Abstract
Background
The Tokyo Guidelines were published in 2007 and updated in 2013 and 2018, with recommendations for the diagnosis and management of acute cholecystitis. We assessed guideline adherence at our academic centre and its impact on patient outcomes.
Methods
This is a retrospective chart review of patients with acute calculous cholecystitis who underwent cholecystectomy at our institution between November 2013 and March 2015. Severity of cholecystitis was graded retrospectively if it had not been documented preoperatively. Compliance with the Tokyo Guidelines’ recommendations on antibiotic use and time to operation was recorded. Cholecystitis severity groups were compared statistically, and logistic regression was used to determine predictors of complications.
Results
One hundred and fifty patients were included in the study. Of these, 104 patients were graded as having mild cholecystitis, 45 as having moderate cholecystitis, and 1 as having severe cholecystitis. Severity was not documented preoperatively for any patient. Compliance with antibiotic recommendations was poor (18.0%) and did not differ by cholecystitis severity (p = 0.90). Compliance with the recommendation on time to operation was 86.0%, with no between-group differences (p = 0.63); it improved when an acute care surgery team was involved (91.0% v. 76.0%, p = 0.025). On multivariable analysis, comorbidities (odds ratio [OR] 1.47, 95% confidence interval [CI] 1.19–1.85, p < 0.001) and conversion to laparotomy (OR 13.45, 95% CI 2.16–125.49, p = 0.01) predicted postoperative complications, while severity of cholecystitis, antibiotic compliance and time to operation had no effect.
Conclusion
In this study, compliance with the Tokyo Guidelines was acceptable only for time to operation. Although the poor compliance with recommendations relating to documentation of severity grading and antibiotic use did not have a negative affect on patient outcomes, these recommendations are important because they facilitate appropriate antibiotic use and patient risk stratification.
Abstract
Contexte
Les Tokyo Guidelines, publiées en 2007, puis mises à jour en 2013 et en 2018, contiennent des recommandations sur le diagnostic et la prise en charge de la cholécystite aiguë. Nous avons évalué le respect de ces lignes directrices dans notre centre universitaire et son incidence sur les issues pour les patients.
Méthodes
Ce document est une revue rétrospective de dossiers des patients atteints de cholécystite aiguë calculeuse qui ont subi une cholécystectomie dans notre établissement entre novembre 2013 et mars 2015. La gravité de la cholécystite a été établie de manière rétrospective si elle n’avait pas été documentée avant l’opération. Le respect des recommandations des Tokyo Guidelines concernant le recours à des antibiotiques et la durée de l’opération a été étudié. Nous avons comparé statistiquement les groupes de gravité de la cholécystite, et avons utilisé une régression logistique pour déterminer les prédicteurs de complications.
Résultats
Au total, 150 patients ont été inclus dans l’étude. Parmi eux, 104 avaient une cholécystite légère, 45, une cholécystite modérée et 1, une cholécystite grave. La gravité de la maladie n’avait été documentée avant l’opération pour aucun patient. Le respect des recommandations sur les antibiotiques était faible (18,0 %) et ne variait pas selon la gravité de la cholécystite (p = 0,90). Le respect des recommandations sur la durée de l’opération était de 86,0 %, sans différence entre les groupes (p = 0,63); il était toutefois plus élevé lorsqu’une équipe de soins chirurgicaux aigus participait aux soins (91,0 % c. 76,0 %, p = 0,025). L’analyse multivariée a permis de déterminer que les comorbidités (rapport des cotes [RC] 1,47, intervalle de confiance [IC] de 95 % 1,19–1,85, p < 0,001) et la conversion en laparotomie (RC 13,45, IC de 95 % 2,16–125,49, p = 0,01) étaient des prédicteurs de complications postopératoires, alors que la gravité de la cholécystite et le respect des recommandations sur les antibiotiques et la durée de l’opération n’avaient pas d’effet.
Conclusion
Dans cette étude, le respect des Tokyo Guidelines était acceptable seulement pour la durée de l’opération. Bien qu’un faible respect des recommandations quant à la documentation de la gravité et à l’utilisation d’antibiotiques n’ait pas eu d’effets négatifs sur les issues pour les patients, ces recommandations sont importantes parce qu’elles favorisent l’utilisation appropriée des antibiotiques et une bonne stratification du risque pour le patient.
Acute calculous cholecystitis is a common illness requiring surgery, and it is the reason for approximately 120 000 cholecystectomies performed annually in the United States.1 In spite of the prevalence of cholecystitis, variation in its diagnosis and treatment, both medical and surgical, has been demonstrated.2,3 In response, the Tokyo Guidelines were published in 20074 (with updates in 2013 and 2018)5,6 to provide evidence-based definitions and treatment recommendations. One of the novel concepts introduced in the Tokyo Guidelines was a severity rating for cholecystitis, based on clinical, biochemical and radiologic findings.4 In the Tokyo Guidelines, it is recommended that clinicians should take into account the severity of illness as well as patientspecific factors when determining the appropriate treatment pathway.
According to the 2013 update of the Tokyo Guidelines, patients with grade I (mild) and grade II (moderate) cholecystitis should undergo early cholecystectomy, within 72 hours of symptom onset. Grade III (severe) cholecystitis is characterized by end-organ failure and should be managed with urgent gallbladder drainage and delayed chole-cystectomy, if tolerated by the patient (Appendix 1, available at canjsurg.ca/002719-a1).7
Variability in the treatment pathway at our institution (McMaster University) has been noted, raising questions about the level of awareness of, and adherence to, the Tokyo Guidelines at our centre. Guidelines for clinical practice have the potential to significantly improve efficiency and quality of care, but the degree of improvement depends on the manner and context in which they are introduced.8 Systematic reviews have demonstrated that compliance with guidelines, although generally cost-effective, tends to be poor overall. Strategies such as passive dissemination are ineffective; strategies that engage physicians and facilitate implementation are associated with better uptake and improved outcomes for patients.9
This study had 2 principal aims. The first aim was to determine compliance with guidelines and, if there was noncompliance, to identify areas for improvement. The second aim was to evaluate the impact of guideline compliance on patient outcomes.
Methods
Patient selection
The McMaster University academic network comprises 3 adult hospitals. Two of these hospitals have acute care surgery (ACS) teams, which exclusively manage urgent and emergent cases, and for whom dedicated operating room time is reserved. A sample of 150 patients was taken, divided evenly between the 3 hospital sites in reverse chronological order from the date of initiation of the study. The study period was November 2013 to March 2015. Inclusion criteria were an admission diagnosis of acute calculous cholecystitis and cholecystectomy during the index admission. Exclusion criteria were age younger than 18 years, cholecystectomy for a reason other than acute cholecystitis (biliary colic, chronic cholecystitis, gallstone pancreatitis) and elective cholecystectomy. Data were derived from physical records, electronic medical records and surgeon office charts. The study proposal was approved by the Hamilton Integrated Research Ethics Board as a quality assessment and improvement initiative before commencement.
Study variables
Three categories of patient information were collected: preoperative, operative and postoperative. Preoperative data included demographic information, such as date and time of admission, age and sex, comorbidities, American Society of Anesthesiologists (ASA) score (as documented by the attending anesthesiologist)10 and ultrasound or imaging findings. The age-adjusted Charlson Comorbidity Index score (hereafter termed the Charlson score)11 was calculated from age and comorbidity data. We reviewed admission notes (written notes, and dictated notes that are transcribed and added to the electronic health record) in their entirety, noting whether severity grading was documented. We also noted whether bile and blood cultures were performed, and their results (if applicable). Use of pre- and perioperative antibiotics was recorded, along with timing and dosing. Preoperative administration of nonsteroidal antiinflammatory drugs was noted. Time from arrival at the emergency department to admission was also recorded.
Operative variables included surgeon, time from hospital arrival to operation, duration of operation, surgical approach (i.e., laparoscopic, open or converted), whether intraoperative cholangiography was performed, and intraoperative complications. Data collected on postoperative care encompassed length of stay, postoperative in-hospital complications, need for postoperative antibiotics, complications following discharge, readmission and pathology results. Postoperative complications were retrospectively classified using the Clavien–Dindo classification system12 to facilitate comparison.
Outcomes
The primary outcome was compliance with the Tokyo Guidelines in 3 domains: documentation of cholecystitis severity, antibiotic treatment and time to operation. The secondary outcomes were postoperative complications and length of stay.
Severity grading and definitions of compliance
Compliance definitions were derived from the 2013 update of the Tokyo Guidelines.7,13–15 Documentation of cholecystitis severity, whether descriptive or in explicit reference to the guidelines, was regarded as adherence to the grading recommendations. Grade I (mild) severity was assigned if the patient did not meet the criteria for grade II or III cholecystitis. Grade II (moderate) cholecystitis was associated with any of the following conditions: white blood count greater than 18 000 per cubic millimetre, palpable tender right upper quadrant mass, symptom duration greater than 72 hours or marked local inflammation, usually discovered on imaging, such as gangrenous cholecystitis. Grade III (severe) cholecystitis was associated with end-organ dys-function, as denoted by clinical or laboratory criteria, and generally required admission to a monitored unit.5,15
Antibiotic administration was considered to be compliant with the guidelines only if the patient was given an antibiotic regimen that was appropriate for the severity of their illness and if this was given at the correct time. All patients with cholecystitis, regardless of severity, require antibiotics upon diagnosis as per the management pathways in the Tokyo Guidelines.7 Choice of antibiotics is determined by severity. For example, a second-generation cephalosporin is appropriate for grade I cholecystitis, but a third or higher generation cephalosporin is required for grade II and III cholecystitis. Some antibiotics are permitted only in certain situations; metronidazole, for instance, is to be given only to patients with prior biliary-enteric anastomosis or culture-proven anaerobic infection.13
The 2013 update of the Tokyo Guidelines recommends that patients with mild and moderate cholecystitis undergo early surgery,7 defined as laparoscopic cholecystectomy performed within 72 hours of symptom onset.14 Patients with severe cholecystitis are to undergo urgent gallbladder drainage with delayed or elective cholecystectomy.7 Because it was difficult to uniformly and accurately assess timing of symptom onset, we defined early surgery as surgery performed within 72 hours of admission. Of note, there are scenarios outlined in the 2013 update of the Tokyo Guidelines in which gallbladder drainage and delayed cholecystectomy are recommended for grade II cholecystitis, such as when severe inflammation is suspected;7 however, our selection criteria included only patients who underwent surgery on index admission and thus we could not assess adherence to this aspect of the recommendations.
Statistical analysis
Continuous data are reported as means with standard deviations if normally distributed and medians with interquartile ranges (IQR) if skewed. Comparison of means between continuous variables was performed using independent samples Student t tests for normally distributed data and medians with Mann–Whitney U tests for non-normally distributed data. For categorical variables, between-group differences were assessed using the Fisher exact test and reported as counts and percentages. Logistic regression was used to identify predictors of binary outcomes. Model building was conducted on a scientific basis. Odds ratios with 95% confidence intervals (CIs) and p values are reported. The C statistic is reported as a measure of model discrimination ability. All tests were 2-sided, and statistical significance was set at a threshold of p < 0.05. Statistical analysis was conducted using R version 3.3 (R Foundation for Statistical Computing).
Results
Demographic characteristics
One hundred and fifty patients were included in the analysis, divided equally among the 3 hospitals. Their baseline characteristics are presented in Table 1. More than half of the patients were women (60.7%), with a mean age of 51.5 years. Patients were generally healthy, with a median age-adjusted Charlson score of 1 (range 0–9). More patients with mild cholecystitis than with moderate cholecystitis had asthma (6.7% v. 0%, p = 0.032) and fewer had type 2 diabetes (10.6% v. 26.1%, p = 0.025). The baseline characteristics of patients with mild and moderate cholecystitis were otherwise similar.
Table 1.
Patient demographic and clinical characteristics
| Characteristic | No. (%) of patients* | p value | ||
|---|---|---|---|---|
| All n = 150 |
Grade I cholecystitis n = 104 |
Grade II and III cholecystitis n = 46 |
||
| Sex, female | 91 (60.7) | 68 (65.4) | 23 (50.0) | 0.11 |
| Age, yr, mean (range)† | 51.5 (20–90) | 50.2 (20–87) | 54.4 (24–90) | 0.14 |
| Comorbidities‡ | ||||
| Obesity | 58 (39.3) | 41 (39.4) | 17 (37.0) | 0.86 |
| CAD | 13 (8.6) | 9 (8.7) | 4 (8.7) | 1.00 |
| COPD | 5 (3.3) | 4 (3.8) | 1 (2.2) | 1.00 |
| OSA | 7 (4.7) | 5 (4.8) | 2 (4.3) | 1.00 |
| Asthma | 10 (6.7) | 10 (6.7) | 0 (0) | 0.032 |
| Current smoker | 39 (26.0) | 25 (24.0) | 14 (30.4) | 0.42 |
| Type 2 diabetes | 23 (15.3) | 11 (10.6) | 12 (26.1) | 0.025 |
| AACCI score‡ | ||||
| 0 | 47 (31.3) | 33 (31.7) | 14 (30.4) | 1.00 |
| 1–5 | 97 (64.7) | 66 (63.5) | 31 (67.4) | 1.00 |
| 6–10 | 6 (4.0) | 5 (4.8) | 1 (2.2) | 0.67 |
| Median (range)§ | 1 (0–9) | 1 (0–7) | 1.5 (0–9) | 0.22 |
| ASA score‡ | ||||
| 1 | 5 (3.3) | 3 (2.8) | 2 (4.3) | 0.64 |
| 2 | 41 (27.3) | 29 (27.9) | 12 (26.1) | 1.00 |
| 3 | 89 (59.3) | 63 (60.6) | 26 (56.5) | 0.72 |
| 4 | 15 (10.0) | 9 (8.7) | 6 (13.0) | 0.39 |
AACCI = Age-adjusted Charlson Comorbidity Index; ASA = American Society of Anesthesiologists; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; OSA = obstructive sleep apnea.
Unless indicated otherwise.
Independent samples t test.
Fisher exact test.
Mann–Whitney U test.
Severity of cholecystitis
One hundred and four patients were rated as having grade I cholecystitis, 45 as having grade II cholecystitis, and 1 as having grade III cholecystitis. For the purposes of analysis, the patient with grade III cholecystitis was included with the patients with grade II cholecystitis.
Compliance with guidelines
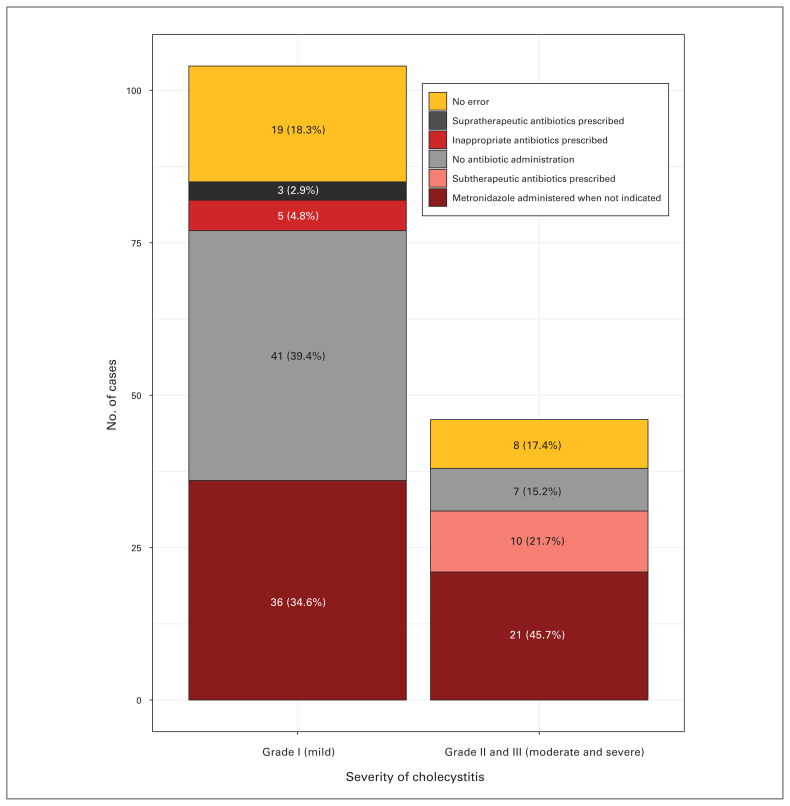
No patient included in the study had received documentation of a severity assessment or grading (Table 2). Compliance with antibiotic administration was poor, at an overall rate of 18.0%. This was not related to cholecystitis severity (mild 18.3%, moderate 17.4%; p = 0.90). Noncompliance was due to the following factors (Fig. 1): metronidazole administration when not indicated (n = 68 patients), no antibiotic administration (n = 48), subtherapeutic antibiotics prescribed (i.e., use of cefazolin in the case of moderate or severe cholecystitis; n = 10), antibiotics chosen against local antibiogram recommendations (n = 6) and supratherapeutic prescription (i.e., piperacillin-tazobactam used for mild cholecystitis; n = 3). Multiple errors in antibiotic choice were made for 11 patients. Failure to prescribe antibiotics was more frequent for patients with mild cholecystitis than for those with moderate cholecystitis (48.2% of errors among patents with mild cholecystitis v. 18.4% among those with moderate cholecystitis, p = 0.004). Patients with moderate cholecystitis were more frequently subjected to metronidazole overadministration than those with mild cholecystitis (47.1% of errors among patients mild cholecystitis v. 73.7% of errors among those with moderate cholecystitis, p = 0.013).
Table 2.
Compliance with guideline recommendations
| Compliance indicator | No. (%) of patients | p value | ||
|---|---|---|---|---|
| All n = 150 |
Grade I cholecystitis n = 104 |
Grade II and III cholecystitis n = 46 |
||
| Documentation of severity grading | 0 (0) | 0 (0) | 0 (0) | — |
| Compliance with antibiotic recommendations* | 27 (18.0) | 19 (18.3) | 8 (17.4) | 0.90 |
| Inappropriate metronidazole administration | 68 (55.3)† | 40 (47.1)‡ | 28 (73.7)‡ | 0.013 |
| No antibiotics given | 48 (39.0) | 41 (48.2) | 7 (18.4) | 0.004 |
| Time to operation < 72 h* | 129 (86.0) | 88 (84.6) | 41 (89.1) | 0.61 |
| Treated at an ACS hospital | 91 (91.0) | 62 (59.6) | 29 (63.0) | 0.72 |
| Treated at a non-ACS hospital | 38 (76.0) | 26 (25.0) | 12 (26.1) | 1.00 |
| p value | 0.025 | 0.06 | 0.10 | |
ACS = acute care surgery.
Fisher exact test.
Reported as the percentage of all study patients who received metronidazole inappropriately.
Reported as the percentage of patients with grade I cholecystitis or with grade II or III cholecystitis who received metronidazole inappropriately, respectively.
Fig. 1.
Reasons for noncompliance with the recommendations for antibiotic use in the Tokyo Guidelines. Although multiple errors were made in 11 instances, this chart displays only the most important error made for each patient.
Compliance with the guidelines in terms of time to operation was 86.0%; compliance was higher at hospitals with an ACS team (91.0% for patients in an ACS hospital v. 76.0% for those in a non-ACS hospital, p = 0.025). Severity of cholecystitis did not affect compliance with time to operation (84.6% for patients with mild cholecystitis v. 89.1% for those with moderate cholecystitis, p = 0.61).
Operative factors
Median time from admission to operation was 27 hours; it did not differ between patients with mild and moderate cholecystitis (Table 3). Median operative length for mild cases was 78 minutes; this increased to 98 minutes for moderate cases (p = 0.005). All procedures were initiated laparoscopically. A total of 141 procedures (94%) proceeded entirely laparoscopically and 9 (6.0%) were converted to laparotomy; there was no difference between patients with mild versus moderate cholecystitis (p = 0.46). Intraoperative cholangiogram was performed in 17 (11.3%) of cases, with no difference by severity grade (p = 0.59).
Table 3.
Operative factors
| Factor | No. (%) of patients* | p value | ||
|---|---|---|---|---|
| All n = 150 |
Grade I cholecystitis n = 104 |
Grade II and III cholecystitis n = 46 |
||
| Time from admission to operation, h, median (IQR)* | 27.0 (14.0–56.8) | 27.5 (11.8–58.5) | 26 (18–52.5) | 0.78 |
| Treated at an ACS hospital | 19.5 (9.8–33.0) | 19.0 (9.8–34.0) | 19.5 (10.0–36.0) | 0.68 |
| Treated at a non-ACS hospital | 55.5 (35.5–70.3) | 49.0 (33.0–71.0) | 58.0 (40.0–69.5) | 0.77 |
| p value | < 0.001 | < 0.001 | < 0.001 | |
| Operative length, min, median (IQR)† | 85.5 (64.3–110.0) | 78.0 (60.0–107.0) | 98.0 (80.0–123.5) | 0.005 |
| Surgical approach‡ | 0.46 | |||
| Laparoscopic | 141 (94.0) | 99 (95.2) | 42 (91.3) | |
| Converted | 9 (6.0) | 5 (4.8) | 4 (8.7) | |
| Open | 0 (0) | 0 (0) | 0 (0) | |
| Intraoperative cholangiography‡ | 17 (11.3) | 13 (12.5) | 4 (8.7) | 0.59 |
ACS = acute care surgery; IQR = interquartile range.
Unless indicated otherwise.
Mann–Whitney U test.
Fisher exact test.
Postoperative outcomes
Length of stay was significantly lower among patients who underwent a fully laparoscopic cholecystectomy than among those requiring conversion to laparotomy (median 3 d v. 11 d, p < 0.001); this held true for patients with both mild and moderate cholecystitis (Table 4). Similarly, patients treated at a hospital with an ACS service had a median length of stay of 3 days, compared with 3.5 days for patients treated at a non-ACS hospital (p = 0.001); this remained consistent across severity levels. Patients with mild cholecystitis were discharged in 3 days whereas those with moderate cholecystitis were discharged in 4 days (p = 0.041). Postoperative complications occurred in 34 cases (22.7%). Twenty-five of these were low-grade (Clavien–Dindo grade I–II) complications, 7 were grade IIIa complications and 2 were grade IVa complications. Severity was not associated with grade of cholecystitis or frequency of complications (Table 4). Thirteen patients (8.7%) required readmission, with no difference in frequency between patients with mild and moderate cholecystitis.
Table 4.
Postoperative factors
| Factor | No. (%) of patients* | |||
|---|---|---|---|---|
| All n = 150 |
Grade I cholecystitis n = 104 |
Grade II and III cholecystitis n = 46 |
p value | |
| Length of stay, d, median (IQR)† | 3 (2–5) | 3 (2–4) | 4 (2–5) | 0.041 |
| Surgical approach | ||||
| Laparoscopic | 3 (2–4) | 3 (2–4) | 3 (2–5) | 0.054 |
| Converted | 11 (10–17) | 17 (12–37) | 10 (9.8–10.3) | 0.18 |
| p value | < 0.001 | < 0.001 | 0.001 | |
| Hospital type | ||||
| ACS hospital | 3 (2–4) | 2 (1–4) | 3 (2–4.5) | 0.10 |
| Non-ACS hospital | 3.5 (3–5) | 3 (3–5) | 5 (3–6) | 0.18 |
| p value | 0.001 | 0.009 | 0.046 | |
| Postoperative complication‡ | ||||
| No | 116 (77.3) | 83 (79.8) | 33 (71.7) | |
| Yes (all CD grades) | 34 (22.7) | 21 (20.2) | 13 (28.3) | 0.30 |
| CD grade I | 14 (9.3) | 9 (8.7) | 5 (10.9) | 0.76 |
| CD grade II | 11 (7.3) | 7 (6.7) | 4 (8.7) | 0.74 |
| CD grade IIIa | 7 (4.7) | 4 (3.8) | 3 (6.5) | 0.68 |
| CD grade IIIb | 0 (0) | 0 (0) | 0 (0) | — |
| CD grade IVa | 2 (1.3) | 1 (0.9) | 1 (2.2) | 0.52 |
| CD grade IVb | 0 (0) | 0 (0) | 0 (0) | — |
| CD grade V (death) | 0 (0) | 0 (0) | 0 (0) | — |
| Readmission‡ | 13 (8.7) | 11 (7.3) | 2 (4.3) | 0.35 |
ACS = acute care surgery; CD = Clavien–Dindo; IQR = interquartile range.
Unless indicated otherwise.
Mann–Whitney U test.
Fisher exact test was used for the analysis of complications of all Clavien–Dindo grades.
Univariable analysis (Table 5) demonstrated that the risk of postoperative complication increased with elevated Charlson score (odds ratio [OR] 1.51, 95% confidence interval [CI] 1.23–1.88, p < 0.001) and conversion to laparotomy (OR 14.78, 95% CI 3.35–103.03, p = 0.001). These remained significant after adjustment (OR 1.47, 95% CI 1.19–1.85, p < 0.001, and OR 13.45, 95% CI 2.16–125.49, p = 0.010, respectively). Severity of cholecystitis, compliance with antibiotic recommendations and time to operation were not associated with postoperative complications, if all other factors were held constant.
Table 5.
Predictors of postoperative complications
| Predictor | Unadjusted analysis | Adjusted analysis | ||
|---|---|---|---|---|
|
|
|
|||
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| Charlson Comorbidity Index score | 1.51 (1.23–1.88) | < 0.001 | 1.47 (1.19–1.85) | < 0.001 |
|
| ||||
| Severity of cholecystitis | 1.32 (0.57–2.92) | 0.51 | 0.98 (0.36–2.49) | 0.96 |
|
| ||||
| Compliance with guidelines for antibiotic use (reference: noncompliance) | 1.57 (0.59–3.90) | 0.34 | 1.80 (0.61–4.98) | 0.27 |
|
| ||||
| Time to operation, h | 1.01 (1.00–1.02) | 0.13 | 1.00 (0.99–1.02) | 0.62 |
|
| ||||
| Operative approach* | 14.78 (3.35–103.03) | 0.001 | 13.45 (2.16–125.49) | 0.010 |
|
| ||||
| Duration of operation, min* | 1.01 (1.00–1.02) | 0.14 | 1.00 (0.99–1.01) | 0.76 |
C statistic for the logistic regression analysis: 0.757. CI = confidence interval.
Collinear.
Discussion
In this retrospective review, compliance with the Tokyo Guidelines was poor, and variability was observed between and within compliance metrics. Clinical documentation was entirely devoid of cholecystitis severity grading, whether in specific reference to the Tokyo Guidelines or in general terms. This probably reflects a lack of awareness of the guidelines at our institution, despite their decade-long existence and the frequency with which cholecystitis is encountered. Although antibiotics were administered to 69% of patients, there was compliance with the antibiotic recommendations in the Tokyo Guidelines in less than a quarter of cases; half of the instances of non-compliance were attributable to metronidazole overprescription. The low burden of biliary anaerobic bacterial flora in the context of acute cholecystitis is well described,16–18 leading to recommendations13,19 to exclude metronidazole from the treatment of mild and moderate cases, except for patients with biliary-enteric bypass (none in our series), patients who are also being given ciprofloxacin (not recommended according to our local antibiogram) 20 or patients whose bile culture result is positive for anaerobes (none in our series). This finding indicates that there is an ongoing issue with antimicrobial administration at our institution, which would be addressed if guidelines were effectively implemented. Compliance with the guidelines’ recommendation for time to operation was 86% overall; it was significantly higher at the hospitals with an ACS service than at the one without an ACS service.
Unlike the first 2 compliance metrics (documentation of severity rating and antibiotic choice), which are entirely under the control of the physician treating the patient, time to operation is also subject to hospital factors. In our system, hospitals with ACS services have dedicated operating time for urgent cases; this may partly explain the higher rate of compliance with the recommendation for time to operation at these hospitals. Nevertheless, surgeons are able to upgrade the priority level of a case to meet time-based criteria, which should enable an operation to be performed within 72 hours of admission.
More importantly, we must consider whether compliance with the guidelines affects patient outcomes. Our data show that postoperative complication rate is not affected by the degree of cholecystitis severity, and length of stay only mildly so. Thus, recognition of severity, as evidenced by documentation of a severity rating, may not cians implicitly recognized which cases of cholecystitis were more severe and therefore managed them differently, reducing the effect of severity toward null; we could not adequately assess this possibility with our data. Compliance with recommendations regarding antibiotic treatment and time to operation did not affect complication rate on univariate or multivariate analysis.
Similar findings have been reported in other studies,21 including a recent prospective study of 952 patients that found that use of the Tokyo Guidelines’ definitions of acute cholecystitis resulted in a diagnostic sensitivity of only 53%, with 47% of patients being undiagnosed on the basis of the Tokyo Guidelines’ criteria.22 This has resulted in a call to develop guidelines for acute cholecystitis that better reflect meaningful outcomes for patients.3 There may be be other benefits to using the guidelines that may not be directly related to patient outcomes. Examples from this study include identifying antibiotic stewardship targets and ensuring consistent treatment of patients with cholecystitis among surgeons and health care institutions. Severity of cholecystitis, while shown here only to affect length of stay, should be recognized and documented to make explicit the degree of illness, thereby guiding clear and timely clinical management. Finally, setting targets for time to operation will help us advocate for our patients and for systems, such as ACS services, that facilitate effective care.
Our results present 2 meaningful targets for quality improvement processes: antibiotic prescribing and time to operation. Recognition of cholecystitis severity may have little impact on patient outcomes, but it is a necessary preliminary step in choosing appropriate antibiotics. Measures to improve adherence to guidelines for appropriate prescribing may greatly improve antibiotic stewardship. Awareness of local resistance patterns and appropriate antibiotic choice will prevent needless development of further antimicrobial resistance.23
A concerted effort on the part of the surgical team and the hospital is required to reduce time to operation. Incorporating ACS services is an effective strategy in this regard,24,25 and it has been shown to improve outcomes for patients.26–28 It requires a commitment on the part of the hospital administration to ensure acute care services have timely access to operating facilities.29 Surgeons must remain cognizant of time since symptom onset and be prepared to advocate for an expedited operation if necessary. In addition, index cholecystectomy has been advocated as the standard of care,30 and with the growing evidence that timely cholecystectomy improves patient outcomes31 the rate of index cholecystectomy may evolve to become an indicator of appropriate management.
To enact these changes, particularly the physiciandependent element of antibiotic prescribing, we must revisit the means by which practice change is implemented. The Tokyo Guidelines were largely disseminated passively, through academic publication and inclusion in text-books. 32 Although there are reports of the guidelines changing practice,33,34 this literature is primarily from Japan. Furthermore, these articles focus on use of early laparoscopic cholecystectomy as an indicator of changing practice, which is now well established as the preferred approach.1,31,35 As has been demonstrated, adherence to the Tokyo Guidelines in our institution has been poor. The literature on guideline effectiveness is clear that passive strategies are ineffectual at driving change.8,9 Quality improvement in this scenario will require multimodal engagement of the physicians responsible for treating patients with cholecystitis. 8 This would include a review of this audit combined with education on the content and role of the guidelines; this strategy was effective at the Aga Khan University Hospital.36 We additionally plan to provide printed material in the emergency department and consultation areas guiding severity grading and appropriate antibiotic administration. Where electronic health systems are used, the standard admission forms will be modified to restrict antibiotic choices on the basis of severity grade in accordance with the guidelines and local resistance patterns. The third hospital in this study has implemented an ACS team in response to these and similar concerns; we hope this will result in improved care for patients through timely access to surgery.
Limitations
Limitations of this study include the retrospective design and the relatively small sample size. The post hoc assessment of severity additionally restricts our ability to draw conclusions about the relationship between guideline adherence and postoperative complications. Nonetheless, the lack of adherence to the guidelines was clearly demonstrated in this study, and it needs to be addressed. The revised management pathways in the 2018 update of the Tokyo Guidelines37 may alter the outcomes reported in this study.
Conclusion
Compliance with guidelines regarding documentation of severity grading and antibiotic prescribing was poor in this study. Compliance with guidelines regarding time to operation was reasonable, but it varied between hospitals. Guideline compliance had little impact on patient outcomes, aside from the recognition that patients with mild cholecystitis experienced reduced length of stay. Documentation of severity grading and antibiotic prescribing could be improved through quality improvement initiatives, and instituting an ACS service and ensuring adequate operating room allocation could decrease variability in time to cholecystectomy. Other institutions may find value in performing a similar audit to identify targets for quality improvement. Change will need to be multimodal and will require engagement from physician leaders and hospital administration.
Footnotes
Presented at the Canadian Surgery Forum 2017, Sept. 14–16, 2017, Victoria, B.C.
Competing interests: None declared.
Contributors: A. Giles, S. Godzisz, R. Nenshi, F. Farrokhyar and C. Eskicioglu designed the study. A. Giles and S. Godzisz acquired the data, which all authors analyzed. A. Giles and J. Lee wrote the article. All authors critically reviewed the article and provided final approval for publication.
References
- 1.Strasberg SM. Acute calculous cholecystitis. N Engl J Med. 2008;358:2804–11. doi: 10.1056/NEJMcp0800929. [DOI] [PubMed] [Google Scholar]
- 2.Badia JM, Nve E, Jimeno J, et al. Surgical management of acute cholecystitis. Results of a nation-wide survey among Spanish surgeons [article in English, Spanish] 2014;92:517–24. doi: 10.1016/j.ciresp.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Campanile FC, Catena F, Coccolini F, et al. The need for new “patient-related” guidelines for the treatment of acute cholecystitis. World J Emerg Surg. 2011;6:44–7. doi: 10.1186/1749-7922-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takada T, Kawarada Y, Nimura Y, et al. Background: Tokyo Guidelines for the management of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Surg. 2007;14:1–10. doi: 10.1007/s00534-006-1150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokoe M, Takada T, Strasberg SM, et al. New diagnostic criteria and severity assessment of acute cholecystitis in revised Tokyo Guidelines. J Hepatobiliary Pancreat Sci. 2012;19:578–85. doi: 10.1007/s00534-012-0548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokoe M, Hata J, Takada T, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos) J Hepatobiliary Pancreat Sci. 2018;25:41–54. doi: 10.1002/jhbp.515. [DOI] [PubMed] [Google Scholar]
- 7.Miura F, Takada T, Strasberg SM, et al. TG13 flowchart for the management of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:47–54. doi: 10.1007/s00534-012-0563-1. [DOI] [PubMed] [Google Scholar]
- 8.Prior M, Guerin M, Grimmer-Somers K. The effectiveness of clinical guideline implementation strategies — a synthesis of systematic review findings. J Eval Clin Pract. 2008;14:888–97. doi: 10.1111/j.1365-2753.2008.01014.x. [DOI] [PubMed] [Google Scholar]
- 9.Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet. 1993;342:1317–22. doi: 10.1016/0140-6736(93)92244-n. [DOI] [PubMed] [Google Scholar]
- 10.Daabiss M. American Society of Anaesthesiologists physical status classification. Indian J Anaesth. 2011;55:111–5. doi: 10.4103/0019-5049.79879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomi H, Solomkin JS, Takada T, et al. TG13 antimicrobial therapy for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:60–70. doi: 10.1007/s00534-012-0572-0. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita Y, Takada T, Strasberg SM, et al. TG13 surgical management of acute cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:89–96. doi: 10.1007/s00534-012-0567-x. [DOI] [PubMed] [Google Scholar]
- 15.Yokoe M, Takada T, Strasberg SM, et al. TG13 diagnostic criteria and severity grading of acute cholecystitis (with videos) J Hepatobiliary Pancreat Sci. 2013;20:35–46. doi: 10.1007/s00534-012-0568-9. [DOI] [PubMed] [Google Scholar]
- 16.Csendes A, Burdiles P, Maluenda F, et al. Simultaneous bacteriologic assessment of bile from gallbladder and common bile duct in control subjects and patients with gallstones and common duct stones. Arch Surg. 1996;131:389–94. doi: 10.1001/archsurg.1996.01430160047008. [DOI] [PubMed] [Google Scholar]
- 17.Galili O, Eldar S, Matter I, et al. The effect of bactibilia on the course and outcome of laparoscopic cholecystectomy. Eur J Clin Microbiol Infect Dis. 2008;27:797–803. doi: 10.1007/s10096-008-0504-8. [DOI] [PubMed] [Google Scholar]
- 18.Mazeh H, Mizrahi I, Dior U, et al. Role of antibiotic therapy in mild acute calculus cholecystitis: a prospective randomized controlled trial. World J Surg. 2012;36:1750–9. doi: 10.1007/s00268-012-1572-6. [DOI] [PubMed] [Google Scholar]
- 19.Fuks D, Cossé C, Régimbeau J-M. Antibiotic therapy in acute calculous cholecystitis. J Visc Surg. 2013;150:3–8. doi: 10.1016/j.jviscsurg.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Antiobiograms. Hamilton: Hamilton Health Sciences; 2010. [accessed 2015 Jan 21]. Hamilton Regional Laboratory Medicine Program: Lab Connections. Available: https://fhs.mcmaster.ca/hrlmp/microbiologylab/labrpt.htm. [Google Scholar]
- 21.Lee S-W, Yang S-S, Chang C-S, et al. Impact of the Tokyo Guidelines on the management of patients with acute calculous cholecystitis. J Gastroenterol Hepatol. 2009;24:1857–61. doi: 10.1111/j.1440-1746.2009.05923.x. [DOI] [PubMed] [Google Scholar]
- 22.Joseph B, Jehan F, Dacey M, et al. Evaluating the relevance of the 2013 Tokyo Guidelines for the diagnosis and management of cholecystitis. J Am Coll Surg. 2018;227:38–43.e1. doi: 10.1016/j.jamcollsurg.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibioticresistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–64. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 24.Lau B, DiFronzo LA. An acute care surgery model improves timeliness of care and reduces hospital stay for patients with acute cholecystitis. Am Surg. 2011;77:1318–21. [PubMed] [Google Scholar]
- 25.Lehane CW, Jootun RN, Bennett M, et al. Does an acute care surgical model improve the management and outcome of acute cholecystitis? ANZ J Surg. 2010;80:438–42. doi: 10.1111/j.1445-2197.2010.05312.x. [DOI] [PubMed] [Google Scholar]
- 26.Gutt CN, Encke J, Köninger J, et al. Acute cholecystitis: early versus delayed cholecystectomy, a multicenter randomized trial (ACDC Study, NCT00447304) Ann Surg. 2013;258:385–93. doi: 10.1097/SLA.0b013e3182a1599b. [DOI] [PubMed] [Google Scholar]
- 27.Papi C, Catarci M, D’Ambrosio L, et al. Timing of cholecystectomy for acute calculous cholecystitis: a meta-analysis. Am J Gastroenterol. 2004;99:147–55. doi: 10.1046/j.1572-0241.2003.04002.x. [DOI] [PubMed] [Google Scholar]
- 28.Wilson E, Gurusamy K, Gluud C, et al. Cost-utility and value-ofinformation analysis of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg. 2010;97:210–9. doi: 10.1002/bjs.6872. [DOI] [PubMed] [Google Scholar]
- 29.Murphy PB, DeGirolamo K, Van Zyle TJ, et al. Impact of the acute care surgery model on disease- and patient-specific outcomes in appendicitis and biliary disease: a meta-analysis. J Am Coll Surg. 2017;225:763–77.e13. doi: 10.1016/j.jamcollsurg.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Murphy PB, Vogt KN, Mele TS, et al. Timely surgical care for acute biliary disease: an indication of quality. Ann Surg. 2016;264:913–4. doi: 10.1097/SLA.0000000000001704. [DOI] [PubMed] [Google Scholar]
- 31.De Mestral C, Laupacis A, Rotstein OD, et al. Early cholecystectomy for acute cholecystitis: a population-based retrospective cohort study of variation in practice. CMAJ Open. 2013;1:E62–7. doi: 10.9778/cmajo.20130001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takada T, Strasberg SM, Solomkin JS, et al. TG13: updated Tokyo Guidelines for the management of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:1–7. doi: 10.1007/s00534-012-0566-y. [DOI] [PubMed] [Google Scholar]
- 33.Shinya S, Yamashita Y, Takada T. The impact of the Japanese clinical guidelines on the clinical management of patients with acute cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:611–9. doi: 10.1007/s00534-013-0603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asai K, Watanabe M, Kusachi S, et al. Changes in the therapeutic strategy for acute cholecystitis after the Tokyo Guidelines were published. J Hepatobiliary Pancreat Sci. 2013;20:348–55. doi: 10.1007/s00534-012-0536-4. [DOI] [PubMed] [Google Scholar]
- 35.Ambe P, Weber SA, Christ H, et al. Cholecystectomy for acute cholecystitis. How time-critical are the so called “golden 72 hours”? Or better “golden 24 hours” and “silver 25–72 hour”? A case control study. World J Emerg Surg. 2014;9:60–5. doi: 10.1186/1749-7922-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bari H, Khan MR, Shariff AH. Antibiotics in acute calculous cholecystitis — Do Tokyo Guidelines influence the surgeons’ practices? J Pak Med Assoc. 2017;67:670–6. [PubMed] [Google Scholar]
- 37.Okamoto K, Suzuki K, Takada T, et al. Tokyo Guidelines 2018: flowchart for the management of acute cholecystitis. J Hepatobiliary Pancreat Sci. 2018;25:55–72. doi: 10.1002/jhbp.516. [DOI] [PubMed] [Google Scholar]