, DMN and FPN in patients with OCD.11–13 With respect to interconnectivity alterations in OCD, Stern and colleagues14 reported increased connectivity between the anterior insula (part of the FPN and SN) and several regions of the DMN, such as the posterior cingulate cortex and the medial frontal cortex. Similarly, Posner and colleagues15 reported increased connectivity between the DMN and SN, specifically between the anterior medial frontal cortex and the anterior insular cortex.
In an attempt to reconcile inconsistent findings, we recently conducted a meta-analysis of 18 resting-state fMRI studies, finding decreased connectivity within the FPN and SN, and between the SN, FPN and DMN, contributing evidence to the triple network model in OCD.16 However, all of these studies employed hypothesis-driven analysis methods (i.e., seed-based connectivity analyses). There is limited research using data-driven methods, such as independent component analysis (ICA).17,18 In ICA, data are decomposed into spatially independent components without a priori knowledge or hypotheses.19 This type of analysis produces spatially independent z maps of functionally connected brain areas called independent components and their corresponding time courses.
Up to now, 2 studies have used ICA to investigate connectivity alterations in adults with OCD. For instance, Fan and colleagues18 investigated connectivity alterations in OCD by splitting the DMN, SN and FPN into subsystems. They found increased connectivity between the SN and anterior parts of the DMN, as well as between the SN and the dorsal FPN. Cheng and colleagues17 also employed ICA and reported decreased connectivity in the DMN in adults with OCD. Two other groups have explored connectivity alterations in pediatric patients with OCD: Weber and colleagues20 found alterations in the auditory and cingulate networks, and Gruner and colleagues21 detected aberrances in parts of the cingulate network.
Although these studies provide a solid basis for a better understanding of connectivity alterations in OCD, none of them has taken the temporal variability of functional connectivity into consideration.22 Intrinsic brain networks are usually analyzed by averaging time courses, assuming that connectivity stays stationary over time. However, recent studies have shown that this may be an oversimplified idea of brain network organization, and that the networks show dynamic changes over time.23,24 In addition, the correlation and anti-correlation of different states may cancel each other in a static time-averaging analysis, and the temporal dynamics of the brain states may be overlooked.
A recently developed method called the “sliding time window approach” takes the temporal dynamics of resting-state changes into account.25 This method subdivides the time course into windows and compares these windows between networks while shifting through the time domain, allowing for an assessment of the dynamic interplay between networks. The sliding time window approach enables us to explore the peak correlation of a time window between networks rather than compare the overall mean correlation of the entire time course.
To extend the current limited literature on data-driven analyses of iFC alterations in OCD, we performed group ICA on resting-state functional images in a moderate sample of patients with OCD to investigate connectivity alterations. Based on the ICA studies reported above, as well as our meta-analysis, we expected to find within-DMN and -FPN alterations on the one hand, and alterations between the DMN and the FPN, the DMN and the SN, and the FPN and the SN on the other. Finally, using the sliding time window approach, which allows for a more detailed investigation of network behaviour across time, we expected to find peak dysconnectivity (i.e., the highest correlation between 2 networks at a specific point in time, different in the patient group compared to healthy controls) between the FPN and the SN. This hypothesis was supported by the fact that this pattern of altered connectivity has been reported by a previous study that used ICA and is a relatively common finding with seed-based functional connectivity.6,16
Methods
Participants
We enrolled 50 patients with OCD and 42 healthy controls matched for age and sex in the study. All participants gave informed consent according to the Human Research Committee guidelines of Klinikum rechts der Isar, Technische Universität München. Patients were recruited from the Psychosomatic Hospital Windach and Tagesklinik of Klinikum rechts der Isar. All patients were diagnosed according to DSM-V criteria. All patients were screened for drug use, and none met the criteria for drug abuse. Healthy controls were recruited through online platforms and newspaper advertisements. All participants were scanned using a 9-minute resting-state fMRI sequence and instructed to keep their eyes closed. The patient group completed several clinical surveys, including the Yale–Brown Obsessive Compulsive Scale (YBOCS)26 and the Obsessive–Compulsive Inventory–Revised (OCI-R).27 Of the patient group, 22 had comorbidities, including depression, attention-deficit/hyperactivity disorder, general anxiety and social anxiety. Thirty-one patients were medicated, and 18 were unmedicated.
MRI data acquisition
We obtained imaging data using a 3T Philips MRI scanner with a 32-channel head coil.
We obtained high-resolution anatomic T1-weighted images using a magnetization-prepared rapid acquisition gradient echo sequence with the following scanning parameters: repetition time 11.08 ms, echo time 5.1 ms, flip angle 8°,; matrix size 368 × 318, number of slices 230, resolution 0.7 × 0.7 × 0.7 mm.
We obtained T2*-weighted functional MRI resting-state data images using echo-planar imaging with the following parameters: repetition time 2.7 s, echo time 33 ms, flip angle Gürsel et al. 90°, field of view 192 × 192 × 141 mm, matrix size 96 × 94. We acquired 64 transverse slices with 2.0 mm thickness, covering the entire brain with a resolution of 3 × 3 × 3 mm. We recorded a series of 200 whole-brain volumes.
Data analysis
fMRI data preprocessing
Functional and structural fMRI data were preprocessed using SPM 12 (Wellcome Institute of Cognitive Neurology). Preprocessing included motion correction, coregistration of functional data to the structural T1-weighted image, extraction of non-brain tissue, normalization to the Montreal Neurological Institute template, resampling into 2 × 2 × 2 mm voxel size, and smoothing with a 6 × 6 × 6 mm3 Gaussian kernel. Excessive head motion was established with frame-wise displacement, calculated as the sum of the absolute values of the derivatives of the 6 motion parameters derived from SPM 12.28 Two participants (1 healthy control and 1 patient with OCD) were excluded due to excessive head motion (mean frame-wise displacement > 0.2 mm). We found no significant differences in mean frame-wise displacement (p = 0.73) between the remaining healthy controls (mean ± standard deviation [SD] = 0.12 ± 0.03) or patients with OCD (0.11 ± 0.04).
Default mode and selection of networks
We implemented a high-model-order ICA approach in the GIFT toolbox (http://icatb.sourceforge.net). Unlike seed-based methods, ICA is a data-driven method in which the data are decomposed into spatially independent components without a priori knowledge or hypothesis.19
Following the approach from Smith and colleagues,29 we ran group ICA using 20 independent components on the preprocessed data. This number of independent components has been shown to be sufficient for identifying the DMN and task-positive networks such as the SN and FPN.30
After concatenating the resting-state fMRI data from all participants and reducing the data set using 2-step principal component analysis, we estimated 20 independent components using the Infomax algorithm. We repeated the ICA 20 times (ICASSO) to ensure the stability of the components. The group-averaged independent components were then back-reconstructed to the single-subject space using the group ICA algorithm. Each back-reconstructed independent component was depicted by a z map, which reflected the functional connectivity of the networks. The associated time courses, representing the time-dependent activity of each component, were de-trended, de-spiked and low-pass-filtered (Butterworth cutoff frequency: 0.15 Hz).
We focused our analyses on the 3 networks of interest (i.e., the FPN, the SN and the DMN). To identify these networks, we correlated each of the 20 components with templates from Yeo and colleagues,31 based on a 7-network parcellation. These templates are created from a whole-brain parcellation of 1000 participants and are very robust representations of the intrinsic brain networks. The templates do not differentiate between the left and right FPN, but we were interested in lateralization effects, so we kept the left and right FPN components separate, as implemented in the group ICA algorithm. This step provided us with 4 networks of interest in our data set.
Statistical analysis
Participant demographics
We analyzed differences in demographic and clinical characteristics between the 2 groups (healthy controls v. patients with OCD) using 2-sample t tests and χ2 tests.
Intra-iFC
We conducted statistical analysis of the independent components using 1-sample t tests for each group. We performed the 1-sample t tests (SPM 12) on the group ICA–derived and reconstructed spatial maps of all participants (p < 0.05, family-wise error (FWE)–corrected for multiple comparisons). To detect group differences, we compared spatial maps using 2-sample t tests (SPM 12), with age and sex as covariates of no interest. To identify medication effects, we also compared the spatial maps of medicated and unmedicated patients using 2-sample t tests.
Inter-iFC
To measure between-network functional connectivity, the time-courses of networks were de-trended, de-spiked and filtered (high cutoff: 0.15 Hz) and entered into pair-wise Pearson correlations. Group differences were detected using 2-sample t tests of the networks’ Fisher correlation coefficients (r to z transformed; Bonferroni correction to p < 0.05/6 = 0.0083). To identify medication effects, we also compared medicated and unmedicated patients’ correlation values using 2-sample t tests.
Sliding time window analysis
Following the approach by Franzmeier and colleagues,25 we performed sliding time window analysis using the temporal dFNC toolbox in GIFT. We applied a sliding time window with a width of 30 repetition times (81 s of magnetic resonance acquisition).
We overlaid the time window on the time course of each intrinsic brain network and shifted it in steps of 1 repetition time. We applied a Gaussian σ of 3 repetition times to the rectangular time window to reach tapering near the edges. We correlated each time window along the time course from 1 network with the time windows from a second network of interest, yielding 170 correlation values for each participant. We found the peak correlation value between 2 networks for each participant and consolidated it with the 10 surrounding correlation values (40 repetition times, 108 seconds of resting-state fMRI). To account for the size of the time window integration, we repeated the procedure with a size of 5 and 15 consecutive time windows. We found no significant differences in the results for the size of the merged time windows, and results are reported for the previously established time window selection of 10. We then sorted the participant-specific mean correlation coefficients into the 2 groups (patients with OCD v. healthy controls) and compared them with 2-sample t tests. Because we were interested in 3 networks (DMN, FPN and SN) that were divided into 4 spatial maps from the group ICA (DMN, left FPN, right FPN and SN), we repeated this procedure 6 times to correlate each network with another. Finally, to identify medication effects, we also compared medicated and unmedicated patients’ correlation values using 2-sample t tests.
IFC correlations with symptom scores
We used Spearman correlations (because iFC z values were not normally distributed) to analyze the relationship between connectivity alterations of intra-iFC results and symptom scores such as Y-BOCS, OCI-R and the Hamilton Rating Scale for Depression (HAM-D).32
Next, we used Pearson correlations to analyze the relationship between connectivity alterations in the inter-iFC and sliding time window results and symptom scores on scales such as Y-BOCS, OCI-R and HAM-D.
Results
Demographic and clinical characteristics
Healthy controls (mean ± SD = 35.07 ± 10.04) and patients with OCD (34.42 ± 12.07) did not differ in terms of age (p = 0.86) or sex (p = 0.51). The demographic and clinical characteristics of the participants are shown in Table 1 and Appendix 1, Table S1, available at jpn.ca/190038-a1.
Table 1.
Participant demographic and clinical characteristics
| Characteristic | Healthy controls (n = 41) | OCD (n = 49)* | p value |
|---|---|---|---|
| Demographic characteristics | |||
| Age, mean ± SD | 35.07 ± 10.04 | 34.42 ± 12.07 | 0.86 |
| Male, n (%) | 19 (46) | 16 (32) | 0.51 |
| Clinical characteristics | |||
| Medication, yes/no | — | 31/18 | — |
| Comorbidities, n | — | 22 | — |
| Depression | — | 14 | — |
| Attention-deficit/hyperactivity disorder | — | < 5 | — |
| Generalized anxiety disorder | — | < 5 | — |
| Social anxiety disorder | < 5 | — | |
| Panic attacks | — | < 5 | — |
| Borderline personality disorder | — | < 5 | — |
| Mean age of OCD onset, yr (min/max) | — | 18.3 (7/58) | — |
| Y-BOCS total, mean ± SD | — | 20.95 ± 6.1 | — |
| Y-BOCS obsessions, mean ± SD | — | 10.65 ± 3.46 | — |
| Y-BOCS compulsions, mean ± SD | — | 10.30 ± 3.83 | — |
| OCI-R total, mean ± SD | — | 27 ± 10.19 | — |
| HAM-D, mean ± SD | — | 13.3 ± 5.46 | — |
HAM-D = Hamilton Rating Scale for Depression; OCD = obsessive–compulsive disorder; OCI-R = Obsessive–Compulsive Inventory–Revised; SD = standard deviation; Y-BOCS = Yale–Brown Obsessive Compulsive Scale.
Populations of fewer than 5 have been rounded to protect participant privacy.
Group ICA
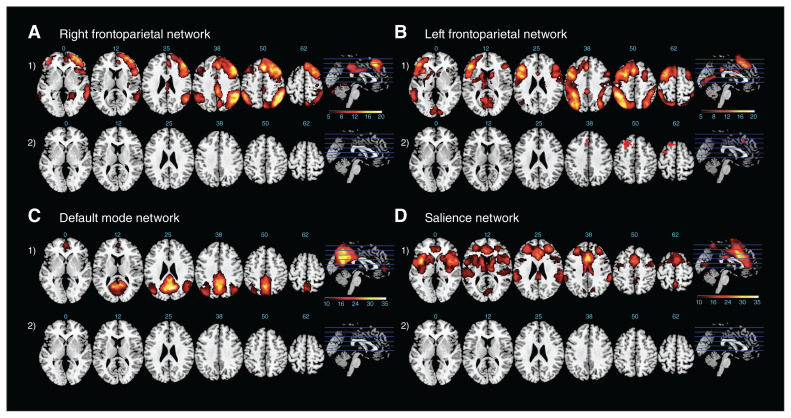
Independent components representing the networks of interest were consistent across patients with OCD and healthy controls (see Fig. 1 for spatial maps). We identified the networks by correlating our components with the templates from Yeo and colleagues.31 Spatial correlation scores between the components and the network templates ranged from 0.30 to 0.44.
Fig. 1.
Right and left frontoparietal network, default mode network and salience network overlaid on a Montreal Neurological Institute template. Axial slices of each network are at the following ascending z coordinates: 0, 12, 25, 38, 50 and 62. (1) Results for 1-sample t tests of the respective network for all participants (pFWE < 0.05). The colour scale for the right and left frontoparietal networks is shown for t values. (2) Results for the 2-sample t test between groups (healthy controls and patients with obsessive–compulsive disorder; OCD). Red highlights the changes for patients with OCD > healthy controls. The colour scale represents t values, and all maps are corrected with the extent threshold (p < 0.001; cluster > 111). FWE = family-wise error.
Group differences in intra-iFC
Compared with healthy controls, patients with OCD showed altered functional connectivity only in the left FPN (Table 2). Specifically, patients showed increased functional connectivity in the superior and middle frontal gyrus (cluster-based correction, p < 0.01; cluster size = 551; Fig. 1, B2, second row). We observed no significant group differences for the right FPN, SN or DMN. Medicated and unmedicated patients showed no significant differences in any of the networks.
Table 2.
Intranetwork functional connectivity differences — OCD v. healthy controls
| Anatomic region | Cluster size, voxels | t value | p value* | MNI (x, y, z) |
|---|---|---|---|---|
| Left frontoparietal network | ||||
| Middle frontal gyrus | 791 | 4.5 | 0.001 (extent threshold) | −26, 24, 50 |
| Right frontoparietal network | ||||
| Default mode network | ||||
| Salience network | ||||
MNI = Montreal Neurological Institute; OCD = obsessive–compulsive disorder.
Significant for 2-sample t test.
Group differences in inter-iFC using ICA
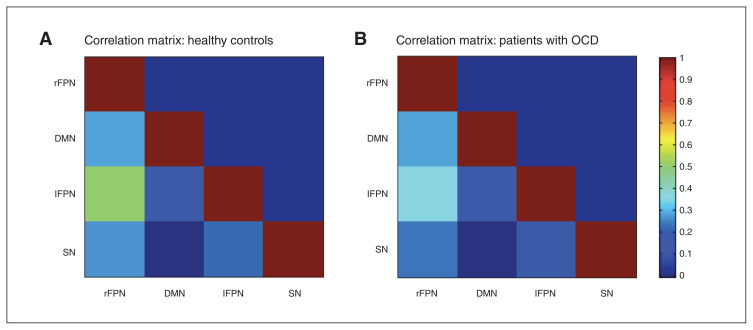
Patients with OCD showed decreased connectivity between the left and right FPN (Fig. 2 and Table 3). We found no other significant group differences. Significant results were due to lower positive correlations in patients with OCD compared to healthy controls (healthy controls 0.498 v. OCD 0.342, p < 0.001). Medicated and unmedicated patients showed no significant differences in any of the networks.
Fig. 2.
Internetwork intrinsic functional connectivity matrices for healthy controls and patients with obsessive–compulsive disorder (OCD). Pairwise Pearson correlations were performed between the 4 networks of interest. The average correlation values of the z scores are presented here; the colours represent the intensity of the correlations. lFPN = left frontoparietal network; rFPN = right frontoparietal network; DMN = default mode network; SN = salience network.
Table 3.
Correlation values between healthy controls and patients with OCD
| Region | Healthy controls (n = 41) | OCD (n = 49) | 2-sample t test direction | p value* |
|---|---|---|---|---|
| Inter-iFC | ||||
| rFPN–DMN | 0.27 | 0.28 | 0.76 | |
| rFPN–IFPN | 0.49 | 0.33 | Healthy controls > OCD | < 0.001‡ |
| rFPN–SN | 0.25 | 0.23 | 0.76 | |
| DMN–IFPN | 0.15 | 0.14 | 0.75 | |
| DMN–SN | −0.02 | −0.01 | 0.76 | |
| IFPN–SN | 0.21 | 0.13 | 0.08 | |
| Sliding time window† | ||||
| rFPN–DMN | 0.71 | 0.77 | 0.30 | |
| rFPN–IFPN | 1.01 | 0.80 | Healthy controls > OCD | < 0.001‡ |
| rFPN–SN | 0.74 | 0.71 | 0.65 | |
| DMN–IFPN | 0.59 | 0.59 | 0.92 | |
| DMN–SN | 0.44 | 0.45 | 0.88 | |
| IFPN–SN | 0.73 | 0.55 | Healthy controls > OCD | 0.007‡ |
DMN = default mode network; iFC = intrinsic functional connectivity; lFPN = left frontoparietal network; OCD = obsessive–compulsive disorder; rFPN = right frontoparietal network; SN = salience network.
From 2-sample t tests reduced to p > 0.0083 after Bonferroni correction for 6 t tests
Fisher z-transformed mean peak correlation values between healthy controls and patients with OCD.
Significant at p < 0.05.
Group differences in inter-iFC using sliding time window analysis
Sliding time window analysis revealed significant differences between 2 network pairs. Patients with OCD showed decreased connectivity between the left and right FPN, and between the left FPN and SN (Table 3). Significant results were due to lower positive correlations in patients with OCD in both cases (healthy controls 1.013 v. OCD 0.806; healthy controls 0.732 v. OCD 0.561; p < 0.008). Medicated and unmedicated patients showed no significant differences in any of the networks.
Next, we investigated the behaviour of the left and right FPN and the left FPN and SN over time in more detail. Specifically, we plotted correlation values of network pairs for all time windows included in the analysis, for both patients with OCD and healthy controls. The plots demonstrated that there was more variance in the OCD group than in the healthy control group. They also showed a specific temporal pattern for the group difference in connectivity: on one hand, a similar time course of the network pairs for both groups, and on the other, only minimal differences between groups in certain time windows (i.e., between time windows 100 to 120; see Appendix 1, Figure S1).
Correlations between altered iFC and symptom scores
First, we tested for intra-iFC correlations with symptom scores. We found no significant correlations between the middle frontal gyrus iFC and Y-BOCS (r = −0.14, p = 0.31), OCI-R (r = −0.006, p = 0.96) or HAM-D (r = −0.04, p = 0.76) scores.
Next, we tested for inter-iFC correlations with symptom scores. We found no significant correlations between the left and right FPN iFC and Y-BOCS (r = 0.02, p = 0.99), OCI-R (r = −0.005, p = 0.97) or HAM-D (r = −0.02, p = 0.87) scores.
Finally, we tested for correlations between sliding time window results and symptom scores. We found no correlation between the left and right FPN and Y-BOCS (r = 0.21, p = 0.13), OCI-R (r = 0.06, p = 0.6) or HAM-D (r = −0.006, p= 0.96) scores. We also found no correlation between the left FPN and SN and Y-BOCS (r = −0.18, p = 0.19), OCI-R (r = 0.04, p = 0.77) or HAM-D (r = −0.19, p = 0.17) scores.
Discussion
The present study investigated functional connectivity alterations in large-scale networks in OCD. To extend the current literature, we focused our analyses on alterations in the triple network model, including the DMN, FPN and SN.16 We employed one of the most established data-driven analysis methods, namely ICA. In addition, we applied a relatively novel analysis method: the sliding time window approach. These analyses allowed us to investigate both static and dynamic alterations of intrinsic brain networks in patients with OCD. We found increased intra-FPN iFC in patients with OCD. We also found internetwork alterations in the form of decreased connectivity between the left and right FPN. Regarding dynamic iFC, we found decreased connectivity between the left and right FPN, and between the left FPN and SN.
Our first finding of increased iFC in the left FPN, peaking in the middle frontal gyrus (Fig. 1, B2 and Table 2), matched previous reports from functional connectivity studies. For example, Göttlich and colleagues33 found increased connectivity for unmedicated patients with OCD in the FPN, specifically in the middle frontal gyrus and superior parietal cortex, using graphical analysis methods. In their patient sample, this increased connectivity was positively correlated with Y-BOCS scores. Anticevic and colleagues4 also reported altered connectivity in the superior, middle and inferior gyri in OCD using a global brain connectivity method.
Our second finding regarding inter-iFC analyses revealed reduced connectivity between the left and right FPN (Fig. 2 and Table 3). Alterations in this network are the most commonly reported findings in the OCD literature.16 The FPN is an attention network that plays a role in goal-directed behaviour. Alterations in FPN can be linked to excessive monitoring of thoughts in OCD,33 which could explain some of the symptoms. In fact, it is known from several task-based fMRI studies that patients with OCD have difficulty with various tasks that require attention, such as response inhibition, inference or attention switching.34–36
Finally, the sliding time window analyses revealed reduced connectivity between the left and right FPN, supporting the inter-iFC analysis, and between the left FPN and SN (Table 3). Our recent meta-analysis16 and a study by Harrison and colleagues6 pointed to hypoconnectivity between the FPN and SN in patients with OCD. As conceptualized in the triple network model, the SN has a role in switching between the DMN and FPN — in other words, switching between self-referential thoughts or internal attention and external attention or goal-directed behaviour. Disturbances in the FPN and SN may indicate a regulation problem in the triple network in OCD and explain why patients have problems disengaging from irrational internal thoughts or correcting their internal concept (e.g., “still not clean”) with external evidence. We also found that between-network connectivity patterns, specifically between the left and right FPN and the left FPN and SN, behaved similarly over the time windows for each group separately. However, the difference in connectivity patterns of the 2 groups followed a specific temporal pattern, ranging from minimal difference to maximal difference in certain time windows. This finding suggests complex patterns of dysconnectivity in patients with OCD, mainly focused on the FPN.
In summary, our current group-based ICA and sliding time window analyses revealed that the main disruptions in iFC are focused on the FPN. This was in line with several studies that found alterations in this network, as well as with our own meta-analysis on seed-based resting-state fMRI in OCD.16,33,37 Furthermore, beyond FPN iFC alterations, our results showed altered connectivity between the FPN and SN in a dynamic manner. Such alterations have been reported before6,16 and are in line with the so-called triple network model of psychopathology, which includes the DMN as well as the FPN and SN.10 However, we did not find alterations between the FPN and DMN or the SN and DMN in this patient sample. With respect to symptomatology, OCD is a heterogeneous disorder, and findings related to altered iFC and intrinsic brain networks are equivocal.17,38
Limitations
Several limitations should be taken into account for the current study. First, there is no gold standard for defining the number of independent components. Using a higher number of components (such as 75) usually provides more detailed networks covering the subcomponents (i.e., anterior, posterior, superior and ventral parts of the networks). Because we decided to investigate connectivity alterations focusing on the 3 networks of interest (DMN, SN and FPN), we chose 20 as our predefined component number. However, future studies might want to investigate the basal ganglia network as well, because alterations have been found between the FPN and nuclei of the basal ganglia, such as the striatum.6,16,39 Second, although noise is a problem inherent to all fMRI analyses, it plays an even larger role in windowed analyses, because the data are considerably more sensitive to noise if multiple time windows are considered, instead of the whole time series. Hence, we could not exclude the possibility that noise confounded the fluctuations over time. However, given that longer windows yield less noise-affected estimates of connectivity, our relatively long window duration of 81 s should at least have minimized this limitation. Third, with respect to symptoms, our OCD sample was heterogeneous, which could theoretically have confounded our results and minimized specificity. Fourth, we included both medicated and unmedicated patients in our study. Medication status5,40 can have an effect on brain connectivity changes. To tackle this issue, we separated our patients into medicated and unmedicated patient subgroups and reran the intra-, inter-and sliding time window iFC analyses, but we found no differences between the subgroups. This finding suggests that medication did not confound our results for the FPN, but we cannot exclude its effect holistically. Fifth, we did not acquire information about intelligence parameters or years of education, so we were unable to control for putative effects of intelligence or education on altered iFC. Finally, almost half of the OCD sample had comorbidities, which might have confounded results as well (e.g., we found no effect in the DMN).
Conclusion
We applied group independent component analysis and, to our knowledge for the first time in OCD research, a sliding window approach to the resting-state fMRI data of patients with OCD and healthy controls. Our findings indicated both static and dynamic functional connectivity alterations in patients with OCD, mainly focused on the FPN: within the FPN and between the FPN and SN. These findings were in line with previous reports that have suggested FPN alterations to be at the core of OCD psychopathology.
Acknowledgements
This study was supported by a Deutsche Forschungsgemeinschaft (DFG) grant to K. Koch (KO 3744/7-1).
Footnotes
Competing interests: None declared.
Contributors: D. Gürsel, L. Reinholz and K. Koch designed the study. D. Gürsel, L. Reinholz, B. Bremer and B. Schmitz-Koep acquired the data, which D. Gürsel, L. Reinholz, N. Franzmeier, M. Avram and K. Koch analyzed. D. Gürsel and M. Avram wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 2.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 3.Greicius MD, Kiviniemi V, Tervonen O, et al. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp. 2008;29:839–47. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anticevic A, Hu S, Zhang S, et al. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:595–605. doi: 10.1016/j.biopsych.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beucke JC, Sepulcre J, Talukdar T, et al. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry. 2013;70:619–29. doi: 10.1001/jamapsychiatry.2013.173. [DOI] [PubMed] [Google Scholar]
- 6.Harrison BJ, Soriano-Mas C, Pujol J, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:1189–200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- 7.Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodds CM, Morein-Zamir S, Robbins TW. Dissociating inhibition, attention, and response control in the frontoparietal network using functional magnetic resonance imaging. Cereb Cortex. 2011;21:1155–65. doi: 10.1093/cercor/bhq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Shin DJ, Jung WH, He Y, et al. The effects of pharmacological treatment on functional brain connectome in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:606–14. doi: 10.1016/j.biopsych.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Fan Q, Zhang H, et al. Altered intrinsic insular activity predicts symptom severity in unmedicated obsessive-compulsive disorder patients: a resting state functional magnetic resonance imaging study. BMC Psychiatry. 2016;16:104. doi: 10.1186/s12888-016-0806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beucke JC, Sepulcre J, Eldaief MC, et al. Default mode network subsystem alterations in obsessive-compulsive disorder. Br J Psychiatry. 2014;205:376–82. doi: 10.1192/bjp.bp.113.137380. [DOI] [PubMed] [Google Scholar]
- 14.Stern ER, Fitzgerald KD, Welsh RC, et al. Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PLoS One. 2012;7:e36356. doi: 10.1371/journal.pone.0036356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Posner J, Song I, Lee S, et al. Increased functional connectivity between the default mode and salience networks in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp. 2017;38:678–87. doi: 10.1002/hbm.23408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gürsel DA, Avram M, Sorg C, et al. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity. Neurosci Biobehav Rev. 2018;87:151–60. doi: 10.1016/j.neubiorev.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y, Xu J, Nie B, et al. Abnormal resting-state activities and functional connectivities of the anterior and the posterior cortexes in medication-naive patients with obsessive-compulsive disorder. PLoS One. 2013;8:e67478. doi: 10.1371/journal.pone.0067478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan J, Zhong M, Gan J, et al. Altered connectivity within and between the default mode, central executive, and salience networks in obsessive-compulsive disorder. J Affect Disord. 2017;223:106–14. doi: 10.1016/j.jad.2017.07.041. [DOI] [PubMed] [Google Scholar]
- 19.Calhoun VD, Adali T, Pearlson GD, et al. Spatial and temporal independent component analysis of functional MRI data containing a pair of task-related waveforms. Hum Brain Mapp. 2001;13:43–53. doi: 10.1002/hbm.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber AM, Soreni N, Noseworthy MD. A preliminary study of functional connectivity of medication naive children with obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53:129–36. doi: 10.1016/j.pnpbp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Gruner P, Vo A, Argyelan M, et al. Independent component analysis of resting state activity in pediatric obsessive-compulsive disorder. Hum Brain Mapp. 2014;35:5306–15. doi: 10.1002/hbm.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen EA, Damaraju E, Plis SM, et al. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex. 2014;24:663–76. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. 2010;50:81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zalesky A, Fornito A, Cocchi L, et al. Time-resolved resting-state brain networks. Proc Natl Acad Sci U S A. 2014;111:10341–6. doi: 10.1073/pnas.1400181111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franzmeier N, Buerger K, Teipel S, et al. Cognitive reserve moderates the association between functional network anti-correlations and memory in MCI. Neurobiol Aging. 2017;50:152–62. doi: 10.1016/j.neurobiolaging.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Goodman WK, Price LH, Rasmussen SA, et al. The Yale–Brown Obsessive Sompulsive Scale: I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 27.Foa EB, Huppert JD, Leiberg S, et al. The Obsessive-Compulsive Inventory: development and validation of a short version. Psychol Assess. 2002;14:485. [PubMed] [Google Scholar]
- 28.Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di X, Biswal BB. Modulatory interactions between the default mode network and task positive networks in resting-state. Peer J. 2014;2:e367. doi: 10.7717/peerj.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gottlich M, Kramer UM, Kordon A, et al. Decreased limbic and increased fronto-parietal connectivity in unmedicated patients with obsessive-compulsive disorder. Hum Brain Mapp. 2014;35:5617–32. doi: 10.1002/hbm.22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abramovitch A, Abramowitz JS, Mittelman A. The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clin Psychol Rev. 2013;33:1163–71. doi: 10.1016/j.cpr.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Eng GK, Sim K, Chen SH. Meta-analytic investigations of structural grey matter, executive domain-related functional activations, and white matter diffusivity in obsessive compulsive disorder: an integrative review. Neurosci Biobehav Rev. 2015;52:233–57. doi: 10.1016/j.neubiorev.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Remijnse PL, van den Heuvel OA, Nielen MM, et al. Cognitive inflexibility in obsessive-compulsive disorder and major depression is associated with distinct neural correlates. PLoS One. 2013;8:e59600. doi: 10.1371/journal.pone.0059600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison BJ, Pujol J, Cardoner N, et al. Brain corticostriatal systems and the major clinical symptom dimensions of obsessive-compulsive disorder. Biol Psychiatry. 2013;73:321–8. doi: 10.1016/j.biopsych.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Hou J, Wu W, Lin Y, et al. Localization of cerebral functional deficits in patients with obsessive-compulsive disorder: a resting-state fMRI study. J Affect Disord. 2012;138:313–21. doi: 10.1016/j.jad.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 39.Fitzgerald KD, Welsh RC, Stern ER, et al. Developmental alterations of frontal-striatal-thalamic connectivity in obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2011;50:938–948. doi: 10.1016/j.jaac.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Posner J, Marsh R, Maia TV, et al. Reduced functional connectivity within the limbic cortico-striato-thalamo-cortical loop in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp. 2014;35:2852–60. doi: 10.1002/hbm.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]