Abstract
Background: The objective of this analysis was to systematically review studies employing wearable technology in patients with dementia by quantifying differences in digitally captured physiological endpoints.
Methods: This systematic review and meta-analysis was based on web searches of Cochrane Database, PsycInfo, Pubmed, Embase, and IEEE between October 25–31st, 2017. Observational studies providing physiological data measured by wearable technology on participants with dementia with a mean age ≥50. Data were extracted according to PRISMA guidelines and methodological quality assessed independently using Downs and Black criteria. Standardized mean differences between cases and controls were estimated using random-effects models.
Results: Forty-eight studies from 18,456 screened abstracts (Dementia: n = 2,516, Control: n = 1,224) met inclusion criteria for the systematic review. Nineteen of these studies were included in one or multiple meta-analyses (Dementia: n = 617, Control: n = 406). Participants with dementia demonstrated lower levels of daily activity (standardized mean difference (SMD), −1.60; 95% CI, −2.66 to −0.55), decreased sleep efficiency (SMD, −0.52; 95% CI, −0.89 to −0.16), and greater intradaily circadian variability (SMD, 0.46; 95% CI, 0.27 to 0.65) than controls, among other measures. Statistical between-study heterogeneity was observed, possibly due to variation in testing duration, device type or patient setting.
Conclusions and Relevance: Digitally captured data using wearable devices revealed that adults with dementia were less active, demonstrated increased fragmentation of their sleep-wake cycle and a loss of typical diurnal variation in circadian rhythm as compared to controls.
Keywords: technology, geriatrics, cognition, sleep, wearable
Introduction and Background
Dementia has been identified by the World Health Organization as a global priority for public health and social care in the twenty-first century (1). Advances in the molecular and genetic understanding of neurodegenerative disease has contributed to improved diagnostic paradigms and helped to foster a new era of personalized medicine for patients with dementia. This has coincided with the advancement in biological drug development for targeted therapies. These therapies have reflected the maturation in the scientific understanding of dementia that goes beyond raw measurement of cognitive performance. As a case in point a recent review of active clinical trials had shown that 14 biological treatments have targeted neuropsychiatric and behavioral symptoms as primary end-points. Challenges remain in capturing the heterogeneity of the clinical course experienced by individuals with dementia and translating these into meaningful end-points.
Technological advances using accelerometers, gyroscopes, and other motion detectors housed in mobile platforms may eventually present a cost-effective way to measure disease burden and deploy personalized treatments (2). Wearable devices that can continuously monitor physiological measures over extended periods, for example in the patient's home, provide unique information not attainable with traditional in-clinic monitoring and hold particular appeal in dementia populations (3). Advances in technology have made these devices increasingly affordable and user friendly but have been limited by methodological challenges. Specifically, their high resolution and sensitivity leaves them susceptible to noisy interference, complicated and time-consuming analytical techniques are required to derive clinically meaningful endpoints from the large amounts of data they produce, and the lack of standards has led to isolated “islands of expertise” (4).
The flexibility of wearable platforms has resulted in a variety of different uses including monitoring of gait, motion tracking, and sleep and circadian rhythm assessment (5). The ability to identify objective measurements of specific endpoints with respect to individual and group-wise subject performance, captured in real-time at various settings including at home, provides ecological validity that would otherwise be lost in laboratory settings. The main question that we had aimed to address was the potential for wearable devices to provide information on the behavioral and neuropsychiatric fluctuations inherent in the clinical course of dementia. The ability to accurately and objectively measure these fluctuations can provide researchers with viable digital surrogate end-points for use in clinical trials. We undertook a systematic review and meta-analysis to evaluate the utility of wearable technology in patients with dementia for the measurement of these neurophysiological parameters. The objective of this analysis was to systematically review studies employing wearable technology in patients with dementia by quantifying differences in digitally captured neurophysiological endpoints.
Literature Selection Criteria
Data Sources and Search Strategy
Five electronic databases were searched including Cochrane, EMBASE, PubMed, PsycInfo, and IEEE. Searches were performed for Cochrane, PsycInfo, and IEEE on October 31, 2017. A PubMed search was performed on October 25, 2017, and an Embase search on October 27, 2017. A combination of Medical Subject Headings and search terms were constructed by the authors (RP, AC, and JB) in collaboration with a librarian. The Search Terms provides an outline of the search strategy for PubMed only.
Search Terms. Systematic Review Search Strategy: PubMed
“Dementia”[Mesh]
dementia
(frontotemporal dementia)
(vascular dementia)
(Alzheimer* disease)
(Parkinson* disease)
(lewy body)
Creutzfeldt-Jakob
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
“Technology”[Mesh]
(wearable device)
(assistive technology)
(wearable technology)
on-body
bracelet
GPS
actigraphy
accelerometer
(galvanic skin response)
biosensor
sensor
gyroscope
watch
necklace
harness
strap
patch
camera
chip
(step counter)
pouch
armband
node
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33
9 and 3.
Types of Studies
We included observational studies reporting primary data in a peer-reviewed scientific journal. Studies had to include participants with a mean age ≥50 years and did not include any direct intervention (i.e., drug, vitamin, supplement, exercise, cognitive, or behavioral intervention). Studies published before 1970 or translated to English were excluded. Studies that did not provide descriptive statistics for a physiological outcome were excluded. Conference abstracts, review papers, case reports, letters, opinion pieces, editorials, article comments, or corrections were excluded.
Type of Exposure
We included all-cause dementia (any dementia subtype) as our exposure (6). Exact search terms for dementia subtypes included can be found in the search strategy (Search Terms). As we included studies from 1970 onwards, diagnostic criteria for the diagnosis of dementia differed between studies and is summarized for each study in Table 1.
Table 1.
Patient setting and diagnostic criteria for 48 observational studies testing wearable technology in participants with dementia.
| Source | Diagnostic criteria | Setting |
|---|---|---|
| Aharon-Peretz et al. (7) | DSM III-R (8)/NINCDS-ADRDA (9) | Not stated |
| Ahmed et al. (10) | McKhann et al. (11)/Gorno-Tempini et al. (12)/Rascovsky et al. (13) | Community (home)/In Lab |
| Anderson et al. (14) | Neary criteria (15) | Community (home) |
| Brown et al. (16) | Medical record diagnosis | Nursing home |
| Carvalho-Bos et al. (17) | NINCDS-ADRDA (9)/DSM-IV (18) | Nursing home |
| David et al. (19) | DSM-IV (18) | Out-patient clinic |
| David et al. (20) | NINCDS-ADRDA (9) | Out-patient clinic |
| Eggermont and Scherder (21) | Medical record diagnosis | Nursing home |
| Fetveit and Bjorvatn (22) | Clinical Dementia Rating (CDR) scale (23) | Nursing home |
| Fleiner et al. (24) | ICD-10 (25) | Psychiatric hospital |
| Gehrman et al. (26) | Medical record diagnosis/NINCDS-ARDA (9) | Nursing home |
| Ghali et al. (27) | DSM-III-R (8) | Dementia treatment Evaluation facility |
| Harper et al. (28) | NINCDS-ADRDA (9) | Hospital/clinical research center |
| Harper et al. (29) | NINCDS-ADRDA (9) | Hospital/clinical research center |
| Hatfield et al. (30) | DSM-IV (18)/ NINCDS-ADRDA (9) | Community (home) |
| Hooghiemstra et al. (31) | DSM-V (32)/NINCDS-ADRDA (9)/Neary Criteria (15)/McKeith et al. (33)/Pohjasvaara et al. (34) | Not stated |
| Ijmker and Lamoth (35) | Clinician/ medical record diagnosis | In laboratory |
| Iwata et al. (36) | DSM-IV (18)/NINCDS-ARDA (9) | Not stated |
| James et al. (37) | NINCDS-ARDA (9) | Community (home) |
| Kodama et al. (38) | DSM-III-R (8) | Community (home) |
| König et al. (39) | International working group−2 criteria (IWG-2) (40) | Memory clinic |
| Kuhlmei et al. (41) | NINCDS-ADRDA (9)/NINDS-AIREN (42) | Not stated |
| La Morgia et al. (43) | NINCDS-ADRDA (9) | Not stated |
| Lamoth et al. (44) | Alzheimer's association criteria | Clinic/hospital |
| Landolt et al. (45) | Autopsy or biopsy confirmation | Hospital/ nursing home |
| Lee et al. (46) | NINCDS-ADRDA (9) | Not stated |
| Leger et al. (47) | DSM-V (32)/ NINCDS-ADRDA (9)/MMSE ≤ 25 and ≥15 (48)/CDR (Score of 0.5, 1, or 2) (23) | Out-patient clinic |
| McCurry et al. (49) | Family physician/medical record diagnosis | Community (home) |
| Merrilees et al. (50) | Neary criteria for frontotemporal lobar degeneration (51) | Community (home) |
| Most et al. (52) | NINCDS- ADRDA (9) | Not stated |
| Moyle et al. (53) | Medical record diagnosis | Long-term care facility |
| Mulin et al. (54) | NINCDS- ADRDA (9) | Community (home) |
| Murphy et al. (55) | MMSE < 23 (48) | Nursing home |
| Olsen et al. (56) | Medical record diagnosis or MMSE < 25 (48) | Nursing home/community (home) |
| Paavilainen et al. (57) | CDR > 0.5 (23)/ MMSE < 20 (48) | Nursing home |
| Pollak and Stokes (58) | Mattis dementia rating scale total score < 123 (59) Mattis dementia rating scale memory score < 19 (59) | Community (home) |
| Rindlisbacher and Hopkins (60) | DSM-III-R (8) | Hospital |
| Schwenk et al. (61) | NINCDS-ADRDA (9)/NINDS-AIREN (42) | Community (home) |
| Valembois et al. (62) | DSM-IV (18) | Hospital |
| van Alphen et al. (63) | Medical record diagnosis | Community (home)/nursing home |
| van Someren et al. (64) | DSM-III-R (8)/NINCDS-ADRDA (9) | Community (home)/nursing home |
| Varma and Watts (65) | NINCDS-ADRDA (9) | Community (home) |
| Viegas et al. (66) | DSM-IV (18)/MMSE ≤ 24 (48) | Nursing home |
| Volicer et al. (67) | NINCDS-ADRDA (9)/DSM-III-R (8) | Hospital |
| Wams et al. (68) | NINCDS-ADRDA (9) | Community (home) |
| Weissova et al. (69) | NINCDS-ADRDA (9) | Community (home) |
| Wirz-Justice et al. (70) | DSM –IV (18) | Hospital |
| Yesavage et al. (71) | NINCDS-ADRDA (9) | Community (home) |
Types of Outcome Measures
We included studies which provided physiological data as measured by wearable technology. Wearable technology was defined as a non-implantable, body-fixed sensor technology designed to monitor for >24 h and to not interfere with the wearer's normal activity (5, 72). By this definition, studies using finger-based pulse oximeters, blood pressure monitors, galvanic skin response sensors, functional near-infrared spectroscopy (fNIRS), and electroencephalograms (EEG) were excluded. Where studies included measurement devices other than a wearable device, only data from the wearable device was included in the final analysis.
Methods for Literature Secondary Screening
First Selection: Abstract Screening
Two authors (RP and AC) independently screened each record by title and abstract according to eligibility criteria. Eighteen thousand four hundred fifty-six abstracts were included in the initial screening process. There were 525 disagreements in abstract selection between the two reviewers. Conflicts were resolved by two additional authors (NS and JB) using the inclusion and exclusion criteria and definitions outlined in Figure 1.
Figure 1.
Literature search flow diagram.
Final Selection
Two hundred thirty articles were eligible for full text review. Two authors (RP and AC) independently determined eligibility of each article for inclusion. In cases of disagreement or conflict, senior authors (NS, JB, and KT) determined whether the study met eligibility criteria. Forty-eight articles were included in the final systematic review.
Data Collection
Data was extracted by three authors (JB, RP, and AC). Information extracted from each publication is provided in Table 6. To assess the methodological quality of included studies, we used the checklist provided by Downs and Black (73). A total quality score is provided for each study in Table 6 (maximum score = 32).
Statistical Analyses
Data analysis was performed using Stata/SE (StataCorp LP, Texas, Version 15). The age and number of included participants per study, as well as general study results are provided in Table 6. Initial synthesis of qualitative data revealed a number of common endpoints reported consistently by authors. Subsequent meta-analyses included only observational case-control or cross-sectional studies that presented data for these commonly reported endpoints (Table 7).
Meta-analyses were conducted using the standardized mean difference (Hedges' g). Hedges' g values ≤0.20, >0.20 but <0.80, and ≥0.80 were considered small, moderate, or large, respectively between controls and participants with dementia (74). For each single or combined effect size, a positive value indicated a higher mean value of that variable in participants with dementia than in healthy volunteers. Some publications contained two subgroups of dementia participants. A fixed effects meta-analysis was performed on the dementia subgroups within each of these studies to compute a composite effect size and variance (75). This composite effect was used in the across-study random effects analysis.
Across-study heterogeneity was investigated using the Cochran's Q-test and I2 statistic. Cochran's Q test was performed using the weighted method of moments method (75). Cochran's Q statistic was considered significant at p < 0.10. I2-values of 25, 50, and 75% were considered indicative of low, moderate, and high heterogeneity, respectively (76).
For each analysis, a funnel plot of standardized mean differences was constructed, and the risk of publication bias evaluated through funnel plot asymmetry and Egger tests. We acknowledge that many other factors including heterogeneity, differences in methodological quality, and selective reporting may produce funnel plot asymmetry (77).
The influence of each study on a meta-analysis estimate was investigated through influence analysis, where each individual study is omitted in turn and the meta-analysis re-estimated using a random effects model. For publications that included more than one subgroup of participants with dementia, the largest subgroup was included in the influence analysis.
There was no funding source for this study and the corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Results
Systematic Review
Five database searches resulted in 18,456 retrieved abstracts after removal of duplicates (Figure 1). Two hundred thirty of these publications qualified for full-text screening after examination by title and abstract. Forty-eight studies qualified for inclusion in the final qualitative analysis and 19 of these publications qualified for inclusion in one or multiple meta-analyses (Dementia: n = 617, Control: n = 406) (Table 7).
Nineteen studies (39%) enrolled participants only with ad-related diagnoses. Table 2 describes the technical specifications of devices used in individual studies. Thirty-four studies (70%) tested participants using a wrist-worn actigraph. The average assigned duration of wear was 8.26 days (range: 6 min−28 days). Forty (83%) studies used accelerometry as the main measurement of activity. One study used an accelerometer with a gyroscope, while one further study used an accelerometer, gyroscope and magnetometer. Six studies (12%) used activity monitors which did not state the type of measurement modality.
Table 2.
Technical specifications of wearable devices used in individual studies.
| Source | Measurement method [Author reported] | Product/company name |
• Device placement/ • Length of monitoring |
|---|---|---|---|
| Aharon-Peretz et al. (7) | Actigraph | Ambulatory Monitoring Inc., Ardsley, NY | • Wrist • 8 days |
| Ahmed et al. (10) | Tri-axial accelerometer | Actiheart device–CamNtech | • Chest/left mid-clavicular line • 7 days |
| Anderson et al. (14) | Actigraph | Actiwatch; Cambridge Neurotechnology, Cambridge, UK | • Wrist • 28 days |
| Brown et al. (16) | Actigraph | ActiGraph ActiSleep monitor (ActiGraph LLC, 2013) | • Non-dominant wrist • 72 h |
| Carvalho-Bos et al. (17) | Actigraph | Actiwatch; Cambridge Neurotechnology, Cambridge, UK | • Non-dominant wrist • 2 consecutive weeks |
| David et al. (19) | Actigraph | Actiwatch-L/MiniMitter | • Non-dominant wrist • 75 consecutive minutes |
| David et al. (20) | Actigraph | Micro- Mini MotionLogger, Ambulatory-Monitoring, Inc., Ardsley, NY | • Non-dominant wrist • Seven consecutive 24-hour periods |
| Eggermont and Scherder (21) | Actigraph | Actigraph activity monitor; Cambridge Neurotechnology Ltd. Cambridge, England | • Wrist • 4 consecutive days |
| Fetveit and Bjorvatn (22) | Actigraph | Actiwatch portable recorder (Cambridge Neurotechnology Ltd, UK) | • Dominant hand (Due to paralysis in the dominant arm, two residents wore the actigraph on the non-dominant arm) • 14 Days |
| Fleiner et al. (24) | Triaxial accelerometer, gyroscope and magnetometer | uSense sensors (FARSEEING EU-Consortium, 2015) | • Lower back • 72 consecutive hours |
| Gehrman et al. (26) | Actigraph/photometric transducer | Actillume Monitor (Ambulatory Monitoring, Ardsley, NY) | • Dominant wrist • 3 Days |
| Ghali et al. (27) | Electronic motion detection monitor | Not stated | • Above left elbow • 48 h |
| Harper et al. (28) | Activity monitor | AM-16, Ambulatory Monitoring, Inc. Ardsley, NY | • Ankle • 72 h |
| Harper et al. (29) | Activity monitor | AM-16, Ambulatory Monitoring, Inc. Ardsley, NY | • Nondominant ankle • 72 h |
| Hatfield et al. (30) | Actigraph | Actiwatch; Cambridge Neurotechnology, Cambridge, UK | • Wrist • 28 days |
| Hooghiemstra et al. (31) | Actigraph | The Actiwatch-4 (AW4) activity monitor (Cambridge Neurotechnology Ltd, Cambridge, UK) | • Dominant wrist • 7 consecutive days |
| Ijmker and Lamoth, (35) | Tri-axial accelerometer | DynaPort MiniMod | • Lower Back • Two 3-min periods |
| Iwata et al. (36) | Tri-axial accelerometer | HJA-350IT; Omron, Kyoto, Japan | • 2–3 months during waking hours |
| James et al. (37) | Actigraph | Actical, Mini Mitter | • Non-dominant wrist • 2–16 days |
| Kodama et al. (38) | Activity monitor | The Actiwatch-2 (AW2) Philips Respironics Inc. | • Non-dominant wrist • 7 days |
| König et al. (39) | Actigraph | Prototype, Philips Research Laboratories Europe | • Wrist • Duration of walking tasks |
| Kuhlmei et al. (41) | Actigraph | Actiwatch Mini, Cambridge Neurotechnology | • Wrist • 5 days |
| La Morgia et al. (43) | Actigraph | Actigraph Mini Motionlogger, Ambulatory Monitoring, Inc. Ardsley, NY | • Non-dominant wrist • 7 days |
| Lamoth et al. (44) | Tri-axial accelerometer | DynaPort MiniMod | • Lower back • Duration of testing |
| Landolt et al. (45) | Actigraph | Actiwatch, Cambridge Technology | • Wrist • 2 weeks |
| Lee et al. (46) | activity monitor | Mini-Logger (Mini-Mitter company) | • Wrist • 96 h |
| Leger et al. (47) | Actigraph | Motionwatch 8 (MW8, Camntech, Cambridge, UK) | • Non-dominant wrist • 14 days |
| McCurry et al. (49) | Actigraph | Actillume wrist-movement recorder (Ambulatory Monitoring, Inc., Ardsley, NY) | • Wrist • 7 days |
| Merrilees et al. (50) | Actigraph | MiniMitter Actiwatch monitors (AW-64) | • Non-dominant wrist • 2 weeks |
| Most et al. (52) | Actigraph | Actiwatch; Cambridge Neurotechnology, Cambridge, UK | • Non-dominant wrist • 2 weeks |
| Moyle et al. (53) | Tri-axial accelerometer | SenseWear® Professional 8.0 activity armband (Temple Healthcare, BodyMedia, Inc) | • Upper non-dominant arm • Monday to Saturday |
| Mulin et al. (54) | Actigraph | Micro- Mini MotionLogger, Ambulatory-Monitoring, Inc., Ardsley, NY | • Non-dominant wrist • 7 days |
| Murphy et al. (55) | Tri-axial accelerometer | Sensewear Armband, Body Media | • Upper left arm • 7 days |
| Olsen et al. (56) | Actigraph | ActiSleep+, Actigraph, Pensacola, USA | • Left wrist • 7 days |
| Paavilainen et al. (57) | Telemonitoring and actigraphy system | Information Security Technology (IST) Vivago system | • Wrist • 9–113 days |
| Pollak and Stokes, (58) | Activity Monitor | MiniMotionlogger recorder (Ambulatory Monitoring, Inc.) | • Non-dominant wrist • 9 days |
| Rindlisbacher and Hopkins, (60) | Ambulatory monitoring device | Not stated | • Above left elbow (in shirt) • Four consecutive days |
| Schwenk et al. (61) | Accelerometer/gyroscope | Physilog (BioAGM, CH) | • Chest • 24 h |
| Valembois et al. (62) | Actigraph | Vivago, Vivago Oy, Espoo, Finland | • Non-dominant wrist • 10 days |
| Van Alphen et al. (63) | Tri-axial accelerometer | The Actiwatch-4 (AW4) activity monitor (Cambridge Neurotechnology Ltd, Cambridge, UK) | • Dominant wrist • 6 days |
| van Someren et al. (64) | Actigraph | Not stated | • Wrist • 155 h |
| Varma and Watts, (65) | Actigraph | Actigraph GT3X+ | • Dominant hip • 7 days |
| Viegas et al. (66) | Actigraph | Basic Mini-Motionlogger Actigraph, Ambulatory Monitoring, Inc. | • Wrist • Five 24-h periods |
| Volicer et al. (67) | Activity monitor | AM-16 Ambulatory Monitoring, Ardsley NY | • Waist • 72 h |
| Wams et al. (68) | Actigraph | Actiwatch 7, CamNTech Ltd. | • Non-dominant wrist • 3 weeks |
| Weissova et al. (69) | Actigraph | Actiwatch, AW4 model, Cambridge Neurotechnology Ltd. | • Non-dominant wrist • 21 days |
| Wirz-Justice et al. (70) | Actigraph with luxmeter | Actiwatch-L, Cambridge Neurotechnologies | • Non-dominant wrist • 10–26 days |
| Yesavage et al. (71) | Actigraph | Ambulatory Monitoring Systems, Inc. Ardsley, NY | • Non-dominant wrist • 6 days |
Daily Activity
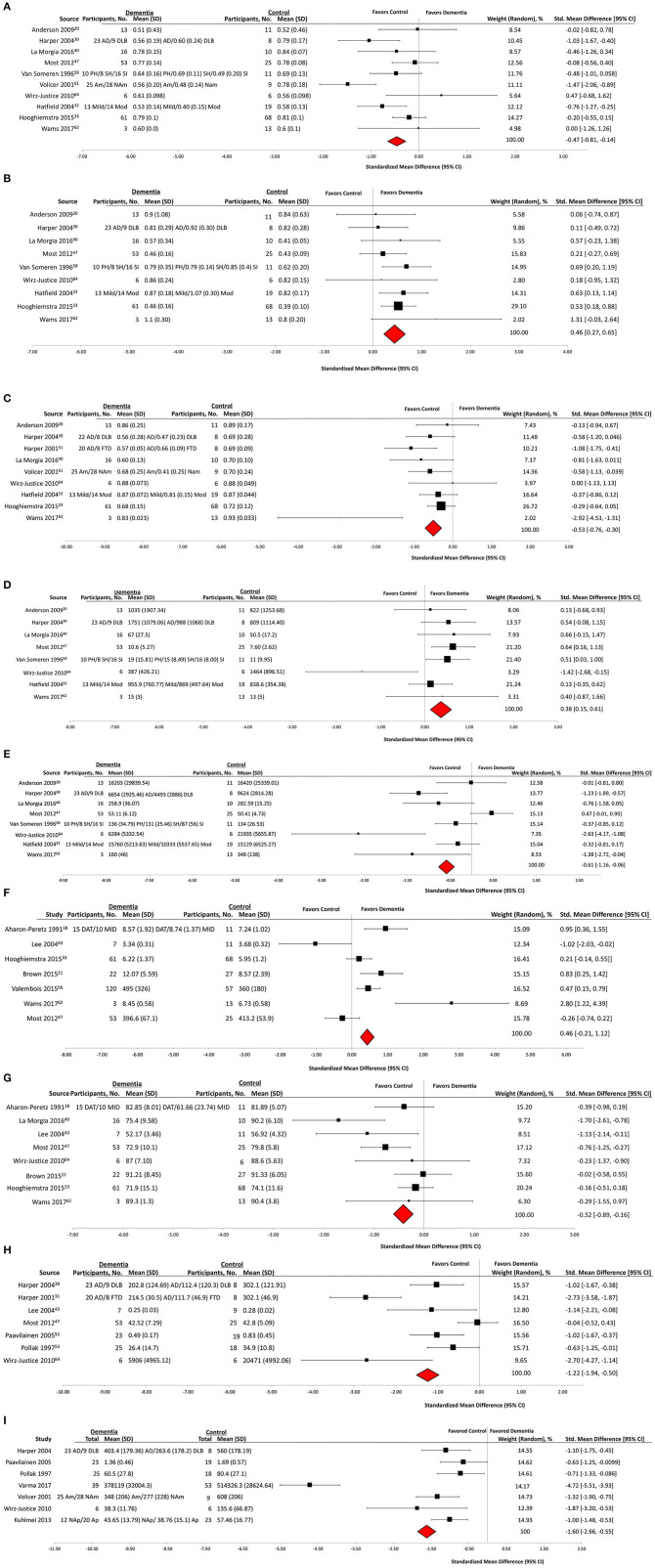
Of the 48 included studies, 23 (47%) groups reported outcome data on daily activity counts as measured by actigraphy. Qualitative analysis showed that activity counts were presented in a number of different ways (Table 3). Significant associations of activity counts with other measures, or differences in activity between individuals with dementia and control groups, were reported for the measures of daily activity (eight groups, 34%), peak daily activity (two groups, 8%), mean activity counts (five groups, 21%), daytime activity (five groups, 21%), night time activity (one group, 4%), number of immobile hours (one group, 4%), and activity patterns (three groups, 13%). Quantitative analysis demonstrated that participants with dementia had a significantly lower mean daytime activity counts compared to controls (mean difference, −1.60; 95% CI, −2.66 to −0.55) (Dementia: n = 210, Control: n = 136) (Figure 2I).
Table 3.
Specific outcome measures of daily activity reported by included studies.
| Source | Daily activity | Peak daily activity | Mean activity counts | Daytime activity | Nighttime activity | Immobile hours | Activity patterns |
|---|---|---|---|---|---|---|---|
| Aharon-Peretz et al. (7) | + | ||||||
| Ahmed et al. (10) | + | ||||||
| Carvalho-Bos et al. (17) | + | ||||||
| David et al. (19) | + | ||||||
| David et al. (20) | + | - | |||||
| Eggermont and Scherder (21) | + | - | |||||
| Ghali et al. (27) | + | ||||||
| Harper et al. (28) | + | – | |||||
| Harper et al. (29) | + | + | |||||
| James et al. (37) | + | ||||||
| Kuhlmei et al. (41) | + | ||||||
| Merrilees et al. (50) | + | ||||||
| Moyle et al. (53) | + | ||||||
| Mulin et al. (54) | + | ||||||
| Olsen et al. (53) | + | ||||||
| Paavilainen et al. (57) | + | ||||||
| Pollak and Stokes. (58) | + | – | |||||
| Rindlisbacher and Hopkins (60) | + | ||||||
| Wirz-Justice et al. (70) | + | ||||||
| Valembois et al. (62) | + | ||||||
| van Alphen et al. (63) | + | ||||||
| Volicer et al. (67) | + | ||||||
| Varma and Watts. (65) | + | + |
+ indicates a significant association or difference reported.
–indicates no significant association or difference reported.
Figure 2.
Actigraphy outcomes in observational case control studies of wearable technology. (A) Interdaily stability, (B) interdaily variability, (C) relative amplitude, (D) activity of least active 5 h, (E) Activity of most active 10 h, (F) total sleep time, (G) sleep efficiency, (H) amplitude, and (I) daytime activity.
Wearable Actigraphy for Sleep Derived Measures
Of the 48 included studies, 31 (64%) groups reported outcome data on sleep characteristics as measured by actigraphy (Table 4). Wake after sleep onset (WASO) was reported by 11 groups: six (55%) reported a significant association or difference in dementia subjects. Total sleep time (TST) was reported by 16 groups: nine (56%) reported a significant association or difference in dementia subjects. Sleep efficiency (SE) was reported by 12 groups: five (42%) reported a significant association or difference in dementia subjects. Participants with dementia had statistically significant lower mean sleep efficiency than controls (mean difference, −0.52; 95% CI, −0.89 to −0.16) (Dementia: n = 193, Control: n = 171) (Figure 2G), and no significant difference in mean total sleep time (mean difference, 0.46; 95% CI, −0.21 to 1.12) (Dementia: n = 291, Control: n = 212) (Figure 2F).
Table 4.
Specific outcome measures of sleep and circadian rhythm reported by included studies.
| Source | WASO | TST | SE | IV | IS | RA | M10 | L5 | Mesor | Acrophase | Amplitude |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aharon-Peretz et al. (7) | – | + | |||||||||
| Anderson et al. (14) | – | – | – | – | – | ||||||
| Brown et al. (16) | + | – | |||||||||
| Carvalho-Bos et al. (17) | + | + | + | + | + | ||||||
| Eggermont and Scherder (21) | + | + | + | ||||||||
| Fetveit and Bjorvatn (22) | + | + | – | – | – | – | |||||
| Gehrman et al. (26) | – | – | – | ||||||||
| Harper et al. (28) | – | + | – | + | + | + | + | + | |||
| Harper et al. (29) | + | + | – | + | + | + | + | ||||
| Hatfield et al. (30) | + | + | + | + | – | ||||||
| Hooghiemstra et al. (31) | + | + | – | + | – | + | |||||
| Kodama et al. (38) | + | + | + | ||||||||
| La Morgia et al. (43) | + | – | – | + | + | – | |||||
| Landolt et al. (45) | + | ||||||||||
| Lee et al. (46) | – | – | – | – | – | ||||||
| Leger et al. (47) | – | + | + | – | – | ||||||
| McCurry et al. (49) | – | ||||||||||
| Most et al. (52) | + | – | + | + | + | – | – | – | |||
| Mulin et al. (50) | + | – | |||||||||
| Murphy et al. (55) | + | ||||||||||
| Olsen et al. (56) | – | – | |||||||||
| Paavilainen et al. (57) | – | + | |||||||||
| Pollak and Stokes (58) | – | + | |||||||||
| van Someren et al. (64) | + | + | + | + | |||||||
| Viegas et al. (66) | + | + | |||||||||
| Volicer et al. (67) | + | + | – | ||||||||
| Wams et al. (68) | – | + | – | ||||||||
| Weissova et al. (69) | – | + | – | ||||||||
| Wirz-Justice et al. (70) | – | – | – | – | – | + | + | + | |||
| Yesavage et al. (71) | + | – | + | – | – | – |
+indicates a significant association or difference reported.
–indicates no significant association or difference reported.
IS, Interdaily stability; IV, Intradaily variability; L5, Activity of least active 5 h; M10, Activity of most active 10 h; RA, Relative amplitude; SE, Sleep efficiency; TST, Total sleep time; WASO, Wake after sleep onset.
Non-parametric Measurements of Circadian Rhythm Using Wearable Devices
Sixteen (33%) of 48 studies reported non-parametric measurements of circadian rhythm (Table 4). Qualitative analysis revealed that intradaily variability (IV) was reported by 13 groups: eight (61%) reported an association or difference in dementia groups. Interdaily stability (IS) was reported by 14 groups: nine (64%) reported an association or difference in dementia subjects. Relative amplitude (RA) was reported by 12 groups: seven (58%) reported an association or difference in dementia subjects. Activity of most active 10 h (M10) was reported by nine groups: seven (77%) reported an association or difference in dementia subjects. Activity of least active 5 h (L5) was reported by eight groups: four (50%) reported an association or difference in dementia groups.
Participants with dementia had significantly lower mean values than controls on IS (mean difference, −0.47; 95% CI, −0.81 to −0·14) (Dementia: n = 298, Control: n = 180) (Figure 2A), RA (mean difference, −0.53; 95% CI, −0.76 to −0.30) (Dementia: n = 237, Control: n = 152) (Figure 2C), and M10 (mean difference, −0.61; 95% CI, −1.16 to −0.06) (Dementia: n = 184, Control: n = 103) (Figure 2E) outcomes. Participants with dementia had statistically significantly higher mean values than controls on IV (mean difference, 0.46; 95% CI, 0.27–0.65) (Dementia: n = 245, Control: n = 171) (Figure 2B) and L5 (mean difference, 0.38; 95% CI, 0.15–0.61) (Dementia: n = 184, Control: n = 103) (Figure 2D) outcomes.
Cosinor Analysis of Circadian Rhythm Using Wearable Devices
Nine (19%) out of the 48 groups reported a cosinor analysis of circadian rhythm. Qualitative analysis (Table 4) showed that midline estimating statistic of rhythm (mesor) was reported by five groups: two (40%) reported a significant association or difference in dementia subjects. Amplitude of the cosinor wave was reported by 10 groups: five (50%) reported an association or difference in dementia subjects. Acrophase was reported by eight groups: two (25%) reported an association or difference in dementia subjects. Quantitative analysis was only performed on the amplitude of the cosinor wave. It revealed that subjects with dementia had a significantly lower mean amplitude than controls (mean difference, −1.22; 95% CI, −1.94 to −0.50) (Dementia: n = 174, Control: n = 93) (Figure 2H).
Wearable Actigraphy for Gait Derived Measures
Of the 48 included studies six (12%) groups reported outcome data on actigraphy to measure posture and gait characteristics (Table 5). Qualitative analysis showed that all six (100%) reported an association or difference in dementia subjects. These studies each reported a different measure of gait or walking activity, and thus a meta-analysis was not possible.
Table 5.
Specific outcome measures of gait and walking activity reported by included studies.
| Source | Gait | Gait speed | Walking speed | Cadence | Step variance | Dual tasking | Walking duration | Physical activity |
|---|---|---|---|---|---|---|---|---|
| Aharon-Peretz et al. (7) | + | |||||||
| Harper et al. (29) | + | |||||||
| Iwata et al. (36) | + | – | – | |||||
| La Morgia et al. (43) | + | |||||||
| Van Alphen et al. (63) | + | |||||||
| Volicer et al. (67) | + |
+, Indicates a significant association or difference reported.
–, Indicates no significant association or difference reported.
Risk of Bias Within Studies
Average rating of methodological quality of included studies was 15·54 points (SD = 1·47). The median and mode were both 16 points, with a range of 12–18 (Table 6).
Table 6.
Characteristics and major findings of included studies.
| Source | Study design | Participants (n) | Age, mean (SD or range), y | Major findings | Quality score† |
|---|---|---|---|---|---|
| Aharon-Peretz et al. (7)‡ | Prospective Case control |
MID (10) AD (15) Control (11) |
MID 75.9 (8.2) AD 72.8 (6.3) Control 69.0 (3.4) |
Groups with dementia demonstrated significant differences in sleep efficiency and total daily activity but not total sleep time. | 13 |
| Ahmed et al. (10) | Prospective case control | FTD (19) AD (13) Control (16) |
Not Stated | Decreased activity levels observed in dementia groups compared to controls. Increased stressed and resting heart rates in dementia groups compared to controls. | 17 |
| Anderson et al. (14)‡ | Prospective case control | FTD (13) Control (11) |
FTD 63.9 (8.8) Control 66.8 (5.7) |
Increase in nocturnal activity and decrease in morning activity in dementia group compared to controls. No significant overall difference in non-parametric analysis of circadian rhythm between dementia group and controls. | 18 |
| Brown et al. (16)‡ | Prospective cross sectional | DEM AC (22) Control (27) |
Not Stated | Less robust sleep wake rhythms, increased total sleep time, and increased time spent in bed in group with dementia but no difference in sleep efficiency as compared to participants without dementia. | 17 |
| Carvalho-Bos et al. (17) | Prospective cohort | AD (57) VaD (13) DEM AC (10) |
85.5 (5.9) | A lower level of cognitive functioning as measured by the MMSE and higher functional impairment were associated with a less stable rest-activity rhythm. | 17 |
| David et al. (19) | Prospective Case control |
AD (32) Control (15) |
AD 78.6 (7.4) Control 73.1 (6.0) | Lower activity levels in dementia group compared to controls. | 13 |
| David et al. (20) | Prospective cohort | AD (107) | AD 77.2 (6.7) | Participants with dementia and apathy had lower daytime activity levels than those without apathy. | 16 |
| Eggermont and Scherder (21) | Prospective cohort | DEM AC (76) | DEM AC 84.9 | No association between cognition and motor activity. | 17 |
| Fetveit and Bjorvatn (22) | Prospective cross sectional | DEM AC (23) | DEM AC 86.1 (7.0) | Consistent association between decreased cognition as measured by the MMSE and reduced activity level as well as fragmented sleep. | 18 |
| Fleiner et al. (24) | Prospective cross sectional | DEM AC (45) | DEM AC 79 (7) | Low activity levels observed with a wide range of activity patterns in groups with dementia. | 16 |
| Gehrman et al. (26) | Retrospective Cross sectional |
DEM AC (150) | DEM AC 84.1 (7.8) | No association between rest activity rhythm and severity of dementia as measured by the MMSE, but changes in circadian rhythm observed in those with dementia. | 16 |
| Ghali et al. (27) | Prospective cohort | AD (18) | AD 78.8 (6.4) | Time of nocturnal activity peak levels associated with duration of illness (measured in years) in groups with dementia. | 16 |
| Harper et al. (28)‡ | Prospective case control | AD (32) Control (8) |
AD 70.2 (1.0) Control 72.8 (2.1) |
Increasing AD pathology associated with greater disturbances in circadian activity. Difference in rest-activity between dementia and control groups. | 17 |
| Harper et al. (29)‡ | Prospective case control | DEM AC (38) Control (8) |
DEM AC 70.2 (1.0) Control 72.8 (2.1) |
Increased nocturnal activity with circadian phase delay observed in participants with AD compared to controls. | 15 |
| Hatfield et al. (30)‡ | Prospective cross sectional | AD (27) Control (19) |
AD 68.5 (60–82) Control 71.8 (1.2) |
Moderately demented participants show rest activity cycle disturbance when compared to controls. No correlation seen between severity of dementia as measured by the MMSE and rest-activity rhythm. | 14 |
| Hooghiemstra et al. (31)‡ | Prospective cross sectional | DEM AC (61) Control (68) |
DEM AC Median 62.5 Control Median 63.0 |
More rest-activity rhythm fragmentation, more time in bed, more time to transition from wake to sleep in those with early onset dementia than controls. | 15 |
| Ijmker and Lamoth (35) | Prospective case control | DEM AC (15) Control (26) |
DEM AC 81.7 (6.3) Control 70.6 |
Changes in gait acceleration in dementia compared to controls | 13 |
| Iwata et al. (36) | Prospective case control | DEM AC (14) Control (16) |
DEM AC 74.8 Control 73.7 |
Decreased physical activity in female subjects with dementia as compared to controls | 14 |
| James et al. (37) | Retrospective cross-sectional | DEM AC (70) Control (624) |
Not stated | Lower levels of total daily activity in subjects with dementia | 16 |
| Kodama et al. (38) | Prospective case control | DEM AC (52) Control (66) |
DEM AC 78.5 (10.7) Control 72.4 (6.7) |
Circadian rhythm parameters significantly differed in subjects with dementia compared to controls | 17 |
| König et al. (39) | Prospective case control | AD (23) Control (22) |
AD 77 (9) Control 73 (7) |
A difference in gait speed under dual task conditions was observed between dementia subjects and controls | 17 |
| Kuhlmei et al. (41)‡ | Retrospective cross-sectional | DEM AC (32) Control (23) | DEM AC 81 Control 78 |
Reduced daytime activity levels seen in subjects with dementia | 12 |
| La Morgia et al. (43)‡ | Prospective case control | AD (16) Control (10) |
AD 70.2 (10.2) Control 65.8 (7.5) |
Reduced sleep efficiency seen in subjects with AD as compared to controls | 14 |
| Lamoth et al. (44) | Prospective case control | AD (13) Control (13) |
AD 82.6 (4.2) Control 79.3 (5.5) |
Changes in gait variability in AD compared to controls | 16 |
| Landolt et al. (45) | Prospective case control | sCJD (7) | sCJD 65.8 (3.8) | High frequency of sleep wake changes seen in those with sCJD. | 15 |
| Lee et al. (46)‡ | Prospective case control | AD (7) Control (11) |
AD 77.0 (4.3) Control 74.2 (5.2) |
Mean phase difference (MESOR) was different between those with AD and controls. No significant change was seen in mean acrophase or mean amplitude of temperature. | 15 |
| Leger et al. (47) | Retrospective cross sectional | AD (208) | AD 73 (11.6) | Increased time spent in bed in those with moderate AD as measured by the MMSE compared to those with mild AD. | 16 |
| McCurry et al. (49) | Prospective cohort | AD (44) | AD 78.8 (7.2) | Significant variation seen in all sleep measures both between and within all subjects | 14 |
| Merrilees et al. (50) | Prospective cohort | FTD (22) | FTD 63.8 | In patients with FTD, apathy was associated with lower activity levels and greater number of bouts of immobility | 15 |
| Most et al. (52)‡ | Prospective case control | AD (55) Control (26) |
AD 70.4 (3.2) Control 73.0 (4.4) |
Longer sleep onset latency and decreased sleep efficiency was seen in subjects with AD compared to controls. | 15 |
| Moyle et al. (53) | Retrospective cross sectional | DEM AC (192) | DEM AC 85.5 (7.7) | No significant correlation seen between level of cognitive impairment as measured by the MMSE and activity and sleep patterns over 24 h. | 16 |
| Mulin et al. (54) | Prospective cohort | AD (103) | AD 76.9 (7.2) | Subjects with apathy demonstrated more time spent in bed during the night, and lower daytime motor activity than those without apathy. | 14 |
| Murphy et al. (55) | Retrospective cross-sectional | DEM AC (20) | DEM AC 78.7 (1.8) | Energy expenditure inversely related to time spent lying down and sleep duration. | 15 |
| Olsen et al. (56) | Retrospective cross-sectional | DEM AC (193) | DEM AC 83.6 | Decreased activity in nursing home subjects with dementia compared to home dwelling subjects with dementia. | 16 |
| Paavilainen et al. (57)‡ | Prospective case control | DEM AC (23) Control (19) |
DEM AC 84.3 (9.5) Control 81.5 (9.0) |
Subject with dementia demonstrated lower daytime and higher nocturnal activity than controls | 16 |
| Pollak and Stokes (58)‡ | Prospective case control | DEM AC (25) Control (18) |
DEM AC 80.7 (7.9) Control 73.7 (7.2) |
Less activity and flat cosine analysis of circadian rhythm in groups with dementia when compared to controls | 18 |
| Rindlisbacher and Hopkins (60) | Prospective cohort | AD (12) | AD 79.4 | Variability in 24-h peaks of activity correlated with years of illness | 16 |
| Schwenk et al. (61) | Prospective cohort | DEM AC (77) | DEM AC 81.8 (6.3) | Actigraph derived “walking bouts average duration” demonstrated a positive predictive value for future falls in subjects with dementia | 17 |
| Valembois et al. (62)‡ | Prospective cross sectional | DEM AC (126) Control (57) |
All Participants 84.9 (6.8) |
Decreased motor activity in subjects with dementia who demonstrate apathy and anxiety. No association between agitation and motor activity. | 15 |
| Van Alphen et al. (63) | Retrospective cross-sectional | DEM AC (146) | DEM AC 83.0 (7.6) | Increased sedentary levels and decreased physical activity levels in subjects with dementia who were institutional dwelling | 17 |
| van Someren et al. (64)‡ | Prospective case control | DEM AC (34) Control (11) |
DEM AC 74.7 Control 72 (1.2) |
Less stable rest-activity rhythm in institutionalized subjects with dementia compared to subjects cared for at home and controls | 15 |
| Varma and Watts (65)‡ | Prospective case control | AD (39) Control (53) |
AD 73.5 (7.9) Control 73.2 (6.5) |
Decreased physical activity and changes in activity patterns seen in dementia subjects compared to controls | 15 |
| Viegas et al. (66) | Retrospective cross sectional | DEM AC (104) | DEM AC 82.9 (8.4) | Average of 476min sleep per 24 h in subjects with dementia. | 18 |
| Volicer et al. (67)‡ | Prospective case control | AD (25) Control (9) |
AD 71.0 (60–88) Control 73.4 (67–83) |
A high percentage of nocturnal activity and less diurnal motor activity in subjects with AD compared to controls | 17 |
| Wams et al. (68)‡ | Retrospective cross sectional | AD (29) Control (14) |
AD 77.7 (7.6) Control 73.8 (4.6) |
AD patients demonstrated longer time in bed, longer sleep duration, and lower amplitude than controls. No difference between groups in sleep quality | 17 |
| Weissová et al. (69) | Prospective case control | AD (4) Control (4) |
Not stated | No difference in sleep parameters in participants with AD compared to controls | 16 |
| Wirz-Justice et al. (70)‡ | Prospective cross sectional | KP (6) Control (6) |
KP 66.8 Control Not Stated |
Longer nocturnal rest duration and lower daytime activity level in participants with KP compared to controls | 14 |
| Yesavage et al. (71) | Retrospective cross sectional | AD (61) | AD 71.4 (8.1) | AD participants show worsening in parameters of nocturnal sleep but no change in rest/activity circadian rhythm over time | 13 |
AD, Alzheimer's Disease; DEM AC, Dementia All Cause; FTD, Frontotemporal Dementia; KP, Korsakoff Psychosis; MID, Multi-Infarct Dementia; MMSE, Mini-Mental State Examination; sCJD, sporadic Creutzfeldt-Jakob Disease; SD, Standard Deviation; VaD, Vascular Dementia; y, Years.
Refers to endpoints reported by authors.
Study included in one or multiple meta-analyses.
Meta-Analysis and Heterogeneity
Low between study heterogeneity (I2 < 50%) was observed for analyses of IV, RA, and L5 variables (Table 7). Moderate to high between study heterogeneity (I2 > 50%) was observed for analyses of IS, TST, amplitude, M10, SE, and daytime activity. Meta-regression or subgroup analyses were performed for all actigraphy measures with a moderate to high heterogeneity (I2 > 50%) which included IS, TST, Amplitude, M10, SE, and daytime activity. Type of dementia, mean age, study design and quality score were all investigated as explanatory variables. Subgroup analyses indicate that effect estimates vary markedly between dementia subtypes for variables M10 and SE, suggesting differences in dementia type between studies may account for some of the heterogeneity observed in meta-analyses of M10 and SE measurements.
Table 7.
Combined effect estimates and heterogeneity for actigraphy outcomes between dementia and control samples.
| Actigraphy measure | Included studies, no. | Dementia subjects, no. | Healthy subjects, no. | Pooled mean difference, random-effects model (95% CI) | Q | I2 (95% CI) |
|---|---|---|---|---|---|---|
| IS | 10 | 298 | 180 | −0.47 (−0.81, −0.14)† | 24.86 (9 df)§ | 64 (29, 82) |
| IV | 9 | 245 | 171 | 0.46 (0.27, 0.65)† | 6.61 (8 df) | 0 (0, 65) |
| RA | 9 | 237 | 152 | −0.53 (−0.76, −0.30)† | 15.39 (8 df) | 48 (0, 76) |
| L5 | 8 | 184 | 103 | 0.38 (0.15, 0.61)§ | 11.21 (7 df) | 38 (0, 72) |
| M10 | 8 | 184 | 103 | −0.61 (−1.16, −0.06)§ | 30.32 (7 df)† | 77 (54, 88) |
| TST | 7 | 291 | 212 | 0.46 (−0.21, 1.12) | 30.31 (6 df)† | 80 (60, 90) |
| SE | 8 | 193 | 171 | −0.52 (−0.89, −0.16)§ | 15.44 (7 df)§ | 55 (0, 80) |
| Amplitude | 7 | 174 | 93 | −1.22 (−1.94, −0.50)‡ | 36.34 (6 df)† | 83 (67, 92) |
| Daytime Activity | 7 | 210 | 136 | −1.60 (−2.66, −0.55)§ | 81.82 (6 df)† | 93 (87, 96) |
df, degrees of freedom; IS, interdaily stability; IV, intradaily variability; I2, percentage of variation across studies due to heterogeneity; L5, activity of least active 5 h; M10, activity of most active 10 h; No., number; Q, Cochran's Q weighted sum of squares of effect size estimates subtracted by their mean; RA, relative amplitude; SE, sleep efficiency; TST, total sleep time.
p < 0.0001.
p < 0.001.
p < 0.05.
Risk of Publication Bias Across Studies for Meta-Analysis
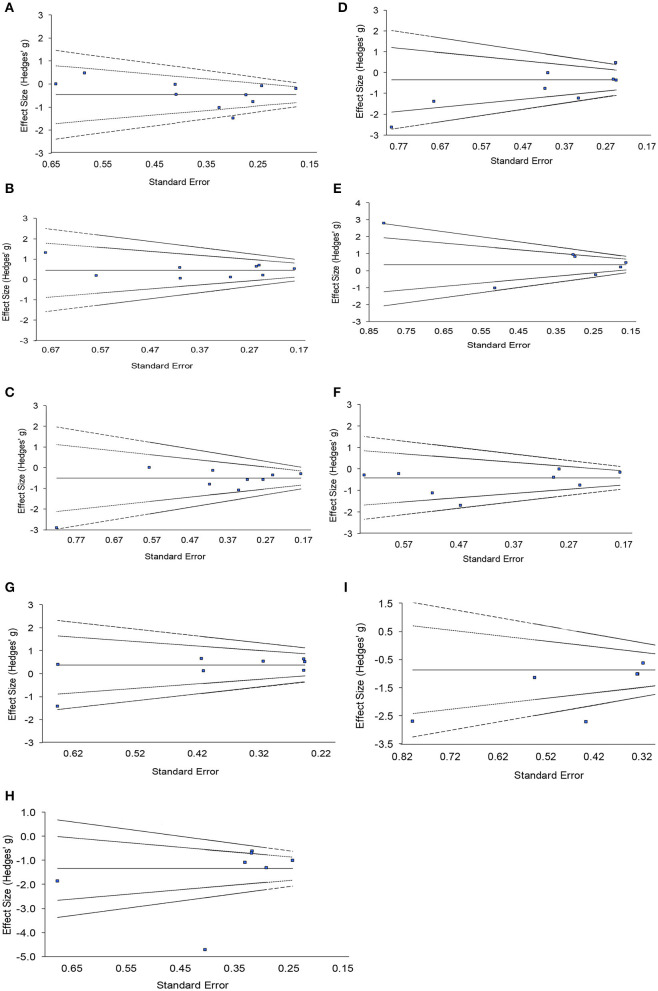
Funnel plots for each variable investigated using random effects meta-analysis are provided in Figure 3. These plots were constructed with a measure of study size on the x-axis and a measure of effect size on the y-axis. Dashed lines represent the pseudo 95 and 99.7% confidence limits about the effect estimate (solid line). Funnel plot asymmetry was observed for all but two variables (IV and RA), and significant Egger tests observed for M10 (p = 0.0057) and amplitude variables (p = 0.0078), suggesting evidence of publication bias for these measurements.
Figure 3.
Funnel plots with pseudo 95 and 99.7% confidence intervals assessing publication bias of included studies for nine actigraphy measures. (A) Interdaily stability, (B) intradaily variability, (C) relative amplitude, (D) activity of most active 10h, (E) total sleep time, (F) sleep efficiency, (G) activity of least active 5h, (H) daytime activity, (I) amplitude.
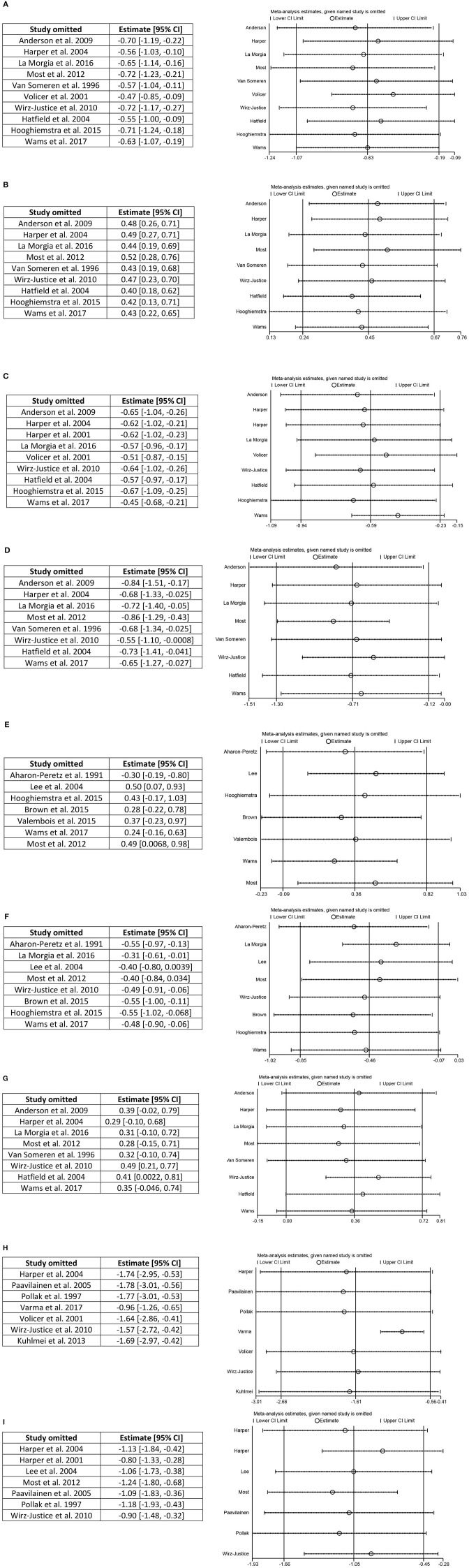
Investigation of Influential Studies
The impact of each study on a meta-analysis estimate was investigated through influence analysis. Influence analysis shows that meta-analysis estimates are generally robust (Figure 4), excluding meta-analysis of daytime activity, where the pooled estimate decreases in magnitude markedly and precision of the estimate improves with exclusion of Varma and Watts (65). Even with exclusion of this influential study, the pooled estimate remains significant and shows the same direction of effect as in the full meta-analysis.
Figure 4.
Influence analysis. (A) Interdaily stability, (B) intradaily variability, (C) relative amplitude, (D) activity of most active 10 h, (E) total sleep time, (F) sleep efficiency, (G) activity of least active 5 h, (H) daytime activity, and (I) amplitude.
Discussion
From our systematic review of the literature we found 48 articles which met our inclusion criteria of wearable technology use in patients with dementia for the measurement of physiological parameters. Wearable devices were utilized most extensively to measure circadian rhythm, measurement of the sleep wake cycle and daily activity. In the studies which were analyzed using forest plots, groups of participants with dementia were less active then controls, had a difference in their sleep wake cycle and showed differences in their circadian rhythms when compared to control groups. To our knowledge, this study is the first systematic review and meta-analysis of wearable device testing in participants with dementia.
Wearable Devices to Measure Sleep and Circadian Rhythm
The use of actigraphy to measure sleep was the most commonly reported outcome. Participants with dementia demonstrated reduced sleep efficiency as compared to controls. There was also a significant difference between individuals with dementia and controls on non-parametric measures of circadian rhythm including IV, IS, and RA, however it should be noted that for some measures the combined effects were substantially weighted by the results of Hooghiemstra et al. (31). Meta-analysis of the amplitude measure of circadian rhythm cosinor analysis also demonstrated a moderate but statistically significant difference between groups. Again, a high level of heterogeneity between studies was observed for this outcome measure. Despite evidence of the utility of wearable actigraphy in sleep monitoring, consistent outcome measures and methods of analyzing sleep data and circadian rhythm have not been universally agreed upon (2). In order for actigraphy to become routinely used in clinical and drug treatment trials, consistent outcome measures are needed and, as shown in this meta-analysis, may provide a useful endpoint for patients with dementia.
Wearable Devices and Daily Activity
When using wearable devices to measure daily activity, those with dementia had significantly lower daily activity counts than controls. This effect was demonstrated despite across-study variation in methods of calculating daytime activity including peak activity counts, mean activity, and daily activity. A meta-analysis of studies measuring daily activity showed that subjects with dementia demonstrate significantly less daily activity as compared to controls. Four groups reported no differences in nocturnal activity between subjects with dementia and controls. It should be noted that two of these studies did not recruit a control group, but instead compared participants with dementia to their caregivers [McCurry et al. (49) and Merrilees et al. (50)]. Physical activity has been examined in longitudinal studies and found to be associated with both development of dementia as well as disease progression (78). There is increasing evidence that physical activity and exercise as part of multi-domain interventions holds benefit for patients with dementia (79). However, as demonstrated in this review, definitions of physical activity differ significantly between studies and daily activity counts measured by wearable devices are not definite indicators of beneficial exercise, but merely of movement. Some researchers have attempted to quantify daily activity counts into variables such as energy expenditure, and this measure was also reduced in participants with dementia as compared to controls (55). With the growing availability of consumer wrist worn devices for movement and activity tracking, the use of daily activity measurements provides a potential novel end point for large scale clinical trials in dementia.
Wearable Devices and Gait
Analysis of gait behavior was studied by six groups. Significant differences between controls and those with dementia were reported by all groups for multiple aspects of the gait cycle and behavior. However, due to the variation in reported outcomes, a quantitative analysis could not be performed and conclusions regarding the use of wearable devices for the study of gait could not be reliably made. It is important to note that gait speed and walking speed were reported as significantly different in subjects with dementia when compared to controls, while cadence and step variance were not. Lower gait speed in particular has been shown in numerous longitudinal studies to correlate with increased fall risk in older adults (80). Further work to replicate these findings in subjects with dementia is warranted.
Limitations
The main limitation of the meta-analysis was the between-study heterogeneity (Table 7). Given differences in characteristics of study design such as duration of testing, wearable device type, and diagnosis, statistical heterogeneity was expected between publications included in each meta-analysis. Despite this, effect size comparisons between healthy volunteers and participants with dementia were generally consistent in direction between studies. Methodological considerations specifically for actigraphy testing in dementia have been more thoroughly addressed in a clinical review (81). Also, all papers included in this review corresponded to definitions of both all cause dementia and wearable devices which were agreed upon by the author group. As a result, studies which did not conform to these definitions have been excluded and the effect these may have had on the analysis cannot be quantified. Lastly not all devices used have been compared to gold—standard clinical testing and their methods of measurement may differ and therefore their reported differences should be interpreted with caution.
Conclusions and Implications
In conclusion this systematic review and meta-analysis has shown that the wearable devices studied demonstrate differences in those with dementia when compared to controls. Specifically, it provides evidence that wearable devices demonstrate a utility in measuring levels of activity, changes in circadian rhythm, and changes in the sleep wake cycle. Included studies were limited by their heterogeneity, the lack of classification of dementia sub-type and stage, as well as the lack of confirmatory clinical trials. Further work is warranted to correlate these findings with clinical changes which may represent surrogate digital end-points such as the neuro-psychiatric manifestations associated with circadian rhythm changes and the loss of mobility associated with decreased activity.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
JB: concept and design. JB, AC, and RP: acquisition, analysis, or interpretation of data, and drafting of the manuscript. JB, AC, RP, NK, and KT: critical revision of the manuscript for important intellectual content, administrative, technical, or material support. AC: statistical analysis. KT: obtained funding. JB and KT: supervision. AC had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Alissa Link, Education and Information Services Librarian, for her assistance in the construction of database search terms.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.501104/full#supplementary-material
References
- 1.Alzheimer's Disease International (ADI) . Dementia: a Public Health Priority. World Health Organization; (2013). [Google Scholar]
- 2.Shelgikar AV, Anderson PF, Stephens MR. Sleep tracking, wearable technology, and opportunities for research and clinical care. Chest. (2016) 150:732–43. 10.1016/j.chest.2016.04.016 [DOI] [PubMed] [Google Scholar]
- 3.Vasunilashorn S, Steinman BA, Liebig PS, Pynoos J. Aging in place: evolution of a research topic whose time has come. J Aging Res. (2012) 2012:1–6. 10.1155/2012/120952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espay AJ, Bonato P, Nahab F, Maetzler W, Horak F, Lang AE, et al. Technology in parkinson disease: challenges and opportunities. Mov Disord. (2017) 31:1272–82. 10.1002/mds.26642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Bruin ED, Hartmann A, Uebelhart D, Murer K, Zijlstra W. Wearable systems for monitoring mobility-related activities in older people: a systematic review. Clin Rehabil. (2008) 22:878–95. 10.1177/0269215508090675 [DOI] [PubMed] [Google Scholar]
- 6.McCleery J, Cohen DA, Sharpley AL. Pharmacotherapies for sleep disturbances in dementia. Cochrane Database Syst Rev. (2016) 11:CD009178. 10.1002/14651858.CD009178.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aharon-Peretz J, Masiah A, Pillar T, Epstein R, Tzischinsky O, Lavie P. Sleep-wake cycles in multi-infarct dementia and dementia of the Alzheimer type. Neurology. (1991) 41:1616–9. 10.1212/WNL.41.10.1616 [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd ed, revised. Washington, DC: (1987). [Google Scholar]
- 9.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. (1984) 34:939–44. 10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- 10.Ahmed RM, Landin-Romero R, Collet TH, van der Klaauw AA, Devenney E, Henning E, et al. Energy expenditure in frontotemporal dementia: a behavioural and imaging study. Brain. (2017) 140:171–83. 10.1093/brain/aww263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ, et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol. (2001) 58:1803–9. 10.1001/archneur.58.11.1803 [DOI] [PubMed] [Google Scholar]
- 12.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. (2011) 76:1006–14. 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. (2011) 134:2456–77. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson KN, Hatfield C, Kipps C, Hastings M, Hodges JR. Disrupted sleep and circadian patterns in frontotemporal dementia. Eur J Neurol. (2009) 16:317–23. 10.1111/j.1468-1331.2008.02414.x [DOI] [PubMed] [Google Scholar]
- 15.Neary D. Overview of frontotemporal dementias and the consensus applied. Dement Geriatr Cogn Disord. (1999) 10:6–9. 10.1159/000051205 [DOI] [PubMed] [Google Scholar]
- 16.Brown DT, Westbury JL, Schüz B. Sleep and agitation in nursing home residents with and without dementia. Int Psychogeriatrics. (2015) 27:1945–55. 10.1017/S1041610215001568 [DOI] [PubMed] [Google Scholar]
- 17.Carvalho-Bos SS, Riemersma-van der Lek RF, Waterhouse J, Reilly T, Van Someren EJ. Strong association of the rest – activity rhythm with well-being in demented elderly women. Am J Geriatr Psychiatry. (2007) 15:92–100. 10.1097/01.JGP.0000236584.03432.dc [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: (1994). [Google Scholar]
- 19.David R, Rivet A, Robert PH, Mailland V, Friedman L, Zeitzer JM, et al. Ambulatory actigraphy correlates with apathy in mild Alzheimer's disease. Dementia. (2010) 9:509–16. 10.1177/1471301210381678 [DOI] [Google Scholar]
- 20.David R, Mulin E, Friedman L, Le Duff F, Cygankiewicz E, Deschaux O, et al. Decreased daytime motor activity associated with apathy in Alzheimer disease: an actigraphic study. Am J Geriatr Psychiatry. (2012) 20:806–14. 10.1097/JGP.0b013e31823038af [DOI] [PubMed] [Google Scholar]
- 21.Eggermont LHP, Scherder EJA. Ambulatory but sedentary : impact on cognition and the rest – activity rhythm in nursing home residents with dementia. J Gerontol B Psychol Sci Soc Sci. (2008) 63:279–87. 10.1093/geronb/63.5.P279 [DOI] [PubMed] [Google Scholar]
- 22.Fetveit A, Bjorvatn B. Sleep duration during the 24-hour day is associated with the severity of dementia in nursing home patients. Int J Geriatr Psychiatry. (2006) 21:945–50. 10.1002/gps.1587 [DOI] [PubMed] [Google Scholar]
- 23.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. (1993) 43:2412–4. 10.1212/WNL.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 24.Fleiner T, Haussermann P, Mellone S, Zijlstra W. Sensor-based assessment of mobility-related behavior in dementia: feasibility and relevance in a hospital context. Int Psychogeriatr. (2016) 28:1687–94. 10.1017/S1041610216001034 [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization . The ICD-10 Classification of Mental and Behavioral Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization; (1997). [Google Scholar]
- 26.Gehrman P, Marler M, Martin JL, Shochat T, Corey-Bloom J, Ancoli-Israel S. The relationship between dementia severity and rest/activity circadian rhythms. Neuropsychiatr Dis Treat. (2005) 1:155–63. 10.2147/nedt.1.2.155.61043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghali LM, Hopkins RW, Rindlisbacher P. Ghali. 1995 Temporal shifts.pdf. Int J Geriatr Psychiatry. (1995) 10:517–21. 10.1002/gps.930100612 [DOI] [Google Scholar]
- 28.Harper DG, Stopa EG, McKee AC, Satlin A, Fish D, Volicer L. Dementia severity and lewy bodies affect circadian rhythms in Alzheimer disease. Neurobiol Aging. (2004) 25:771–81. 10.1016/j.neurobiolaging.2003.04.009 [DOI] [PubMed] [Google Scholar]
- 29.Harper DG, Stopa EG, Mckee AC, Satlin A, Patricia C, Goldstein R, et al. Differential circadian rhythm disturbances in men with alzheimer disease and frontotemporal degeneration. Arch Gen Psychiatry. (2001) 58:353–60. 10.1001/archpsyc.58.4.353 [DOI] [PubMed] [Google Scholar]
- 30.Hatfield CF, Herbert J, Van Someren EJW, Hodges JR, Hastings MH. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer's dementia. Brain. (2004) 127:1061–74. 10.1093/brain/awh129 [DOI] [PubMed] [Google Scholar]
- 31.Hooghiemstra AM, Eggermont LHP, Scheltens P, Van Der Flier WM, Scherder EJA. The rest-activity rhythm and physical activity in early-onset dementia. Alzheimer Dis Assoc Disord. (2015) 29:45–9. 10.1097/WAD.0000000000000037 [DOI] [PubMed] [Google Scholar]
- 32.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: (2013). [Google Scholar]
- 33.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, et al. Diagnosis and management of dementia with Lewy bodies —third report of the DLB consortium. Neurology. (2005) 65:1863–72. 10.1212/01.wnl.0000187889.17253.b1 [DOI] [PubMed] [Google Scholar]
- 34.Pohjasvaara T, Mäntylä R, Ylikoski R, Kaste M, Erkinjuntti T. Comparison of different clinical criteria (DSM-III, ADDTC, ICD-10, NINDS- AIREN, DSM-IV) for the diagnosis of vascular dementia. Stroke. (2000) 31:2952–7. [DOI] [PubMed] [Google Scholar]
- 35.IJmker T, Lamoth CJC. Gait and cognition: the relationship between gait stability and variability with executive function in persons with and without dementia. Gait Posture. (2012) 35:126–30. 10.1016/j.gaitpost.2011.08.022 [DOI] [PubMed] [Google Scholar]
- 36.Iwata A, Kowa H, Tsuji S. Monitoring daily life activity shows less activity among female dementia patients. Neurol Clin Neurosci. (2013) 1:91–5. 10.1111/j.2049-4173.2013.00029.x [DOI] [Google Scholar]
- 37.James BD, Boyle PA, Bennett DA, Buchman AS. Total daily activity measured with actigraphy and motor function in community-dwelling older persons with and without dementia. Alzheimer Dis Assoc Disord. (2012) 26:238–45. 10.1097/WAD.0b013e31822fc3cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kodama A, Kume Y, Tsugaruya M, Ishikawa T. Deriving the reference value from the circadian motor active patterns in the “non-dementia” population, compared to the “dementia” population: what is the amount of physical activity conducive to the good circadian rhythm. Chronobiol Int. (2016) 33:1056–63. 10.1080/07420528.2016.1196696 [DOI] [PubMed] [Google Scholar]
- 39.König A, Klaming L, Pijl M, Demeurraux A, David R, Robert P. Objective measurement of gait parameters in healthy and cognitively impaired elderly using the dual-task paradigm. Aging Clin Exp Res. (2017) 29:1181–9. 10.1007/s40520-016-0703-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. (2014) 13:614–29. 10.1016/S1474-4422(14)70090-0 [DOI] [PubMed] [Google Scholar]
- 41.Kuhlmei A, Walther B, Becker T, Müller U, Nikolaus T. Actigraphic daytime activity is reduced in patients with cognitive impairment and apathy. Eur Psychiatry. (2013) 28:94–7. 10.1016/j.eurpsy.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 42.Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular Dementia Diagnostic criteria for research studies: Report of the NINDS-AIREN International Workshop. Neurology. (1993) 43:250–60. 10.1212/WNL.43.2.250 [DOI] [PubMed] [Google Scholar]
- 43.La Morgia C, Ross-Cisneros FN, Koronyo Y, Hannibal J, Gallassi R, Cantalupo G, et al. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol. (2016) 79:90–109. 10.1002/ana.24548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamoth CJ, Van Deudekom FJ, Van Campen JP, Appels BA, De Vries OJ, Pijnappels M. Gait stability and variability measures show effects of impaired cognition and dual tasking in frail people. J Neuroeng Rehabil. (2011) 8:2. 10.1186/1743-0003-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landolt H, Glatzel M, Bla T, Achermann P, Roth C, Mathis J, et al. Sleep-wake disturbances in sporadic Creutzfeldt-Jakob disease. Neurology. (2006) 66:1418–24. 10.1212/01.wnl.0000210445.16135.56 [DOI] [PubMed] [Google Scholar]
- 46.Lee JH, Friedland R, Whitehouse PJ, Woo JI. Rhythms of sleep-wake cycle and temperature in alzheimer ' s disease. J Neuropsychiatr. (2004) 16:192–8. 10.1176/jnp.16.2.192 [DOI] [PubMed] [Google Scholar]
- 47.Leger D, Elbaz M, Dubois A, Rio S, Mezghiche H, Carita P, et al. Alzheimer's disease severity is not significantly associated with short sleep: survey by actigraphy on 208 mild and moderate alzheimer's disease patients. J Alzheimers Dis. (2016) 55:321–31. 10.3233/JAD-160754 [DOI] [PubMed] [Google Scholar]
- 48.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. [DOI] [PubMed] [Google Scholar]
- 49.McCurry SM, Pike KC, Vitiello MV, Logsdon RG, Teri L. Factors associated with concordance and variability of sleep quality in persons with Alzheimer's disease and their caregivers. Sleep. (2008) 31:741–8. 10.1093/sleep/31.5.741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merrilees J, Dowling GA, Hubbard E, Mastick J, Ketelle R, Miller BL. Characterization of apathy in persons wth frontotemporal dementia and the impact on family caregivers. Alzheimer Dis Assoc Disord. (2012) 00:1–6. 10.1097/WAD.0b013e3182471c54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration—a consensus on clinical diagnostic criteria. Neurology. (1998) 51:1546–54. 10.1212/WNL.51.6.1546 [DOI] [PubMed] [Google Scholar]
- 52.Most EIS, Aboudan S, Scheltens P, Van Someren EJW. Discrepancy between subjective and objective sleep disturbances in early- and moderate-stage alzheimer disease. Am J Geriatr Psychiatry. (2012) 20:460–7. 10.1097/JGP.0b013e318252e3ff [DOI] [PubMed] [Google Scholar]
- 53.Moyle W, Jones C, Murfield J, Draper B, Beattie E, Shum D, et al. Levels of physical activity and sleep patterns among older people with dementia living in long-term care facilities: A 24-h snapshot. Maturitas. (2017) 102:62–8. 10.1016/j.maturitas.2017.05.015 [DOI] [PubMed] [Google Scholar]
- 54.Mulin E, Zeitzer JM, Friedman L, Le Duff F, Yesavage J, Robert PH, et al. Relationship between apathy and sleep disturbance in mild and moderate Alzheimer's disease: An actigraphic study. J Alzheimers Dis. (2011) 25:85–91. 10.3233/JAD-2011-101701 [DOI] [PubMed] [Google Scholar]
- 55.Murphy J, Holmes J, Brooks C. Measurements of daily energy intake and total energy expenditure in people with dementia in care homes: the use of wearable technology. J Nutr Health Aging. (2017) 21:927–32. 10.1007/s12603-017-0870-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olsen C, Pedersen I, Bergland A, Enders-Slegers M-J, Jøranson N, Calogiuri G, et al. Differences in quality of life in home-dwelling persons and nursing home residents with dementia – a cross-sectional study. BMC Geriatr. (2016) 16:137. 10.1186/s12877-016-0312-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paavilainen P, Korhonen I, Lötjönen J, Cluitmans L, Jylhä M, Särelä A, et al. Circadian activity rhythm in demented and non-demented nursing-home residents measured by telemetric actigraphy. J Sleep Res. (2005) 14:61–8. 10.1111/j.1365-2869.2004.00433.x [DOI] [PubMed] [Google Scholar]
- 58.Pollak CP, Stokes PE. Circadian rest-activity rhythms in demented and nondemented older community residents and their caregivers. J Am Geriatr Soc. (1997) 45:446–52. 10.1111/j.1532-5415.1997.tb05169.x [DOI] [PubMed] [Google Scholar]
- 59.Mattis . Mental status examination for organic mental syndrome in the elderly patient. In: Karasu E. editor. Geriatric Psychiatry. New York, NY: Grune and Stratton; (1976). [Google Scholar]
- 60.Rindlisbacher P, Hopkins RW. An investigation of the sundowning syndrome. Int J Geriatr Psychiatry. (1992) 7:15–23. 10.1002/gps.930070104 [DOI] [Google Scholar]
- 61.Schwenk M, Hauer K, Zieschang T, Englert S, Mohler J, Najafi B. Sensor-derived physical activity parameters can predict future falls in people with dementia. Gerontology. (2014) 60:483–92. 10.1159/000363136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valembois L, Oasi C, Pariel S, Jarzebowski W, Lafuente-Lafuente C, Belmin J. Wrist actigraphy: a simple way to record motor activity in elderly patients with dementia and apathy or aberrant motor behavior. J Nutr Heal Aging. (2015) 19:759–64. 10.1007/s12603-015-0530-z [DOI] [PubMed] [Google Scholar]
- 63.Van Alphen HJM, Volkers KM, Blankevoort CG, Scherder EJA, Hortobágyi T, Van Heuvelen MJG. Older adults with dementia are sedentary for most of the day. PLoS ONE. (2016) 11:1–16. 10.1371/journal.pone.0152457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Someren EJ, Hagebeuk EE, Lijzenga C, Scheltens P, de Rooij SE, Jonker C, et al. Circadian rest-activity rhythm disturbances in Alzheimer's disease. Biol Psychiatry. (1996) 40:259–70. 10.1016/0006-3223(95)00370-3 [DOI] [PubMed] [Google Scholar]
- 65.Varma VR, Watts A. Daily physical activity patterns during the early stage of Alzheimer's disease. J Alzheimers Dis. (2017) 55:659–67. 10.3233/JAD-160582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Viegas SM, Richards KC, Beck CK, Lambert CW, O'Sullivan PS, Cole CS, et al. Predictors of daytime sleep of nursing home residents with dementia. J Am Psychiatr Nurses Assoc. (2006) 12:286–93. 10.1177/1078390306295071 [DOI] [Google Scholar]
- 67.Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A. Sundowning and circadian rhythms in alzheimer ' s disease. Am J Psychiatry. (2001) 158:704–11. 10.1176/appi.ajp.158.5.704 [DOI] [PubMed] [Google Scholar]
- 68.Wams EJ, Wilcock GK, Foster RG, Wulff K. Sleep-wake patterns and cognition of older adults with amnestic mild cognitive impairment (aMCI): a comparison with cognitively healthy adults and moderate alzheimer's disease patients. Curr Alzheimer Res. (2017) 14:1030–41. 10.2174/1567205014666170523095634 [DOI] [PubMed] [Google Scholar]
- 69.Weissová K, Bartoš A, Sládek M, Nováková M, Sumová A. Moderate changes in the circadian system of Alzheimer's disease patients detected in their home environment. PLoS ONE. (2016) 11:1–19. 10.1371/journal.pone.0146200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wirz-Justice A, Schröder CM, Gasio PF, Cajochen C, Savaskan E. The circadian rest-activity cycle in korsakoff psychosis. Am J Geriatr Psychiatry. (2010) 18:33–41. 10.1097/JGP.0b013e3181b0467a [DOI] [PubMed] [Google Scholar]
- 71.Yesavage JA, Friedman L, Kraemer HC, Noda A, Wicks D, Bliwise DL, et al. A follow-up study of actigraphic measures in home-residing Alzheimer's disease patients. J Geriatr Psychiatry Neurol. (1998) 11:7–10. 10.1177/089198879801100103 [DOI] [PubMed] [Google Scholar]
- 72.Lukowicz P, Kirstein T, Tröster G. Wearable systems for health care applications. Methods Inf Med. (2004) 43:232–8. 10.1055/s-0038-1633863 [DOI] [PubMed] [Google Scholar]
- 73.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Commun Health. (1998) 52:377–84. 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Routledge; (1988). Available from: https://www.taylorfrancis.com/books/9780203771587 (accessed September 8, 2018). [Google Scholar]
- 75.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1:97–111. 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 76.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Chichester: The Cochrane Collaboration and John Wiley & Sons Ltd. (2008). p. 1–649. 10.1002/9780470712184 [DOI] [Google Scholar]
- 77.Ioannidis JPA. Perfect study, poor evidence: interpretation of biases preceding study design. Semin Hematol. (2008) 45:160–6. 10.1053/j.seminhematol.2008.04.010 [DOI] [PubMed] [Google Scholar]
- 78.Rovio S, Kåreholt I, Viitanen M, Winblad B, Kivipelto M, Soininen NH, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. (2005). 4:705–11. 10.1016/S1474-4422(05)70198-8 [DOI] [PubMed] [Google Scholar]
- 79.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. (2015) 385:2255–63. 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 80.Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci. (2013) 68:39–46. 10.1093/gerona/gls174 [DOI] [PubMed] [Google Scholar]
- 81.Camargos EF, Louzada FM, Nóbrega OT. Wrist actigraphy for measuring sleep in intervention studies with Alzheimer's disease patients: application, usefulness, and challenges. Sleep Med Rev. (2013) 17:475–88. 10.1016/j.smrv.2013.01.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.