Highlights
-
•
Peutz-Jeghers syndrome (PJS) is an inherited polyposis syndrome.
-
•
PJS-characteristic hamartoma polyps occur in the gastrointestinal (GI) tract.
-
•
In patients with intussusception, the presence of a hamartoma polyp may have been the trigger.
-
•
Hamartoma polyps in the GI mucosa and hyperdense macular lesions on the lip and buccal mucosa are pathognomonic signs of PJS.
Keywords: Peutz-Jeghers syndrome, Intussusception, Hamartoma, Polyps, Case report
Abstract
Introduction
Peutz-Jeghers syndrome (PJS) is an uncommon autosomal dominant syndrome with a variable to high penetrance that leads to the development of polyps within the gastrointestinal mucosa. Here we report a case of an adult female suffering jejunoileal intussusception due to PJS.
Presentation of case
A 30-year-old woman came to an emergency department with a small bowel obstruction caused by intussusception. The patient underwent an emergency exploratory laparotomy. An intussusception at the level of 60 cm from the ligamentum treitz was revealed, and the intussusception small bowel segment was not viable; we decided to perform segmental jejunoileal resection with the Bishop-Koop procedure, and the specimen histopathology of the segmental jejunoileal resection showed a typical hamartomatous polyp features. Two month later, diagnostic endoscopy showed multiple polyps (between 5 and 15 mm) in the large bowel. The polyps were removed with endoscopic polypectomy and examined histopathologically, showing characteristics of PJS. Further detailed family history was obtained, and similar skin lesions were detected on our patient’s child (since birth). Although endoscopy screening identified multiple polyps in the child’s ileum and large bowel, he was not suffering from abdominal symptoms.
Conclusion
In patients with intussusception at a young age, PJS can be caused by the presence of a hamartoma polyp as a trigger for intussusception. If there are multiple polyps found in the gastrointestinal mucosa and other pathognomonic signs are found, such as hyperdense macular lesions on the lip and buccal mucosa, such cases should be confirmed as PJS.
1. Introduction
Peutz-Jeghers syndrome (PJS) is an uncommon autosomal dominant syndrome with a variable to high penetrance that leads to the development of polyps within the colon and rectum, and only a small number of cases have been reported in the literature [1]. PJS affects around 1 in 8300 to 200,000 births [2]. PJS was first reported by Peutz in 1921 [3,4], and subsequently, detailed case PJS by Jeghers, McKusick, and Katz in 1949 [5]. This syndrome consists of the association of gastrointestinal polyps mucocutaneous pigmentation and a familial incidence [6]. In PJS, the gastrointestinal (GI) tract develops hamartomatous polyps, which occur most commonly in the small bowel, colon, and rectum (≥ 90% of cases), and less commonly in the stomach or urinary tract [7]. Hamartomas can range from 5 to 50 mm in diameter (median size, 35 mm). Hamartomas are associated with intussusception, bleeding, anemia, and obstruction [8].
Here, we report the uncommon case of a female suffering jejunoileal intussusception due to PJS, in line with the updated consensus-based surgical case report (SCARE) guidelines [9].
2. Case presentation
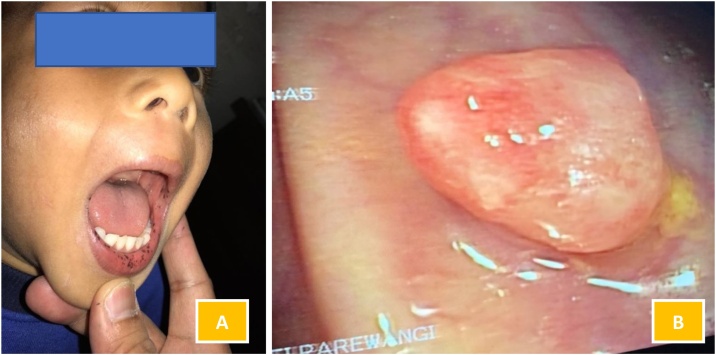
A 30-year-old woman came to an emergency department with the chief complaint of intermittent episodes of colicky abdominal pain with tarry stool 4 weeks before admission. From the vital signs, the patient was tachycardic and hypotensive. On physical examination, she had an of pigmentation in the oral cavity (Fig. 1). The abdomen was less distended, with increased bowel sound, tenderness, and hypertimpanic on percussion. The digital rectal examination revealed normal limits. Blood investigation showed anemia (hemoglobin level, 7.6 g/dl). An abdominal X-ray showed a small bowel obstruction.
Fig. 1.
Mucocutaneous pigmentation on the buccal mucosa and lips.
The patient underwent an emergency exploratory laparotomy. We (the digestive surgeon and residents) detected a jejunoileal intussusception caused by multiple polyps at ∼60 cm from the ligamentum treitz (Fig. 2A and B), and the milking procedure was successfully performed, but a 40-cm segment of the jejunoileal intussusception was not viable. Therefore, we decided to perform segmental resection and, through this, found multiple polyps in the borderline resection of the mucosa proximal jejunum and distal ileum. We decided to extend the resection until we no longer found polyps. We decided to perform the Bishop-Koop procedure (Fig. 3A). The specimen was sent for histopathological examination and described as a typical hamartomatous polyp (Fig. 3B). She was given an intravenous broad-spectrum antibiotic, and analgesics drugs were administered for 5 days.
Fig. 2.
(A) Intussusception ileojejenal and (B) hamartoma of the ileum.
Fig. 3.
(A) The Bishop-Koop procedure of the jejunum; (B) The histopathological findings reveal hamartoma, with a typical PJS polyp demonstrating an arborizing pattern of growth of smooth-muscle (HE staining, magnification 4×); and (C) Histopathological findings revealed an edematous appearance of the intestinal mucosa with inflammatory lymphocytes, histiocytes, and very dense plasma cells. In the layer below, there appears pigment hemosiderin. There is no evidence of dysplasia or malignancy (HE staining, magnification 40×).
Postoperatively, the patient had satisfactory progress, tolerating oral feeding, and was discharged on the seventh postoperative day. Two month later, we performed diagnostic endoscopy; there were multiple polyps in the large bowel measuring 5–15 mm, without any features of intussusceptions (Fig. 4). This polyp was removed with endoscopic polypectomy. Histopathological examination revealed a polyp characteristic of PJS consisting of a branching framework of connective tissue and smooth muscle lined by the normal intestinal epithelium. Further detailed family history was obtained, and similar skin lesions were detected on her child (since birth). Although endoscopy screening performed identified multiple polyps (< 5 mm) in the ileum and large bowel, the child was not suffering from abdominal symptoms (Fig. 5).
Fig. 4.
Colonoscopy with more than 50 polyps scattered throughout the colon and rectum.
Fig. 5.
On the patient’s male child, we detected: (A) pigmentations of the lips and oral mucosa; and (B) polyp in the large bowel detected by endoscopy screening.
3. Discussion
PJS is an inherited polyposis syndrome in which multiple characteristic polyps occur in the gastrointestinal (GI) tract, associated with mucocutaneous pigmentation, especially of the vermilion border of the lips. PJS is an autosomal dominant disease caused by a germline mutation in the STK11 (LKB1) gene [8]. The estimation of the population prevalence of PJS differs between studies, ranging from 1 in 8300 to 1 in 280,000 individuals. The probable prevalence is around 1 in 100,000 people [2].
The diagnosis of PJS can be made in patients with hamartomatous polyp(s) with at least two of the following clinical criteria also present: labial melanin deposits, a family history of the syndrome, and small bowel polyposis. The syndrome appears equally in males and females and is found in all racial groups [6]. The clinical manifestation of PJS is characterized by asymptomatic periods interspersed with complications, such as abdominal pain, intussusception often leading to intestinal obstruction, polyp extrusion through the rectum, and bleeding, which is often occult [7,10,11]. Small bowel obstruction is the presenting complaint in half of the cases, and relaparotomy due to polyp-induced complications occurs commonly and might do so at quite short intervals [10].
In addition to polyposis, previous studies have reported an increased risk of GI and extra‑GI malignancies in PJS patients, compared with that of the general population [7]. The PJS is associated with an increased risk of gastrointestinal and non-gastrointestinal malignancies. A meta-analysis involving six studies and 210 patients showed a cumulative risk of 93% from 15 to 64 years for all types of malignancies. Thus, the relative risk of an individual with SPJ to present neoplasia in any region, compared with the general population, is up to 15 times higher [11,12]. The most frequent neoplasm in patients with PJS is the colonic tumor (57%), followed by breast (45%), pancreas (36%), stomach (29%), ovary (21%), small intestine (13%), and uterus (10%) tumors [11,13]. Among gastrointestinal cancers, increased cancer risk was indicated for the colon, stomach, small intestine, and pancreas. Also, female PJS patients are at greater risk of gastrointestinal and gynecological cancers, ovarian cancer, cervical cancer, uterine cancer, and breast cancer. Meanwhile, the high risk of pulmonary cancer, renal cancer, prostatic cancer, bone cancer, and leukemia has also been reported. There is wide variability in cancer risk estimates, as reviewed in a recent meta-analysis study [14].
Until recently, PJS was considered a benign condition and was treated conservatively. Repeated laparotomies were considered risky and ineffective because of the continuous growth of the polyps. Patients were, therefore, treated surgically only when acute bowel obstruction occurred [10].
More recently, the management approach has changed, becoming more aggressive. When the diagnosis of PJS is histologically confirmed, we suggest that all polyps should be removed with the aim of preventing intussusception with gangrene requiring resection, which might lead to short-bowel syndrome [10].
Most publications recommend polypectomy for polyps in the stomach or colon that are greater than 1 cm in size noted during endoscopic surveillance. Surgery has been recommended for symptomatic or rapidly growing small intestinal polyps or asymptomatic polyps greater than 1–1.5 cm in size. Some experts suggest a clean sweep. This can be facilitated by concomitant interoperative endoscopy with polypectomy or, in the case of larger polyps, enterotomy. The clean sweep approach appears to decrease the need for recurrent small bowel surgery. Recently, the use of double-balloon enteroscopy for removal of small bowel PJS polyps has been reported and might decrease the need for laparotomy [14].
In our case, the patient was admitted to the hospital with intussusception. The exploratory laparotomy revealed intussusception with the small bowel segment not viable. Small bowel resection was performed and continued with the Bishop-Koop procedure due to prevent short bowel syndrome. We did not perform an end-to-end anastomosis because the patient had hypovolemic shock preoperative and anemia, and we found edema in the small bowel mucosa.
4. Conclusion
In patients with intussusception at a young age, a hamartoma polyp might have been the trigger for intussusception. If there are multiple polyps found in the GI mucosa together with other pathognomonic signs, such as hyperdense macular lesions on the lip and buccal mucosa, such cases should be confirmed as PJS.
Declaration of Competing Interest
The authors declare that they have no conflict of interests.
Funding
No funding or sponsorship was received for this study or publication of this article.
Ethical Approval
The study is exempt from ethical approval in our institution.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Erwin Syarifuddin, Julianus A. Uwuratuw, and Muhammad Faruk: study concept, surgical therapy for this patient. Erwin Syarifuddin, Rina Masadah, and Muhammad Faruk: Data collection, Writing-Original draft preparation. Ronald E. Lusikooy and Warsinggih: senior author and the manuscript reviewer. Erwin Syarifuddin and Muhammad Faruk: Editing and Writing. All authors read and approved the final manuscript.
Registration of research studies
Not applicable.
Guarantor
Erwin Syarifuddin.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgment
We acknowledge Robert Christeven, M.D, for her help in providing us with the linguistic assistance for this case report.
Contributor Information
Erwin Syarifuddin, Email: erwinsyarifuddin@yahoo.com.
Rina Masadah, Email: r.masadah@med.unhas.ac.id.
Ronald Erasio Lusikooy, Email: ronaldlusikooy@gmail.com.
Warsinggih, Email: kbd.warsinggih@gmail.com.
Julianus Aboyaman Uwuratuw, Email: boyuwuratuw@gmail.com.
Muhammad Faruk, Email: faroex8283@gmail.com.
References
- 1.Duan S.-X., Wang G.-H., Zhong J., Ou W.-H., Fu M.-X., Wang F.-S., Ma S.-H., Li J.-H. Peutz–Jeghers syndrome with intermittent upper intestinal obstruction. Medicine (Baltimore) 2017;96:e6538. doi: 10.1097/MD.0000000000006538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopacova M., Tacheci I., Rejchrt S., Bures J. Peutz-Jeghers syndrome: diagnostic and therapeuticapproach. World J. Gastroenterol. 2009;15:5397. doi: 10.3748/wjg.15.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tweedie J.H., McCann B.G. Peutz-Jeghers syndrome and metastasising colonic adenocarcinoma. Gut. 1984;25:1118–1123. doi: 10.1136/gut.25.10.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chowdhry S., Umrigar D.D., Yadav N. Peutz-jeghers syndrome in a child presenting with acute abdomen: a case report. Asian J. Dermatol. 2015;8:20–24. doi: 10.3923/ajd.2016.20.24. [DOI] [Google Scholar]
- 5.Foley T.R., McGarrity T.J., Abt A.B. Peutz-Jeghers syndrome: a clinicopathologic survey of the “Harrisburg family” with a 49-year follow-up. Gastroenterology. 1988;95:1535–1540. doi: 10.1016/s0016-5085(88)80074-x. [DOI] [PubMed] [Google Scholar]
- 6.Giardiello F.M., Trimbath J.D. Peutz-Jeghers syndrome and management recommendations. Clin. Gastroenterol. Hepatol. 2006;4:408–415. doi: 10.1016/j.cgh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Butt N., Salih M., Khan M., Ahmed R., Haider Z., Shah S.A. An incidentally discovered asymptomatic para-aortic paraganglioma with Peutz-Jeghers syndrome. Saudi J. Gastroenterol. 2012;18:388. doi: 10.4103/1319-3767.103432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latchford A., Cohen S., Auth M., Scaillon M., Viala J., Daniels R., Talbotec C., Attard T., Durno C., Hyer W. Management of Peutz-Jeghers syndrome in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2019;68:442–452. doi: 10.1097/MPG.0000000000002248. [DOI] [PubMed] [Google Scholar]
- 9.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A., Beamish A.J., Noureldin A., Rao A., Vasudevan B., Challacombe B., Perakath B., Kirshtein B., Ekser B., Pramesh C.S., Laskin D.M., Machado-Aranda D., Miguel D., Pagano D., Millham F.H., Roy G., Kadioglu H., Nixon I.J., Mukhejree I., McCaul J.A., Chi-Yong Ngu J., Albrecht J., Rivas J.G., Raveendran K., Derbyshire L., Ather M.H., Thorat M.A., Valmasoni M., Bashashati M., Chalkoo M., Teo N.Z., Raison N., Muensterer O.J., Bradley P.J., Goel P., Pai P.S., Afifi R.Y., Rosin R.D., Coppola R., Klappenbach R., Wynn R., De Wilde R.L., Surani S., Giordano S., Massarut S., Raja S.G., Basu S., Enam S.A., Manning T.G., Cross T., Karanth V.K., Kasivisvanathan V., Mei Z. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 10.Rebsdorf Pedersen I., Hartvigsen A., Fischer Hansen B., Toftgaard C., Konstantin-Hansen K., Büllow S. Management of Peutz-Jeghers syndrome. Experience with patients from the Danish Polyposis Register. Int. J. Colorectal Dis. 1994;9:177–179. doi: 10.1007/BF00292244. [DOI] [PubMed] [Google Scholar]
- 11.Loureiro J., Menegazzo G.L., Vergamini L., Pestana R.C., Formiga F.B., de Sousa M.G.C., Takahashi T.Y., Silveira F., de A.P. Candelária P., Filho D.M., Bin F.C. Diagnostic difficulty in Peutz–Jeghers syndrome. Colorectal Dis. 2015;35:67–71. doi: 10.1016/j.jcol.2014.08.012. [DOI] [Google Scholar]
- 12.Giardiello F.M. Early Diagnosis Treat. Cancer Ser. Color. Cancer. Elsevier; 2011. Hereditary colorectal cancer and polyp syndromes; pp. 21–30. [DOI] [Google Scholar]
- 13.Jansen M., Brosens L.A.A., Offerhaus G.J.A. Pathobiol. Hum. Dis. Elsevier; 2014. Gastrointestinal polyposis syndromes: early tumor evolution through the looking glass; pp. 1319–1331. [DOI] [Google Scholar]
- 14.Chen H.-Y., Jin X.-W., Li B.-R., Zhu M., Li J., Mao G.-P., Zhang Y.-F., Ning S.-B. Cancer risk in patients with Peutz-Jeghers syndrome: a retrospective cohort study of 336 cases. Tumour Biol. 2017;39 doi: 10.1177/1010428317705131. 1010428317705131. [DOI] [PubMed] [Google Scholar]