Highlights
-
•
Abscopal effect is the ability of local RT to induce systemic antitumor effect.
-
•
Abscopal effect is increasingly frequent with concomitant immunotherapy and RT.
-
•
Abscopal effect remains a rare observation.
-
•
We report an abscopal effect in MPM treated with concomitant anti-PD-L1 and RT.
Keywords: Abscopal effect, Malignant pleural mesothelioma, Radiotherapy, Immunotherapy, Checkpoint inhibitors
Abstract
The abscopal effect describes the ability of locally administered radiotherapy to induce systemic antitumor effects. Although mentioned for the first time in the 1950s, records of abscopal effects, considered to be immune-mediated, are scarce with radiotherapy alone. However, with the continued development and use of immunotherapy, reports on the abscopal effect have become increasingly frequent during the last decade. Here, we report a patient with advanced malignant pleural mesothelioma who had progressive disease while on the anti-PDL1 inhibitor pembrolizumab and showed an abscopal response after palliative radiotherapy.
1. Introduction
The introduction of immune-checkpoint inhibitors (ICI) has led to a paradigm shift in the treatment of patients with metastatic cancer. However, only a minority of patients across tumor types respond to these therapies [1]. Recently, several phase II studies investigating programmed death-ligand 1 PD-(L) 1 inhibitors in malignant pleural mesothelioma (MPM) show overall response rates (ORR) between 10 and 29% [2] and efforts are underway to improve therapeutic outcomes by combining immunotherapy with traditional oncological interventions like chemotherapy, anti-angiogenic therapy and radiotherapy (RT).
The abscopal effect refers to a rare phenomenon of tumor regression at a site distant from the primary site of RT. Localized RT induces abscopal effects in several types of cancer, including melanoma, lymphoma, and renal-cell carcinoma [3], [4]. The biologic characteristics underlying this effect are not fully understood, but in pre-clinical models and in patients, local irradiation can promote the release of danger-associated molecular patterns leading to the recruitment of immune cells, thereby inducing a systemic response to tumor antigens that protect against local disease relapse and also mediate distant antineoplastic effects [3], [4].
We report here a patient with advanced MPM who had progressive disease while on the anti-PD-L1 inhibitor pembrolizumab and showed an abscopal response after palliative RT.
2. Case report
A 72-year-old male patient with a history of hypertension, ischemic heart disease, chronic obstructive pulmonary disease, and an occupational exposure to asbestos was admitted to our Hospital in April 2015 with dyspnoea class IV (according to the New York Heart Association – NYHA), cough and weight loss of 5 kgs in 3 months. A chest CT scan was conducted and revealed a significant pleural effusion, calcified pleural plaques and mediastinal pleural thickening. 18Flourodeoxyglucose positron emission tomography/computed tomography (18FDG PET/CT) showed a diffuse heterogeneous, and moderate uptake of the right pleura with nodular thickening. Thoracoscopic biopsy and talc pleurodesis were performed. The pathology showed superficial atypical mesothelial proliferation without infiltrative pattern. In November 2015, a CT scan revealed an increase of the right pleural nodular thickening, extending next to the trachea and pericardium of the right auricle with increased uptake on 18FDG PET/CT. Endobronchial ultrasound transbronchial needle aspiration confirmed atypical cells compatible with MPM. Tumor staging was T3 N2 M0. The patient was treated with carboplatin area under the curve (AUC) 5, pemetrexed on day 1 (500 mg/m2) every three weeks (q3W) for 4 cycles and bevacizumab (15 mg/kg) q3W. After 4 cycles, a CT scan showed partial response (PR). He continued three cycles of maintenance chemotherapy with pemetrexed and bevacizumab at the same doses with reported stable disease (SD). Subsequently, he experienced grade 3 oral mucositis and watering eyes, and grade 2 nephrotoxicity, prompting chemotherapy discontinuation.
In July 2016, the patient started immunotherapy with pembrolizumab 200 mg intravenously (IV) q3W and the follow-up scan showed an initial SD followed by a PR documented in December 2016.
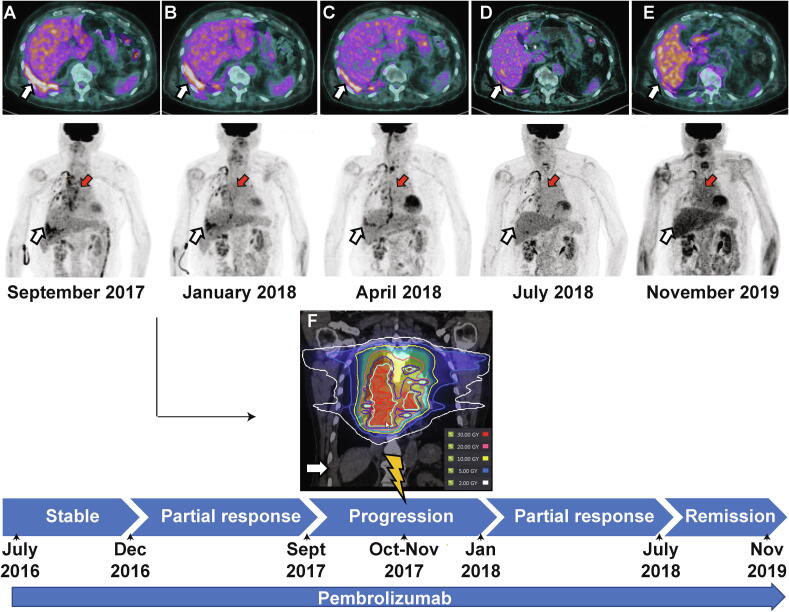
In September 2017, after 20 cycles of pembrolizumab, there was no clinically relevant treatment-related toxicity, but 18FDG PET/CT showed progressive enlargement and increased uptake of right pleural and paramediastinal lesions (Fig. 1A). While pursuing pembrolizumab, a 2 arcs volumetric modulated arc therapy was used to deliver 30 Gy in 10 fractions with 10-MV photons to the paramediastinal lesion over two weeks to relieve grade 2 dyspnoea. Right pleural lesions were not included in the RT volume (Fig. 1F).
Fig 1.
Diagnostic and radiotherapy simulation imaging throughout the disease course. Axial and coronal (maximum intensity projection – MIP) 18FDG PET/CT scans corresponding to the timeline for treatment and disease status (from panel A to E). Red arrows indicate the mediastinal lesions that have been irradiated. White arrows indicate unirradiated right pleural lesions. Panel A (top) reflects the pre-RT status of a progressive disease on pembrolizumab (SUVmax 7.3). Panel B shows images 3 months after RT when the response to the irradiated and unirradiated lesions has begun (SUVmax 6.5) Panel C shows images 6 months after RT with complete response of the targeted mediastinal mass and partial response of the abscopal right pleural lesion (SUVmax 4). Panel D-E shows images 9 months and 24 months after the first RT with the patient in complete remission (SUVmax 2.8). Panel F shows the CT simulation image for radiotherapy planning, and the target volume in the mediastinum. Isodose lines represent total dose of 30 Gy (red), 20 Gy (orange), 10 Gy (yellow). The 2 Gy isodose line in white is far from the right abscopal pleural lesion. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Three months after RT, the 18FDG PET/CT scan revealed PR in both irradiated para-mediastinal and unirradiated right pleural lesions. (Fig. 1B, C). The response of the unirradiated right pleural lesion suggested a remarkable abscopal effect.
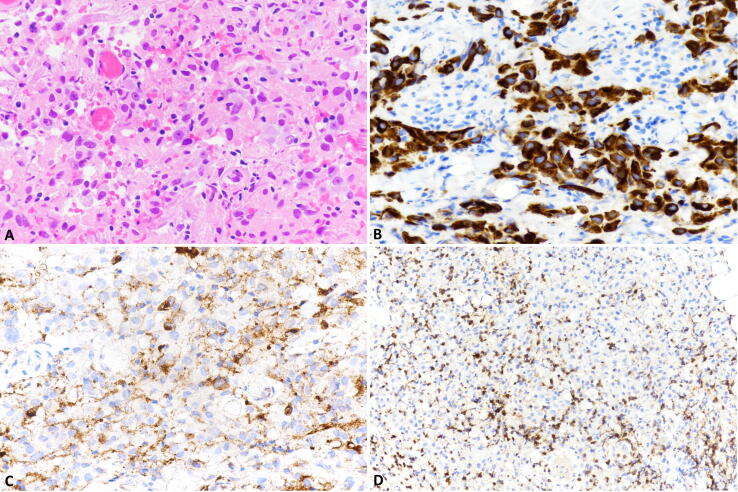
However, further oligometastatic progression of the disease with retropharyngeal metastasis, was confirmed by histology. Immunohistochemical expression of PD-L1 evaluated in the retropharyngeal metastases, was positive in about 10% of tumor cells (TPS = 10%) and in several inflammatory cells (CPS = 30). Also, moderate intratumoral infiltration of CD3-positive T lymphocytes, was observed and CD8-positive cells accounted for about 25% of the T-cell population (Fig. 2). In April 2018, retropharyngeal metastases were irradiated using the same radiation dose and technique.
Fig 2.
Immunohistochemistry analysis of the retropharyngeal metastasis. The retropharyngeal metastasis was composed of cuboidal or polygonal cells with eosinophilic cytoplasm and monotonous nuclei with visible nucleoli (A). Tumor cells were positive for cytokeratin (B) and PD-L1, which was also observe in some inflammatory cells (C). A moderate intratumoral infiltration of CD3-positive T lymphocytes was observed and CD8-positive cells (D) represented about 25% of the T-cell population.
In July 2018, while still on pembrolizumab, his paramediastinal lesion as well as the retropharyngeal mass were in complete remission and the abscopal right pleural lesion continued to shrink (Fig. 1D). Nevertheless, a retro-crural metastatic lymph node emerged and was treated with the same radiation dose and technique in conjunction with pembrolizumab. The follow-up 18FDG PET/CT, 24 months after RT revealed a significant decline in the SUVmax (2.8) of the right pleural lesion (Fig. 1E).
3. Discussion
Upon relapse to platinum-based chemotherapy, there are no other treatment options demonstrating improved survival in MPM. However, gemcitabine and vinorelbine are widely considered as second line option [2]. There is growing interests in the use of ICI in the management of MPM. For example, the phase I expansion cohort of KEYNOTE-028 trial showed a 20% objective response rate (ORR) and a median progression-free survival (PFS) of 5.4 months with pembrolizumab [5]. More recently, the phase III PROMISE-meso trial showed no improved PFS with pembrolizumab over chemotherapy in patients with MPM. In this trial, 144 patients with relapsed MPM were randomized 1:1 to receive 200 mg of fixed-dose pembrolizumab IV on day 1 of q3W cycle (n = 73) or physician’s choice chemotherapy of gemcitabine at 1000 mg/m2 on days 1 and 8 q3W, vinorelbine at 30 mg/m2on days 1 and 8 q3W, or vinorelbine at 60 or 80 mg/m2on days 1 and 8 q3W (n = 71). PFS was 2.5 months for pembrolizumab compared with 3.4 months for chemotherapy (HR, 1.06; 95% CI, 0.73–1.53; P = 0.76) [6].
Our patient has been treated in a similar way than in the pembrolizumab arm of the PROMISE-meso trial. One year after an initial PR to pembrolizumab, he progressed in the right mediastinum and pleura. While continuing with pembrolizumab, palliative RT was performed to the mediastinum and the patient developed a systemic abscopal response in unirradiated pleural areas. We believe that the response observed is an abscopal effect as it was observed after progression to 20 cycles of pembrolizumab suggesting a systemic immune response triggered by RT. The unirradiated pleural lesions received less than 0.5 Gy, further supporting that disease regression at this distant site was due to an enhanced systemic response to localized irradiation. Subsequently, the patient developed extra-thoracic oligometastatic disease and pembrolizumab was continued with interim localized RT. At every turn of RT, the patient achieved a genuine and durable clinical response. Five years after diagnosis, the patient remains clinically and radiologically stable and received his 70th cycle of pembrolizumab, in November 2020. As far as we know, this is the first MPM abscopal effect reported in the literature.
Our clinical observation is consistent with previous reports demonstrating that mobilization of antitumor immunity is an important determinant of the overall clinical efficacy of RT in both targeted and distant tumors [7], [8]. Beside its direct tumoricidal effects, moderate to high-doses of conventional hypofractionated RT can produce important immunomodulatory effects [3], [4], including the increase exposure of tumor-associated antigen through in situ vaccination effects, and the tumor microenvironment (TME) reprogramming through the upregulation of cytokines and chemokines, normalization of the tumor vasculature, and activation of dendritic cells (DCs). In essence, RT can promote T cell homing, migration into the tumor, tumor cell recognition, and effector function [9]. However, several immunosuppressive loops in the TME may be obstacles for the abscopal effect. Upregulation of PD-L1-following RT was documented in several preclinical studies and occurred in the first 24 h following 10 Gy in 5 fractions irradiation [10]. Radiation induced-PD-L1 upregulation is mediated via increased production of IFNγ by T cells infiltrating the TME following RT, which in turn induces PD-L1 expression on tumor cells. Therefore, the concomitant administration of RT with anti PD-L1 antibody, as it was performed in our patient, could have overcome tumor T cell exhaustion favoring an effective antitumor immune response. The optimal dose and fractionation may be an important determinant of the abscopal effect. It has been shown that a single 20 Gy dose of radiation is less effective than a 24 Gy in three fractions or 30 Gy in five fractions regimens administered concomitantly with ICI [11]. Vanpouille-Box et al demonstrated that the abscopal effect is abrogated with radiation dose per fraction greater than 18 Gy due to the upregulation of Trex1, an enzyme that shuttles DNA outside the cell impeding the activation of the inducible IFN-I pathway, cGAS/STING which is essential for the activation of antigen presenting cells [12]. We used radiation doses of 3 Gy delivered in 10 fractions, which has proven to be safe and other groups have demonstrated abscopal effects using a similar fractionation [13].
4. Conclusion
Abscopal effect may be observed in MPM treated with concomitant anti-PD-L1 and RT. However, this is a rare observation and does not justify implementation in routine clinical practice; and robust hypothesis-testing clinical trials with study of patients’ biopsies are required to determine the appropriate radiation and immunotherapy approach to be implemented in the clinical setting. It is possible that enhancing innate and adaptive immunity by combining RT and immunotherapy could induce more abscopal effects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Thoracic oncology unit of Lausanne university Hospital. Ludwig Institute for Cancer Research.
Contributor Information
Wambaka Ange Mampuya, Email: ange.mampuya@chuv.ch.
Hasna Bouchaab, Email: hasna.bouchaab@chuv.ch.
Niklaus Schaefer, Email: niklaus.schaefer@chuv.ch.
Remy Kinj, Email: remy.kinj@chuv.ch.
Stefano La Rosa, Email: stefano.larosa@chuv.ch.
Igor Letovanec, Email: igor.letovanec@chuv.ch.
Mahmut Ozsahin, Email: esat-mahmut.ozsahin@chuv.ch.
Jean Bourhis, Email: jean.bourhis@chuv.ch.
George Coukos, Email: george.coukos@chuv.ch.
Solange Peters, Email: solange.peters@chuv.ch.
Fernanda G. Herrera, Email: fernanda.herrera@chuv.ch.
References
- 1.Hegde P.S., Chen D.S. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52:17–35. doi: 10.1016/j.immuni.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Scherpereel A., Wallyn F., Albelda S.M., Munck C. Novel therapies for malignant pleural mesothelioma. Lancet Oncol. 2018;19:e161–e172. doi: 10.1016/S1470-2045(18)30100-1. [DOI] [PubMed] [Google Scholar]
- 3.Herrera F.G., Bourhis J., Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice: radiation-immunotherapy combinations. CA Cancer J Clin. 2017;67:65–85. doi: 10.3322/caac.21358. [DOI] [PubMed] [Google Scholar]
- 4.Reynders K., Illidge T., Siva S., Chang J.Y., De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41(6):503–510. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alley E.W., Lopez J., Santoro A., Morosky A., Saraf S., Piperdi B. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017;18:623–630. doi: 10.1016/S1470-2045(17)30169-9. [DOI] [PubMed] [Google Scholar]
- 6.Popat S., Curioni-Fontecedro A., Polydoropoulou V., Shah R., O’Brien M., Pope A. A multicentre randomized phase III trial comparing pembrolizumab (P) vs single agent chemotherapy (CT) for advanced pre-treated malignant pleural mesothelioma (MPM): results from the European Thoracic Oncology Platform (ETOP 9-15) PROMISE-meso trial. Ann Oncol. 2019;30:v851. doi: 10.1016/j.annonc.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Postow M.A., Callahan M.K., Barker C.A., Yamada Y., Yuan J., Kitano S. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demaria S., Ng B., Devitt M.L., Babb J.S., Kawashima N., Liebes L. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Weichselbaum R.R., Liang H., Deng L., Fu Y.-X. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14:365–379. doi: 10.1038/nrclinonc.2016.211. [DOI] [PubMed] [Google Scholar]
- 10.Dovedi S.J., Adlard A.L., Lipowska-Bhalla G., McKenna C., Jones S., Cheadle E.J. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 11.Dewan M.Z., Galloway A.E., Kawashima N., Dewyngaert J.K., Babb J.S., Formenti S.C. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti–CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanpouille-Box C., Alard A., Aryankalayil M.J., Sarfraz Y., Diamond J.M., Schneider R.J. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko E.C., Benjamin K.T., Formenti S.C. Generating antitumor immunity by targeted radiation therapy: role of dose and fractionation. Adv Radiat Oncol. 2018;3:486–493. doi: 10.1016/j.adro.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]