Abstract
Studies using whole blood platelet aggregometry as a laboratory research tool, provided important insights into the mechanism and modulators of platelet aggregation. Subsequently, a number of point-of-care (POC) platelet function tests (PFTs) were developed for clinical use, based on the concept that an individual’s thrombotic profile could be assessed in vitro by assessing the response to stimulation of platelet aggregation by specific, usually solo agonists such as adenosine diphosphate (ADP), collagen and thrombin. However, adjusting antiplatelet medication in order to improve the results of such POC PFTs has not translated into a meaningful reduction in cardiovascular events, which may be attributable to important differences between the POC PFT techniques and in vivo conditions, including patient-to-patient variability. Important limitations of most tests include the use of citrate-anticoagulated blood. Citrate directly and irreversibly diminishes platelet function and even after recalcification, it may result in altered platelet aggregation in response to ADP, epinephrine or collagen, and interfere with thrombin generation from activated platelets. Furthermore, most tests do not employ flowing blood and therefore do not assess the effect of high shear forces on platelets that initiate, propagate and stabilize arterial thrombi. Finally, the effect of endogenous thrombolysis, due to fibrinolysis and dislodgement, which ultimately determines the outcome of a thrombotic stimulus, is mostly not assessed. In order to accurately reflect an individual’s predisposition to arterial thrombosis, future tests of thrombotic status which overcome these limitations should be used, to improve cardiovascular risk prediction and to guide pharmacotherapy.
Keywords: Platelet aggregation, Platelet function test, Thrombosis, Fibrinolysis, Citrate, Shear
Highlights
Commonly used point-of-care platelet function tests have many limitations and do not accurately reflect the in vivo situation.
To assess overall thrombotic status, a global thrombotic stimulus (like high shear) should be used, rather than assessing the response to individual agonist(s) (ADP, collagen, thrombin).
Native blood is preferable to citrated blood, as the latter may alter platelet aggregation and thrombin generation.
Accurate thrombotic status assessment should also assess endogenous thrombolysis.
Tests of thrombotic status which overcome these limitations may better reflect physiology, improve cardiovascular risk prediction and help tailor pharmacotherapy.
Introduction
The discovery that platelet aggregation is inducible in vitro, and the development of an instrument to measure aggregation quantitatively, began a flourishing era in thrombosis research [1–3]. Platelets are highly reactive cells and aggregation can be induced by a variety of substances including chemicals, proteins, foreign surfaces and altered flow conditions. Among these, collagen, adenosine diphosphate (ADP) and thrombin-induced platelet aggregation generated greatest interest due to their physiological significance and potential use for the development of targeted therapies. As platelet aggregometry evolved, from testing platelet-rich plasma to whole blood, and replacement of a chart recorder with automated recording, it gained popularity in clinical research. While aggregometry has remained an extremely useful laboratory tool, point-of-care (POC) instruments such as the platelet function analyser (PFA-100/PFA-200; Siemens Diagnostic Deerfield, Illinois, USA), VerifyNow (Werfen, Barcelona, Spain), Plateletworks (Helena Laboratories Beaumont, Texas, USA), TEG the thrombelastography (TEG; Hemoscope Corporation, Niles, Illinois, USA), the rotational thromboelastometry (ROTEM; Werfen, Barcelona, Spain) [4–6], and more recently the Global Thrombosis Test (Thromboquest Ltd., London, UK) [7] have been increasingly used in clinical settings. Early POC techniques were considered highly pathologically-relevant, since the release of agonists, such as ADP, from activated platelets was regarded as the single most important mechanism initiating arterial thrombosis. However, having been used in a number of clinical studies for more than a decade, many early POC techniques have not found a use in routine clinical care and may be considered as research tools with limited clinical utility.[8, 9]. Despite strong evidence for an association between persistent high on-treatment platelet reactivity (HTPR) and an increased risk of adverse cardiovascular events, several large studies using POC techniques did not show more favourable clinical outcomes when antithrombotic treatment was either altered or titrated to reduce platelet aggregation using these techniques, and therefore POC platelet function tests (PFTs) are currently not recommended for routine clinical use [10–13]. In most such trials, POC PFTs did not improve clinical outcome compared to standard antiplatelet therapy without monitoring [14, 15]. More recently, POC PFT have been used to support de-escalation of antiplatelet therapy in patients with acute coronary syndrome (ACS). In elderly ACS patients, similar ischaemic and bleeding rates were observed whether patients were treated with conventional low dose prasugrel (5 mg/day), or with PFT-guided prasugrel escalation to 10 mg daily or de-escalation to clopidogrel [16]. Amongst patients with ACS treated with PCI, PFT-guided de-escalation from prasugrel to clopidogrel was non-inferior to standard prasugrel with respect to net clinical benefit (combined ischaemic and bleeding events) at 1 year [17]. However, these PFT-guided de-escalation studies were underpowered to assess safety with respect to major clinical events such as myocardial infarction and stent thrombosis, so this approach cannot be adopted into clinical care as assumed safe practice.
It is important to consider why POC-guided therapy failed to translate into a meaningful reduction in major adverse thrombotic events. [9, 18]. One can postulate that this was due to either trial design, assessment of low risk populations, variability in the HTPR definition and possible failure to adequately overcome HTPR. However, it is also possible that POC PFT lacked direct pathological relevance to arterial thrombosis in vivo (Table 1). This review will focus on pathologically-relevant issues pertaining to the assessment of arterial thrombosis and consider how future tests could be tailored to more accurately reflect clinical conditions, in order to improve the assessment of thrombotic risk and personalize pharmacotherapy.
Table 1.
Comparative features of point-of-care tests of platelet function
| Instrument | Manufacturer | Technique | Anticoagulant | Agonist used to stimulate thrombus formation | Assesses contribution of RBC/WBC | Flowing blood | Shear*/gradient | Assesses endogenous thrombolysis/fibrinolysis |
|---|---|---|---|---|---|---|---|---|
|
PFA-100 PFA-200 |
Siemens Healthcare GmbH, Erlangen, Germany | Measures closure time (occlusion of a coated membrane aperture) | Citrate |
Three assays with different agonist: Collagen/EPI assay, Collagen/ADP assay, and Innovance P2Y test |
No |
Yes Low flow |
Sub-pathologic ~ 5000 s−1 | No |
| VerifyNow | Werfen, Barcelona, Spain | Measures optical signal of light transmitted through whole blood which depends on the degree of platelet aggregation around fibrinogen coated beads | Citrate |
Three assays vary in platelet agonist: -Aspirin assay uses AA -P2Y12 assay uses ADP -Gp IIb/IIIa assay |
No | No | No | No |
| Plateletworks | Helena Laboratories Beaumont, Texas, USA | Measures platelet count before and after addition of platelet agonist | None in agonist tubes, but EDTA in reference tube | ADP, Collagen or AA agonists | No | No | No | No |
| Impact R | DANED SA, Beersel, Belgium | Cone and plate viscometry- Measures platelet inhibition and aggregation induced by extracellular matrix and very low shear stress | Citrate | Extracellular matrix | Yes | Yes | Sub-Pathologic ~ 1800 s−1 | No |
| Multiplate | Roche Diagnostics, Basel, Switzerland | Impedance aggregometry | Hirudin | ADP test, ASPI test (AA), TRAP test | No | No | No | No |
| TEG/ROTEM |
TEG; Haemonetics Corporation, Braintree, MA, USA ROTEM; Werfen, Barcelona, Spain |
Measurement of whole blood clotting (not platelet function), in response to rotation and measurement of viscoelastic clot characteristics reflected by changes in impedance | Citrate |
Low shear and external agonists (eg kaolin, ellagic acid, tissue factor) More akin to venous thrombosis/clotting than arterial thrombosis |
Yes | No | No shear 0.1 s−1 | Clot lysis, not thrombolysis or fibrinolysis |
| Global thrombosis test |
Thromboquest Ltd, London, UK |
Measurement of thrombus formation in response to high shear, and subsequent measurement of spontaneous thrombolysis | None | High shear only (no external agonists) | Yes | Yes | Pathologic ~ 12,000 s−1 | Yes |
AA arachidonic acid, ADP adenosine diphosphate, EDTA Ethylenediaminetetraacetic acid, EPI epinephrine, Gp glycoprotein, RBC red blood cells, TRAP thrombin receptor activating peptide, WBC white blood cells
*Pathological shear rate > 10,000 s−1
Determinants of arterial thrombosis
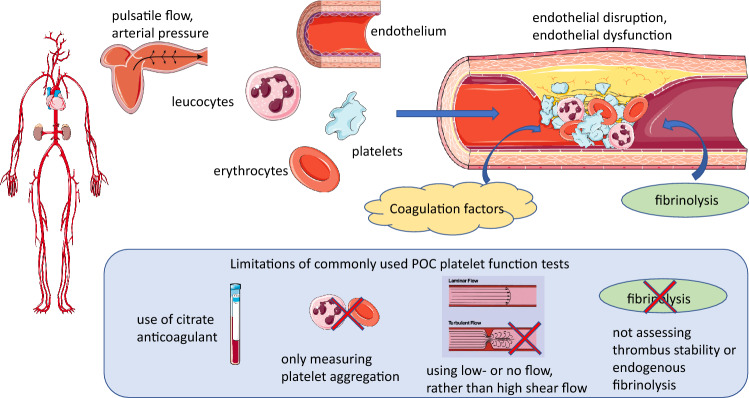
There are many components of arterial blood that determine thrombosis, some of which can be replicated in vitro (Fig. 1). Whilst thrombosis can clearly occur in an otherwise healthy vessel, the vast majority of local thrombus formation (in contrast to embolization) occurs in atherosclerotic arteries, with some degree of endothelial injury or dysfunction. Cellular components together with plasma proteases, specifically components of the coagulation and the fibrinolytic pathways, as well as the characteristics of blood flow impact on the development and stability of the growing thrombus. The ideal test to assess thrombosis should incorporate these features as much as possible, to simulate in vivo conditions.
Fig. 1.
Main pathophysiological determinants of arterial thrombosis and limitations of current point-of-care (POC) platelet function tests. Arterial thrombosis under pathological conditions is driven by shear gradient-mediated platelet aggregation and activation of coagulation, resulting in an occlusive fibrin mesh, in which entrapped erythrocytes and leucocytes make important contributions to thrombus stability, as well as fibrinolysis. Rheological flow characteristics and effectiveness of endogenous fibrinolysis determine thrombus stability and lysis. Important limitations of many POC PFTs include (i) use of citrate-anticoagulated blood, which even after recalcification, may result in impaired platelet aggregation and thrombin generation from activated platelets, (ii) not assessing the contribution of erythrocytes, leucocytes and NETs, (iii) use of stagnant conditions or laminar blood flow and therefore not assessing the effect of high shear gradient on platelets and (iv) failing to assess the effect of endogenous thrombolysis
Cellular components
Platelets are the predominant mediator of arterial thrombosis, through platelet activation, adhesion and aggregation. Platelets contribute to both coagulation and thrombolysis, mainly through thrombin generation and release of plasminogen activator inhibitor 1 (PAI-1). Leucocytes and erythrocytes are important mediators of thrombus stability, which impart both structural stability as well as mediate resistance to fibrinolysis. Neutrophils release proteases such as elastase and cathepsin G, which exert a plasmin-independent fibrinolytic effect, including inactivating the major plasmin inhibitor α2-PI [19]. Such proteases can directly alter platelet function and/or participate in coagulation cascade reactions on the platelet or neutrophil surface to enhance fibrin formation. In addition, neutrophils contribute to thrombosis through the formation of neutrophil extracellular traps (NETs), which promote thrombosis by providing a scaffold to entrap platelets, erythrocytes, and procoagulant molecules, as well as enhancing coagulation through the intrinsic and extrinsic pathways [19].
Coagulation and fibrinolytic pathways
Activation of coagulation is necessary for fibrin formation, which is essential for cross linking platelets within the thrombus, as well as the stimulation of fibrinolytic enzymes such as plasmin and antithrombin. Not only is it important to assess the pro-coagulant tendencies, but also to consider the effectiveness of endogenous thrombolytic/fibrinolytic pathways, which can determine the outcome of a pro-thrombotic stimulus [19].
Haemodynamic conditions
The characteristics of blood flow, including laminar flow, pulsatility, arterial pressure and local haemodynamic/shear forces, impact the development and stability of the thrombus. Rheological disturbances have an important role in promoting occlusive thrombus formation, as thrombus propagation and vessel occlusion tend to occur on plaques causing marked disturbance of laminar blood flow, rather than on non-stenotic lesions [20]. In severely stenotic arteries, pathological wall shear rate can exceed 10,000 s−1, and may reach 100,000–1 [21]. High shear forces that occur in areas of accelerated flow at the site of maximal stenosis potentiate platelet activation, whilst the turbulence in the low-flow, low-shear, deceleration zone in the post-stenotic region provides an ideal flow environment for the progressive accrual and aggregation of platelets. At the same time, faster flow through the stenosis as well as flow pulsation also favour thrombus embolization.
Endothelium
The healthy endothelium can exert antithrombotic effects, through release of prostacyclin and nitric oxide, potent inhibitors of platelet and monocyte activation. Endothelial cells can secrete tissue plasminogen activator, which breaks down fibrin, as well as von Willebrand factor (vWF), which can initiate platelet aggregation and contribute to blood coagulation [22]. While endothelial cells do not express much tissue factor (TF), they have important anticoagulant effects since they are the predominant source of TF pathway inhibitor (TFPI), as well as through surface expression of thrombomodulin, which binds thrombin, acting as a potent anticoagulant. Pathological arterial injury with plaque erosion or rupture results in expose of subendothelial contents including collagen, which activates the contact system, resulting in FXIa-driven thrombin generation, leading to platelet activation and TF that is a strong stimulator of the prothrombinase complex, thrombin generation and fibrin formation. Contact activation can also be induced by blood contact with negatively charged surfaces of biomaterials. Most patients at thrombosis risk exhibit endothelial dysfunction, which manifests in loss of protective molecules and expression of adhesive molecules, and increased secretion of PAI-1 and vWF.
Limitations of commonly used point-of-care PFTs
Advances in research in the past few decades have shed light on the mechanism of occlusive arterial thrombosis, revealing important new determinants that were previously unrecognised, including altered flow conditions at high shear rates (shear gradients), local thrombin generation by shear-activated platelets, the contribution of coagulation factors and NETs, and the importance of fibrin clot stability and fibrinolysis. This new knowledge has relegated to secondary significance the role of specific agonist-induced platelet aggregation in mediating thrombus formation. However, these newer determinants of thrombosis are not reflected in or assessed by many of the POC tests commonly in use in clinical settings.
Assessment of platelets function in whole blood
Whilst most tests use whole blood, some such as Platelet Works, the IMPACT: Cone and Plate(Let) Analyzer (Image Analysis Monitoring Platelet Adhesion Cone and Plate Technology) (CPA) (DANED SA, Beersel, Belgium) and Multiplate Analyzer (Roche, Basel, Switzerland) although employing whole blood, give results that reflect only platelet aggregation specific to a particular platelet agonist, and do not take account of the contribution of other blood constituents to thrombus formation.
Use of a single agonist to initiate platelet activation
Many physiologically-relevant agonists (such as ADP, epinephrine, collagen) can cause platelet activation, adhesion and aggregation both in vivo and in vitro. However, in a severely stenosed artery, it is shear-gradient dependent platelet aggregation mechanism which drives thrombus formation [23–25] while soluble agonists released from platelets play a secondary role, mainly in stabilizing formed aggregates. Despite this, the most commonly used tests such as the PFA and the VerifyNow still employ single agonists such as ADP or collagen to initiate platelet aggregation to determine the likelihood of arterial thrombosis, such as the PFA-100 and the VerifyNow. These tests do not reflect arterial thrombogenesis under high shear conditions.
Use of anticoagulant interferes with thrombus formation
Effect of citrate on ionized calcium concentration
Most POC PFTs in current clinical use are performed on anticoagulated blood. There are very few exceptions, notably the Global Thrombosis Test and Plateletworks, which employ native non-anticoagulated whole blood. Sodium citrate is the most commonly used anticoagulant for POC PFTs and for platelet aggregometry in platelet-rich plasma or in whole blood. Citrate acts by chelating extracellular ionized calcium (iCa2+), reducing the concentration in the blood sample from ~ 1.2 to ~ 0.1 mmol/L. Citrate anticoagulation is a very convenient way to handle blood samples, allowing storage for a few hours and transfer of samples from the patient to the laboratory for subsequent analysis. However, the “convenience” of anticoagulation comes at a price; namely the rendering of the blood sample highly non-physiological, such that it lacks relevance to the function of whole blood and platelets under thrombotic or haemorrhagic conditions in vivo. The serious drawbacks of citrate anticoagulation have been known for a long time, but there has been no viable alternative proposed and it has not been considered possible to overcome the logistic need for storage of blood prior to analysis. Citrate is added to blood in a fixed concentration and the final plasma iCa concentration after citrate is slightly above the threshold required for activation and aggregation of platelets without triggering blood coagulation. Citrate anticoagulation assumes that plasma iCa concentration in individuals is standard, but this is not the case [26]. Stratification of subjects based on iCa concentration identified that approximately 15% of subjects had a serum iCa above, 2% below, and only 69% had iCa within the normal range (1.12–1.30 mmol/L).[27] Sustained hyper- or hypocalcaemic disorders have significant physiological effects that can contribute to onset and progression of cardiovascular disorders or bleeding, respectively.
Considerable evidence shows that hypercalcaemia is associated with an increased risk of developing cardiovascular disease, arterial thrombosis and death [28–30]. As extracellular iCa concentration determines the rate of platelet reactivity to various agonists [31], variation in plasma iCa concentration results in highly variable aggregation response to agonists, especially if the agonist is applied in submaximal concentration [32]. Other factors can also influence plasma iCa [26]. The inverse relationship between serum iCa concentration and parathyroid hormone levels is well established. In patients receiving dual antiplatelet therapy, higher parathyroid hormone levels were associated with an increase in ADP-mediated platelet reactivity. Dietary factors such as citrate consumption can cause transient hypocalcaemia. It is therefore possible that measured platelet reactivity may vary with factors affecting plasma iCa concentration, rather than simply reflecting pharmacological resistance to antiplatelet therapy.[10, 33]
Effects of citrate on coagulation and thrombus stability
Citrate anticoagulation directly impairs coagulation dynamics [34] and the activation and adhesion of platelets and leukocytes in a concentration-dependent manner [35]. Extensive aggregation of human platelets in response to ADP, followed by secondary aggregation due to the release of platelet granule contents, occurs only in citrated blood. The main agent responsible for ADP-induced secondary aggregation and granule release appears to be sodium citrate [36, 37]. In citrated blood, the extent of aggregation did not change for up to 2 h after blood collection, but subsequently dramatically decreased. It was therefore recommended that platelet aggregation studies should be completed within 2 h of sampling, instead of the current practice of 4 h [38]. Relatively small increments in citrate concentration markedly inhibited platelet aggregation in response to ADP, epinephrine or collagen, and the effects of these agonists were much more marked in citrate-anticoagulated than in hirudin/PPACK-anticoagulated blood [37, 38]. Furthermore, the use of citrate anticoagulant significantly impaired platelet aggregation compared to anticoagulation with a direct thrombin inhibitor or unfractionated heparin, even when platelet aggregation was assessed immediately after blood draw; however with increasing sample storage, even direct thrombin inhibitors and heparin affected aggregation [39].
Observation of thrombus formation ex vivo in perfusion chambers showed that compared to native blood, citrate significantly inhibited platelet attachment kinetics, reduced adhesion, nearly abolished platelet aggregation and reduced thrombus stability [40]. Citrated blood did not reliably reflect fresh whole blood coagulability measured using thromboelastography [41–43]. Because of variations in calcium concentration, recalcification of citrate-anticoagulated blood does not yield a reliable depiction of natural blood coagulation dynamics [34, 44].
Perhaps the most important problem with use of citrate anticoagulation is that at the very low resultant plasma iCa concentration, activated platelets do not generate thrombin. Thrombin generation by activated platelets is one of the most important drivers of thrombus growth and stabilization. Since thrombin is the most potent platelet agonist, locally-generated thrombin accelerates platelet aggregation and through the formation of a stabilizing fibrin network, renders the thrombus resistant to dislodgement by arterial pressure. Plasma iCa concentration in citrated blood is ~ 0.1 mM while the threshold concentration of free calcium required for thrombin generation is 0.25 ± 0.05 mM [45], such that in citrate-anticoagulated blood, activated platelets do not generate thrombin. Variation in iCa concentration of citrate-anticoagulated plasma may be responsible for the high variation, low reproducibility and low sensitivity to coagulation deficiencies observed with thrombin generation tests [34, 44].
Slightly elevated or high-normal iCa concentrations (but still within the normal range) were independently associated with increased levels of fibrinogen and a higher risk of cardiovascular disease [46]. Furthermore, chelation of calcium leads to conformational changes in coagulation factors V and VIII, resulting in loss of procoagulant activity. Calcium is also required at many points within the coagulation cascade, the tenase and prothrombinase complexes to function, and also mediates the binding of FXa and FIXa to the phospholipid surface of platelets and may all be impacted by fluctuations caused by citrate and recalcification.
Alternative anticoagulants
The aforementioned adverse effects of citrate highlight the need to consider alternative anticoagulants in POC PFTs. However, heparin enhances primary aggregation and induces aggregation by potentiating platelet release reaction. Akin to that observed in citrated blood, platelets tend to clump in samples collected into unfractionated or low molecular weight heparin, precluding the use of these as anticoagulants in PFTs [47–49]. Because of the pivotal role of thrombin in thrombosis, the use of thrombin antagonists (hirudin, PPACK, direct thrombin inhibitors) would make any PFT highly nonphysiological and use of these anticoagulants is limited to research. Native, non-anticoagulated blood should therefore be used.
Static versus pathological flow conditions
Arterial flow and haemodynamic forces have dramatic impact on thrombus formation, coagulation and lysis of an arterial thrombus [21, 50]. In a severely stenotic artery, thrombus formation mainly results from shear-dependent platelet aggregation [23–25], with soluble agonists released from platelets playing a secondary role in stabilizing formed aggregates [51, 52]. By extending the range of shear at which thrombi are formed, shear gradients (stenoses) dramatically change platelet thrombi formation compared to constant base shear alone. Furthermore, individual healthy donors displayed quantifiable differences in extent/formation of thrombi within varying shear gradients [53].
The effect of high shear forces on platelets in initiating, propagating and stabilizing arterial thrombi is two-fold. At pathological shear rates (threshold > 10,000 s−1), conformational extension of vWF and its interaction with platelets initiates thrombus formation by causing an explosion of thrombus growth with rapid platelet aggregation, independent of activation [54, 55].The importance of high shear in initiating a thrombotic reaction in a severely stenotic artery is exemplified in a recent study which showed that amongst patients with > 70% luminal stenosis of the internal carotid artery, those with a shear rate in excess of 8000 s−1 were 12 times more likely to experience cerebrovascular ischemic events than those with lower shear rates [56].
In vivo or in extracorporeal flow chambers with circulating flow loops, the repeated exposure of platelets to lower shear rates (< 10,000 s−1) during recirculation, so called “platelet hammering”, can trigger persistent platelet aggregation. However, in ex vivo devices where platelets are exposed very briefly to high shear while passing through the stenotic region only once, stable aggregate formation requires exposure to much higher shear (> 20,000 s−1) [52].
Blood flow and haemodynamic forces also affect localized thrombin generation, fibrin formation and structure. Upon activation by collagen, thrombin or high shear, a subpopulation of platelets externalize the procoagulant phospholipid phosphatidylserine (PS) and release PS-exposed microvesicles, resulting in localized thrombin generation and fibrin formation [57, 58]. In contrast, platelet activation by ADP, vWF, or thromboxane A2 does not generate procoagulant platelets. Some studies report that platelet pretreatment with aspirin or a P2Y12 inhibitor has minimal effect on the generation of procoagulant platelets and their thrombin-generating potential [59]. In the absence of plaque rupture and abundance of TF, most thrombin generated in and around the growing thrombus is formed in situ by this mechanism. Localized thrombin-generation rate within the growing thrombus and on thrombogenic surfaces has been described as a function of shear stress/rate [59–61]. High thrombin concentrations produce dense networks of fibrin fibers, rendering the thrombus relatively resistant to fibrinolysis. In contrast, low thrombin concentrations produce coarse networks of fibrin fibers, and such thrombi are relatively susceptible to fibrinolysis [52].
The importance of testing blood under pathologically-relevant flow conditions is further reflected by the lack of effect of aspirin at high shear rates. Although the antiplatelet effects of aspirin are well recognized, at shear rates corresponding to an 80% arterial luminal stenosis, aspirin did not affect either the growth rate of thrombus or the thrombotic occlusion time [62, 63]. This is further exemplified by the observation that antiplatelet agents in routine clinical use have very limited efficacy in modulating the hypershear-mediated platelet activation that is associated with mechanical circulatory support [64]. Individuals with diabetes have heightened platelet reactivity, resulting in a pro-thrombotic state [65]. This is partly attributable to endothelial dysfunction, resulting in reduction in nitric oxide and prostacyclin synthesis and increased release of vWF. Hyperglycemia has been shown to enhance shear stress-induced platelet activation, which may explain why individuals with diabetes respond less well to standard antiplatelet therapy. It has been suggested that optimal monitoring of antiplatelet therapy using vWF/high shear PFT may provide a better guide to adjustment of antiplatelet treatment in diabetic individuals than other PFTs [66].
Most POC PFTs do not employ flowing blood under high shear or shear gradient, making these not only nonphysiological but notably, since platelet deposition onto reactive surfaces induced by shear gradients is not prevented by aspirin, clopidogrel or thrombin inhibitors so the effect of these drugs may be overestimated by low-shear tests [23]. Whilst some employ static conditions (VerifyNow, Multiplate, ROTEM/TEG), others employ slightly higher shear (such as the PFA), whilst some employ pathological high shear conditions (Global Thrombosis Test).
Assessment of endogenous fibrinolysis
The clinical outcome following an ACS is determined by the interplay between pro-thrombotic and endogenous thrombolytic activities in blood [19]. When the balance is altered in favour of platelet activation and/or coagulation, or if endogenous fibrinolysis becomes less efficient, thrombosis can occur, resulting in vessel occlusion [19, 52]. Assessment of the true global thrombotic status therefore requires measurement of not only platelet reactivity and coagulation, but also of the endogenous fibrinolytic activity. Recent evidence shows that endogenous fibrinolysis is significantly impaired in a number of patients with ACS and is a strong and independent predictor of recurrent adverse cardiovascular events [7, 67, 68].
The intrinsic clot lysis theory was put forward 60 years ago by Alkjaersig, Fletcher, and Sherry in 1959. As such, endogenous thrombolysis is regarded as an intrinsic process, where activators, inhibitors and modulators of fibrinolysis are already localized within the developing thrombus. Major factors distinguishing lysis of an arterial thrombus from plasma or whole blood clot lysis are arterial flow, the presence of platelets, locally-generated thrombin and adherent leukocytes. While it is fibrinolysis which plays the major role in endogenous thrombolysis, the stability of the thrombus in withstanding dislodgement by arterial flow [52] and clot retraction [52, 69] are also important determinants of lasting vessel occlusion.
Blood flow is necessary to accurately assess endogenous thrombolysis. Flow localizes plasminogen during thrombus formation [70] and promotes thrombolysis by enhancing the transport, binding and penetration of plasminogen activators into the thrombus core [71, 72]. Flow promotes accumulation and adherence of leucocytes in the thrombus, where they are key modulators of fibrin structure and lysis. Both monocytes and neutrophils can modulate the activity of the fibrinolytic pathway and impact the susceptibility of formed fibrin to fibrinolysis [73]. Neutrophils can cause hyperfibrinolysis by releasing the proteolytic enzyme elastase, but under different conditions (“fibrinolysis shutdown”) inhibit fibrinolysis by degrading plasminogen [74]. Platelets are the main modulators of thrombolysis [75, 76] by accumulating plasminogen from the circulation in the core of thrombus and supplying the major inhibitors PAI-1, alpha 2-antiplasmin, protease nexin-1 and thrombin activatable fibrinolysis inhibitor (TAFI). Thrombin generated locally by activated platelets plays the major role in the resistance of thrombus to lysis. In addition to determining the size of fibrin fibres with promotion of thin, less porous and more compact fibrin fibre network, locally-generated thrombin has a bidirectional function in endogenous fibrinolysis by facilitating fibrinolysis via inactivation of PAI-1 and through inhibition of fibrinolysis by inactivating pro-urokinase or activation of TAFI.
As the above factors are not present in the static systems commonly used to assess plasma or whole blood fibrinolysis, such tests are not relevant to in vivo endogenous thrombolysis and not suitable to assess endogenous thrombolytic potential in individual patients. Only a test which can form autologous, platelet-rich thrombi from native blood under ex vivo pathological flow conditions and detect the restoration of flow after occlusive thrombus formation can claim to have pathological relevance. Such animal models of thrombolysis have been used for some time in research [77] but the principle of these techniques did not manifest in POC test, although early data are promising with the Global Thrombosis Test [19, 52]. It is proposed that high shear/vWF-based tests may provide more useful information on adjusting antiplatelet therapy in individual patients with atherosclerosis.
The ideal point-of-care thrombosis test
Based on the importance of the need to reproduce a physiological milieu and the need to avoid the complications of anticoagulation, the ideal POC PFT should employ non-anticoagulated whole blood, which necessitates immediate assessment and avoidance of storage. The test should not only assess platelet activation and aggregation but reflect whole blood thrombus formation. To replicate arterial thrombosis, the test should employ conditions of high shear gradient, during which thrombin is the driving force for thrombus formation and also the main determinant of thrombus stability. Whilst the contribution of the endothelium is difficult to replicate, contact activation due to the artificial surfaces of PFTs contributes toward simulation of coagulation activation by endothelial disruption.
Whilst complex ex vivo laboratory models in use and in development, use of animal models of atherosclerosis, flow circuits, platelet-like particles or nanovesicles may better reflect arterial thrombosis than POC tests [78, 79], such techniques do not replace the need for a POC, clinically-friendly test of thrombosis.
The ideal test should employ blood that is flowing under constant arterial pressure, which is subjected to a site of pathological high shear gradient, that leads to occlusive thrombus formation. Such a test should not only assess thrombus growth, but also the susceptibility of the thrombus to endogenous thrombolysis, as well as stability and propensity to embolization.
Conclusion
Recognition of the importance of altered flow conditions, hemodynamic forces, procoagulant activity of platelets and endogenous thrombolytic activity is not reflected in most POC PFTs currently in mainstream use. Most tests still rely on specific agonist-induced platelet aggregation using citrate-anticoagulated blood under static conditions, while the significance of high flow, shear and locally-generated thrombin is ignored. To reflect physiological processes, POC tests should assess non-anticoagulated blood at high shear, to reflect and assess the dynamics of thrombus formation, platelet reactivity and thrombus stability, as well as the rate of endogenous thrombolysis of the occlusive thrombus. Future tests should incorporate all these features and physicians employing POC PFTs should chose the most physiological tests to best reflect the thrombotic and thrombolytic status of patients, and to better assess response to pharmacotherapy.
Abbreviations
- ACS
Acute coronary syndrome
- ADP
Adenosine diphosphate
- iCa
Ionized calcium concentration
- HTPR
High on-treatment platelet reactivity
- NETs
Neutrophil extracellular trapsV
- PAI-1
Plasminogen activator inhibitor
- PFT
Platelet function test
- POC
Point-of-care
- PS
Phospholipid phosphatidylserine
- TAFI
Thrombin-activatable fibrinolysis inhibitor
- TF
Tissue factor
- TFPI
Tissue factor pathway inhibitor
- vWF
Von Willebrand factor
Funding
This manuscript was not externally funded.
Compliance with ethical standards
Conflicts of interest
D.A.G. has received institutional research grants from Bayer and Astra Zeneca, and is a co-recipient of a research grant from Werfen. She has received honoraria/speaker’s bureau fees from Pfizer, Bayer and Astra Zeneca. She is related through family to a company director in Thromboquest Ltd, which manufactures the Global Thrombosis Test, but neither she, nor her spouse, nor children have financial involvement or equity interest in and have received no financial assistance, support, or grants from Thromboquest Ltd. R.C.B Becker has received support from National Institutes of Health grants R01-HL065222, U54-HL112307 and American Heart Association 14FRN24110000. He serves on scientific advisory boards for: Janssen and DSMB Committees for Ionis Pharmaceuticals, Akcea Therapeutics and Novartis.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien JR. Platelet aggregation: part I Some effects of the adenosine phosphates, thrombin, and cocaine upon platelet adhesiveness. J Clin Pathol. 1962;15:446–452. doi: 10.1136/jcp.15.5.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorog DA, Geisler T. Platelet Inhibition in acute Coronary syndrome and percutaneous coronary intervention: insights from the past and present. Thromb Haemost. 2020;1–14:565–578. doi: 10.1055/s-0040-1702920. [DOI] [PubMed] [Google Scholar]
- 4.Sweeny JM, Gorog DA, Fuster V. Antiplatelet drug “resistance”. Part 1: mechanisms and clinical measurements. Nat Rev Cardiol. 2009;6:273–282. doi: 10.1038/nrcardio.2009.10. [DOI] [PubMed] [Google Scholar]
- 5.Gorog DA, Sweeny JM, Fuster V. Antiplatelet drug “resistance”. Part 2: laboratory resistance to antiplatelet drugs-fact or artifact? Nat Rev Cardiol. 2009;6:365–373. doi: 10.1038/nrcardio.2009.13. [DOI] [PubMed] [Google Scholar]
- 6.Gorog DA, Fuster V. Platelet function tests in clinical cardiology: unfulfilled expectations. J Am Coll Cardiol. 2013;61:2115–2129. doi: 10.1016/j.jacc.2012.11.080. [DOI] [PubMed] [Google Scholar]
- 7.Farag M, Spinthakis N, Gue YX, et al. Impaired endogenous fibrinolysis in ST-segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention is a predictor of recurrent cardiovascular events: the RISK PPCI study. Eur Heart J. 2019;40:295–305. doi: 10.1093/eurheartj/ehy656. [DOI] [PubMed] [Google Scholar]
- 8.Lansky AJ, Ng VG (2016) Platelet function testing: a research tool with limited clinical utility (expert analysis). www.acc.org
- 9.Gorog DA, Jeong Y-H. Platelet function tests: why they fail to guide personalized antithrombotic medication. J Am Heart Assoc. 2015 doi: 10.1161/JAHA.115.002094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olędzki S, Kornacewicz-Jach Z, Safranow K, et al. Variability of platelet response to clopidogrel is not related to adverse cardiovascular events in patients with stable coronary artery disease undergoing percutaneous coronary intervention. Eur J Clin Pharmacol. 2017;73:1085–1094. doi: 10.1007/s00228-017-2271-x. [DOI] [PubMed] [Google Scholar]
- 11.Michelson AD, Bhatt DL. How I use laboratory monitoring of antiplatelet therapy. Blood. 2017;130:713–721. doi: 10.1182/blood-2017-03-742338. [DOI] [PubMed] [Google Scholar]
- 12.Senzel L, Ahmed T, Spitzer ED. Laboratory monitoring of platelet P2Y12 receptor inhibitors and reversal of antiplatelet agents: an ACLPS critical review. Am J Clin Pathol. 2018;152:1–6. doi: 10.1093/ajcp/aqy151. [DOI] [PubMed] [Google Scholar]
- 13.Angiolillo DJ. Dual antiplatelet therapy guided by platelet function testing. Lancet. 2017;390:1718–1720. doi: 10.1016/S0140-6736(17)32279-1. [DOI] [PubMed] [Google Scholar]
- 14.Collet J-P, Cuisset T, Rangé G, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. 2012;367:2100–2109. doi: 10.1056/NEJMoa1209979. [DOI] [PubMed] [Google Scholar]
- 15.Sinhal A, Aylward PE. New antiplatelet agents and the role of platelet function testing in acute coronary syndromes. Clin Ther. 2013;35:1064–1068. doi: 10.1016/j.clinthera.2013.07.429. [DOI] [PubMed] [Google Scholar]
- 16.Cayla G, Cuisset T, Silvain J, et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet. 2016;388:2015–2022. doi: 10.1016/S0140-6736(16)31323-X. [DOI] [PubMed] [Google Scholar]
- 17.Sibbing D, Aradi D, Jacobshagen C, et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet. 2017;390:1747–1757. doi: 10.1016/S0140-6736(17)32155-4. [DOI] [PubMed] [Google Scholar]
- 18.Messas N, Tanguay JF, Lordkipanidze M. Tailored antiplatelet therapy in high-risk ACS patients treated with PCI stenting: lessons from the ANTARCTIC trial. J Thorac Dis. 2017;9:E440–443. doi: 10.21037/jtd.2017.04.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varjú I, Kolev K. Networks that stop the flow: a fresh look at fibrin and neutrophil extracellular traps. Thromb Res. 2019;182:1–11. doi: 10.1016/j.thromres.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Gorog DA, Lip GYHR. Impaired spontaneous/endogenous fibrinolytic status as new cardiovascular risk factor? JACC review topic of the week. J Am Coll Cardiol. 2019;74:1366–1375. doi: 10.1016/j.jacc.2019.07.030. [DOI] [PubMed] [Google Scholar]
- 21.Falk E. Morphologic features of unstable atherothrombotic plaques underlying acute coronary syndromes. Am J Cardiol. 1989;63:E114–E120. doi: 10.1016/0002-9149(89)90242-7. [DOI] [PubMed] [Google Scholar]
- 22.Hathcock JJ. Flow effects on coagulation and thrombosis. Arterioscler Thrombosis Vasc Biol. 2006;26:1729–1737. doi: 10.1161/01.ATV.0000229658.76797.30. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Hao H, Leeper NJ, et al. Thrombotic regulation from the endothelial cell perspectives. Arterioscler Thromb Vasc Biol. 2018;38:e90–e95. doi: 10.1161/atvbaha.118.310367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nesbitt WS, Westein E, Tovar-Lopez FJ, et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15:665–673. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- 25.Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109:5087–5095. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- 26.Ruggeri ZM. The role of von Willebrand factor in thrombus formation. Thromb Res. 2007;120(Suppl 1):S5–9. doi: 10.1016/j.thromres.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peace AJ, Egan K, Kavanagh GF, et al. Reducing intra-individual variation in platelet aggregation: implications for platelet function testing. J Thromb Haemost. 2009;7:1941–1943. doi: 10.1111/j.1538-7836.2009.03593.x. [DOI] [PubMed] [Google Scholar]
- 28.Cho H, Lee S, Shin J, et al. Association of serum calcium concentrations with fibrinogen and homocysteine in nondiabetic Korean subjects. Medicine (Baltimore) 2016;95(24):e3899. doi: 10.1097/MD.0000000000003899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rohrmann S, Garmo H, Malmström H, et al. Association between serum calcium concentration and risk of incident and fatal cardiovascular disease in the prospective AMORIS study. Atherosclerosis. 2016;251:85–93. doi: 10.1016/j.atherosclerosis.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 30.de Moerloose P, Boehlen F, Neerman-Arbez M. Fibrinogen and the risk of thrombosis. Semin Thromb Hemost. 2010;36:7–17. doi: 10.1055/s-0030-1248720. [DOI] [PubMed] [Google Scholar]
- 31.Lind L, Skarfors E, Berglund L, et al. Serum calcium: a new, independent, prospective risk factor for myocardial infarction in middle-aged men followed for 18 years. J Clin Epidemiol. 1997;50:967–973. doi: 10.1016/s0895-4356(97)00104-2. [DOI] [PubMed] [Google Scholar]
- 32.Harfenist EJ, Packham MA, Kinlough-Rathbone RL, et al. Effect of calcium ion concentration on the ability of fibrinogen and von Willebrand factor to support the ADP-induced aggregation of human platelets. Blood. 1987;70:827–831. doi: 10.1182/blood.V70.3.827.827. [DOI] [PubMed] [Google Scholar]
- 33.Verdoia M, Pergolini P, Rolla R, et al. Parathyroid hormone levels and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with acetylsalicylic acid and clopidogrel or ticagrelor. Cardiovasc Ther. 2016;34:209–215. doi: 10.1111/1755-5922.12188. [DOI] [PubMed] [Google Scholar]
- 34.Linden M, Tran H, Woods R, et al. High platelet reactivity and antiplatelet therapy resistance. Semin Thromb Haemost. 2012;38:200–2012. doi: 10.1055/s-0032-1301417. [DOI] [PubMed] [Google Scholar]
- 35.Mann KG, Whelihan MF, Butenas S, Orfeo T. Citrate anticoagulation and the dynamics of thrombin generation. J Thromb Haemost. 2007;5:2055–2061. doi: 10.1111/j.1538-7836.2007.02710.x. [DOI] [PubMed] [Google Scholar]
- 36.Weiss R, Fischer MB, Weber V. The impact of citrate concentration on adhesion of platelets and leukocytes to adsorbents in whole blood lipoprotein apheresis. J Clin Apheresis. 2017;32:375–383. doi: 10.1002/jca.21519. [DOI] [PubMed] [Google Scholar]
- 37.Mustard JF, Perry DW, Kinlough-Rathbone RL, Packham MA. Factors responsible for ADP-induced release reaction of human platelets. Am J Physiol. 1975;228:1757–1765. doi: 10.1152/ajplegacy.1975.228.6.1757. [DOI] [PubMed] [Google Scholar]
- 38.Glusa E, Markwardt F. Platelet functions in recombinant hirudin-anticoagulated blood. Haemostasis. 1990;20:112–118. doi: 10.1159/000216116. [DOI] [PubMed] [Google Scholar]
- 39.Germanovich K, Femia EA, Cheng CY, et al. Effects of pH and concentration of sodium citrate anticoagulant on platelet aggregation measured by light transmission aggregometry induced by adenosine diphosphate. Platelets. 2018;29:21–26. doi: 10.1080/09537104.2017.1327655. [DOI] [PubMed] [Google Scholar]
- 40.Kalb ML, Potura L, Scharbert G, Kozek-Langenecker SA. The effect of ex vivo anticoagulants on whole blood platelet aggregation. Platelets. 2009;20:7–11. doi: 10.1080/09537100802364076. [DOI] [PubMed] [Google Scholar]
- 41.Baumgartner HR, Turitto V, Weiss HJ. Effect of shear rate on platelet interaction with subendothelium in citrated and native blood. II. Relationships among platelet adhesion, thrombus dimensions, and fibrin formation. J Lab Clin Med. 1980;95:208–221. [PubMed] [Google Scholar]
- 42.Gilman EA, Koch CD, Santrach PJ, et al. Fresh and citrated whole-blood specimens can produce different thromboelastography results in patients on extracorporeal membrane oxygenation. Am J Clin Pathol. 2013;140:165–169. doi: 10.1309/AJCPYIQ9JNNSEN4Q. [DOI] [PubMed] [Google Scholar]
- 43.Silverberg E, Tornqvist F, Kander T, et al. Comparison of citrated and fresh whole blood for viscoelastic coagulation testing during elective neurosurgery. Thromb Res. 2017;156:73–79. doi: 10.1016/j.thromres.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 44.Roche AM, James MF, Grocott MP, Mythen MG. Citrated blood does not reliably reflect fresh whole blood coagulability in trials of in vitro hemodilution. Anesth Analg. 2003;96:58–66. doi: 10.1097/00000539-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Parunov LA, Surov SS, Liang Y, et al. Can the diagnostic reliability of the thrombin generation test as a global haemostasis assay be improved? The impact of calcium chloride concentration. Haemophilia. 2017;23:466–475. doi: 10.1111/hae.13174. [DOI] [PubMed] [Google Scholar]
- 46.Ataullakhanov FI, Pohilko AV, Sinauridze EI, Volkova RI. Calcium threshold in human plasma clotting kinetics. Thromb Res. 1994;75:383–394. doi: 10.1016/0049-3848(94)90253-4. [DOI] [PubMed] [Google Scholar]
- 47.Lam V, Dhaliwal S, Mamo JC. (2013) Adjustment of ionized calcium concentration for serum pH is not a valid marker of calcium homeostasis: implications for identifying individuals at risk of calcium metabolic disorders. Ann Clin Biochem. 2013;50:224–229. doi: 10.1177/0004563212473747. [DOI] [PubMed] [Google Scholar]
- 48.Kingston JK, Bayly WM, Sellon DC, et al. Effects of sodium citrate, low molecular weight heparin, and prostaglandin E1 on aggregation, fibrinogen binding, and enumeration of equine platelets. Am J Vet Res. 2001;62:547–554. doi: 10.2460/ajvr.2001.62.547. [DOI] [PubMed] [Google Scholar]
- 49.Nelles NJ, Chandler WL. Platelet mapping assay interference due to platelet activation in heparinized samples. Am J Clin Pathol. 2014;142:331–338. doi: 10.1309/AJCPQ9BYJ0OEENGA. [DOI] [PubMed] [Google Scholar]
- 50.Sugawara E, Shimizu M, Yamamoto M, et al. Influence of platelet aggregate formation in blood samples on light transmission aggregometry results. J Stroke Cerebrovasc Dis. 2019;28:1001–1006. doi: 10.1016/j.jstrokecerebrovasdis.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 51.Brass LF, Diamond SL. Transport physics and biorheology in the setting of hemostasis and thrombosis. J Thromb Haemost. 2016;14:906–917. doi: 10.1111/jth.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nergiz-Unal R, Cosemans JMEM, Feijge MA, et al. Stabilizing role of platelet P2Y(12) receptors in shear-dependent thrombus formation on ruptured plaques. PLoS ONE. 2010;5:e10130. doi: 10.1371/journal.pone.0010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorog DA, Fayad ZA, Fuster V. Arterial thrombus stability: does it matter and can we detect it? J Am Coll Cardiol. 2017;70:2036–2047. doi: 10.1016/j.jacc.2017.08.065. [DOI] [PubMed] [Google Scholar]
- 54.Lui M, Gardiner EE, Arthur JF, et al. Novel stenotic microchannels to study thrombus formation in shear gradients: influence of shear forces and human platelet-related factors. Int J Mol Sci. 2019 doi: 10.3390/ijms20122967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casa LDCD, Ku DN. Thrombus formation at high shear rates. Annu Rev Biomed Eng. 2017;19:415–433. doi: 10.1146/annurev-bioeng-071516-044539. [DOI] [PubMed] [Google Scholar]
- 56.Ruggeri ZM, Orje JN, Habermann R, et al. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood. 2006;108:1903–1910. doi: 10.1182/blood-2006-04-011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hariri N, Russell T, Kasper G, Lurie F. Shear rate is a better marker of symptomatic ischemic cerebrovascular events than velocity or diameter in severe carotid artery stenosis. J Vasc Surg. 2019;69:448–452. doi: 10.1016/j.jvs.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 58.Pang A, Cui Y, Chen Y, et al. Shear-induced integrin signaling in platelet phosphatidylserine exposure, microvesicle release, and coagulation. Blood. 2018;132:533–543. doi: 10.1182/blood-2017-05-785253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Didelot M, Docq C, Wahl D, et al. Platelet aggregation impacts thrombin generation assessed by calibrated automated thrombography. Platelets. 2018;29:156–161. doi: 10.1080/09537104.2017.1356452. [DOI] [PubMed] [Google Scholar]
- 60.Yin W, Bond K, Rouf F, Rubenstein DA. altered flow changes thrombin generation rate of circulating platelets. Ann Biomed Eng. 2015;43:2827–2837. doi: 10.1007/s10439-015-1346-z. [DOI] [PubMed] [Google Scholar]
- 61.Diquélou A, Lemozy S, Dupouy D, et al. Effect of blood flow on thrombin generation is dependent on the nature of the thrombogenic surface. Blood. 1994;84:2206–2213. doi: 10.1182/blood.V84.7.2206.2206. [DOI] [PubMed] [Google Scholar]
- 62.Nagy M, Heemskerk JWMW, Swieringa F. Use of microfluidics to assess the platelet-based control of coagulation. Platelets. 2017;28:441–448. doi: 10.1080/09537104.2017.1293809. [DOI] [PubMed] [Google Scholar]
- 63.Li M, Hotaling NA, Ku DN, Forest CR. Microfluidic thrombosis under multiple shear rates and antiplatelet therapy doses. PLoS ONE. 2014;9:e82493. doi: 10.1371/journal.pone.0082493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barstad RM, Orvim U, Hamers MJ, et al. Reduced effect of aspirin on thrombus formation at high shear and disturbed laminar blood flow. Thromb Haemost. 1996;75:827–832. doi: 10.1055/s-0038-1650374. [DOI] [PubMed] [Google Scholar]
- 65.Valerio L, Sheriff J, Tran PL, et al. Routine clinical anti-platelet agents have limited efficacy in modulating hypershear-mediated platelet activation associated with mechanical circulatory support. Thromb Res. 2018;163:162–171. doi: 10.1016/j.thromres.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gresele P, Guglielmini G, De Angelis M, et al. Acute, short-term hyperglycemia enhances shear stress-induced platelet activation in patients with type II diabetes mellitus. J Am Coll Cardiol. 2003;41(6):1013–1020. doi: 10.1016/s0735-1097(02)02972-8. [DOI] [PubMed] [Google Scholar]
- 67.Westein E, Hoefer T, Calkin AC. Thrombosis in diabetes: a shear flow effect? Clin Sci. 2017;131:1245–1260. doi: 10.1042/CS20160391. [DOI] [PubMed] [Google Scholar]
- 68.Sumaya W, Wallentin L, James SK, et al. Fibrin clot properties independently predict adverse clinical outcome following acute coronary syndrome: a PLATO substudy. Eur Heart J. 2018;39:1078–1085. doi: 10.1093/eurheartj/ehy013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saraf S, Christopoulos C, Salha IB, et al. Impaired endogenous thrombolysis in acute coronary syndrome patients predicts cardiovascular death and nonfatal myocardial infarction. J Am Coll Cardiol. 2010;55:2107–2115. doi: 10.1016/j.jacc.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 70.Samson AL, Alwis I, Maclean JAAA, et al. Endogenous fibrinolysis facilitates clot retraction in vivo. Blood. 2017;130:2453–2462. doi: 10.1182/blood-2017-06-789032. [DOI] [PubMed] [Google Scholar]
- 71.Whyte CS, Swieringa F, Mastenbroek TG, et al. Plasminogen associates with phosphatidylserine-exposing platelets and contributes to thrombus lysis under flow. Blood. 2015;125:2568–2578. doi: 10.1182/blood-2014-09-599480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rijken D. Sakharov DV (2000) Molecular transport during fibrin clot lysis. Fibrinolysis Proteolysis. 2000;14(98–113):72. [Google Scholar]
- 73.Sakharov D, Sakharov DV. The effect of flow on lysis of plasma clots in a plasma environment. Thromb Haemost. 2000;83:469–474. doi: 10.1055/s-0037-1613838. [DOI] [PubMed] [Google Scholar]
- 74.Swystun LL, Liaw PC. The role of leukocytes in thrombosis. Blood. 2016;128:753–762. doi: 10.1182/blood-2016-05-718114. [DOI] [PubMed] [Google Scholar]
- 75.Barrett CD, Moore HB, Banerjee A, et al. Human neutrophil elastase mediates fibrinolysis shutdown through competitive degradation of plasminogen and generation of angiostatin. J Trauma Acute Care Surg. 2017;83:1053–1061. doi: 10.1097/TA.0000000000001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okafor ON, Gorog DA. Endogenous fibrinolysis an important mediator of thrombus formation and cardiovascular risk. J Am Coll Cardiol. 2015;65:1683–1699. doi: 10.1016/j.jacc.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 77.Kim PY. Platelets: connecting clotting and lysis. Blood. 2015;125:2459. doi: 10.1182/blood-2015-03-632158. [DOI] [PubMed] [Google Scholar]
- 78.Gold HK, Yasuda T, Jang IK, et al. Animal models for arterial thrombolysis and prevention of reocclusion. Erythrocyte-rich versus platelet-rich thrombus. Circulation. 1991;83:426–440. [PubMed] [Google Scholar]
- 79.Becker RC, Sadayappan S. Designing human in vitro models for drug development. J Am Coll Cardiol. 2020;75:587–589. doi: 10.1016/j.jacc.2019.12.013. [DOI] [PubMed] [Google Scholar]