Abstract
BACKGROUND:
Visual problems can negatively affect visual development and learning but often go undetected. We assessed the feasibility of scaling up a school-based screening program to identify and treat kindergarten children with visual problems.
METHODS:
We conducted a prospective cohort study offering vision screening to junior (JK) and senior kindergarten (SK) children attending 43 schools in 15 Ontario communities. Screening comprised photoscreeners and tests of visual acuity, stereoacuity and eye alignment. Children who failed any test were referred for a comprehensive eye examination, with treatment as needed (e.g., glasses).
RESULTS:
Using a passive consent model, 89% of children were screened compared with 62% using an active consent model (p < 0.001). Referral rates to an optometrist varied across schools (mean referral rate for children in JK 53%, range 25%–83%; mean referral rate for children in SK 34%, range 12%–61%). Among 4811 children who were screened, a visual problem was detected in 516 (10.7%), including 164 (3.4%) with amblyopia and 324 (6.7%) with clinically significant refractive errors. For 347 (67.2%) of the children with a visual problem, this was their first eye examination. Rescreening in Year 2 did not lead to detection of additional problems among children who passed screening in Year 1. Regardless of location (child’s school or optometrist’s office), 1563 (68.9%) of children attended the follow-up optometry examination. Most of the children who were surveyed (291 of 322, 90.4%) indicated that they enjoyed vision screening.
INTERPRETATION:
Many children in Ontario with a visual problem were not being identified by the status quo in 2015–2017. We found that in-school vision screening with follow-up eye examinations is an effective strategy for identifying at-risk children and placing them in eye care before grade 1.
Two previous Canadian studies suggested that about 1 in 5 children aged 3–6 years have a visual problem such as amblyopia or clinically significant refractive errors (i.e., hyperopia, myopia, astigmatism or anisometropia).1,2 Population-based studies in the United States and other countries have reported similar prevalence.3–15 The United States Preventive Services Task Force recommends at least 1 vision screening in children between the ages of 3 and 5 years.16 The Canadian Paediatric Society recommends screening for visual problems at well-child visits for children aged 3–5 years;17 however, this often does not occur.18 One report from Ontario showed that only 14% of children under 6 years of age had a comprehensive eye examination in 2013, even though examinations are paid for by provincial health insurance.19 The underutilization of vision care means that children with amblyopia are not receiving treatment when it is most effective (i.e., before 8 yr of age).20–22 In addition, early visual problems can negatively affect learning — children with amblyopia read more slowly than those with normal vision,23,24 and refractive errors are associated with poor reading by grade 1.25–31
We are unaware of any studies to date that have examined the scaling and implementation issues associated with a school-based, vision-screening program in kindergarten, or whether such a program could successfully identify and offer treatment (e.g., glasses) to children with previously undiagnosed visual problems.32–34 In a study1 in 1 school (n = 709 children), we investigated what combination of screening tools provided the highest sensitivity and specificity. In the current study, we used this information to test the feasibility of scaling up vision screening to multiple schools in diverse Ontario communities. We investigated whether screening should be offered in both junior kindergarten (JK) and senior kindergarten (SK), whether using an active or passive consent model makes a difference, whether offering follow-up eye examinations at the child’s school can reduce barriers to access, and whether children are receptive to a screening program. These are practical details needed by funders to make decisions about implementing school-based vision screening.
Methods
Study participants and design
Participants were JK (4 yr of age) and SK (5 yr of age) children who attended 43 schools in 15 communities in Ontario (n = 5884 children) from October 2015 to June 2017. The sample population was diverse: urban, suburban and rural communities; schools with small and large enrolment; and public and separate school boards. For 9 school boards (31 schools), we used a passive consent model (children were screened unless parents submitted an opt-out form), whereas 3 school boards (12 schools) required active consent (children were screened only when parents returned a signed consent form).
Based on previous studies35–40 and a concurrent study,1 we used an acuity test (Cambridge Crowded Acuity Cards), 2 photoscreeners (Plusoptix S12 and Spot Vision Screener photoscreeners) and 2 measures of binocular vision (Preschool Randot Stereoacuity test and Pediatric Vision Scanner [PVS]) because they have high accuracy in identifying children with visual problems1 (Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.191085/-/DC1).
We collaborated with pre-existing screening programs whenever possible. If no pre-existing program was available, we hired screeners. Training was provided by authors M.N. and D.M. to ensure compliance with the research protocol. We hired a local coordinator in each community for administrative duties who was trained by author M.N. or the central coordinator from our concurrent study.1
We considered children who met any referral criterion (Table 1) as a “refer” and only children who passed all 5 tests as a “pass.” We based referral criteria on the 2013 guidelines of the American Association of Pediatric Ophthalmology and Strabismus (AAPOS),41 previously published norms42 and our own data.1 Children who did not understand or were uncooperative were considered a refer, because such children have a higher risk of having a vision disorder,43 and we reported the frequency for each screening test. In August 2018, the Ontario Ministry of Health and Long-Term Care (MOHLTC) released a protocol for vision screening in children in SK as part of the Ontario Public Health Standards (2017). We reanalyzed data from Year 1 using only the data from acuity, stereoacuity and 1 photoscreener, the tests mandated by this protocol, using the referral criteria in Table 2, and reported these findings separately.
Table 1:
Referral criteria for screening tools for children 31–48 months and more than 48 months of age
| Screening test | Criteria for children aged 31–48 mo | Criteria for children aged > 48 mo |
|---|---|---|
| Cambridge Crowded Acuity | Worse than 6/12 in either eye* | Worse than 6/9 in either eye |
| Plusoptix and Spot photoscreeners | ||
| Hyperopia | > 4.0 D | > 3.5 D |
| Myopia | < −3.0 D | < −1.5 D |
| Astigmatism | > 2.0 D | > 1.5 D |
| Anisometropia IOD | > 2.0 | > 1.5 |
| Randot Preschool Stereoacuity | Worse than 100 arcsec | |
| Pediatric Vision Scanner | Binocularity < 0.60 | |
Note: D = diopters, IOD = interocular difference.
Children aged 48 months were tested against the “worse than 6/9” criterion.
Table 2:
Definitions of visual problems in children
| Disorder | Definition | |
|---|---|---|
| Amblyopia | ≥ 2-line difference in best corrected acuity and worse than 20/40 in 1 eye | |
| Binocular vision | ||
| Strabismus | Tropias > 10 D | |
| Reduced stereoacuity | > 100 arcsec | |
| Refractive errors | At 31–48 mo | At > 48 mo |
| Hyperopia (sph) | > 4.0 D | > 3.5 D |
| Astigmatism (cyl) | > 2.0 D | > 1.5 D |
| Anisometropia (SE) | > 2.0 D IOD | > 1.5 D IOD |
| Myopia (sph) | < −2.0 D | < −1.5 D |
| Other problems | Nystagmus, vitreous abnormalities, optic nerve abnormalities | |
Note: cyl = cylindrical error, D = diopters, IOD = interocular difference, SE = spherical equivalent, sph = spherical error.
Children who did not pass screening were booked for a comprehensive eye examination with a licensed optometrist. For all schools in Year 1 and a subset in Year 2, the eye examination was at the school. For 19 schools, the appointment was at the school in Year 1 and at the optometrist’s office in Year 2, allowing a comparison of compliance. Optometrists were masked to the screening results to reduce bias.
The definition of amblyopia and its risk factors (binocular vision disorders and clinically significant refractive errors) are shown in Table 2 and they follow the AAPOS (2013) guideline41 for children aged 31–72 months and the clinical judgment of author A.W. For children screened in both JK and SK, we included only the findings from JK in the calculation of the number of children with visual problems.
In 6 schools in Year 2, we collected responses from 322 children about what they thought of the “vision games.” They were given a private space to circle the face (Appendix 1, Supplemental Figure 1) that matched their assessment (happy = fun, neutral or sad = not fun).
We estimated the costs of screening for 1 city from those in this feasibility study (Appendix 1, Supplementary Table 1).
Statistical analysis
We used descriptive statistics to analyze trends observed in implementing vision screening: referral rates, opt-out rates, participation rates in follow-up eye examinations and frequency of finding a visual problem. Rates were analyzed at the school level and summarized for the overall cohort as the mean and range of school-level rates. Because the PVS was a prototype not commercially available, we analyzed the results from screening with PVS data included and excluded. We used SPSS Statistics software (IBM, version 25) to conduct paired-samples t tests to compare means between groups in the same schools (i.e., JK v. SK; in-school v. in-office follow-up examinations). We used an independent-samples t test to compare the percentage of students who were screened in active versus passive consent models (equal variances were not assumed). We also conducted post hoc analyses using information available from the Internet to determine whether family income or immigration status might explain the variability in referral rates across schools.
Ethics approval
The study was approved by the Research Ethics Boards of The Hospital for Sick Children and McMaster University, as well as school board research ethics committees when required. The PVS was used with authorization from Health Canada.
Results
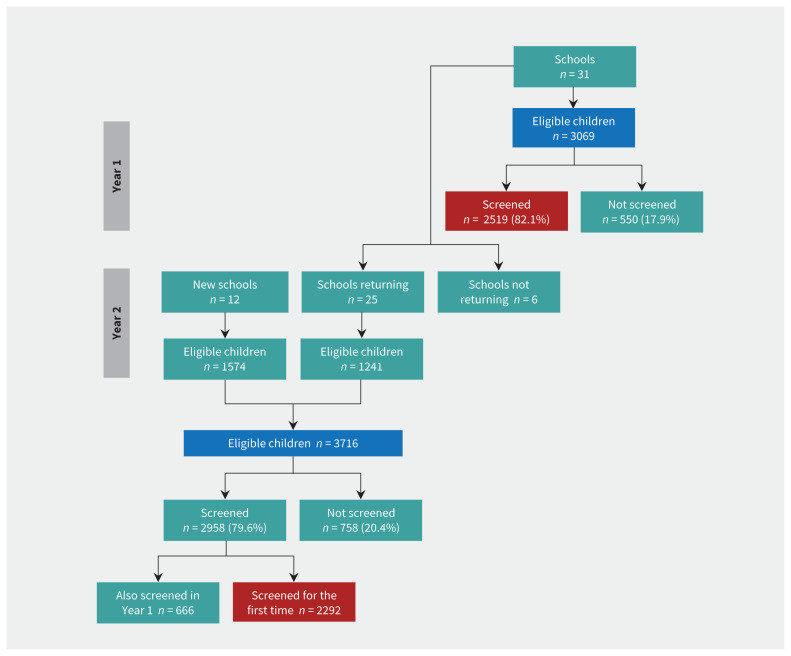
We screened children at 31 schools in Year 1 and 37 schools in Year 2 (25 of which participated in Year 1; Figure 1) and a summary of the results is provided in Table 3 (from the first year in which we screened at each school). We report the mean and range per school, across the 43 schools.
Figure 1:
Flow diagram of participating schools and children. Blue boxes represent number of eligible children and red boxes represent children participating for the first time.
Table 3:
Number of children enrolled per school who were screened and referred in the first year screening was offered
| School | No. of children eligible | No. of children (% of enrolled children) | No. of children (% of screened children) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Absent | Opted out | Other* | Screened | Referred | Referred (no PVS) | ||
| 1 | 109 | 6 (5.5) | 7 (6.4) | 0 (0.0) | 96 (88.1) | 62 (64.6) | 50 (52.1) |
|
| |||||||
| 2 | 38 | 1 (2.6) | 0 (0.0) | 0 (0.0) | 37 (97.4) | 19 (51.4) | 13 (35.1) |
|
| |||||||
| 3 | 142 | 9 (6.3) | 5 (3.5) | 0 (0.0) | 128 (90.1) | 40 (31.3) | 40 (31.3) |
|
| |||||||
| 4 | 169 | 6 (3.6) | 13 (7.7) | 4 (2.4) | 146 (86.4) | 78 (53.4) | 49 (33.6) |
|
| |||||||
| 5 | 54 | 5 (9.3) | 23 (42.6)† | 0 (0.0) | 26 (48.1) | 20 (76.9) | 10 (38.5) |
|
| |||||||
| 6 | 63 | 4 (6.3) | 23 (36.5)† | 1 (1.6) | 35 (55.6) | 27 (77.1) | 22 (62.9) |
|
| |||||||
| 7 | 87 | 5 (5.7) | 10 (11.5)† | 1 (1.1) | 71 (81.6) | 57 (80.3) | 47 (66.2) |
|
| |||||||
| 8 | 92 | 5 (5.4) | 36 (39.1)† | 0 (0.0) | 51 (55.4) | 33 (64.7) | 20 (39.2) |
|
| |||||||
| 9 | 118 | 16 (13.6) | 48 (40.7)† | 0 (0.0) | 54 (45.8) | 28 (51.9) | 20 (37.0) |
|
| |||||||
| 10 | 103 | 10 (9.7) | 35 (34.0)† | 0 (0.0) | 57 (55.3) | 17 (29.8) | 15 (26.3) |
|
| |||||||
| 11 | 21 | 2 (9.5) | 0 (0.0) | 1 (4.8) | 18 (85.7) | 8 (44.4) | 4 (22.2) |
|
| |||||||
| 12 | 50 | 4 (8.0) | 1 (2.0) | 0 (0.0) | 45 (90.0) | 29 (64.4) | 27 (60.0) |
|
| |||||||
| 13 | 53 | 4 (7.5) | 0 (0.0) | 0 (0.0) | 49 (92.5) | 32 (65.3) | 25 (51.0) |
|
| |||||||
| 14 | 80 | 6 (7.5) | 2 (2.5) | 0 (0.0) | 72 (90.0) | 40 (55.6) | 37 (51.4) |
|
| |||||||
| 15 | 63 | 9 (14.3) | 2 (3.2) | 0 (0.0) | 52 (82.5) | 31 (59.6) | 23 (44.2) |
|
| |||||||
| 16 | 64 | 9 (14.1) | 0 (0.0) | 0 (0.0) | 55 (85.9) | 40 (72.7) | 30 (54.5) |
|
| |||||||
| 17 | 84 | 6 (7.1) | 10 (11.9) | 0 (0.0) | 68 (81.0) | 26 (38.2) | 25 (36.8) |
|
| |||||||
| 18 | 96 | 12 (12.5) | 4 (4.2) | 0 (0.0) | 80 (83.3) | 61 (76.3) | 51 (63.8) |
|
| |||||||
| 19 | 134 | 6 (4.5) | 11 (8.2) | 0 (0.0) | 117 (87.3) | 61 (52.1) | 39 (33.3) |
|
| |||||||
| 20 | 135 | 7 (5.2) | 8 (5.9) | 2 (1.5) | 118 (87.4) | 44 (37.0) | 33 (27.7) |
|
| |||||||
| 21 | 145 | 9 (6.2) | 21 (14.5) | 1 (0.7) | 114 (78.6) | 60 (52.6) | 33 (28.9) |
|
| |||||||
| 22 | 177 | 4 (2.3) | 6 (3.4) | 0 (0.0) | 167 (94.4) | 71 (42.5) | 54 (32.3) |
|
| |||||||
| 23 | 119 | 7 (5.9) | 9 (7.6) | 1 (0.8) | 102 (85.7) | 49 (48.0) | 22 (21.6) |
|
| |||||||
| 24 | 110 | 6 (5.5) | 25 (22.7)† | 0 (0.0) | 79 (71.8) | 38 (48.1) | 36 (45.6) |
|
| |||||||
| 25 | 120 | 11 (9.2) | 37 (30.8)† | 0 (0.0) | 72 (60.0) | 29 (40.3) | 25 (34.7) |
|
| |||||||
| 26 | 132 | 5 (3.8) | 46 (34.8)† | 1 (0.8) | 80 (60.6) | 33 (41.3) | 33 (41.3) |
|
| |||||||
| 27 | 26 | 1 (3.8) | 0 (0.0) | 0 (0.0) | 25 (96.2) | 12 (48.0) | 8 (32.0) |
|
| |||||||
| 28 | 103 | 7 (6.8) | 2 (1.9) | 1 (1.0) | 93 (90.3) | 43 (46.2) | 34 (37.0) |
|
| |||||||
| 29 | 34 | 3 (8.8) | 3 (8.8) | 0 (0.0) | 28 (82.4) | 11 (39.3) | 10 (35.7) |
|
| |||||||
| 30 | 78 | 9 (11.5) | 4 (5.1) | 0 (0.0) | 65 (83.3) | 39 (60.0) | 35 (53.8) |
|
| |||||||
| 31 | 78 | 5 (6.4) | 1 (1.3) | 0 (0.0) | 72 (92.3) | 40 (55.6) | 25 (34.7) |
|
| |||||||
| 32 | 97 | 2 (2.1) | 4 (4.1) | 0 (0.0) | 91 (93.8) | 57 (62.6) | 49 (53.8) |
|
| |||||||
| 33 | 98 | 12 (12.2) | 1 (1.0) | 0 (0.0) | 85 (86.7) | 52 (61.2) | 32 (38.1) |
|
| |||||||
| 34 | 177 | 7 (4.0) | 0 (0.0) | 2 (1.1) | 168 (94.9) | 117 (69.6) | 92 (54.8) |
|
| |||||||
| 35 | 181 | 4 (2.2) | 3 (1.7) | 0 (0.0) | 174 (96.1) | 108 (62.1) | 93 (53.4) |
|
| |||||||
| 36 | 196 | 3 (1.5) | 1 (0.5) | 0 (0.0) | 192 (98.0) | 102 (53.1) | 95 (49.5) |
|
| |||||||
| 37 | 210 | 10 (4.8) | 3 (1.4) | 0 (0.0) | 197 (93.8) | 93 (47.2) | 81 (41.1) |
|
| |||||||
| 38 | 238 | 10 (4.2) | 1 (0.4) | 7 (2.9) | 220 (92.4) | 158 (71.8) | 140 (63.6) |
|
| |||||||
| 39 | 249 | 13 (5.2) | 0 (0.0) | 2 (0.8) | 234 (94.0) | 149 (63.7) | 131 (56.0) |
|
| |||||||
| 40 | 138 | 12 (8.7) | 12 (8.7) | 1 (0.7) | 113 (81.9) | 43 (38.1) | 26 (23.0) |
|
| |||||||
| 41 | 45 | 4 (8.9) | 8 (17.8)† | 0 (0.0) | 33 (73.3) | 25 (75.8) | 10 (30.3) |
|
| |||||||
| 42 | 53 | 5 (9.4) | 6 (11.3)† | 0 (0.0) | 42 (79.28) | 31 (73.8) | 18 (42.9) |
|
| |||||||
| 43 | 84 | 3 (3.6) | 13 (15.5)† | 1 (1.2) | 67 (79.8) | 48 (71.6) | 25 (37.3) |
|
| |||||||
| Total (%) | 4643 | 284 (6.1) | 421 (9.1) | 26 (0.5) | 3889 (83.8) | 2206 (47.5) | 1680 (36.2) |
|
| |||||||
| School-level mean (range) | 6.9% (1.5%–14.3%) | 10.5% (0.0%–42.6%) | 0.5% (0.0%–4.8%) | 82.1% (45.8%–98.0%) | 56.2% (29.8%–80.3%) | 43.5% (21.8%–72.9%) | |
Note: PVS = Pediatric Vision Scanner.
Other reasons for missing screening included extended vacations, family moving away or missing data.
Active consent schools (opted out includes those not returning the consent form).
Screening typically took 15–20 minutes per child. On average, 6.9% (range 1.5%–14.3%) of children missed screening because of absence. In schools using passive consent (n = 31), 4% of parents opted out of screening and an average of 82.1% of children were screened (range 45.8%–98.0%). In schools requiring active consent (n = 12), 8% of parents opted out but 22% failed to return the consent form and, hence, prevented the child from being screened. As a result, an average of only 64.0% of children were screened (range 45.8%–81.6%). Therefore, more children were screened when a passive consent model was used (p < 0.001).
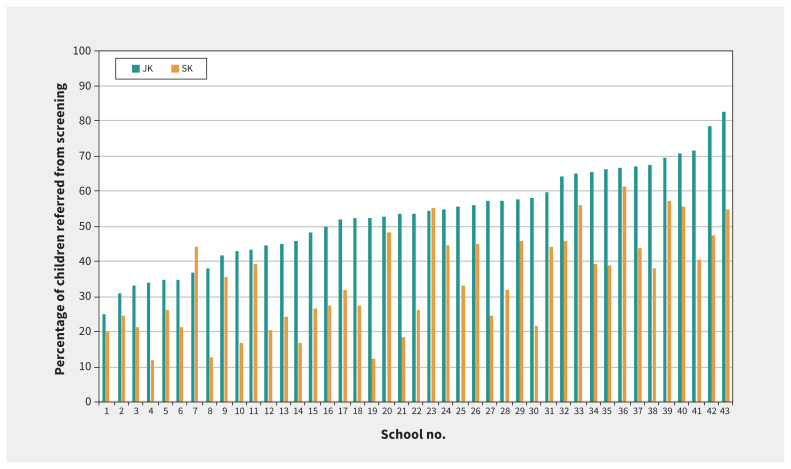
Refer rates varied across schools (Table 3). The mean was 56.2% (range 29.8%–80.3%) when PVS data were included and 43.5% (range 21.8%–72.9%) when they were excluded. Consistent with our concurrent study,1 the mean referral rate was significantly higher for children in JK than SK (p < 0.001), both with PVS data included (JK mean 64.2%; range 34.5%–96.6% v. SK mean 48.2%, range 21.6%–72.2%; Table 4), and with PVS data excluded (JK mean 53.3%, range 25.0%–82.8% v. SK mean 34.4%, range 11.9%–61.3%; Figure 2).
Table 4:
Referral rates at 43 schools (n = 3886) among junior (JK) and senior kindergarten (SK) children (PVS data included)
| School | Pass | Refer (% screened) | ||
|---|---|---|---|---|
|
|
|
|||
| JK | SK | JK | SK | |
| 1 | 10 | 24 | 39 (79.6) | 23 (48.9) |
|
| ||||
| 2 | 8 | 10 | 13 (61.9) | 6 (37.5) |
|
| ||||
| 3 | 49 | 39 | 26 (34.7) | 14 (26.4) |
|
| ||||
| 4 | 34 | 34 | 49 (59.0) | 29 (46.0) |
|
| ||||
| 5 | 2 | 4 | 10 (83.3) | 10 (71.4) |
|
| ||||
| 6 | 3 | 5 | 14 (82.4) | 13 (72.2) |
|
| ||||
| 7 | 1 | 13 | 28 (96.6) | 29 (69.0) |
|
| ||||
| 8 | 7 | 11 | 16 (69.6) | 17 (60.7) |
|
| ||||
| 9 | 7 | 19 | 14 (66.7) | 14 (42.4) |
|
| ||||
| 10 | 15 | 25 | 8 (34.8) | 9 (26.5) |
|
| ||||
| 11 | 5 | 5 | 3 (37.5) | 5 (50.0) |
|
| ||||
| 12 | 6 | 10 | 14 (70.0) | 15 (60.0) |
|
| ||||
| 13 | 7 | 10 | 19 (73.1) | 13 (56.5) |
|
| ||||
| 14 | 17 | 15 | 24 (58.5) | 16 (51.6) |
|
| ||||
| 15 | 9 | 12 | 19 (67.9) | 12 (50.0) |
|
| ||||
| 16 | 5 | 10 | 16 (76.2) | 21 (67.7) |
|
| ||||
| 17 | 12 | 30 | 14 (53.8) | 12 (28.6) |
|
| ||||
| 18 | 5 | 14 | 37 (88.1) | 24 (63.2) |
|
| ||||
| 19 | 27 | 29 | 30 (52.6) | 31 (51.7) |
|
| ||||
| 20 | 38 | 37 | 20 (34.5) | 24 (39.3) |
|
| ||||
| 21 | 16 | 38 | 32 (66.7) | 28 (42.4) |
|
| ||||
| 22 | 40 | 56 | 44 (52.4) | 27 (32.5) |
|
| ||||
| 23 | 18 | 35 | 26 (59.1) | 23 (39.7) |
|
| ||||
| 24 | 24 | 17 | 30 (55.6) | 8 (32.0) |
|
| ||||
| 25 | 24 | 19 | 14 (36.8) | 15 (44.1) |
|
| ||||
| 26 | 18 | 29 | 25 (58.1) | 8 (21.6) |
|
| ||||
| 27 | 5 | 8 | 9 (64.3) | 3 (27.3) |
|
| ||||
| 28 | 19 | 31 | 29 (60.4) | 14 (31.1) |
|
| ||||
| 29 | 6 | 11 | 5 (45.5) | 6 (35.3) |
|
| ||||
| 30 | 15 | 11 | 20 (57.1) | 19 (63.3) |
|
| ||||
| 31 | 8 | 24 | 23 (74.2) | 17 (41.5) |
|
| ||||
| 32 | 13 | 21 | 36 (73.5) | 21 (50.0) |
|
| ||||
| 33 | 12 | 21 | 28 (70.0) | 24 (53.3) |
|
| ||||
| 34 | 20 | 31 | 57 (74.0) | 60 (65.9) |
|
| ||||
| 35 | 25 | 41 | 67 (72.8) | 41 (50.0) |
|
| ||||
| 36 | 35 | 55 | 50 (58.8) | 52 (48.6) |
|
| ||||
| 37 | 40 | 64 | 54 (57.4) | 39 (37.9) |
|
| ||||
| 38 | 25 | 37 | 90 (78.3) | 68 (64.8) |
|
| ||||
| 39 | 32 | 53 | 92 (74.2) | 57 (51.8) |
|
| ||||
| 40 | 24 | 46 | 26 (52.0) | 17 (27.0) |
|
| ||||
| 41 | 4 | 4 | 17 (81.0) | 8 (66.7) |
|
| ||||
| 42 | 5 | 6 | 22 (81.5) | 9 (60.0) |
|
| ||||
| 43 | 9 | 10 | 32 (78.0) | 16 (61.5) |
Note: PVS = Pediatric Vision Scanner.
Figure 2:
Referral rates (% of screened children) per school for children in junior kindergarten (JK) and senior kindergarten (SK) from the first year in which we screened. Schools have been ordered from lowest to highest JK referral rate. (Pediatric Vision Scanner data excluded.)
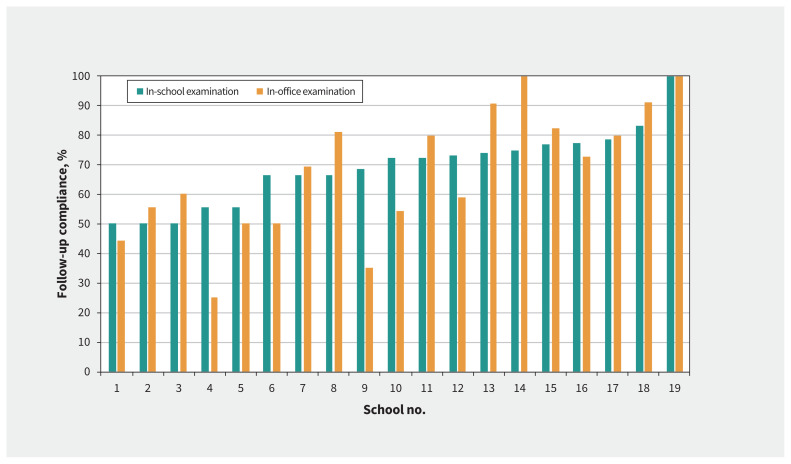
We compared compliance with follow-up eye examinations in 2 cohorts of children in JK who were referred for follow-up examinations (n = 778; Appendix 1): those who had in-school examinations (Year 1) versus those who had in-office examinations (Year 2). Among them, 155 of 778 (19.9%) children did not attend the follow-up examination but were already under the care of an optometrist. Therefore, we excluded their data from this analysis. Attendance was similar in the 2 models (Figure 3): 69.1% of students who were referred attended in-school appointments (range 50.0%–100.0% per school) and 69.4% attended in-office appointments (range 25.0%–100.0%). Thus, regardless of the location of the follow-up examination, at least two-thirds of the referred children not already under care had an eye examination as a result of our program.
Figure 3:
Percentage of referred junior kindergarten children who attended the follow-up optometry examination for each of the 19 schools when the examinations were offered at the child’s school or at the local optometrist’s office, excluding children already under care.
Because we found no difference between in-school and in-office examinations, we evaluated compliance with follow-up examinations in all referred children over 2 years for all 43 schools (counting only the JK results for students screened twice). Of 2662 referrals, 15% of parents indicated that their child was already under care (similar to previously published rates19). When we excluded the 392 children already under care, 1563 of the remaining 2270 referred children (68.9%) attended the optometry examinations, 222 (9.8%) had parental consent but did not show up for the examination, 88 (3.9%) opted out and 397 (17.5%) did not return the permission form.
The percentages of screened children who were found to have visual problems through the vision screening program (when screened for the first time, n = 4811) are shown in Table 5. In our sample population, 516 children (10.7% of children who were screened) were identified as having a visual problem; of those children, the parents of 347 (67.2%) reported that this was their child’s first eye examination. Amblyopia was diagnosed in 164 children (3.4%) for which they received treatment. A total of 458 children were prescribed glasses (9.5% of screened children). Astigmatism was the most common reason for prescribing glasses and was diagnosed by the optometrist in 227 (4.7%) screened children.
Table 5:
Prevalence of eye problems among children screened (for the first time) and among those undergoing their first eye examination
| Disorder | No. (%) of children n = 4811 |
|
|---|---|---|
| With eye problems | With eye problems and undergoing first examination | |
| Any problem | 516 (10.7) | 347 (7.2) |
| Amblyopia | 164 (3.4) | 121 (2.5) |
| Amblyopia risk factors | 455 (9.5) | 301 (6.3) |
| Strabismus | 45 (0.9) | 22 (0.5) |
| Reduced stereoacuity | 172 (3.6) | 92 (1.9) |
| Any refractive error | 324 (6.7) | 237 (4.9) |
| Hyperopia | 124 (2.6) | 84 (1.7) |
| Astigmatism | 227 (4.7) | 167 (3.5) |
| Anisometropia | 44 (0.9) | 29 (0.6) |
| Myopia | 13 (0.3) | 11 (0.2) |
| Other problems | 17 (0.4) | 7 (0.1) |
Note: Prevalence was calculated from all children screened for the first time in Year 1 (n = 2519) and in Year 2 (n = 2292). If a child had more than 1 eye problem, they were counted in each applicable disorder category. Other problems that we identified were 6 cases of opaque media, 5 cases of limited/overactive ocular motility, 3 cases of nystagmus, 2 cases of optic nerve abnormality and 1 case of narrow angles.
The results from a single cohort of 747 children who were eligible in both JK and SK were compared in 22 schools where we offered screening over 2 consecutive years and for which individual data were available for both years. In Year 1, 78 children (10.4%) were identified during the follow-up eye examination as having a visual problem. In Year 2, that number was reduced to 23 children (3.1%), 18 of whom were not screened in Year 1 (because of absences or opting out of screening). Of the 277 children who passed screening in Year 1, 41 were referred in Year 2 and, of those 41 who were referred, 16 were examined by an optometrist and did not have a visual problem.
We reanalyzed data from Year 1 using only the data from acuity, stereoacuity and Spot photoscreener tests (Table 6; results were similar when Plusoptix data were used instead). We excluded the results for 77 children who were already wearing glasses (3.1%) from this analysis because they were not tested with the photoscreener. If only children who failed acuity, stereoacuity and the photoscreener testing were referred, this would have reduced the number of referrals from 928 of 2434 in Year 1 (38.1% of screened children) to just 106 (4.3% of screened children). When children failed all 3 tests, 62 of the 71 children who were examined by an optometrist had a visual problem (87.3%), compared with 53 of 237 (22.4%) children who were examined and who failed only acuity.
Table 6:
Screening results for acuity, stereoacuity and Spot photoscreener* tests for children (in Year 1) who were examined by a licensed optometrist
| Acuity | Stereoacuity | Photoscreener (Spot) | No. of observed cases (% screened) n = 2434 |
No. of children examined by an optometrist (% of observed cases) | No. of children with a visual problem (% of those examined) |
|---|---|---|---|---|---|
| Pass | Pass | Pass | 1506 (61.9) | NA | NA |
| Refer | Refer | Refer | 106 (4.4) | 71 (67.0) | 62 (87.3) |
| Refer | Refer | Pass | 132 (5.4) | 81 (61.4) | 30 (37.0) |
| Refer | Pass | Refer | 93 (3.8) | 62 (66.7) | 42 (67.7) |
| Pass | Refer | Refer | 20 (0.8) | 14 (70.0) | 8 (57.1) |
| Refer | Pass | Pass | 390 (16.0) | 237 (60.8) | 53 (22.4) |
| Pass | Refer | Pass | 143 (5.9) | 84 (58.7) | 27 (32.1) |
| Pass | Pass | Refer | 44 (1.8) | 30 (68.2) | 13 (43.3) |
| Total | 2434 | 579 (23.8) | 235 (40.6) |
Note: NA = not applicable.
We obtained similar results when the Plusoptix photoscreener results were used instead of those for the Spot photoscreener.
Only 0.9%–4.2% (depending on the test) of screened children were unable to complete a test (because the child did not understand the test, did not give the correct response even at the easiest test levels or the electronic device was unable to take a measurement; Table 7). Among these children who were examined by an optometrist, 25.0%–51.4% (3–29 children, depending on the test) were found to have a visual problem, a rate higher than the 35.3% (442 of 1251 children) observed among all children who were examined (except for screening by using the Spot photoscreener).
Table 7:
Screening results by test
| Screening test | No. (%) of children screened | No. of children unable to complete a test | ||||
|---|---|---|---|---|---|---|
| Pass | Refer | Unable to complete a test | Total Year 1 | Total examined by an optometrist | No. (%) of those examined and found to have a visual problem | |
| Cambridge Crowded Acuity | 1755 (69.7) | 703 (27.9) | 61 (2.4) | 2519 | 36 | 14 (38.9) |
| Randot Preschool Stereoacuity | 2074 (82.6) | 359 (14.3) | 79 (3.1) | 2512 | 37 | 19 (51.3) |
| Plusoptix photoscreener | 2129 (87.5) | 202 (8.3) | 103 (4.2) | 2434 | 65 | 29 (44.6) |
| Spot photoscreener | 2173 (89.2) | 241 (9.9) | 22 (0.90) | 2436 | 12 | 3 (25.0) |
Note: Shaded area: children in Year 1 who passed, were referred or were unable to complete the test (children already wearing glasses were excluded from the photoscreener tests). Unshaded area: among those unable to complete a test, the number of children examined by an optometrist and found to have a visual problem.
Of the 322 children who were surveyed on their feelings about playing the vision games, 291 (90.4%) chose the happy face (indicating that they found the testing fun), 23 (7.1%) chose the neutral face and 8 (2.4%) chose the sad face.
The screening tests have been reliable over the 5 years we have used them, with minimal repairs (e.g., replacing broken 3-dimensional polarized glasses). Based on a projection over 5 years for the city of Hamilton, Ontario, we estimated the cost of vision screening to be roughly $10 per child (Appendix 1, Supplementary Table 1).
Our post hoc analyses showed large variations in the average household income across the 43 neighbourhoods in which the schools were located (mean household income $86 639, range $39 509–$282 073). We also found large variations in the percentage of students who did not speak English as their first language in each school (mean 24.3%, range 0.0%–85.0% per school) or were born in Canada (mean 89.0%, range 55.0%–100.0% per school). We determined that none of these measures correlated with referral rates: average income (r = −0.17, p = 0.3), percentage of children whose first language was not English (r = 0.16, p = 0.3) and those born in Canada (r = −0.26, p = 0.09).
Interpretation
Our objective for this study was to determine if a school-based screening program can be scaled up across diverse communities to identify children in kindergarten with visual problems and arrange for them to receive follow-up eye examinations and treatment. Most parents provided consent for their child to participate in vision screening. Although referral rates varied across schools, just under half of the children who were screened were referred for follow-up eye examinations, and referral rates were higher for children in JK than for those in SK. As a result of the follow-up examinations, more than 10% of the children who were screened were identified as having a visual problem, most for the first time. Thus, a school-based vision screening program can be an effective population intervention for reducing untreated visual problems that otherwise might lead to amblyopia or affect learning.
Our study provides several important pieces of information for optimizing implementation. We found little added value in requiring a child to undergo screening in both JK and SK, because this doubles the needed resources for screening and increases the number of follow-up examinations and few children with problems would be newly identified. We chose an arbitrarily strict criterion that only children who passed all tests were considered a “pass” — all other children were referred for an eye examination. One consideration to minimize overreferrals would be to arrange follow-up examinations (i.e., actually book the appointment) for children who fail multiple screening tests and simply suggest (with a letter) eye examinations for children who fail a single test.
Two-thirds of referred students attended in-school and in-office examinations. Our experience suggests that a key to our success was to prebook the appointments for parents and to ensure that there was no cost to the parents should the child require glasses. It is important that most of the children reported enjoying the “vision games,” which is needed if the program is to be supported by parents and school staff. Additional considerations for optimizing implementation are discussed in Appendix 1.
Limitations
We did not collect individual demographic data (e.g., socioeconomic status, ethnicity and parental education) that might have explained the variability in referral rates across schools and cohorts. In addition, there were 23 instances (0.4% of all screenings) of erroneous pass letters that were sent home when the child had failed screening. Because no screening is perfect, our pass letters explained that screenings do not replace comprehensive eye examinations and included the office information of our collaborating optometrist to facilitate booking of examinations by parents, especially if their child had never had one.
Conclusion
Through our program, about 1 in 10 children who were screened were identified as having a visual problem, with most identified for the first time. These numbers show that the status quo (in 2015–2017) was insufficient in identifying and treating young children with a visual problem before grade 1. The willingness of school boards and principals to participate in our study underlines the recognized need for better access to visual health care for children in kindergarten. The success of any vision screening program depends on the extent of the follow-up care that is provided (i.e., access to eye care professionals and glasses, reminders for follow-up and information about the importance of early vision care). Implementation can be facilitated by collaborating with existing associations with a mandate to improve children’s eye health and community eye care professionals with offices located close to the school. Despite our efforts, however, nearly one-third of referred children not identified as already under care did not receive the follow-up eye examination we offered, and further research is needed to explore strategies for reducing this number. Nevertheless, our program was successful in assuring vision care for many children with previously unidentified visual problems. A visual screening program for children in SK has been implemented in Ontario, in part as a result of our program.44 Our findings indicate the value of implementing this type of program universally across Canada.
Acknowledgements
The authors thank the Ontario Association of Optometrists for their collaboration in many of the communities, including the recruitment of optometrists and provision of glasses through the Eye See…Eye Learn program (lenses were donated by Nikon and frames were donated by OGI). They thank the Toronto Foundation for Student Success and its Gift of Sight and Sound program for organizing the screeners, optometrists and opticians, as well as for arranging free glasses through their partners for children within the Toronto District School Board (TDSB). They also thank the volunteer screeners who worked with them in many of the communities: Lions Clubs from District A15 in Rockwood, Fergus, Cambridge and Wellesley, Ont.; University of Ottawa’s IScreen Ottawa Interest Group (medical students at the University of Ottawa); personnel from the Timiskaming Health Unit in Kirkland Lake, Ont.; personnel from Lambton Public Health in Sarnia, Ont.; personnel from Southwestern Public Health (formerly Oxford County Public Health) in Woodstock and Ingersoll, Ont.; and third- and fourth-year trainees from the School of Optometry and Vision Science, University of Waterloo. The authors also thank Lisa W. Christian, O.D., (Associate Clinical Professor at the University of Waterloo), Paul Chris, O.D. (Executive Director at Vision Institute of Canada) and Maria Yau, (Research Co-ordinator at Research & Development, TDSB), for their contributions to the design of the study. They thank Wylie Tan, O.D., for supervising the screening conducted by third-year trainees from the University of Waterloo and providing additional optometry examinations when needed. They thank David Hunter and Justin Shaka for the loan of an early Pediatric Vision Scanner prototype, and Mano Chandrakumar and Sally Stafford for their assistance with the logistics of the study.
Footnotes
Competing interests: Agnes Wong has received a grant from the PSI Foundation. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Mayu Nishimura and Daphne Maurer contributed to data acquisition. Mayu Nishimura performed the data analysis. Helen Dimaras contributed expert advice about knowledge translation in communicating with stakeholders and how to collect feedback from children. Mayu Nishimura drafted the manuscript. All authors contributed to the study design and to data interpretation, revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Data sharing: The full data set is available on request from Mayu Nishimura (mayu.nishimura@sickkids.ca) or Daphne Maurer (maurer@mcmaster.ca) until 7 years after publication, at which time the data will be destroyed and deleted securely in accordance with the data management policies of The Hospital for Sick Children, Toronto.
Funding: This research was supported by a Collaborative Health Research Project grant from the Canadian Institutes of Health Research and the Natural Sciences and Engineering Research Council of Canada to Daphne Maurer and Agnes Wong, a Joanne Angle Investigator Award from Prevent Blindness America to Agnes Wong and private donations.
References
- 1.Nishimura M, Wong A, Cohen A, et al. Choosing appropriate tools and referral criteria for vision screening of 4- and 5-year-old children. BMJ Open 2019;9:e032138. 10.1136/bmjopen-2019-032138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drover JR, Kean PG, Courage ML, et al. Prevalence of amblyopia and other vision disorders in young Newfoundland and Labrador children. Can J Ophthalmol 2008;43:89–94. [DOI] [PubMed] [Google Scholar]
- 3.Multi-ethnic Pediatric Eye Disease Study Group. Prevalence of amblyopia and strabismus in African American and Hispanic children ages 6 to 72 months: the multi-ethnic pediatric eye disease study. Ophthalmology 2008;115:1229–36.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKean-Cowdin R, Cotter SA, Tarczy-Hornoch K, et al. Multi-ethnic Pediatric Eye Disease Study Group. Prevalence of amblyopia or strabismus in Asian and non-Hispanic white preschool children: multi-ethnic pediatric eye disease study. Ophthalmology 2013;120:2117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman DS, Repka MX, Katz J, et al. Prevalence of amblyopia and strabismus in white and African American children aged 6 through 71 months. Ophthalmology 2009;116:2128–34.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chia A, Dirani M, Chan YH, et al. Prevalence of amblyopia and strabismus in young Singaporean Chinese children. Invest Ophthalmol Vis Sci 2010;51:3411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Fu Z, Yu J, et al. Prevalence of amblyopia and strabismus in Eastern China: results from screening of preschool children aged 36–72 months. Br J Ophthalmol 2016;100:515–9. [DOI] [PubMed] [Google Scholar]
- 8.Pascual M, Huang J, Maguire MG, et al. Vision in Preschoolers (VIP) Study Group. Risk factors for amblyopia in the Vision in Preschoolers Study. Ophthalmolology 2014;121:622–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Leeuwen R, Eijkemans MJC, Vingerling JR, et al. Risk of bilateral visual impairment in individuals with amblyopia: the Rotterdam study. Br J Ophthalmol 2007;91:1450–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahi J, Logan S, Timms C, et al. Risk, causes, and outcomes of visual impairment after loss of vision in the non-amblyopic eye: a population-based study. Lancet 2002;360:597–602. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt P, Maguire M, Dobson V, et al. Vision in Preschoolers (VIP) Study Group. Comparison of preschool vision screening tests as administered by licensed eye care professionals in the Vision in Preschoolers Study. Am Acad Ophthalmology 2004;111:637–50. [DOI] [PubMed] [Google Scholar]
- 12.Huang J, Maguire MG, Ciner E, et al. Vision in Preschoolers (VIP) Study Group. Risk factors for astigmatism in the Vision in Preschoolers Study. Optom Vis Sci 2014;91:514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen G, Tarczy-Hornoch K, McKean-Cowdin R, et al. Multi-ethnic Pediatric Eye Disease Study Group. Prevalence of myopia, hyperopia and astigmatism in non-Hispanic White and Asian children: multi-ethnic pediatric eye disease study. Ophthalmology 2013;120:2109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giordano L, Friedman DS, Repka MX, et al. Prevalence of refractive error among preschool children in an urban population: the Baltimore Pediatric Eye Disease Study. Ophthalmology 2009;116:739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dirani M, Chan YH, Gazzard G, et al. Prevalence of refractive error in Singaporean Chinese Children: the strabismus, amblyopia, and refractive error in young Singaporean children (STARS) study. Invest Ophthalmol Vis Sci 2010;51: 1348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.United States Preventive Services Task Force Grossman DC, Curry SJ, Owens DK, et al. Vision screening in children aged 6 months to 5 years: U.S. Preventive Services Task Force Recommendation Statement. JAMA 2017;318:836–44. [DOI] [PubMed] [Google Scholar]
- 17.Amit MCanadian Paediatric Society, Community Paediatrics Committee. Vision screening in infants, children, and youth [position statement]. Paediatr Child Health 2009;14:246–8 [reaffirmed 2018 Feb. 28]. Available: www.cps.ca/en/documents/authors-auteurs/community-paediatrics-committee (accessed 2020 June 5).20357924 [Google Scholar]
- 18.Le TD, Raashid RA, Colpa L, et al. Paediatric vision screening in the primary care setting in Ontario. Paediatr Child Health 2018;23:e33–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Optimizing optometry’s role in Ontario [a white paper by the Ontario Association of Optometrists]. Toronto: Ontario Association of Optometrists; 2015. [Google Scholar]
- 20.American Academy of Ophthalmology Pediatric Ophthalmology/Strabismus Panel. Amblyopia Preferred Practice Pattern. San Francisco: American Academy of Opthalmology; 2017. Available: www.aao.org/preferred-practice-pattern/amblyopia-ppp-2017 (accessed 2020 June 18). [Google Scholar]
- 21.Levi DM. Visual processing in amblyopia: human studies. Strabismus 2006;14: 11–9. [DOI] [PubMed] [Google Scholar]
- 22.Amblyopia: challenges and opportunities. The Lasker/IRRF Initiative for Innovation in Vision Science; 2017. Available: www.laskerfoundation.org/new-noteworthy/articles/amblyopia-challenges/ (accessed 2020 June 24).
- 23.Kelly KR, Jost RM, De La Cruz A, et al. Amblyopic children read more slowly than controls under natural, binocular reading conditions. J AAPOS 2015;19:515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stifter E, Burggasser G, Hirmann E, et al. Monocular and binocular reading performance in children with microstrabismic amblyopia. Br J Ophthalmol 2005;89:1324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulp MT, Schmidt PP. Visual predictors of reading performance in kindergarten and first grade children. Optom Vis Sci 1996;73:255–62. [DOI] [PubMed] [Google Scholar]
- 26.Shankar S, Evans MA, Bobier WR. Hyperopia and emergent literacy of young children: pilot study. Optom Vis Sci 2007;84:1031–8. [DOI] [PubMed] [Google Scholar]
- 27.VIP-HIP Study Group Kulp MT, Ciner E, Maguire M, et al. Uncorrected hyperopia and preschool early literacy: results of the Vision in Preschoolers-Hyperopia in Preschoolers (VIP-HIP) Study. Ophthalmology 2016;123:681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart-Brown S, Haslum MN, Butler N. Educational attainment of 10-year-old children with treated and untreated visual defects. Dev Med Child Neurol 1985;27:504–13. [DOI] [PubMed] [Google Scholar]
- 29.Hopkins S, Sampson GP, Hendicott PL, et al. Vision problems and reduced reading outcomes in Queensland schoolchildren. Optom Vis Sci 2017;94: 345–52. [DOI] [PubMed] [Google Scholar]
- 30.Harvey EM, Miller JM, Twelker JD, et al. Reading fluency in school-aged children with bilateral astigmatism. Optom Vis Sci 2016;93:118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roch-Levecq AC, Brody BL, Thomas RG, et al. Ametropia, preschoolers’ cognitive abilities, and effects of spectacle correction. Arch Ophthalmol 2008;126:252–8. [DOI] [PubMed] [Google Scholar]
- 32.Bennett KP, Maloney W. Weighing in on Canadian school-based vision screening: a call for action. Can J Public Health 2017;108:e421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonas DE, Amick HR, Wallace IF, et al. Vision screening in children aged 6 months to 5 years: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2017;318:845–58. [DOI] [PubMed] [Google Scholar]
- 34.Ontario Agency for Health Protection and Promotion (Public Health Ontario). Effectiveness of vision screening programs for children aged one to six years. Toronto: Queen’s Printer for Ontario; 2016. [Google Scholar]
- 35.Schmidt P, Maguire M, Dobson V, et al. Vision in Preschoolers (VIP) Study Group. Comparison of preschool vision screening tests as administered by licensed eye care professionals in the Vision in Preschoolers Study. Ophthalmology 2004;111:637–50. [DOI] [PubMed] [Google Scholar]
- 36.Clarke MP, Wright CM, Hrisos S, et al. Randomised controlled trial of treatment of unilateral visual impairment detected at preschool vision screening. BMJ 2003;327:1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moseley MJ, Fielder AR. Preschool vision screening. Br J Ophthalmol 2003; 87:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cotter SA, Cyert LA, Miller JM, et al. Vision screening for children 36 to < 72 months: recommended practices. Optom Vis Sci 2015;92:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moganeswari D, Thomas J, Srinivasan K, et al. Test re-test reliability and validity of different visual acuity and stereoacuity charts used in preschool children. J Clin Diagn Res 2015;9:NC01–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langeslag-Smith MA, Vandal AC, Briane V, et al. Preschool children’s vision screening in New Zealand: a retrospective evaluation of referral accuracy. BMJ Open 2015;5:e009207. 10.1136/bmjopen-2015-009207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donahue SP, Arthur B, Neely DE, et al. Guidelines for automated preschool vision screening: a 10-year, evidence-based update. J AAPOS 2013;17:4–8. [DOI] [PubMed] [Google Scholar]
- 42.Birch E, Williams C, Drover J, et al. Randot Preschool Stereoacuity Test: normative data and validity. J AAPOS 2008;12:23–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maguire MGVision in Preschoolers (VIP) Study Group. Children unable to perform screening test in Vision in Preschoolers Study: proportion with ocular conditions and impact on measures of test accuracy. Invest Ophthalmol Vis Sci 2007;48:83–7. [DOI] [PubMed] [Google Scholar]
- 44.Ontario public health standards: requirements for programs, services, and accountability Toronto: Ministry of Health and Long-Term Care Ontario; revised 2018 July 1:50–2. [Google Scholar]