Abstract
Cardiovascular diseases (CVDs) are the leading cause of death worldwide. Heart attack and stroke cause irreversible tissue damage. The currently available treatment options are limited to “damage-control” rather than tissue repair. The recent advances in nanomaterials have offered novel approaches to restore tissue function after injury. In particular, carbon nanomaterials (CNMs) have shown significant promise to bridge the gap in clinical translation of biomaterial based therapies. This family of carbon allotropes (including graphenes, carbon nanotubes and fullerenes) have unique physiochemical properties, including exceptional mechanical strength, electrical conductivity, chemical behaviour, thermal stability and optical properties. These intrinsic properties make CNMs ideal materials for use in cardiovascular theranostics. This review is focused on recent efforts in the diagnosis and treatment of heart diseases using graphenes and carbon nanotubes. The first section introduces currently available derivatives of graphenes and carbon nanotubes and discusses some of the key characteristics of these materials. The second section covers their application in drug delivery, biosensors, tissue engineering and immunomodulation with a focus on cardiovascular applications. The final section discusses current shortcomings and limitations of CNMs in cardiovascular applications and reviews ongoing efforts to address these concerns and to bring CNMs from bench to bedside.
Keywords: Carbon nanomaterials, Cardiovascular disease, Drug delivery, Biosensors, Cardiac tissue engineering, Immunomodulation
Graphical abstract
Highlights
-
•
Unique properties of the carbon nanomaterials (CNM) for cardiovascular theranostics.
-
•
Applications of CNMs for cardiovascular medicine as drug carriers, biosensors, cardiac tissue engineering constructs and immunomodulation.
-
•
Limitations of CNMs in cardiovascular theranostics applications.
-
•
Strategies to mitigate the challenges to bring CNMs from bench to bedside for cardiovascular theranostics.
1. Introduction
Cardiovascular diseases (CVDs) are the leading cause of death world-wide. The primary cause of CVDs is atherosclerosis that can result in ischemic heart disease (IHD), cerebrovascular disease, mesenteric ischemia, renovascular disease and peripheral vascular disease (PAD) [[1], [2], [3]]. In the heart, atherosclerosis causes blockage of epicardial coronary arteries by plaque deposition or blood clot formation, reducing and/or interrupting blood flow to the heart muscle. This ultimately results in a myocardial infarction (MI), commonly known as a heart attack which can cause permanent damage to the heart due to a massive loss of functional cardiomyocytes. Intrinsic repair mechanisms prevent cardiac rupture through the creation of a fibrotic scar at the infarct site. However, the scar tissue is non-contractile and MI can result in heart failure due to insufficient pump function [4,5]. Heart failure is associated with high mortality rates and immense financial strain for patients. Currently available pharmacologic and procedural interventions are entirely palliative measures that do not address the fundamental problem of cardiomyocyte loss and reduced contractile area [[6], [7], [8]]. This is a huge gap that needs to be bridged through regenerative medicine and tissue engineering [9].
Tissue engineering uses a combination of biomaterials, signalling molecules and cells to promote regeneration of a target tissue/organ. Biomaterials, in particular, are rapidly gaining popularity in biomedical research and development. These specialized materials can directly interact with the biological system and support tissue formation by mimicking the native extracellular matrix [10,11]. The advancements in nanotechnology to generate nanoscale biomaterials has further increased the theranostic potential of this field [12,13].
Carbon nanomaterials (CNMs) are an elite class of biomaterials with distinctive structural and physiochemical properties. CNMs have been studied as potential drug carriers, biosensors, and in tissue engineering applications. Some of the prominent CNMs used in biomedical engineering are graphene, carbon nanotubes (CNTs), fullerene and carbon dots [[14], [15], [16]]. In this review, CNMs (including graphene, CNTs, and their derivatives) will be discussed in the context of cardiovascular theranostics. First, we will review the synthesis and the unique physicochemical properties of each material. Next, we will discuss existing efforts to use these materials in the field of cardiovascular theranostics. Finally, we will discuss the shortcomings of these materials and the current efforts underway to address these concerns.
2. Carbon nanomaterials
The synthesis of first carbon nanomaterial, fullerene, in 1985 revolutionized the application of carbon-based materials in all areas of research. It was quickly realized that different carbon allotropes have different arrangements of sp2 hybridized carbon atoms, giving each of them unique physical, electrical, and optical properties. The other carbon allotropes, CNTs and graphene were first synthesized in 1991 and 2004 respectively [17,18]. The CNMs have large surface area, high mechanical integrity, and excellent electrical and thermal conductivity [16,19]. These characteristics make them excellent choice compared to other biomaterials for cardiovascular theranostics. In the following sections, some of features of the most commonly employed carbon allotropes such as graphene and CNTs as well as their derivatives are discussed with respect to their potential applications in cardiovascular theranostics.
2.1. Graphene
Graphene is one of the most widely used carbon allotrope in biomedical applications [20]. Its characteristic structure contains a uniform 2D arrangement of carbon atoms in a honeycomb-like pattern. The physiochemical properties of graphene arise from the long range π-conjugation bonds which yield excellent mechanical, thermal, and electrical properties. Graphene is 1 atom thick with an elastic modulus ranging from 0.5 to 1 TPa and has an ultimate tensile strength of 130 GPa. In addition, weak Van Der Waals force of attraction among different layers of graphene makes it soft and lightweight, increasing its potential for application in medical nanotechnology [18,[21], [22], [23]].
Graphene also has many derivatives which are gaining recognition for their importance in various applications. Graphene oxide (GO), reduced graphene oxide (rGO), and graphene quantum dots (GQDs) are examples of commonly available graphene derivatives [24,25]. The major limitation of pristine graphene is its hydrophobic nature and its unstable homogenous dispersion, which prevents its application in many areas of biomedical research [25]. Therefore, GO and rGO are more commonly utilized in medical applications. GO is most commonly obtained through the modified Hummers' method, which involves the oxidation process of the base material ‘graphite’ and its subsequent exfoliation. Upon oxidation, hydroxyl groups are functionalized on the GO sheets increasing the interlayer space and thereby allowing them to be hydrophilic in nature. This property permits them to interact electrostatically in an aqueous solution, making it distinct from the hydrophobic pristine graphene [26,27] Furthermore, the open hydroxyl groups in the edges and periphery of GO nanosheets provide functionalization capabilities, flexible processability, amphiphilic nature, and fluorescent quencher ability, which help in creating better composites for various biomedical applications including cardiovascular theranostics [28].
In the reduction process of GO, functional oxygen groups are removed and its reduced form, rGO, is obtained. This is mostly done to mimic the properties of pristine graphene along with GO's hydrophilicity. But the defects in rGO during synthesis makes it a distinct carbon allotrope of its own not resembling either of the two carbon materials [29,30]. Finally, another derivative of graphene is graphene quantum dots (GQD), which are synthesized either by the top-down or bottom up approach. They have superior electrical and optical properties compared to pristine graphene [31,32].
Graphene nanomaterials have high surface area and the presence of interactive functional groups such as –COOH, –OH, and –COC allows its functionalization with other organic or inorganic molecules. They can also be covalently or non-covalently functionalized with a wide range of FDA approved polymers to reduce their toxicity and improve their specificity/selectively to interact with cells of interest including cardiac cells [24]. Table 1 shows some of the applications of graphene and its derivatives in cardiovascular tissue engineering over the past 5 years. The multifaceted advantages and unique properties of graphene and its derivatives make them better candidates than other biomaterials for drug/gene delivery, biosensing, tissue engineering, and immunomodulatory applications in CVDs.
Table 1.
Graphene and its derivatives for cardiovascular applications over the past 5 years.
| Graphene composite | Field of application | in vitro/in vivo validation | Significant Outcomes | References |
|---|---|---|---|---|
| Glassy carbon electrodes (GCE)/Nitrogen doped porous rGO | Biosensor | Human serum | The detection range of cardiac troponin I was between 0.001 and 100 ng/ml | [33] |
| Indium–Tin Oxides electrode/Manganese doped CdS sensitized graphene/Cu2MoS4 | Biosensor | Human serum | The linear detection range of cardiac troponin I was 0.005–1000 ng/ml | [34] |
| Cu nanoparticles/laser induced graphene | Biosensor | Human serum | The detection limit was between 0.001 and 6 mM for glucose | [35] |
| Carbon coated screen-printed electrodes/Au nanoparticles/rGO/Myoglobin Ab | Biosensor | Human serum | The range of detection for cardiac myoglobin was between 1 ng/ml to 1400 ng/ml | [36] |
| GCE/CeO2–Au nanofibers/GO | Biosensor | Human serum | A wide detection range of 0.01–1000 μmol/l for amlodipine drug | [37] |
| GCE/N-GNRs-Fe-MOF@AuNPs and AuPt-Methylene blue nanorod/Gal-3 | Biosensor | Human serum | The biosensor has a better linear range (0.0001–50 ng/ml) of galectin-3 detection when compared to ELISA. | [38] |
| rGO/Gelatin methacryloyl (GelMA) hybrid hydrogel | Tissue engineering | Neonatal rat ventricular cardiomyocytes (NRVMs) |
Better mechanical, electrical properties, cellular attachment and maturation. | [39] |
| GO-Reverse Thermal Gel | Tissue engineering | NRVMs | Gelation at 37 °C with improved cellular adhesion, proliferation and survival. | [40] |
| rGO–reinforced gellan gum hydrogel | Tissue engineering | Rat myoblast (H9C2) | Mechanical properties similar to the native myocardium with no cellular toxicity. | [41] |
| GO/silk fibroin hydrogel | Tissue engineering | Cardiac progenitor cells & human coronary artery endothelial cells |
Cytocompatibility, improved proliferation and survival on the hydrogel | [42] |
| Partially reduced graphene oxide foam chip | Tissue engineering | NRVMs | Improved cell attachment, spreading, organization and beating function | [43] |
| Heparin loaded polycaprolactone/chitosan/polypyrrole/functionalized graphene cardiac patch | Tissue engineering | Embryonic stem cell derived embryoid bodies | Improved mechanical and electrical properties with biocompatibility | [44] |
2.2. CNTs
CNTs are another major carbon allotrope which are being used in industry, medical and electronic applications due to their peculiar mechanical and electrical properties [45,46]. CNTs were discovered by Sumio Iijima, a Japanese scientist in 1991 using the arc discharge method [47]. After years of optimization, chemical vapor deposition and laser ablation techniques are currently used to develop CNTs with high purity and yield [48]. While both methods use amorphous carbon as a starting material, due to difference in the source of energy, the end product varies in physiochemical properties. Other less commonly used methods for synthesizing CNT include the flame synthesis method and the ‘high pressure CO’ process [49,50]. All the methods rely on recombination of individual carbon atoms to form carbon tubes as the final product.
CNTs have high aspect ratio with diameter of around 1 nm and length in micron scale [51]. CNTs can exhibit three different configurations: armchair, chiral and zigzag. The specific structural configuration of CNTs confers their electrical properties, ranging from pseudo-metallic to semi-conductive depending on their chirality [52]. Pristine CNTs are hydrophobic in nature with poor aqueous solubility, thereby having a high tendency to aggregate due to van der Waals's interaction between neighbouring nanotubes. Surface functionalization of CNTs with OH, COOH or NH2 groups enhances their solubility and dispersion in aqueous media. Further functionalization with biocompatible polymers can also address the toxicity concerns raised by pristine CNT usage in biomedical applications [53].
Geometrically, CNTs are classified into two types: single walled CNTs (SWCNTs) and multi-walled CNTs (MWCNTs). SWCNTs have only a single outer wall with a diameter ranging 1–2 nm whereas MWCNTs have multiple graphene sheets wrapped around the inner core, with an outer diameter ranging from 2 to 100 nm and an inner diameter of about 1–3 nm [54]. The major advantage with MWCNT over SWCNT is that the former can be functionalized with ease due to abundant defects present in the concentric layers [55]. Overall, the high elastic moduli, light weight, stability, and electrical conductivity of CNTs make them an ideal class of materials for various applications of cardiovascular tissue engineering. Table 2 shows some of the applications of CNT composites in cardiovascular tissue engineering over the past 5 years.
Table 2.
CNTs and their derivative for cardiovascular applications.
| CNT composite | Field of application | In vitro/in vivo validation | Significant Outcomes | Reference |
|---|---|---|---|---|
| MWCNTs/dipyridamole | Drug delivery | Varied pH solutions (Hydrochloric acid & PBS) | Improved dissolution rate and controlled release of the drug | [56] |
| GCE coated poly styrene/Carboxylated-MWCNT/whiskered nanofibers/HRP-anti-cTnI | Biosensor | Human serum & PBS solution | The linear detection limit of cTnI was between 0.5 ng/ml – 100 ng/ml | [57] |
| GCE/carboxyl-terminated ionic liquid and helical carbon nanotubes/anti-cTnI antibodies | Biosensor | Human serum | The linear range limit for cTnI antigens was between 0.01 and 60.0 ng/ml | [58] |
| Au/ZnO/MWCNTs | Biosensor | Human serum | The detection range of cholesterol was between 0.1 and 100 μM | [59] |
| Chitosan/polyvinyl alcohol/MWCNTs nanofibers | Tissue engineering | Somatic stem cells | Improved tensile and electrical properties and increased cardiac gene expressions (troponin I, CX43) | [60] |
| SWCNT/Collagen hydrogel | Tissue engineering | NRVMs | Better mechanical strength and electrical conductivity with improved cellular assembly & alignment | [61] |
| SWCNT/gelatin with methacrylate anhydride (GelMA) | Tissue engineering | NRVMs | Improved cellular alignment, assembly, adhesion and maturation | [62] |
| Hydrazide functionalized/MWCNT/pericardial matrix hydrogel | Tissue engineering | Human mesenchymal stem cells (hMSCs) & human induced pluripotent stem cell derived cardiomyocytes (iPSC-CMs) | Promoted proliferation of hMSCs and improved the maturation of iPSC-CMs | [63] |
| MWCNTs/silk fibroin electrospun fibers | Tissue engineering | NRVMs | Enhanced conductivity and tensile strength with improved cell alignment and Cx43 protein expression | [64] |
| 3D printable SWCNTs ink/bacterial nanocellulose cardiac patch | Tissue engineering | Canine model | Similar conductivity and elasticity when compared to the native myocardium Restored the conduction in the disrupted myocardial conduction system |
[65] |
| Tissue–engineered blood vessels/C-SWCNT/Resveratrol | Immune modulation | Vascular smooth Muscle cells (VSMCs) /SD rats |
Polarized the shift of M1 inflammatory macrophage to M2 reparative macrophages along with preserved contractile phenotype of VSMCs | [66] |
3. Application of CNMs in cardiovascular tissue engineering
3.1. Drug/biomolecule delivery
Drug delivery is an important area of research in cardiovascular medicine. Pharmacologic therapy, given orally or intravenously, is a mainstay therapy for the prevention and treatment of cardiovascular diseases [67]. Common problems faced by the conventional drug delivery approaches include poor bioavailability of the drug, frequent dosing to achieve therapeutic efficacy and off-target effects. These factors pose significant challenges in optimizing the therapeutic benefits of drug therapies. Therefore, novel nanoscale drug delivery systems are highly desirable to address these concerns.
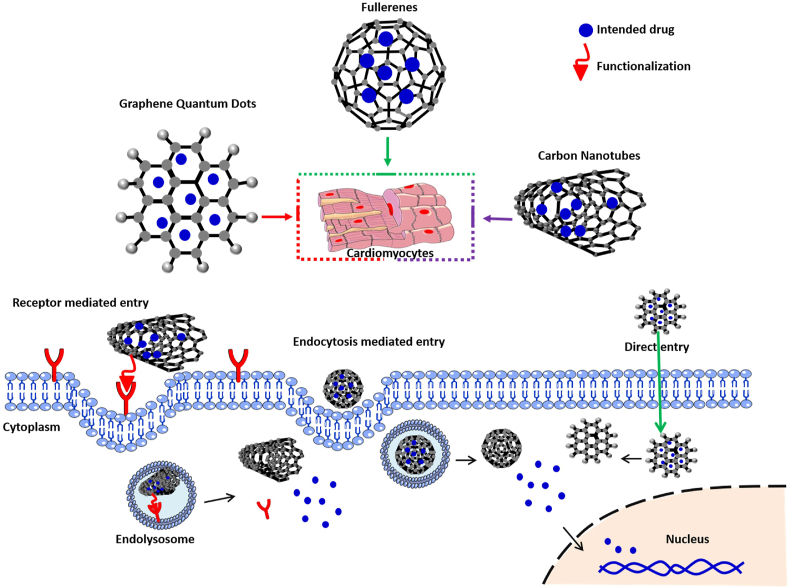
Nanomaterials have been explored as drug delivery carriers for localized and controlled delivery of drugs to the target site. In cardiovascular medicine, conventional drugs or novel synthetic compounds are delivered to reduce atherosclerotic lesion formation, promote angiogenesis in ischemic tissues and improve the remodelling process in the damaged organs [[68], [69], [70]]. In the emerging area of nanotechnology, carbon allotropes are considered as one of the cardinal classes, which have gained significant attention in drug delivery applications [71,72]. The advantages of CNMs over many other biomaterials as drug carriers are their nanoscale size range and facile processability to conjugate large amount of therapeutics [73]. Fig. 1 shows commonly used CNMs for drug delivery. This figure also emphasizes on various transport approaches CNMs undertake in order to effectively deliver the biomolecule of interest to the target cells in the heart. The mechanisms of entry of these biomaterials into the cell depends mainly on their size and surface chemistry. For instance, bulky CNMs such as CNTs undergo receptor mediated cell entry whereas GQDs can directly enter the cell membrane due to their small size [[74], [75], [76]]. In this section, carbon-based nanomaterials such as graphene, CNT and their derivatives will be discussed in detail as drug/biomolecule carriers for cardiovascular engineering.
Fig. 1.
Schematic representation of different mechanistic approaches employed by CNMs as carriers to deliver drugs/gene into the cardiomyocytes.
3.1.1. Graphene and its derivatives as drug/biomolecule carriers
Graphene derivatives possess ultra-high surface area, high mechanical strength, facile hydrophilic and hydrophobic drug loading capabilities [77,78]. Further, graphene derivatives are capable of facilitating targeted localized delivery of the drugs without losing the efficacy. These properties make graphene and its derivatives better candidates than many other biomaterials for drug delivery. While pristine graphene is hydrophobic and has limited ability to be used as a drug carrier, GO and rGO are preferred as carriers to load drugs. These materials have easy processability to functionalize drugs with high loading capacity compared to other graphene-based biomaterials. These properties are utilized to effectively deliver both organic and inorganic biomolecules to the target areas [79]. In a study by Kaya et al., an electroconductive hydrogel was prepared by reinforcing rGO into a hyaluronic acid (HyA)-gelatin-poly (ethylene oxide) (PEO) hydrogel to study the drug release kinetics of irbesartan. Irbesartan belongs to the class of angiotensin receptor blockers which are used to treat high blood pressure in CVD patients. The study involves the application of mathematical models to simulate and understand the release kinetics of irbesartan from the electroconductive hydrogel. It was reported that the hydrogel containing 20% rGO was able to effectively release irbesartan in a controlled fashion for a 10-day period. This composite shows the efficacy of rGO in the hydrogel as a drug carrier for the treatment of CVDs [80]. Similarly, in a study by Sarkar et al., an effective drug carrier membrane was prepared with GO and methylcellulose (MC) to study the release kinetics of diltiazem. Diltiazem is a calcium channel blocker used to manage arrhythmias and treat hypertension. This study demonstrated the importance of GO/MC composite for efficient and controlled drug release [81].
Occlusion of blood vessels is a serious concern arising as a result of atherosclerosis. Therapeutic angiogenesis is one such treatment that offers development of new blood vessels from the pre-existing one [82]. Vascular endothelial growth factor (VEGF) is a soluble factor demonstrated to exert therapeutic benefits by inducing angiogenesis in ischemic cardiac tissue [83,84]. To facilitate effective delivery of VEGF, it was functionalized with GO to improve the half-life and availability of VEGF to improve angiogenesis in ischemic muscle tissue. In this study, hindlimb ischemia model in Balb/c mice was used to mimic conditions found in peripheral arterial disease (PAD) [85]. VEGF was delivered with or without GO, it was reported that there was a significant increase in blood vessel density and oxygen availability in the ischemic muscle in VEGF-GO group compared to only VEGF group. This study also reported that VEGF loaded GO specifically targeted VEGF receptors in the ischemic tissues of the hind limb demonstrating the therapeutic benefits of presence of GO in the formulation.
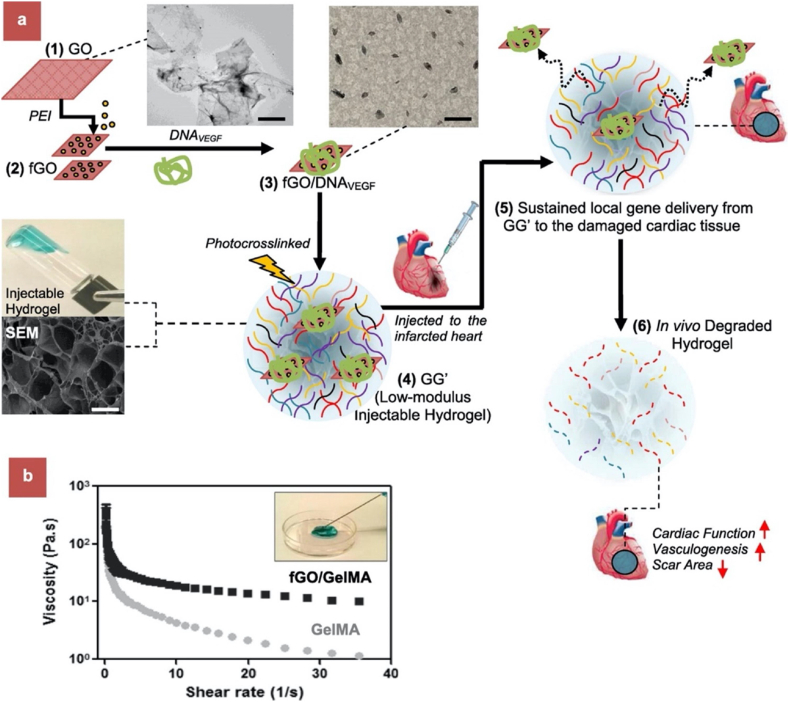
In a similar study, Paul et al. [86] demonstrated controlled and localized delivery of a plasmid containing VEGF-165 pro-angiogenic gene. Fig. 2 depicts the overall process of the study from the drug carrier design to its functional evaluation in a MI model for improved cardiac performance. Basically, the gene carrier was composed of polyethyleneimine (PEI) to bind VEGF gene, functionalized GO (fGO) and methacrylated gelatin (GelMA) composite for its application in acute myocardial infarction. A rat MI model was employed to evaluate the cytotoxic and the therapeutic effect of the fGOVEGF/GelMA hydrogel when injected intramyocardially into the infarct region for 14 days. The fGOVEGF/GelMA group had increased capillary density formation when compared to sham and DNAVEGF/GelMA groups. There was also a decrease in scar size and an increase in fractional shortening (FS) after 14 days in the fGOVEGF/GelMA group in this study.
Fig. 2.
Preparation of injectable hydrogel for acute myocardial infarction (AMI) therapy. (a) Schematic of stepwise formulation process of bioactive hydrogel and subsequent injection to treat damaged heart with acute myocardial infarction. (1) First, GO nanosheets are functionalized by amide bond with branched PEI to form cationic fGO. (2) fGO is then surface functionalized with anionic plasmids (DNAVEGF) to form fGO/DNAVEGF as shown in TEM images. (3) These bioactive hybrids are then suspended in prepolymer of GelMA hydrogel and UV cross-linked under optimized condition to form hydrogel (4) injectable fGO/DNAVEGF carrying GelMA hydrogel (GG′). (5) The latter is then intramyocardially injected in rat heart with AMI for local gene delivery of incorporated fGO/DNAVEGF nanocomplexes. (6) The hydrogel exhibits therapeutic effects by promoting myocardial vasculogenesis, which leads to reduced scar area and improved cardiac function. (b) Injectability of the developed GO carrying GelMA hydrogel. The viscosity of GO/GelMA nanocomposite hydrogels was monitored at different shear rates. At low shear rate, both fGO/GelMA and GelMA hydrogels had high viscosity. However, at higher shear rate, fGO/GelMA and GelMA hydrogels showed decreased viscosity. This suggests that both GelMA and fGO/GelMA were able to flow at higher shear rate and were easily injectable. The results also indicate that the addition of surface functionalized fGO to GelMA results in higher viscosity of fGO/GelMA at higher shear rate compared to GelMA. In other words, fGO reinforces the GelMA hydrogel network. Scale bar: 1 μm(reproduced with permission from Ref. [86], © American Chemical Society, 2020).
One of the major disadvantages associated with GO-based drug delivery is their toxicity towards cells. This can be overcome by functionalizing them with other biocompatible nanocomposites to make a well-dispersed and nontoxic drug carrier [87,88]. Ginsenoside is a natural glycosidic nanocomposite derived from ginseng. It possesses intrinsic anti-inflammatory and antihypertensive properties [89]. Zardini et al. functionalized GO with ginsenoside (Rh2) and evaluated the toxicity of GO in cardiac tissue after transplantation in a rat model. It was reported that presence of ginsenoside mitigated the toxic effects of GO and retained its therapeutic value [90]. These studies demonstrate that GO and rGO hold excellent potential as drug carriers for cardiovascular applications. This is due to the unique properties such as high drug loading capacity because of large surface area, hydrophilicity, ease of functionalization and their ability to effectively internalize into the target cells [82]. However, future studies focusing on further improvements in terms of biocompatibility of these materials will add tremendous value to the therapeutic potential of these biomaterials.
3.1.2. CNTs and their derivatives as drug/biomolecule carriers
CNTs are another class of biomaterials which are being exploited for drug delivery. The high aspect ratio of CNTs is responsible for their efficient drug loading capacity and controlled release of drug to the target site [91]. In addition to these properties, the ease of functionalization with covalent or non-covalent modification in CNTs offers ways to engineer superior structural and chemical complexes which can be employed in delivering drug/biomolecules [92]. Pristine SWCNTs and MWCNTs are not employed because of their poor dispersibility with minimal drug loading capacity. Among the two CNTs, SWCNTs have a much better drug loading efficiency due to their high surface area compared to MWCNTs [93]. There are many strategies to functionalize drugs in CNTs, including doping, a common strategy to improve the adsorption of drugs on the surfaces of CNTs [94,95]. In a study Namin et al. used density functional theory (DFT) to study the adsorption efficiency of a drug, metformin, in gaseous and aqueous environments in pristine SWCNT, silicon (Si), and aluminium (Al)-doped (5, 5) SWCNTs. Metformin is a very effective antihyperglycemic drug, which is widely used to treat type 2 diabetic patients. It was reported that, Al-doped SWCNT and Si-doped SWCNTs showed the maximal absorption rate and maximal sensitivity towards metformin when compared to pristine CNT [96]. Similarly, in another study, the adsorption of resveratrol (RSV) on CNT was evaluated using DFT in gaseous phase [97]. RSV is a polyphenolic compound which has been reported as a cardioprotective agent [98,99]. In this study, it was reported that the CNTs have high sensitivity towards RSV through non bonded interactions which makes them a suitable drug carrier for safe and controlled release of RSV [97]. CNTs are also reported as carriers to deliver angiogenic factors to the ischemic tissues to promote angiogenesis [100,101].
In another study Masotti et al. functionalized CNT with PEI/polyamidoamine dendrimer (PAMAM) to deliver miR-503 oligonucleotides to promote angiogenesis during CVD. It was reported that CNTs were able to effectively deliver miR-503 and improve angiogenesis [102]. Further, CNTs have also been used to coat stents to deliver angiogenic molecules to prevent in-stent restenosis. After stent implantation at the site of blockage in the coronary artery, there is a high chance of occurrence of endothelial dysfunction along with increased smooth muscle cell proliferation around the stent. This results in rapid reocclusion of the coronary artery and can be associated with repeated heart attacks or recurrent symptoms. To overcome this complication, Paul et al. devised a polyacrylic acid (PAA) coated SWCNT in a fibrin hydrogel that contained VEGF and angiopoietin-1 with endosomolytic Tat peptide. The efficacy of this composite was tested in a canine model. This biohybrid stent improved reendothelization, halted neointima formation and prevented stenosis at the injured artery segment [103]. In a study Renyun et al. compared various CNMs such as MWCNTs, GO and carbon black as drug carriers. This study reported that GO had the highest loading capacity compared to other CNMs. This study also demonstrated that other factors such as pH and functionalization played significant role in deciding release profile of the drug [104]. More comparative studies like this one are needed to understand the role of different CNMs in drug delivery and how they can be optimized effectively for controlled and safe release of drugs to the cardiovascular system.
3.2. Biosensors
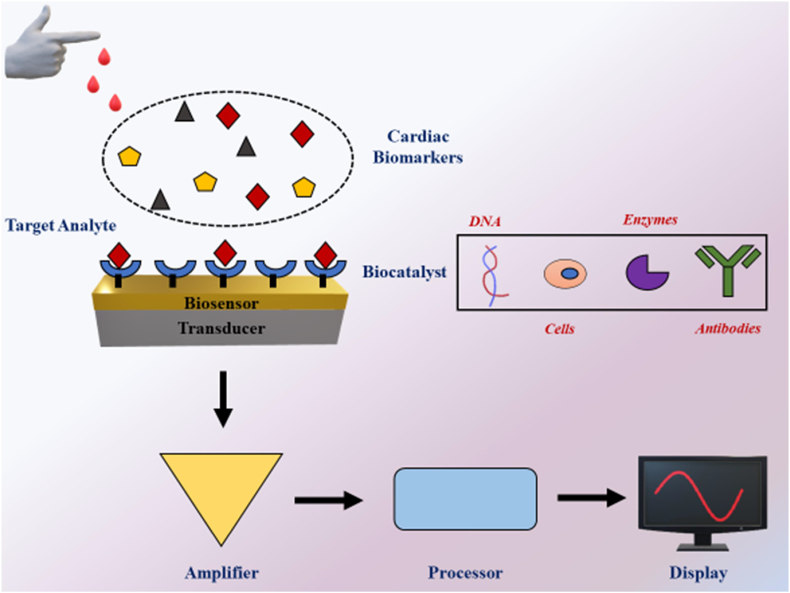
Biosensors are promising tools that can be used for early detection of cardiac biomarkers and continuous monitoring of CVDs to allow for the timely treatment and preservation of heart function [[105], [106], [107]]. A wide range of biochemical molecules such as metabolites, proteins, and nucleic acids can be detected using biosensors [108]. Biosensors are composed of a biocatalyst and a transducer. The former is used to detect biological analyte of interest and the latter is used to record the interaction of the biocatalyst and the target biological analyte. A biocatalyst can be any biomolecule (including nucleic acids, antibodies, proteins and immobilized cells) that has sensitivity towards a given target analyte. Electrochemical and optical sensors are the two major types of transducers employed to quantify the concentration of target analytes [109,110]. The use of nanotechnology in biosensors has improved their sensitivity and robustness by providing a larger surface-to-volume ratio for better conjugation of biocatalysts. Furthermore, electroconductive nanomaterials can be used as both a sensing element and a transducer in a biosensor [111,112]. Some of the nanomaterials commonly used in the management of cardiovascular diseases as biosensors are gold nanoparticles, CNMs and zinc oxide. Due to their low cost, high surface to volume ratio, smaller size, and unique electrical and optical properties, CNMs are ideal candidate to be used as sensing materials in the diagnosis of CVD [[113], [114], [115], [116]]. Fig. 3 shows the schematic representation of CNMs in diagnosis of CVD.
Fig. 3.
Application of CNMs in biosensors for diagnosis of CVDs. Model demonstrates the detection of cardiac biomarkers in blood of CVD patients. The sensors have biocatalysts such as DNA, enzymes, antibodies present on the surface which are used to detect specific cardiac biomarkers. The signals are then amplified and processed to display the value.
3.2.1. Graphene and its derivatives as biosensors
The movement of free electrons and the arrangement of carbon atoms in 2D hexagonal planar structure of graphene is responsible for its electrical conductivity, high surface area and robust mechanical strength [117,118]. These properties collectively provide graphene the biosensing ability to detect biomarkers under disease conditions.
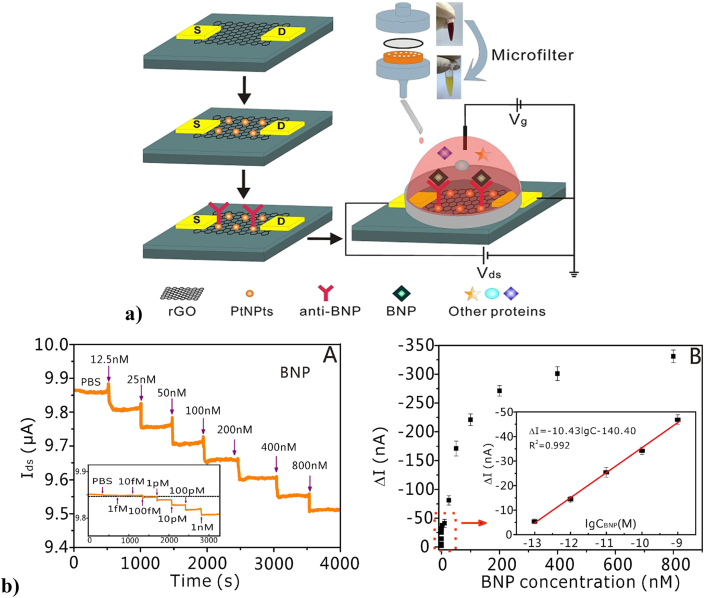
In AMI, several macromolecules (biochemical markers) such as myoglobin, creatine kinase, B type-natriuretic peptides (BNP), and cardiac troponins, are released from dying cardiomyocytes during a stress or injury [120,121]. The robustness and sensitivity of biosensors to detect minute levels of these circulating biomarkers is the key in early diagnosis of the disease. In a study by Demirbakan et al., an ultrasensitive graphite paper electrode was modified by hydrochloric acid and functionalized with cardiac troponin T antibody (cTnT) to detect circulating levels of cTnT in human serum. This electrode demonstrated excellent sensitivity and has surface stability similar to a gold electrode and was able to detect cTnT in sub-femtogram level as well [122]. Similarly, Kazemi et al., prepared a glassy carbon electrode (GCE) coated with porous GO (pGO) which was further functionalized with human cTnI antibodies to detect cTnI antigens in human serum. The GE/pGO/cTnI immunosensor was found to be sensitive and specific to the human cTnI antigen where the dynamic linear range was around 0.1–10 ng/ml. Though, the sensitivity of the immunosensor towards cTnI was similar to the conventional enzyme-linked fluorescence assay, the detection speed was much faster in the biosensor [123]. Another important derivative of graphene, rGO, was also used as a sensor in a study by Lei et al., Fig. 4a depicts a schematic representation of the design of biosensor in this study. The biosensor was composed of reduced graphene oxide and was decorated with platinum nanoparticles and anti-BNP antibodies to detect BNPs in whole human blood. This immunosensor was able to detect BNP at as low as 100 fM. Fig. 4b shows the sensitivity of the biosensor towards different amounts of BNP antigen in PBS. The sensitivity of this biosensor was better than the other commonly available conventional methods such as ELISA, surface plasmon resonance and electrochemical method. Further, this biosensor was highly specific towards BNP in human blood samples of CVD patients [119].
Fig. 4.
a) Schematic illustration of the PtNPs-decorated rGO FET biosensor with a custom-made microfilter for BNP detection. b). (A) Real-time electrical detection at different concentrations of BNP solution in PBS (12.5 nM, 25 nM, 50 nM, 100 nM, 200 nM, 400 nM, 800 nM). Inset: Real-time electrical detection at lower concentrations of BNP. (B) The response of the PtNPs-decorated rGO biosensors to BNP at different concentrations. Inset: The response of the biosensors to BNP at low concentration range (100 fM, 1 pM, 10 pM, 100 pM, 1 nM) (Reproduced with permission from Ref. [119].© Elsevier Ltd 2020).
Graphene quantum dots (GQD) are nano sized zero dimensional derivative of graphene with excellent electrical properties which are required for sensors in CVD [124]. Lakshmanakumar et al., coated functionalized GQDs with acetic acid over a gold electrode to synthesize an electronic immunosensor to detect cTnI for AMI. Analytical techniques such as cyclic voltammetry and amperometry were used to evaluate the characteristics of the immunosensors. This study reported that the immunosensor was highly sensitive and was able to detect levels of cTnI in the range of 0.17–3 ng/ml in human serum. This study did not use antibodies to detect cTnI and addressed the limitations of antibody mediated antigen detection, including lower efficiency, poor recovery, high cost due to antibody use, and instability at high temperatures [125]. Metals and nanostructured metal chalcogenides have excellent electrocatalytic activity, mechanical stiffness, and fast charge transfer kinetics, making them ideal materials to dope or functionalize with graphene electrodes to improve their sensitivity and selectivity to detect cardiac biomarkers in CVD [126,127]. In a study by Chauhan et al., rGO was embedded with metal chalcogenide (molybdenum tetraselenide, nMo3Se4) in an indium tin oxide (ITO) coated glass electrode, which was further functionalized with anti-cTnI using bovine serum albumin (BSA) for detection of cTnI. The biosensing ability of the fabricated composite electrochemical immunosensor was validated with cyclic voltammetry studies with analytes from human serum. The result showed that the immunosensor had a wide linear range from 1 fg/ml-100 ng/ml and was stable for up to 35 days at room temperature. It also showed that the fabricated electrode was 9 times more sensitive compared to other ZrO2 based electrodes commonly used to detect cTnI [128]. These studies suggest that graphene and its derivatives have tremendous potential as diagnostic tools for early detection of heart failure in CVD patients. Graphene based biosensors’ possess unique electrochemical properties, and the ease of biomolecule adsorption through π–π stacking or electrostatic bonding makes them ideal for cardiovascular biosensing applications [129]. Future studies are needed to compare the sensitivity and robustness of different graphene based biosensors to find the best fit products for any specific application. The outcome of comparative analysis will also help the researchers and engineers to improve the existing products and manufacture new need based tailored products for biomedical applications.
3.2.2. CNTs and their derivatives as biosensors
The CNTs have drawn significant attention as biosensors for various medical applications owing to their unique properties. CNTs have extremely sensitive surfaces which can be used to detect specific analytes. They can also be attached with bioactive molecules on the surface to increase the sensitivity towards cardiac biomarkers. The high surface area, excellent electrical and mechanical strength, and electrochemical stability in fluids make CNTs a good candidate for nano biosensor applications [130,131]. However, the intermolecular π-π interactions among the carbon layers in CNTs make these composites hydrophobic in nature. To this end, functionalization of CNTs by chemical adsorption or doping can be used to generate stable and hydrophilic nanomaterials suitable for biosensor applications [[132], [133], [134]]. In 2014, Freitas et al., developed an amino-functionalized MWCNT based carbon electrode to measure cTnT levels. The amino group allowed proper immobilization of the anti-cTnT antibodies on the surface of the CNT to improve its sensitivity to detect cTnT antigens. In human serum samples, the electrochemical immunosensor had a broad detection range of 0.02–0.32 ng/ml and a LOD of 0.016 ng/ml making it suitable to detect cTnT levels in vitro [135].
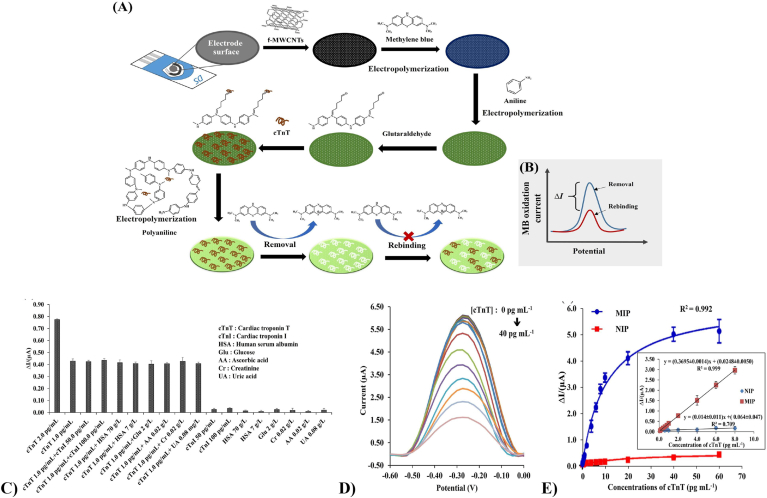
Similarly, Phonklam et al., devised an electrochemical biosensor where the carbon electrode was coated with MWCNTs and polymethylene blue (PMB) to improve the electrode surface area for cTnT attachment with improved redox reaction detection. In this study, polyaniline (PANI) was coated around cTnT antibody embedded onto the MWCNT/PMB immune sensor. Fig. 5A and B show step by step construction of the biosensor along with its voltage potential. Fig. 5C &E show the robustness and specificity of the biosensor towards cTnT antigens. Also, The PMB/MWCNTs sensor was reported to have a linear detection range of 0.10–8.0 pg/ml with a LOD of 0.040 pg/ml to measure the cTnT levels in human serum as shown in Fig. 5D [136].
Fig. 5.
Schematic illustration showing (A) the fabrication of the MIP sensor and (B) the binding detection. Sensor performance: (C) effects of interfering substances on the response of the cTnT-MIP sensors after the addition of cTnI, HSA, Glu, AA, Cr and UA in a solution containing 1.0 pg mL-1 cTnT and the different concentrations of cTnI, HSA, Glu, AA, Cr and UA were tested using the developed MIP sensor. (D) differential pulse voltammograms (DPVs) of the developed MIP sensor obtained with 0.0–40 pg mL−1 of cTnT in 0.10 mol l−1 PBS (pH 7.00). (E) plot of the change in current response (ΔI) versus concentration of cTnT of the MIP and NIP sensors fitted with the Langmuir isotherm using GraphPad Prism 8 and the inset showing the linear range (n = 3) (Reproduced with permission from Ref. [136].© Elsevier Ltd 2020).
Different CNMs (e.g. graphene and CNTs) based biosensors have unique properties that defines their performance and make them suitable for different applications. In a study, Shimaa et al., compared the performance of six commercial CNMs based electrodes (Carbon, carbon nanofibers, MWCNT, SWCNT, pristine graphene and GO) for detection of glycosylated haemoglobin levels. Even though all the biosensors showed good selectivity and sensitivity towards biomarkers, SWCNT based biosensor had the overall best performance [137]. More studies like this one on comparative analysis of different CNMs as biosensors for cardiovascular applications are needed to select the best product that can be effectively used for any particular type of CVD diagnosis. Furthermore, considering the advantages of CNT based biosensors, more in depth studies are required to understand the mechanisms of CNTs mediated CVD diagnosis so that the commercial products with improved efficacy can be manufactured for CVD diagnosis and management.
3.3. Tissue engineering
At the onset of myocardial infarction, ischemia results in massive cell death amongst functional cardiomyocytes in the infarct area. Even after restoration of normal blood circulation, the heart muscle does not have the ability to regenerate itself. Thus, disease progression often results in a permanent non-conductive and non-contractile scar in the infarct region. If large enough, this can adversely affect the pumping function of the heart that leads towards heart failure [138,139]. Conventional therapies do not provide a permanent solution to repair the damaged myocardium. As a last resort, heart transplantation can be employed for end-stage heart failure, but it is associated with many potentially life-threatening complications.
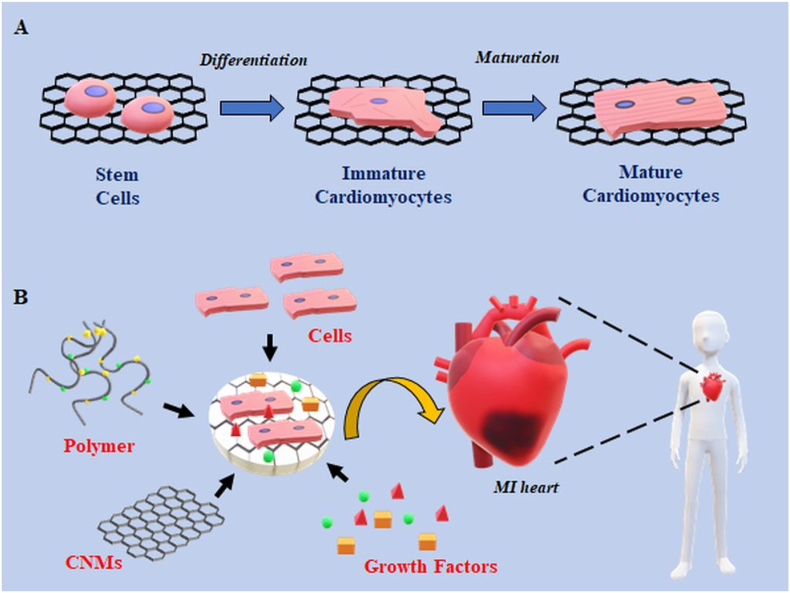
Cell-based therapies and tissue engineering approaches have the potential to offer permanent solutions to this problem. Tissue engineering utilizes cells and biomaterials to regenerate and/or replace the lost muscle tissue during a myocardial infarction. Advancements in the field of nanotechnology have further improved the prospects of cardiac tissue engineering by generating superior scaffolds, injectable hydrogels, and conductive cell-laden cardiac patches. For the latter, biomaterials also play an important role in providing the correct signalling cues and extracellular matrix (ECM) to support differentiation and maturation of stem cells into mature cardiomyocytes [140,141]. A successful scaffold mimics the structural and chemical composition of native ECM of the myocardium. The CNMs provide structural strength similar to collagen in the ECM and their conductive properties help to improve electrical conduction among remaining cardiomyocytes in the myocardium and transplanted stem cell derived cardiac cells [[142], [143], [144]]. Fig. 6 displays a model of CNMs based cardiac tissue engineering.
Fig. 6.
Overview of CNMs in cardiac tissue engineering. A) Application of CNMs in differentiation and maturation of stem cells derived cardiomyocytes. CNMs serve as reinforcers in scaffold/hydrogels which promotes differentiation of stem cells and maturation of stem cells derived cardiomyocytes. B) Construction of CNMs-based cardiac constructs for treatment in MI. CNMs provide mechanical strength and electrical conductivity to a cardiac patch which is comprised of cells, polymers and growth factors for effective myocardial repair during MI.
3.3.1. Graphene and its derivatives for cardiac tissue engineering
The extraordinary mechanical strength of graphene coupled with other characteristics such as electrical conductivity, stiffness and large surface areas provide cues for the stem cells to proliferate and differentiate. Graphene materials are also being used to prepare scaffolds to generate cardiac tissue constructs that can electromechanically couple with the host myocardium [145,146]. The structural arrangement of carbon in graphene nanosheets provides a large surface area which allows cells to grow and differentiate into cardiac cells. Graphene nanosheets also possess the ability to promote maturation of implanted stem cells derived cardiomyocytes. In a study by Ahadian et al., aqueous graphene was incorporated into embryoid bodies (EB–graphene) to induce cardiac differentiation. Embryoid bodies are formed from 3D aggregates of pluripotent stem cells. They can be differentiated into the three germ layers upon addition of appropriate growth factors. In this study, it was reported that the EB-graphene group had improved Young's modulus and electrical conductivity, which promoted better cardiac differentiation compared to the EB group alone. In addition, upon electrical stimulation, the EB-graphene group showed upregulation of the cardiac specific genes cardiac such as muscle alpha actin (ACTC1), myosin heavy chain alpha and beta (MYH6, MYH7), and cardiac muscle troponin T (TNNT2), with a significant improvement in spontaneous beat rates [147].
The electrical properties of an ideal CNM-based scaffold should mimic the electrical network of native myocardium. This will facilitate impulse propagation among adjacent cardiomyocytes and promote efficient contraction of the myocardium. Therefore, incorporation of electroactive materials in addition to CNMs in a nonconductive polymer/scaffold results in better electromechanical coupling of the cardiomyocytes, thereby improving the performance of the injured myocardium [144,148,149]. In a study by Nazari et al., reduced graphene oxide-silver (rGO-Ag) nanoparticles were embedded in a polyurethane (PU) scaffold to improve the mechanoelectrical properties of the composite. The scaffold was then seeded with human cardiac progenitor cells (hCPCs). In this study, it was found that the rGO-Ag/PU scaffold improved the survival and growth of hCPCs compared to PU group. It upregulated the expression of cardiac specific genes GATA4, TBX18, cTnT, and α-MHC compared to the PU composite [150]. Similarly, our lab recently synthesized a conductive scaffold by incorporating GO-AU nanoparticles in a chitosan (CS) scaffold, to improve its mechanical strength and electrical conduction. This GO-Au/CS scaffold showed no toxicity towards fibroblasts and smooth muscle cells in vitro. Also, in a rat MI model, GO-Au/CS scaffold implantation improved the conduction velocity and contractility with increased levels of connexin 43 (Cx43) expression in cardiomyocytes [151]. The outcome of these studies show the importance of conductive materials in scaffolds developed for cardiac tissue engineering.
In the myocardium, the cardiac ECM is highly elastic and flexible, which allows for cardiomyocytes to contract and relax upon electrical stimulation. Injectable hydrogels are 3D interconnected polymers which provides a natural environment for the cardiomyocytes to grow and beat synchronously. In addition, the shear thinning property of the injectable hydrogels allows them to safely inject the encapsulated cells into the damaged myocardium and effectively facilitate the repair process in MI [152,153].
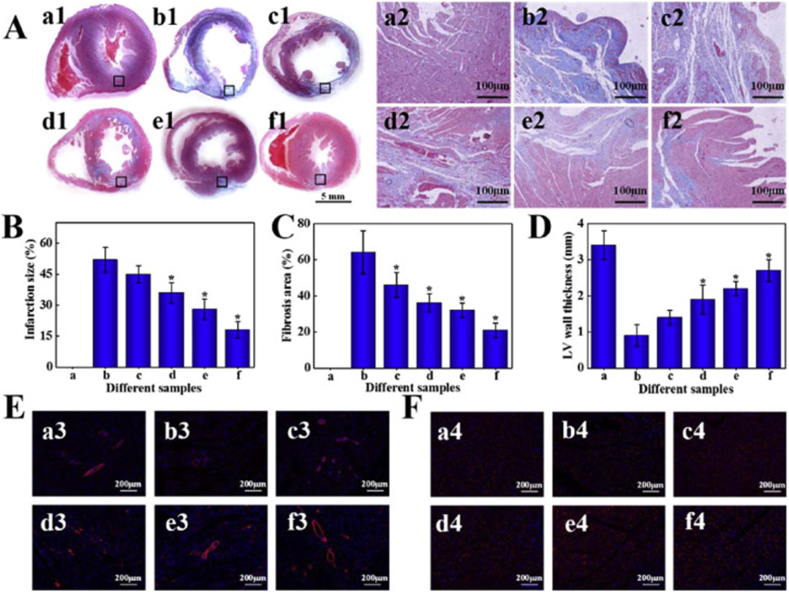
In a study by Bao et al., a soft injectable hydrogel was synthesized using melamine which was crosslinked to thiol-modified hyaluronic acid (Hya-SH) using a crosslinker PEGDA700. The PEG-MEL/Hya-SH hydrogel was then incorporated with GO to make it conductive. The PEG-MEL/Hya-SH/GO hydrogel was found to exhibit anti-fatigue mechanical and electrical properties, mimicking the myocardium. The electroconductive hydrogel was then encapsulated with adipose tissue-derived stromal cells (ADSCs) and injected intramyocardially into the infarct region in a rat MI model. As shown in Fig. 7, after 4 weeks, the cells in the hydrogel group showcased improved α-smooth muscle actin (α-SMA) and Cx43 expression when compared to the control groups. Also, there was a significant improvement in the ejection fraction, blood vessel density levels, reduced infarct size and fibrosis. Overall, the hydrogel effectively improved the electromechanical coupling of the injected cells thereby improving the cardiac performance in the infarcted heart [154]. In a similar study, GO was reinforced into oligo (poly(ethylene glycol) fumarate) (OPF) hydrogels to improve the mechanical strength and the electrical conduction among remaining healthy cardiomyocytes in an infarcted heart. The GO/OPF hydrogel was able to improve cardiac fibroblast attachment when compared to the nonconductive OPF hydrogel. In a rat MI model, the intramyocardial injection of GO/OPF hydrogel upregulated the expression of gap junctions related genes including Cx43, Cx40, Cx37 and Cx45 [155]. These examples suggest that graphene increases the mechanical and electrical properties of the polymer composites allowing functional cardiomyocytes to grow and mature for tissue engineering applications.
Fig. 7.
Cardiac repair by different treatments after 28 days (a, a1-a4: Sham; b, b1-b4: PBS; c, c1-c4: PEG-MEL/HA-SH; d, d1-d4: PEG-MEL/HA-SH/GO; e, e1-e4: PEG-MEL/HA-SH/ADSCs; f, f1-f4: PEG-MEL/HA-SH/GO/ADSCs). (A) Masson's trichrome staining for collagen (blue) and muscle (red); a2-f2 is magnification of the corresponding black box labelled in a1-f1. (B) Infarction size. (C) Fibrosis area. (D) LV wall thickness. (E) Immunofluorescence staining for α-SMA. (F) Immunofluorescence staining for Cx43. * indicates a significant difference between the experimental group and PBS treated group (Reproduced with permission from Ref. [154].© Elsevier Ltd 2020).
Biomaterial-mediated stem cell delivery has also been reported to promote angiogenesis and protect implanted cells from reactive oxygen species (ROS) in the infarct zone [156,157]. Norahan et al. synthesized a collagen and graphene oxide (Col–GO) patch to induce angiogenesis after MI. The addition of GO improved the tensile strength to mimic native myocardium, it also improved the conductivity of the Col-GO cardiac patch. The Col-GO patch had no toxicity towards human umbilical vein endothelial cells (HUVECs) after 96 h. Furthermore, the Col-GO patch showed enhanced gene expression of Cx43, Actn4 and TrpT-2 in rat neonatal cardiomyocytes [158]. Therefore, the presence of graphene and its derivatives in scaffolds and hydrogels promote cell survival, differentiation and induce angiogenesis after implantation. These are ideal characteristics of a biomaterial to synthesize a tissue construct for cardiac repair.
3.3.2. CNTs and their derivatives for cardiac tissue engineering
CNTs are electrically stable materials, can interact with electroactive tissue such as neural, cardiac and bone tissues [[159], [160], [161], [162]]. This property is extensively investigated in cardiac tissue engineering applications. This section majorly covers the role of CNTs in promoting cardiomyocyte differentiation and maturation of cardiomyocytes.
In a study by Ahadian et al., CNTs incorporated in a scaffold were able to enhance differentiation of embryoid bodies toward cardiomyocytes. This study reported a significant decrease in the expression of pluripotency markers (Nanog, Pou5f1, Klf4) and an increase in cardiac lineage markers (Nkx2–5, Tnnt2, Myl7, Actc1 and Actc2) in the presence of CNTs [163]. One limitation with the above-mentioned approach is the possibility of randomly dispersed CNTs to aggregate, thereby causing uneven mechanical strength and improper conductivity throughout the scaffold, which may later affect the growth conditions for the implanted cells. To address this issue, Ren et al. prepared a super aligned CNT (SA-CNT) in poly dimethylsiloxane (PDMS) to mimic the ECM of native myocardium. When neonatal rat ventricular cardiomyocytes (NRVMs) were cultured in the presence of SA-CNT, it led to improvements in the alignment and morphology of cells. In addition, when paced with electrical stimulation, the SA-CNT was able to reduce the spontaneous beating of CMs with improved contractility [164]. Furthermore, incorporation of CNTs in hydrogels and scaffolds creates electrically conductive next generation constructs for cardiac tissue repair. Both natural and synthetic polymers can be employed in these biomaterials to provide the necessary elasticity to support the cells and mimic native myocardium [165,166].
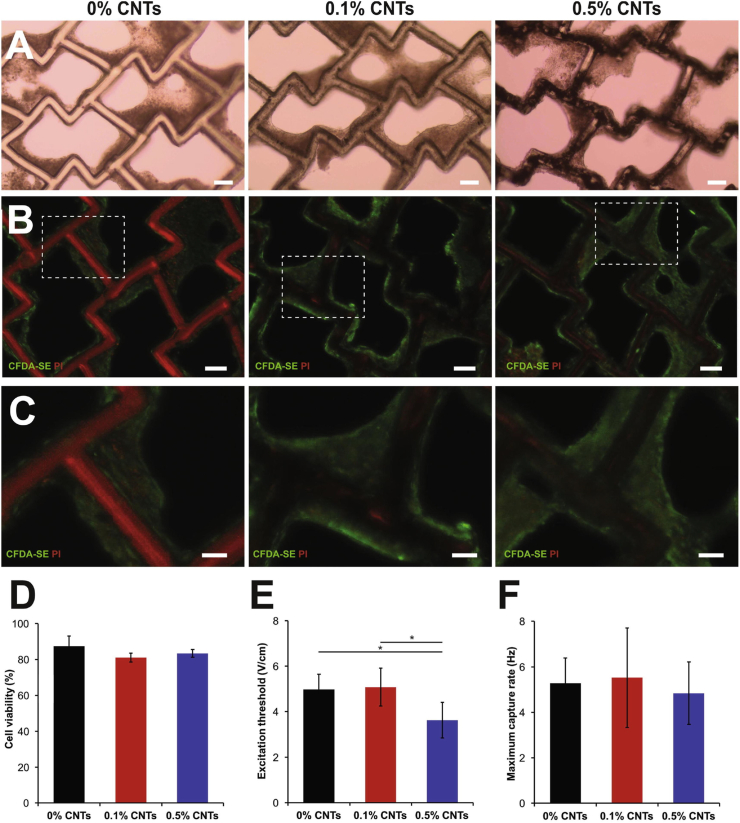
In a study by Ahadian et al., an elastic and conductive scaffold was prepared using a synthetic polymer (124 polymer) along with electroconductive CNT to mimic the native ECM. Fig. 8 shows different 124 polymer-CNT constructs with cells encapsulated in the scaffold. The hybrid 124 polymer-CNT scaffold offered better mechanical support and improved cell viability after 7 days when neonatal rat ventricular myocytes (NRVMs) were seeded into the constructs compared to the controls. Additionally, the 124 polymer-CNT cardiac patch exhibited better contractility with uniform syncytium of the seeded NRVMs compared to non-conductive controls [167]. Similarly, another study reinforced collagen, a natural polymer, with CNT to prepare a conductive hydrogel. The NRVMs had better compatibility and rhythmic contractions in the collagen-CNT hydrogel compared to collagen only hydrogel [168]. Both above-mentioned studies demonstrated the importance of synthetic and natural polymers in creating biomimetic cardiac constructs for cardiac tissue engineering. The combination of natural and synthetic polymers can offer even better characteristics to the constructs compared to using only one type of polymer [169]. Mombini et al. prepared an electromechanical composite comprised of the synthetic polymer, polyvinyl alcohol (PVA) and a natural polymer chitosan embedded with CNTs to drive differentiation of MSCs to cardiomyocytes. In this study, the tensile strength and the conductivity of the chitosan/PVA/CNT scaffold was found to be enhanced compared to individual polymer groups without CNT. Furthermore, the cardiac specific gene expression levels (Nkx2.5, Troponin I, and β–MHC) at day 10 and day 20 in cardiomyocytes were significantly upregulated in the chitosan/PVA/CNT scaffold group compared to the controls [170]. These studies illustrate the benefits of incorporating CNTs in scaffolds and hydrogels when constructing patches for cardiac tissue engineering.
Fig. 8.
Composite scaffolds in cardiac tissue engineering. (A–C) Images of rat cardiac tissue constructs with 124 polymer-CNT scaffolds containing 0.1% and 0.5% CNTs compared to pure polymer scaffolds. (A) Bright field images of polymers seeded with rat CMs at day 7 of culture demonstrate tissue compaction around scaffold struts (Scale bars: 100 μm). (B) Tissues were imaged for viability with live/dead staining assay (Live cells: green, dead cells: red), where the scaffold exhibits autofluorescence in the red channel (Scale bars: 100 μm). (C) High magnification excerpts demonstrate the wrapping of viable cells around scaffold struts (Scale bars: 50 μm). (D) The quantification of live-dead assay images present no difference in cell viability among material groups. (E and F) Comparison of excitation threshold (E) and maximum capture rate (F) suggest improved tissue properties with 0.5% CNTs in polymer scaffolds in comparison to pure polymer controls (∗p < 0.05) (Reproduced with permission from Ref. [167].© Elsevier Ltd 2020).
The literature reviewed in above sections suggest that each and every CNM has distinct mechanoelectrical properties, therefore comparative analysis is required to determine the best suited CNM for any particular function. In a study by Junmin et al. the performance of different CNMs (GO, rGO and C-MWCNTs) in GelMA scaffolds was evaluated individually for cardiac tissue engineering applications. The cardiomyocytes grown in CNT-GelMA based scaffold had the best electrophysiological properties and enhanced capabilities to promote maturation of cardiomyocytes compared to other CNM composites [171]. Therefore, future studies focussed on in depth analysis of different synthesis approaches, functionalization strategies of different CNMs will improve our understanding with respect to the choice of materials for cardiac repair.
3.4. Immunomodulatory agents
After MI, the infiltration of immune cells in the myocardium is a process that initially helps in cardiac tissue repair process [172,173]. The cytokines released from the immune cells clear the dead cells and debris from the damaged myocardium to initiate the next step in the myocardial repair process. This inflammatory phase in the cardiac tissue repair process must be tightly regulated as excessive inflammation can cause second degree injury around the infarct while insufficient inflammation prevents proper scar tissue formation and interrupts postinjury cardiac repair process. Therefore, homeostasis must be maintained to prevent prolonged immune response in the infarct region. The currently available treatment options to regulate inflammation in the heart are mostly devised to target specific cell types like B cells or T cells, or factors such as reactive oxygen species (ROS) [[174], [175], [176]]. Recently, the application of nanomaterials is also being explored in immunomodulation. The nanomaterials can induce both immune suppression and immune stimulation depending upon their surface chemistry [177]. In this section, we will focus on the application of CNMs such as graphene and CNTs to explore their immunomodulatory and anti-inflammatory behaviours for the treatment of CVD.
3.4.1. Graphene and its derivatives in immunomodulation
Several studies have reported the immunomodulatory effects of graphene [178]. The innate immune system that comprises macrophages, dendritic cells (DC) and neutrophils acts as body's first line of defence. Graphene has been reported to be immunomodulatory due to its ability to suppress these cells. Malanagahalli et al. prepared few-layer graphene (FLG), a derivative of graphene, and studied its immunomodulatory effects on mouse bone marrow-derived macrophages. This study reported that FLG exerted its immunomodulatory effects after entering into the macrophages by passive diffusion [179]. Graphene is also reported to suppress dendritic cells which are responsible to stimulate adaptive immune cells such as T and B cells in response to a foreign antigen [180]. In another study, Tomić et al. synthesized GQDs and studied their immunomodulatory properties in peripheral blood mononuclear cells (PBMCs). It was reported that GQDs at a concentration of 200 μg/ml were able to inhibit the proliferation of activated human PBMCs and suppress proinflammatory (IL-1β, lymphotoxin-α, IL-6, IL-8) and Th1 cytokines (interferon (IFN)-γ, IL-12) [181]. Functionalization of graphene and its derivatives with polymers can improve their biocompatibility and immune evasive properties towards immune cells. In a study, Wang et al. prepared two different composites of GO by functionalizing it with PEI and polyethylene glycol (PEG). Both composites were nontoxic to macrophages even at 100 μg/ml and suppressed the levels of pro-inflammatory cytokines [182].
During a myocardial infarction, the presence of M1 macrophages increases the production of proinflammatory cytokines thereby aggravating the infarct zone expansion and ultimately resulting in the inflammation induced failure of the heart. The immunotherapies to polarize proinflammatory macrophages M1 to regenerative M2 macrophages have gained significant attention for cardiac repair [183]. Han et al. prepared a macrophage-targeting/polarizing GO complex (MGC) made up of GO, PEI, PEG and folic acid to specifically bind the IL-4 gene to target the polarization of M1 to M2 macrophages. In the design of the MGC complex, GO was used a carrier, while PEI was used to bind the IL-4 gene and folic acid was used to specifically target M1 macrophages. The results showed that MGC complex showed no signs of cellular toxicity in macrophages. In a mouse model of myocardial infarction, the delivery of MGC complex was able to polarize M1 into M2 macrophages along with improved ROS scavenging properties, fibrosis mitigation, and improvement in angiogenesis with preserved cardiac functions [184]. Some graphene derivatives have also been used as antioxidants to remove ROS in the cardiac tissue post myocardial infarction. In a study, GQDs were tested for their ability to scavenge free radicals in vitro in cardiomyocytes. It was found that GQDs at a concentration of 1 μg/ml were able to protect cardiomyocytes from H2O2-mediated cell injury [185].
In cardiac stem cell therapy, poor survival of transplanted cells is often a major issue due to the hypoxic and ROS-concentrated environment in the infarct region. Graphene is reported to protect the transplanted stem cells from ROS-mediated death and improve survival of implanted cells in the heart. Park et al. synthesized GO flakes which prevented ROS-mediated death of MSCs and promoted therapeutic effects of transplanted MSCs in a MI model. GO flakes were surface modified with ECM proteins and MSCs where attached to the GO flakes. An ischemia reperfusion model was used to evaluate the antioxidant behaviour of MSC-GO composites against H2O2-mediated cell death. It was reported that the presence of GO promoted the survival of MSCs in ROS concentrated environment in vitro compared to MSCs alone. Under in vivo conditions they found an improved survival and engraftment of MSCs in the MSC-GO group, and an overall improvement in cardiac performance compared to MSC transplantation alone [186]. Similarly, Choe et al. prepared an antioxidant hydrogel composed of rGO/alginate encapsulated with MSCs to prevent ROS-mediated cell death. The microgel prevented death of MSCs under H2O2-mediated oxidative stress [187]. Therefore, above-mentioned studies show that GO and its derivatives can serve as immunomodulatory and antioxidant agents during inflammation related complications in the injured heart.
3.4.2. CNTs and their derivatives in immunomodulation
The strategies based on CNTs-mediated immunomodulation may offer great benefits due to their potential application in immunotherapy against inflammatory disorders, infectious diseases and cancer. The limiting factor which prevents CNTs from being utilized in immunomodulation is their intrinsic immunogenicity when injected into the body. However, this can be overcome by fine-tuning their size, altering their physicochemical properties and surface functionalization to make them less immunogenic [188,189]. This section will cover immunomodulatory behaviour of CNTs and their interaction with immune cells and how these properties can be exploited to treat cardiovascular diseases.
The innate immune system offers the first line of defence against foreign bodies and pathogens.
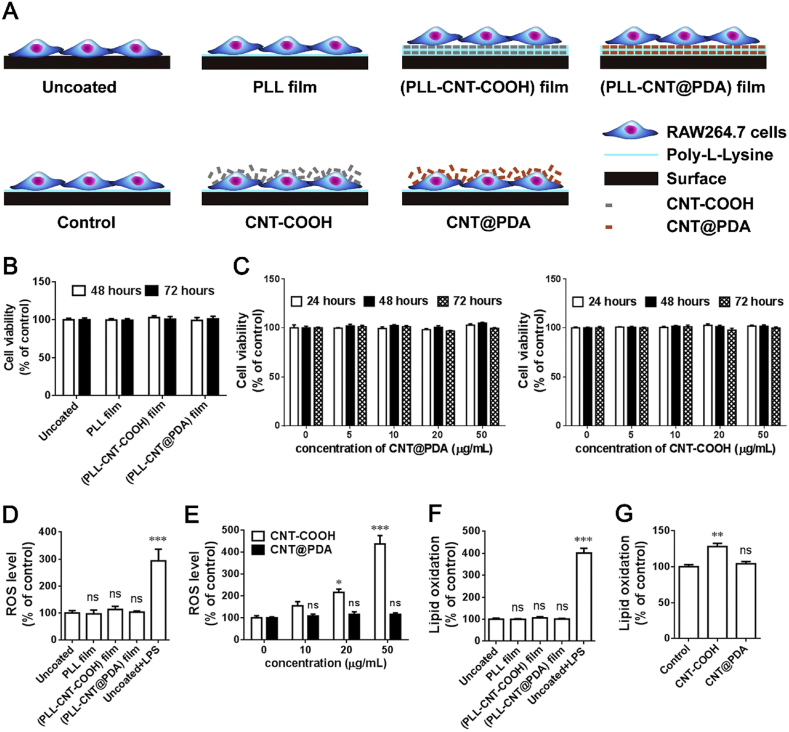
Macrophages and DCs are two major immune cell types which effectively eliminate foreign bodies through phagocytosis or complement activation. The interaction of CNTs with these immune cells is essential for their immunomodulatory action. Sun et al. prepared a composite comprised of polydopamine (PDA) functionalized MWCNTs and poly-l-lysine (PLL) and studied the immunomodulatory effects in macrophages. As shown in Fig. 9, the composite was nontoxic towards the RAW264.7 macrophages with no change in ROS or lipid oxidation. Also, there was no change in the morphology of immune cells and secretion of inflammatory cytokines. Therefore, the intrinsic immunogenicity of CNTs can be modified by functionalization of these biomaterials [190]. Furthermore, the dendritic cells are responsible for presenting the antigens to T-cells for their activation and secondary immune response. Aldinucci et al. studied the immunomodulatory effects of CNTs on DCs, in this study MWCNTs were functionalized with COOH groups and tested on human DCs for their toxicity and immunological properties. They found no significant effect of the biomaterial on cytoskeleton organization, gene expression and cytokine levels, demonstrating the tolerance of DCs towards MWCNTs [191]. In another study Mitchell et al. investigated the interaction of MWCNT with T cells as well as systemic immune function in C57Bl/6 mice. MWCNT was able to suppress T cell activation in a dose dependent manner [192]. The application of CNMs to evade immune cells in the body is promising technique which can be exploited to develop therapeutic strategies to modulate myocardial inflammation during cardiac injury. Even though, biomaterials based immunomodulation is an emerging field and has witnessed a significant progress, however, the application of CNMs in immunomodulation is very limited. But the available evidences strongly suggest that CNMs based strategies hold huge potential in terms developing therapeutic interventions for cardiovascular repair. Therefore, extensive basic research studies focused on understanding the effects of CNMs on the immune system are needed to broaden the scope of CNMs in clinical applications.
Fig. 9.
(A). Schematic diagram of experimental design to evaluate material biocompatibility with RAW264.7 macrophages. The cells were cultured on (PLL-CNT-COOH) film or (PLL-CNT@PDA) film, and then examined for cellular functions. The cells grown on the surface without modification (Uncoated) and PLL-coated surface (PLL film) were applied as controls. RAW264.7 cells were also directly treated with dispersed CNTs (CNT-COOH or CNT@PDA), using untreated cells as control. B, The cells were seeded onto different films for 48 and 72 h. Cell viability examined by the CCK-8 assay was comparable among these groups. C, The cells were treated with CNT@PDA or CNT-COOH at different concentrations for 24, 48 and 72 h. No viability change was observed. D, ROS levels were detected by the DCFH-DA assay. No change in ROS levels was found in RAW264.7 cells following a 72-h culture on the specified films. E, A 24 h treatment of CNT-COOH increased ROS levels in a concentration-dependent manner, whereas CNT@PDA had no significant effect even at a concentration of 50 μg/mL. F, Treatments with none of the films for 72 h affected lipid oxidation, as determined by the MDA assay. G, CNT-COOH, but not CNT@PDA treatment for 24 h raised cellular lipid oxidation. n = 4–6; *P < 0.05, **P < 0.01, ***P < 0.001 versus uncoated or specified controls by one-way ANOVA; ns: not significant (Reproduced with permission from Ref. [190].© Elsevier Ltd 2020).
3.5. Other CNMs for cardiovascular theranostics
Fullerenes and carbon nanodiamonds are some of the other commonly employed CNMs in several biomedical applications due to their unique physicochemical properties [[193], [194], [195], [196]]. This section will discuss about the application of these CNMs in cardiovascular theranostics.
Fullerene is a truncated icosahedron which contains 60 carbon atoms (C60) with C5–C5 single bonds forming pentagons and C5–C6 double bonds forming hexagons. A single fullerene molecule has a diameter of 0.7 nm. Also, fullerenes are commonly termed as buckyball or C60. The physical properties such as density and electrical resistivity of fullerenes are around 1.65 kg/cm3 and 1014 Ω/m respectively [197]. Fullerenes are hydrophobic in nature which results in poor solubility. However, functionalization and modification approaches can improve the water solubility and increase their potential use in drug delivery and tissue engineering applications. The application of fullerenes and nanodiamonds are studied for bio-imaging, targeted drug delivery, and photodynamic therapy [198]. Tong et al. reported their potential as scaffold reinforcers in cardiovascular tissue engineering applications. This group demonstrated the ability of C60 fullerene in enhancing cell survival and proliferation of brown adipose derived stem cells (BADSCs) through modulation of MAPK signalling pathway [199]. Another major application of these caged carbon molecules is their ability to act as ROS scavengers during heart failure. Another study by Tong et al. demonstrated the cytoprotective capability of fullerenes during oxidative stress mediated cell death. The fullerenol conjugated alginate hydrogel was able to effectively protect BADSCs from H2O2 mediated ROS. Also, the survival and retention of BADSCs in fullerenol/alginate composite was significantly improved when injected into ischemic areas of the MI rats [200]. Therefore fullerene has the potential to be applied in cardiovascular theranostics.
Similarly, carbon nanodiamonds are an essential class of biomaterials which consist of sp3 carbon atoms arranged in a diamond crystal structure, with an average size of around 5 nm. Excellent optical and mechanical properties, high surface area, tuneable surface characteristics and low toxicity make them a good candidate for biological applications. Furthermore superior intrinsic fluorescence capabilities increase their potential use in biomedical imaging applications [201]. However, so far, the application of carbon nanodiamonds in cardiovascular field is limited only to biosensing. In this regard, Zhao et al., designed a biosensor for electrochemical detection of glucose. In this study, a gold electrode was pre-treated with nanocrystalline diamonds and subsequently conjugated with glucose oxidase enzyme. The resultant sensor displayed high selectivity for glucose with minimal crossover effect and a detection limit of 5 μM was achieved [202]. Similarly, in a recent study, nanodiamond and graphdiyne heterostructure based biosensor was devised to detect cTnI and myoglobin levels in human serum. The biosensor was able to detect very minute levels of myoglobin (0.01–1000 pg/ml), when compared to other commonly available biosensors such as black phosphorous and gold nanoparticle based biosensors [203]. However, in spite of these exciting reports the application of nanodiamonds and fullerenes in biomedical field face a lot of obstacles, including off target effects and toxicity. Therefore, extensive basic research needs to be done in terms of developing new modifications and functionalization strategies to pave the way for these carbon nanomaterials to emerge as candidate material of choice for cardiovascular theranostics in future.
In this review, the biomedical applications of different CNMs have been discussed in the field of drug delivery, tissue engineering, bio-sensing and immunomodulation. We have also reviewed different types and derivatives of CNMs. Overall, it is clear from the literature reviewed here that CNMs have the potential to act as outstanding candidates for cardiovascular applications. However, the clinical use of CNMs is still in its infancy, there are many challenges such as improvement in bioavailability, stability and biocompatibility of CNMs. Therefore, for successful clinical translation of CNMs based strategies these challenges need to be dealt with. In the following section we have discussed some of the barriers involved in clinical translation CNMs based strategies.
4. Barrier for clinical translation of CNMs
The translation of nanomaterials based strategies from bench to clinical application has witnessed some progress in the past few years. Currently, there are over 50 nanomaterial-based drug formulations approved for clinical use by FDA for different disease conditions. Also, there are more than 100 ongoing clinical trials testing the efficacy of nanomaterials as biosensors, tracers in biomedical imaging and for many other applications [[204], [205], [206], [207]]. However, there are several challenges involved in bringing nanomaterials-based approaches to the clinic and in this section, we will discuss some of these challenges.
The unique physicochemical properties of CNMs and promising outcome of preclinical studies have motivated the investigators to test their efficacy in clinical trials. Presently, the CNMs are being tested in clinical trials as biosensors for neurological disorders and stomach diseases, tracers for imaging tumours, enhancers in dental tissue engineering, in bioinstrumentation for prosthesis development in medical biotechnology [[208], [209], [210], [211], [212], [213]]. In cardiovascular field the potential of GQDs as biosensors is currently being validated as photo electrochemical immunosensor for early diagnosis of cardiac troponin I in AMI patients. The focus of this clinical trial will be to test the precision and sensitivity of biosensors in AMI patients. This study is being led by the Xinhua Hospital in China and is expected to be completed by 2023 [214]. Even though the numbers of nanomaterials based clinical trials has grown recently, the CNMs based clinical studies have come across several challenges in terms of approvals for application in patients. This is mainly due to their intrinsic cytotoxicity to all major organs in the body [14,[215], [216], [217], [218]]. Therefore, in order to develop safe CNM based strategies for cardiovascular repair, the toxic effects of these materials need to be thoroughly investigated.
4.1. Toxicity of CNMs in cardiovascular tissues
There are several factors including size, shape, method of synthesis, surface chemistry, dosage and delivery route which are reported to contribute to the toxicity of CNMs. Basically these factors influence the way CNMs interact with the cells or tissues. The complications such as excessive inflammation and genotoxicity are some of the effects of their interaction with cells or tissues [[219], [220], [221]]. In this section, the toxicity of graphene and CNTs toward cardiovascular system is discussed. This will provide researchers the insights on how to tune CNMs toward biocompatible materials for cardiovascular theranostics.
4.2. Toxicity of graphene and its derivatives
Several studies have reported the toxicity of graphene and its derivatives in different cells and animal models. The toxicity of graphene in biological systems is mediated by its planar dimensions, concentration of the particles and their surface properties [222,223]. The long-term exposure and accumulation of graphene in the cells causes inflammation, permanent genetic damage and cell death [224]. The size and proper dispersion of graphene nanosheets are major concerns which must be regulated to minimize the toxicity in a biological system. Duch et al. reported that GO and hydrophobic graphene caused oxidative stress in the lungs whereas hydrophobic graphene dispersed in pluronic was biocompatible and elicited no toxicity in vivo. This study highlighted the importance of surface functionalization and dispersion which improved the biocompatibility of graphene [225]. In another study by Chen et al. different sizes of GO (50–200 nm, <500 nm and >500 nm) with different concentrations (0.1,1,10 and 100 mg/l) were employed to study their effects on embryonic zebrafish development. This study reported that GO at 100 mg/l concentration led to a slower hatching time of the zebrafish embryos, reduced body length, change in the heart rate and blood flow along with increase in apoptotic gene expression. This study also reported that the toxicity was not dependent on the size of GO when it reached above 10 mg/l concentration [226]. Therefore, choosing the size and dose of CNMs is very important for its safety and efficacy for therapeutic applications.
Next, the exposure time and the dose of graphene and its derivatives are highly important. Arbo et al. found that nano-GO was highly toxic for H9c2 cell line at a dose of 20 μg/ml for 24hrs. However, GO at 10 μg/ml was biocompatible with H9c2 cells emphasizing the importance of dose [227]. Similarly, in another study, pristine graphene was evaluated for its genotoxicity on cardiovascular system in zebrafish. This study reported that graphene at a concentration greater than 15 μg/l caused apoptosis, reduced blood vessel formation and early developmental heart defects. But at lower dosage including 5–10 μg/l, graphene were nontoxic to the cells [228]. Therefore optimization of dosage and treatment protocol is very critical and important for biomedical applications of graphene.
4.3. Toxicity of CNT and its derivatives
CNTs are also reported to exert toxicity when employed in biological systems. This limits the application of CNTs in drug delivery, biosensors, tissue engineering applications for various tissues especially sensitive organs like heart and brain [229,230]. Short term pulmonary exposure of CNTs in animal models is an effective way to check their initial toxicity. Lung is the primary organ that is severely affected through inhalation of the CNTs thereafter indirectly affecting the functioning of cardiac tissues [231,232]. In a study in rats it was reported that inhalation of MWCNTs decreased the blood pressure and the heart rate [233]. The strategies to minimize the toxic effects are focussed on the alterations in functional groups and surface chemistry of the CNTs. Conjugating chemical group which are nontoxic or removing the reactive functional groups on the surface of graphene based composite are mostly the preferred ways to make it biocompatible. In a study, the toxicity of SWCNTs and acid functionalized SWCNTs was evaluated in mice through oropharyngeal aspiration. It was reported that exposure to acid functionalized SWCNTs increased neutrophil and edema formation in the lungs in a dose dependent manner [234]. CNTs tend to aggregate in aqueous solutions due to their bulky and large dimensions compared to smaller CNMs which can be easily dispersed. This is the main reason for CNM mediated toxicity in cells [235]. In a study, long (L)-MWCNTs in micron size which tend to form aggregates and small (S)-MWCNTs in nano size which are well dispersed in the aqueous environment were used to evaluate the toxicity in a zebrafish developmental model. Exposure to L-MWCNTs led to a significant reduction in heart rate and caused heart developmental defects in zebrafish when compared to S-MWCNTs. Therefore the size and dimensions of CNMs is one of the major reasons for cardiotoxicity [236].
5. Future directions and conclusions
This review summarizes promises and challenges associated with the application of CNMs for drug delivery, biosensors, tissue engineering applications and immunomodulation for cardiovascular diseases. Even though CNMs possess several properties which make them attractive candidates for cardiovascular theranostics. However, as discussed in this review there are several challenges which we need to overcome for successful translation of these materials for clinical applications. In order to thoroughly understand the effect of size, shape and surface chemistry of the materials on their function, more extensive studies on the synthesis, physicochemical characterization and functionalization techniques of CNMs are needed. The biocompatibility of materials is very important parameter in deciding their applicability in the clinic. Therefore, more studies on the validation of these materials in different in vitro models and small as well as large animal models are needed to understand the safety profile and basic mechanisms of biomaterials mediated tissue repair and beneficial effects. The pharmacokinetics and pharmacodynamics profiles of CNMs such as their effective absorption, distribution in the cardiovascular tissue and effective excretion from the body are required. In depth studies focussed on understanding the best administration route, optimum dosage for therapeutic effects, biodegradability and nontoxic behaviour of CNMs will help in developing procedures to synthesize next generation biomaterials for cardiovascular theranostics. Another area of focus in future for successful clinical translation of CNMs based biomaterials is the development of facilities for large scale production of CNM products. Some of the challenges involved with the large scale production of GMP grade CNMs are inefficient reproducibility of CNM products, lack of expertise in large scale manufacturing, expensive raw materials and impurities in the synthesized materials. Therefore, future efforts should be directed toward finding solutions for these issues.
CRediT authorship contribution statement
Keshav Narayan Alagarsamy: Conceptualization, Writing - original draft, Conception, Designing, Literature survey, Writing and Final Approval of Manuscript. Sajitha Mathan: Writing - original draft, Literature survey, Writing and Final Approval of Manuscript. Weiang Yan: Writing - original draft, Literature survey, Writing and Final Approval of Manuscript. Alireza Rafieerad: Writing - original draft, Writing and Final Approval of Manuscript. Saravanan Sekaran: Writing - original draft, Literature survey, Writing and Final Approval of Manuscript. Hanna Manego: Writing - original draft, Writing and Final Approval of Manuscript. Sanjiv Dhingra: Conceptualization, Writing - original draft, Conception, Designing, Literature survey, Writing and Final Approval of Manuscript.
Declaration of competing interest
Authors declare no conflict of interest.
Acknowledgment
This work was supported by Canadian Institutes of Health Research Grant MOP142265 (to S. Dhingra).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Flora G.D., Nayak M.K. A brief review of cardiovascular diseases, associated risk factors and current treatment regimes. Curr. Pharmaceut. Des. 2019;25:4063–4084. doi: 10.2174/1381612825666190925163827. [DOI] [PubMed] [Google Scholar]
- 2.Barquera S., Pedroza-Tobías A., Medina C., Hernández-Barrera L., Bibbins-Domingo K., Lozano R., Moran A.E. Global Overview of the epidemiology of atherosclerotic cardiovascular disease. Arch. Med. Res. 2015;46:328–338. doi: 10.1016/j.arcmed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Nabel E.G. Cardiovascular disease. N. Engl. J. Med. 2003;349:60–72. doi: 10.1056/NEJMra035098. [DOI] [PubMed] [Google Scholar]
- 4.Burke A.P., Virmani R. Pathophysiology of acute myocardial infarction. Med. Clin. 2007;91:553–572. doi: 10.1016/j.mcna.2007.03.005. ix. [DOI] [PubMed] [Google Scholar]
- 5.Frangogiannis N.G. Comprehensive Physiology. American Cancer Society; 2015. Pathophysiology of myocardial infarction; pp. 1841–1875. [DOI] [PubMed] [Google Scholar]
- 6.Hobbs F.D.R. Cardiovascular disease: different strategies for primary and secondary prevention? Heart. 2004;90:1217–1223. doi: 10.1136/hrt.2003.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foëx P. Innovations in management of cardiac disease: drugs, treatment strategies and technology. Br. J. Anaesth. 2017;119:i23–i33. doi: 10.1093/bja/aex327. [DOI] [PubMed] [Google Scholar]
- 8.Karwalajtys T., Kaczorowski J. An integrated approach to preventing cardiovascular disease: community-based approaches, health system initiatives, and public health policy. Risk Manag. Healthc. Pol. 2010;3:39–48. doi: 10.2147/RMHP.S7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domenech M., Polo-Corrales L., Ramirez-Vick J.E., Freytes D.O. Tissue engineering strategies for myocardial regeneration: acellular versus cellular scaffolds? Tissue Eng. B Rev. 2016;22:438–458. doi: 10.1089/ten.teb.2015.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berthiaume F., Maguire T.J., Yarmush M.L. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng. 2011;2:403–430. doi: 10.1146/annurev-chembioeng-061010-114257. [DOI] [PubMed] [Google Scholar]
- 11.Dhandayuthapani B., Kumar D.S. Biomedical Applications of Polymeric Materials and Composites. John Wiley & Sons, Ltd; 2016. Biomaterials for biomedical applications; pp. 1–20. [DOI] [Google Scholar]
- 12.Taylor-Pashow K.M.L., Rocca J.D., Huxford R.C., Lin W. Hybrid nanomaterials for biomedical applications. Chem. Commun. 2010;46:5832–5849. doi: 10.1039/C002073G. [DOI] [PubMed] [Google Scholar]
- 13.Salata O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004;2:3. doi: 10.1186/1477-3155-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erol O., Uyan I., Hatip M., Yilmaz C., Tekinay A.B., Guler M.O. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2018;14:2433–2454. doi: 10.1016/j.nano.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Ku S.H., Lee M., Park C.B. Carbon-based nanomaterials for tissue engineering. Adv. Healthcare Mater. 2013;2:244–260. doi: 10.1002/adhm.201200307. [DOI] [PubMed] [Google Scholar]
- 16.Maiti D., Tong X., Mou X., Yang K. Carbon-based nanomaterials for biomedical applications: a recent study. Front. Pharmacol. 2019;9 doi: 10.3389/fphar.2018.01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dresselhaus M.S. Fifty years in studying carbon-based materials. Phys. Scripta. 2012;T146 doi: 10.1088/0031-8949/2012/T146/014002. [DOI] [Google Scholar]
- 18.Nasir S., Hussein M.Z., Zainal Z., Yusof N.A. Carbon-based nanomaterials/allotropes: a glimpse of their synthesis, properties and some applications. Materials. 2018;11 doi: 10.3390/ma11020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cha C., Shin S.R., Annabi N., Dokmeci M.R., Khademhosseini A. Carbon-based nanomaterials: multifunctional materials for biomedical engineering. ACS Nano. 2013;7:2891–2897. doi: 10.1021/nn401196a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geim A.K., Novoselov K.S. The rise of graphene. Nat. Mater. 2007;6:183–191. doi: 10.1038/nmat1849. [DOI] [PubMed] [Google Scholar]
- 21.Lakshmanan R., Maulik N. Graphene-based drug delivery systems in tissue engineering and nanomedicine. Can. J. Physiol. Pharmacol. 2018;96:869–878. doi: 10.1139/cjpp-2018-0225. [DOI] [PubMed] [Google Scholar]
- 22.Huang L., Wu B., Yu G., Liu Y. Graphene: learning from carbon nanotubes. J. Mater. Chem. 2011;21:919–929. doi: 10.1039/C0JM02225J. [DOI] [Google Scholar]
- 23.Allen M.J., Tung V.C., Kaner R.B. Honeycomb carbon: a review of graphene. Chem. Rev. 2010;110:132–145. doi: 10.1021/cr900070d. [DOI] [PubMed] [Google Scholar]
- 24.Zhao H., Ding R., Zhao X., Li Y., Qu L., Pei H., Yildirimer L., Wu Z., Zhang W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov. Today. 2017;22:1302–1317. doi: 10.1016/j.drudis.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee A.N. Graphene and its derivatives as biomedical materials: future prospects and challenges. Interface Focus. 2018;8:20170056. doi: 10.1098/rsfs.2017.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paredes J.I., Villar-Rodil S., Martínez-Alonso A., Tascón J.M.D. Graphene oxide dispersions in organic solvents. Langmuir. 2008;24:10560–10564. doi: 10.1021/la801744a. [DOI] [PubMed] [Google Scholar]
- 27.Song J., Wang X., Chang C.-T. Preparation and characterization of graphene oxide. J. Nanomater. 2014 https://doi-org.uml.idm.oclc.org/10.1155/2014/276143 [Google Scholar]
- 28.Yang K., Feng L., Shi X., Liu Z. Nano-graphene in biomedicine: theranostic applications. Chem. Soc. Rev. 2012;42:530–547. doi: 10.1039/C2CS35342C. [DOI] [PubMed] [Google Scholar]
- 29.Xu C., Shi X., Ji A., Shi L., Zhou C., Cui Y. Fabrication and characteristics of reduced graphene oxide produced with different green reductants. PloS One. 2015;10 doi: 10.1371/journal.pone.0144842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pei S., Cheng H.-M. The reduction of graphene oxide. Carbon. 2012;50:3210–3228. doi: 10.1016/j.carbon.2011.11.010. [DOI] [Google Scholar]
- 31.Qu D., Zheng M., Zhang L., Zhao H., Xie Z., Jing X., Haddad R.E., Fan H., Sun Z. Formation mechanism and optimization of highly luminescent N-doped graphene quantum dots. Sci. Rep. 2014;4:1–11. doi: 10.1038/srep05294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu R., Wu D., Feng X., Müllen K. Bottom-up fabrication of photoluminescent graphene quantum dots with uniform morphology. J. Am. Chem. Soc. 2011;133:15221–15223. doi: 10.1021/ja204953k. [DOI] [PubMed] [Google Scholar]
- 33.Chekin F., Vasilescu A., Jijie R., Singh S.K., Kurungot S., Iancu M., Badea G., Boukherroub R., Szunerits S. Sensitive electrochemical detection of cardiac troponin I in serum and saliva by nitrogen-doped porous reduced graphene oxide electrode. Sensor. Actuator. B Chem. 2018;262:180–187. doi: 10.1016/j.snb.2018.01.215. [DOI] [Google Scholar]
- 34.Huitong Chi, Qingzhi Han, Tianhe Chi, Xing Bin, Ning Ma, Dan Wu, Qin Wei. Manganese doped CdS sensitized graphene/Cu2MoS4 composite for the photoelectrochemical immunoassay of cardiac troponin I. Biosens. Bioelectron. 2019;132:1–7. doi: 10.1016/j.bios.2019.02.048. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y., Li N., Xiang Y., Wang D., Zhang P., Wang Y., Lu S., Xu R., Zhao J. A flexible non-enzymatic glucose sensor based on copper nanoparticles anchored on laser-induced graphene. Carbon. 2020;156:506–513. doi: 10.1016/j.carbon.2019.10.006. [DOI] [Google Scholar]
- 36.Singh S., Tuteja S.K., Sillu D., Deep A., Suri C.R. Gold nanoparticles-reduced graphene oxide based electrochemical immunosensor for the cardiac biomarker myoglobin. Microchim Acta. 2016;183:1729–1738. doi: 10.1007/s00604-016-1803-x. [DOI] [Google Scholar]
- 37.Arvand M., Samie H.A. Electrospun CeO2–Au nanofibers/graphene oxide 3D nanonetwork structure for the electrocatalytic detection of amlodipine. Ionics. 2018;24:1813–1826. doi: 10.1007/s11581-017-2321-5. [DOI] [Google Scholar]
- 38.Tang Z., He J., Chen J., Niu Y., Zhao Y., Zhang Y., Yu C. A sensitive sandwich-type immunosensor for the detection of galectin-3 based on N-GNRs-Fe-MOFs@AuNPs nanocomposites and a novel AuPt-methylene blue nanorod. Biosens. Bioelectron. 2018;101:253–259. doi: 10.1016/j.bios.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 39.Shin S.R., Zihlmann C., Akbari M., Assawes P., Cheung L., Zhang K., Manoharan V., Zhang Y.S., Yüksekkaya M., Wan K.-T., Nikkhah M., Dokmeci M.R., Tang X.S., Khademhosseini A. Reduced graphene oxide-GelMA hybrid hydrogels as scaffolds for cardiac tissue engineering. Small. 2016;12:3677–3689. doi: 10.1002/smll.201600178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao L. A novel graphene oxide polymer gel platform for cardiac tissue engineering application. 3 Biotech. 2019;9:401. doi: 10.1007/s13205-019-1912-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zargar S.M., Mehdikhani M., Rafienia M. Reduced graphene oxide–reinforced gellan gum thermoresponsive hydrogels as a myocardial tissue engineering scaffold. J. Bioact. Compat Polym. 2019;34:331–345. doi: 10.1177/0883911519876080. [DOI] [Google Scholar]
- 42.Yuan Z., Qin Q., Yuan M., Wang H., Li R. Development and novel design of clustery graphene oxide formed Conductive Silk hydrogel cell vesicle to repair and routine care of myocardial infarction: investigation of its biological activity for cell delivery applications. J. Drug Deliv. Sci. Technol. 2020;60:102001. doi: 10.1016/j.jddst.2020.102001. [DOI] [Google Scholar]
- 43.Wang Y., Dong Y., Chen P., Chen R., Li Y., Du W., Liu B.-F. Reduced graphene oxide foam templated by nickel foam for organ-on-a-chip engineering of cardiac constructs. Mater. Sci. Eng. C. 2020;117:111344. doi: 10.1016/j.msec.2020.111344. [DOI] [PubMed] [Google Scholar]
- 44.Talebi A., Labbaf S., Karimzadeh F., Masaeli E., Nasr Esfahani M.-H. Electroconductive graphene-containing polymeric patch: a promising platform for future cardiac repair. ACS Biomater. Sci. Eng. 2020;6:4214–4224. doi: 10.1021/acsbiomaterials.0c00266. [DOI] [PubMed] [Google Scholar]
- 45.Simon J., Flahaut E., Golzio M. Overview of carbon nanotubes for biomedical applications. Materials. 2019;12 doi: 10.3390/ma12040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdalla S., Al-Marzouki F., Al-Ghamdi A.A., Abdel-Daiem A. Different technical applications of carbon nanotubes. Nanoscale Res. Lett. 2015;10 doi: 10.1186/s11671-015-1056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. doi: 10.1038/354056a0. [DOI] [Google Scholar]
- 48.Sinha N., Yeow J.T.W. Carbon nanotubes for biomedical applications. IEEE Trans. NanoBioscience. 2005;4:180–195. doi: 10.1109/tnb.2005.850478. [DOI] [PubMed] [Google Scholar]
- 49.Bronikowski M.J., Willis P.A., Colbert D.T., Smith K.A., Smalley R.E. Gas-phase production of carbon single-walled nanotubes from carbon monoxide via the HiPco process: a parametric study. J. Vac. Sci. Technol. 2001;19:1800–1805. doi: 10.1116/1.1380721. [DOI] [Google Scholar]
- 50.Saito K., Gordon A.S., Williams F.A., Stickle W.F. A study of the early history of soot formation in various hydrocarbon diffusion flames. Combust. Sci. Technol. 1991;80:103–119. doi: 10.1080/00102209108951779. [DOI] [Google Scholar]
- 51.Alshehri R., Ilyas A.M., Hasan A., Arnaout A., Ahmed F., Memic A. Carbon nanotubes in biomedical applications: factors, mechanisms, and remedies of toxicity. J. Med. Chem. 2016;59:8149–8167. doi: 10.1021/acs.jmedchem.5b01770. [DOI] [PubMed] [Google Scholar]
- 52.He H., Pham-Huy L.A., Dramou P., Xiao D., Zuo P., Pham-Huy C. Carbon nanotubes: applications in pharmacy and medicine. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/578290. https://doi-org.uml.idm.oclc.org/10.1155/2013/578290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bianco A., Kostarelos K., Partidos C.D., Prato M. Biomedical applications of functionalised carbon nanotubes. Chem. Commun. 2005:571–577. doi: 10.1039/b410943k. [DOI] [PubMed] [Google Scholar]
- 54.Sajid M.I., Jamshaid U., Jamshaid T., Zafar N., Fessi H., Elaissari A. Carbon nanotubes from synthesis to in vivo biomedical applications. Int. J. Pharm. 2016;501:278–299. doi: 10.1016/j.ijpharm.2016.01.064. [DOI] [PubMed] [Google Scholar]
- 55.Simon J., Flahaut E., Golzio M. Overview of carbon nanotubes for biomedical applications. Materials. 2019;12 doi: 10.3390/ma12040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu W., Huang H., Dong Y., Han C., Sui X., Jian B. Multi-walled carbon nanotube-based systems for improving the controlled release of insoluble drug dipyridamole. Exp. Therap. Med. 2019;17:4610–4616. doi: 10.3892/etm.2019.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rezaei B., Shoushtari A.M., Rabiee M., Uzun L., Mak W.C., Turner A.P.F. An electrochemical immunosensor for cardiac Troponin I using electrospun carboxylated multi-walled carbon nanotube-whiskered nanofibres. Talanta. 2018;182:178–186. doi: 10.1016/j.talanta.2018.01.046. [DOI] [PubMed] [Google Scholar]
- 58.Yan H., Tang X., Zhu X., Zeng Y., Lu X., Yin Z., Lu Y., Yang Y., Li L. Sandwich-type electrochemical immunosensor for highly sensitive determination of cardiac troponin I using carboxyl-terminated ionic liquid and helical carbon nanotube composite as platform and ferrocenecarboxylic acid as signal label. Sensor. Actuator. B Chem. 2018;277:234–240. doi: 10.1016/j.snb.2018.09.010. [DOI] [Google Scholar]
- 59.Ghanei Agh Kaariz D., Darabi E., Elahi S.M. Fabrication of Au/ZnO/MWCNTs electrode and its characterization for electrochemical cholesterol biosensor. J. Theor. Appl. Phys. 2020;14:339–348. doi: 10.1007/s40094-020-00390-5. [DOI] [Google Scholar]
- 60.Abedi A., Bakhshandeh B., Babaie A., Mohammadnejad J., Vahdat S., Mombeiny R., Moosavi S.R., Amini J., Tayebi L. Concurrent application of conductive biopolymeric chitosan/polyvinyl alcohol/MWCNTs nanofibers, intracellular signaling manipulating molecules and electrical stimulation for more effective cardiac tissue engineering. Mater. Chem. Phys. 2021;258:123842. doi: 10.1016/j.matchemphys.2020.123842. [DOI] [Google Scholar]
- 61.Sun H., Zhou J., Huang Z., Qu L., Lin N., Liang C., Dai R., Tang L., Tian F. Carbon nanotube-incorporated collagen hydrogels improve cell alignment and the performance of cardiac constructs. Int. J. Nanomed. 2017;12:3109–3120. doi: 10.2147/IJN.S128030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun H., Tang J., Mou Y., Zhou J., Qu L., Duval K., Huang Z., Lin N., Dai R., Liang C., Chen Z., Tang L., Tian F. Carbon nanotube-composite hydrogels promote intercalated disc assembly in engineered cardiac tissues through β1-integrin mediated FAK and RhoA pathway. Acta Biomater. 2017;48:88–99. doi: 10.1016/j.actbio.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 63.Roshanbinfar K., Mohammadi Z., Mesgar A.S.-M., Dehghan M.M., Oommen O.P., Hilborn J., Engel F.B. Carbon nanotube doped pericardial matrix derived electroconductive biohybrid hydrogel for cardiac tissue engineering. Biomater. Sci. 2019;7:3906–3917. doi: 10.1039/C9BM00434C. [DOI] [PubMed] [Google Scholar]
- 64.Zhao G., Zhang X., Li B., Huang G., Xu F., Zhang X. Solvent-free fabrication of carbon nanotube/silk fibroin electrospun matrices for enhancing cardiomyocyte functionalities. ACS Biomater. Sci. Eng. 2020;6:1630–1640. doi: 10.1021/acsbiomaterials.9b01682. [DOI] [PubMed] [Google Scholar]
- 65.Pedrotty Dawn M., Volodymyr Kuzmenko, Erdem Karabulut, Sugrue Alan M., Christopher Livia, Vaidya Vaibhav R., McLeod Christopher J. Asirvatham samuel J., gatenholm Paul, kapa suraj, three-dimensional printed biopatches with conductive ink facilitate cardiac conduction when applied to disrupted myocardium, circulation. Arrhythmia Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.118.006920. [DOI] [PubMed] [Google Scholar]
- 66.Ding N., Dou C., Wang Y., Liu F., Guan G., Huo D., Li Y., Yang J., Wei K., Yang M., Tan J., Zeng W., Zhu C. Antishear stress bionic carbon nanotube mesh coating with intracellular controlled drug delivery constructing small-diameter tissue–engineered vascular grafts. Adv. Healthcare Mater. 2018;7:1800026. doi: 10.1002/adhm.201800026. [DOI] [PubMed] [Google Scholar]
- 67.Eltaher H.M., Said S.S., El-Khordagui L.K. Applications of Nanocomposite Materials in Drug Delivery. Elsevier; 2018. Drug delivery for cardiac regeneration; pp. 283–321. [DOI] [Google Scholar]
- 68.Deng Y., Zhang X., Shen H., He Q., Wu Z., Liao W., Yuan M. Application of the nano-drug delivery system in treatment of cardiovascular diseases. Front. Bioeng. Biotechnol. 2020;7 doi: 10.3389/fbioe.2019.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prajnamitra R.P., Chen H.-C., Lin C.-J., Chen L.-L., Hsieh P.C.-H. Nanotechnology approaches in tackling cardiovascular diseases. Molecules. 2019;24 doi: 10.3390/molecules24102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh A.P., Biswas A., Shukla A., Maiti P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles, Signal Transduction and Targeted Therapy. 2019. 4, 1-21. [DOI] [PMC free article] [PubMed]
- 71.Liu Z., Robinson J.T., Tabakman S.M., Yang K., Dai H. Carbon materials for drug delivery & cancer therapy. Mater. Today. 2011;14:316–323. doi: 10.1016/S1369-7021(11)70161-4. [DOI] [Google Scholar]
- 72.Tripathi A.C., Saraf S.A., Saraf S.K. Carbon nanotropes: a contemporary paradigm in drug delivery. Materials. 2015;8:3068–3100. doi: 10.3390/ma8063068. [DOI] [Google Scholar]
- 73.Tripathi A.C., Saraf S.A., Saraf S.K. Carbon nanotropes: a contemporary paradigm in drug delivery. Materials. 2015;8:3068–3100. doi: 10.3390/ma8063068. [DOI] [Google Scholar]
- 74.Cheng R., Xue Y. Carbon nanomaterials for drug delivery. In: Zhang M., Naik R.R., Dai L., editors. Carbon Nanomaterials for Biomedical Applications. Springer International Publishing; Cham: 2016. pp. 31–80. [DOI] [Google Scholar]
- 75.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M. del P., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., Habtemariam S., Shin H.-S. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caldorera-Moore M., Guimard N., Shi L., Roy K. Designer nanoparticles: incorporating size, shape, and triggered release into nanoscale drug carriers. Expet Opin. Drug Deliv. 2010;7:479–495. doi: 10.1517/17425240903579971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goenka S., Sant V., Sant S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Contr. Release. 2014;173:75–88. doi: 10.1016/j.jconrel.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y., Nayak T.R., Hong H., Cai W. Graphene: a versatile nanoplatform for biomedical applications. Nanoscale. 2012;4:3833–3842. doi: 10.1039/C2NR31040F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCallion C., Burthem J., Rees-Unwin K., Golovanov A., Pluen A. Graphene in therapeutics delivery: problems, solutions and future opportunities. Eur. J. Pharm. Biopharm. 2016;104:235–250. doi: 10.1016/j.ejpb.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 80.Kaya D., Küçükada K., Alemdar N. Modeling the drug release from reduced graphene oxide-reinforced hyaluronic acid/gelatin/poly(ethylene oxide) polymeric films. Carbohydr. Polym. 2019;215:189–197. doi: 10.1016/j.carbpol.2019.03.041. [DOI] [PubMed] [Google Scholar]
- 81.Sarkar G., Saha N.R., Roy I., Bhattacharyya A., Adhikari A., Rana D., Bhowmik M., Bose M., Mishra R., Chattopadhyay D. Cross-linked methyl cellulose/graphene oxide rate controlling membranes for in vitro and ex vivo permeation studies of diltiazem hydrochloride. RSC Adv. 2016;6:36136–36145. doi: 10.1039/C5RA26358A. [DOI] [Google Scholar]
- 82.Deveza L., Choi J., Yang F. Therapeutic angiogenesis for treating cardiovascular diseases. Theranostics. 2012;2:801–814. doi: 10.7150/thno.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rufaihah A.J., Vaibavi S.R., Plotkin M., Shen J., Nithya V., Wang J., Seliktar D., Kofidis T. Enhanced infarct stabilization and neovascularization mediated by VEGF-loaded PEGylated fibrinogen hydrogel in a rodent myocardial infarction model. Biomaterials. 2013;34:8195–8202. doi: 10.1016/j.biomaterials.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 84.Rufaihah A.J., Johari N.A., Vaibavi S.R., Plotkin M., Di Thien D.T., Kofidis T., Seliktar D. Dual delivery of VEGF and ANG-1 in ischemic hearts using an injectable hydrogel. Acta Biomater. 2017;48:58–67. doi: 10.1016/j.actbio.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 85.Sun Z., Huang P., Tong G., Lin J., Jin A., Rong P., Zhu L., Nie L., Niu G., Cao F., Chen X. VEGF-loaded graphene oxide as theranostics for multi-modality imaging-monitored targeting therapeutic angiogenesis of ischemic muscle. Nanoscale. 2013;5:6857–6866. doi: 10.1039/c3nr01573d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paul A., Hasan A., Kindi H.A., Gaharwar A.K., Rao V.T.S., Nikkhah M., Shin S.R., Krafft D., Dokmeci M.R., Shum-Tim D., Khademhosseini A. Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano. 2014;8:8050–8062. doi: 10.1021/nn5020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee X.J., Lim H.N., Abdul Rahman M.B., Che Abdullah C.A., Muthoosamy K. Chapter 7 - functionalization of graphene for nanodelivery of drugs. In: Rashid S.A., Raja Othman R.N.I., Hussein M.Z., editors. Synthesis, Technology and Applications of Carbon Nanomaterials. Elsevier; 2019. pp. 157–176. [DOI] [Google Scholar]
- 88.Martín C., Kostarelos K., Prato M., Bianco A. Biocompatibility and biodegradability of 2D materials: graphene and beyond. Chem. Commun. 2019;55:5540–5546. doi: 10.1039/C9CC01205B. [DOI] [PubMed] [Google Scholar]
- 89.Wang H., Yu P., Gou H., Zhang J., Zhu M., Wang Z.-H., Tian J.-W., Jiang Y.-T., Fu F.-H. Cardioprotective effects of 20(S)-Ginsenoside Rh2 against doxorubicin-induced cardiotoxicity in vitro and in vivo. Evid Based Complement Alternat Med. 2012:506214. doi: 10.1155/2012/506214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zare-Zardini H., Taheri-Kafrani A., Ordooei M., Amiri A., Karimi-Zarchi M. Evaluation of toxicity of functionalized graphene oxide with ginsenoside Rh2, lysine and arginine on blood cancer cells (K562), red blood cells, blood coagulation and cardiovascular tissue: in vitro and in vivo studies. J. Taiwan Inst. Chem. Eng. 2018;93:70–78. doi: 10.1016/j.jtice.2018.08.010. [DOI] [Google Scholar]
- 91.Rastogi V., Yadav P., Bhattacharya S.S., Mishra A.K., Verma N., Verma A., Pandit J.K. Carbon nanotubes: an emerging drug carrier for targeting cancer cells. J. Drug Deliv. 2014;2014 doi: 10.1155/2014/670815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mehra N.K., Palakurthi S. Interactions between carbon nanotubes and bioactives: a drug delivery perspective. Drug Discov. Today. 2016;21:585–597. doi: 10.1016/j.drudis.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 93.Madani S.Y., Naderi N., Dissanayake O., Tan A., Seifalian A.M. A new era of cancer treatment: carbon nanotubes as drug delivery tools. Int. J. Nanomed. 2011;6:2963–2979. doi: 10.2147/IJN.S16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song C., Xia Y., Zhao M., Liu X., Li F., Huang B., Zhang H., Zhang B. Functionalization of silicon-doped single walled carbon nanotubes at the doping site: an ab initio study. Phys. Lett. 2006;358:166–170. doi: 10.1016/j.physleta.2006.05.003. [DOI] [Google Scholar]
- 95.Mirmotahari M., Sani E., Rad A.S., Khalilzadeh M.A. Calcium-doped single-wall nanotubes (Ca/SWCNTs) as a superior carrier for atropine drug delivery: a quantum-chemical study in gas and solvent phases. J. Biomol. Struct. Dyn. 2019;37:4267–4273. doi: 10.1080/07391102.2018.1546233. [DOI] [PubMed] [Google Scholar]
- 96.Hoseininezhad-Namin M.S., Pargolghasemi P., Alimohammadi S., Rad A.S., Taqavi L. Quantum Chemical Study on the adsorption of metformin drug on the surface of pristine, Si- and Al-doped (5, 5) SWCNTs. Phys. E Low-dimens. Syst. Nanostruct. 2017;90:204–213. doi: 10.1016/j.physe.2017.04.002. [DOI] [Google Scholar]
- 97.Sheikhi M., Shahab S., Khaleghian M., Hajikolaee F.H., Balakhanava I., Alnajjar R. Adsorption properties of the molecule resveratrol on CNT(8,0-10) nanotube: geometry optimization, molecular structure, spectroscopic (NMR, UV/Vis, excited state), FMO, MEP and HOMO-LUMO investigations. J. Mol. Struct. 2018;1160:479–487. doi: 10.1016/j.molstruc.2018.01.005. [DOI] [Google Scholar]
- 98.Bonnefont-Rousselot D. Resveratrol and cardiovascular diseases. Nutrients. 2016;8:250. doi: 10.3390/nu8050250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheng C.K., Luo J.-Y., Lau C.W., Chen Z.-Y., Tian X.Y., Huang Y. Pharmacological basis and new insights of resveratrol action in the cardiovascular system. Br. J. Pharmacol. 2020;177:1258–1277. doi: 10.1111/bph.14801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu W., Zhang G., Wu J., Zhang Y., Liu J., Luo H., Shao L. Insights into the angiogenic effects of nanomaterials: mechanisms involved and potential applications. J. Nanobiotechnol. 2020;18 doi: 10.1186/s12951-019-0570-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ho Y.T., Poinard B., Kah J.C.Y. Nanoparticle drug delivery systems and their use in cardiac tissue therapy. Nanomedicine. 2016;11:693–714. doi: 10.2217/nnm.16.6. [DOI] [PubMed] [Google Scholar]
- 102.Masotti A., Miller M.R., Celluzzi A., Rose L., Micciulla F., Hadoke P.W.F., Bellucci S., Caporali A. Regulation of angiogenesis through the efficient delivery of microRNAs into endothelial cells using polyamine-coated carbon nanotubes. Nanomedicine. 2016;12:1511–1522. doi: 10.1016/j.nano.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paul A., Shao W., Shum-Tim D., Prakash S. The attenuation of restenosis following arterial gene transfer using carbon nanotube coated stent incorporating TAT/DNAAng1+Vegf nanoparticles. Biomaterials. 2012;33:7655–7664. doi: 10.1016/j.biomaterials.2012.06.096. [DOI] [PubMed] [Google Scholar]
- 104.Zhang R., Olin H. Carbon nanomaterials as drug carriers: Real time drug release investigation. Mater. Sci. Eng. C. 2012;32:1247–1252. doi: 10.1016/j.msec.2012.03.016. [DOI] [Google Scholar]
- 105.Malima A., Siavoshi S., Musacchio T., Upponi J., Yilmaz C., Somu S., Hartner W., Torchilin V., Busnaina A. Highly sensitive microscale in vivo sensor enabled by electrophoretic assembly of nanoparticles for multiple biomarker detection. Lab Chip. 2012;12:4748–4754. doi: 10.1039/C2LC40580F. [DOI] [PubMed] [Google Scholar]
- 106.Gupta S., Sharma A., Verma R.S. Polymers in biosensor devices for cardiovascular applications. Curr. Opin. Biomed. Eng. 2020;13:69–75. doi: 10.1016/j.cobme.2019.10.002. [DOI] [Google Scholar]
- 107.Bakirhan N.K., Ozcelikay G., Ozkan S.A. Recent progress on the sensitive detection of cardiovascular disease markers by electrochemical-based biosensors. J. Pharmaceut. Biomed. Anal. 2018;159:406–424. doi: 10.1016/j.jpba.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 108.Shafiee A., Ghadiri E., Kassis J., Pourhabibi Zarandi N., Atala A. Biosensing technologies for medical applications, manufacturing, and regenerative medicine. Curr. Stem Cell Rep. 2018;4:105–115. doi: 10.1007/s40778-018-0123-y. [DOI] [Google Scholar]
- 109.Metkar S.K., Girigoswami K. Diagnostic biosensors in medicine – a review. Biocatal. Agric. Biotechnol. 2019;17:271–283. doi: 10.1016/j.bcab.2018.11.029. [DOI] [Google Scholar]
- 110.Bhattarai P., Hameed S. Nanobiosensors. John Wiley & Sons, Ltd; 2020. Basics of biosensors and nanobiosensors; pp. 1–22. [DOI] [Google Scholar]
- 111.Patel S., Nanda R., Sahoo S., Mohapatra E. Biosensors in health care: the milestones achieved in their development towards lab-on-chip-analysis. Biochem. Res. Int. 2016;2016 doi: 10.1155/2016/3130469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shedden L. 11 - biosensor technology in the treatment of cardiovascular disease. In: Gourlay T., Black R.A., editors. Biomaterials and Devices for the Circulatory System. Woodhead Publishing; 2010. pp. 286–308. [DOI] [Google Scholar]
- 113.Rezaee M., Behnam B., Banach M., Sahebkar A. The Yin and Yang of carbon nanomaterials in atherosclerosis. Biotechnol. Adv. 2018;36:2232–2247. doi: 10.1016/j.biotechadv.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 114.Esmaeil Heydari-Bafrooei, Ensafi Ali A. Chapter 4 - typically used carbon-based nanomaterials in the fabrication of biosensors. In: Ensafi A.A., editor. Electrochemical Biosensors. Elsevier; 2019. pp. 77–98. [DOI] [Google Scholar]
- 115.Negahdary M. Aptamers in nanostructure-based electrochemical biosensors for cardiac biomarkers and cancer biomarkers: a review. Biosens. Bioelectron. 2020;152:112018. doi: 10.1016/j.bios.2020.112018. [DOI] [PubMed] [Google Scholar]
- 116.Kumar S., Ahlawat W., Kumar R., Dilbaghi N. Graphene, carbon nanotubes, zinc oxide and gold as elite nanomaterials for fabrication of biosensors for healthcare. Biosens. Bioelectron. 2015;70:498–503. doi: 10.1016/j.bios.2015.03.062. [DOI] [PubMed] [Google Scholar]
- 117.Bai Y., Xu T., Zhang X. Graphene-based biosensors for detection of biomarkers. Micromachines. 2020;11 doi: 10.3390/mi11010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang H., Su S., Wu N., Wan H., Wan S., Bi H., Sun L. Graphene-based sensors for human health monitoring. Front. Chem. 2019;7 doi: 10.3389/fchem.2019.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lei Y.-M., Xiao M.-M., Li Y.-T., Xu L., Zhang H., Zhang Z.-Y., Zhang G.-J. Detection of heart failure-related biomarker in whole blood with graphene field effect transistor biosensor. Biosens. Bioelectron. 2017;91:1–7. doi: 10.1016/j.bios.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 120.Garg P., Morris P., Fazlanie A.L., Vijayan S., Dancso B., Dastidar A.G., Plein S., Mueller C., Haaf P. Cardiac biomarkers of acute coronary syndrome: from history to high-sensitivity cardiac troponin. Intern. Emerg. Med. 2017;12:147–155. doi: 10.1007/s11739-017-1612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Danese E., Montagnana M. An historical approach to the diagnostic biomarkers of acute coronary syndrome. Ann. Transl. Med. 2016;4 doi: 10.21037/atm.2016.05.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Demirbakan B., Kemal Sezgintürk M. A novel ultrasensitive immunosensor based on disposable graphite paper electrodes for troponin T detection in cardiovascular disease. Talanta. 2020;213:120779. doi: 10.1016/j.talanta.2020.120779. [DOI] [PubMed] [Google Scholar]
- 123.Kazemi S.H., Ghodsi E., Abdollahi S., Nadri S. Porous graphene oxide nanostructure as an excellent scaffold for label-free electrochemical biosensor: detection of cardiac troponin I. Mater. Sci. Eng. C. 2016;69:447–452. doi: 10.1016/j.msec.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 124.Mansuriya B.D., Altintas Z. Applications of graphene quantum dots in biomedical sensors. Sensors. 2020;20 doi: 10.3390/s20041072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lakshmanakumar M., Nesakumar N., Sethuraman S., Rajan K.S., Krishnan U.M., Rayappan J.B.B. Functionalized graphene quantum dot interfaced electrochemical detection of cardiac troponin I: an antibody free approach. Sci. Rep. 2019;9:17348. doi: 10.1038/s41598-019-53979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Y.-H., Huang K.-J., Wu X. Recent advances in transition-metal dichalcogenides based electrochemical biosensors: a review. Biosens. Bioelectron. 2017;97:305–316. doi: 10.1016/j.bios.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 127.Nezami A., Dehghani S., Nosrati R., Eskandari N., Taghdisi S.M., Karimi G. Nanomaterial-based biosensors and immunosensors for quantitative determination of cardiac troponins. J. Pharmaceut. Biomed. Anal. 2018;159:425–436. doi: 10.1016/j.jpba.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 128.Chauhan D., Pooja, Nirbhaya V., Srivastava C.M., Chandra R., Kumar S. Nanostructured transition metal chalcogenide embedded on reduced graphene oxide based highly efficient biosensor for cardiovascular disease detection. Microchem. J. 2020;155:104697. doi: 10.1016/j.microc.2020.104697. [DOI] [Google Scholar]
- 129.Foo M.E., Gopinath S.C.B. Feasibility of graphene in biomedical applications. Biomed. Pharmacother. 2017;94:354–361. doi: 10.1016/j.biopha.2017.07.122. [DOI] [PubMed] [Google Scholar]
- 130.Volder M.F.L.D., Tawfick S.H., Baughman R.H., Hart A.J. Carbon nanotubes: present and future commercial applications. Science. 2013;339:535–539. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- 131.Sireesha M., Babu V.J., Kiran A.S.K., Ramakrishna S. A review on carbon nanotubes in biosensor devices and their applications in medicine. Nanocomposites. 2018;4:36–57. doi: 10.1080/20550324.2018.1478765. [DOI] [Google Scholar]
- 132.Vardharajula S., Ali S.Z., Tiwari P.M., Eroğlu E., Vig K., Dennis V.A., Singh S.R. Functionalized carbon nanotubes: biomedical applications. Int. J. Nanomed. 2012;7:5361–5374. doi: 10.2147/IJN.S35832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhu Z. An Overview of carbon nanotubes and graphene for biosensing applications. Nano-Micro Lett. 2017;9:25. doi: 10.1007/s40820-017-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang N., Chen X., Ren T., Zhang P., Yang D. Carbon nanotube based biosensors. Sensor. Actuator. B Chem. 2015;207:690–715. doi: 10.1016/j.snb.2014.10.040. [DOI] [Google Scholar]
- 135.Freitas T.A., Mattos A.B., Silva B.V.M., Dutra R.F. Amino-functionalization of carbon nanotubes by using a factorial design: human cardiac troponin T immunosensing application. BioMed. Res. Int. 2014 doi: 10.1155/2014/929786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Phonklam K., Wannapob R., Sriwimol W., Thavarungkul P., Phairatana T. A novel molecularly imprinted polymer PMB/MWCNTs sensor for highly-sensitive cardiac troponin T detection. Sensor. Actuator. B Chem. 2020;308:127630. doi: 10.1016/j.snb.2019.127630. [DOI] [Google Scholar]
- 137.Eissa S., Almusharraf A.Y., Zourob M. A comparison of the performance of voltammetric aptasensors for glycated haemoglobin on different carbon nanomaterials-modified screen printed electrodes. Mater. Sci. Eng. C. 2019;101:423–430. doi: 10.1016/j.msec.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 138.Heusch G., Gersh B.J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur. Heart J. 2017;38:774–784. doi: 10.1093/eurheartj/ehw224. [DOI] [PubMed] [Google Scholar]
- 139.Gabriel-Costa D. The pathophysiology of myocardial infarction-induced heart failure. Pathophysiology. 2018;25:277–284. doi: 10.1016/j.pathophys.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 140.Nguyen A.H., Marsh P., Schmiess-Heine L., Burke P.J., Lee A., Lee J., Cao H. Cardiac tissue engineering: state-of-the-art methods and outlook. J. Biol. Eng. 2019;13:57. doi: 10.1186/s13036-019-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Coffee M., Biswanath S., Bolesani E., Zweigerdt R. Heart muscle tissue engineering. In: Brand-Saberi B., editor. Essential Current Concepts in Stem Cell Biology. Springer International Publishing; Cham: 2020. pp. 99–121. [DOI] [Google Scholar]
- 142.Loh K.P., Ho D., Chiu G.N.C., Leong D.T., Pastorin G., Chow E.K.-H. Clinical applications of carbon nanomaterials in diagnostics and therapy. Adv. Mater. 2018;30 doi: 10.1002/adma.201802368. [DOI] [PubMed] [Google Scholar]
- 143.Dozois M.D., Bahlmann L.C., Zilberman Y., Shirley X.(, Tang Carbon nanomaterial-enhanced scaffolds for the creation of cardiac tissue constructs: a new frontier in cardiac tissue engineering. Carbon. 2017;120:338–349. doi: 10.1016/j.carbon.2017.05.050. [DOI] [Google Scholar]
- 144.Ashtari K., Nazari H., Ko H., Tebon P., Akhshik M., Akbari M., Alhosseini S.N., Mozafari M., Mehravi B., Soleimani M., Ardehali R., Ebrahimi Warkiani M., Ahadian S., Khademhosseini A. Electrically conductive nanomaterials for cardiac tissue engineering. Adv. Drug Deliv. Rev. 2019;144:162–179. doi: 10.1016/j.addr.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hitscherich P., Aphale A., Gordan R., Whitaker R., Singh P., Xie L., Patra P., Lee E.J. Electroactive graphene composite scaffolds for cardiac tissue engineering. J. Biomed. Mater. Res. 2018;106:2923–2933. doi: 10.1002/jbm.a.36481. [DOI] [PubMed] [Google Scholar]
- 146.Qu Y., He F., Yu C., Liang X., Liang D., Ma L., Zhang Q., Lv J., Wu J. Advances on graphene-based nanomaterials for biomedical applications. Mater. Sci. Eng. C. 2018;90:764–780. doi: 10.1016/j.msec.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 147.Ahadian S., Zhou Y., Yamada S., Estili M., Liang X., Nakajima K., Shiku H., Matsue T. Graphene induces spontaneous cardiac differentiation in embryoid bodies. Nanoscale. 2016;8:7075–7084. doi: 10.1039/C5NR07059G. [DOI] [PubMed] [Google Scholar]
- 148.Pfeiffer E.R., Tangney J.R., Omens J.H., McCulloch A.D. Biomechanics of cardiac electromechanical coupling and mechanoelectric feedback. J. Biomech. Eng. 2014;136 doi: 10.1115/1.4026221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jiang L., Chen D., Wang Z., Zhang Z., Xia Y., Xue H., Liu Y. Preparation of an electrically conductive graphene oxide/chitosan scaffold for cardiac tissue engineering. Appl. Biochem. Biotechnol. 2019;188:952–964. doi: 10.1007/s12010-019-02967-6. [DOI] [PubMed] [Google Scholar]
- 150.Nazari H., Azadi S., Hatamie S., Zomorrod M.S., Ashtari K., Soleimani M., Hosseinzadeh S. Fabrication of graphene-silver/polyurethane nanofibrous scaffolds for cardiac tissue engineering. Polym. Adv. Technol. 2019;30:2086–2099. doi: 10.1002/pat.4641. [DOI] [Google Scholar]
- 151.Saravanan S., Sareen N., Abu-El-Rub E., Ashour H., Sequiera G.L., Ammar H.I., Gopinath V., Shamaa A.A., Sayed S.S.E., Moudgil M., Vadivelu J., Dhingra S. Graphene oxide-gold nanosheets containing chitosan scaffold improves ventricular contractility and function after implantation into infarcted heart. Sci. Rep. 2018;8:15069. doi: 10.1038/s41598-018-33144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Alagarsamy K.N., Yan W., Srivastava A., Desiderio V., Dhingra S. Application of injectable hydrogels for cardiac stem cell therapy and tissue engineering. Rev. Cardiovasc. Med. 2019;20:221–230. doi: 10.31083/j.rcm.2019.04.534. [DOI] [PubMed] [Google Scholar]
- 153.Freytes D.O., O'Neill J.D., Duan-Arnold Y., Wrona E., Vunjak-Novakovic G. Native cardiac extracellular matrix hydrogels for cultivation of human stem cell-derived cardiomyocytes. Methods Mol. Biol. 2014;1181:69–81. doi: 10.1007/978-1-4939-1047-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bao R., Tan B., Liang S., Zhang N., Wang W., Liu W. A π-π conjugation-containing soft and conductive injectable polymer hydrogel highly efficiently rebuilds cardiac function after myocardial infarction. Biomaterials. 2017;122:63–71. doi: 10.1016/j.biomaterials.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 155.Zhou J., Yang X., Liu W., Wang C., Shen Y., Zhang F., Zhu H., Sun H., Chen J., Lam J., Mikos A.G., Wang C. Injectable OPF/graphene oxide hydrogels provide mechanical support and enhance cell electrical signaling after implantation into myocardial infarct. Theranostics. 2018;8:3317–3330. doi: 10.7150/thno.25504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gandhimathi C., Muthukumaran P., Srinivasan D.K. 17 - nanofiber composites in cardiac tissue engineering. In: Ramalingam M., Ramakrishna S., editors. Nanofiber Composites for Biomedical Applications. Woodhead Publishing; 2017. pp. 411–453. [DOI] [Google Scholar]
- 157.Hasan A., Waters R., Roula B., Dana R., Yara S., Alexandre T., Paul A. Engineered biomaterials to enhance stem cell-based cardiac tissue engineering and therapy. Macromol. Biosci. 2016;16:958–977. doi: 10.1002/mabi.201500396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Norahan M.H., Amroon M., Ghahremanzadeh R., Mahmoodi M., Baheiraei N. Electroactive graphene oxide-incorporated collagen assisting vascularization for cardiac tissue engineering. J. Biomed. Mater. Res. 2019;107:204–219. doi: 10.1002/jbm.a.36555. [DOI] [PubMed] [Google Scholar]
- 159.Newman P., Minett A., Ellis-Behnke R., Zreiqat H. Carbon nanotubes: their potential and pitfalls for bone tissue regeneration and engineering. Nanomed. Nanotechnol. Biol. Med. 2013;9:1139–1158. doi: 10.1016/j.nano.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 160.Redondo-Gómez C., Leandro-Mora R., Blanch-Bermúdez D., Espinoza-Araya C., Hidalgo-Barrantes D., Vega-Baudrit J. Recent advances in carbon nanotubes for nervous tissue regeneration. Adv. Polym. Technol. 2020 https://doi-org.uml.idm.oclc.org/10.1155/2020/6861205 [Google Scholar]
- 161.Ho C.M.B., Mishra A., Lin P.T.P., Ng S.H., Yeong W.Y., Kim Y.-J., Yoon Y.-J. 3D printed polycaprolactone carbon nanotube composite scaffolds for cardiac tissue engineering. Macromol. Biosci. 2017;17 doi: 10.1002/mabi.201600250. [DOI] [PubMed] [Google Scholar]
- 162.Bosi S., Ballerini L., Prato M. Carbon nanotubes in tissue engineering. Top. Curr. Chem. 2014;348:181–204. doi: 10.1007/128_2013_474. [DOI] [PubMed] [Google Scholar]
- 163.Ahadian S., Yamada S., Estili M., Liang X., Banan Sadeghian R., Nakajima K., Shiku H., Matsue T., Khademhosseini A. Carbon nanotubes embedded in embryoid bodies direct cardiac differentiation. Biomed. Microdevices. 2017;19:57. doi: 10.1007/s10544-017-0184-1. [DOI] [PubMed] [Google Scholar]
- 164.Ren J., Xu Q., Chen X., Li W., Guo K., Zhao Y., Wang Q., Zhang Z., Peng H., Li Y.-G. Superaligned carbon nanotubes guide oriented cell growth and promote electrophysiological homogeneity for synthetic cardiac tissues. Adv. Mater. 2017;29:1702713. doi: 10.1002/adma.201702713. [DOI] [PubMed] [Google Scholar]
- 165.Kankala R.K., Zhu K., Sun X.-N., Liu C.-G., Wang S.-B., Chen A.-Z. Cardiac tissue engineering on the nanoscale. ACS Biomater. Sci. Eng. 2018;4:800–818. doi: 10.1021/acsbiomaterials.7b00913. [DOI] [PubMed] [Google Scholar]
- 166.Coenen A.M.J., Bernaerts K.V., Harings J.A.W., Jockenhoevel S., Ghazanfari S. Elastic materials for tissue engineering applications: natural, synthetic, and hybrid polymers. Acta Biomater. 2018;79:60–82. doi: 10.1016/j.actbio.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 167.Ahadian S., Davenport Huyer L., Estili M., Yee B., Smith N., Xu Z., Sun Y., Radisic M. Moldable elastomeric polyester-carbon nanotube scaffolds for cardiac tissue engineering. Acta Biomater. 2017;52:81–91. doi: 10.1016/j.actbio.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 168.Yu H., Zhao H., Huang C., Du Y. Mechanically and electrically enhanced CNT–collagen hydrogels as potential scaffolds for engineered cardiac constructs. ACS Biomater. Sci. Eng. 2017;3:3017–3021. doi: 10.1021/acsbiomaterials.6b00620. [DOI] [PubMed] [Google Scholar]
- 169.Camci-Unal G., Annabi N., Dokmeci M.R., Liao R., Khademhosseini A. Hydrogels for cardiac tissue engineering. NPG Asia Mater. 2014;6 doi: 10.1038/am.2014.19. e99–e99. [DOI] [Google Scholar]
- 170.Mombini S., Mohammadnejad J., Bakhshandeh B., Narmani A., Nourmohammadi J., Vahdat S., Zirak S. Chitosan-PVA-CNT nanofibers as electrically conductive scaffolds for cardiovascular tissue engineering. Int. J. Biol. Macromol. 2019;140:278–287. doi: 10.1016/j.ijbiomac.2019.08.046. [DOI] [PubMed] [Google Scholar]
- 171.Lee J., Manoharan V., Cheung L., Lee S., Cha B.-H., Newman P., Farzad R., Mehrotra S., Zhang K., Khan F., Ghaderi M., Lin Y.-D., Aftab S., Mostafalu P., Miscuglio M., Li J., Mandal B.B., Hussain M.A., Wan K., Tang X.S., Khademhosseini A., Shin S.R. Nanoparticle-based hybrid scaffolds for deciphering the role of multimodal cues in cardiac tissue engineering. ACS Nano. 2019;13:12525–12539. doi: 10.1021/acsnano.9b03050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Talman V., Ruskoaho H. Cardiac fibrosis in myocardial infarction—from repair and remodeling to regeneration. Cell Tissue Res. 2016;365:563–581. doi: 10.1007/s00441-016-2431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Min Nian, Lee Paul, Neelam Khaper, Liu Peter. Inflammatory cytokines and postmyocardial infarction remodeling. Circ. Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 174.Sattler S., Fairchild P., Watt F.M., Rosenthal N., Harding S.E. The adaptive immune response to cardiac injury—the true roadblock to effective regenerative therapies? Npj Regen. Med. 2017;2:1–5. doi: 10.1038/s41536-017-0022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wagner M.J., Khan M., Mohsin S. Healing the broken heart; the immunomodulatory effects of stem cell therapy. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Panahi M., Papanikolaou A., Torabi A., Zhang J.-G., Khan H., Vazir A., Hasham M.G., Cleland J.G.F., Rosenthal N.A., Harding S.E., Sattler S. Immunomodulatory interventions in myocardial infarction and heart failure: a systematic review of clinical trials and meta-analysis of IL-1 inhibition. Cardiovasc. Res. 2018;114:1445–1461. doi: 10.1093/cvr/cvy145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Allen S., Liu Y.-G., Scott E. Engineering nanomaterials to address cell-mediated inflammation in atherosclerosis. Regen. Eng. Transl. Med. 2016;2:37–50. doi: 10.1007/s40883-016-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Orecchioni M., Ménard-Moyon C., Delogu L.G., Bianco A. Graphene and the immune system: challenges and potentiality. Adv. Drug Deliv. Rev. 2016;105:163–175. doi: 10.1016/j.addr.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 179.Malanagahalli S., Murera D., Martín C., Lin H., Wadier N., Dumortier H., Vázquez E., Bianco A. Few layer graphene does not affect cellular homeostasis of mouse macrophages. Nanomaterials. 2020;10:228. doi: 10.3390/nano10020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mukherjee S.P., Bottini M., Fadeel B. Graphene and the immune system: a romance of many dimensions. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tomić S., Janjetović K., Mihajlović D., Milenković M., Kravić-Stevović T., Marković Z., Todorović-Marković B., Spitalsky Z., Micusik M., Vučević D., Čolić M., Trajković V. Graphene quantum dots suppress proinflammatory T cell responses via autophagy-dependent induction of tolerogenic dendritic cells. Biomaterials. 2017;146:13–28. doi: 10.1016/j.biomaterials.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 182.Wang B., Su X., Liang J., Yang L., Hu Q., Shan X., Wan J., Hu Z. Synthesis of polymer-functionalized nanoscale graphene oxide with different surface charge and its cellular uptake, biosafety and immune responses in Raw264.7 macrophages. Mater. Sci. Eng. C. 2018;90:514–522. doi: 10.1016/j.msec.2018.04.096. [DOI] [PubMed] [Google Scholar]
- 183.Ma Y., Mouton A.J., Lindsey M.L. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl. Res. 2018;191:15–28. doi: 10.1016/j.trsl.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Han J., Kim Y.S., Lim M.-Y., Kim H.Y., Kong S., Kang M., Choo Y.W., Jun J.H., Ryu S., Jeong H., Park J., Jeong G.-J., Lee J.-C., Eom G.H., Ahn Y., Kim B.-S. Dual roles of graphene oxide to attenuate inflammation and elicit timely polarization of macrophage phenotypes for cardiac repair. ACS Nano. 2018;12:1959–1977. doi: 10.1021/acsnano.7b09107. [DOI] [PubMed] [Google Scholar]
- 185.Li Y.R., Santo A., Zhu H., Jia Z., Trush M.A. Graphene quantum dots protect against copper redox-mediated free radical generation and cardiac cell injury. React. Oxyg. Species (Apex) 2018;6:338–348. doi: 10.20455/ros.2018.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Park J., Kim B., Han J., Oh J., Park S., Ryu S., Jung S., Shin J.-Y., Lee B.S., Hong B.H., Choi D., Kim B.-S. Graphene oxide flakes as a cellular adhesive: prevention of reactive oxygen species mediated death of implanted cells for cardiac repair. ACS Nano. 2015;9:4987–4999. doi: 10.1021/nn507149w. [DOI] [PubMed] [Google Scholar]
- 187.Choe G., Kim S.-W., Park J., Park J., Kim S., Kim Y.S., Ahn Y., Jung D.-W., Williams D.R., Lee J.Y. Anti-oxidant activity reinforced reduced graphene oxide/alginate microgels: mesenchymal stem cell encapsulation and regeneration of infarcted hearts. Biomaterials. 2019;225:119513. doi: 10.1016/j.biomaterials.2019.119513. [DOI] [PubMed] [Google Scholar]
- 188.Orecchioni M., Bedognetti D., Sgarrella F., Marincola F.M., Bianco A., Delogu L.G. Impact of carbon nanotubes and graphene on immune cells. J. Transl. Med. 2014;12:138. doi: 10.1186/1479-5876-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pondman K.M., Salvador-Morales C., Paudyal B., Sim R.B., Kishore U. Interactions of the innate immune system with carbon nanotubes. Nanoscale Horizons. 2017;2:174–186. doi: 10.1039/C6NH00227G. [DOI] [PubMed] [Google Scholar]
- 190.Sun X., Shao H., Xiang K., Yan Y., Yu X., Li D., Wu W., Zhou L., So K.-F., Ren Y., Ramakrishna S., Li A., He L. Poly(dopamine)-modified carbon nanotube multilayered film and its effects on macrophages. Carbon. 2017;113:176–191. doi: 10.1016/j.carbon.2016.11.040. [DOI] [Google Scholar]
- 191.Aldinucci A., Turco A., Biagioli T., Toma F.M., Bani D., Guasti D., Manuelli C., Rizzetto L., Cavalieri D., Massacesi L., Mello T., Scaini D., Bianco A., Ballerini L., Prato M., Ballerini C. Carbon nanotube scaffolds instruct human dendritic cells: modulating immune responses by contacts at the nanoscale. Nano Lett. 2013;13:6098–6105. doi: 10.1021/nl403396e. [DOI] [PubMed] [Google Scholar]
- 192.Mitchell L.A., Lauer F.T., Burchiel S.W., McDonald J.D. Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat. Nanotechnol. 2009;4:451–456. doi: 10.1038/nnano.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pochkaeva E.I., Podolsky N.E., Zakusilo D.N., Petrov A.V., Charykov N.A., Vlasov T.D., Penkova A.V., Vasina L.V., Murin I.V., Sharoyko V.V., Semenov K.N. Fullerene derivatives with amino acids, peptides and proteins: from synthesis to biomedical application. Prog. Solid State Chem. 2020;57:100255. doi: 10.1016/j.progsolidstchem.2019.100255. [DOI] [Google Scholar]
- 194.Goodarzi S., Da Ros T., Conde J., Sefat F., Mozafari M. Fullerene: biomedical engineers get to revisit an old friend. Mater. Today. 2017;20:460–480. doi: 10.1016/j.mattod.2017.03.017. [DOI] [Google Scholar]
- 195.Rehman A., Houshyar S., Wang X. Nanodiamond in composite: biomedical application. J. Biomed. Mater. Res. 2020;108:906–922. doi: 10.1002/jbm.a.36868. [DOI] [PubMed] [Google Scholar]
- 196.Gao G., Guo Q., Zhi J. Nanodiamond-based theranostic platform for drug delivery and bioimaging. Small. 2019;15:1902238. doi: 10.1002/smll.201902238. [DOI] [PubMed] [Google Scholar]
- 197.Kroto H.W., Heath J.R., O'Brien S.C., Curl R.F., Smalley R.E. C 60 : buckminsterfullerene. Nature. 1985;318:162–163. doi: 10.1038/318162a0. [DOI] [Google Scholar]
- 198.Melanko J.B., Pearce M.E., Salem A.K., Nanotubes Nanorods. Nanofibers, and fullerenes for nanoscale drug delivery. In: de Villiers M.M., Aramwit P., Kwon G.S., editors. Nanotechnology in Drug Delivery. Springer; New York, NY: 2009. pp. 105–127. [DOI] [Google Scholar]
- 199.Hao T., Zhou J., Lü S., Yang B., Wang Y., Fang W., Jiang X., Lin Q., Li J., Wang C. Fullerene mediates proliferation and cardiomyogenic differentiation of adipose-derived stem cells via modulation of MAPK pathway and cardiac protein expression. Int. J. Nanomed. 2016;11:269–283. doi: 10.2147/IJN.S95863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hao T., Li J., Yao F., Dong D., Wang Y., Yang B., Wang C. Injectable fullerenol/alginate hydrogel for suppression of oxidative stress damage in Brown adipose-derived stem cells and cardiac repair. ACS Nano. 2017;11:5474–5488. doi: 10.1021/acsnano.7b00221. [DOI] [PubMed] [Google Scholar]
- 201.Liu J.-H., Yang S.-T., Chen X.-X., Wang H. Fluorescent carbon dots and nanodiamonds for biological imaging: preparation, application, pharmacokinetics and toxicity. Curr. Drug Metabol. 2012;13:1046–1056. doi: 10.2174/138920012802850083. [DOI] [PubMed] [Google Scholar]
- 202.Zhao W., Xu J.-J., Qiu Q.-Q., Chen H.-Y. Nanocrystalline diamond modified gold electrode for glucose biosensing. Biosens. Bioelectron. 2006;22:649–655. doi: 10.1016/j.bios.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 203.Wang C., Li J., Kang M., Huang X., Liu Y., Zhou N., Zhang Z. Nanodiamonds and hydrogen-substituted graphdiyne heteronanostructure for the sensitive impedimetric aptasensing of myocardial infarction and cardiac troponin I. Anal. Chim. Acta. 2021;1141:110–119. doi: 10.1016/j.aca.2020.10.044. [DOI] [PubMed] [Google Scholar]
- 204.Yan L., Zhao F., Wang J., Zu Y., Gu Z., Zhao Y. A safe-by-design strategy towards safer nanomaterials in nanomedicines. Adv. Mater. 2019;31:1805391. doi: 10.1002/adma.201805391. [DOI] [PubMed] [Google Scholar]
- 205.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic: an update. Bioeng. Transl. Med. 2019;4 doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.D'Mello S.R., Cruz C.N., Chen M.-L., Kapoor M., Lee S.L., Tyner K.M. The evolving landscape of drug products containing nanomaterials in the United States. Nat. Nanotechnol. 2017;12:523–529. doi: 10.1038/nnano.2017.67. [DOI] [PubMed] [Google Scholar]
- 207.Lammers T., Ferrari M. The success of nanomedicine. Nano Today. 2020;31:100853. doi: 10.1016/j.nantod.2020.100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.ClinicalTrials.gov Identifier: NCT02008032 Comparison of stationary breast tomosynthesis and 2-D digital mammography in patients with breast augmentation. 2018. https://clinicaltrials.gov/ct2/show/NCT02008032
- 209.Amal H., Leja M., Funka K., Skapars R., Sivins A., Ancans G., Liepniece-Karele I., Kikuste I., Lasina I., Haick H. Detection of precancerous gastric lesions and gastric cancer through exhaled breath. Gut. 2016;65:400–407. doi: 10.1136/gutjnl-2014-308536. [DOI] [PubMed] [Google Scholar]
- 210.ClinicalTrials.gov Identifier: NCT02123407 Clinical study on the harvesting lymph nodes with carbon nanoparticles for advanced gastric cancer: a prospective randomized study. 2014. https://clinicaltrials.gov/ct2/show/NCT02123407 [DOI] [PMC free article] [PubMed]
- 211.Finberg J.P.M., Schwartz M., Jeries R., Badarny S., Nakhleh M.K., Abu Daoud E., Ayubkhanov Y., Aboud-Hawa M., Broza Y.Y., Haick H. Sensor array for detection of early stage Parkinson's disease before medication. ACS Chem. Neurosci. 2018;9:2548–2553. doi: 10.1021/acschemneuro.8b00245. [DOI] [PubMed] [Google Scholar]
- 212.ClinicalTrials.gov Identifier: NCT03705754 Effect OF endoscopic tattooing with carbon black suspension on the staging of colon cancer. 2020. https://clinicaltrials.gov/ct2/show/NCT03705754
- 213.ClinicalTrials.gov Identifier: NCT02698163 Nanodiamond modified gutta percha (NDGP) composite for non-surgical root canal therapy (RCT) filler material applied by vertical condensation obturation procedure. 2020. https://clinicaltrials.gov/ct2/show/NCT02698163
- 214.ClinicalTrials.gov Identifier: NCT04390490 Clinical trails of photoelectrochemical immunosensor for early diagnosis of acute myocardial infarction. 2020. https://clinicaltrials.gov/ct2/show/NCT04390490
- 215.Kim M., Jang J., Cha C. Carbon nanomaterials as versatile platforms for theranostic applications. Drug Discov. Today. 2017;22:1430–1437. doi: 10.1016/j.drudis.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 216.Hassan A.A., Mansour M.K., Sayed El Ahl R.M.H., El Hamaky A.M.A., Oraby N.H. 23 - toxic and beneficial effects of carbon nanomaterials on human and animal health. In: Abd-Elsalam K.A., editor. Carbon Nanomaterials for Agri-Food and Environmental Applications. Elsevier; 2020. pp. 535–555. [DOI] [Google Scholar]
- 217.Raphey V.R., Henna T.K., Nivitha K.P., Mufeedha P., Sabu C., Pramod K. Advanced biomedical applications of carbon nanotube. Mater. Sci. Eng. C. 2019;100:616–630. doi: 10.1016/j.msec.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 218.Gurunathan S., Kim J.-H. Synthesis, toxicity, biocompatibility, and biomedical applications of graphene and graphene-related materials. Int. J. Nanomed. 2016;11:1927–1945. doi: 10.2147/IJN.S105264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Magrez A., Kasas S., Salicio V., Pasquier N., Seo J.W., Celio M., Catsicas S., Schwaller B., Forró L. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006;6:1121–1125. doi: 10.1021/nl060162e. [DOI] [PubMed] [Google Scholar]
- 220.Madannejad R., Shoaie N., Jahanpeyma F., Darvishi M.H., Azimzadeh M., Javadi H. Toxicity of carbon-based nanomaterials: reviewing recent reports in medical and biological systems. Chem. Biol. Interact. 2019;307:206–222. doi: 10.1016/j.cbi.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 221.Gupta T.K., Budarapu P.R., Chappidi S.R., Y B S.S., Paggi M., Bordas S.P. Advances in carbon based nanomaterials for bio-medical applications. Curr. Med. Chem. 2019;26:6851–6877. doi: 10.2174/0929867326666181126113605. [DOI] [PubMed] [Google Scholar]
- 222.Seabra A.B., Paula A.J., de Lima R., Alves O.L., Durán N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014;27:159–168. doi: 10.1021/tx400385x. [DOI] [PubMed] [Google Scholar]
- 223.Dasari Shareena T.P., McShan D., Dasmahapatra A.K., Tchounwou P.B. A review on graphene-based nanomaterials in biomedical applications and risks in environment and health. Nano-Micro Lett. 2018;10:53. doi: 10.1007/s40820-018-0206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bostan H.B., Rezaee R., Valokala M.G., Tsarouhas K., Golokhvast K., Tsatsakis A.M., Karimi G. Cardiotoxicity of nano-particles. Life Sci. 2016;165:91–99. doi: 10.1016/j.lfs.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 225.Duch M.C., Budinger G.R.S., Liang Y.T., Soberanes S., Urich D., Chiarella S.E., Campochiaro L.A., Gonzalez A., Chandel N.S., Hersam M.C., Mutlu G.M. Minimizing oxidation and stable nanoscale dispersion improves the biocompatibility of graphene in the lung. Nano Lett. 2011;11:5201–5207. doi: 10.1021/nl202515a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chen Z., Yu C., Khan I.A., Tang Y., Liu S., Yang M. Toxic effects of different-sized graphene oxide particles on zebrafish embryonic development. Ecotoxicol. Environ. Saf. 2020;197:110608. doi: 10.1016/j.ecoenv.2020.110608. [DOI] [PubMed] [Google Scholar]
- 227.Arbo M.D., Altknecht L.F., Cattani S., Braga W.V., Peruzzi C.P., Cestonaro L.V., Göethel G., Durán N., Garcia S.C. In vitro cardiotoxicity evaluation of graphene oxide. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019;841:8–13. doi: 10.1016/j.mrgentox.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 228.Manjunatha B., Park S.H., Kim K., Kundapur R.R., Lee S.J. Pristine graphene induces cardiovascular defects in zebrafish (Danio rerio) embryogenesis. Environ. Pollut. 2018;243:246–254. doi: 10.1016/j.envpol.2018.08.058. [DOI] [PubMed] [Google Scholar]
- 229.Ema M., Hougaard K.S., Kishimoto A., Honda K. Reproductive and developmental toxicity of carbon-based nanomaterials: a literature review. Nanotoxicology. 2016;10:391–412. doi: 10.3109/17435390.2015.1073811. [DOI] [PubMed] [Google Scholar]
- 230.Madani S.Y., Mandel A., Seifalian A.M. A concise review of carbon nanotube's toxicology. Nano Rev. 2013;4 doi: 10.3402/nano.v4i0.21521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Snyder-Talkington B.N., Dong C., Porter D.W., Ducatman B., Wolfarth M.G., Andrew M., Battelli L., Raese R., Castranova V., Guo N.L., Qian Y. Multiwalled carbon nanotube-induced pulmonary inflammatory and fibrotic responses and genomic changes following aspiration exposure in mice: a 1-year postexposure study. J. Toxicol. Environ. Health. 2016;79:352–366. doi: 10.1080/15287394.2016.1159635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ema M., Gamo M., Honda K. A review of toxicity studies of single-walled carbon nanotubes in laboratory animals. Regul. Toxicol. Pharmacol. 2016;74:42–63. doi: 10.1016/j.yrtph.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 233.Zheng W., McKinney W., Kashon M.L., Pan D., Castranova V., Kan H. The effects of inhaled multi-walled carbon nanotubes on blood pressure and cardiac function. Nanoscale Res. Lett. 2018;13 doi: 10.1186/s11671-018-2603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tong H., McGee J.K., Saxena R.K., Kodavanti U.P., Devlin R.B., Gilmour M.I. Influence of acid functionalization on the cardiopulmonary toxicity of carbon nanotubes and carbon black particles in mice. Toxicol. Appl. Pharmacol. 2009;239:224–232. doi: 10.1016/j.taap.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 235.Harik V.M. Geometry of carbon nanotubes and mechanisms of phagocytosis and toxic effects. Toxicol. Lett. 2017;273:69–85. doi: 10.1016/j.toxlet.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 236.Biological response and developmental toxicity of zebrafish embryo and larvae exposed to multi-walled carbon nanotubes with different dimension. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02308. [DOI] [PMC free article] [PubMed] [Google Scholar]