Introduction
Prostate cancer (CaP) is the most commonly diagnosed solid malignancy in men, accounting for an estimated 161,360 newly diagnosed cases and 26,730 cancer deaths in the USA in 2017.1 Risk stratification of newly diagnosed CaP is the basis for predicting outcome and for treatment selection. As such, the NCCN has incorporated a stratification paradigm into its guidelines for the purposes of treatment selection.2 In particular, men are classified into low, intermediate, or high risk categories using clinical factors that include PSA, tumor stage, and biopsy Gleason score.2–4 More recently, gene signature platforms have been established as potentially valuable tools to prognosticate and guide treatment selection, and an integrated clinical-genomic risk group classification system has been proposed for localized CaP.5
Multiple investigators have shown that post-treatment factors, including a PSA value at a specified time point following treatment,6–8 PSA nadir,9–11 PSA doubling time,9 and interval to PSA failure11 are prognostic for outcome. Since they reflect primary treatment response and/or failure, such post-treatment factors may potentially afford improved prognostic capability over pretreatment factors. It has also been demonstrated that the time to PSA nadir (TTN) is associated with progression, cancer-specific death, and all-cause mortality in men with metastatic CaP who receive androgen-deprivation therapy (ADT).12–15 However, the prognostic value of TTN in the setting of localized disease amongst men who do not achieve an undetectable PSA (< 0.2 ng/ml) has not been studied.16,17 If shown to be prognostic, then TTN would be a useful pre-randomization stratification and selection factor in future randomized trials, enrolling men who do not achieve an undetectable PSA nadir after undergoing conventional ADT and RT for unfavorable risk CaP. Such a trial would be designed to evaluate the impact of adding an agent shown to improve outcomes in men at high risk for having or known to have castrate-resistant CaP (such as abiraterone, enzalutamide, docetaxel or apalutamide) to conventional ADT on metastasis-free, prostate cancer-specific, and overall survival.18–21 This trial could also assess if time to PSA nadir was predictive of response to these novel agents. These interventions could be implemented at a time before PSA failure, but when castrate resistance is suspected, based on a measurable PSA despite castrate levels of testosterone.22
Therefore, in the current study, we investigate the prognostic significance of TTN, stratified by a detectable versus undetectable PSA nadir and adjusted for age, comorbidity, and known CaP prognostic factors, in men with newly diagnosed unfavorable risk prostate cancer using long term follow-up data from a randomized controlled trial that compared definitive radiation therapy (RT) with or without ADT.23,24
Materials and Methods
Patient Population and Treatment
The study population is comprised of men enrolled on the prospective randomized trial DFCI 95–096, which included men with unfavorable risk CaP (PSA greater than 10 but less than 40, biopsy Gleason score 7 or greater, or MRI evidence of extracapsular invasion and/or seminal vesicle invasion), who were treated at one of three community hospitals (St. Anne’s Hospital, Metrowest Medical Center, and Suburban Oncology Center) or one of three academic centers (Dana-Farber Cancer Institute, Brigham and Women’s Hospital, or Beth Israel Deaconess Medical Center) with external beam radiation therapy (EBRT) to a total dose of 70.20Gy in 1.8Gy fractions with or without six months of ADT between December 1, 1995 and April 15, 2001. Biopsy pathological samples were centrally reviewed. At the time of enrollment, patient comorbidity was assessed using the Adult Comorbidity Evaluation 27 (ACE-27) index. 204 of the enrolled 206 patients who had available data on TTN were included in this analysis.
Follow-up and Determination of Cause of Death
Patients were followed longitudinally following treatment: every three months for two years, every six months for the following three years, and then annually thereafter to the time of death or until October 9, 2016. Time zero (T0) is the day of randomization. Physical exam including a digital rectal exam, as well as serum PSA testing were obtained at each follow up visit. Further diagnostics such as an MRI or bone scan were performed at the time of PSA failure, and in general, salvage ADT with an LHRH agonist was initiated once serum PSA exceeded 10 ng/mL. The cause of death was assigned by the patient’s primary medical oncologist. Assignment of CaP as a cause of death required that the patient have castrate-resistant metastatic disease, rising PSA through several attempts for salvage with ADT, and typically the use of chemotherapy prior to death. The study was approved by Institutional Review Boards at each institution. Informed consent was obtained from the patient at the time of enrollment, and a waiver of consent was obtained for long-term follow up.
Statistical Methods
Distribution and comparison of clinical characteristics and treatment stratified by PSA nadir and TTN
Table 1 illustrates the distribution of clinical and treatment characteristics among men with PSA nadir ≥ 0.2 ng/mL or < 0.2 ng/mL stratified by TTN about the median value. Continuous factors including age, PSA level at diagnosis, and PSA nadir were compared using the Wilcoxon rank-sum test, whereas categorical covariates including AJCC T stage, biopsy Gleason score, treatment with EBRT and ADT, and ACE-27 comorbidity were compared using the Mantel-Haenszel χ2 test. For small sample sizes, a Fisher exact test was used.
Table 1.
Comparison of the distribution of patient clinical characteristics stratified by the median time to PSA nadir
| Clinical Characteristic | PSA Nadir ≥ 0.2 ng/mL (N= 113) |
PSA Nadir < 0.2 ng/ml (N=91) |
||||
|---|---|---|---|---|---|---|
| TTN<median* | TTN≥median | P-value | TTN<median | TTN≥median | P-value | |
| Age, median (IQR), yrs | 72.43 (66.74, 75.71) | 73.39 (70.72, 76.45) | 0.30 | 71.52 (68.75, 74.60) | 72.26 (70.31, 75.43) | 0.46 |
| AJCC clinical tumor category | ||||||
| T1 | 14 (42.42%) | 35 (43.75%) | 0.90 | 38 (55.07%) | 9 (40.91%) | 0.25 |
| T2 | 19 (57.58%) | 45 (56.25%) | 31 (44.93%) | 13 (59.09%) | ||
| PSA at diagnosis, median (IQR), ng/mL | 12.12 (9.5, 20.04) | 11.10 (7.77, 16.08) | 0.14 | 10.96 (7.3, 15.93) | 9.63 (5.1, 12.25) | 0.26 |
| Biopsy Gleason score | ||||||
| ≤6 | 7 (21.21%) | 23 (28.75%) | 0.15 | 23 (33.33%) | 4 (18.18%) | 0.34 |
| 7 | 17 (51.52%) | 45 (56.25%) | 39 (56.52%) | 16 (72.73%) | ||
| 8–10 | 9 (27.27%) | 12 (15.00%) | 7 (10.14%) | 2 (10.14%) | ||
| PSA nadir, median (IQR), ng/mL | 0.9 (0.42, 2.8) | 0.65 (0.40, 0.94) | 0.02 | 0.10 (0.10, 0.10) | 0.08 (0.03, 0.10) | 0.30 |
| Randomized treatment | ||||||
| EBRT alone | 26 (78.79%) | 71 (88.75%) | 0.17 | 0 (0%) | 7 (31.82%) | <0.001** |
| EBRT + ADT | 7 (21.21%) | 9 (11.25%) | 69 (100%) | 15 (68.18%) | ||
| ACE-27 Comorbidity Score | ||||||
| No or minimal | 26 (78.79%) | 62 (77.50%) | 0.88 | 52 (75.36%) | 15 (68.18%) | 0.51 |
| Moderate or severe | 7 (21.21%) | 18 (22.50%) | 17 (24.64%) | 7 (31.82%) | ||
Abbreviations: PSA (prostate specific antigen); TTN (time to PSA nadir); AJCC (American Joint Committee on Cancer); IQR (intraquartile range); ACE-27 (Adult Comorbidity Evaluation 27); EBRT (external beam radiation therapy); ADT (androgen deprivation therapy); N (number).
median = 12 months for the study cohort with 204 men,
Fisher exact test
Risk of prostate cancer-specific mortality
We evaluated whether an association exists between TTN and the risk of prostate cancer-specific mortality (PCSM) in men with PSA nadir <0.2 ng/ml or ≥ 0.2 ng/ml using a Fine and Gray competing risk regression model. We adjusted this risk assessment for patient factors including age and comorbidity, defined using the ACE-27 metric at randomization, as well as known CaP prognostic factors including PSA, biopsy Gleason score, and AJCC tumor stage. An interaction term25 between PSA nadir and TTN (PSA nadir ≥ vs <0.2 ng/mL x TTN stratified by the median of 12 months) was included in the model in order to assess the impact of TTN stratified by the median within the subgroups of PSA nadir (<0.2 ng/mL versus ≥ 0.2 ng/mL). We also included an interaction term between comorbidity and treatment. In a distinct adjusted interaction model, we assessed the impact of TTN ≥ or < the median among men who underwent RT alone or RT and ADT. The baseline/referent categories for categorical covariates were biopsy Gleason score ≤ 6, AJCC T1, and no or minimal comorbidity. Given that T0 was set as the time of randomization, PSA nadir and TTN were treated as time-dependent covariates. Unadjusted and adjusted hazard ratios (HR/AHR) were calculated for each covariate in the model and the respective 95% confidence intervals were reported (CI). A two-sided P-value of < 0.05 was considered statistically significant.
Estimates of prostate cancer-specific mortality
Estimates of PCSM were calculated using the extended Kaplan-Meier method with time-dependent covariates, stratified by the median TTN for men with a PSA nadir < 0.2 ng/mL versus ≥ 0.2 ng/mL. These estimates were adjusted for significant covariates shown in the competing-risks regression model in Table 2. R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria) was used for all calculations pertaining to cumulative incidence functions with time-dependent covariates. SAS version 9.4 (SAS Institute, Cary, North Carolina) was used for all other calculations.
Table 2.
Prostate cancer-specific mortality (PCSM) unadjusted and adjusted hazard ratios
| Clinical characteristic | Number of Men | Number of PC Deaths | Univariable | Multivariable* | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | AHR (95% CI) | P-value | |||
| Interaction term: PSA nadir and TTN# | ||||||
| PSA nadir ≥ 0.2 ng/mL (t) | ||||||
| TTN < median (t) | 33 | 14 | 3.98 (1.82, 8.70) | <0.001 | 5.07 (2.10, 12.23) | <0.001 |
| TTN ≥ median (t) | 80 | 10 | 1.0 (Ref) | - | 1.0 (Ref) | - |
| PSA nadir < 0.2 ng/mL (t) | ||||||
| TTN < median(t) | 69 | 5 | 2.11 (0.25, 17.96) | 0.49 | 9.91 (0.23, 433.8) | 0.23 |
| TTN ≥ median (t) | 22 | 1 | 1.0 (Ref) | - | 1.0 (Ref) | - |
| PSA nadir ≥ 0.2 vs < 0.2 (t) x TTN < median vs ≥median (t) | 204 | 30 | 1.89 (0.19, 18.37) | 0.59 | 0.51 (0.01, 22.36) | 0.73 |
| Interaction term: Treatment and Comorbidity | ||||||
| No or minimal ACE-27 comorbidity | ||||||
| Randomized Treatment Arm EBRT + ADT | 76 | 5 | 0.22 (0.08, 0.57) | 0.002 | 0.11 (0.01, 0.98) | 0.048 |
| EBRT | 79 | 21 | 1.0 (Ref) | - | 1.0 (Ref) | - |
| Moderate or severe ACE-27 comorbidity | ||||||
| Randomized Treatment Arm EBRT + ADT | 25 | 1 | 0.34 (0.04, 3.33) | 0.36 | 0.12 (0.01, 1.91) | 0.13 |
| EBRT | 24 | 3 | 1.0 (Ref) | - | 1.0 (Ref) | - |
| Treatment x Comorbidity | 204 | 30 | 1.59 (0.14, 18.60) | 0.71 | 1.06 (0.09, 12.92) | 0.96 |
| Additional clinical characteristics | ||||||
| Age, yrs (continuous) | 204 | 30 | 1.01 (0.96, 1.07) | 0.66 | 1.03 (0.97, 1.10) | 0.32 |
| Log PSA, ng/mL (continuous) | 204 | 30 | 1.29 (0.54, 3.08) | 0.56 | 1.27 (0.59, 2.77) | 0.54 |
| AJCC tumor category | ||||||
| T2 | 108 | 21 | 2.22 (1.03, 4.80) | 0.04 | 2.06 (0.93, 4.55) | 0.07 |
| T1 | 96 | 9 | 1.0 (Ref) | - | 1.0 (Ref) | - |
| Biopsy Gleason score | ||||||
| 8 to 10 | 30 | 8 | 6.08 (1.61, 22.82) | 0.008 | 4.70 (1.45, 15.29) | 0.01 |
| 7 | 117 | 19 | 3.32 (1.002, 11.01) | 0.05 | 2.40 (0.79, 7.25) | 0.12 |
| 6 or less | 57 | 3 | 1.0 (Ref) | - | 1.0 (Ref) | - |
Abbreviations: PSA (prostate specific antigen); TTN (time to PSA nadir); AJCC (American Joint Committee on Cancer); REF (referent); ACE-27 (Adult Comorbidity Evaluation 27); EBRT (external beam radiation therapy); ADT (androgen deprivation therapy); AHR (adjusted hazard ratio); CI (confidence interval); (t) (time-dependent covariate).
Note. The adjusted risk of PCSM among men whose TTN was < median as compared to ≥ median was increased, regardless if they received RT alone (AHR 5.99, 95%CI 2.7,13.3, p<0.001) or RT and ADT (AHR 1.86, 95%CI 0.18, 19.45, p=0.60).
Results
Distribution and comparison of clinical characteristics and treatment stratified by PSA nadir and TTN
As shown in Table 1, there were no significant differences in the distribution of the patients’ clinical characteristics and assigned treatments for men who achieved a post-treatment PSA nadir ≥ 0.2, with one exception being median PSA nadir, which was higher in men whose TTN was <12 months as opposed to ≥12 months (0.9 ng/ml vs. 0.65 ng/ml, p=0.02). Amongst men who reached an undetectable PSA, the only significant difference was that among men assigned to receive EBRT plus ADT as compared to EBRT alone, TTN < 12 months was significantly more likely (100% vs 0%, p<0.001).
Risk of prostate cancer-specific mortality
After a median follow up of 18.17 years [Interquartile range (IQR)]: 16.96 to 19.37], 160 men died and 30 (18.75%) of prostate cancer. As shown in Table 2, amongst men with a PSA nadir ≥ 0.2 ng/ml, a TTN < 12 months was significantly associated with an increased risk of PCSM as compared to men whose TTN was 12 months or more (AHR 5.07, 95%CI 2.10–12.23, p<0.001); whereas this association was not observed among men with a PSA nadir of <0.2 ng/ml, (AHR 9.9, 95%CI 0.23–433.8, p=0.23). Moreover, this association was observed among men undergoing RT (AHR 5.99, 95%CI 2.7,13.3, p<0.001) or RT and ADT (AHR 1.86, 95%CI 0.18, 19.45, p=0.60). Gleason score 8–10 (AHR 4.70, 95%CI 1.45–15.29, p=0.01) as compared to 6, and randomized treatment with EBRT plus ADT versus EBRT alone in men with no or minimal comorbidity as measured by the ACE-27 index (AHR 0.11, 95%CI 0.01–0.98, p=0.048) were also significantly associated with the risk of PCSM.
Estimates of prostate cancer-specific mortality
As shown in Figure 1, estimates of PCSM were significantly lower amongst men with a TTN ≥ 12 months as compared to those with TTN < 12 months, for men with a detectable PSA nadir >0.2 ng/ml (p=0.0019) whereas no significant difference in these estimates was observed among men with an undetectable PSA nadir <0.2 ng/ml (p=0.6895). Specifically, 15-year-estimates of PCSM (95%CI) were 12.77% (6.25%−25.11%) versus 46.27% (26.04%−72.17%) for men with TTN ≥ 12 months versus with a TTN < 12 months, respectively when PSA nadir ≥0.2 ng/ml; these estimates were 12.50% (1.86%−61.30%) versus 10.15% (4.2%−23.15%) for men with a PSA nadir of <0.2 ng/ml.
Figure 1.
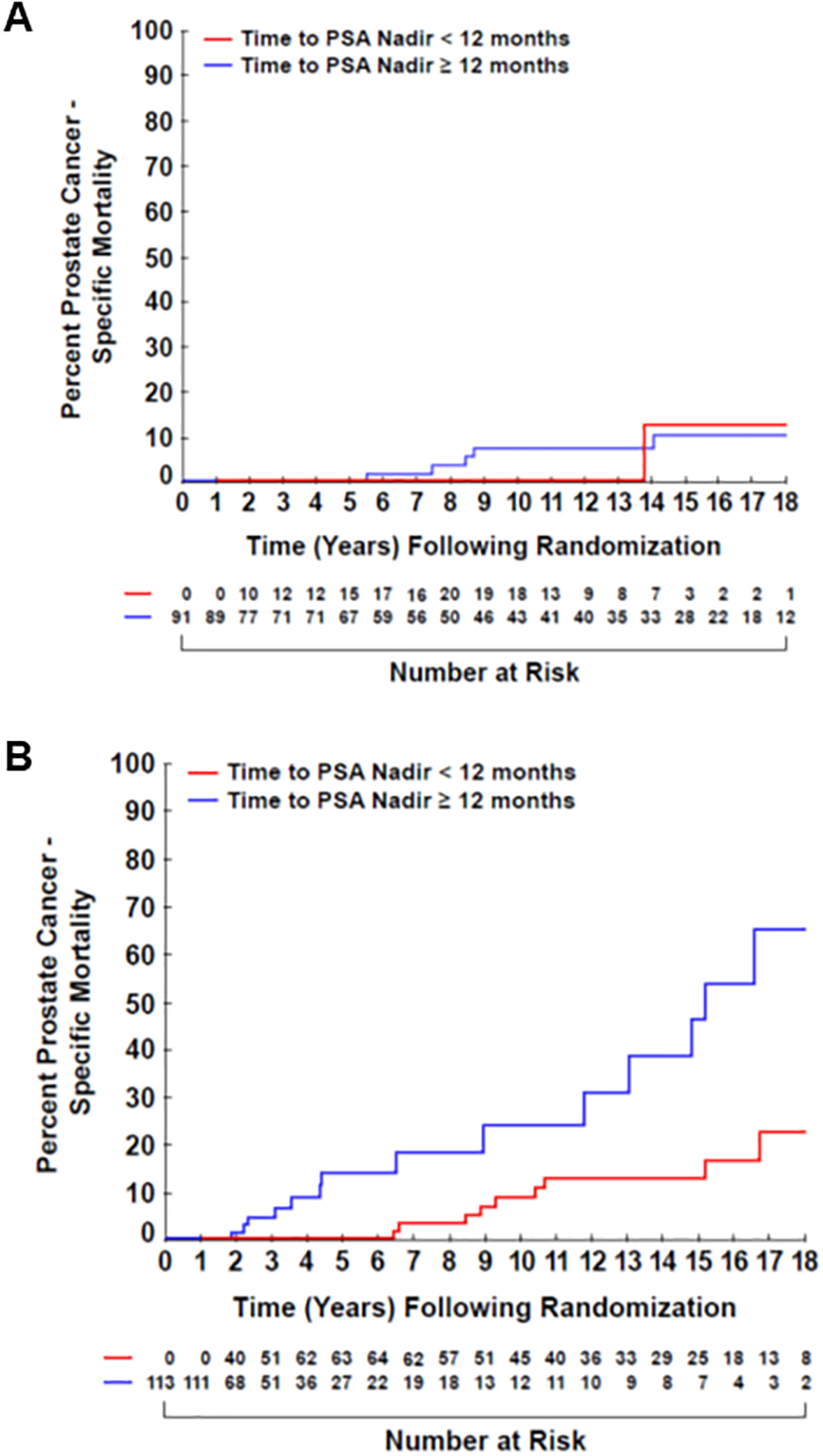
Prostate cancer-specific mortality over time of patients with an (A) undetectable or (B) detectable PSA nadir, stratified by time to PSA nadir.
Discussion
We found that a shorter TTN in men with a detectable PSA nadir (≥0.2ng/mL) was associated with an increased risk of PCSM, as compared to a longer TTN (greater than the median of 12 months). However, this observation was not seen in men with an undetectable PSA nadir (<0.2 ng/mL). The clinical relevance of these observations is that they infer a high likelihood of the presence of clinically occult castrate-resistant CaP in men with a detectable PSA nadir and a short TTN. Supporting the assertion that these patients are more likely to be castrate-resistant are prior reports showing the prognostic significance of PSA nadir at three months following RT,26 as well as its potential for being a surrogate for all-cause mortality when the PSA nadir value is greater than 0.5 ng/mL.27 Since ongoing or completed randomized controlled trials demonstrate the efficacy of novel agents such as abiraterone, enzalutamide or docetaxel in prolonging survival in castrate-resistant metastatic CaP,18–20 it would be reasonable to consider similar studies in men who are at high risk of harboring castrate-resistant disease prior to PSA failure. Specifically, we would suggest selecting men for a randomized trial testing treatment escalation who have a detectable PSA nadir and who reached the nadir value in less than 1 year.
Several points require clarification. First, the design of a clinical trial that enrolls patients at the time of a rapidly attained and detectable PSA nadir and then randomizes them to completion of standard of care ADT with or without an agent shown to prolong survival in castrate-resistant CaP is a new paradigm for the timing of intervention. Specifically, we would be studying novel salvage therapy before the time of documented PSA failure (as per the Phoenix definition of a rise of 2ng/mL above nadir). Men with high risk CaP, who by today’s standard should receive at least 18 months of ADT,2 will still be receiving ADT at the time of a PSA nadir should this occur in less than 12 months. Thus, the randomization would be to ADT versus ADT plus a novel agent18–21 for the remainder of planned ADT duration. Second, though most prior studies in patients with metastatic CaP have demonstrated a similar finding of worse outcome in those patients with a detectable PSA nadir and short TTN,14,28,29 two prior studies have linked a short TTN to improved PCSM17 and overall survival,15 respectively. These studies differed from ours, however, in that they did not define time 0 as the date of randomization or utilize time-dependent covariates for TTN (the first study utilized landmark analysis, while the latter did not correct for possible lead-time bias) as was done in the current study by using a time-dependent covariate for TTN. Third, the primary endpoint of our study is PCSM, which we believe is reliable because it was validated in the setting of a prospective randomized controlled trial where death was confirmed by the principal investigator or treating oncologist who followed the patient until death. Finally, the impact of TTN on the risk of PCSM appears to be independent of the initial treatment received, although the association was only significant for men undergoing RT given the small absolute number of men whose PSA nadir was >0.2 ng/mL and who underwent RT and ADT. Given that the RT dose used in our study was only 70 Gy and that many of these men had intermediate and not high risk CaP, further work is needed to determine which men with intermediate-risk CaP and a TTN < 1 year would have had decreased risk of PCSM if given a higher RT dose without ADT versus a higher dose RT and ADT. However, for men who present with high risk or very high risk prostate cancer, it is likely that occult micrometastatic disease that is castrate-resistant exist in some of these men at presentation. Our results of rapid time to nadir and prior results of high PSA nadir being associated with an increased risk of PCSM are consistent with this hypothesis. Therefore, we support studies of the addition of agents shown to prolong survival in castrate-resistant metastatic and non-metastatic prostate cancer in such men using the trial design we have suggested. Thus, our study’s strengths include the use of prospectively collected data, long median follow up, consistency in defining cause of death by a single expert and utilization of time-dependent covariates to accurately evaluate for a potential association between TTN and the risk of PCSM. Finally, while our observation of the prognostic impact of TTN in the setting of detectable PSA nadir is hypothesis generating and warrants prospective validation by others, the observation that TTN does not have a significant association with the risk of PCSM in men who achieve an undetectable nadir has not been previously reported and serves to define high and low risk subsets of men with respect to the future risk of PCSM.
In conclusion, we support a novel randomized trial design in men with a high risk of PCSM, namely those with high risk CaP selected to undergo RT and at least 18 months of conventional ADT and a detectable PSA nadir who reach this value in less than 1 year. Such men would be randomized based on the biomarker of TTN to either continued standard of care with conventional ADT or standard of care with the addition of a novel agent such a abiraterone, enzalutamide, docetaxel or apalutamide to evaluate the impact of these additional agents on metastasis-free, cancer-specific and overall survival in the definitive management of localized high-risk prostate cancer.
Acknowledgements
Thank you to Dr. William Oh for helpful discussions leading to the inception of this study (Department of Hematology and Medical Oncology, Mount Sinai Hospital, New York, NY).
Footnotes
Funding and conflict of interest statement: The authors have no relevant conflicts of interest. There were no funding sources for this work.
Comment: Time to PSA nadir prognosticates for cancer specific mortality in men with detectable PSA following definitive treatment for localized prostate cancer
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw. 2016;14(1):19–30. http://www.ncbi.nlm.nih.gov/pubmed/26733552. Accessed April 13, 2018. [DOI] [PubMed] [Google Scholar]
- 3.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical Outcome After Radical Prostatectomy, External Beam Radiation Therapy, or Interstitial Radiation Therapy for Clinically Localized Prostate Cancer. JAMA. 1998;280(11):969. doi: 10.1001/jama.280.11.969 [DOI] [PubMed] [Google Scholar]
- 4.Zumsteg ZS, Spratt DE, Pei I, et al. A New Risk Classification System for Therapeutic Decision Making with Intermediate-risk Prostate Cancer Patients Undergoing Dose-escalated External-beam Radiation Therapy. Eur Urol. 2013;64(6):895–902. doi: 10.1016/j.eururo.2013.03.033 [DOI] [PubMed] [Google Scholar]
- 5.Spratt DE, Zhang J, Santiago-Jiménez M, et al. Development and Validation of a Novel Integrated Clinical-Genomic Risk Group Classification for Localized Prostate Cancer. J Clin Oncol. 2018;36(6):581–590. doi: 10.1200/JCO.2017.74.2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray ME, Thames HD, Levy LB, et al. PSA nadir predicts biochemical and distant failures after external beam radiotherapy for prostate cancer: A multi-institutional analysis. Int J Radiat Oncol. 2006;64(4):1140–1150. doi: 10.1016/j.ijrobp.2005.07.006 [DOI] [PubMed] [Google Scholar]
- 7.Hanlon AL, Diratzouian H, Hanks GE. Posttreatment prostate-specific antigen nadir highly predictive of distant failure and death from prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53(2):297–303. http://www.ncbi.nlm.nih.gov/pubmed/12023133. Accessed October 24, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Zelefsky MJ, Shi W, Yamada Y, et al. Postradiotherapy 2-Year Prostate-Specific Antigen Nadir as a Predictor of Long-Term Prostate Cancer Mortality. Int J Radiat Oncol. 2009;75(5):1350–1356. doi: 10.1016/j.ijrobp.2008.12.067 [DOI] [PubMed] [Google Scholar]
- 9.Zietman AL, Tibbs MK, Dallow KC, et al. Use of PSA nadir to predict subsequent biochemical outcome following external beam radiation therapy for T1-2 adenocarcinoma of the prostate. Radiother Oncol. 1996;40(2):159–162. doi: 10.1016/0167-8140(96)01770-7 [DOI] [PubMed] [Google Scholar]
- 10.Ray ME, Thames HD, Levy LB, et al. PSA nadir predicts biochemical and distant failures after external beam radiotherapy for prostate cancer: a multi-institutional analysis. Int J Radiat Oncol Biol Phys. 2006;64(4):1140–1150. doi: 10.1016/j.ijrobp.2005.07.006 [DOI] [PubMed] [Google Scholar]
- 11.Stewart AJ, Scher HI, Chen M-H, et al. Prostate-Specific Antigen Nadir and Cancer-Specific Mortality Following Hormonal Therapy for Prostate-Specific Antigen Failure. J Clin Oncol. 2005;23(27):6556–6560. doi: 10.1200/JCO.2005.20.966 [DOI] [PubMed] [Google Scholar]
- 12.Choueiri TK, Xie W, D’Amico AV, et al. Time to prostate-specific antigen nadir independently predicts overall survival in patients who have metastatic hormone-sensitive prostate cancer treated with androgen-deprivation therapy. Cancer. 2009;115(5):981–987. doi: 10.1002/cncr.24064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang S-P, Bao B-Y, Wu M-T, et al. Impact of prostate-specific antigen (PSA) nadir and time to PSA nadir on disease progression in prostate cancer treated with androgen-deprivation therapy. Prostate. 2011;71(11):1189–1197. doi: 10.1002/pros.21334 [DOI] [PubMed] [Google Scholar]
- 14.Teoh JYC, Tsu JHL, Yuen SKK, et al. Prognostic significance of time to prostate-specific antigen (PSA) nadir and its relationship to survival beyond time to PSA nadir for prostate cancer patients with bone metastases after primary androgen deprivation therapy. Ann Surg Oncol. 2015;22(4):1385–1391. doi: 10.1245/s10434-014-4105-8 [DOI] [PubMed] [Google Scholar]
- 15.Sasaki T, Onishi T, Hoshina A. Nadir PSA level and time to PSA nadir following primary androgen deprivation therapy are the early survival predictors for prostate cancer patients with bone metastasis. Prostate Cancer Prostatic Dis. 2011;14(3):248–252. doi: 10.1038/pcan.2011.14 [DOI] [PubMed] [Google Scholar]
- 16.D’Amico AV, McLeod DG, Carroll PR, Cullen J, Chen M-H. Time to an undetectable prostate-specific antigen (PSA) after androgen suppression therapy for postoperative or postradiation PSA recurrence and prostate cancer-specific mortality. Cancer. 2007;109(7):1290–1295. doi: 10.1002/cncr.22550 [DOI] [PubMed] [Google Scholar]
- 17.Chung CS, Chen M-H, Cullen J, McLeod D, Carroll P, D’Amico AV. Time to Prostate-Specific Antigen Nadir After Androgen Suppression Therapy for Postoperative or Postradiation PSA Failure and Risk of Prostate Cancer-Specific Mortality. Urology. 2008;71(1):136–140. doi: 10.1016/J.UROLOGY.2007.08.028 [DOI] [PubMed] [Google Scholar]
- 18.James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377(4):338–351. doi: 10.1056/NEJMoa1702900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720 [DOI] [PubMed] [Google Scholar]
- 20.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N Engl J Med. 2014;371(5):424–433. doi: 10.1056/NEJMoa1405095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith MR, Saad F, Chowdhury S, et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med. 2018;378(15):1408–1418. doi: 10.1056/NEJMoa1715546 [DOI] [PubMed] [Google Scholar]
- 22.Atkins KM, Chen M-H, Wu J, et al. Low testosterone at first prostate-specific antigen failure and assessment of risk of death in men with unfavorable-risk prostate cancer treated on prospective clinical trials. Cancer. 2018;124(7):1383–1390. doi: 10.1002/cncr.31204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Amico AV, Chen M-H, Renshaw AA, Loffredo M, Kantoff PW. Androgen Suppression and Radiation vs Radiation Alone for Prostate Cancer. JAMA. 2008;299(3):289–295. doi: 10.1001/jama.299.3.289 [DOI] [PubMed] [Google Scholar]
- 24.D’Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-Month Androgen Suppression Plus Radiation Therapy vs Radiation Therapy Alone for Patients With Clinically Localized Prostate Cancer. JAMA. 2004;292(7):821. doi: 10.1001/jama.292.7.821 [DOI] [PubMed] [Google Scholar]
- 25.Klein JP, Moeschberger ML. Survival Analysis. New York: Springer-Verlag; 2003. doi: 10.1007/b97377 [DOI] [Google Scholar]
- 26.Bryant AK, D’Amico AV, Nguyen PL, et al. Three-month posttreatment prostate-specific antigen level as a biomarker of treatment response in patients with intermediate-risk or high-risk prostate cancer treated with androgen deprivation therapy and radiotherapy. Cancer. May 2018. doi: 10.1002/cncr.31400 [DOI] [PubMed] [Google Scholar]
- 27.Royce TJ, Chen M-H, Wu J, et al. Surrogate End Points for All-Cause Mortality in Men With Localized Unfavorable-Risk Prostate Cancer Treated With Radiation Therapy vs Radiation Therapy Plus Androgen Deprivation Therapy: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2017;3(5):652–658. doi: 10.1001/jamaoncol.2016.5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choueiri TK, Xie W, D’Amico AV, et al. Time to prostate-specific antigen nadir independently predicts overall survival in patients who have metastatic hormone-sensitive prostate cancer treated with androgen-deprivation therapy. Cancer. 2009;115(5):981–987. doi: 10.1002/cncr.24064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang S-P, Bao B-Y, Wu M-T, et al. Significant associations of prostate-specific antigen nadir and time to prostate-specific antigen nadir with survival in prostate cancer patients treated with androgen-deprivation therapy. Aging Male. 2012;15(1):34–41. doi: 10.3109/13685538.2011.580398 [DOI] [PubMed] [Google Scholar]


