Abstract
Glyphosate, the most commonly used pesticide worldwide, blocks aromatic amino acid biosynthetic pathways and inhibits growth in plants. Although the specific mode of action of glyphosate in animals remains unclear, adverse effects during embryonic development have been reported, including epiboly delays, morphological alterations, and changes in central nervous system development and cardiogenesis. In this study, we suggest a possible toxicity mechanism for this herbicide related to changes in microtubule stability, which could alter the distribution and dynamics of cytoskeleton components. Using zebrafish embryos to evaluate in vivo effects of glyphosate exposure (5, 10, and 50 μg/ml), we found significant reductions in the levels of acetylated α-tubulin (50 μg/ml) and in the polymeric tubulin percentage in zebrafish embryos that had been exposed to 10 and 50 μg/ml glyphosate, without any changes in either the expression patterns of α-tubulin or the stability of actin filaments. These results indicate that high concentrations of glyphosate were associated with reduced levels of acetylated α-tubulin and altered microtubule stability, which may explain some of the neurotoxic and cardiotoxic effects that have been attributed to this herbicide.
Keywords: Glyphosate, Microtubule stability, Zebrafish
Glyphosate, Microtubule stability, Zebrafish.
1. Introduction
Microtubules are polymeric structures composed of α,β-tubulin heterodimers, which are essential components of the cytoskeleton. They play important roles in numerous processes, including cell shape maintenance, intracellular transport, cell motility, and cell division. Microtubules undergo constant assembly and disassembly, the dynamics of which are highly regulated by several cellular factors that interact with these structures and affect their stability [1]. Microtubule-associated proteins (MAPs), such as MAP2, MAP4, and Tau, are key factors that regulate microtubule stability [2]. These proteins are activated and regulated by various serine/threonine kinases, such as calcium/calmodulin-dependent protein kinase II (CaMKII) [3], which plays a key role in the activation of calmodulin, a calcium-binding protein implicated in rapid microtubule disassembly by a calcium-calmodulin-induced mechanism [4]. Another key factor in microtubule stabilization is Wnt signaling, which has been implicated in cell migration, cell polarization, and neurite outgrowth. Wnt signaling directly regulates microtubule stability [5] by the activation or inhibition of Gsk3, a serine/threonine protein kinase that is involved in the phosphorylation of Tau and other MAPs, which can result in tauopathy and microtubule depolymerization [6].
Glyphosate (N-phosphomethyl glycine) is a broad-spectrum herbicide that specifically inhibits 5-enol-pyruvilshikimato-3-phosphatosintase (EPSP), a key enzyme required for the production of aromatic amino acids in photosynthetic organisms [7, 8]. Despite the lack of glyphosate targets in metazoans and the low acute toxicity of glyphosate [9], multiple studies have suggested that this compound and its several commercial preparations produce toxic effects [9, 10], including modifying the endocrine system, altering the antioxidant defense system, inducing morphological defects, altering cardiac development, and structurally and functionally modifying the central nervous system during development [11, 12, 13].
The ability of glyphosate to modify calcium dynamics within cells [14, 15, 16] has been linked to changes in cellular architecture and the distribution of cytoskeleton components as well as to variations in cell adhesion, cell morphology, and the organization and distribution of microtubules and MAPs. For instance, in oocytes, exposure to glyphosate at less than 100 μM has been found to alter the configuration of the achromatic spindle, whereas concentrations above 100 μM have been found to result in a notable loss of microtubules from the mitotic spindle, suggesting that components of the cytoskeleton and microtubule organization centers are being affected [17]. Likewise, exposure to glyphosate has also been found to affect the regulatory mechanisms of neuronal cytoskeleton stability, which has been associated with changes in the expression patterns of CaMKIIA, CaMKIIB, Wnt3a, Wnt5a, and tubulin beta class III (TUBB3) [14,18]. Moreover, exposure to glyphosate-based herbicides (GBH) has been found to alter the levels of proteins associated with microtubule stability like the MAP protein S100B, which has been implicated in the Ca2+-dependent regulation of microtubule disassembly [19]. Despite evidence of cytoskeleton changes, the mechanisms of the glyphosate interactions that affect the distribution and dynamics of cytoskeleton components remain unclear.
Currently, zebrafish are considered to be powerful vertebrate models to study the effects of xenobiotics on embryonic development. Previous reports have evaluated the effects of glyphosate exposure in zebrafish embryo development, finding that early exposure to this herbicide can generate neurotoxicity, cardiotoxicity, and morphological abnormalities during development [11, 12, 20]. In embryonic zebrafish, microtubule dynamics are vital to many developmental processes. In addition to their well-known role in mitosis, microtubules are required for epiboly, furrow formation, the cohesion of post-cytokinesis blastomeres, and nervous system development [21, 22, 23]. We suggest a possible toxicity mechanism of this herbicide regarding modifications in microtubule stability during embryonic development. This was achieved by isolating the microtubule-enriched fraction from zebrafish embryos and assessing modifications in the composition and stability of the microtubules using specific antibodies.
2. Materials and methods
2.1. Fish maintenance and embryo production
Wild-type adult zebrafish (Danio rerio) were obtained from a commercial pet store and maintained in compliance with the guidelines of the Zebrafish Book [24]. Zebrafish embryos were generated by massive mating, and the embryos that reached the 16-cell stage were placed in petri dishes that contained medium for zebrafish embryo (EM buffer; 15 mM NaCl, 0.5 mM KCl, 1 mM CaCl2, 1 mM MgSO4, 0.15 mM KH2PO4, 0.05 mM Na2HPO4, and 0.7 mM NaHCO3 at pH 7 ± 0.2) and used in subsequent experiments. All procedures were approved and met the ethical standards of the Institutional Animal Care and Use Committee of CIAD (Centro de Investigación en Alimentación y Desarrollo, A.C.).
2.2. Glyphosate exposure
Zebrafish embryos were loaded into 6-well plates (10 embryos per well) and exposed to glyphosate (CAS Number 1071-83-6; Sigma-Aldrich, St. Louis, USA) resuspended in EM buffer at one of three concentrations: 5, 10, or 50 μg/ml. In each treatment, 5 ml of the solution was added to each well, and the plates were incubated at 28.5 °C. An EM buffer without glyphosate was used in the control treatment. The exposure period began at the 50% epiboly stage and ended 96 h post-fertilization (hpf). Survival and hatching were evaluated every 24 h until 96 hpf. Each treatment was carried out in quadruplicate.
2.3. Isolation of the microtubule-enriched fraction (P)
The extraction of the microtubule-enriched fraction (P) was carried out following the methodology of Bershadsky et al. [25] and Gómez de León et al. [26]. Briefly, zebrafish embryos at 96 hpf were anesthetized using tricaine (Sigma-Aldrich Co) and washed with phosphate-buffered saline (PBS). The embryos were lysed with Triton X-100 (Sigma-Aldrich) in PHEM buffer (100 mM HEPES pH 6.9, 10 mM EGTA pH 7.0, 1 mM MgCl2, and 0.5% Triton X-100 supplemented with a protease inhibitor cocktail) and centrifuged at 30,000 x g for 45 min at 4 °C. The pellet, corresponding to the insoluble microtubule enriched fraction (P), was washed with PHEM solution without Triton X-100 and then centrifuged at 15,000 x g for 10 min. The soluble proteins from the non-polymer fraction (SN) were precipitated with isopropanol at -20 °C overnight and centrifuged at 15,000 x g for 15 min. Both fractions were suspended in lysis buffer (2% β mercaptoethanol, 1% SDS, 20 mM EGTA, and 2 mM Tris HCl at pH 7.5 and supplemented with a protease inhibitor cocktail) and frozen until use. To prepare whole embryo extracts, the embryos were homogenized in the presence of lysis buffer. The lysate was centrifuged at 6000 x g for 10 min at 4 °C, and the supernatant was recovered. The total protein concentration was estimated using Bradford reagent (Bio-Rad Laboratories Inc., CA, USA).
2.4. Western blot
The protein samples (50 μg per lane) were separated under reducing conditions with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 10% gels) and transferred to PVDF membranes. The membranes were blocked with 6% milk and incubated with the corresponding primary antibody, after which they were incubated with a secondary antibody conjugated to horseradish peroxidase (HRP). Detection was performed by chemiluminescence in a ChemiDoc XRS system (Bio-Rad Laboratories Inc., CA, USA) using Image Lab Software (BIO-RAD). Data analyses were carried out with R v. 3.6.3 [27]. A one-way analysis of variance (ANOVA) and a post-hoc Tukey multiple comparison test were used to evaluate significant differences in protein levels between the glyphosate treatments and those of the control. P-values ≤ 0.05 were considered to be statistically significant. The results were plotted using the means and standard deviations.
2.5. Antibodies
The primary antibody against actin (SC-8432) was obtained from Santa Cruz Biotechnology (Santa Cruz, USA). The anti-alpha (α) tubulin antibody (T6199) was obtained from Sigma-Aldrich, while the anti-GAPDH antibody (MA5-15738) and the anti-acetyl-alpha (α) Tubulin (Lys40) antibody (32–2700) were obtained from Thermo Fisher Scientific (San Jose, USA). The secondary antibodies conjugated to HRP and goat-anti-rabbit IgG (NB7160) were obtained from Novus Biologicals (Littleton, USA), while Goat anti-mouse IgG (A-10677) was obtained from Thermo Fisher Scientific.
3. Results
3.1. Glyphosate exposure reduces α-tubulin acetylation in zebrafish embryos
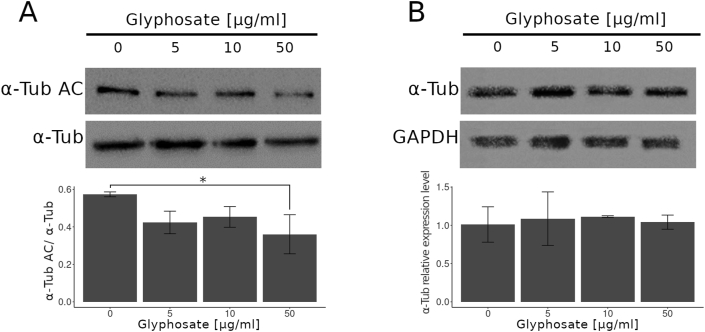
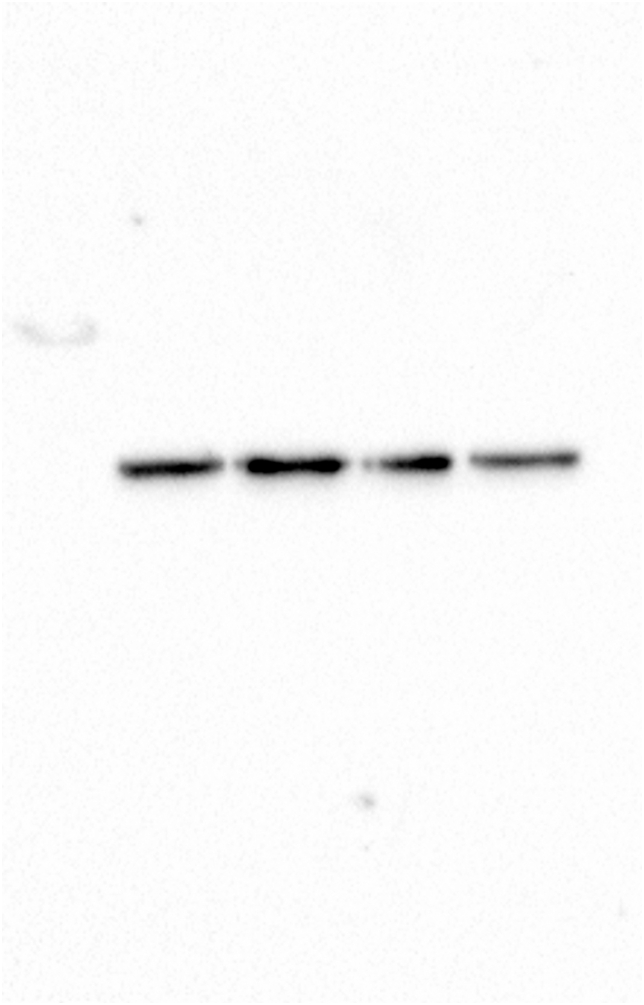
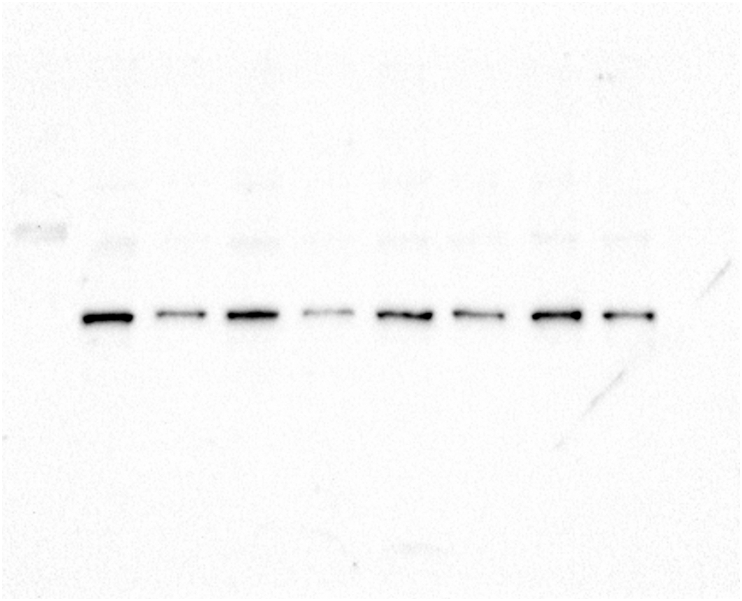
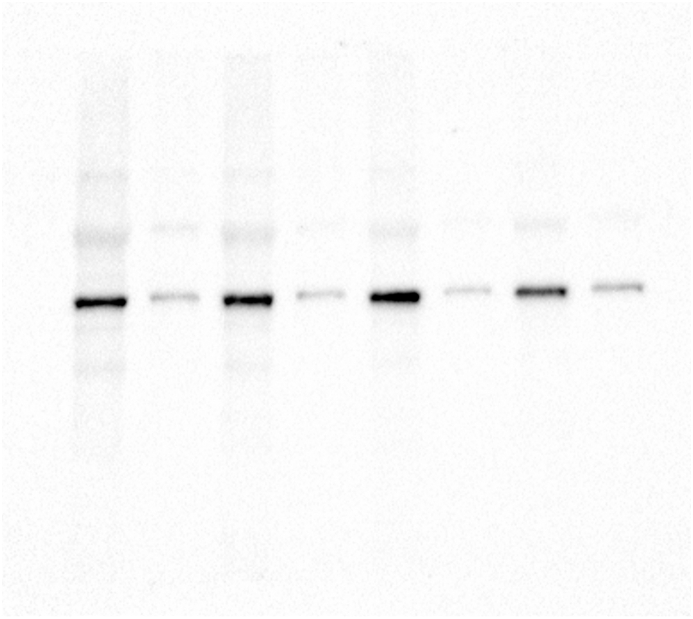
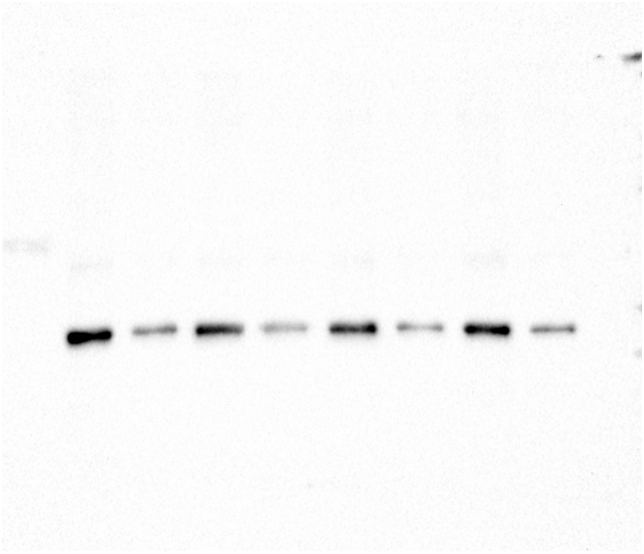
The analysis of whole zebrafish embryo extracts indicated that exposure to glyphosate induced a reduction in acetylated α-tubulin levels, and a significant difference was observed between the 50 μg/ml treatment and the control (Figure 1A; p < 0.05). Glyphosate exposure of up to 50 μg/ml was not able to induce changes in the patterns of total α-tubulin levels (Figure 1B).
Figure 1.
Glyphosate exposure reduces α-tubulin acetylation in zebrafish embryos. (A) Immunoblot of zebrafish embryo whole protein extract probed with antibody against Acetylated α-tubulin. A quantification analysis of the ratio α-Tub AC/total α-Tub shows that the level of acetylated α-tubulin in embryos exposed to glyphosate (50 μg/ml) was significantly lower than that of the control group. (B) Immunoblot of embryo whole protein extract probed with antibody against α-Tub. The quantification analysis relative to that of the control shows that the expression level of the α-tubulin protein in the whole embryo extracts did not change significantly in any of the treatments after glyphosate exposure. Original images of the western blots were provided as supplementary material: (A) Fig_S1A_alphaTubAC.jpg and Fig_S1A_alphaTub.jpg; (B) Fig_S1B_alphaTub.jpg and Fig_S1B_GAPDH.jpg.
3.2. Glyphosate exposure induced microtubule instability
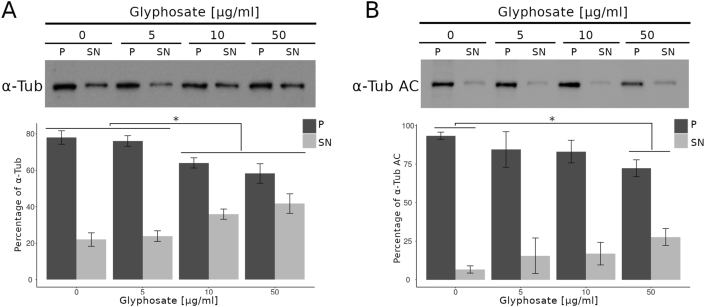
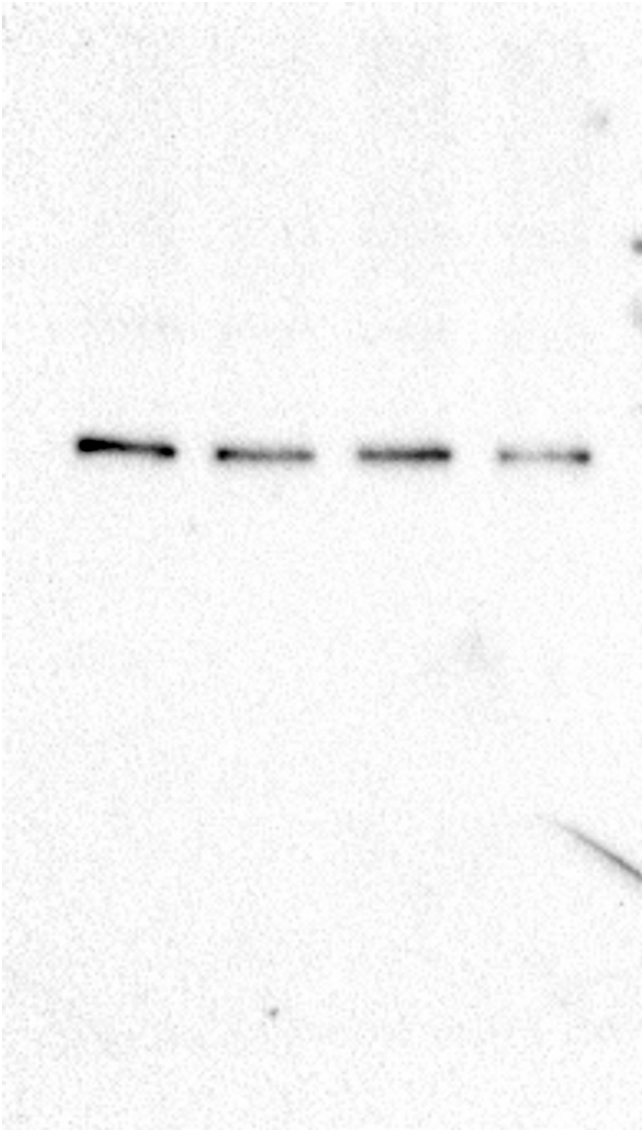
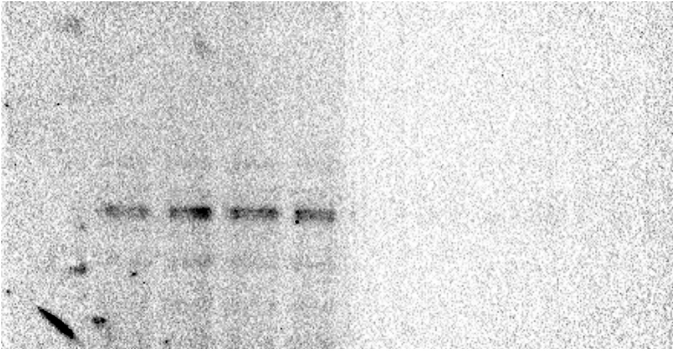
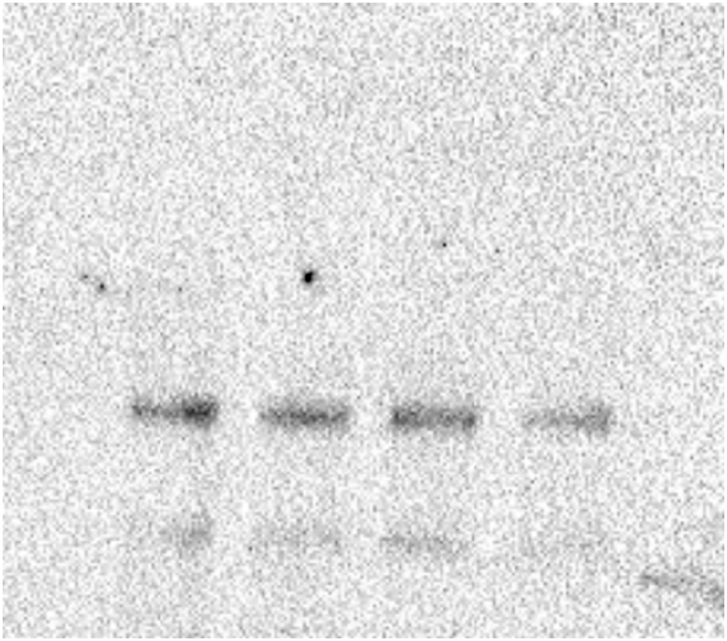
To determine the effect of glyphosate exposure on microtubule stability, we carried out an in vivo microtubule sedimentation assay and analyzed the distribution of α-tubulin via Western blot. An analysis of the polymer to soluble tubulin ratio in the zebrafish embryos exposed to glyphosate (Figure 2A) indicated a reduction in α-tubulin levels in the polymer fraction (P) and significant differences in α-tubulin levels were observed between that of the control and those of the 10 and 50 μg/ml treatments (p < 0.05). This change in the polymeric tubulin percentage was accompanied by an increase in the level of α-tubulin in the non-polymer fraction (SN), and significant differences in α-tubulin levels were observed between the control and the 10 and 50 μg/ml treatments (p < 0.05). Likewise, a reduction in the level of acetylated α-tubulin was observed in the P of all glyphosate-exposed groups (Figure 2B). However, this reduction was only significant for the 50 μg/ml glyphosate treatment (p < 0.05). Interestingly, a significant increase in acetylated α-tubulin levels was observed in the SN fraction of the embryos exposed to this same glyphosate concentration (p < 0.05).
Figure 2.
Glyphosate induced changes in microtubule stability between the microtubule-enriched fraction (P) and the non-polymer fraction (SN). (A) Western blot analysis against α-tubulin antibody. The quantification of the percentage of polymeric and non-polymeric tubulin with regard to total tubulin (polymer + soluble dimer) indicated that the percentage of polymeric tubulin in the embryos exposed to glyphosate at either 10 or 50 μg/ml was significantly lower than that of the control group (p < 0.05). (B) Western blot analysis against acetylated α-tubulin antibody. The quantification analysis indicated that the percentage of acetylated α-tubulin in the polymeric fraction (P) from the embryos exposed to glyphosate at 50 μg/ml was significantly lower than that of the control group (p < 0.05). Original images of the western blots were provided as supplementary material: (A) Fig_S2A_alphaTub.jpg; (B) Fig_S2B_alphaTubAC.jpg.
3.3. Glyphosate exposure does not appear to affect the stability of actin filaments
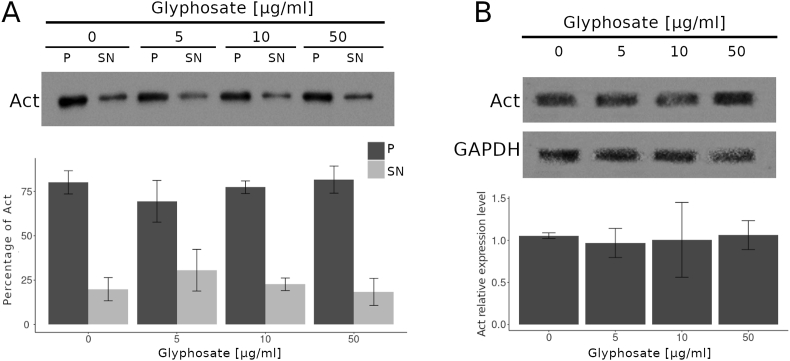
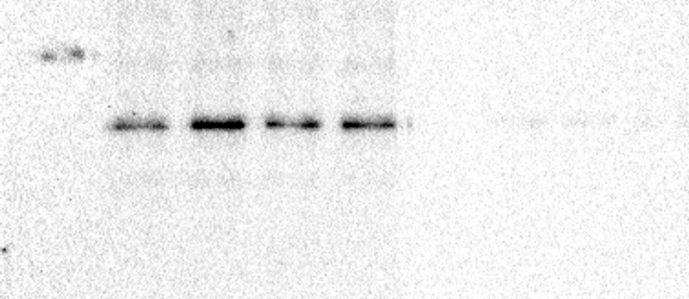
No changes were observed in the actin levels in the embryos of any treatment (Figure 3A). Also, the actin filament stability analysis did not reveal any changes in actin distribution between the P and SN fractions in any treatment (Figure 3B) (see Figure 4).
Figure 3.
Glyphosate exposure does not affect the stability of actin filaments. (A) The Western blot analysis of actin partitioning between the P fraction and the SN fraction in zebrafish embryos exposed to glyphosate indicated that the percentage of actin in the P and SN fractions did not significantly change in any of the groups exposed to glyphosate compared to that of the control group. (B) Western blot analysis against actin antibody. The quantification analysis relative to that of the control indicated that after glyphosate exposure, actin levels in the whole embryo extracts did not significantly change in any of the groups. Original images of the western blots were provided as supplementary material: (A) Fig_S3A_Act.jpg; (B) Fig_S3B_Act.jpg and Fig_S3B_GAPDH.jpg.
Figure 4.
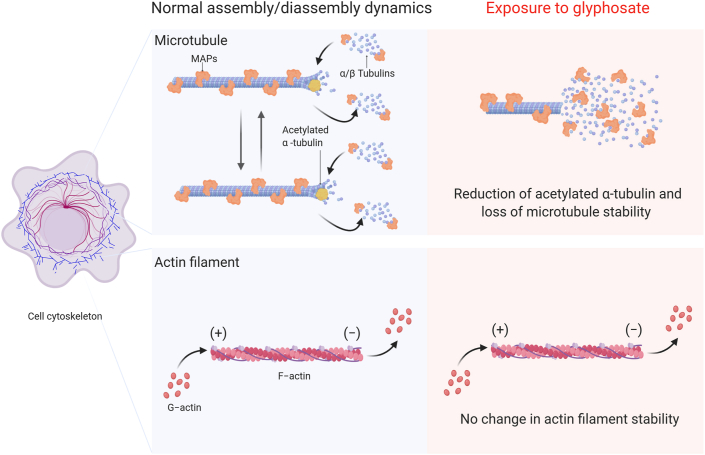
The effect of glyphosate exposure on microtubule stability. Microtubule dynamics are vital to developmental processes, and these dynamics are kept in equilibrium by several mechanisms that involve post-translational modifications (α-tubulin acetylation) and microtubule-associated proteins (MAPs). Glyphosate exposure during embryonic development in zebrafish can result in a reduction of the levels of acetylated α-tubulin and a loss of microtubule stability without generating any apparent changes in the stability of actin filaments, suggesting that glyphosate can modify the function of the cytoskeleton by altering microtubule stability. Created with BioRender.com.
4. Discussion
All exposed embryos reached normal development without morphological abnormalities like body malformations, spinal curvature, pericardial, or yolk sac edemas. An increase in these morphological abnormalities has been previously found, especially in embryos exposed to 100 mg/L glyphosate [28]. Several studies have found that exposure to glyphosate can induce changes in cellular architecture and the distribution of cytoskeleton components in diverse models [16, 17, 29]. In zebrafish embryos, studies have shown that glyphosate exposure can result in an epiboly delay during early embryo development, in addition to affecting motility, heart development, and the formation and function of nervous system components, which suggests that cytoskeleton modifications may be implicated in glyphosate toxicity mechanisms [11, 12, 13, 20].
In this study, changes in the levels of acetylated α-tubulin in the zebrafish embryos exposed to glyphosate at all concentrations suggests that a change in microtubule stability was induced by exposure to this herbicide. In vivo assessments of acetylated microtubules have indicated that they are more resistant to mild-cold, nocodazole, or colchicine treatments when compared to non-acetylated microtubules, suggesting that microtubule stability is related to α-tubulin acetylation [30, 31]. Likewise, multiple studies have suggested that reduced levels of K40 acetylation in α-tubulin cause axonal transport defects associated with Huntington's disease, amyotrophic lateral sclerosis, and Parkinson's disease [32]. Microtubule stability is known to be mediated by several intracellular mechanisms, among which, Wnt5a signaling has been found to be crucial for microtubule stabilization during cell division through disheveled 2 (Dvl2) [5], which is associated with axonal microtubules and regulates microtubule stability in processes like cell migration, cell polarity, and axonal remodeling in the nervous system [33]. Interestingly, it has been observed that the exposure of hippocampal neurons to glyphosate at a concentration of 4 mg/ml reduced both Wnt5a expression and CaMKII activity, accompanied by a redistribution of the microtubules in the forming axons and changes in the location of Tau proteins [14]. This suggests that changes in the expression of the components of the Wnt signaling pathway may be involved in the glyphosate-induced microtubule instability observed in the in vivo microtubule sedimentation assay in this study.
The enzyme responsible for regulating tubulin deacetylation is histone deacetylase 6 (HDAC6), a member of the HDAC family that not only participates in histone acetylation and deacetylation but also targets several nonhistone substrates, such as α-tubulin and heat shock protein 90 (HSP90) while participating in multiple processes, such as cell proliferation, metastasis, invasion, and mitosis in tumors [32, 33, 34]. It has been observed that the expression of HDAC6 may be mediated through the estrogen signaling pathway, as the activation of the estrogen receptor in breast cancer MCF-7 cells induces an overexpression of HDAC6, suggesting that HDAC6 is an estrogen-regulated gene [35, 36]. This is interesting given that it has been suggested that glyphosate exposure in estrogen-dependent cancer cells can induce the activation of the estrogen response element (ERE) mediated via estrogen receptors ERα and ERβ [37, 38, 39]. This relationship between the estrogenic activity of glyphosate and the regulation of HDAC6 expression via ER may explain the reduction in acetylated α-tubulin in the zebrafish embryos exposed to glyphosate.
Further evidence suggests that some of the effects of glyphosate on the development of the nervous system could be related to changes in microtubule stability. For instance, exposure of the neuroblastoma cell line SH-SY5Y to glyphosate (5 mM) and Aminomethylphosphonic acid (AMPA; 10 mM), one of the main degradation products of glyphosate, up-regulated the expression of CaMKIIA and CaMKIIB and the Wnt proteins Wnt3a, Wnt5a, and Wnt7a [18]. CaMKII is a serine/threonine kinase that is regulated by the calcium-calmodulin complex, which plays a key role in the activation of MAP2 and Tau (key regulators of microtubule dynamics in the axons and dendrites, respectively) [3, 34]. Exposure to GBHs at 1% (corresponding to 0.36% of glyphosate), has been found to directly modify the levels of S100B [19], a Ca2+-binding protein implicated in the regulation of microtubule stability during nervous system development [4]. It is worth noting that the regulation of these proteins is particularly important during nervous system development, which requires a precise regulation of microtubule stability [35].
It has also been reported that glyphosate exposure (50 and 100 μg/ml) in zebrafish embryos can induce the selective downregulation of L-type calcium channel (Cacana1C) and ryanodine receptor (ryr2a) genes [36]. In addition, it has also been found that both glyphosate and the commercial preparation Roundup at 0.036 g/L were able to activate multiple stress-response pathways in Sertoli cells, increasing the intracellular Ca2+ concentration by opening L-type voltage-dependent Ca2+ channels as well as endoplasmic reticulum IP3 and ryanodine receptors, leading to Ca2 + overload within the cells [37]. Similarly, in the hippocampi of rats, it has been found that acute exposure to GBHs (corresponding to 0.38% glyphosate), increased the activity of both N-methyl-D-aspartate (NMDA) receptors and voltage-dependent Ca2+ channels, leading to a Ca2+ influx and the activation of CaMKII and ERK [38]. This implies that the changes in microtubule stability observed in our study could correspond to possible changes in calcium levels inside the cell and to the activity of CaMKII. In this sense, it has been observed that microtubules can be rapidly disassembled into their subunits in the presence of high concentrations of Ca2+ by a microtubule disassembly mechanism that is induced by the activity of calcium-calmodulin [4, 39].
Interestingly, no changes in the actin levels of the whole embryo extracts or in the percentages of actin in the polymeric fraction were observed in any of the glyphosate treatments, which contrasts with what was observed by Hedberg et al. [16], who found that both glyphosate and Roundup inhibited melanosome transport and altered cytoskeleton morphology and the distribution of actin filaments in Xenopus laevis melanophores exposed to glyphosate (0.5–5 mM), which was found to be related to glyphosate-induced pH changes. Further evidence suggests that actin dynamics may be sensitive to pH through the modulation of intracellular actin polymerization and the function of actin-related proteins [40, 41]. However, in our experiment, the pH effect of glyphosate was counteracted by the EM buffer. After ruling out the effect of pH, our results indicate that the effects that have been observed in the cytoskeletons of several animal models [14, 15, 17] may be related to changes in microtubule stability in a non pH-dependent manner. This is consistent with the results of Yahfoufi et al. [17] who reported oocyte deterioration in a pH-controlled medium, observing via immunofluorescence modifications of the microtubule organization center and a shortening of the microtubules of the spindle fibers.
The significant reduction in the acetylation of the microtubules in the polymeric fraction of the embryos exposed to glyphosate can be related to both changes in HDAC6 and to changes in the function of the canonical wnt signal pathway. It has been suggested that the canonical Wnt pathway is directly regulated by the expression of HDAC6, as it has been observed that histone deacetylase 6 directly modulates β-catenin protein levels in the Wnt/β-catenin signaling pathway [41]. Interestingly, an analysis of the prefrontal cortex of mice exposed to glyphosate during pregnancy and lactation showed abnormalities in the expression patterns of proteins related to the Wnt/β-catenin signal pathway, which is involved in the regulation of microtubule stability through cyclin-dependent kinase 11 (CDK11) [[42], [43]]. In the same way, another key component in the acetylation of microtubules, α-tubulin N-acetyltransferase 1 (αTAT1), which is the major α-tubulin acetyltransferase enzyme in vertebrates, can mediate the Wnt/β-catenin signaling pathway through the regulation of the location of β-catenin to the plasma membrane and nucleus, suggesting a relationship between microtubule acetylation and the function of the Wnt/β-catenin pathway that implicates the stability of microtubules [[44], [45]].
In conclusion, we have determined that exposure to glyphosate during embryonic development in zebrafish can generate changes in the levels of acetylated α-tubulin and result in microtubule instability without generating apparent changes in the relative expression of α-tubulin, which may be due to its effects on the Wnt signaling pathway and to changes in calcium dynamics.
Declarations
Author contribution statement
Rubén D. Díaz-Marín: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Jesús D. Valencia-Hernández: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Miguel Betancourt-Lozano, Beatriz Yáñez-Rivera: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
M. Betancourt-Lozano was supported by the Mexican National Council of Science and Technology (CONACYT) project (CB2016 288306). R.D. Díaz-Marín was supported by a CONACYT postdoctoral fellowship (28548). J.D. Valencia-Hernández was supported by a CONACYT fellowship (29448).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Beatriz Ibarra Mendoza for her support in the construction of the diagrams. We thank Andrea Lievana MacTavish for English language editing.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Fig_S1A_ alphaTub.
Fig_S1A_alphaTubAC.
Fig_S1B_GAPDH.
Fig_ S1B_ alphaTub.
Fig_S2A_alphaTub.
Fig_S2B_alphaTubAC.
Fig_S3A_Act.
Fig_S3B_Act.
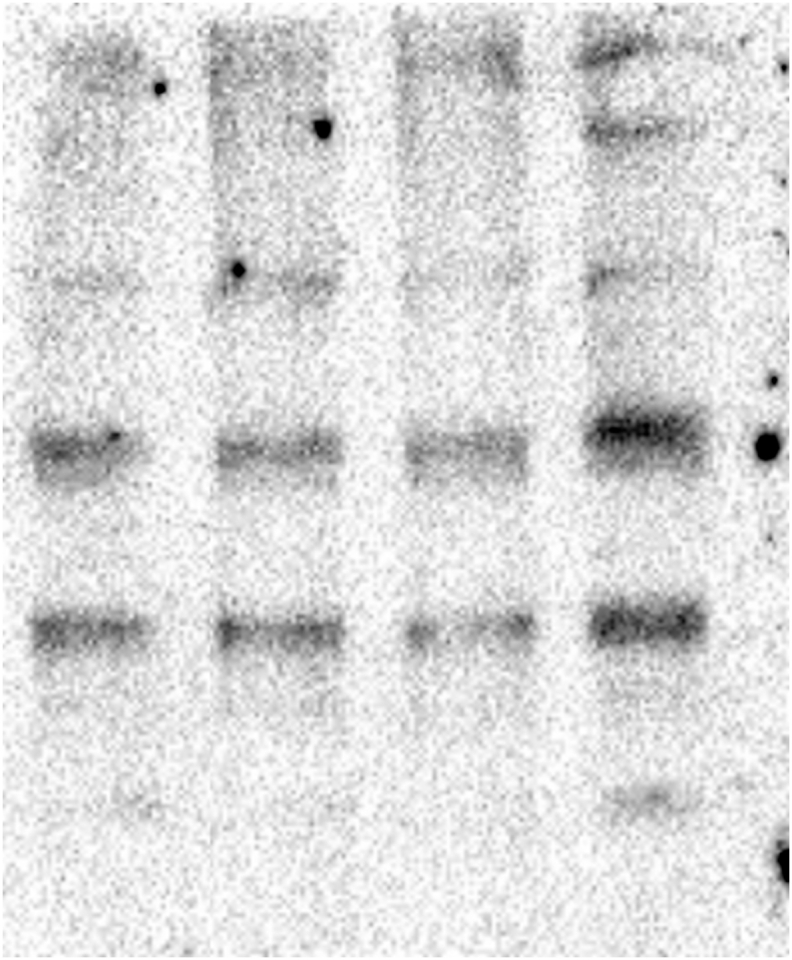
Fig_S3B_GAPDH.
References
- 1.Drewes G., Ebneth A., Mandelkow E.M. MAPs, MARKs and microtubule dynamics. Trends Biochem. Sci. 1998;23:307–311. doi: 10.1016/s0968-0004(98)01245-6. [DOI] [PubMed] [Google Scholar]
- 2.Andersen S.S.L. Trends Cell Biol. Trends Cell Biol; 2000. Spindle assembly and the art of regulating microtubule dynamics by MAPs and Stathmin/Op18; pp. 261–267. [DOI] [PubMed] [Google Scholar]
- 3.Mcvicker D.P., Millette M.M., Dent E.W. Signaling to the microtubule cytoskeleton: an unconventional role for CaMKII. Dev. Neurobiol. 2015;75:423–434. doi: 10.1002/dneu.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baudier J., Briving C., Deinum J., Haglid K., Sörskog L., Wallin M. Effect of S-100 proteins and calmodulin on Ca2+-induced disassembly of brain microtubule proteins in vitro. FEBS Lett. 1982;147:165–167. doi: 10.1016/0014-5793(82)81033-8. [DOI] [PubMed] [Google Scholar]
- 5.Fumoto K., Kikuchi K., Gon H., Kikuchi A. Wnt5a signaling controls cytokinesis by correctly positioning ESCRT-III at the midbody. J. Cell Sci. 2012;125:4822–4832. doi: 10.1242/jcs.108142. [DOI] [PubMed] [Google Scholar]
- 6.Xu W., Ge Y., Liu Z., Gong R. Glycogen synthase kinase 3β orchestrates microtubule remodeling in compensatory glomerular adaptation to podocyte depletion. J. Biol. Chem. 2015;290:1348–1363. doi: 10.1074/jbc.M114.593830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boocock M.R., Coggins J.R. Kinetics of 5-enolpyruvylshikimate-3-phosphate synthase inhibition by glyphosate. FEBS Lett. 1983;154:127–133. doi: 10.1016/0014-5793(83)80888-6. [DOI] [PubMed] [Google Scholar]
- 8.Zablotowicz R.M., Reddy K.N. Impact of glyphosate on the symbiosis with glyphosate-resistant transgenic soybean. J. Environ. Qual. 2004;33:825. doi: 10.2134/jeq2004.0825. [DOI] [PubMed] [Google Scholar]
- 9.Solomon K.R., Thompson D.G. Ecological risk assessment for aquatic organisms from over-water uses of glyphosate. J. Toxicol. Environ. Health B Crit. Rev. 2003;6:289–324. doi: 10.1080/10937400306468. [DOI] [PubMed] [Google Scholar]
- 10.Van Bruggen A.H.C., He M.M., Shin K., Mai V., Jeong K.C., Finckh M.R., Morris J.G. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018;616–617:255–268. doi: 10.1016/j.scitotenv.2017.10.309. [DOI] [PubMed] [Google Scholar]
- 11.Roy N.M., Carneiro B., Ochs J. Glyphosate induces neurotoxicity in zebrafish. Environ. Toxicol. Pharmacol. 2016;42:45–54. doi: 10.1016/j.etap.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Roy N.M., Ochs J., Zambrzycka E., Anderson A. Glyphosate induces cardiovascular toxicity in Danio rerio. Environ. Toxicol. Pharmacol. 2016;46:292–300. doi: 10.1016/j.etap.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Bridi D., Altenhofen S., Gonzalez J.B., Reolon G.K., Bonan C.D. Glyphosate and Roundup® alter morphology and behavior in zebrafish. Toxicology. 2017;392:32–39. doi: 10.1016/j.tox.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Coullery R.P., Ferrari M.E., Rosso S.B. Neuronal development and axon growth are altered by glyphosate through a WNT non-canonical signaling pathway. Neurotoxicology. 2016;52:150–161. doi: 10.1016/j.neuro.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Szekacs I., Farkas E., Gemes B.L., Takacs E., Szekacs A., Horvath R. Integrin targeting of glyphosate and its cell adhesion modulation effects on osteoblastic MC3T3-E1 cells revealed by label-free optical biosensing. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-36081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedberg D., Wallin M. Effects of Roundup and glyphosate formulations on intracellular transport, microtubules and actin filaments in Xenopus laevis melanophores. Toxicol. Vitro. 2010;24:795–802. doi: 10.1016/j.tiv.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Yahfoufi Z.A., Bai D., Khan S.N., Chatzicharalampous C., Kohan-Ghadr H.R., Morris R.T., Abu-Soud H.M. Glyphosate induces metaphase II oocyte deterioration and embryo damage by zinc depletion and overproduction of reactive oxygen species. Toxicology. 2020;439 doi: 10.1016/j.tox.2020.152466. [DOI] [PubMed] [Google Scholar]
- 18.Martínez M.A., Rodríguez J.L., Lopez-Torres B., Martínez M., Martínez-Larrañaga M.R., Maximiliano J.E., Anadón A., Ares I. Use of human neuroblastoma SH-SY5Y cells to evaluate glyphosate-induced effects on oxidative stress, neuronal development and cell death signaling pathways. Environ. Int. 2020;135 doi: 10.1016/j.envint.2019.105414. [DOI] [PubMed] [Google Scholar]
- 19.Cattani D., Cesconetto P.A., Tavares M.K., Parisotto E.B., De Oliveira P.A., Rieg C.E.H., Leite M.C., Prediger R.D.S., Wendt N.C., Razzera G., Filho D.W., Zamoner A. Developmental exposure to glyphosate-based herbicide and depressive-like behavior in adult offspring: implication of glutamate excitotoxicity and oxidative stress. Toxicology. 2017;387:67–80. doi: 10.1016/j.tox.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S., Xu J., Kuang X., Li S., Li X., Chen D., Zhao X., Feng X. Biological impacts of glyphosate on morphology, embryo biomechanics and larval behavior in zebrafish (Danio rerio) Chemosphere. 2017;181:270–280. doi: 10.1016/j.chemosphere.2017.04.094. [DOI] [PubMed] [Google Scholar]
- 21.Sarmah S., Muralidharan P., Curtis C.L., McClintick J.N., Buente B.B., Holdgrafer D.J., Ogbeifun O., Olorungbounmi O.C., Patino L., Lucas R., Gilbert S., Groninger E.S., Arciero J., Edenberg H.J., Marrs J.A. Ethanol exposure disrupts extraembryonic microtubule cytoskeleton and embryonic blastomere cell adhesion, producing epiboly and gastrulation defects. Biol. Open. 2013;2:1013–1021. doi: 10.1242/bio.20135546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bercier V., Rosello M., Del Bene F., Revenu C. Zebrafish as a model for the study of live in vivo processive transport in neurons. Front. Cell Dev. Biol. 2019;7:17. doi: 10.3389/fcell.2019.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J., Lessman C.A. Soluble tubulin complexes, γ-tubulin, and their changing distribution in the zebrafish (Danio rerio) ovary, oocyte and embryo. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007;147:56–73. doi: 10.1016/j.cbpb.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Westerfield M. fifth ed. 2007. The Zebrafish Book : a Guide for the Laboratory Use of Zebrafish (Danio rerio) Printed by the University of Oregon Press; Distributed by the Zebrafish International Resource Center ;Distributed by the Zebrafish International Resource Center, Eugene OR. [Google Scholar]
- 25.Bershadsky A.D., Gelfand V.I., Svitkina T.M., Tint I.S. Microtubules in mouse embryo fibro blasts extracted with Triton X-100. Cell Biol. Int. Rep. 1978;2:425–432. doi: 10.1016/0309-1651(78)90093-0. [DOI] [PubMed] [Google Scholar]
- 26.Gómez de León C.T., Díaz Martín R.D., Mendoza Hernández G., González Pozos S., Ambrosio J.R., Mondragón Flores R. Proteomic characterization of the subpellicular cytoskeleton of Toxoplasma gondii tachyzoites. J. Proteomics. 2014;111:86–99. doi: 10.1016/j.jprot.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 27.R Core Team . References - Scientific Research Publishing; Vienna: 2018. R A Language and Environment for Statistical Computing. R Foundation for Statistical Computing.https://www.scirp.org/(S(lz5mqp453edsnp55rrgjct55))/reference/ReferencesPapers.aspx?ReferenceID=2342186 (n.d.) [Google Scholar]
- 28.Sulukan E., Köktürk M., Ceylan H., Beydemir Ş., Işik M., Atamanalp M., Ceyhun S.B. An approach to clarify the effect mechanism of glyphosate on body malformations during embryonic development of zebrafish (Daino rerio) Chemosphere. 2017;180:77–85. doi: 10.1016/j.chemosphere.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Yasuor H., Abu-Abied M., Belausov E., Madmony A., Sadot E., Riov J., Rubin B. Glyphosate-induced anther indehiscence in cotton is partially temperature dependent and involves cytoskeleton and secondary wall modifications and auxin accumulation. Plant Physiol. 2006;141:1306–1315. doi: 10.1104/pp.106.081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wloga D., Joachimiak E., Fabczak H. Tubulin post-translational modifications and microtubule dynamics. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18102207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., Yoshida M., Wang X.-F., Yao T.-P. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 32.Eshun-Wilson L., Zhang R., Portran D., Nachury M.V., Toso D.B., Löhr T., Vendruscolo M., Bonomi M., Fraser J.S., Nogales E. Effects of α-tubulin acetylation on microtubule structure and stability. Proc. Natl. Acad. Sci. U. S. A. 2019;116:10366–10371. doi: 10.1073/pnas.1900441116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciani L., Krylova O., Smalley M.J., Dale T.C., Salinas P.C. A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules. J. Cell Biol. 2004;164:243–253. doi: 10.1083/jcb.200309096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zha X.M., Dailey M.E., Green S.H. Role of Ca2+/calmodulin-dependent protein kinase II in dendritic spine remodeling during epileptiform activity in vitro. J. Neurosci. Res. 2009;87:1969–1979. doi: 10.1002/jnr.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baas P.W., Rao A.N., Matamoros A.J., Leo L. Stability properties of neuronal microtubules. Cytoskeleton. 2016;73:442–460. doi: 10.1002/cm.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaur H., Bhargava A. Glyphosate induces toxicity and modulates calcium and NO signaling in zebrafish embryos. Biochem. Biophys. Res. Commun. 2019;513:1070–1075. doi: 10.1016/j.bbrc.2019.04.074. [DOI] [PubMed] [Google Scholar]
- 37.De Liz Oliveira Cavalli V.L., Cattani D., Heinz Rieg C.E., Pierozan P., Zanatta L., Benedetti Parisotto E., Wilhelm Filho D., Mena Barreto Silva F.R., Pessoa-Pureur R., Zamoner A. Roundup disrupts male reproductive functions by triggering calcium-mediated cell death in rat testis and Sertoli cells. Free Radic. Biol. Med. 2013;65:335–346. doi: 10.1016/j.freeradbiomed.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 38.Cattani D., de Liz Oliveira Cavalli V.L., Heinz Rieg C.E., Domingues J.T., Dal-Cim T., Tasca C.I., Mena Barreto Silva F.R., Zamoner A. Mechanisms underlying the neurotoxicity induced by glyphosate-based herbicide in immature rat hippocampus: involvement of glutamate excitotoxicity. Toxicology. 2014;320:34–45. doi: 10.1016/j.tox.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Job D., Fischer E.H., Margolis R.L. Rapid disassembly of cold-stable microtubules by calmodulin. Proc. Natl. Acad. Sci. U. S. A. 1981;78:4679–4682. doi: 10.1073/pnas.78.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wioland H., Jegou A., Romet-Lemonne G. Quantitative variations with pH of actin depolymerizing factor/cofilin’s multiple actions on actin filaments. Biochemistry. 2019;58:40–47. doi: 10.1021/acs.biochem.8b01001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crevenna A.H., Naredi-Rainer N., Schonichen A., Dzubiella J., Barber D.L., Lamb D.C., Wedlich-Söldner R. Electrostatics control actin filament nucleation and elongation kinetics. J. Biol. Chem. 2013;288:12102–12113. doi: 10.1074/jbc.M113.456327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji H., Xu L., Wang Z., Fan X., Wu L. Differential microRNA expression in the prefrontal cortex of mouse offspring induced by glyphosate exposure during pregnancy and lactation. Exp. Ther. Med. 2018;15:2457–2467. doi: 10.3892/etm.2017.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ou D., Chen L., He J., Rong Z., Gao J., Li Z., Liu L., Tang F., Li J., Deng Y., Sun L. CDK11 negatively regulates Wnt/β-catenin signaling in the endosomal compartment by affecting microtubule stability. Cancer Biol. Med. 2020;17:328–342. doi: 10.20892/j.issn.2095-3941.2019.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oh S., You E., Ko P., Jeong J., Keum S., Rhee S. Genetic disruption of tubulin acetyltransferase, αTAT1, inhibits proliferation and invasion of colon cancer cells through decreases in Wnt1/β-catenin signaling. Biochem. Biophys. Res. Commun. 2017;482:8–14. doi: 10.1016/j.bbrc.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 45.Kalebic N., Sorrentino S., Perlas E., Bolasco G., Martinez C., Heppenstal P.A. αTAT1 is the major α-tubulin acetyltransferase in mice. Nat. Commun. 2013;4:1962. doi: 10.1038/ncomms2962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.